
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 30 March 2022
Sec. Gynecological Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.809159
This article is part of the Research Topic Women in Gynecological Oncology: 2021 View all 34 articles
Objectives: To assess the risk factors of lymph node metastasis (LNM) in patients with FIGO stage (2009) IB1 cervical cancer (CC).
Methods: Patients with FIGO stage IB1 CC who underwent radical resection between 2012 and 2018 were recruited. The risk factors for LNM were analysed. A recursive partitioning analysis (RPA) was used to divide the patients into risk groups and assess their risk of LNM.
Results: The 5-year overall survival rate was 91.72%, while 80.0% and 93.5% for patients with or without LNM (P<0.05). Multivariable logistic regression analysis showed that lymphovascular invasion (LVI), depth of invasion (DI), tumour size (TS), squamous cell carcinoma (SCC) antigen level were independent risk factors (all P<0.05). Patients were divided into low-risk (no LVI, DI <1/2, TS <2 cm), intermediate-risk (no LVI, DI <1/2, TS ≥2 cm; no LVI, DI ≥1/2, normal SCC level; LVI, DI <1/2, TS <2 cm), and high-risk (no LVI, DI ≥1/2, SCC level ≥1.5 ng/ml; LVI, TS <2 cm, DI ≥1/2; LVI, TS ≥2 cm) groups by RPA according to these four factors. The incidence of LNM among the three groups was 0.00%, 4.40%, and 24.10%, respectively (all P<0.001). The 5-year overall survival rates differed among the groups (98.2%, 92.7%, 83.0%, respectively, P=0.001).
Conclusions: LNM affects the prognosis of patients with FIGO stage IB1 CC. Lymphadenectomy may be avoided for patients in the low-risk group and recommended for those in the high-risk group. Whether dissection is performed in the intermediate-risk group depends on the lymph node biopsy results.
Cervical cancer (CC), the most common gynaecologic malignancy in women worldwide, has the highest incidence and mortality in China and threatens women’s lives and health (1, 2). Lymph node metastasis (LNM) is a critical risk factor for the survival of patients with CC (3, 4). The survival in patients with LNM is obviously worse than that of patients without LNM in the same International Federation of Gynecology and Obstetrics (FIGO) stage. The 5-year overall survival (OS) rate is 90% for early-stage CC without versus less than 50% with LNM (5). According to the 2019 National Comprehensive Cancer Network (NCCN) guideline, radical hysterectomy and pelvic lymph node dissection ± para-aortic lymph node dissection are the standard surgical treatment for FIGO IB1 CC patients without fertility requirements. Fertility-sparing surgery (Radical trachelectomy and pelvic lymph node dissection ± para-aortic lymph node dissection) for stage IB1 has been most validated for tumors ≤2 cm (6). Nevertheless, some patients experience sequelae after lymphadenectomy, such as vascular or nerve injury, pelvic lymphocysts, and lower-limb lymphedema, which can be life-threatening, increase length of hospital stay, and affect quality of life (7–9). The incidence of LNM in early-stage CC is reportedly 15–20% (10, 11) and even lower in FIGO stage IB1 patients at 7–17.4% (12–14). Therefore, exploring the risk factors of LNM in FIGO stage IB1 and classifying patients into different groups to avoid the risk caused by lymph node dissection for low-risk patients is of great clinical significance. Current predictive models for the simultaneous assessment of LNM risk in patients with FIGO stage IB1 CC have not been reported. Therefore, this study explored the available factors for LNM in FIGO stage IB1 CC and stratified the risk of LNM based on recursive partitioning analysis (RPA) to provide a reference for the selection of surgical treatment for early CC.
A total of 284 patients with CC who underwent radical resection at Fujian Provincial Maternity and Children’s Hospital between January 2012 and December 2018 were recruited. The following inclusion criteria were applied: (1) histologically confirmed CC; (2) FIGO stage IB1 with no evidence of tumours invading adjacent organs or distant metastasis; (3) having undergone radical hysterectomy with pelvic lymphadenectomy; and (4) patients had completed fertility or without fertility requirements. The risk of surgery had been fully informed and all patients signed informed consent before operation and required radical hysterectomy. The study excluded patients who had distant metastases in the liver, lung, or peritoneum/pelvic cavity diagnosed before or during the surgery, those who underwent preoperative neoadjuvant radiotherapy and/or chemotherapy, or those whose medical records were incomplete/inaccurate. FIGO staging criteria (2009) were used for tumour staging. According to the postoperative pathological examination results, supplementary chemotherapy, radiotherapy, or concurrent chemoradiotherapy should be performed in cases of high-risk factors such as worse pathological differentiation degree, positive pelvic LNM, parametrial involvement, deep muscle layer infiltration, positive lymphovascular invasion (LVI), or positive surgical resection margins.
A postoperative follow-up assessment was performed every 3 months for 2 years and then every 6 months during years 3–5. Most routine follow-up appointments included a physical examination, vaginal examination, laboratory testing (including cancer antigen 125 (Ca125) and squamous cell carcinoma (SCC) antigen), chest radiography, and pelvic ultrasonography. Lung computed tomography or pelvic magnetic resonance imaging was performed when necessary. OS was defined as the time from surgery to death of any cause or to the time of censoring on the date of the last follow-up. The final follow-up evaluation was conducted in December 2020. The median follow-up period was 54.3 (range, 6.4–97.1) months.
The chi-square test or Fisher’s exact probability method was used to compare the classified variables. A t-test or the Mann-Whitney U test was used to analyse differences in numerical variables. Survival curves were constructed according to the Kaplan–Meier method, and differences between curves were analysed using the log-rank test. Variables with values of P<0.05 on univariate analysis were subjected to a multivariate logistic regression analysis. According to those results, recursive partitioning analysis (RPA) was used to divide the patients into different risk groups. The groups with a similar incidence of LNM were reintegrated into a single risk group. Finally, the low-risk, intermediate-risk, and high-risk groups were determined with the incidence of LNM increasing in turn. The statistical analyses were performed using SPSS for Windows version 26.0 (SPSS Inc., Chicago, IL, USA) and R x64 ver. 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria). All tests were two-sided, and statistical significance was determined at values of P<0.05.
A total of 284 patients were included in the study. Their clinicopathological features are presented in Table 1. The incidence of LNM was 8.4%. The median patient age was 46 (range, 24–66) years. A body mass index (BMI) of <24 and ≥24 was observed in 188 (66.2%) and 96 (33.8%) cases, respectively. Squamous, adenoma, and adenosquamous carcinomas were observed in 214 (75.4%), 59 (20.8%), and 11 (3.9%) cases, respectively. Invasion depths of <1/2 and ≥ 1/2 of the stroma were observed in 168 (59.2%) and 116 (40.8%) cases, respectively. There were 77 (27.1%) and 207 (72.9%) cases of positive LVI versus no LVI, respectively. There were 178 (62.7%) and 106 (37.3%) patients with a tumour size (TS) <2 cm and ≥2 cm, respectively. There were 163 (57.4%) and 121 (42.6%) cases with a normal or high Ca125 level, respectively. There were 187 (65.8%) and 97 (34.2%) cases of a normal and high SCC level, respectively (Table 1).
The median follow-up time was 54.3 (range, 6.4–97.1) months. The 5-year OS rate of all patients and in those with and without LNM were 91.72%, 80.0%, and 93.5%, respectively (P=0.009) (Figure 1).
The univariate analysis showed that TS, depth of invasion (DI), LVI, SCC antigen level, and Ca125 level were associated with LNM (all P<0.05) (Table 1). In the multivariate logistic regression analysis, TS (odds ratio (OR), 0.351; 95% confidence interval (CI), 0.123–0.992; P=0.044), DI (OR, 0.205; 95% CI, 0.054–0.772; P=0.019), LVI (OR, 0.281; 95% CI, 0.100–0.785; P=0.015), SCC antigen level (OR, 0.338; 95% CI, 0.123–0.924; P=0.035) were independent factors for LNM (Table 2).
Based on the results of the multivariable analysis, RPA using the four independent risk factors was performed to classify the patients into different risk groups. The group was divided into subgroups according to the R software prioritisation of the binary variables. Finally, the patients were reclassified into nine groups. Patients with a similar incidence of LNM were merged. The patients were ultimately divided into low-risk, intermediate-risk, and high-risk groups (Figure 2). In the model, there were 110 low-risk patients (38.7%) (no LVI, DI <1/2, TS <2 cm), 91 intermediate-risk patients (32.1%) (no LVI, DI <1/2, TS ≥2 cm; no LVI, DI ≥1/2, normal SCC antigen level; LVI, DI <1/2, TS <2 cm), and 83 high-risk patients (29.2%) (no LVI, DI ≥1/2, SCC antigen level ≥1.5 ng/mL; LVI, TS <2 cm, DI ≥1/2; LVI, TS ≥2 cm).
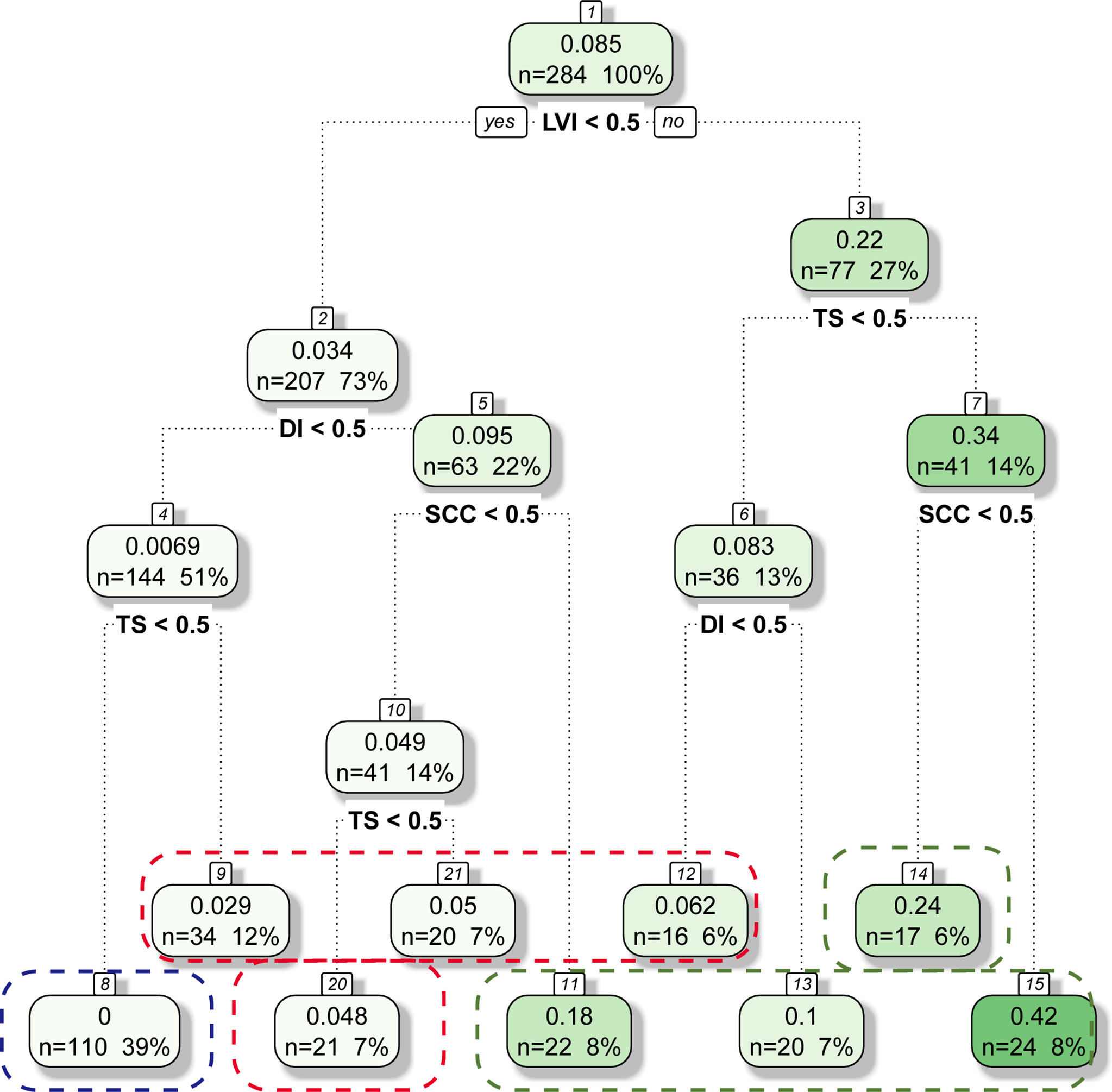
Figure 2 Classification Tree for LNM Status. Blue line, Red line, Green line represents Low-Risk Group, Intermediate-Risk Group and High-Risk Group, respectively.
The incidence of LNM was 0.00%, 4.40%, and 24.10% in the low-risk, intermediate-risk, and high-risk groups, respectively (all P<0.001; Figure 3). The 5-year OS rates were 98.2%, 92.7%, and 82.0%, respectively, which were also significantly different (P=0.001; Figure 4).
CC is a common gynaecologic malignant tumour for which postoperative recurrence and metastasis are the main causes of death (15, 16). Patients with early-stage CC always have a better prognosis after surgery (2) but worse prognosis when proven to be metastatic. LNM, as the main mode of metastasis in patients with CC, is a high-risk factor for recurrence and greatly impacts treatment and prognosis (3, 4). LNM was also formally included in the FIGO staging system in 2018 (17). Therefore, pelvic lymph node dissection is important. According to the NCCN, the standard surgical treatment is radical hysterectomy and pelvic lymphadenectomy in patients with FIGO stage IB1 CC without fertility requirements (6). However, some patients experience sequelae from this procedure (such as vascular or nerve injury, pelvic lymphocyst, and lower-limb lymphedema), which may increase the length of hospital stay and affect quality of life (7–9). In addition, the rate of LNM in FIGO stage IB1 patients was 7–17.4% (12–14). Lymphadenectomy may not have a survival benefit, but it may increase the incidence of postoperative complications in LNM-negative patients. LNM-positive patients not treated with lymphadenectomy may experience recurrence within a short period postoperative. Therefore, it is of great significance to explore the risk factors influencing LNM and conduct a risk assessment in patients with FIGO stage IB1 CC.
This study showed an 8.4% (24/284) rate of LNM in patients with FIGO stage IB1 CC, a finding that is in accordance with those of other studies (12–14). The 5-year OS rate in patients with LNM was significant lower than patients without LNM. As the patients were selected from January 2012 to December 2018 before the results of LACC trails (18), a certain proportion of patients underwent radical hysterectomy via laparoscopy. However, the results showed no prognostic differences between patients via laparoscopy or not (Supplementary Figure 1). This result needed further confirmation in future studies. The rate of LNM also showed no significant difference between the open and laparoscopic surgery in the study. In addition, with the development of technology, robotic surgery has been gradually applied in clinical. Previous studies showed the equivalence of robotic and laparoscopic approaches to radical surgery of early CC patients (19), so did the salvage lymphadenectomy (20). Therefore, it also may be a good choice in clinical practice in the future.
The multivariable logistic regression analysis showed that LVI, DI, TS, and SCC antigen level were independent risk factors, which is also consistent with previous reports (4, 21–23). LVI is closely associated with LNM in CC. Invasion into the space between lymphatic endothelial cells is an indispensable step for the metastasis of cancer cells; thus, it often predicts a poor prognosis. The larger the tumour diameter and the deeper the musculature invasion, the more likely tumour cells will invade the intravascular system, and thus the more likely the development of LNM. The SCC antigen is a specific serum tumour marker for CC that was first discovered by Kato in 1977 (24). The higher the SCC antigen level, the more aggressive the tumour and the higher the probability of LNM (25, 26). However, previous studies (4, 21) analysed only the independent risk factors affecting LNM without further risk grouping, resulting in some limitations in determining whether lymphadenectomy should also be performed. This study was the first to report a risk stratification by RPA based on these factors, and it provides a reference for whether lymphadenectomy should be performed simultaneously in patients with early-stage CC.
RPA is a statistical method for multivariable analyses that divides groups into subgroups according to the priority of several binary independent variables to correctly classify the members of a group. As a result, a concise decision tree is generated intuitively to determine decision rules with higher sensitivity and specificity (27). This method is widely used in medical decision-making. The RPA was first used by Goldman to establish a decision tree for the diagnosis of patients with acute chest pain in 1982 (28). It was also used to group patients with acute decompensated heart failure by Fonarow (29). In this study, the rate of LNM was 0.00% in patients with no LVI, a DI <1/2, and a TS <2 cm (low-risk group) according to the RPA. The negative LVI, less tumor size and less depth of invasion meant the less invasion in the intravascular system and parametrial involvement, leading to the less possibility of LNM (21–23, 30). Lymphadenectomy may be avoided in these patients to reduce postoperative complications. The LNM rate was as high as 24.10% in high-risk patients, including: those with no LVI, a DI ≥ 1/2, and an SCC antigen level ≥1.5 ng/mL; those with LVI, a TS <2 cm, and a DI ≥1/2; and those with LVI and a TS ≥2 cm. Therefore, simultaneous lymphadenectomy is recommended.
Sentinel lymph node biopsy (SLNB) has recently been used in patients with early-stage CC. Compared to pelvic lymph node dissection, SLNB reduces the incidence of postoperative complications and improves quality of life without affecting the survival prognosis (31–34). SLNB could be applied in the intermediate-risk group (no LVI, DI <1/2, TS ≥2 cm; no LVI, DI ≥1/2, normal SCC level; LVI, DI <1/2, TS <2 cm) as the rate of LNM was 4.40%. Whether to perform simultaneous lymphadenectomy could be determined based on the SLNB results. In addition, the LNM rate differed significantly between the three groups here and also on the survival analysis, indicating that risk grouping based on these four risk factors has a certain reference significance in clinical decision-making.
This study had several limitations. First, it was retrospective in design, which inevitably involves data selection bias. Second, the research was conducted in a single centre with small case numbers, and our findings require further confirmation in prospective multicentre studies. However, to our knowledge, this study is the first to explore the risk factors affecting LNM in patients with FIGO stage IB1 CC. Furthermore, the RPA was used to classify the risk groups to make the model more clinically useful. Based on these findings, we have proposed recommendations for lymphadenectomy for different risk groups with early CC, which will aid surgeons make better clinical decisions, showing the important clinical significance of our study.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The present study was approved by the Ethics Committee of the Fujian Provincial Maternity and Children’s Hospital. Written informed consent was obtained from all participants. The patients/participants provided their written informed consent to participate in this study.
MX and XX designed, conceived this study, and wrote the paper. YX and QG contributed to the literature search and collect the data. LC was involved in data extraction and analyzed the data. PS revised the paper. All authors have approved the final edition of the manuscript.
This study was funded by Fujian Provincial Maternity and Children’s Hospital Natural Science Foundation (Grant No. YCXM 20–03).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.809159/full#supplementary-material
Supplementary Figure 1 | The survival between laparoscopic surgery and open surgery.
1. Song B, Ding C, Chen W, Sun H, Zhang M, Chen W. Incidence and Mortality of Cervical Cancer in China, 2013. Chin J Cancer Res (2017) 29(6):471–6. doi: 10.21147/j.issn.1000-9604.2017.06.01
2. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2018. CA Cancer J Clin (2018) 68(1):7–30. doi: 10.3322/caac.21442
3. Mabuchi S, Isohashi F, Yokoi T, Takemura M, Yoshino K, Shiki Y, et al. A Phase II Study of Postoperative Concurrent Carboplatin and Paclitaxel Combined With Intensity-Modulated Pelvic Radiotherapy Followed by Consolidation Chemotherapy in Surgically Treated Cervical Cancer Patients With Positive Pelvic Lymph Nodes. Gynecol Oncol (2016) 141(2):240–6. doi: 10.1016/j.ygyno.2016.02.011
4. Nanthamongkolkul K, Hanprasertpong J. Predictive Factors of Pelvic Lymph Node Metastasis in Early-Stage Cervical Cancer. Oncol Res Treat (2018) 41(4):194–8. doi: 10.1159/000485840
5. Aoki Y, Sasaki M, Watanabe M, Sato T, Tsuneki I, Aida H, et al. High-Risk Group in Node-Positive Patients With Stage IB, IIA, and IIB Cervical Carcinoma After Radical Hysterectomy and Postoperative Pelvic Irradiation. Gynecol Oncol (2000) 77(2):305–9. doi: 10.1006/gyno.2000.5788
6. Koh WJ, Abu-Rustum NR, Bean S, Bradley K, Campos SM, Cho KR, et al. Cervical Cancer, Version 3. 2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw (2019) 17(1):64–84. doi: 10.6004/jnccn.2019.0001
7. Ferrandina G, Pedone Anchora L, Gallotta V, Fagotti A, Vizza E, Chiantera V, et al. Can We Define the Risk of Lymph Node Metastasis in Early-Stage Cervical Cancer Patients? A Large-Scale, Retrospective Study. Ann Surg Oncol (2017) 24(8):2311–8. doi: 10.1245/s10434-017-5917-0
8. Yahata H, Kobayashi H, Sonoda K, Kodama K, Yagi H, Yasunaga M, et al. Prognostic Outcome and Complications of Sentinel Lymph Node Navigation Surgery for Early-Stage Cervical Cancer. Int J Clin Oncol (2018) 23(6):1167–72. doi: 10.1007/s10147-018-1327-y
9. Zhao J, Cai J, Wang H, Dong W, Zhang Y, Wang S, et al. Region-Specific Risk Factors for Pelvic Lymph Node Metastasis in Patients With Stage IB1 Cervical Cancer. J Cancer (2021) 12(9):2624–32. doi: 10.7150/jca.53215
10. Salvo G, Ramirez PT, Levenback CF, Munsell MF, Euscher ED, Soliman PT, et al. Sensitivity and Negative Predictive Value for Sentinel Lymph Node Biopsy in Women With Early-Stage Cervical Cancer. Gynecol Oncol (2017) 145(1):96–101. doi: 10.1016/j.ygyno.2017.02.005
11. Tanaka T, Sasaki S, Tsuchihashi H, Terai Y, Yamamoto K, Yamada T, et al. Which Is Better for Predicting Pelvic Lymph Node Metastases in Patients With Cervical Cancer: Fluorodeoxyglucose-Positron Emission Tomography/Computed Tomography or a Sentinel Node Biopsy? A Retrospective Observational Study. Med (Baltimore) (2018) 97(16):e0410. doi: 10.1097/MD.0000000000010410
12. Wang Y, Yao T, Yu J, Li J, Chen Q, Lin Z. Can Pelvic Lymphadenectomy be Omitted in Patients With Stage IA2, IB1, and IIA1 Squamous Cell Cervical Cancer? Springerplus (2016) 5(1):1262. doi: 10.1186/s40064-016-2927-5
13. Zhou J, Ran J, He ZY, Quan S, Chen QH, Wu SG, et al. Tailoring Pelvic Lymphadenectomy for Patients With Stage IA2, IB1, and IIA1 Uterine Cervical Cancer. J Cancer (2015) 6(4):377–81. doi: 10.7150/jca.10968
14. Rob L, Robova H, Chmel R, Komar M, Halaska M, Skapa P. Surgical Options in Early Cervical Cancer. Int J Hyperthermia (2012) 28(6):489–500. doi: 10.3109/02656736.2012.675116
15. Twu NF, Ou YC, Liao CI, Chang WY, Yang LY, Tang YH, et al. Prognostic Factors and Adjuvant Therapy on Survival in Early-Stage Cervical Adenocarcinoma/Adenosquamous Carcinoma After Primary Radical Surgery: A Taiwanese Gynecologic Oncology Group (TGOG) Study. Surg Oncol (2016) 25(3):229–35. doi: 10.1016/j.suronc.2016.05.028
16. Jeong SY, Park H, Kim MS, Kang JH, Paik ES, Lee YY, et al. Pretreatment Lymph Node Metastasis as a Prognostic Significance in Cervical Cancer: Comparison Between Disease Status. Cancer Res Treat (2020) 52(2):516–23. doi: 10.4143/crt.2019.328
17. Bhatla N, Aoki D, Sharma DN, Sankaranarayanan R. Cancer of the Cervix Uteri. Int J Gynaecol Obstet (2018) 143(Suppl 2):22–36. doi: 10.1002/ijgo.12611
18. Ramirez PT, Frumovitz M, Pareja R, Lopez A, Vieira M, Ribeiro R, et al. Minimally Invasive Versus Abdominal Radical Hysterectomy for Cervical Cancer. N Engl J Med (2018) 379(20):1895–904. doi: 10.1056/NEJMoa1806395
19. Gallotta V, Conte C, Federico A, Vizzielli G, Alletti SG, Tortorella L, et al. Robotic Versus Laparoscopic Radical Hysterectomy in Early Cervical Cancer: A Case Matched Control Study. Eur J Surg Oncol (2018) 44(6):754–9. doi: 10.1016/j.ejso.2018.01.092
20. Gallotta V, Giudice MT, Conte C, Sarandeses AV, D’Indinosante M, Federico A, et al. Minimally Invasive Salvage Lymphadenectomy in Gynecological Cancer Patients: A Single Institution Series. Eur J Surg Oncol (2018) 44(10):1568–72. doi: 10.1016/j.ejso.2018.08.006
21. Huang BX, Fang F. Progress in the Study of Lymph Node Metastasis in Early-Stage Cervical Cancer. Curr Med Sci (2018) 38(4):567–74. doi: 10.1007/s11596-018-1915-0
22. Zhou Z, Li W, Zhang F, Hu K. The Value of Squamous Cell Carcinoma Antigen (Scca) to Determine the Lymph Nodal Metastasis in Cervical Cancer: A Meta-Analysis and Literature Review. PLoS One (2017) 12(12):e0186165. doi: 10.1371/journal.pone.0186165
23. Yang H, Hu H, Gou Y, Hu Y, Li H, Zhao H, et al. Combined Detection of Twist1, Snail1 and Squamous Cell Carcinoma Antigen for the Prognostic Evaluation of Invasion and Metastasis in Cervical Squamous Cell Carcinoma. Int J Clin Oncol (2018) 23(2):321–8. doi: 10.1007/s10147-017-1210-2
24. Kato H, Torigoe T. Radioimmunoassay for Tumor Antigen of Human Cervical Squamous Cell Carcinoma. Cancer (1977) 40(4):1621–8. doi: 10.1002/1097-0142(197710)40:4<1621::aid-cncr2820400435>3.0.co;2-i
25. Salvatici M, Achilarre MT, Sandri MT, Boveri S, Vanna Z, Landoni F. Squamous Cell Carcinoma Antigen (SCC-Ag) During Follow-Up of Cervical Cancer Patients: Role in the Early Diagnosis of Recurrence. Gynecol Oncol (2016) 142(1):115–9. doi: 10.1016/j.ygyno.2016.04.029
26. Xu F, Li Y, Fan L, Ma J, Yu L, Yi H, et al. Preoperative SCC-Ag and Thrombocytosis as Predictive Markers for Pelvic Lymphatic Metastasis of Squamous Cervical Cancer in Early FIGO Stage. J Cancer (2018) 9(9):1660–6. doi: 10.7150/jca.24049
28. Goldman L, Weinberg M, Weisberg M, Olshen R, Cook EF, Sargent RK, et al. A Computer-Derived Protocol to Aid in the Diagnosis of Emergency Room Patients With Acute Chest Pain. N Engl J Med (1982) 307(10):588–96. doi: 10.1056/NEJM198209023071004
29. Fonarow GC, Adams KF Jr, Abraham WT, Yancy CW, Boscardin WJ. ADHERE Scientific Advisory Committee, Et Al. Risk Stratification for in-Hospital Mortality in Acutely Decompensated Heart Failure: Classification and Regression Tree Analysis. JAMA (2005) 293(5):572–80. doi: 10.1001/jama.293.5.572
30. Kato T, Takashima A, Kasamatsu T, Nakamura K, Mizusawa J, Nakanishi T, et al. Clinical Tumor Diameter and Prognosis of Patients With FIGO Stage IB1 Cervical Cancer (JCOG0806-A). Gynecol Oncol (2015) 137(1):34–9. doi: 10.1016/j.ygyno.2015.01.548
31. Lennox GK, Covens A. Can Sentinel Lymph Node Biopsy Replace Pelvic Lymphadenectomy for Early Cervical Cancer? Gynecol Oncol (2017) 144(1):16–20. doi: 10.1016/j.ygyno.2016.08.337
32. Togami S, Kubo R, Kawamura T, Yanazume S, Kamio M, Kobayashi H. Comparison of Lymphatic Complications Between Sentinel Node Navigation Surgery and Pelvic Lymphadenectomy in Patients With Cervical Cancer. Jpn J Clin Oncol (2020) 50(5):543–7. doi: 10.1093/jjco/hyaa001
33. Guani B, Dorez M, Magaud L, Buenerd A, Lecuru F, Mathevet P. Impact of Micrometastasis or Isolated Tumor Cells on Recurrence and Survival in Patients With Early Cervical Cancer: SENTICOL Trial. Int J Gynecol Cancer (2019) 29(3):447–52. doi: 10.1136/ijgc-2018-000089
Keywords: cervical cancer, lymph node metastasis, lymph node dissection, risk factor, prognosis
Citation: Xu M, Xie X, Cai L, Xie Y, Gao Q and Sun P (2022) Risk Factor Assessment of Lymph Node Metastasis in Patients With FIGO Stage IB1 Cervical Cancer. Front. Oncol. 12:809159. doi: 10.3389/fonc.2022.809159
Received: 04 November 2021; Accepted: 07 March 2022;
Published: 30 March 2022.
Edited by:
Patrice Mathevet, Centre Hospitalier Universitaire Vaudois (CHUV), SwitzerlandReviewed by:
Valerio Gallotta, Agostino Gemelli University Polyclinic (IRCCS), ItalyCopyright © 2022 Xu, Xie, Cai, Xie, Gao and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pengming Sun, c3VuZmVteUBob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.