- 1Cancer Center, The Affiliated Changzhou No. 2 People’s Hospital of Nanjing Medical University, Changzhou, China
- 2Department of Oncology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China
- 3Department of Oncology, Graduate School of Dalian Medical University, Dalian, China
The combination of immunotherapy and chemotherapy has a synergic effect in non-small cell lung cancer (NSCLC). However, the elderly are often excluded from clinical trails due to their poor health status and more comorbidities. We sought to assess the efficacy and safety of low-dose nanoparticle albumin-bound paclitaxel (nab-paclitaxel) plus tislelizumab (an anti-PD-1 antibody) in elderly patients with advanced NSCLC. In this phase 2 clinical trail, eligible patients were those aged ≥65 years with metastatic NSCLC who had disease progression after treatment with ≥1 line of chemotherapy or targeted therapy. Patients with epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) variations were eligible if they demonstrated disease progression after treatment with ≥1 corresponding inhibitor. Primary endpoints were progression-free survival and safety/tolerability. Secondary endpoints included objective response rate and overall survival. Among 29 patients enrolled from May 2019 through August 2020, 21 (72.4%) had adenocarcinoma, 17 (58.6%) had a performance status of 2, 8 (27.6%) had asymptomatic brain metastases, and 13 (44.8%) had EGFR/ALK variations. As of the data cutoff point on April 1, 2021, median progression-free survival and overall survival were 9.5 months and 16.5 months, respectively. Ten patients achieved a partial response (objective response rate of 34.5%). Seventeen (58.6%) patients had ≥1 treatment-related adverse event, with grade 3 events seen in 3 patients (10.3%). The most common adverse events were fatigue (20.7%), fever (17.2%), abnormal liver function (17.2%), and rash (17.2%). These results suggest that low-dose nab-paclitaxel plus tislelizumab is well tolerated and effective in elderly patients with advanced NSCLC, including those with EGFR/ALK variations.
Introduction
Lung cancer, which accounts for 11.6% of all new cancer cases worldwide, is the leading cause of cancer-related mortality, with a 5-year survival rate of 4% to 17% (1). Approximately 80% to 85% of lung cancer cases are classified as non-small cell lung cancer (NSCLC) (2, 3). Conventional chemotherapy used to be the standard treatment option for patients with advanced NSCLC, but the 5-year survival rate with this treatment was only 5% (4). Targeted therapy has a good therapeutic outcome in patients with specific molecular subtypes such as epidermal growth factor receptor (EGFR) mutation or anaplastic lymphoma kinase (ALK) positive, but eventual drug resistance is unavoidable in most cases, and many patients do not have the gene mutations targeted by these specific therapies. With the development of immunotherapy, immune checkpoint inhibitors such as programmed cell death-1 (PD-1) and programmed cell death ligand-1 (PD-L1) antibodies have gradually become the standard treatment in patients with advanced NSCLC (5). Several studies, such as KEYNOTE-189, have also suggested that the combination of immunotherapy with standard-dose chemotherapy can have a synergic effect in NSCLC, leading to improved survival outcomes (6–8).
Elderly patients represent a high proportion of the NSCLC population (9). Because these patients are more frequently frail and have more comorbidities, the risks associated with full dose of chemotherapy are higher, thus limiting the treatment options available (10). As a result, they are often excluded from clinical trials. Additionally, no consensus has been reached regarding treatment recommendations or risk/benefit ratios. For these patients, suitable treatments that can effectively control the disease while minimizing the risk of adverse reactions are urgently needed.
Nanoparticle albumin-bound paclitaxel (nab-paclitaxel) is a novel nanoparticulate formulation of paclitaxel bound to human serum albumin that has been found to improve the therapeutic index of paclitaxel (11). In a phase 3 trial, first-line treatment with nab-paclitaxel in combination with carboplatin was effective and well tolerated in patients aged ≥70 years with advanced NSCLC (12). Earlier phase 2 trials also reported good efficacy and safety with nab-paclitaxel monotherapy as second-line treatment for patients with NSCLC who had disease progression after platinum-based chemotherapy (13–15). Nab-paclitaxel may therefore be a useful alternative for elderly patients with advanced NSCLC.
As mentioned earlier, a combination of immunotherapy and chemotherapy may improve outcomes in patients with NSCLC. Tislelizumab, a humanized anti-PD-1 monoclonal antibody, is engineered to minimize binding to FcγR on macrophages to abrogate antibody-dependent cellular phagocytosis, a mechanism of T-cell clearance and potential resistance to anti-PD-1 therapy (16, 17). In studies, tislelizumab has been well tolerated with no unexpected safety concerns in patients with advanced NSCLC (18). Regardless of ethnicity and PD-L1 expression, promising efficacy has been observed in heavily pretreated patients with NSCLC who received tislelizumab as monotherapy and in untreated patients with NSCLC who received tislelizumab in combination with chemotherapy (18).
Because elderly patients with NSCLC have more complications, weaker bone marrow function, and lower tolerance to treatment, we sought to determine whether it would be possible to reduce the dose of chemotherapy and still achieve good outcomes in these patients by combining this treatment with tislelizumab. In this study, we therefore evaluated the efficacy and safety of combination therapy with low-dose nab-paclitaxel and tislelizumab in elderly patients with metastatic NSCLC who had previously been treated with platinum-based chemotherapy or targeted therapy.
Methods
Study Design
This single-arm, open-label, investigator-initiated phase 2 prospective study was performed at the Affiliated Changzhou No. 2 People’s Hospital of Nanjing Medical University in Changzhou, China. The study was designed to evaluate the efficacy and safety of treatment with tislelizumab plus low-dose nab-paclitaxel in patients aged ≥65 years who had metastatic NSCLC with disease progression after platinum-based chemotherapy or targeted therapy. The study was approved by the hospital’s ethics committee and was conducted in accordance with the principles of the Declaration of Helsinki. All patients included in the study provided written informed consent.
Patients
From May 2019 through August 2020, we recruited patients aged ≥65 years with histologically confirmed metastatic NSCLC and with ≥1 measurable lesion who had demonstrated disease progression after treatment with platinum-based chemotherapy or targeted therapy. Patients with EGFR or ALK variations were eligible to participate if they had demonstrated disease progression after treatment with ≥1 approved corresponding inhibitor. Patients with asymptomatic brain metastases were also eligible, regardless of Eastern Cooperative Oncology Group performance status (PS) and PD-L1 expression. Patients who received previous treatment with drugs specifically targeting checkpoint pathways were excluded from the study.
Procedures
Participants included in the study received nab-paclitaxel (130 mg/m2), day 1, every 3 weeks) for ≤6 cycles plus tislelizumab (200 mg, day 2, every 3 weeks), followed by maintenance therapy with tislelizumab (200 mg, every 3 weeks) until confirmed disease progression, death, or unacceptable toxicity.
Study Assessments
The primary endpoints were progression-free survival (PFS) and safety/tolerability. Secondary endpoints included objective response rate (ORR), disease control rate (DCR), and overall survival (OS). Tumor responses were assessed according to the Response Evaluation Criteria in Solid Tumors (RECIST 1.1) and included complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). ORR was defined as the sum of CR and PR. DCR was defined as the sum of CR, PR, and SD. PFS was defined as the time from the first day of nab-paclitaxel until disease progression or death by any cause whichever occurred first. OS was defined as the time from the date of trial enrollment to death or last follow-up. Duration of response (DoR) was defined as the time from first RECIST response to disease progression in patients who demonstrated at least a PR. We assessed response to treatment every 6 weeks using CT based on RECIST 1.1 criteria. Treatment-related adverse events (TRAEs) were monitored and graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0). The data cutoff was April 1, 2021.
Statistical Analysis
Continuous variables were summarized as arithmetic means or medians with standard deviations (SDs) or percentiles for descriptive purposes, and categorical variables were presented as frequencies and percentages. χ2 tests or Fisher exact tests were used to assess the statistical significance of categorical variables. ORR was presented as a percentage with 95% confidence intervals (CIs). PFS, DoR, and OS were analyzed using the Kaplan-Meier method. Subgroups were compared using the log-rank test. For survival curve analysis, all variables were dichotomized according to their medians. Statistical significance was defined as P<0.05 using a 2-tailed test. All statistical analyses were performed using SPSS version 19.0.
Results
Patients
From May 2019 through August 2020, a total of 30 cases of patients with NSCLC were initially identified as eligible for study inclusion. Among these patients, 1 declined to participate; therefore, a total of 29 patients (mean age, 70.3 ± 4.1 years) were enrolled in the study. Among the 29 recruited patients, 14 (48.3%) were men, 12 (41.4%) were current/former smokers, 17 (58.6%) had a PS of 2, and 8 (27.6%) had asymptomatic brain metastases. In total, 13 patients (44.8%) with EGFR/ALK variations were included in the study (11 with EGFR variations and 2 with ALK variations). The most common histologic diagnosis was adenocarcinoma, accounting for 21 (72.4%) patients. The expression of PD-L1 was available for 18 (62.1%) patients; of these patients, 8 demonstrated PD-L1 expression <1%; 7, 1% to 10%, and 3, >10% (Table 1).
Patients had received 1 (13/29, 44.8%), 2 (12/29, 41.4%), or ≥3 (4/29, 13.8%) lines of therapy previously. All 16 patients without driver gene mutations had received platinum-based chemotherapy, most commonly pemetrexed plus carboplatin. All 11 patients with EGFR variations had received a first- or second-generation EGFR tyrosine kinase inhibitor (afatinib, gefitinib, erlotinib, or icotinib). Both of the patients with ALK variations had received crizotinib.
The mean number of nab-paclitaxel chemotherapy cycles was 4.6 ± 1.7 among study patients. Fifteen of the 29 patients (51.7%) received treatment for 6 cycles. As of April 1, 2021, the median follow-up was 14.0 months (95% CI, 8.5-19.6); on this date, 1 patient was still receiving treatment with tislelizumab. The most common reason for treatment discontinuation was progressive disease (n = 22; 75.9%).
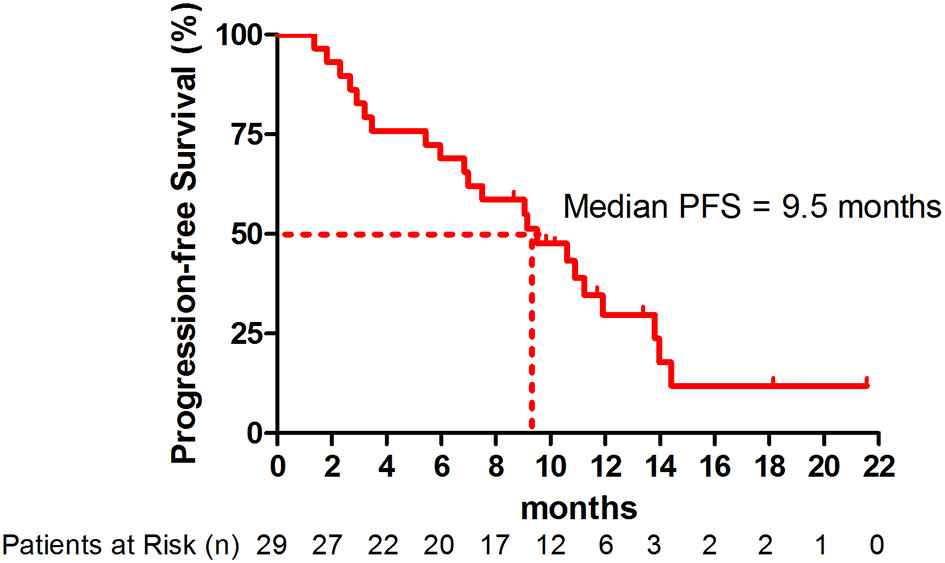
Efficacy
At the point of data cutoff, 22 patients (75.9%) had disease progression. The overall median PFS was 9.5 months (95% CI, 5.8-13.2 months) (Figure 1). The median PFS was 10.9 months (95% CI, 8.6-13.2 months) in patients with adenocarcinoma versus 3.2 months (95% CI, 0-10.4 months) in patients with squamous cell carcinoma (hazard ratio [HR], 0.63; 95% CI, 0.24-1.62; P=0.33) (Figure S1 and Table S1). The median PFS was significantly higher in patients with nonsquamous NSCLC with EGFR/ALK wild type (11.9 months; 95% CI, 9.3-14.5 months) than in those with EGFR/ALK variations (7.0 months; 95% CI. 3.5-10.5 months; HR, 0.56; 95% CI, 0.33-0.97; P=0.032) (Figure S2).
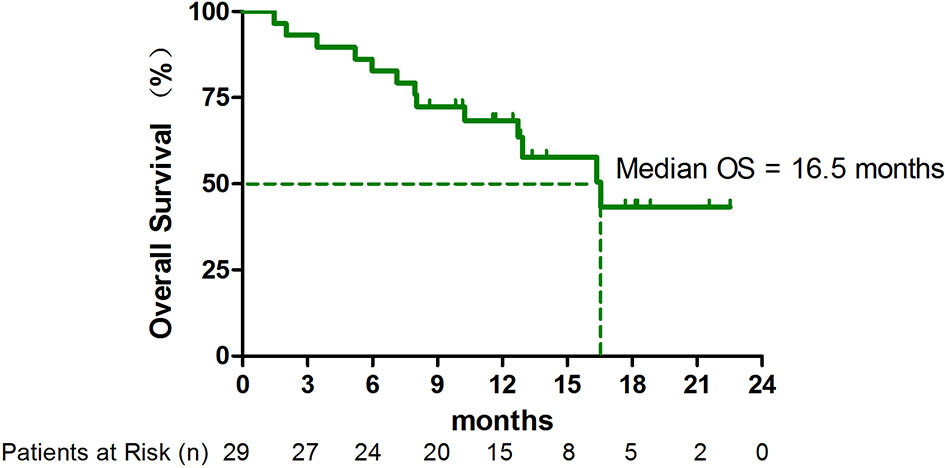
As of April 1, 2021, 13 (44.8%) patients had died. The median OS was 16.5 months (95% CI, 10.8-22.3 months) (Figure 2). In subgroup analysis, the median OS in patients with adenocarcinoma with EGFR/ALK wild type has not yet been reached (Table S1).
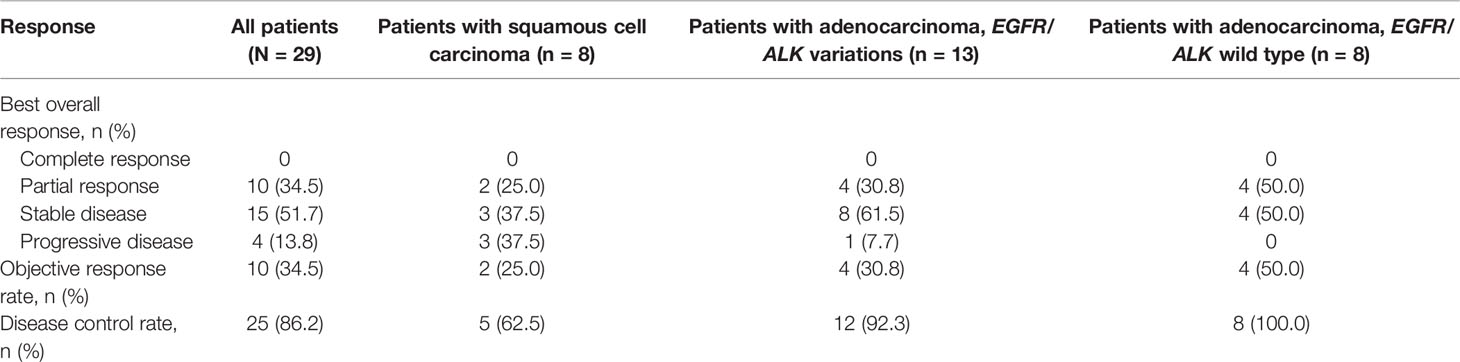
In total, 10 patients achieved PR, leading to an ORR of 34.5% (95% CI, 17.9%-54.3%) (Table 2). As the point of data cutoff, the median duration of these responses was 9.8 months (95% CI, 5.4–14.3 months), with 2 response ongoing (Figure 3). The ORR was 38.1% (95% CI, 18.1%-61.6%) in patients with adenocarcinoma versus 25.0% (95% CI, 3.2%-65.1%; P = 0.419) in patients with squamous cell carcinoma (Table 2). In patients with nonsquamous NSCLC, the ORR was 50.0% (95% CI, 15.7%-84.3%) in patients with EGFR/ALK wild type versus 30.8% (95% CI, 9.1%-61.4%) in those with EGFR/ALK variations (P =0 .336) (Table 2).
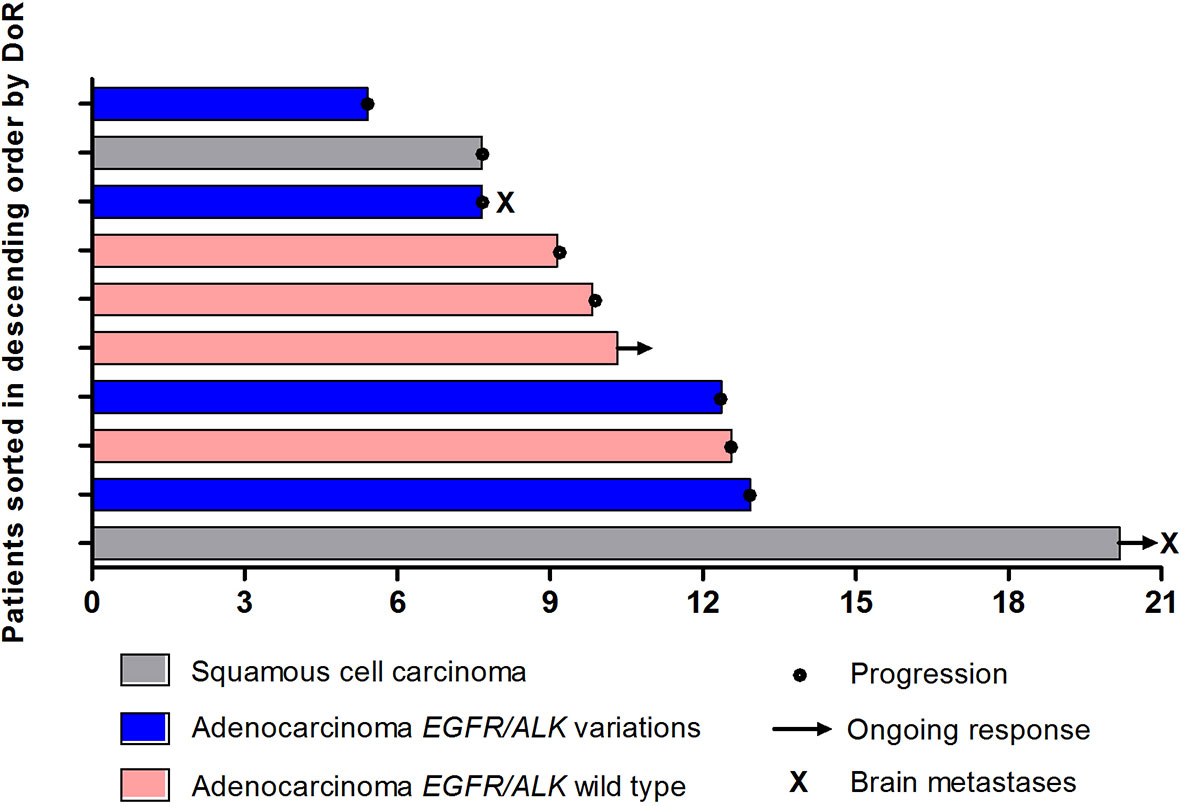
Overall, disease control was reported in 25 patients (86.2%) (Table 2). Additionally, among 8 patients with asymptomatic brain metastases, PR was achieved in 2 patients, and the DCR was 100.0%. Eighteen patients overall (62.1%) had a decrease in tumor size from baseline (median change, −18%) (Figure 4).
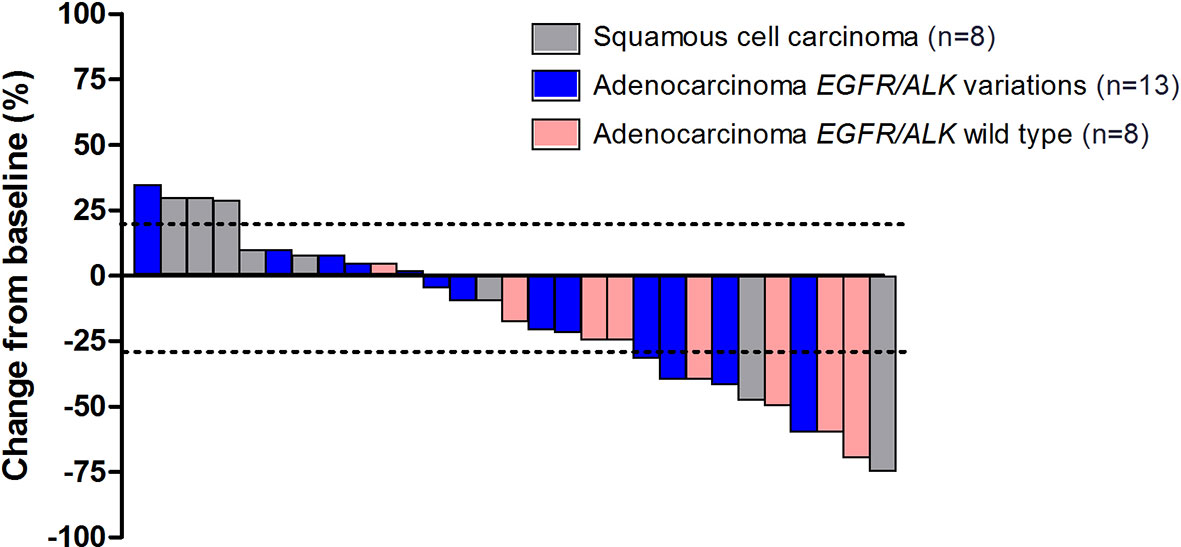
Figure 4 The maximum percentage reduction from baseline in sum of target lesions based on histology and EGFR/ALK status (n = 29).
Safety
A total of 17 patients (58.6%) suffered at least 1 TRAE, with 3 patients (10.3%) experiencing a grade 3 adverse event (Table 3). No grade 4 adverse events were reported. The most common TRAEs were fatigue (6/29; 20.7%), fever (5/29; 17.2%), abnormal liver function (5/29; 17.2%), rash (5/29; 17.2%), and numbness (4/29; 13.8%). Only 1 (3.4%) patient discontinued treatment because of an adverse event (immune-related pneumonitis).
Follow Up
After the disease progressed, 16/22 (72.7%) patients received the follow-up treatment, among which 2 (12.5%) received next-generation EGFR/ALK targeted drugs, 7 (43.75%) received mono antiangiogenic agents, 4 (25.0%) received chemotherapy plus antiangiogenic agents, the last 3 (18.75%) received immunotherapy plus antiangiogenic agents. In general, antiangiogenic targeted therapy played a major role in the posterior treatment.
Discussion
Immunotherapy can dramatically improve clinical outcomes in patients with advanced NSCLC without targetable genetic alterations. Additionally, research has shown that apart from chemotherapy’s direct cytotoxic activity, this treatment exerts immune-potentiating effects such as increasing the immunogenicity of tumor cells, inhibiting negative immune signals, and altering the immune microenvironment (19–21). Chemotherapy has also been shown to induce PD-L1 expression on tumor cells (22, 23). Thus, the combination of immunotherapy and chemotherapy has a synergistic effect. Because elderly patients are less able to tolerate the toxicities associated with standard-dose chemotherapy, we sought to assess the combination of low-dose chemotherapy and immunotherapy in elderly patients with previously treated metastatic NSCLC. We found that low-dose nab-paclitaxel plus immunotherapy [with chemotherapy administered before immunotherapy to enhance antigen liberation and increase the synergistic effects (24, 25)] was safe and effective in these patients.
To our knowledge, this is the first clinical trial to prospectively report that the combination of low-dose nab-paclitaxel and tislelizumab is well tolerated and leads to promising outcomes in elderly patients with advanced NSCLC, including patients with EGFR or ALK variations. A previous study demonstrated a PFS of 7.6 months and an ORR of 74.8% in patients who received tislelizumab plus nab-paclitaxel and carboplatin as first-line treatment for advanced squamous NSCLC, whereas patients treated with paclitaxel plus carboplatin alone had a shorter PFS, highlighting the possible synergistic effect between chemotherapy and immunotherapy (26). Nab-paclitaxel monotherapy has previously been found to be efficacious as second-line treatment for patients with NSCLC, as indicated by ORRs of 14.5% to 31.7%, DCRs of 65.5% to 71.9%, median PFS values of 3.9 to 5.1 months, and median OS values of 9.9 to 13.0 months (13–15). Similar results have been seen with other immunotherapy and chemotherapy combinations. For instance, in a randomized phase 2 trial, the addition of pembrolizumab to docetaxel was found to significantly improve PFS when compared with docetaxel alone (9.5 months vs 3.9 months; HR, 0.24) in patients with disease progression after platinum-based chemotherapy (24). The ORR was also improved with combination therapy (42.5% vs 15.8%).
Though the ORR in our study was not as high as in this previous study, the results are still noteworthy in consideration of the poor physical condition of patients as around 50% of them had a PS of 2. Meanwhile, it translated into a PFS benefit nonetheless, representing a longer duration of response, suggesting that low-dose chemotherapy combined with immunotherapy can also produce good synergy effects with tolerable side effects especially for those patients with poor physical conditions. The molecular mechanism of this potential synergistic effect has not yet been fully explained; however, our C57BL/6 mouse lung cancer model study demonstrated an increase in the expression of PD-L1 and CD8+ T lymphocytes in the immune microenvironment with combination therapy of anti-PD-1 antibody plus chemotherapy, especially in the group of half-dose chemotherapy and anti-PD-1 antibody (data not shown), which may be a factor. One possible explanation is that low-dose chemotherapy was inclined to induce the immunogenicity of tumor cells rather than killing tumor cells. Therefore, low-dose chemotherapy not only maintains antitumor efficacy but also reduces the adverse effects of combination therapy.
One interesting finding in this study was the efficacy of low-dose nab-paclitaxel plus tislelizumab in patients with EGFR/ALK variations, who have generally been underrepresented in previous clinical trials (27, 28). It is well known that the frequency of EGFR variations in the Chinese population is relatively high (29), so immunotherapy options must be considered for patients with these variations whose disease is resistant to EGFR tyrosine kinase inhibitors. A total of 13 patients (44.8%) with EGFR/ALK variations were included in this study. In this patient subgroup, the PFS and ORR were lower than those of patients with EGFR/ALK wild type, but patients with EGFR/ALK variations still demonstrated improvements in PFS and ORR with this treatment combination. Previous studies included only a small number of patients with EGFR variations (24, 30); however, these studies did demonstrate that the addition of immunotherapy (atezolizumab or pembrolizumab) significantly improves PFS in patients with genetically altered EGFR in advanced nonsquamous NSCLC. Taken together, these results suggest that large trials should be conducted to further assess the use of combination therapy after treatment with tyrosine kinase inhibitors in patients with nonsquamous NSCLC with EGFR/ALK variations.
Another interesting finding in this study was the efficacy of this treatment regimen in patients with brain metastases, a population with limited available data. Metastases to the brain, which manifest as neurological disorders, occur in 18% to 61% of patients with lung cancer and are associated with a poor prognosis (31–33). The role of chemotherapeutic agents in these patients is limited by the blood–brain barrier, but the introduction of targeted therapies and immune checkpoint inhibitors has radically changed the treatment algorithm for this patient population (34). Despite these changes, most previous studies have recruited only a small percentage of patients with brain metastases, which is not representative of the overall population. In our study, 8 patients with asymptomatic brain metastases were included, 2 of whom achieved PR. The DCR in this subgroup was 100%, highlighting the efficacy of this treatment combination. These data support the need for well-designed larger trials exploring combination therapy in this patient subgroup.
Because elderly patients have decreased organ function and a higher incidence of comorbidities, they are more likely to experience toxicities related to chemotherapy, making the treatment of these patients challenging (35). In this study, TRAEs were reported in 17 of 29 patients (58.6%), a lower rate than has been reported in previous studies of combination therapy, even with the inclusion of patients with a PS of 2. In addition, most of the TRAEs were grade 1 or 2 and were manageable; no grade 4 adverse events were reported.
This study had several limitations, key among them the small number of patients who met the eligibility criteria within the study period. This led to insufficient statistical power for the data analysis. We will continue to enroll patients to expand the sample size. Second, this was a single-arm study; comparisons with full-dose chemotherapy plus immunotherapy or with immunotherapy alone were not made. Third, data were missing regarding PD-L1 expression and tumor mutation burden for some of the study patients, so we were unable to evaluate these factors as biomarkers. Despite these limitations, this is the first trial to demonstrate that low-dose nab-paclitaxel plus tislelizumab is safe and effective in elderly patients with metastatic NSCLC when used as second- or later-line treatment, even though half of the patients had a PS of 2 and many had NSCLC with EGFR/ALK variations. Indeed, this trial population may be representative of the real-world patient population, as it included patients with higher PS scores, patients with asymptomatic brain metastases, patients with EGFR/ALK variations, and patients with various levels of PD-L1 expression. Further randomized controlled trials, preferably in large patient populations, are needed to confirm these results. Also we can envisage that maybe this combination can even be a valuable first-line option in patients not candidate to platinum-based chemotherapy. Randomized clinical trials evaluating this combination could be run in the setting of first-line for elderly NSCLC patients.
In summary, we found that for elderly patients with advanced NSCLC, including those with high PS scores, brain metastases, or EGFR/ALK variations, combination therapy with low-dose nab-paclitaxel and tislelizumab is safe and has synergistic effects. Our results raise the possibility of using this treatment regimen clinically in this patient population with the goal of prolonging patient survival.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Changzhou No.2 People’s Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas, took part in drafting, revising or critically reviewing the article, gave final approval of the version to be published, have agreed on the journal to which the article has been submitted, and agree to be accountable for all aspects of the work.
Funding
This study was supported by the Research Fund from Chinese Society of Clinical Oncology (Grant No. Y-sy2018-030), and the Young Scientists Foundation of Changzhou No.2 People’s Hospital (2018K004).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.802467/full#supplementary-material
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
2. Hirsch FR, Scagliotti GV, Mulshine JL, Kwon R, Curran WJ Jr., Wu YL, et al. Lung Cancer: Current Therapies and New Targeted Treatments. Lancet (2017) 389(10066):299–311. doi: 10.1016/S0140-6736(16)30958-8
3. Yi M, Li A, Zhou L, Chu Q, Luo S, Wu K. Immune Signature-Based Risk Stratification and Prediction of Immune Checkpoint Inhibitor's Efficacy for Lung Adenocarcinoma. Cancer Immunol Immunother (2021) 70(6):1705–19. doi: 10.1007/s00262-020-02817-z
4. Zhang QY, Wang FX, Jia KK, Kong LD. Natural Product Interventions for Chemotherapy and Radiotherapy-Induced Side Effects. Front Pharmacol (2018) 9:1253. doi: 10.3389/fphar.2018.01253
5. Heymach J, Krilov L, Alberg A, Baxter N, Chang SM, Corcoran RB, et al. Clinical Cancer Advances 2018: Annual Report on Progress Against Cancer From the American Society of Clinical Oncology. J Clin Oncol (2018) 36(10):1020–44. doi: 10.1200/JCO.2017.77.0446
6. Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab Plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med (2018) 378(22):2078–92. doi: 10.1056/NEJMoa1801005
7. Kon E, Benhar I. Immune Checkpoint Inhibitor Combinations: Current Efforts and Important Aspects for Success. Drug Resist Update (2019) 45:13–29. doi: 10.1016/j.drup.2019.07.004
8. Morel D, Jeffery D, Aspeslagh S, Almouzni G, Postel-Vinay S. Combining Epigenetic Drugs With Other Therapies for Solid Tumours - Past Lessons and Future Promise. Nat Rev Clin Oncol (2020) 17(2):91–107. doi: 10.1038/s41571-019-0267-4
9. Bonanno L, Attili I, Pavan A, Sepulcri M, Pasello G, Rea F, et al. Treatment Strategies for Locally Advanced non-Small Cell Lung Cancer in Elderly Patients: Translating Scientific Evidence Into Clinical Practice. Crit Rev Oncol Hematol (2021) 163:103378. doi: 10.1016/j.critrevonc.2021.103378
10. Townsley C, Pond GR, Peloza B, Kok J, Naidoo K, Dale D, et al. Analysis of Treatment Practices for Elderly Cancer Patients in Ontario, Canada. J Clin Oncol (2005) 23(16):3802–10. doi: 10.1200/JCO.2005.06.742
11. Desai N, Trieu V, Yao Z, Louie L, Ci S, Yang A, et al. Increased Antitumor Activity, Intratumor Paclitaxel Concentrations, and Endothelial Cell Transport of Cremophor-Free, Albumin-Bound Paclitaxel, ABI-007, Compared With Cremophor-Based Paclitaxel. Clin Cancer Res (2006) 12(4):1317–24. doi: 10.1158/1078-0432.CCR-05-1634
12. Socinski MA, Langer CJ, Okamoto I, Hon JK, Hirsh V, Dakhil SR, et al. Safety and Efficacy of Weekly Nab(R)-Paclitaxel in Combination With Carboplatin as First-Line Therapy in Elderly Patients With Advanced Non-Small-Cell Lung Cancer. Ann Oncol (2013) 24(2):314–21. doi: 10.1093/annonc/mds461
13. Anzai M, Morikawa M, Okuno T, Umeda Y, Demura Y, Sonoda T, et al. Efficacy and Safety of Nanoparticle Albumin-Bound Paclitaxel Monotherapy as Second-Line Therapy of Cytotoxic Anticancer Drugs in Patients With Advanced Non-Small Cell Lung Cancer. Med (Baltimore) (2017) 96(51):e9320. doi: 10.1097/MD.0000000000009320
14. Sakata S, Saeki S, Okamoto I, Otsubo K, Komiya K, Morinaga R, et al. Phase II Trial of Weekly Nab-Paclitaxel for Previously Treated Advanced non-Small Cell Lung Cancer: Kumamoto Thoracic Oncology Study Group (KTOSG) Trial 1301. Lung Cancer (2016) 99:41–5. doi: 10.1016/j.lungcan.2016.06.009
15. Liu Z, Wei Z, Hu Y, Gao F, Hao L, Fang P, et al. A Phase II Open-Label Clinical Study of Comparing Nab-Paclitaxel With Pemetrexed as Second-Line Chemotherapy for Patients With Stage IIIB/IV non-Small-Cell Lung Cancer. Med Oncol (2015) 32(8):216. doi: 10.1007/s12032-015-0660-5
16. Dahan R, Sega E, Engelhardt J, Selby M, Korman AJ, Ravetch JV. FcgammaRs Modulate the Anti-Tumor Activity of Antibodies Targeting the PD-1/PD-L1 Axis. Cancer Cell (2015) 28(4):543. doi: 10.1016/j.ccell.2015.09.011
17. Zhang T, Song X, Xu L, Ma J, Zhang Y, Gong W, et al. The Binding of an Anti-PD-1 Antibody to FcgammaRIota has a Profound Impact on its Biological Functions. Cancer Immunol Immunother (2018) 67(7):1079–90. doi: 10.1007/s00262-018-2160-x
18. Liu SY, Wu YL. Tislelizumab: An Investigational Anti-PD-1 Antibody for the Treatment of Advanced Non-Small Cell Lung Cancer (NSCLC). Expert Opin Investig Drugs (2020) 29(12):1355–64. doi: 10.1080/13543784.2020.1833857
19. Apetoh L, Ladoire S, Coukos G, Ghiringhelli F. Combining Immunotherapy and Anticancer Agents: The Right Path to Achieve Cancer Cure? Ann Oncol (2015) 26(9):1813–23. doi: 10.1093/annonc/mdv209
20. Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunological Effects of Conventional Chemotherapy and Targeted Anticancer Agents. Cancer Cell (2015) 28(6):690–714. doi: 10.1016/j.ccell.2015.10.012
21. Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of Action of Conventional and Targeted Anticancer Therapies: Reinstating Immunosurveillance. Immunity (2013) 39(1):74–88. doi: 10.1016/j.immuni.2013.06.014
22. Peng J, Hamanishi J, Matsumura N, Abiko K, Murat K, Baba T, et al. Chemotherapy Induces Programmed Cell Death-Ligand 1 Overexpression via the Nuclear Factor-kappaB to Foster an Immunosuppressive Tumor Microenvironment in Ovarian Cancer. Cancer Res (2015) 75(23):5034–45. doi: 10.1158/0008-5472.CAN-14-3098
23. Zhang P, Ma Y, Lv C, Huang M, Li M, Dong B, et al. Upregulation of Programmed Cell Death Ligand 1 Promotes Resistance Response in non-Small-Cell Lung Cancer Patients Treated With Neo-Adjuvant Chemotherapy. Cancer Sci (2016) 107(11):1563–71. doi: 10.1111/cas.13072
24. Arrieta O, Barron F, Ramirez-Tirado LA, Zatarain-Barron ZL, Cardona AF, Diaz-Garcia D, et al. Efficacy and Safety of Pembrolizumab Plus Docetaxel vs Docetaxel Alone in Patients With Previously Treated Advanced Non-Small Cell Lung Cancer: The PROLUNG Phase 2 Randomized Clinical Trial. JAMA Oncol (2020) 6(6):856–64. doi: 10.1001/jamaoncol.2020.0409
25. Chen DS, Mellman I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity (2013) 39(1):1–10. doi: 10.1016/j.immuni.2013.07.012
26. Wang J, Lu S, Yu X, Hu Y, Sun Y, Wang Z, et al. Tislelizumab Plus Chemotherapy vs Chemotherapy Alone as First-Line Treatment for Advanced Squamous Non-Small-Cell Lung Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol (2021) 7(5):709–17. doi: 10.1001/jamaoncol.2021.0366
27. Lee CK, Man J, Lord S, Links M, Gebski V, Mok T, et al. Checkpoint Inhibitors in Metastatic EGFR-Mutated Non-Small Cell Lung Cancer-A Meta-Analysis. J Thorac Oncol (2017) 12(2):403–7. doi: 10.1016/j.jtho.2016.10.007
28. Oya Y, Kuroda H, Nakada T, Takahashi Y, Sakakura N, Hida T. Efficacy of Immune Checkpoint Inhibitor Monotherapy for Advanced Non-Small-Cell Lung Cancer With ALK Rearrangement. Int J Mol Sci (2020) 21(7):2623. doi: 10.3390/ijms21072623
29. Shi Y, Au JS, Thongprasert S, Srinivasan S, Tsai CM, Khoa MT, et al. A Prospective, Molecular Epidemiology Study of EGFR Mutations in Asian Patients With Advanced Non-Small-Cell Lung Cancer of Adenocarcinoma Histology (PIONEER). J Thorac Oncol (2014) 9(2):154–62. doi: 10.1097/JTO.0000000000000033
30. Reck M, Mok TSK, Nishio M, Jotte RM, Cappuzzo F, Orlandi F, et al. Atezolizumab Plus Bevacizumab and Chemotherapy in non-Small-Cell Lung Cancer (IMpower150): Key Subgroup Analyses of Patients With EGFR Mutations or Baseline Liver Metastases in a Randomised, Open-Label Phase 3 Trial. Lancet Respir Med (2019) 7(5):387–401. doi: 10.1016/S2213-2600(19)30084-0
31. Chason JL, Walker FB, Landers JW. Metastatic Carcinoma in the Central Nervous System and Dorsal Root Ganglia. A Prospective Autopsy Study. Cancer (1963) 16:781–7. doi: 10.1002/1097-0142(196306)16:6<781::aid-cncr2820160614>3.0.co;2-m
32. Nussbaum ES, Djalilian HR, Cho KH, Hall WA. Brain Metastases. Histology, Multiplicity, Surgery, and Survival. Cancer (1996) 78(8):1781–8.
33. Zimm S, Wampler GL, Stablein D, Hazra T, Young HF. Intracerebral Metastases in Solid-Tumor Patients: Natural History and Results of Treatment. Cancer (1981) 48(2):384–94. doi: 10.1002/1097-0142(19810715)48:2<384::aid-cncr2820480227>3.0.co;2-8
34. Veccia A, Kinspergher S, Dipasquale M, Caffo O. Management of Brain Metastases From Lung Cancer in the Era of Immunotherapy: A Review of the Literature. Future Oncol (2021) 17(5):597–609. doi: 10.2217/fon-2020-0701
Keywords: low-dose, chemotherapy, immunotherapy, elderly, non-small cell lung cancer
Citation: Zhu W, Geng Q, Peng H, Jin Z, Li D, Pu X, Wang G and Jiang H (2022) Efficacy and Safety of Low-Dose Nab-Paclitaxel Plus Tislelizumab in Elderly Patients With Previously Treated Metastatic Non-Small Cell Lung Cancer. Front. Oncol. 12:802467. doi: 10.3389/fonc.2022.802467
Received: 26 October 2021; Accepted: 24 February 2022;
Published: 17 March 2022.
Edited by:
Clare Y. Slaney, Peter MacCallum Cancer Centre, AustraliaReviewed by:
Francesco Facchinetti, Gustave Roussy Cancer Campus, FranceWenjie Wang, National Institutes of Health (NIH), United States
Copyright © 2022 Zhu, Geng, Peng, Jin, Li, Pu, Wang and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hua Jiang, Y3pleWpoQG5qbXUuZWR1LmNu
†These authors have contributed equally to this work
 Wenyu Zhu1†
Wenyu Zhu1† Hua Jiang
Hua Jiang