- The Cancer Center, First Hospital of Jilin University, Changchun, China
Background/Purpose: Lenvatinib is a first-line treatment for unresectable hepatocellular carcinoma (uHCC). We assessed the value of early alpha-fetoprotein (AFP) response for predicting clinical outcomes with lenvatinib treatment in patients with HBV-related uHCC and elevated AFP levels.
Methods: This retrospective analysis included patients with HBV-related uHCC and baseline AFP levels ≥20 ng/ml who received lenvatinib for >1 month between November 2018 and May 2021. Early AFP response was defined as a >20% decrease in AFP serum level from baseline after 4 weeks of lenvatinib treatment. Radiological response (Response Evaluation Criteria in Solid Tumors v1.1), progression-free survival, and overall survival were assessed in AFP responders and non-responders.
Results: Of the 46 patients analyzed, 30 (65.2%) were early AFP responders and 16 (34.8%) were non-responders. Compared to the non-responders, early AFP responders had a significantly higher objective response rate (34.5% vs 6.3%, p=0.0349), disease control rate (82.8% vs 50.0%; p=0.0203) and longer median progression-free survival (13.0 vs 7.0 months; HR, 0.464; 95% CI, 0.222-0.967; p=0.028). A subsequent multivariate analysis confirmed that early AFP response (HR, 0.387; 95% CI, 0.183-0.992; p=0.0154), Eastern Cooperative Oncology Group Performance Status of 0 (HR, 0.890; 95% CI, 0.811-0.976; p=0.0132) and Albumin-Bilirubin grade 1 (HR, 0.457; 95% CI, 0.269-0.963; p=0.0327) were independent prognostic factors for longer progression-free survival.
Conclusion: AFP is an important prognostic factor and a predictive biomarker for survival benefit with lenvatinib treatment in patients with HBV-related uHCC.
Introduction
Worldwide, liver cancer is the sixth most commonly diagnosed cancer, with an estimated 905,677 new cases and 830,180 deaths in 2020 (1). The most common type of liver cancer is hepatocellular carcinoma (HCC), which accounts for 75–85% of cases (2). As HCC is asymptomatic until late in the natural disease course, most patients present with advanced disease, and fewer than 20% of patients are suitable candidates for radical surgery (3). Hence, systemic therapies are the mainstay of treatment for patients with HCC. Recently, systemic treatment options for HCC have expanded, with the emergence of new therapies, particularly targeted agents (4).
Lenvatinib is an oral multikinase inhibitor that targets vascular endothelial growth factor (VEGF) receptors 1-3, fibroblast growth factor (FGF) receptors 1-4, platelet-derived growth factor (PDGF) receptor-α, rearranged during transfection (RET), and KIT, with antiangiogenic and antiproliferative effects (5–7). Findings from clinical and real-world studies have consistently demonstrated the efficacy and good tolerability of lenvatinib in patients with unresectable HCC (uHCC) (8–11). In the REFLECT study, lenvatinib was the first drug to show noninferiority to sorafenib in patients with uHCC with respect to overall survival (OS), as well as statistically significant improvements in progression-free survival (PFS) and objective response rate (ORR) (12). Two thirds of patients in this study were from the Asia-Pacific region and half had hepatitis B virus-related HCC (12). Based on these results, lenvatinib was introduced into clinical practice as a new therapeutic option for the first-line treatment of uHCC (4, 13). However, there remains a need for non-invasive, convenient, and inexpensive biomarkers to assess treatment response and identify which patients are most likely to benefit from lenvatinib therapy.
Serum alpha-fetoprotein (AFP) is a glycoprotein that is overproduced in approximately 70% of patients with HCC (14). AFP levels can be measured using a simple blood test that is routinely available worldwide. Evaluation of changes in AFP levels over time can improve the performance of this biomarker versus a single assessment of the AFP level (15). The association of AFP response with radiological response and prognosis has been assessed in large cohorts of patients with HCC, including those treated with surgical resection, radiofrequency ablation, transarterial chemoembolization, cytotoxic chemotherapy and molecular targeted therapy, and can potentially guide clinical practice in the majority of cases (16–20). For example, previous studies of patients with HCC receiving sorafenib have shown that AFP response is associated with survival, despite differences in AFP criteria for study entry (>20 or >200 ng/ml) and AFP response definitions (21, 22). However, few studies have investigated the value of AFP as a biomarker in patients with advanced HCC treated with lenvatinib (23–25). In particular, a better understanding of the relationship between early AFP response and clinical outcomes in these patients may facilitate decisions on whether to continue lenvatinib treatment.
We performed a retrospective analysis to examine the association between early AFP response and treatment outcomes in patients with advanced HCC receiving lenvatinib in real-world clinical practice.
Patients and Methods
Study Population
We retrospectively reviewed medical records of consecutive patients with HBV-related uHCC who received lenvatinib between November 2018 and May 2021 at the Cancer Center of the First Hospital of Jilin University. Inclusion criteria were: uHCC diagnosed using contrast enhanced computed tomography (CT), magnetic resonance imaging (MRI), or tumor biopsy; baseline AFP level ≥20 ng/ml; and lenvatinib treatment duration of ≥1 month. The study was conducted in accordance with the recently revised Declaration of Helsinki and approved by the ethics committee in our institution. Written informed consent was waived for this study because of the retrospective nature of the study. AFP assessment and definition of early AFP response
Serum AFP levels were measured at baseline (before the administration of lenvatinib) and at 1 month after starting lenvatinib therapy. There are no standardized cutoff values for AFP response, although thresholds of 20% and 50% change from baseline are frequently used (23, 24). In this study, early AFP response was defined as a >20% decrease in serum AFP levels after 1 month of lenvatinib treatment.
Treatment and Outcome Assessments
All patients received oral lenvatinib at a dose of 8 mg/day (bodyweight <60 kg) or 12 mg/day (bodyweight ≥60 kg). According to the instructions for lenvatinib administration, the dose was reduced or treatment was interrupted if grade ≥3 adverse events (AEs) or any unacceptable grade 2 AEs occurred. If a drug-related grade ≥3 AE or unacceptable grade 2 AE occurred, dose reduction or temporary interruption was maintained until the AE improved to grade 1 or 2. AEs were assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.
Radiological evaluations using enhanced CT or MRI were performed after 1 month of lenvatinib treatment and every 2-3 months thereafter, or whenever there was a sign or symptom suggesting tumor progression. Radiological response was determined according to the Response Evaluation Criteria in Solid Tumors (RECIST) v1.1. ORR was defined as the percentage of patients who achieved complete response (CR) or partial response (PR). The disease control rate (DCR) was defined as the percentage of patients who achieved a CR, PR or stable disease (SD). PFS was defined as the time from the start of lenvatinib treatment until tumor progression or death. OS was defined as the time from the start of lenvatinib treatment until death from any cause.
Statistical Analysis
Continuous variables were summarized using median values with interquartile (IQR) ranges, and intergroup values were compared using Mann–Whitney U tests. Categorical variables were summarized as number and percentage and were compared using a Fisher’s exact test. PFS and OS were calculated using the Kaplan–Meier method and intergroup differences compared using a log–rank test. Radiological responses, PFS, and OS were assessed in all patients and stratified by early AFP responders and AFP non-responders. Potential prognostic factors for PFS and OS were assessed using univariate and multivariate Cox proportional hazards models. All factors exhibiting a significant association with PFS or OS in the univariate analyses were included in the multivariate models. For subgroup analyses of PFS and OS, a univariate Cox proportional hazard model was used to estimate the hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) for AFP responders versus AFP non-responders in specific patient subgroups. For all analyses, p<0.05 was considered statistically significant and all reported p values are two-sided. All statistical analyses were performed using R software version 3.6.3 (http://www.R-project.org/).
Results
Patients
Medical records from a total of 79 patients were screened, of whom 46 were included in the analysis. Reasons for exclusion were diagnosis of non-HBV related HCC (n=4), baseline AFP <20 ng/ml (n=29). At baseline, more than half of patients (65.2%) were classified as Child-Pugh class A. Extrahepatic metastases were present in 52.2% of patients and portal vein thrombosis was noted in 34.8%. The median tumor size was 5.6 cm, with 17.4% and 80.4% of patients classified as Barcelona Clinic Liver Cancer (BCLC) stages B and C, respectively. In total, 82.6% of patients had received lenvatinib as first-line therapy, and the patients in the second-line setting are all progressed after sorafenib. The median follow-up period was 18.0 (IQR, 3-29) months. Overall, 10 (21.7%) patients had a lenvatinib dose reduction and three (6.5%) had a treatment interruption due to AEs.
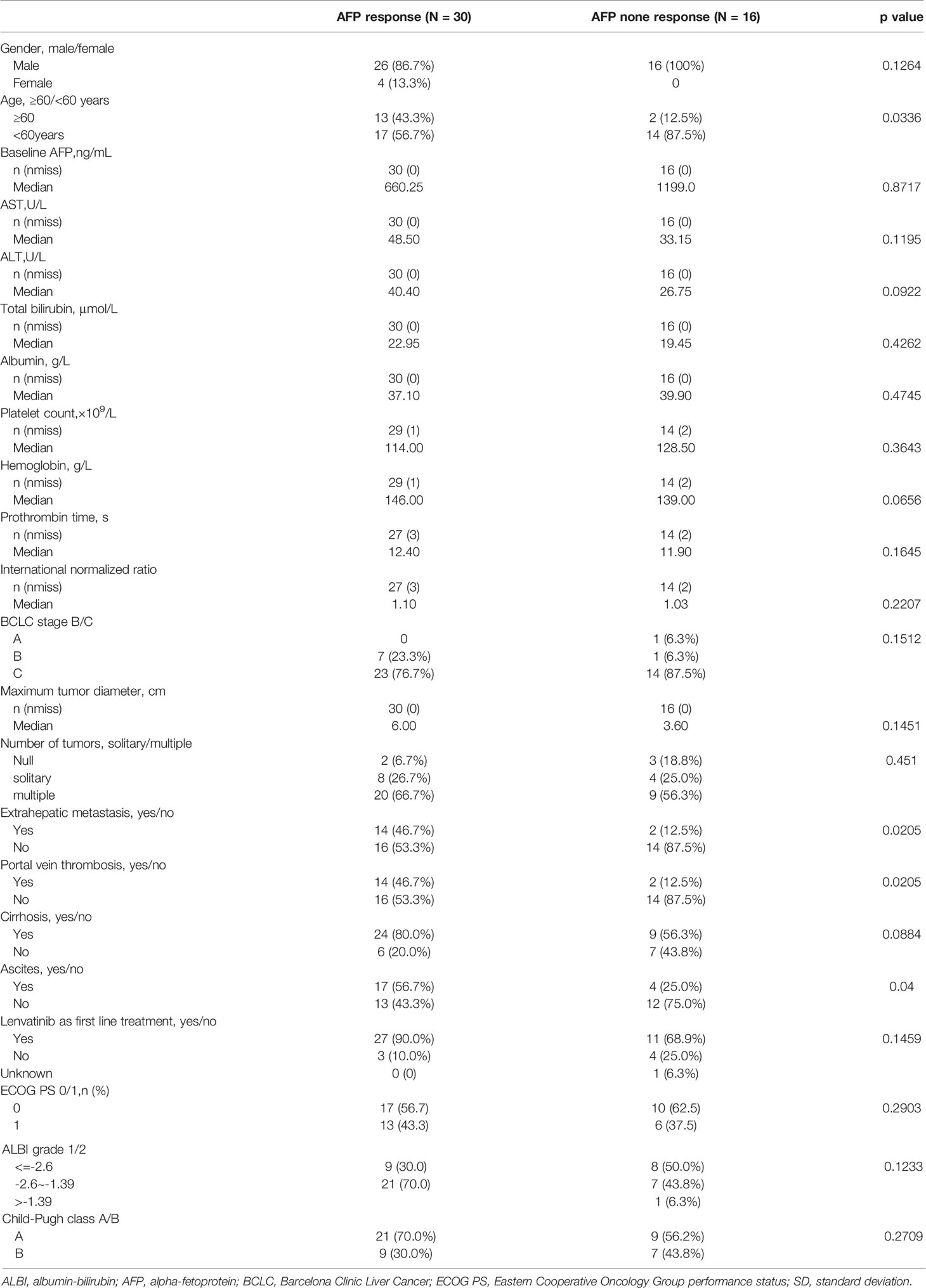
Of the 46 patients analyzed, 30 (65.2%) were early AFP responders and 16 (34.8%) were AFP non-responders. Median baseline AFP levels were 660.25 ng/ml and 1199.0 ng/ml in early AFP responders and non-responders, respectively. Baseline characteristics were similar between early AFP responders and non-responders, except for age (p=0.0336), portal vein thrombosis (p=0.0205) and ascites(p=0.04) (Table 1).
Relationship Between Early AFP Response and Imaging Response
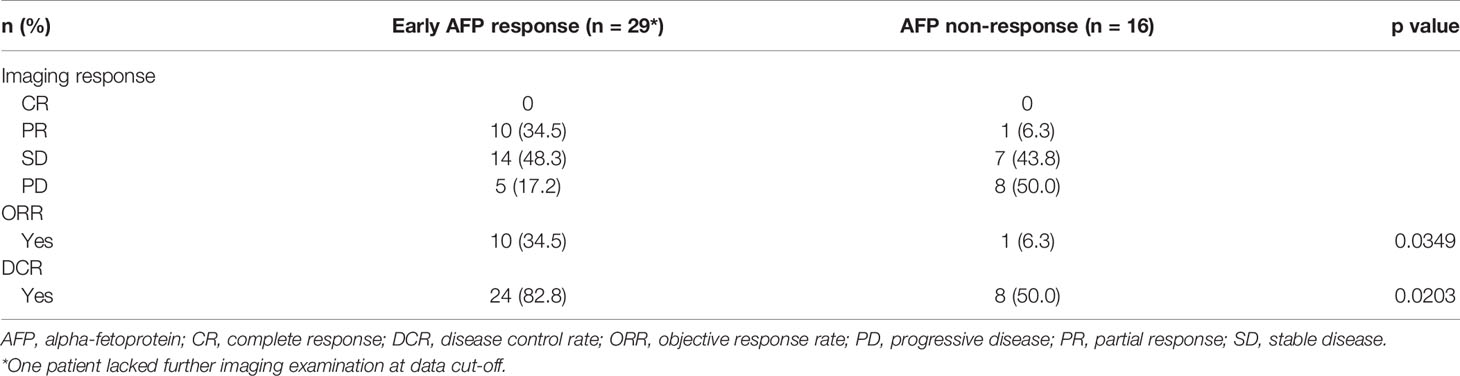
No patients achieved a CR. Among early AFP responders, PR, SD, and PD were observed in 10 (34.5%), 14 (48.3%), and 5 (17.2%) patients, respectively, compared with 1 (6.3%), 7 (43.8%), and 8 (50.0%) patients, respectively, in the non-responder group. Early AFP responders had a significantly higher ORR (34.5% vs 6.3%; p=0.0349) and DCR (82.8% vs 50.0%; p=0.0203) versus non-responders (Table 2).
Relationship Between Early AFP Response and Survival Outcome
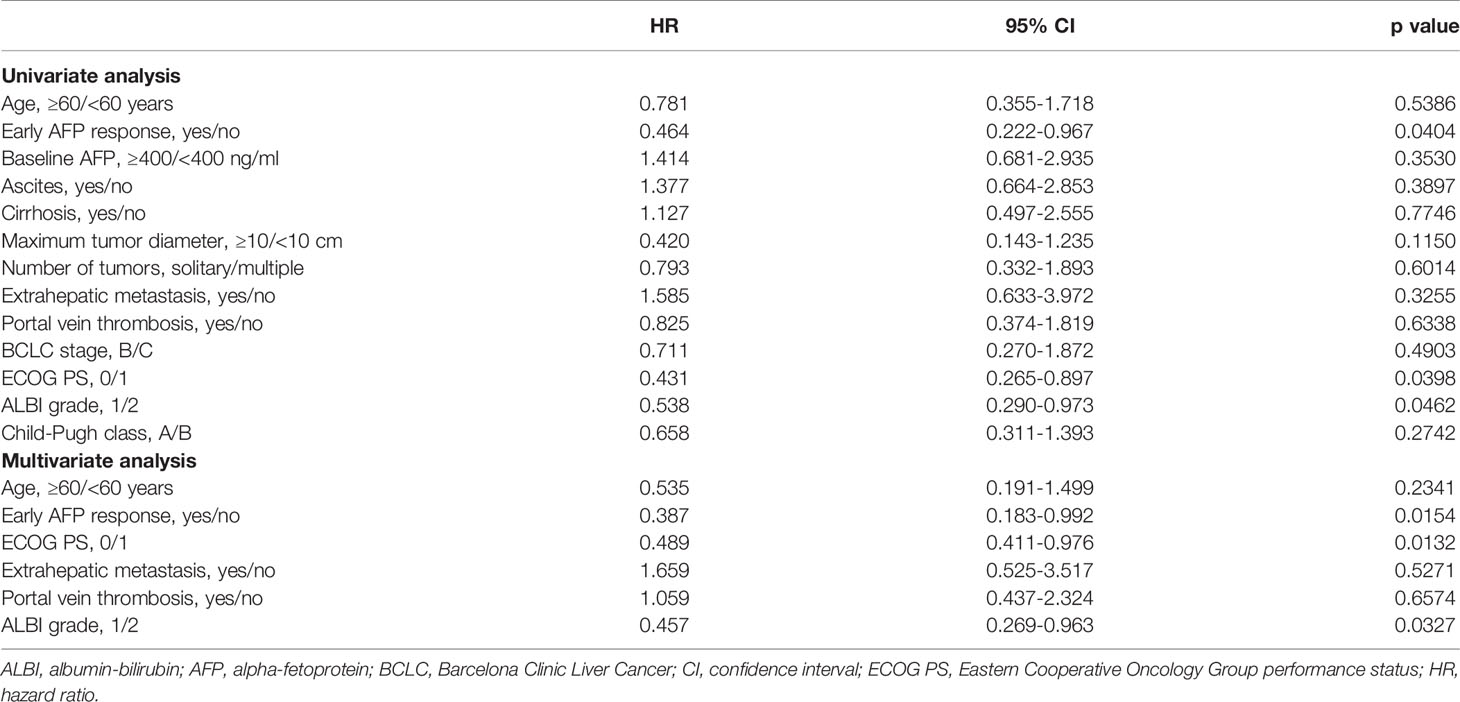
Early AFP responders had a significantly longer median PFS compared with non-responders (13.0 vs 7.0 months; p=0.028; Figure 1A). The results of univariate and multivariate analyses for PFS are presented in Table 3. In the univariate analysis, patients were more likely to have longer PFS if they had an early AFP response (HR, 0.464; 95% CI, 0.222-0.967; p=0.0404), ECOG PS of 0 (HR, 0.431; 95% CI 0.265-0.897; p=0.0398) and ALBI grade 1 (HR, 0.538; 95% CI, 0.290-0.973; p=0.0462).A subsequent multivariate analysis confirmed that early AFP response (HR, 0.387; 95% CI, 0.183-0.992; p=0.0154), Eastern Cooperative Oncology Group Performance Status of 0 (HR, 0.890; 95% CI, 0.811-0.976; p=0.0132) and Albumin-Bilirubin grade 1 (HR, 0.457; 95% CI, 0.269-0.963; p=0.0327) were independent prognostic factors for longer progression-free survival.
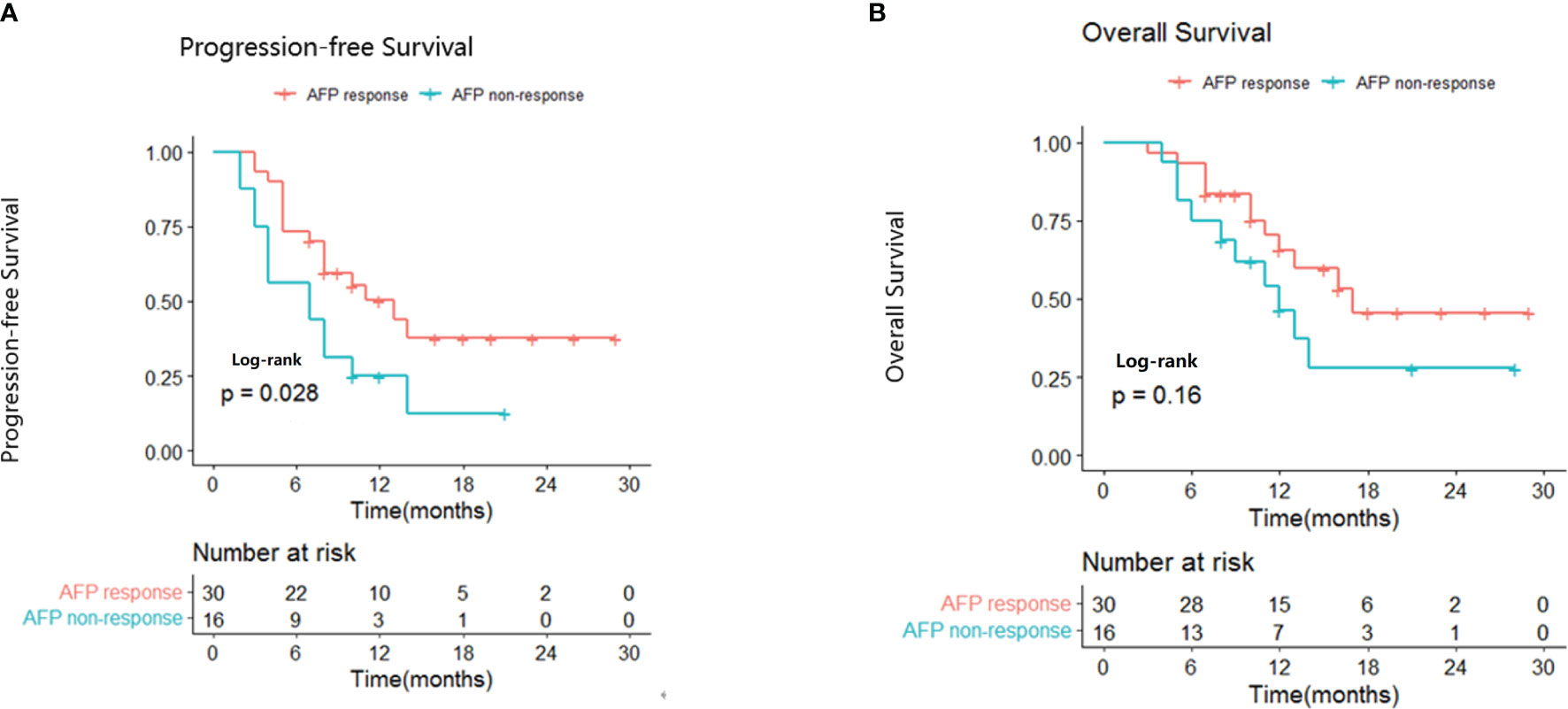
Figure 1 Kaplan-Meier curves for progression-free survival (A) and overall survival (B) in AFP responders and non-responders.
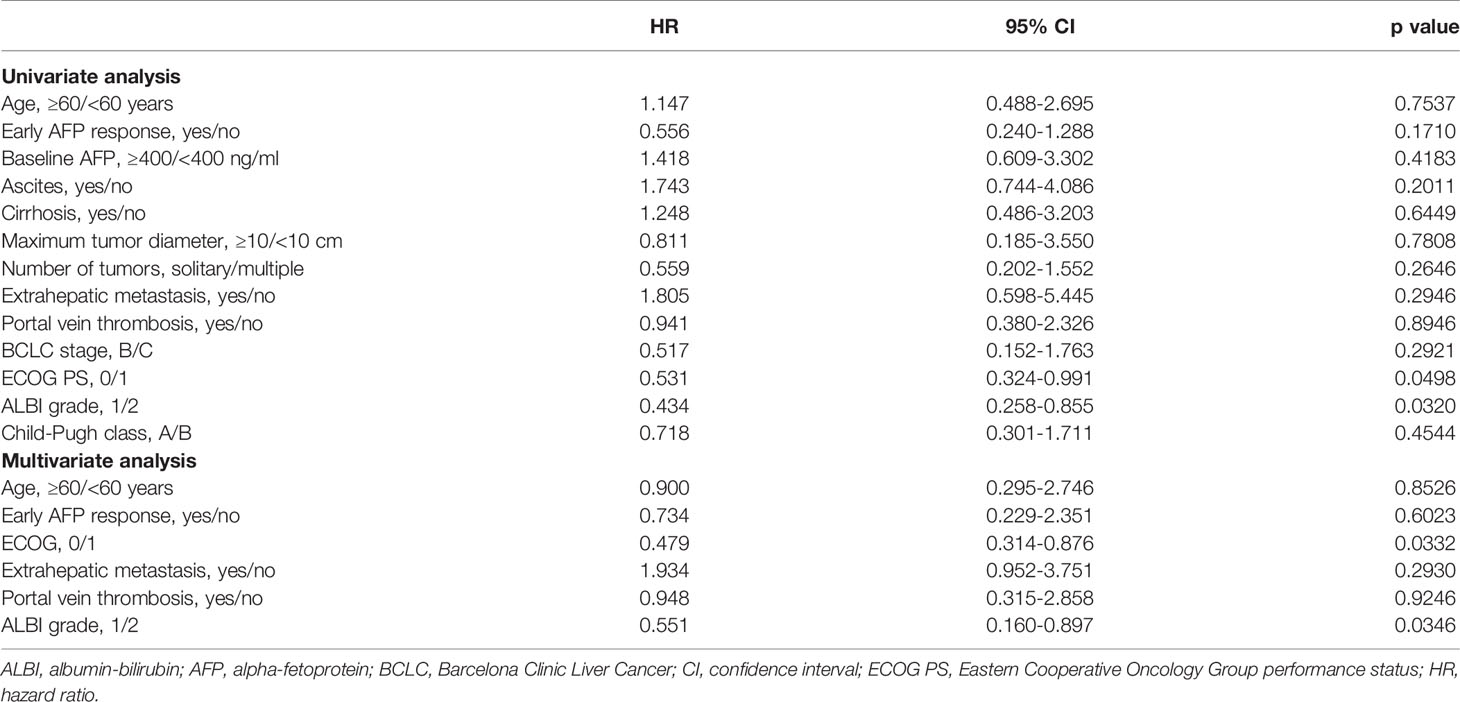
Median OS was 17.0 months for early AFP responders compared with 12.0 months for non-responders (p=0.16; Figure 1B). The results of univariate and multivariate analyses for OS are presented in Table 4. In the univariate analysis, patients were more likely to have longer OS if they had ECOG PS of 0 (HR, 0.531; 95% CI, 0.324-0.991; p=0.0498) and ALBI grade 1 (HR, 0.434; 95% CI 0.258-0.855; p=0.0320). Multivariate analysis showed that ECOG PS of 0 (HR, 0.479; 95% CI, 0.314-0.876; p=0.0332), and ALBI grade 1 (HR, 0.551; 95% CI, 0.160-0.897; p=0.0346) were independent prognostic factors for longer OS.
Relationship Between Liver Function and Survival Outcome
We evaluated the association between AFP response and change in liver function. Deterioration of liver function was defined as a change from Child-Pugh class A to Child-Pugh class B after initiating lenvatinib treatment or, in the case of patients with Child-Pugh class B7, the patient’s Child-Pugh score increased to ≥8 points. Most patients (31/46, 67.4%) showed maintained or improved liver function during lenvatinib therapy. There was no significant association between AFP response and change in liver function (p=0.42). Median PFS was significantly longer in patients with maintained or improved liver function compared with those whose liver function deteriorated during treatment (13.0 months vs 5.0 months; p=0.015; Figure 2A). Median OS was not reached in the maintained or improved liver function group and was 13 months in the deteriorated liver function group (p=0.081; Figure 2B).
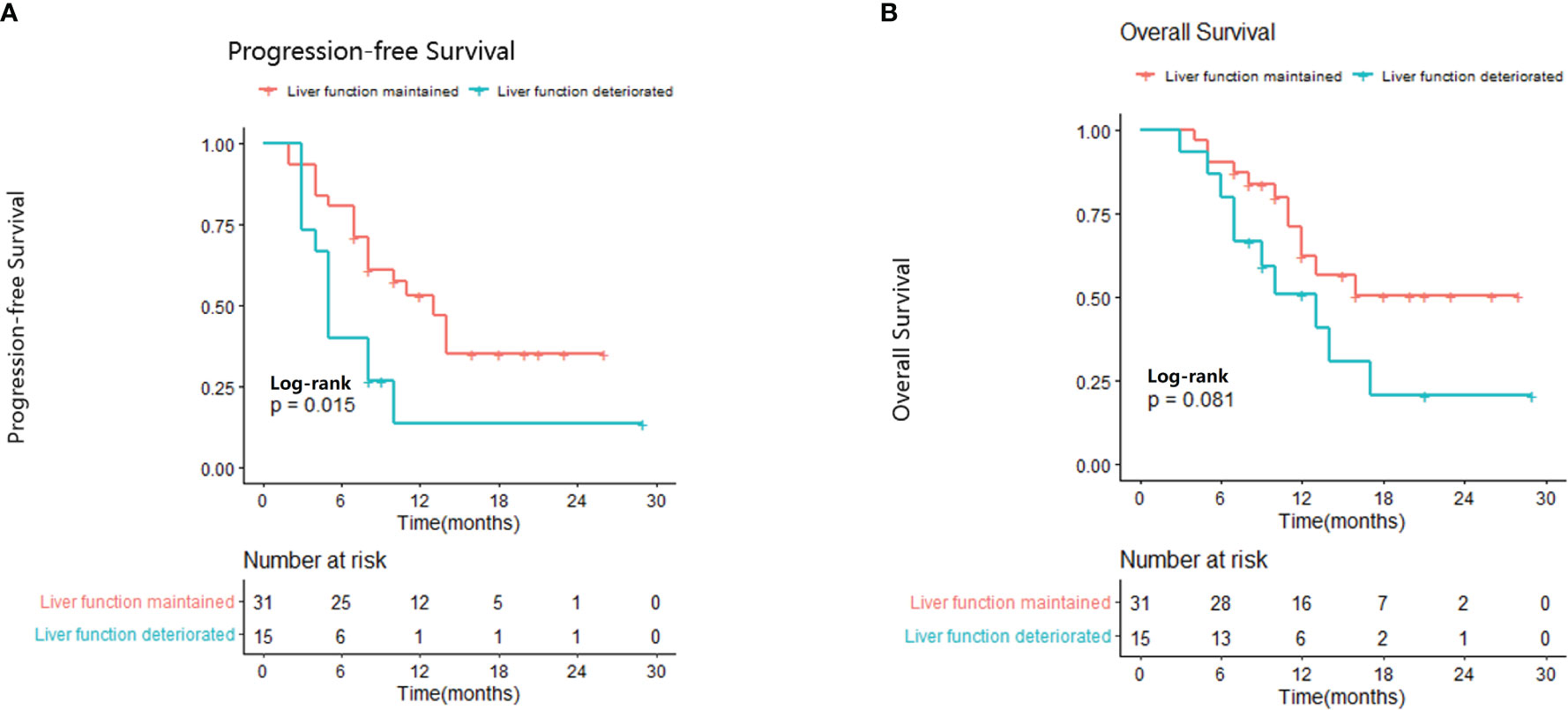
Figure 2 Relationship between liver function and progression-free survival (A) and overall survival (B).
Discussion
Findings from this study in patients with uHCC receiving lenvatinib showed that early AFP responders achieved a significantly higher ORR and DCR compared with AFP non-responders. These results are consistent with previous studies of the relationship between early AFP decline and radiological response in patients with uHCC treated with lenvatinib (23–25). In one study, patients with a sustained reduction of AFP from 2 to 4 weeks after lenvatinib initiation had a higher ORR compared with patients with a non-sustained reduction (67% vs 0%; p=0.02) (24). Another study reported that, among patients with baseline AFP ≥10 ng/ml, AFP responders (defined as those with an AFP reduction of ≥40%) versus non-responders had a significantly higher ORR (68.4% vs 7.1%; p<0.001) and DCR (84.2% vs 36.0%; p=0.009), and AFP response was the only significant predictor of objective response (odds ratio, 51.389; 95% CI, 4.888-540.281; p=0.001) (23). In addition, a multivariate analysis showed that a decrease in AFP level was an independent factor associated with response to lenvatinib (adjusted odds ratio, 10.3; 95% CI, 1.81-58.7; p<0.01) (25). Our results, together with this previous evidence, suggest that early AFP non-response can help to identify patients who are less likely to respond to lenvatinib and may therefore require more frequent imaging assessments.
Moreover, surgical resection remains the most important radical treatment for patients with liver cancer. For patients with advanced liver cancer who do not have an opportunity for surgery, transformational resection (resection after the cancer is reduced or downgraded) has a key role (26). From the perspective of transformational resection, the ORR is one of the most important considerations in the systemic treatment plan (26). However, efficacy predictors for the systemic treatment of liver cancer are lacking. Our research and previous studies have found that patients achieving an early AFP response have a significantly better tumor response compared with non-responders. Therefore, early AFP response may have value in guiding systemic treatment prior to transformational resection.
AFP response has previously been associated with survival in studies of patients with uHCC treated with drugs other than lenvatinib (21, 27). In patients treated with immune checkpoint inhibitors (nivolumab or pembrolizumab), early AFP response (>10% reduction in AFP within 4 weeks of treatment) predicted better objective response and survival (27). Early AFP response was also a significant independent predictor for better PFS and OS following antiangiogenic systemic therapy (21). Furthermore, similar results have been reported in patients receiving locoregional treatments, despite different AFP entry criteria and definitions of AFP response (28, 29). These previous reports support our findings that early AFP response may be a useful predictive marker for survival in patients with uHCC receiving lenvatinib, thereby helping to identify patients with a better prognosis and those who are candidates for other therapy options. The ability of AFP response to predict outcomes in uHCC may be explained by the role of AFP in promoting the growth, proliferation, and metastasis of HCC, and eliciting the escape of HCC from immune surveillance (14, 30, 31). Until now, no studies have investigated the value of AFP response in predicting the survival of patients with uHCC receiving lenvatinib. Our study demonstrated that early AFP responders achieved significantly longer PFS compared with non-responders. In addition, early AFP response was an independent prognostic factor for longer PFS in multivariate analysis. However, our results were inconclusive on whether early AFP response was predictive of OS, as the p-value was not significant in the univariate analysis(HR, 0.556; 95% CI, 0.240-1.288; p=0.1710). This may be related to the limited sample size and short follow-up time.
Previous studies have shown that in some patients with grade 2 albumin-bilirubin at baseline, a decrease in tumor burden in response to treatment leads to an improvement in liver function (32). However, in our study, there was no significant association between AFP response and liver function, which may be related to patient selection. Patients with advanced HCC treated with lenvatinib have previously been shown to have maintained or improved liver functional reserves after 4 and 12 weeks (33). Consistent with this observation, approximately two thirds of patients in the present study had maintained or improved liver function during lenvatinib therapy. As expected, patients with stable liver function had better survival compared with those with worsening liver function.
This study has several limitations. Firstly, it was a retrospective study with a modest sample size and, consequently, the findings require confirmation in further studies with larger numbers of patients. Secondly, as only patients with elevated AFP levels (serum AFP ≥20 ng/ml) at the initiation of lenvatinib therapy were included, the results may not be applicable to patients whose baseline AFP levels are within the normal range. Thirdly, the etiology of HCC in this study was hepatitis B virus (HBV), with a higher proportion of patients with HBV infection than in previous studies conducted in Western countries. As HBV-related hepatitis or cirrhosis may contribute to elevation of AFP, further validation is needed.
In conclusion, early AFP response may be a useful predictor of better tumor response and longer PFS and OS in patients with uHCC receiving lenvatinib. Therefore, early AFP response should be taken into consideration when assessing treatment response to lenvatinib in patients with uHCC, particularly in those with elevated AFP levels prior to treatment initiation.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of the First Hospital of Jilin University Affiliation: First Hospital of Jilin University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
BL and XS drafted the manuscript and contributed for the data analysis. J-YS, G-ZC, and XL critically revised the article. N-YW contributed for study concept and design. All authors read and approved the final manuscript, and all authors have taken due care to ensure the integrity of the work.
Funding
Jilin Provincial Department of Education grant number 3D5196776428 and Beijing Medical and Health Public Welfare Foundation grant number 3R2205401428.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors wish to acknowledge Prof. Yang Tian, Department of Hepatic Surgery, Eastern Hepatobiliary Surgery Hospital, Second Military Medical University, Shanghai, China, for their assistance with this manuscript.
References
1. World Fact Sheet. Available at: https://gco.iarc.fr/today/data/factsheets/populations/900-world-fact-sheets.pdf.Published 2020 (Accessed 10 February, 2021).
2. Bray F, Ferlay J, Soerjomataram I, Siegel R, Torre L, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
3. Poulou L, Botsa E, Thanou I, Ziakas P, Thanos L. Percutaneous Microwave Ablation vs Radiofrequency Ablation in the Treatment of Hepatocellular Carcinoma. World J Hepatol (2015) 7(8):1054–63. doi: 10.4254/wjh.v7.i8.1054
4. Clinical Practice Guidelines EASL. Management of Hepatocellular Carcinoma. J Hepatol (2018) 69(1):182–236. doi: 10.1016/j.jhep.2018.03.019
5. Tohyama O, Matsui J, Kodama K, Hata-Sugi N, Kimura T, Okamoto K, et al. Antitumor Activity of Lenvatinib (E7080): An Angiogenesis Inhibitor That Targets Multiple Receptor Tyrosine Kinases in Preclinical Human Thyroid Cancer Models. J Thyroid Res (2014) 2014:638747. doi: 10.1155/2014/638747
6. Yamamoto Y, Matsui J, Matsushima T, Obaishi H, Miyazaki K, Nakamura K, et al. Lenvatinib, an Angiogenesis Inhibitor Targeting VEGFR/FGFR, Shows Broad Antitumor Activity in Human Tumor Xenograft Models Associated With Microvessel Density and Pericyte Coverage. Vasc Cell (2014) 6:18. doi: 10.1186/2045-824X-6-18
7. Ikeda M, Okusaka T, Mitsunaga S, Ueno H, Tamai T, Suzuki T, et al. Safety and Pharmacokinetics of Lenvatinib in Patients With Advanced Hepatocellular Carcinoma. Clin Cancer Res an Off J Am Assoc Cancer Res (2016) 22(6):1385–94. doi: 10.1158/1078-0432.CCR-15-1354
8. Wang D, Yang X, Lin J, Bai Y, Long J, Yang X, et al. Efficacy and Safety of Lenvatinib for Patients With Advanced Hepatocellular Carcinoma: A Retrospective, Real-World Study Conducted in China. World J Gastroenterol (2020) 26(30):4465–78. doi: 10.3748/wjg.v26.i30.4465
9. Hiraoka A, Kumada T, Kariyama K, Takaguchi K, Itobayashi E, Shimada N, et al. Therapeutic Potential of Lenvatinib for Unresectable Hepatocellular Carcinoma in Clinical Practice: Multicenter Analysis. Hepatol Res Off J Japan Soc Hepatol (2019) 49(1):111–7. doi: 10.1111/hepr.13243
10. Obi S, Sato T, Sato S, Kanda M, Tokudome Y, Kojima Y, et al. The Efficacy and Safety of Lenvatinib for Advanced Hepatocellular Carcinoma in a Real-World Setting. Hepatol Int (2019) 13(2):199–204. doi: 10.1007/s12072-019-09929-4
11. Hiraoka A, Kumada T, Kariyama K, Takaguchi K, Atsukawa M, Itobayashi E, et al. Clinical Features of Lenvatinib for Unresectable Hepatocellular Carcinoma in Real-World Conditions: Multicenter Analysis. Cancer Med (2019) 8(1):137–46. doi: 10.1002/cam4.1909
12. Kudo M, Finn R, Qin S, Han K, Ikeda K, Piscaglia F, et al. Lenvatinib Versus Sorafenib in First-Line Treatment of Patients With Unresectable Hepatocellular Carcinoma: A Randomised Phase 3 non-Inferiority Trial. Lancet (Lond Engl) (2018) 391(10126):1163–73. doi: 10.1016/S0140-6736(18)30207-1
13. Chen L, Martinelli E, Cheng A, Pentheroudakis G, Qin S, Bhattacharyya S, et al. Pan-Asian Adapted ESMO Clinical Practice Guidelines for the Management of Patients With Intermediate and Advanced/Relapsed Hepatocellular Carcinoma: A TOS-ESMO Initiative Endorsed by CSCO, ISMPO, JSMO, KSMO, MOS and SSO. Ann Oncol Off J Eur Soc Med Oncol (2020) 31(3):334–51. doi: 10.1016/j.annonc.2019.12.001
14. Mitsuhashi N, Kobayashi S, Doki T, Kimura F, Shimizu H, Yoshidome H, et al. Clinical Significance of Alpha-Fetoprotein: Involvement in Proliferation, Angiogenesis, and Apoptosis of Hepatocellular Carcinoma. J Gastroenterol Hepatol (2008) 23:e189–197. doi: 10.1111/j.1440-1746.2008.05340.x
15. Yang J, Hainaut P, Gores G, Amadou A, Plymoth A, Roberts L. A Global View of Hepatocellular Carcinoma: Trends, Risk, Prevention and Management. Nat Rev Gastroenterol Hepatol (2019) 16(10):589–604. doi: 10.1038/s41575-019-0186-y
16. Kuzuya T, Ishigami M, Ishizu Y, Honda T, Hayashi K, Katano Y, et al. Early Clinical Response After 2 Weeks of Sorafenib Therapy Predicts Outcomes and Anti-Tumor Response in Patients With Advanced Hepatocellular Carcinoma. PloS One (2015) 10(9):e0138776. doi: 10.1371/journal.pone.0138776
17. Lai Q, Melandro F, Pinheiro R, Donfrancesco A, Fadel A, Levi B, et al. Alpha-Fetoprotein and Novel Tumor Biomarkers as Predictors of Hepatocellular Carcinoma Recurrence After Surgery: A Brilliant Star Raises Again. Int J Hepatol (2012) 2012:893103. doi: 10.1155/2012/893103
18. Liu G, Ouyang Q, Xia F, Fan G, Yu J, Zhang C, et al. Alpha-Fetoprotein Response Following Transarterial Chemoembolization Indicates Improved Survival for Intermediate-Stage Hepatocellular Carcinoma. HPB Off J Int Hepato Pancreato Biliary Assoc (2019) 21(1):107–13. doi: 10.1016/j.hpb.2018.06.1800
19. Lin W, Hsu C, Chen Y, Chang M, Liang K, Huang Y, et al. GALNT14 Genotype, α-Fetoprotein and Therapeutic Side Effects Predict Post-Chemotherapy Survival in Patients With Advanced Hepatocellular Carcinoma. Mol Clin Oncol (2014) 2(4):630–40. doi: 10.3892/mco.2014.294
20. Tsai M, Wang J, Hung C, Kee K, Yen Y, Lee C, et al. Favorable Alpha-Fetoprotein Decrease as a Prognostic Surrogate in Patients With Hepatocellular Carcinoma After Radiofrequency Ablation. J Gastroenterol Hepatol (2010) 25(3):605–12. doi: 10.1111/j.1440-1746.2009.06115.x
21. Shao Y, Lin Z, Hsu C, Shen Y, Hsu C, Cheng A. Early Alpha-Fetoprotein Response Predicts Treatment Efficacy of Antiangiogenic Systemic Therapy in Patients With Advanced Hepatocellular Carcinoma. Cancer (2010) 116(19):4590–6. doi: 10.1002/cncr.25257
22. Personeni N, Bozzarelli S, Pressiani T, Rimassa L, Tronconi M, Sclafani F, et al. Usefulness of Alpha-Fetoprotein Response in Patients Treated With Sorafenib for Advanced Hepatocellular Carcinoma. J Hepatol (2012) 57(1):101–7. doi: 10.1016/j.jhep.2012.02.016
23. Saeki I, Yamasaki T, Yamashita S, Hanazono T, Urata Y, Furutani T, et al. Early Predictors of Objective Response in Patients With Hepatocellular Carcinoma Undergoing Lenvatinib Treatment. Cancers (2020) 12(4):779. doi: 10.3390/cancers12040779
24. Kodama K, Kawaoka T, Namba M, Uchikawa S, Ohya K, Morio K, et al. Correlation Between Early Tumor Marker Response and Imaging Response in Patients With Advanced Hepatocellular Carcinoma Treated With Lenvatinib. Oncology (2019) 97(2):75–81. doi: 10.1159/000499715
25. Ohki T, Sato K, Kondo M, Goto E, Sato T, Kondo Y, et al. Impact of Adverse Events on the Progression-Free Survival of Patients With Advanced Hepatocellular Carcinoma Treated With Lenvatinib: A Multicenter Retrospective Study. Drugs - Real World Outcomes (2020) 7(2):141–9. doi: 10.1007/s40801-020-00179-7
26. Hui-Chuan Sun JZ, Wang Z, Liu X, Xie Q, Jia W, Zhao M, et al. Chinese Expert Consensus on Conversion Therapy in Hepatocellular Carcinoma (2021 Edition). Chin J Digest Surg (2021) 20:600–16. doi: 10.3760/cma.j.cn115610-20210512-00223
27. Lee P, Chao Y, Chen M, Lan K, Lee C, Lee I, et al. Predictors of Response and Survival in Immune Checkpoint Inhibitor-Treated Unresectable Hepatocellular Carcinoma. Cancers (2020) 12(1). doi: 10.3390/cancers12010182
28. Paul S, Sahu P, Sreenivas V, Nadda N, Gamanagatti S, Nayak B, et al. Prognostic Role of Serial Alpha-Fetoprotein Levels in Hepatocellular Carcinoma Treated With Locoregional Therapy. Scand J Gastroenterol (2019) 54(9):1132–7. doi: 10.1080/00365521.2019.1660403
29. Rungsakulkij N, Suragul W, Mingphruedhi S, Tangtawee P, Muangkaew P, Aeesoa S. Prognostic Role of Alpha-Fetoprotein Response After Hepatocellular Carcinoma Resection. World J Clin Cases (2018) 6(6):110–20. doi: 10.12998/wjcc.v6.i6.110
30. Li M, Ma Q, Chen Q, Liu X, Li P, Du G, et al. Alpha-Fetoprotein Triggers Hepatoma Cells Escaping From Immune Surveillance Through Altering the Expression of Fas/FasL and Tumor Necrosis Factor Related Apoptosis-Inducing Ligand and its Receptor of Lymphocytes and Liver Cancer Cells. World J Gastroenterol (2005) 11(17):2564–9. doi: 10.3748/wjg.v11.i17.2564
31. He C, Peng W, Liu X, Li C, Li X, Wen T. Post-Treatment Alpha-Fetoprotein Response Predicts Prognosis of Patients With Hepatocellular Carcinoma: A Meta-Analysis. Medicine (2019) 98(31):e16557. doi: 10.1097/MD.0000000000016557
32. Ying Hao Shen H-CS. Effects of Combination Therapy Using Lenvatinib and an Anti-PD-1 Antibody on Liver Function in Patients With Advanced Hepatocellular Carcinoma. 2021 APPLE . Abstract FV-24.
Keywords: unresectable hepatocellular carcinoma, lenvatinib, AFP response, biomarker, targeted therapy, survival
Citation: Liu B, Shang X, Shi J-Y, Cui G-Z, Li X and Wang N-Y (2022) Early Alpha-Fetoprotein Response Is Associated With Survival in Patients With HBV-Related Hepatocellular Carcinoma Receiving Lenvatinib. Front. Oncol. 12:807189. doi: 10.3389/fonc.2022.807189
Received: 01 November 2021; Accepted: 26 January 2022;
Published: 17 February 2022.
Edited by:
Jia Fan, Fudan University, ChinaReviewed by:
Jiwei Liu, Dalian Medical University, ChinaMing Zhao, Sun Yat-sen University Cancer Center (SYSUCC), China
Copyright © 2022 Liu, Shang, Shi, Cui, Li and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nan-Ya Wang, d2FuZ255QGpsdS5lZHUuY24=; orcid.org/0000-0001-9227-1374
†These authors have contributed equally to this work and share first authorship
 Bo Liu†
Bo Liu† Xiao Shang
Xiao Shang Jin-Yu Shi
Jin-Yu Shi Nan-Ya Wang
Nan-Ya Wang