
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 14 February 2022
Sec. Surgical Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.798016
Background: The impact of primary site surgery on survival remains controversial in female patients with stage IV breast cancer. The purpose of this study was to investigate the role of primary tumor surgery in patients with stage IV breast cancer and concurrently develop a nomogram to identify which patients will benefit from surgery.
Methods: We retrospectively searched the SEER database for female patients newly diagnosed with stage IV breast infiltrating duct carcinoma (BIDC) between 2010 and 2015 and then divided them into surgery and non-surgery groups. The propensity score matching (PSM) method was implemented to eliminate the bias, and Kaplan–Meier survival analysis was generated to compare the overall survival (OS) and cancer-specific survival (CSS) between the two groups. After PSM, Cox regression analyses were performed to determine the independent protective value of primary tumor surgery, while logistic regression analyses were utilized to uncover significant predictors of surgical benefit and establish a screening nomogram for female patients with stage IV BIDC. Nomogram performance was evaluated by calibration curves, receiver operating characteristic (ROC) curves, and decision curve analysis (DCA).
Result: 5,475 patients with stage IV BIDC were included in this study, and 2,375 patients (43.38%) received primary tumor surgery. After PSM, the median CSS was 53 months (95% CI: 46.84–59.16) in the surgery group compared with only 33 months (95% CI: 30.05–35.95) in the non-surgery group. We further found that primary tumor surgery was an independent protective factor for patients with stage IV BIDC. The independent factors affecting the benefit of locoregional surgery in patients with stage IV BIDC included histological grade, T stage, molecular subtype, lung metastasis, liver metastasis, brain metastasis, and marital status. The AUC of the nomogram was 0.785 in the training set and 0.761 in the testing set. The calibration curves and DCA confirmed that the nomogram could precisely predict the possibility of benefit from primary tumor resection.
Conclusion: Our study suggested that primary tumor surgery improved the prognosis of female patients with stage IV BIDC and developed a nomogram to quantify the probability of surgical benefit to help identify surgical candidates clinically.
Breast cancer (BC) is a common malignant tumor and the second most common cause of cancer death among women in the United States (1). Owing to the intense effort in public education and effective screening, approximately 90% of BC patients present early-stage disease at the time of diagnosis (2). Early-stage BC is considered as a curable disease with standard surgical resection and radiation, with a 5-year survival rate of over 90% (3, 4). Although early-stage BC presents an excellent prognosis, it is virtually incurable once tumor cells spread to distant sites (5). Additionally, approximately 25%–30% of early-stage BC metastasizes and progresses to advanced BC, which is the leading cause of death from BC (6), and only 24%–26.5% of patients with stage IV BC survive for more than 5 years (7, 8).
Systemic therapy is the main treatment for stage IV BC patients to relieve symptoms, improve quality of life, and prolong survival, including endocrine therapy, targeted drug therapy, chemotherapy, and radiotherapy (9, 10). Moreover, amplitude-modulated radiofrequency electromagnetic fields (AM RF EMF) at breast cancer-specific frequencies can result in complete and partial responses in patients with stage IV BC (11). However, the efficacy of surgical resection of the primary site in patients with stage IV BC remains controversial. Metastatic BC represents a major clinical problem as it is hard to be surgically resected, unlike the primary tumor (12). In clinical practice, primary tumor resection is not a routine treatment for patients with stage IV BC, but only to relieve chest symptoms such as bleeding, ulcers, and pain due to chest wall invasion (13). Several studies indicated that surgical intervention not only failed to improve the survival rate of patients with metastatic BC but also created a permissive environment for tumor relapses and distant metastases (14). The possible reason for this phenomenon may be that the surgery causes cancer cells to enter the circulation (15). Yu et al. combined prospective clinical multicenter trials and found that locoregional surgery did not prolong overall survival (OS) of stage IV BC patients but had a significantly longer locoregional progression-free survival (PFS) (16). Similarly, the ECOG-E2018 study reported the results of a randomized phase III trial that showed no significant difference in OS or PFS in patients with stage IV BC who received systemic therapy plus early local therapy versus systemic therapy alone. Conversely, Khan et al. and Thomas et al. generated two retrospective population-based studies and the results indicated that local tumor resection had a positive impact on survival in patients with stage IV BC (17, 18). Therefore, it is of great importance to clarify the effect of primary tumor resection on the survival of female patients with stage IV BC and develop a novel model to quantify the probability of surgical benefit to help identify surgical candidates clinically.
It is well known that the histological subtype of BC can affect prognosis, with approximately 70%–80% of BCs being infiltrating duct carcinoma (IDC) (19), so we selected female patients with stage IV breast IDC (BIDC) as research objects. Thus, we identified a large representative stage IV BIDC cohort to evaluate the impact of primary site surgery on survival after propensity score matching (PSM) and explore the independent protective value of locoregional surgery. Then, we established a nomogram to identify candidates and quantify the probability of surgical benefit in female patients with stage IV BIDC.
The data included in this study were obtained from the Surveillance, Epidemiology, and End Results (SEER) database. The analysis of unidentified data in the SEER database did not require informed consent and was exempt from medical ethics review. We retrospectively searched for the data of female patients with stage IV BIDC from 2010 to 2015 and conducted a retrospective study. Patients who met the following inclusion criteria were included into the research: (1) 20 ≤ age ≤80; (2) first tumor; (3) histologically diagnosed as IDC; (4) survival time ≥1 month; (5) demographic variables and tumor characteristics are all available. In addition, patients diagnosed with autopsy or death certificate were excluded from this study. Totally, 5,475 patients (2,375 in the surgery group and 3,100 patients in the non-surgery group) were included to form a PSM cohort to explore the impact of locoregional surgery on survival in female patients with stage IV BIDC. Afterward, 2,375 patients in the surgery group were randomly divided into the training group and testing group in a ratio of 7:3. Patients in the training set were used to develop the model, and patients in the testing set were used to validate the performance of the model.
Statistical analysis was conducted using SPSS 24.0 and R 4.0.2. All statistical tests were bilateral, and p < 0.05 was considered statistically significant. After stage IV BIDC patients were divided into surgery and non-surgery groups, the PSM method was implemented to construct paired matched samples of two treatment groups to balance confounding variables. More specifically, patients in the two groups were 1:1 matched on the logit scale, using variables of p < 0.05 to generate propensity scores, with a caliper value of 0.0001. Then, the differences of variables between the surgery and non-surgery groups (before and after PSM) were evaluated by the chi-square test. We determined overall survival (OS) and cancer-specific survival (CSS) as two primary endpoints in this research. OS was measured as the time from BC diagnosis to the date of death due to any cause (including BC) or the date of last follow-up. CSS was measured as the time from the date of BC diagnosis to the date of BC death or the date of last follow-up. Subsequently, the Kaplan–Meier (K-M) method with the log-rank test was generated to observe the differences in OS and CSS between the surgery and non-surgery groups. Additionally, univariate and multivariate Cox regression analyses were performed to assess the independent protective value of primary tumor resection, and to identify CSS-related independent clinicopathological factors.
We hypothesized that not all female patients with stage IV BIDC would benefit from primary tumor surgery. Under this assumption, patients who received primary tumor resection were divided into two groups, the benefit group and the non-benefit group, based on the median CSS time of the non-surgery group after PSM (33 months). Then, the univariate logistic analysis was used to determine the factors affecting the benefit of locoregional surgery. Significant variables with p < 0.05 in the univariate analysis were incorporated into the multivariate logistic analysis to further reveal the independent predictors of surgical benefit for patients with stage IV BIDC. Subsequently, on the basis of the surgery-benefit-associated factors, we established a novel nomogram with the “rms” package, a simple, multivariate oncology visualization tool for predicting and quantifying outcome rates for individual patients (20), to identify candidates with stage IV BIDC for primary tumor resection and quantify the probability of surgical benefit. To validate the performance of the screening nomogram for patients with stage IV BIDC, we generated the receiver operating characteristic (ROC) curves and compared the corresponding area under the curve (AUC) values of the nomogram and individual surgery-benefit-associated factor in the training and testing sets, respectively. Furthermore, the discrimination and clinical practicability of the nomogram were evaluated by the calibration plot and decision curve analysis (DCA).
Moreover, to further verify the applicability of the model in the absence of prospective research data, we assessed the performance of the nomogram in the PSM cohort. Based on the score of each patient calculated by the nomogram, we divided all female patients with stage IV BIDC into three groups: (1) Surgery-Benefit group; (2) Surgery-Nonbenefit group; and (3) Non-surgery group. Specifically, patients in the surgery group with a probability (calculated by the nomogram score) greater than 50% were assigned to the Surgery-Benefit group, while others were assigned to the Surgery-Nonbenefit group. Finally, K-M survival analysis with the log-rank test (overall and pairwise comparisons) was implemented to compare the CSS status among the above three groups, so as to test whether the model could quantify the probability of surgical benefit and identify surgical candidates.
In total, 13,285 female patients with stage IV BIDC at initial diagnosis between 2010 and 2015 were included in this study and 5,475 met the criteria (Figure 1). As shown in Table 1, 3,100 patients (56.62%) did not receive primary tumor surgery and 2,375 did (43.38%). There were significant differences between these two groups. Patients with locoregional surgery were more likely to be younger, married, and insured, and have higher histologic grade, higher T stage, higher N stage, and higher proportion of bone-only metastasis. Furthermore, those who received chemotherapy and radiation therapy also tended to undergo locoregional surgery. This indicated an imbalance in the baseline characteristics between the two groups.
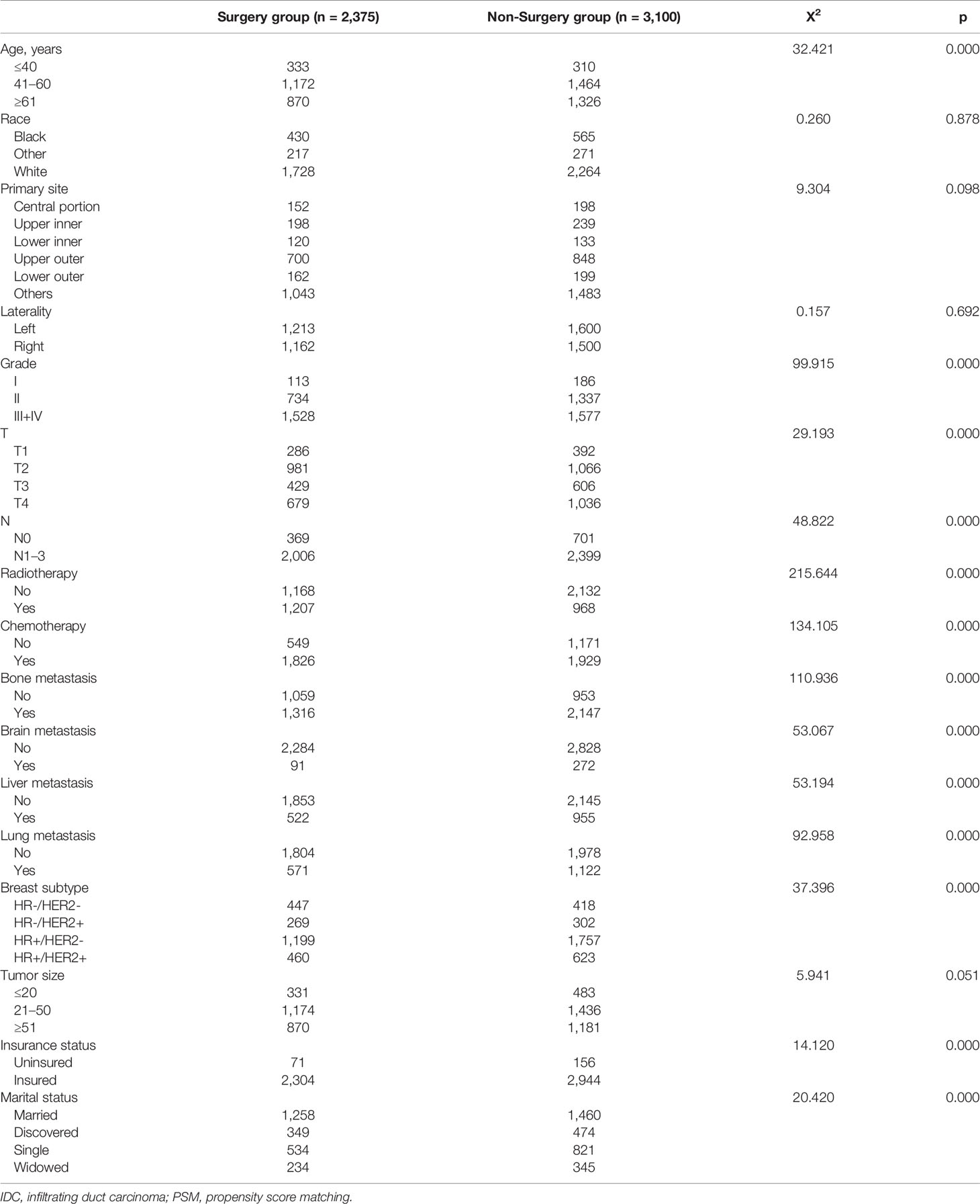
Table 1 Clinical and pathological characteristics for female patients with stage IV IDC of breast before PSM.
To eliminate the patient-dependent bias, the 1:1 matched PSM method was generated, and 2,056 female patients with stage IV BIDC (1,028 in the surgery group and 1,028 in the non-surgery group) were included for the following survival analysis. Baseline characteristics, including age, race, primary site, laterality, histologic grade, T stage, N stage, radiation therapy, chemotherapy, metastatic site (bone, brain, liver, lung), molecular subtype, tumor size, insurance status, and marital status, were all balanced (p > 0.05), as seen in Table 2. Moreover, we compared the characteristics for the surgery/non-surgery group before and after PSM (Supplementary Tables 1, 2). The distributions of grade, N stage, radiotherapy status, chemotherapy status, bone metastasis status, liver metastasis, and molecular subtype were unbalanced for patients in the surgery group before and after PSM, while grade, T stage, N stage, radiotherapy status, chemotherapy status, bone metastasis status, brain metastasis status, liver metastasis status, lung metastasis status, and insurance status were unevenly distributed for the non-surgery group.
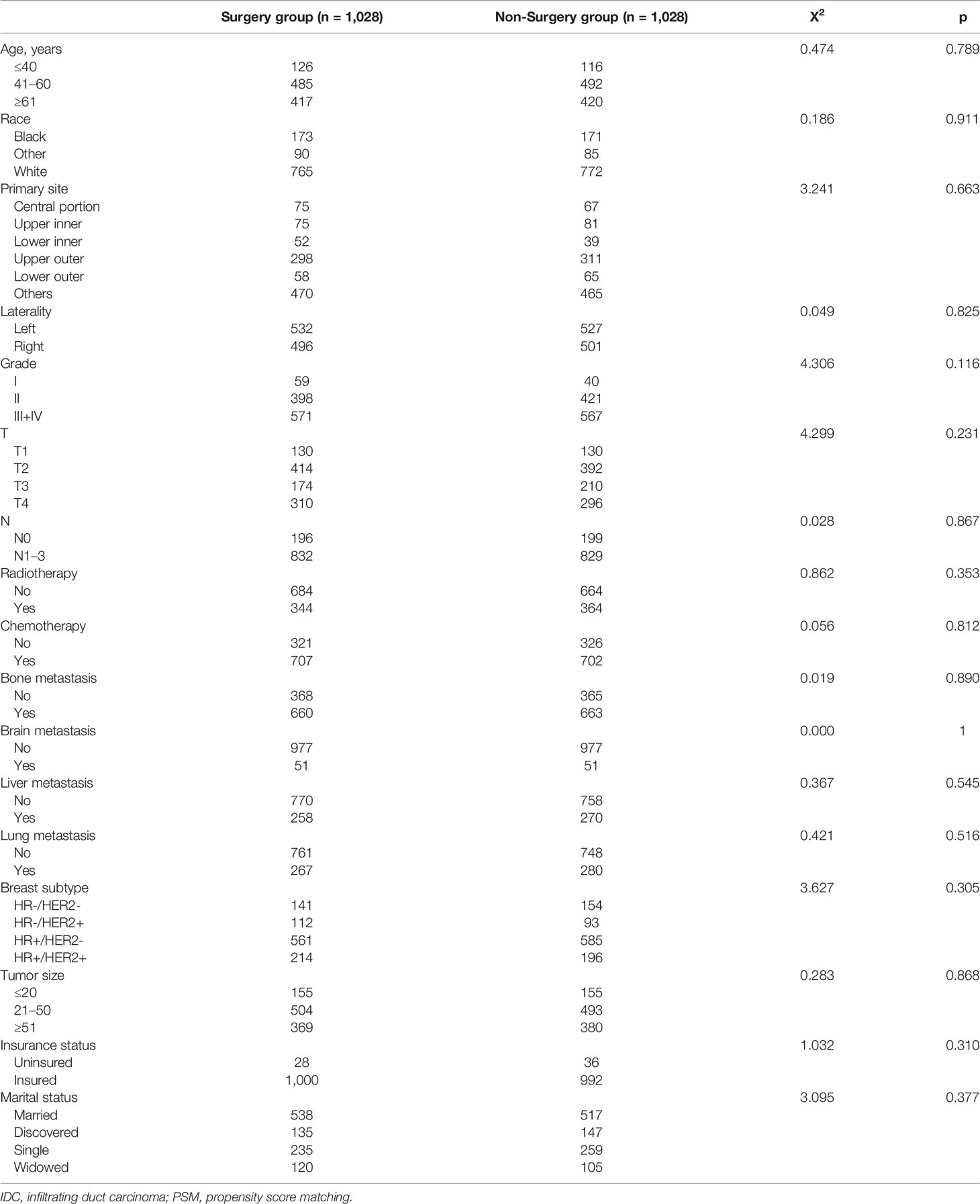
Table 2 Clinical and pathological characteristics for female patients with stage IV IDC of breast after PSM.
The median OS time, median CSS time, and 1–5-year OS/CSS survival rate for the surgery and non-surgery groups (before and after PSM) are shown in Supplementary Table 3. After eliminating the patient-dependent bias of the surgery and non-surgery groups with the help of PSM analysis, the effect of primary tumor resection on the prognosis of patients with stage IV BIDC could be further studied. As shown in Figures 2A–D, the K-M survival analysis indicated that, among patients in the 1:1 matched group, those who underwent primary tumor resection had longer OS and CSS time both before and after PSM than those who did not. The median CSS time was 53 months (95% CI: 46.84–59.16) in the surgery group as compared to only 33 months (95% CI: 30.05–35.95) in the non-surgery group (Table 3). Additionally, the 3-year CSS rate and 5-year CSS rate were 0.641 (95% CI: 0.583–0.648) and 0.459 (95% CI: 0.422–0.501) for the surgery group and 0.473 (95% CI: 0.439–0.510) and 0.281 (95% CI: 0.240-0.330) for the non-surgery group (Table 4). To further investigate the prognostic role of locoregional surgery, univariate and multivariate analyses for CSS were implemented, and the result concluded that primary tumor resection was clearly an independent protective factor (Table 5). Other than that, age, histological grade, T stage, site of metastasis (bone, brain, liver, lung), molecular subtype, and marital status could also independently affect the clinical outcome of patients with stage IV BIDC (Table 5).
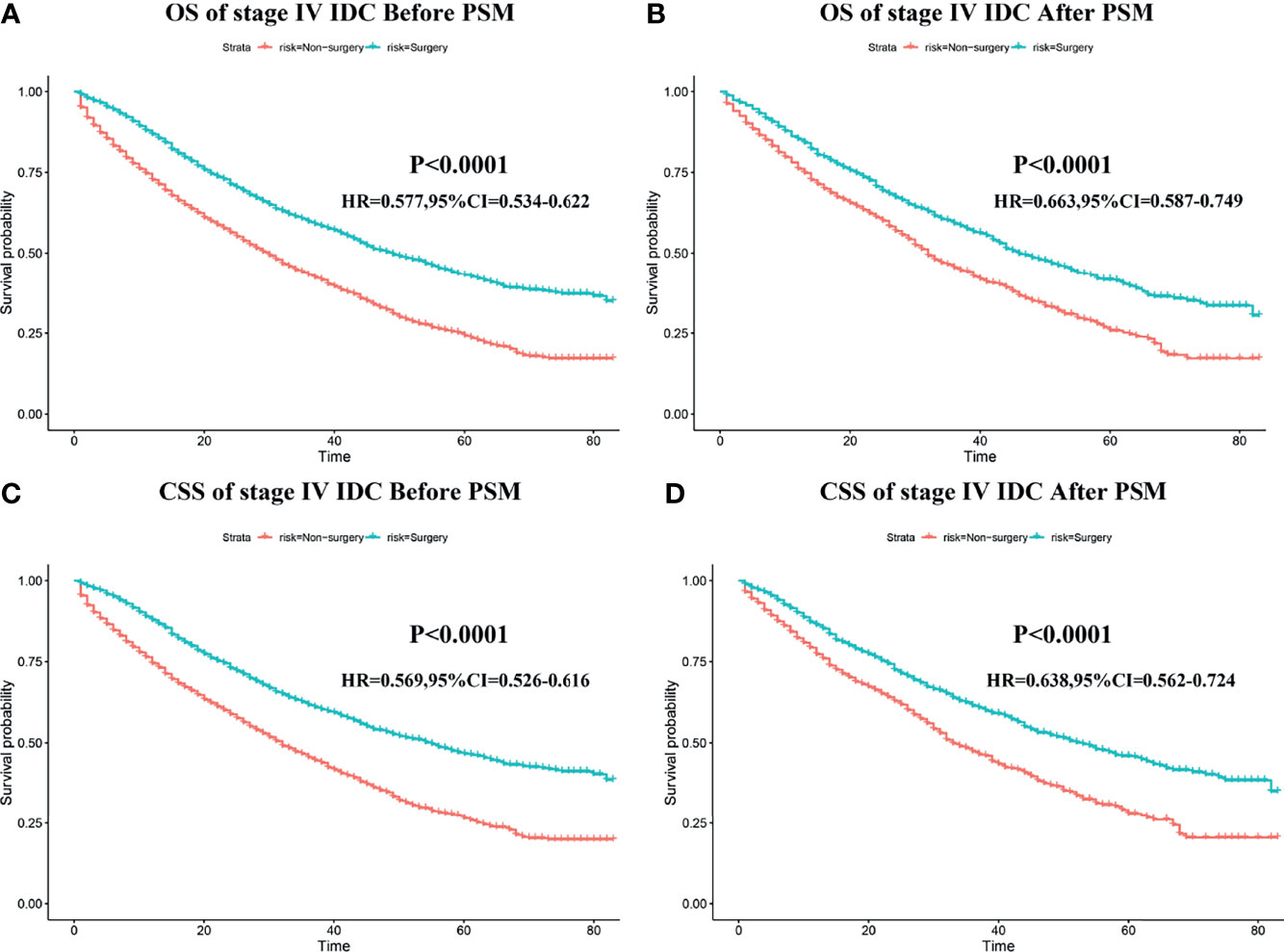
Figure 2 The impart of primary tumor resection on the survival outcomes of patients with stage IV BIDC. Kaplan–Meier (K-M) survival curves of overall survival (OS) before PSM (A) and after PSM (B), and cancer-specific survival (CSS) before PSM (C) and after PSM (D) in the surgery and non-surgery groups.
We hypothesized that not all patients would benefit from primary site surgery and defined that only patients in the surgery group who had a median CSS time longer than the non-surgery group (33 months) would benefit. For the rigor of this study, we deleted two types of patients (n = 563) in the surgery group who could not be determined as benefit or not, as follows: (1) CSS status = 0 and survival time < 33 months and (2) CSS status = 1 and survival time = 33 months. With this assumption, patients in the surgery group with a median CSS time of more than 33 months were categorized as the benefit group (1,064 patients), while those with less than 33 months were categorized as the non-benefit group (748 patients). Thereafter, univariate and multivariate logistic analyses were performed to determine the independent factors influencing the benefit from primary tumor resection of patients with stage IV BIDC. The independent surgery-benefit-associated predictors for primary tumor resection included histologic grade (p < 0.001), T stage (p < 0.001), molecular subtype (p < 0.001), lung metastasis (p = 0.005), liver metastasis (p < 0.001), brain metastasis (p < 0.001), and marital status (p = 0.002) (Table 6). Based on the above seven independent factors, a novel nomogram was established to identify female candidates with stage IV BIDC for locoregional surgery (Figure 3). By adding up the score for each corresponding variable, the resulting total score could quantify the probability of surgical benefit.
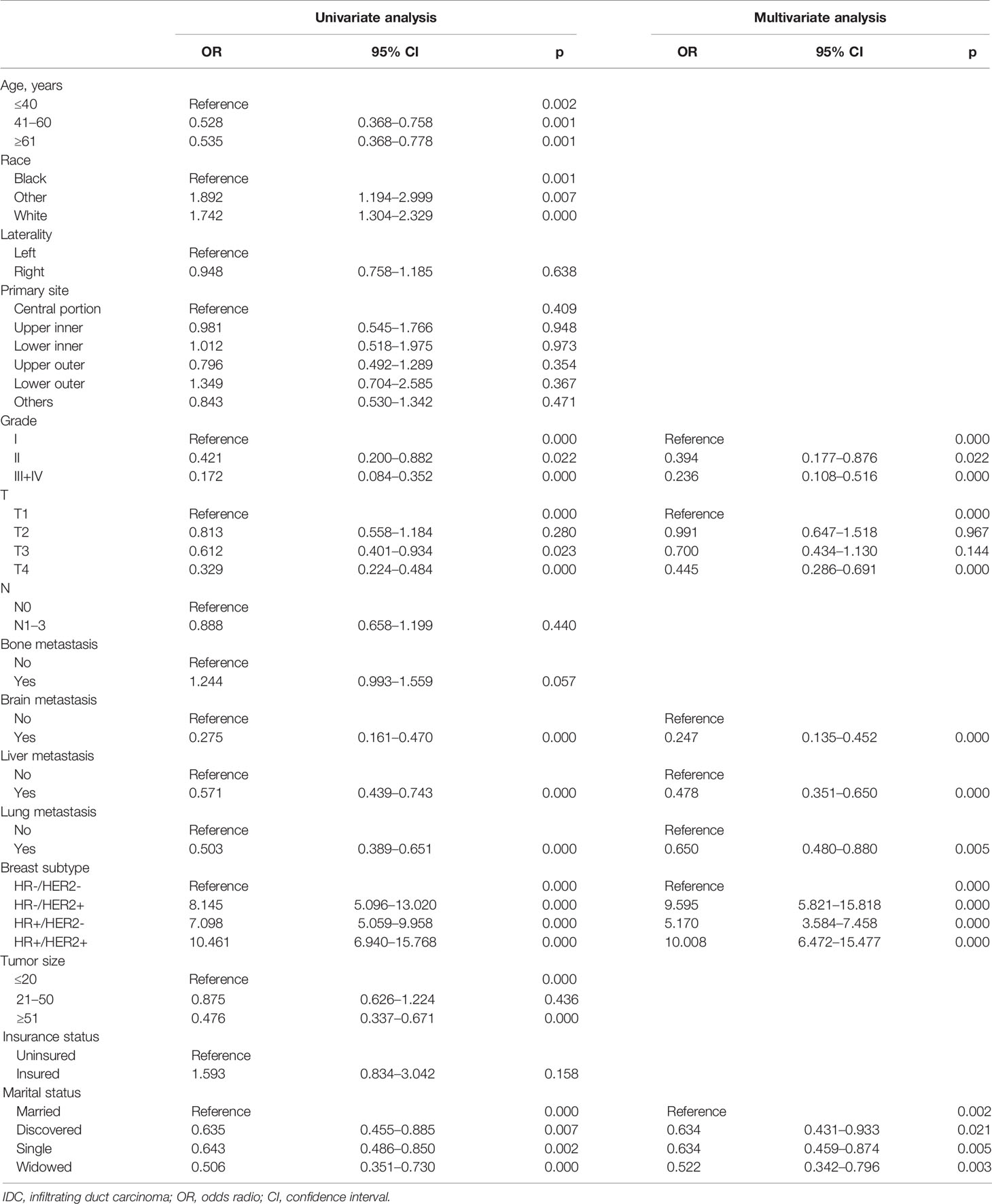
Table 6 Univariate and multivariate logistic analyses of benefit in female patients with stage IV IDC of breast.
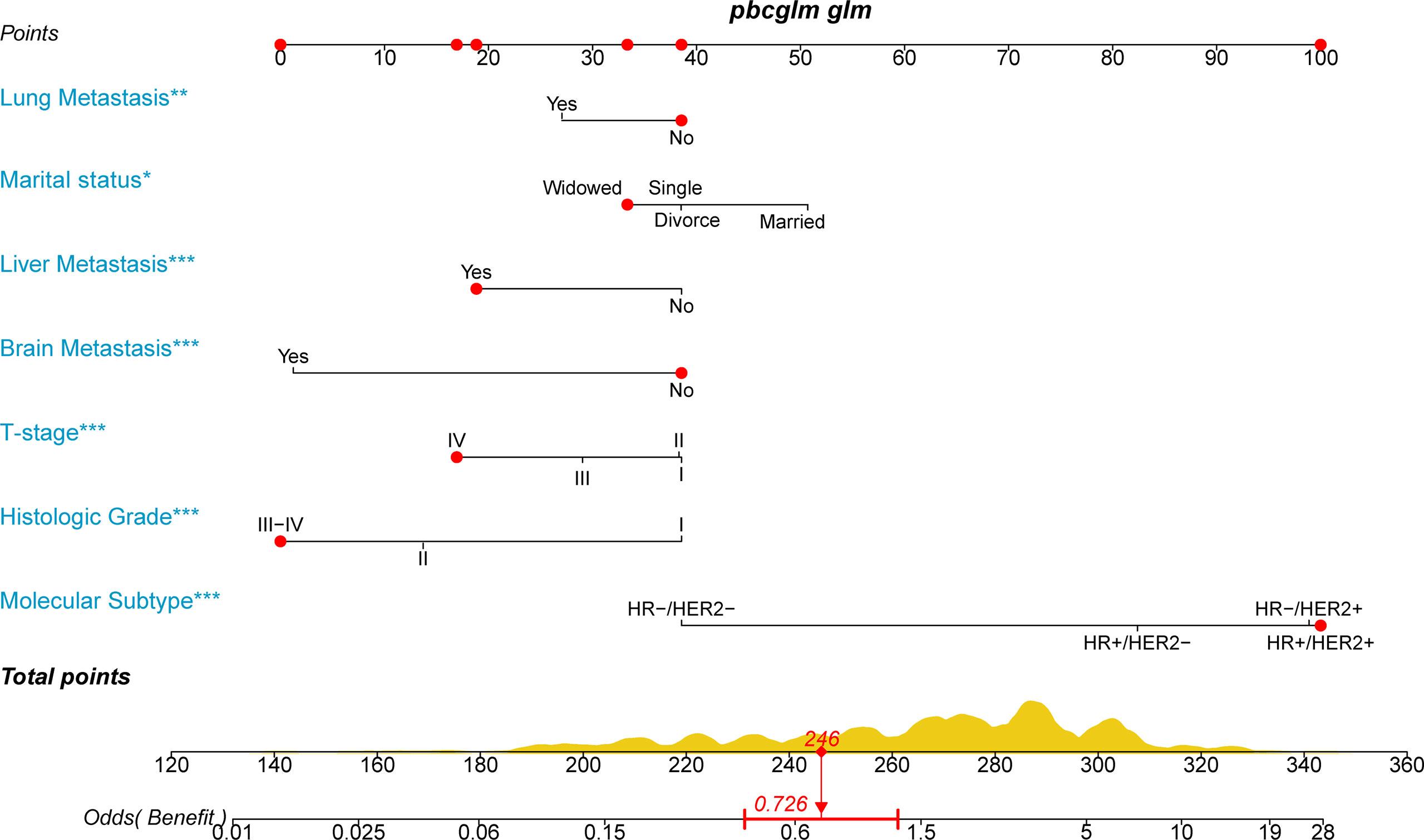
Figure 3 A novel nomogram to select candidates with stage IV BIDC for primary site surgery and predict the possibility of surgical benefit.
We drew ROC curves for the training and testing sets, with the AUC of the nomogram being 0.785 in the training set and 0.761 in the testing set (Figure 4A and Figure 5A). As shown in Figure 4B and Figure 5B, the calibration curves showed a good correlation between predictions and actual observations for patients with stage IV BIDC both in the training and testing sets. Besides, DCA curves also suggested that the nomogram presented good predictive ability and could be a precise tool for identifying surgical candidates clinically (Figure 4C and Figure 5C). In addition, ROC curves were also generated for each independent predictor variable (Figures 6A, B), and the results implied that the AUC of the nomogram was higher than the AUC of all predictors individually, in both the training and testing sets. Additionally, we further validated the screening nomogram in the entire cohort and obtained good results with an AUC of 0.778 (Supplementary Figure 1A). The calibration plot also presented good agreement, and DCA showed the prediction accuracy in a wider range (Supplementary Figures 1B, C).
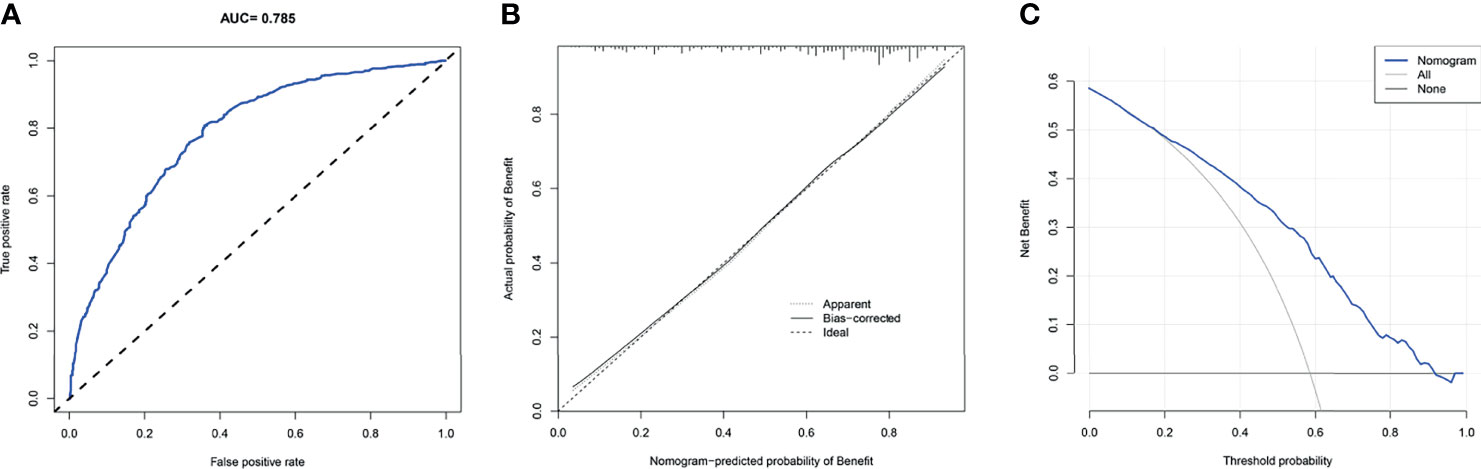
Figure 4 The receiver operating characteristic curve (A), calibration curve (B), and decision curve analysis (C) of the screening nomogram in the training set.
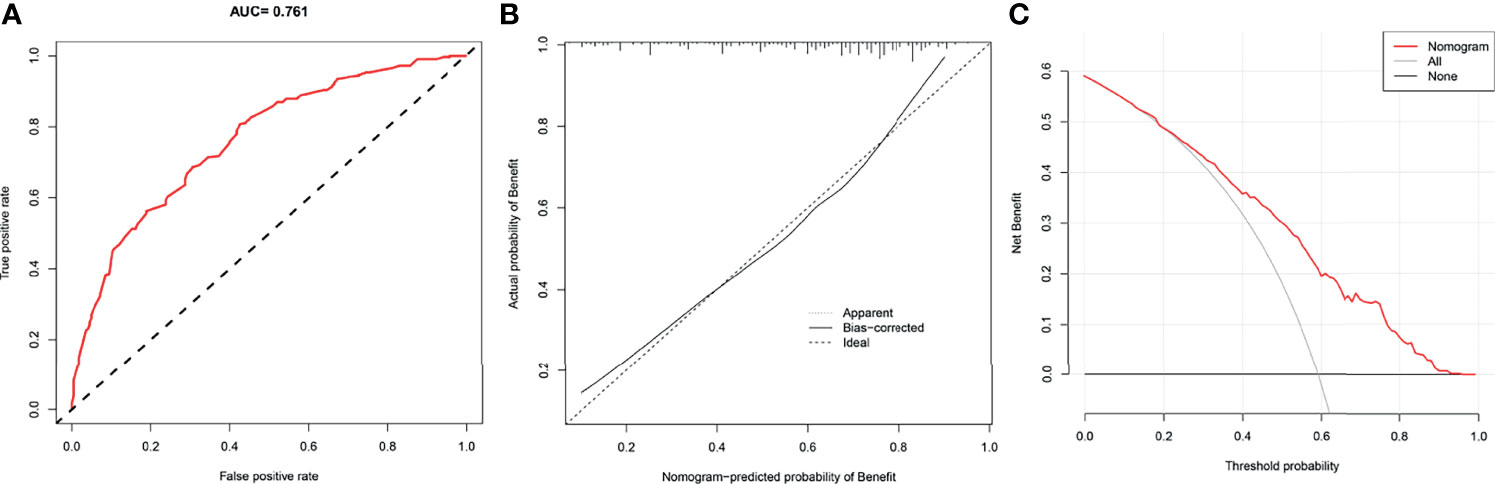
Figure 5 The receiver operating characteristic curve (A), calibration curve (B), and decision curve analysis (C) of the screening nomogram in the testing set.
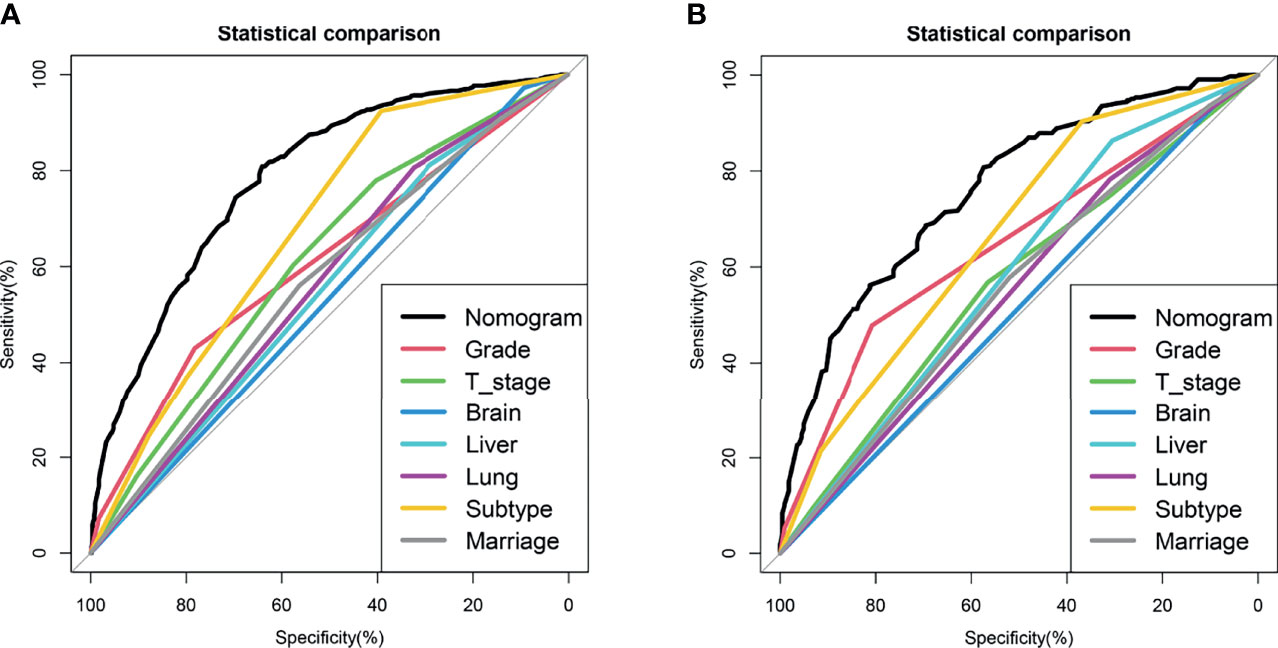
Figure 6 Comparison of area under the receiver operating characteristic curve between nomogram and each independent predictors in the training set (A) and the testing set (B).
Moreover, to further verify the applicability of the model in the absence of prospective research data, we went back to the PSM cohort for application validation. As described before, 728 patients with stage IV BIDC were assigned to the Surgery-Benefit (S-Benefit) group, 300 patients were assigned to the Surgery-Nonbenefit (S-Nonbenefit) group, and the remaining 1,028 were in the Non-Surgery group. Subsequently, we generated the K-M survival curves with the log-rank test to verify the discrimination ability of the screening nomogram (Figure 7). The results showed that patients in the S-Benefit group had a higher survival advantage than patients in the Non-surgery group (HR = 0.437, 95% CI, 0.376–0.508, p < 0.001), whereas patients in the S-Nonbenefit group presented poorer CSS than patients in the Non-Surgery group (HR = 1.535, 95% CI, 1.298–1.815, p < 0.001). These data further confirmed our hypothesis that not all stage IV BIDC patients would benefit from primary tumor surgery, and some patients may even have shorter postoperative survival time.
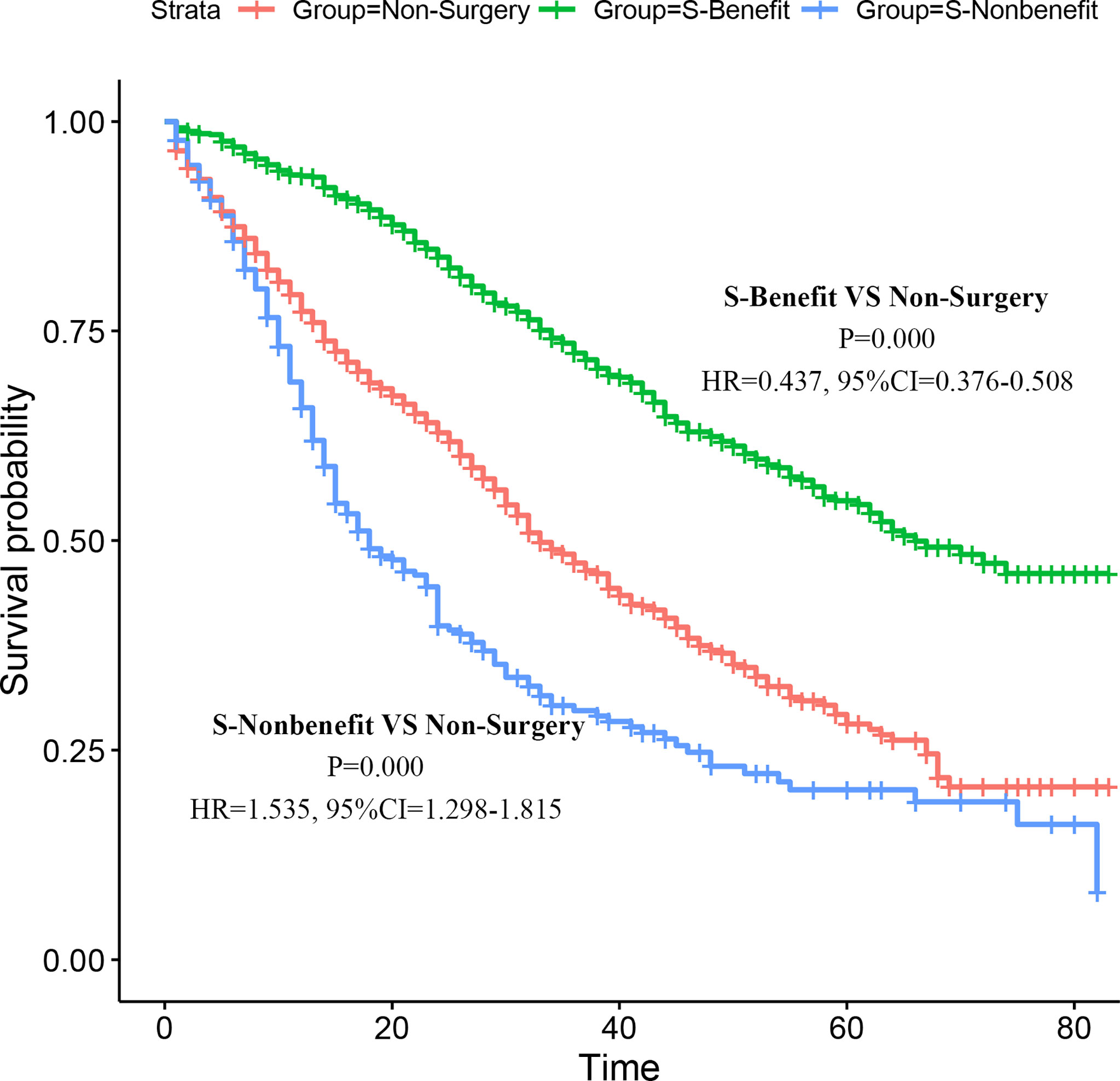
Figure 7 Validation of the nomogram in the PSM cohort. Kaplan–Meier (K-M) survival analysis of the patients in the S-Benefit, S-Nonbenefit, and Non-Surgery groups.
To apply the nomogram, we first draw a vertical line up from the corresponding point of each variable to obtain the corresponding score and then add up the scores of each variable and draw a vertical line down from the total score row to get the probability of benefit from primary tumor resection (Figure 3). For example, if a widow with stage IV BIDC presents liver metastases, T4, histological grade IV, and molecular subtype HR-/HER2+, she will have a total score of 246, corresponding to an OR value of 0.726 (<1), indicating that she may not obtain a survival advantage from primary tumor resection. The novel nomogram is expected to be an effective screening tool for quantifying surgical benefit in female patients with stage IV BIDC, which may help oncologists make clinical decisions.
Approximately 6%–10% of female patients were diagnosed with stage IV BC (18), and about 20%–30% of early-stage patients would develop distant metastasis (21). Stage IV BC occurs when the tumor metastasizes from the breast and axilla to distant sites, most often to the bone (48%), brain (15%), liver, and lungs (21–23). The role of primary site surgery in the treatment of stage IV BC is still controversial. First, our study showed that primary site surgery improved prognosis in female patients with stage IV BIDC. The second aim of this study was to develop a nomogram to select candidates for locoregional surgery in female patients with stage IV BIDC and verify our hypothesis that not all female patients with stage IV BIDC are suitable for primary tumor resection.
The conventional view is that resection of the primary tumor in patients with stage IV BC is not associated with prolonged survival (except in patients with bone disease), and surgery may be only considered for certain patients whose systemic disease is under control, mainly to improve quality of life (QoL) (24–26). However, several recent retrospective population-based studies have shown that primary tumor resection has a positive effect on survival in patients with stage IV BC (18, 27–29). The earliest and largest relevant retrospective study was implemented by Khan and colleagues of the National Cancer Database (NCDB) from 1990 to 1993, in which they included 16,023 patients with stage IV BC. Of the patients, 42.8% did not undergo primary site surgery and 57.2% did (including partial and total mastectomy). The observed 3-year OS rate was 24.9%, including 31.8% for total mastectomy, 27.7% for partial mastectomy, and 17.3% for non-surgery (17). Additionally, a recent retrospective cohort study included 21,372 female patients diagnosed with stage IV BC between 1988 and 2011 and reached the similar result. Patients who underwent surgery had a longer median OS time than those who did not (28 months vs. 19 months) (18). However, the conclusions of these trials may be influenced by selection bias, as patients undergoing surgery tended to be younger or have only one metastatic lesion. Therefore, to address this problem, the propensity score matching (PSM) method was applied in our study to balance the clinical and pathological characteristics in the surgery and non-surgery groups and reduce selection bias. The results of these retrospective studies provided consistent and strong evidence for our finding that patients with stage IV BC who underwent primary tumor resection achieved better survival (30, 31). In addition, as with previous findings, we found that locoregional surgery was apparently an independent protective factor for survival (HR = 0.673, p < 0.001) by multivariate Cox regression analysis. Previous studies indicated that marginal status, systemic therapy, HER2 expression, number of metastatic sites, and type of metastatic disease were independent factors affecting the prognosis of BC patients (32–34). In contrast to these studies, we integrated different variables and further found that older age, higher T stage, higher histologic grade, distant metastases other than bone metastases (especially brain metastases, HR = 2.037), triple-negative breast cancer, and non-married status were associated with poor survival outcomes. Furthermore, in future work, we should compare the risk factors between the surgery group and all patients. The factors that lead to poor prognosis in the surgery group and do not affect the prognosis of all patients can be regarded as the signal of not accepting surgery, which is very valuable. Although retrospective studies have shown that primary tumor resection improves survival in patients with stage IV BC, there is insufficient evidence for prospective studies. The results of two international prospective studies (NCT00193778 trial and MF07-01 trial) on the role of locoregional surgery in the survival of patients with stage IV BC were inconsistent, primarily related to differences in postoperative systemic treatment. Thus, there is an urgent need for larger, multicenter prospective studies.
Although surgical interventions have shown better survival outcomes, whether all patients should undergo locoregional surgery needs further discussion, especially in patients with poor surgical tolerance. The unplanned subgroup of the MF07-01 trial found a survival benefit of breast surgery in young patients (<55 years) with bone metastases only and positive ER/PR status (35). In addition, another retrospective study indicated that stage IV BC patients with only bone metastases had prolonged postoperative survival (30). Existing studies lack a screening model to identify candidates for primary site surgery, so our study developed a nomogram to quantify the probability of surgical benefit. Our research suggested that seven independent indicators are associated with whether patients benefit from locoregional surgery, including histological grade, T stage, molecular subtype, lung metastasis, liver metastasis, brain metastasis, and marital status. Molecular subtype was most strongly associated with the probability of surgical benefit, followed by the histologic grade and brain metastasis. First, patients with stage IV BIDC of the molecular subtype HER2-/ER- were least likely to benefit from locoregional surgery. The reason may be that TNBC is the most aggressive group of BC, with poor prognosis and high recurrence rate. In addition, previous studies have reported that basal BC is prone to visceral metastases, especially brain and lung (36–38). Besides, our study suggested that patients with higher histologic grade of tumor were less likely to benefit from surgery. This can be explained by the fact that histologic grade is widely recognized as an important prognostic factor and that tumors with higher histologic grade are more prone to metastasize and relapse. We found that patients with brain metastases were less likely to benefit from surgery, possibly due to rapid disease progression, poor quality of life, and high mortality (21). In addition, Li et al. confirmed that patients only with brain metastases had a lower survival rate than patients with multisite metastases (excluding brain metastases) (39). Moreover, advanced T stage was found to have a negative effect on surgical benefit, which is consistent with the previous conclusion that advanced tumors suitable for surgical treatment should be small. It may be that the larger the tumor, the more likely it is to invade the chest wall, the more difficult it is to ensure a negative surgical margin (40). Hence, our screening nomogram incorporating these predictors may be useful for quantifying the probability of surgical benefit, providing guidance for further personalized clinical management.
To our knowledge, this is the first screening nomogram to quantify the probability of surgical benefit in female patients with stage IV BIDC and identify surgical candidates clinically. However, our study has some shortcomings. First, the information in the SEER database is not complete, such as surgical method, endocrine or targeted therapy status, and general health status of patients. Second, although the screening nomogram was established in the training set, and verified in the testing set, entire cohort, and PSM cohort, prospective studies are still needed. Third, as a retrospective study, only patients with stage IV BIDC at initial diagnosis were included, and metastatic disease that occurred in the latter stage cannot be identified, which may reduce some clinical guidance value.
Our study suggested that primary tumor resection improved the survival of female patients with stage IV BIDC and developed a nomogram to quantify the probability of surgical benefit to help identify surgical candidates, which may help oncologists make clinical decisions.
Publicly available datasets were analyzed in this study. These data can be found here: https://seer.cancer.gov/.
ZQW, BC, and XXD conceived of and designed the study. JYC, ZXW, and HYG performed the literature search. BC and YW generated the figures and tables. ZQW and BC analyzed the data and wrote the manuscript. XXD critically reviewed the manuscript and supervised the research. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.798016/full#supplementary-material
Supplementary Figure 1 | The receiver operating characteristic curve (A), calibration curve (B), and decision curve analysis (C) of the screening nomogram in the entire cohort.
Supplementary Table 1 | Comparison of clinical characteristics for the surgery group between before and after PSM.
Supplementary Table 2 | Comparison of clinical characteristics for the non-surgery group between before and after PSM.
Supplementary Table 3 | Comparison of median survival time and survival rate between the surgery and non-surgery groups.
Supplementary Table 4 | The data of stage IV BIDC patients in the surgery and non-surgery groups before PSM.
Supplementary Table 5 | The data of stage IV BIDC patients in the surgery and non-surgery groups after PSM.
1. DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A, et al. Breast Cancer Statistics, 2019. CA Cancer J Clin (2019) 69(6):438–51. doi: 10.3322/caac.21583
2. Garcia-Murillas I, Chopra N, Comino-Méndez I, Beaney M, Tovey H, Cutts R, et al. Assessment of Molecular Relapse Detection in Early-Stage Breast Cancer. JAMA Oncol (2019) 5(10):1473–8. doi: 10.1001/jamaoncol.2019.1838
3. Liu Q, Cheng R, Kong X, Wang Z, Fang Y, Wang J. Molecular and Clinical Characterization of PD-1 in Breast Cancer Using Large-Scale Transcriptome Data. Front Immunol (2020) 11:558757. doi: 10.3389/fimmu.2020.558757
4. Li Y, Shui L, Wang X, Sun Y, Zhong R, Shui P, et al. Long-Term Results of Partial Breast Irradiation After Breast-Conserving Surgery for Early Stage Breast Cancer: A Prospective Phase II Trial in China. Front Oncol (2020) 10:550950. doi: 10.3389/fonc.2020.550950
5. Higgins M, Wolff A. Therapeutic Options in the Management of Metastatic Breast Cancer. Oncol (Williston Park NY) (2008) 22(6):614–23; discussion 23, 27-9.
6. Redig A, McAllister S. Breast Cancer as a Systemic Disease: A View of Metastasis. J Intern Med (2013) 274(2):113–26. doi: 10.1111/joim.12084
7. Roy A, Zhao Y, Yang Y, Szeitz A, Klassen T, Li S. Selective Targeting and Therapy of Metastatic and Multidrug Resistant Tumors Using a Long Circulating Podophyllotoxin Nanoparticle. Biomaterials (2017) 137:11–22. doi: 10.1016/j.biomaterials.2017.05.019
8. Siegel R, Miller K, Jemal A. Cancer Statistics, 2018. CA Cancer J Clin (2018) 68(1):7–30. doi: 10.3322/caac.21442
9. Tyagi NK, Dhesy-Thind S. Clinical Practice Guidelines in Breast Cancer. Curr Oncol (2018) 25(Suppl 1):S151–s60. doi: 10.3747/co.25.3729
10. Sánchez-Muñoz A, Pérez-Ruiz E, Ribelles N, Márquez A, Alba E. Maintenance Treatment in Metastatic Breast Cancer. Expert Rev Anticancer Ther (2008) 8(12):1907–12. doi: 10.1586/14737140.8.12.1907
11. Sharma S, Wu S, Jimenez H, Xing F, Zhu D, Liu Y, et al. Ca and CACNA1H Mediate Targeted Suppression of Breast Cancer Brain Metastasis by AM RF EMF. EBioMedicine (2019) 44:194–208. doi: 10.1016/j.ebiom.2019.05.038
12. Kaya P, Lee S, Lee Y, Kwon S, Yang H, Lee H, et al. Curcumae Radix Extract Decreases Mammary Tumor-Derived Lung Metastasis via Suppression of C-C Chemokine Receptor Type 7 Expression. Nutrients (2019) 11(2):410. doi: 10.3390/nu11020410
13. Łukasiewicz S, Czeczelewski M, Forma A, Baj J, Sitarz R, Stanisławek A. Breast Cancer-Epidemiology, Risk Factors, Classification, Prognostic Markers, and Current Treatment Strategies-An Updated Review. Cancers (Basel) (2021) 13(17):4287. doi: 10.3390/cancers13174287
14. Tohme S, Simmons R, Tsung A. Surgery for Cancer: A Trigger for Metastases. Cancer Res (2017) 77(7):1548–52. doi: 10.1158/0008-5472.can-16-1536
15. Krall J, Reinhardt F, Mercury O, Pattabiraman D, Brooks M, Dougan M, et al. The Systemic Response to Surgery Triggers the Outgrowth of Distant Immune-Controlled Tumors in Mouse Models of Dormancy. Sci Trans Med (2018) 10(436):eaan3464. doi: 10.1126/scitranslmed.aan3464
16. Yu Y, Hong H, Wang Y, Fu T, Chen Y, Zhao J, et al. Clinical Evidence for Locoregional Surgery of the Primary Tumor in Patients With De Novo Stage IV Breast Cancer. Ann Surg Oncol (2021) 28(9):5059–70. doi: 10.1245/s10434-021-09650-3
17. Khan SA, Stewart AK, Morrow M. Does Aggressive Local Therapy Improve Survival in Metastatic Breast Cancer? Surgery (2002) 132(4):620–6; discussion 6-7. doi: 10.1067/msy.2002.127544
18. Thomas A, Khan SA, Chrischilles EA, Schroeder MC. Initial Surgery and Survival in Stage IV Breast Cancer in the United States, 1988-2011. JAMA Surg (2016) 151(5):424–31. doi: 10.1001/jamasurg.2015.4539
19. Cheung Y, Chen K, Yu C, Ueng S, Li C, Chen S. Contrast-Enhanced Mammographic Features of In Situ and Invasive Ductal Carcinoma Manifesting Microcalcifications Only: Help to Predict Underestimation? Cancers (Basel) (2021) 13(17):4371. doi: 10.3390/cancers13174371
20. Balachandran V, Gonen M, Smith J, DeMatteo R. Nomograms in Oncology: More Than Meets the Eye. Lancet Oncol (2015) 16(4):e173–80. doi: 10.1016/s1470-2045(14)71116-7
21. Diossy M, Reiniger L, Sztupinszki Z, Krzystanek M, Timms K, Neff C, et al. Breast Cancer Brain Metastases Show Increased Levels of Genomic Aberration-Based Homologous Recombination Deficiency Scores Relative to Their Corresponding Primary Tumors. Ann Oncol (2018) 29(9):1948–54. doi: 10.1093/annonc/mdy216
22. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin (2017) 67(1):7–30. doi: 10.3322/caac.21387
23. Chen M, Sun H, Zhao Y, Fu W, Yang L, Gao S, et al. Comparison of Patterns and Prognosis Among Distant Metastatic Breast Cancer Patients by Age Groups: A SEER Population-Based Analysis. Sci Rep (2017) 7(1):9254. doi: 10.1038/s41598-017-10166-8
24. Santa-Maria C, Gradishar W. Changing Treatment Paradigms in Metastatic Breast Cancer: Lessons Learned. JAMA Oncol (2015) 1(4):528–34; quiz 49. doi: 10.1001/jamaoncol.2015.1198
25. Bleicher R, Ruth K, Sigurdson E, Beck J, Ross E, Wong Y, et al. Time to Surgery and Breast Cancer Survival in the United States. JAMA Oncol (2016) 2(3):330–9. doi: 10.1001/jamaoncol.2015.4508
26. Cardoso F, Paluch-Shimon S, Senkus E, Curigliano G, Aapro M, André F, et al. 5th ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 5). Ann Oncol (2020) 31(12):1623–49. doi: 10.1016/j.annonc.2020.09.010
27. Lang J, Tereffe W, Mitchell M, Rao R, Feng L, Meric-Bernstam F, et al. Primary Tumor Extirpation in Breast Cancer Patients Who Present With Stage IV Disease Is Associated With Improved Survival. Ann Surg Oncol (2013) 20(6):1893–9. doi: 10.1245/s10434-012-2844-y
28. Warschkow R, Güller U, Tarantino I, Cerny T, Schmied B, Thuerlimann B, et al. Improved Survival After Primary Tumor Surgery in Metastatic Breast Cancer: A Propensity-Adjusted, Population-Based SEER Trend Analysis. Ann Surg (2016) 263(6):1188–98. doi: 10.1097/sla.0000000000001302
29. Arciero C, Liu Y, Gillespie T, Subhedar P. Surgery and Survival in Patients With Stage IV Breast Cancer. Breast J (2019) 25(4):644–53. doi: 10.1111/tbj.13296
30. Lin Y, Huang K, Zeng Q, Zhang J, Song C. Impact of Breast Surgery on Survival of Patients With Stage IV Breast Cancer: A SEER Population-Based Propensity Score Matching Analysis. PeerJ (2020) 8:e8694. doi: 10.7717/peerj.8694
31. Huang Z, Tan Q, Qin Q, Mo Q, Wei C. Impact of Primary Site Surgery on Survival of Patients With De Novo Stage IV Breast Cancer. Cancer Manag Res (2021) 13:319–27. doi: 10.2147/cmar.s280470
32. Xiong Z, Deng G, Wang J, Li X, Xie X, Shuang Z, et al. Could Local Surgery Improve Survival in De Novo Stage IV Breast Cancer? BMC Cancer (2018) 18(1):885. doi: 10.1186/s12885-018-4767-x
33. Blanchard D, Shetty P, Hilsenbeck S, Elledge R. Association of Surgery With Improved Survival in Stage IV Breast Cancer Patients. Ann Surg (2008) 247(5):732–8. doi: 10.1097/SLA.0b013e3181656d32
34. Choi S, Kim J, Choi J, Sohn J, Kim S, Park S, et al. Locoregional Treatment of the Primary Tumor in Patients With De Novo Stage IV Breast Cancer: A Radiation Oncologist's Perspective. Clin Breast Cancer (2018) 18(2):e167–78. doi: 10.1016/j.clbc.2017.06.002
35. Soran A, Ozmen V, Ozbas S, Karanlik H, Muslumanoglu M, Igci A, et al. Randomized Trial Comparing Resection of Primary Tumor With No Surgery in Stage IV Breast Cancer at Presentation: Protocol MF07-01. Ann Surg Oncol (2018) 25(11):3141–9. doi: 10.1245/s10434-018-6494-6
36. Fulford L, Reis-Filho J, Ryder K, Jones C, Gillett C, Hanby A, et al. Basal-Like Grade III Invasive Ductal Carcinoma of the Breast: Patterns of Metastasis and Long-Term Survival. Breast Cancer Res BCR (2007) 9(1):R4. doi: 10.1186/bcr1636
37. Crabb S, Cheang M, Leung S, Immonen T, Nielsen T, Huntsman D, et al. Basal Breast Cancer Molecular Subtype Predicts for Lower Incidence of Axillary Lymph Node Metastases in Primary Breast Cancer. Clin Breast Cancer (2008) 8(3):249–56. doi: 10.3816/CBC.2008.n.028
38. Smid M, Wang Y, Zhang Y, Sieuwerts A, Yu J, Klijn J, et al. Subtypes of Breast Cancer Show Preferential Site of Relapse. Cancer Res (2008) 68(9):3108–14. doi: 10.1158/0008-5472.can-07-5644
39. Li Y, Wang S, Yang W, Liu H. Prognostic Significance of Molecular Subtype, Metastatic Site and Primary Tumor Surgery for Survival in Primary Metastatic Breast Cancer: A SEER-Based Study. Med (Baltimore) (2021) 100(27):e26619. doi: 10.1097/md.0000000000026619
Keywords: stage IV breast cancer, propensity score matching, surgery, prognosis, nomogram
Citation: Wang Z, Chen B, Chen J, Wu Z, Gu H, Wang Y and Dai X (2022) A Novel Nomogram Model to Identify Candidates and Predict the Possibility of Benefit From Primary Tumor Resection Among Female Patients With Metastatic Infiltrating Duct Carcinoma of the Breast: A Large Cohort Study. Front. Oncol. 12:798016. doi: 10.3389/fonc.2022.798016
Received: 20 October 2021; Accepted: 13 January 2022;
Published: 14 February 2022.
Edited by:
Marco Scarpa, University Hospital of Padua, ItalyReviewed by:
Miguel J. Gil Gil, Catalan Institute of Oncology, SpainCopyright © 2022 Wang, Chen, Chen, Wu, Gu, Wang and Dai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuanxuan Dai, ZGFvc2hpZGFpeHVhbnh1YW5AMTI2LmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.