- 1Department of Obstetrics and Gynecology, First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China
- 2School of Finance, Xi’an Eurasia University, Xi’an, China
- 3Department of Epidemiology and Biostatistics, School of Public Health, Xi’an Jiaotong University Health Science Center, Xi’an, China
Introduction: This meta-analysis evaluated the efficacy and safety of placebo during the maintenance therapy of ovarian cancer (OC) patients in randomized controlled trials (RCTs).
Methods: A comprehensive literature review was performed for RCTs published up to and including August 2020 from four electronic databases. We analyzed the efficacy and safety in the control arms of the maintenance therapy in advanced OC patients. Hazard ratios (HRs) and the corresponding 95% confidence intervals (CIs) of progression-free survival (PFS) and overall survival (OS) were estimated in the placebo arms and the observation arms, respectively, using the Frequency Framework method. We also calculated the incidences of common adverse effects (AEs) in the placebo arms.
Results: In total, 41 articles with 20,099 (4,787 in the placebo arms, 3,420 in the observation arms, and 11,892 in the experiment arms) patients were included in this meta-analysis. Compared with observation, placebo did not improve or reduce PFS (HR, 1.02; 95% CI, 0.87–1.20; P = 0.81) and OS (HR, 1.02; 95% CI, 0.89–1.16; P = 0.76) of OC patients, while other treatments, except for radiotherapy, significantly improved PFS and OS (all P < 0.05). The incidences of AEs produced by placebo were 94.03% in all grades and 20.22% in grade ≥3. The incidences of AEs were 29.75% in fatigue, 26.38% in nausea, 24.34% in abdominal pain, 18.92% in constipation, 16.65% in diarrhea, 14.55% in vomiting, 13.89% in hypertension, and 13.14% in headache.
Conclusions: Placebo did not improve or reduce the PFS and OS benefits of OC patients in RCTs but increased the incidences of AEs.
Introduction
Ovarian cancer (OC) is one of the most common malignant reproductive tumors in women, with high recurrence rate and high mortality. Its median progression-free survival (PFS) and median overall survival (OS) range from 16 to 21 months and from 24 to 60 months, respectively (1). OC is the main cause of death among women in the USA and worldwide, accounting for the fifth and eighth, respectively (2, 3). In 2021, a total of 21,410 newly diagnosed cases and 13,770 deaths due to OC were estimated in America (2).
The standard first-line therapy of OC is debulking surgery in combination with chemotherapy based on paclitaxel and platinum, which can relieve the symptoms of the patients and achieve no evidence of disease progression temporarily. About 70% of the patients encounter a recurrence within 3 years (4). Therefore, many researchers put forward maintenance therapy, including extra-chemotherapy, immunotherapy, antiangiogenic inhibitors (AIs), selective small-module (SSM) inhibitors, and poly-ADP-ribose polymerase (PARP) inhibitors (4–8). All of them were added following complete or partial remission of chemotherapy in order to eschew disease progression and increase PFS and OS. Many randomized controlled trials (RCTs) further confirmed the efficacy and the safety of these maintenance therapies (4, 9–11). Some maintenance drugs, such as olaparib and niraparib, have shown significant positive effects on PFS and OS (4, 9). Most clinical trials on immunotherapy (7, 12–15), chemotherapy (6, 16–18), and SSM inhibitors (8) exhibited negative effects on PFS and OS, with a few studies gaining opposite results (16, 19). However, whether the control arms of RCTs, including placebo or observation, basically have effects on survival in the maintenance process is undefined. Until now, scarce reports have been published on the positive effects of placebo in RCTs.
Placebo, with a long history, mainly contains three forms: pharmacologic (a tablet), physical (a manipulation), and psychological (a conversation) (20). Recently, placebo tablets, an inert substance, have been administrated blindly to patients in clinical trials with an expectation for such to produce clinical benefits through the interaction with a caregiver and healthcare systems (21). The positive effects produced by placebo in clinical trials are not affected by its pharmacologic or physiologic properties (22). The positive effects are the evolution of the disease process altered in a positive direction (22). Researchers compared the differences between placebo and observation directly in the aspects of subjective and objective outcomes, including pain, psychopathy, hypertension, and so on (20). They found that placebo exhibited a few benefits on continuous subjective outcomes and the treatment of pain, but it did not have effects on objective or binary outcomes. Jonas et al. found that the placebo effect was related to the size, color, and label of tablets (23). I Požgain et al. showed that placebo in RCTs was effective for the health status of patients because of their own beliefs (24). Julia W. Haas et al. concerned about the effect of blindness caused by placebo on the treatment of patients with irritable bowel syndrome. The results found that patients with double-blind placebo possessed more enthusiasm; however, those with open-label placebo were contradicted, attributing the improvement of symptoms more to psychological function instead of the treatment itself (25). About 25% patients (26) and 19% healthy volunteers (27) taking a placebo experienced adverse events (AEs). Maxine de la Cruz et al. found that there was a placebo response in clinical trials about the treatment of fatigue in advanced cancer patients (28). However, no comparative study has been established to prove the differences between placebo and observation in the efficacy and AEs of the maintenance therapy of OC RCTs yet.
In this meta-analysis, we mainly compared the differences of median PFS and median OS between patients in the placebo and observation arms and illustrated the safety of placebo in RCTs with the maintenance therapy of advanced OC.
Patients and Methods
Search Strategy
According to the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guidelines (29), electronic databases (PubMed, Web of Science, Embase, and Cochrane Library) were searched from their inception to August 2020 to obtain relevant RCTs. The search terms included “ovarian cancer” or “ovarian neoplasms”, “placebo” or “maintenance” or “consolidation” or “observation” or “natural history”, and “randomized controlled trial”. We also performed a manual search to find potential relative RCTs by using the reference lists of key articles. The language of all RCTs was limited to English.
Inclusion and Exclusion Criteria
The inclusion criteria were as follows: (1) population: patients with FIGO stage IIB–IV epithelial ovarian, fallopian tube, primary peritoneal cancer or platinum-sensitive recurrent ovarian cancer (ROC) who are receiving maintenance therapy; (2) study design and comparators: phase II or III RCTs with control arms, including placebo or observation; (3) interventions: no other anticancer treatments except for standard front-line chemotherapy added to placebo or observation arms; (4) outcomes: mature data of median PFS or median OS reported as hazard ratios (HRs) and 95% confidence intervals (CIs), the number of patients with common AEs in the placebo arm, and the total number of patients receiving placebo; and (5) the latest articles were applied when duplicate publications existed or when publications were continuously updated.
The exclusion criteria were as follows: (1) not RCTs, including abstract, meeting, case, editorial, review, and so on; (2) platinum-resistant advanced OC or ROC; (3) animal trials; (4) other antitumor agents in the control arms; (5) no long-term outcomes covered in the study; and (6) unavailable research data.
No ethical approval and patient consent were required because the meta-analysis was performed based on previously published studies.
Outcome Measures
The primary outcome of this meta-analysis was median PFS. Median OS and AEs were the secondary outcomes. The AEs were in accordance with the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.
Assessment of the Risk of Bias and Data Extraction
We assessed the potential risk of bias in the trials using the Cochrane Collaboration Risk of Bias Assessment tool, which involved the following domains: (1) random sequence generation (selection bias); (2) allocation concealment (selection bias); (3) blinding of participants, personnel, and outcome assessment (performance bias and detection bias); (4) incomplete outcome data (attrition bias); and (5) selective reporting data (reporting bias) (30). The risks were divided into three levels: high, unclear, and low. Two reviewers (Wang and Sun) completed the review independently. Disagreements were resolved by a discussion.
Two reviewers (Wang and Sun) independently extracted the baseline information from each study, including data on author, year of publication, RCT phase, and the number of experimental arms and control arms. The primary and secondary endpoints included median PFS, median OS, the corresponding HR and 95% CIs, and the number of common AEs.
Statistical Analysis
We indirectly compared the median PFS and median OS between the placebo arms and the observation arms by using HR and 95% CIs in view of the Frequency Framework method. We calculated the corresponding HR and 95% CIs by combining the HR with the 95% CIs of all subgroups in view of the generic inverse of variance method with a fixed-effect model (31). The incidences of common AEs were calculated by IBM SPSS Statistics (version 20.0) using the number of patients with common AEs in the placebo arms and the total number of patients receiving placebo. Heterogeneity among the studies was evaluated by the inconsistency index (I2) value. If I2 ≥ 50% or P < 0.1, a random-effect model was used to reduce the heterogeneity and increase the reliability; if I2 < 50% or P > 0.1, a fixed-effect model was used. All statistical tests were two-sided, P < 0.05 was considered statistically significant (22). We used Review Manager (version 5.3, the Cochrane library) for the assessment of the risk of bias and R software (version 3.4.4, the R Foundation for Statistical Computing) for network meta-analyses.
Results
Study Selection and Characteristics
A total of 4,102 studies were retrieved through searching the electronic databases and other sources. Forty-one studies with 20,099 patients (experiment arms = 11,892; placebo arms = 4,787; observation arms = 3,420) that met the inclusion criteria were retained for comparison analysis. The PRISMA flow chart summarizing the process of evidence acquisition is shown in Figure 1. The flow chart mapped out the number of studies identified, screened, included, and excluded as well as the reasons for exclusions. The included studies were published between 2003 and 2020. The control arms of these RCTs consisted of 21 placebos (10 placebo maintenance after chemotherapy and 11 placebo through maintenance with and after chemotherapy) and 14 observations. Among 21 placebo studies (1, 5, 8, 9, 11, 12, 14, 15, 32–49), which all analyzed mature median PFS, 15 studies calculated the median OS, with 9 results being mature. There were 14 studies in the observation arms (6, 7, 13, 16–19, 50–57), all of which analyzed the mature median PFS and 13 studies analyzed the median OS, with 9 studies acquiring mature results. One study with secondary cytoreduction in the observation arms (57), one study with only a subgroup analysis (40), and one phase Ib/II study after extracting phase II data (8) were included in this meta-analysis. Except for one study (8), the other placebo arms with 2,665 patients all reported AEs in detail. The main characteristics and outcomes of the included RCTs are summarized in Table 1. Figure 2 shows the results about the assessment of the risk of bias in the included RCTs according to the Cochrane Collaboration Risk of Bias Assessment tool. The network plots of the direct comparisons in the maintenance therapy of OC for PFS and OS are displayed in Figures 3A, B.
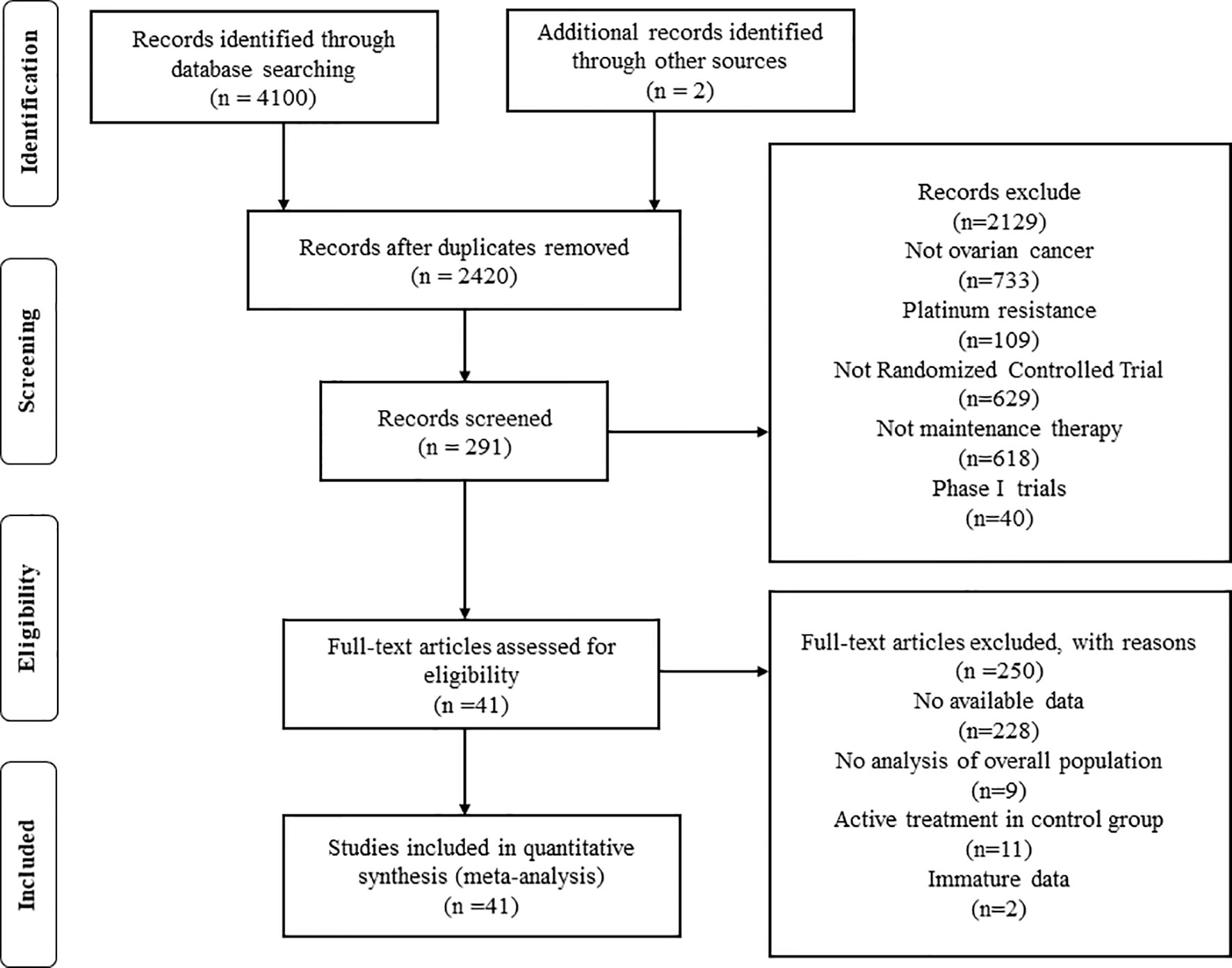
Figure 1 Preferred Reporting Items for Systematic Review and Meta-analysis flow chart of the study selection process.
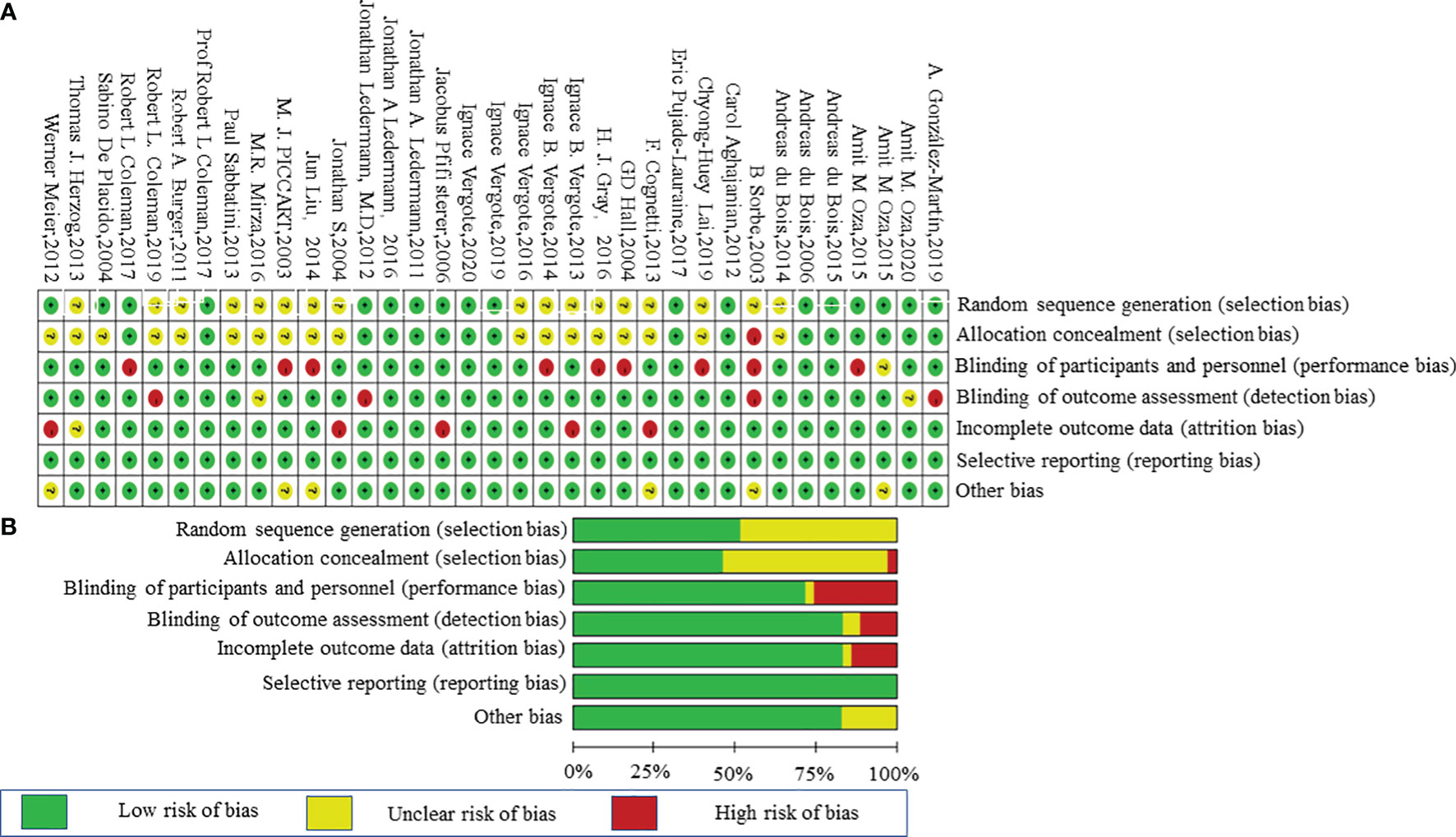
Figure 2 Risk-of-bias graph. (A) Review authors’ judgments about each risk-of-bias item presented as percentages across all included studies. (B) Review of authors’ judgments about each risk-of-bias item for each included study.
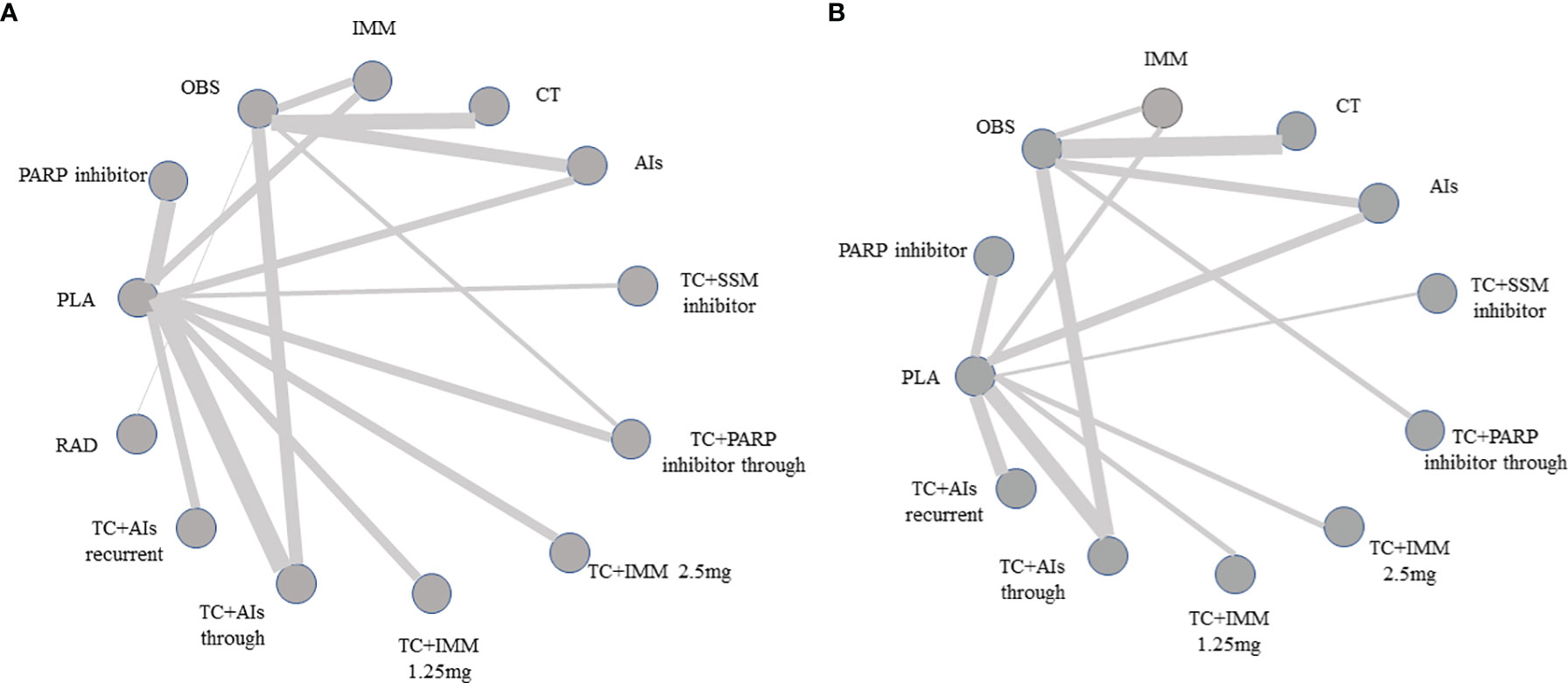
Figure 3 Network of treatment comparisons for overall efficacy. (A) Network plot of treatment comparisons of progression-free survival. (B) Network of treatment comparisons of overall survival. Directly comparable treatments are linked with a line, the thickness of which corresponds to the number of trials that assessed the comparison. AIs, angiogenesis inhibitors; PLA, placebo; TC, platinum plus paclitaxel; CT, chemotherapy; OBS, observation; PARP inhibitors, poly (ADP-ribose) polymerase inhibitors; IMM, immunotherapy; RAD, radiotherapy; SSM inhibitors, selective small-module inhibitors.
Progression-Free Survival
A total of 35 RCTs (4 RCTs: AIs with 1,058 patients; 10 RCTs: TC + AIs through with 3,990 patients; 1 RCT: TC + AIs current with 625 patients; 5 RCTs: PARPi with 1,566 patients; 2 RCTs: TC + PARPi through with 463 patients; 5 RCTs: immunotherapy with 890 patients; 1 RCT: TC + immunotherapy at 1.25 mg with 370 patients; 1 RCT: TC + immunotherapy at 2.5 mg with 366 patients; 6 RCTs: chemotherapy with 1,576 patients; 1 RCT: radiotherapy with 32 patients; 2 RCTs: TC + selective small-molecule inhibitor through with 117 patients; 21 RCTs: placebo with 4,606 patients; and 14 RCTs: observation with 3,420 patients) were included, which reported mature data on the PFS of OC patients with maintenance therapy. There was significant heterogeneity among RCTs (overall: I2 = 49.6%; P = 0.002), so the pooled HR was calculated by using a random-effect model. Except for placebo (HR, 1.02; 95% CI, 0.87–1.20; P = 0.81) and radiotherapy (HR, 0.59; 95% CI, 0.30–1.19; P = 0.13), other treatments significantly improved the PFS when compared with observation (all P <0.05) (Figure 4A). Compared with PARP inhibitors indirectly, PFS was significantly improved in AIs (HR, 0.61; 95% CI, 0.48–0.77; P < 0.001), chemotherapy (HR, 0.57; 95% CI, 0.44–0.75; P < 0.001), immunotherapy (HR, 0.59; 95% CI, 0.46–0.75; P < 0.001), chemotherapy combined with AIs recurrent (HR, 0.61; 95% CI, 0.45–0.83; P < 0.002), chemotherapy combined with AIs through (HR, 0.70; 95% CI, 0.58–0.84; P < 0.001), chemotherapy combined with immunotherapy at 1.25 mg (HR, 0.56; 95% CI, 0.40–0.79; P < 0.001), and chemotherapy combined with immunotherapy at 2.5 mg (HR, 0.64; 95% CI, 0.45–0.91; P = 0.012) (Figure 5).
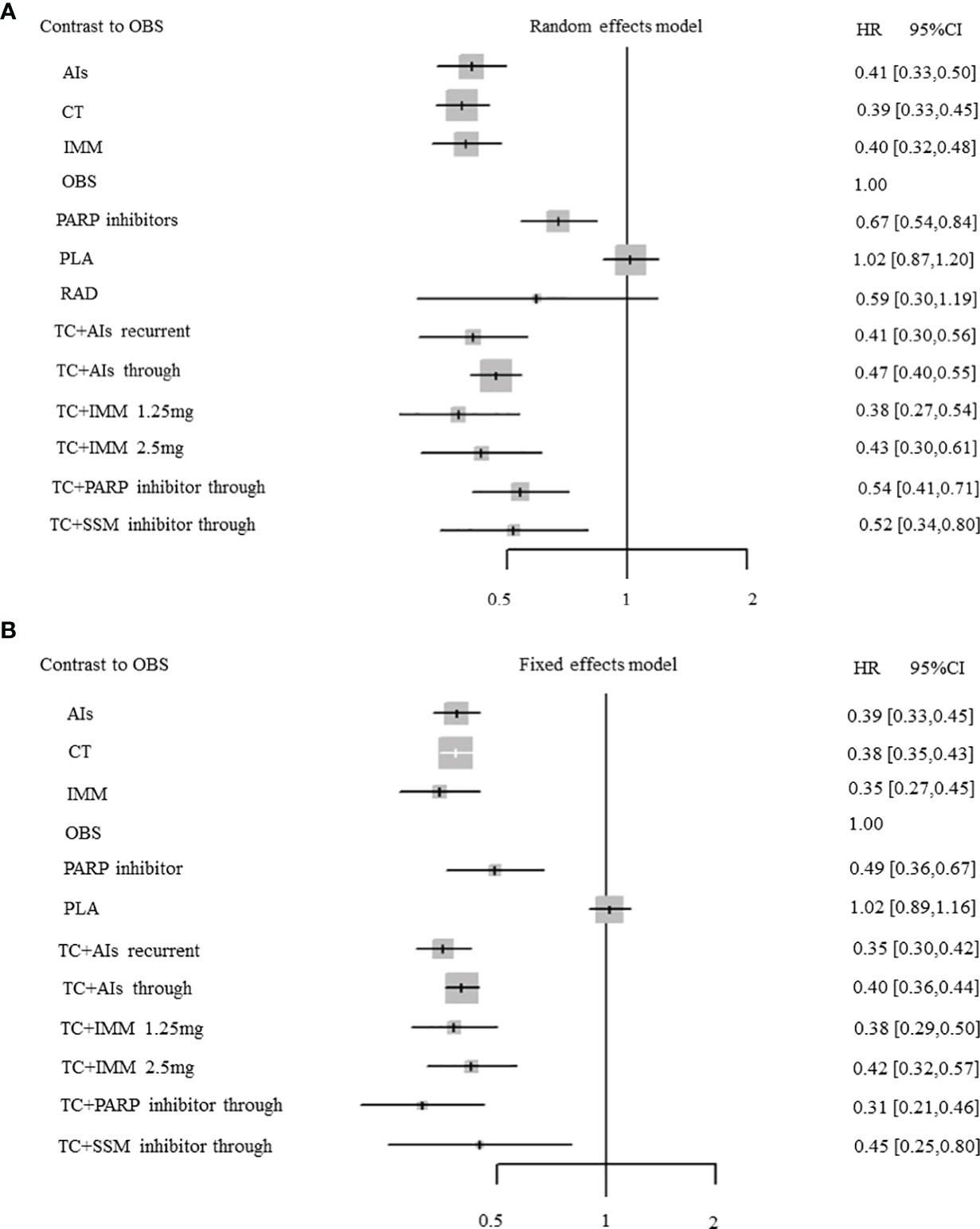
Figure 4 Forest plots. (A) Progression-free survival (PFS). (B) Overall survival. Hazard ratios and 95% confidence intervals (95% CIs) of each treatment versus observation in the maintenance therapy of ovarian cancer. Central dots represent medians; lines represent 95% CIs.
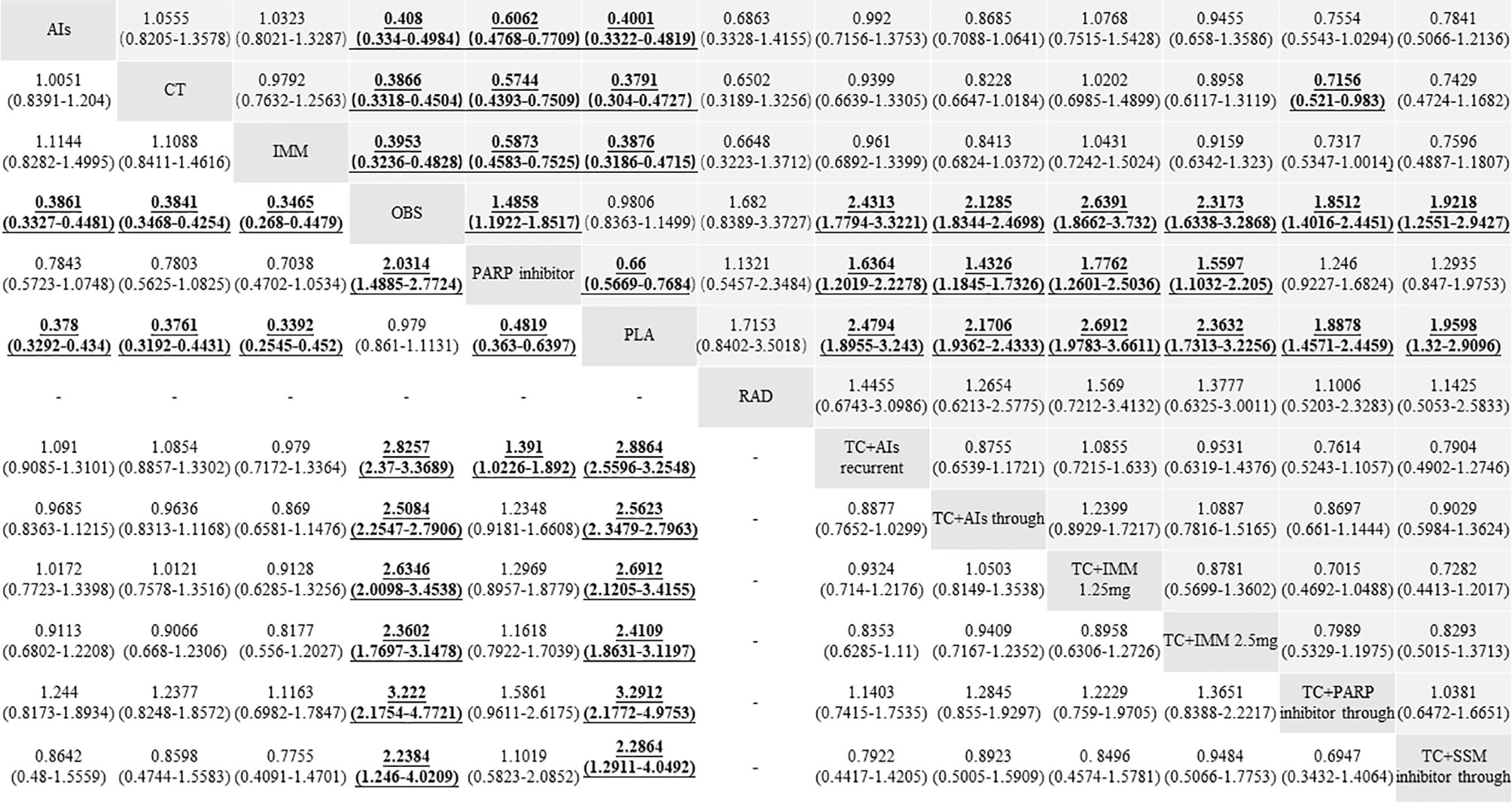
Figure 5 Hazard ratios (HRs) and 95% confidence intervals (95% CIs) of efficacy among the maintenance therapies of ovarian cancer patients between progression-free survival (PFS, up) and overall survival (OS, down). Comparisons between treatments should be read from left to right, and the estimate is in the cell in common between the column-defining treatment and the row-defining treatment. For efficacy, HR lower than 1 and 95% CI not including 1 favor the row-defining treatment of PFS or the column-defining treatment of OS. To obtain the HRs for comparisons in the opposite direction, reciprocals should be taken. Significant results are in bold and underlined.
Overall Survival
Among 28 RCTs about median OS, 18 studies (3 RCTs: AIs with 935 patients; 7 RCTs: TC+AIs through with 2,861 patients; 1 RCT: TC + AIs recurrent with 625 patients; 1 RCT: PARPi with 136 patients; 1 RCT: TC + PARPi through with 81 patients; 1 RCT: immunotherapy with 149 patients; 1 RCT: TC + immunotherapy at 1.25 mg with 370 patients; 1 RCT: TC + immunotherapy at 2.5 mg with 366 patients; 3 RCTs: chemotherapy with 1,381 patients; 1 RCT: TC + selective small-molecule inhibitor through with 58 patients; 9 RCTs: placebo with 2,375 patients; 9 RCTs: observation with 3,159 patients) reported mature data on OS of OC patients with maintenance therapy. No significant heterogeneity existed in RCTs (overall: I2 = 0%; P = 0.73), so the pooled HR was calculated by using a fixed-effect model. Except for placebo (HR, 1.02; 95% CI, 0.89–1.16; P = 0.76), other treatments significantly improved OS when compared with observation (all P <0.05) (Figure 4B). Compared with PARP inhibitors indirectly, OS was significantly improved in chemotherapy combined with AIs recurrent (HR, 0.72; 95% CI, 0.53–0.98; P = 0.036) (Figure 5).
AEs
Considering the accuracy of the results, we only analyzed the toxicity profiles of placebo maintenance therapy after completing chemotherapy to avoid its effect. Results regarding the patients’ all grades and grade ≥ 3 toxicity profiles were pooled for only 10 placebo maintenance therapy in all included studies. Except for a study with grade ≥ 3 toxicity profiles (45), other RCTs reported all grades and grade ≥ 3 toxicity profiles in detail. Toxicity profiles were classified into total toxicity, hematological toxicities, gastrointestinal toxicities, and other toxicities. In all, 5 studies (9, 14, 41, 42, 46) with 954 patients reported the number of all grades AEs (897 patients) and 6 studies (9, 14, 41, 42, 45, 46) with 994 patients reported the number of grade ≥ 3 AEs (201 patients); the incidences of all grades AEs and grade ≥ 3 AEs were 94.03% (95% CI, 92.53%–95.53%) and 20.22% (95% CI, 17.72%–22.72%), respectively (Table 2).
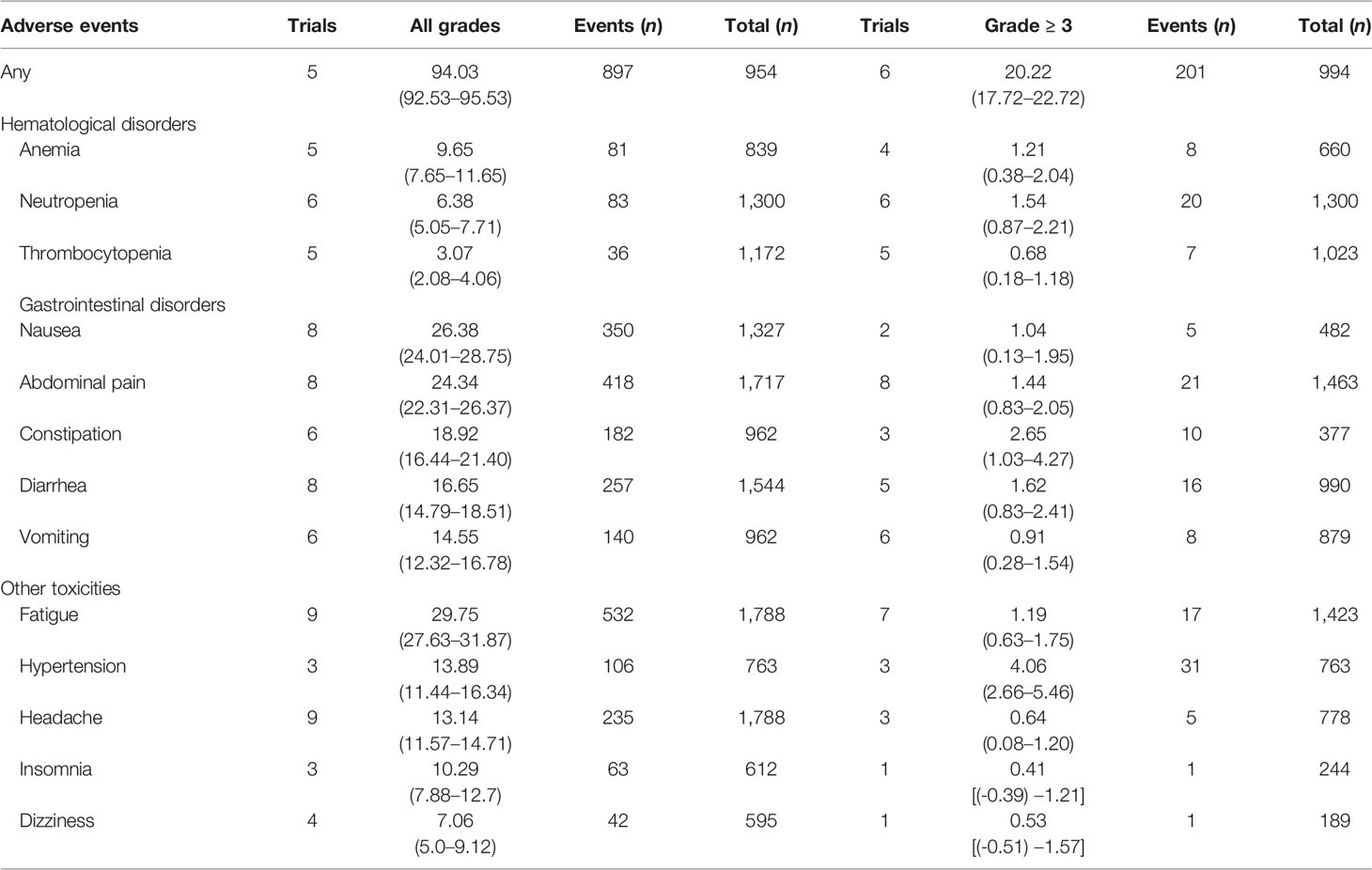
Table 2 Overall incidences (%) and 95% confidence intervals of common adverse events in patients with placebo maintenance.
Hematological Toxicities
We assessed three common hematological toxicities, including anemia, neutropenia, and thrombocytopenia, in this meta-analysis. The incidences of all grades and grade ≥ 3 toxicities were 9.65% (95% CI, 7.65%–11.65%) and 1.21% (95% CI, 0.38%–2.04%) in anemia, 6.38% (95% CI, 5.05%–7.71%) and 1.54% (95% CI, 0.87%–2.21%) in neutropenia, and 3.07% (95% CI, 2.08%–4.06%) and 0.68% (95% CI, 0.18%–1.18%) in thrombocytopenia, respectively (Table 2).
Gastrointestinal Toxicities
We also assessed several common gastrointestinal toxicities, such as nausea, abdominal pain, constipation, diarrhea, and vomiting. The incidences of all grades and grade ≥ 3 gastrointestinal toxicities were 26.38% (95% CI, 24.01%–28.75%) and 1.04% (95% CI, 0.13%–1.95%) in nausea, 24.34% (95% CI, 22.31%–26.37%) and 1.44% (95% CI, 0.83%–2.05%) in abdominal pain, 18.92% (95% CI, 16.44%–21.4%) and 2.65% (95% CI, 1.03%–4.27%) in constipation, 16.65% (95% CI, 14.79%–18.51%) and 1.62% (95% CI, 0.83%–2.41%) in diarrhea, and 14.55% (95% CI, 12.32%–16.78%) and 0.91% (95% CI, 0.28%–1.54%) in vomiting, respectively (Table 2).
Other Toxicities
Other toxicities like fatigue, hypertension, headache, insomnia, and dizziness were also analyzed in this meta-analysis. The incidences of all grades and grade ≥ 3 toxicities were 29.75% (95% CI, 27.63%–31.87%) and 1.19% (95% CI, 0.63%–1.75%) in fatigue, 13.89% (95% CI, 11.44%–16.34%) and 4.06% (95% CI, 2.66%–5.46%) in hypertension, 13.14% (95% CI, 11.57%–14.71%) and 0.64% (95% CI, 0.08%–1.2%) in headache, 10.29% (95% CI, 7.88%–12.7%) and 0.41% [95% CI, (-0.39%)-1.21%] in insomnia, and 7.06% (95% CI, 5.0%–9.12%) and 0.53% [95% CI, (-0.51%)-1.57%] in dizziness successively (Table 2).
Discussion
Lots of meta-analyses about experimental drugs were performed to estimate the effect on survival. Ours was the first one concentrating on RCTs to assess the placebo effect of maintenance therapy in primary and recurrent OC settings. In this meta-analysis, we proved no statistically significant differences in the survival, whether PFS or OS, of OC patients between placebo and observation (all P > 0.05). Until now, no research has focused on this point. The National Comprehensive Cancer Network Guideline believes that participating in clinical trials for any cancer patients is the best management, which is positively encouraged (the corresponding website: nccn.org/clinical trials/member_institutions.aspx.). The ratios of the amount of participants between the experimental and the control arms of the included RCTs were 2:1 (9, 14, 36, 40, 42, 46, 48), 1:1 (1, 5–8, 11–13, 16–19, 32, 34, 38, 39, 41, 43–45, 50–53, 55–57, 58), or 1:1:1 (15, 35, 37, 49, 54). That implied that the participants had the opportunity of 1/3 or 1/2 to take placebo, but our results proved that it did not have an effect on survival. Therefore, it is safe to be ignored when designing patients’ composition in RCTs.
However, placebo produced some AEs—the incidences of all grades and grade ≥ 3 were 94.03% (95% CI, 92.53%–95.53%) and 20.22% (95% CI, 17.72%–22.72%), respectively, which were higher than those of the observation arms and the study of Matías Rodrigo Chacón et al. (85.1% in all grades and 18% in grade ≥ 3) (59). The reason of the difference was that our study only included OC patients, while the study of Matías Rodrigo Chacón et al. contained cases of melanoma, non-small cell lung cancer, gastrointestinal stromal tumor, and renal cell carcinoma. SOLO2 (42), focusing on OC, reported the incidences of placebo-related AEs as 94.95% in all grades and 18.18% in grade ≥ 3, which was similar to our results. Fatigue was the most common AEs, followed by gastrointestinal toxicities. A. Hrobjartsson et al. suggested that subjective symptoms, such as pain and anxiety, were affected more easily by placebo effect than objective measures like blood pressure (20). Julia W. Haas et al. found that patients with irritable bowel syndrome in a double-blind placebo experiment possessed more enthusiasm. However, those in an open-label placebo research were contradicted and thought that the improvement of symptoms rarely came from the treatment itself but that it was more like a psychological function (25).
The results compared with PARP inhibitors in this study were different from the study of Feng et al. (60), the only meta-analysis comparing PARP inhibitors, AIs, and chemotherapy, and showed that PARP inhibitors were superior to AIs and chemotherapy. We considered the following several reasons: (1) our study only included platinum-sensitive OC patients, while Feng’s study included those cases which are platinum-sensitive and platinum-resistant; (2) comprehensive maintenance therapy models were illustrated in this meta-analysis, and the trials’ numbers of immunotherapy were obviously less than those of other treatments, which might affect the weight of data; and (3) Feng’s study merged placebo and observation into one arm, but some results continued to be debatable in the maintenance therapy of RCTs with OC—for example, immunotherapy did not improve the patients’ survival, but PARP inhibitors did so, while in this indirect meta-analysis immunotherapy was prior to PARP inhibitors. We considered that sample sizes and the weight of data produced conflicting results. In the future, a large number of direct comparative clinical trials are needed to confirm the relation between immunotherapy and PARP inhibitors.
Several highlights existed in this meta-analysis, which are as follows: firstly, it was conducted according to PRISMA and included all well-designed and high-quality phase II or phase III RCTs to reduce the risk of bias among trials and increase the reliability of the results. Secondly, it included comprehensive models of maintenance treatment of OC, such as chemotherapy, AIs, PARP inhibitors, immunotherapy, and SSM inhibitors. Thirdly, network meta-analysis was used to indirectly compare the efficacy between placebo and observation and among experimental drugs due to no direct RCTs. Lastly, it firstly stated the incidences of AEs produced by placebo tablets in RCTs with OC and was not only limited to the fatigue in advanced cancer patients (28).
Some limitations were stated in our meta-analysis. First, performing a stratified pooled analysis to reduce the risk of bias among clinical trials according to disease setting (primary vs. recurrent OC) was difficult because of the limited clinical trials. Different endpoints existed in the studies, and the number of clinical trials of all kinds of maintenance therapies was less than 10. Second, the data used were based on the clinical trial level rather than the individual patient; data on survival and AEs were not assessed accurately or incorporated into the analysis due to lacking original data which were not available, which were supposed to be more sensitive for toxicity analysis. Third, we only evaluated the incidences of AEs in placebo arms without combination with chemotherapy, which could not better represent all patients included in the RCTs. Lastly, we did not calculate the relative risk ratio of AEs between placebo and observation due to insufficient data on AEs about observation.
Conclusions
This network meta-analysis indicated that the maintenance therapy of OC improved PFS and partial OS benefits. Compared with observation, placebo did not improve or reduce the PFS or OS benefits, but it increased the incidences of AEs in OC patients. In the future, more clinical trials should be designed to directly confirm the placebo effect.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author Contributions
QL conceived the study idea and wrote the manuscript. JW performed the search in the electronic databases, organized the data, and wrote the manuscript. LH and FC analyzed the data. CS performed the search in the electronic databases and organized the data. YW and XF checked the data again. LZ, YB, KZ, and JJ made modifications in the paper. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Clinical Research Award of the First Affiliated Hospital of Xi’an Jiaotong University, China (XJTU1AF-2018-017 and XJTU1AF-CRF-2019-002), the Natural Science Basic Research Program of Shaanxi (2018JM7073 and 2017ZDJC-11), the Key Research and Development Program of Shaanxi (2017ZDXM-SF-068 and 2019QYPY-138), the Innovation Capability Support Program of Shaanxi (2017XT-026 and 2018XT-002), and the Medical Research Project of Xi’an Social Development Guidance Plan (2017117SF/YX011-3). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Vergote IB, Chekerov R, Amant F, Harter P, Casado A, Emerich J, et al. Randomized, Phase II, Placebo-Controlled, Double-Blind Study With and Without Enzastaurin in Combination With Paclitaxel and Carboplatin as First-Line Treatment Followed by Maintenance Treatment in Advanced Ovarian Cancer. J Clin Oncol (2013) 31(25):3127–32. doi: 10.1200/JCO.2012.44.9116
2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin (2021) 71(1):7–33. doi: 10.3322/caac.21654
3. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
4. Moore K, Colombo N, Scambia G, Kim BG, Oaknin A, Friedlander M, et al. Maintenance Olaparib in Patients With Newly Diagnosed Advanced Ovarian Cancer. N Engl J Med (2018) 379(26):2495–505. doi: 10.1056/NEJMoa1810858
5. Aghajanian C, Blank SV, Goff BA, Judson PL, Teneriello MG, Husain A, et al. OCEANS: A Randomized, Double-Blind, Placebo-Controlled Phase III Trial of Chemotherapy With or Without Bevacizumab in Patients With Platinum-Sensitive Recurrent Epithelial Ovarian, Primary Peritoneal, or Fallopian Tube Cancer. J Clin Oncol (2012) 30(17):2039–45. doi: 10.1200/JCO.2012.42.0505
6. De Placido S, Scambia G, Di Vagno G, Naglieri E, Lombardi AV, Biamonte R, et al. Topotecan Compared With No Therapy After Response to Surgery and Carboplatin/Paclitaxel in Patients With Ovarian Cancer: Multicenter Italian Trials in Ovarian Cancer (MITO-1) Randomized Study. J Clin Oncol (2004) 22(13):2635–42. doi: 10.1200/JCO.2004.09.088
7. Hall GD, Brown JM, Coleman RE, Stead M, Metcalf KS, Peel KR, et al. Maintenance Treatment With Interferon for Advanced Ovarian Cancer: Results of the Northern and Yorkshire Gynaecology Group Randomised Phase III Study. Br J Cancer (2004) 91(4):621–6. doi: 10.1038/sj.bjc.6602037
8. Vergote I, Heitz F, Buderath P, Powell M, Sehouli J, Lee CM, et al. A Randomized, Double-Blind, Placebo-Controlled Phase 1b/2 Study of Ralimetinib, A P38 MAPK Inhibitor, Plus Gemcitabine and Carboplatin Versus Gemcitabine and Carboplatin for Women With Recurrent Platinum-Sensitive Ovarian Cancer. Gynecol Oncol (2020) 156(1):23–31. doi: 10.1016/j.ygyno.2019.11.006
9. González-Martín A, Pothuri B, Vergote I, DePont Christensen R, Graybill W, Mirza MR, et al. Niraparib in Patients With Newly Diagnosed Advanced Ovarian Cancer. N Engl J Med (2019) 381(25):2391–402. doi: 10.1056/NEJMoa1910962
10. Hirte H, Vergote IB, Jeffrey JR, Grimshaw RN, Coppieters S, Schwartz B, et al. A Phase III Randomized Trial of BAY 12-9566 (Tanomastat) as Maintenance Therapy in Patients With Advanced Ovarian Cancer Responsive to Primary Surgery and Paclitaxel/Platinum Containing Chemotherapy: A National Cancer Institute of Canada Clinical Trials Group Study. Gynecol Oncol (2006) 102(2):300–8. doi: 10.1016/j.ygyno.2005.12.020
11. Herzog TJ, Scambia G, Kim BG, Lhommé C, Markowska J, Ray-Coquard I, et al. A Randomized Phase II Trial of Maintenance Therapy With Sorafenib in Front-Line Ovarian Carcinoma. Gynecol Oncol (2013) 130(1):25–30. doi: 10.1016/j.ygyno.2013.04.011
12. Berek JS, Taylor PT, Gordon A, Cunningham MJ, Finkler N, Orr J Jr., et al. Randomized, Placebo-Controlled Study of Oregovomab for Consolidation of Clinical Remission in Patients With Advanced Ovarian Cancer. J Clin Oncol (2004) 22(17):3507–16. doi: 10.1200/JCO.2004.09.016
13. Gray HJ, Benigno B, Berek J, Chang J, Mason J, Mileshkin L, et al. Progression-Free and Overall Survival in Ovarian Cancer Patients Treated With CVac, a Mucin 1 Dendritic Cell Therapy in a Randomized Phase 2 Trial. J Immunother Cancer (2016) 4:34. doi: 10.1186/s40425-016-0137-x
14. Sabbatini P, Harter P, Scambia G, Sehouli J, Meier W, Wimberger P, et al. Abagovomab as Maintenance Therapy in Patients With Epithelial Ovarian Cancer: A Phase III Trial of the AGO OVAR, COGI, GINECO, and GEICO–the MIMOSA Study. J Clin Oncol (2013) 31(12):1554–61. doi: 10.1200/JCO.2012.46.4057
15. Vergote I, Armstrong D, Scambia G, Teneriello M, Sehouli J, Schweizer C, et al. A Randomized, Double-Blind, Placebo-Controlled, Phase III Study to Assess Efficacy and Safety of Weekly Farletuzumab in Combination With Carboplatin and Taxane in Patients With Ovarian Cancer in First Platinum-Sensitive Relapse. J Clin Oncol (2016) 34(19):2271–8. doi: 10.1200/JCO.2015.63.2596
16. Piccart MJ, Floquet A, Scarfone G, Willemse PH, Emerich J, Vergote I, et al. Intraperitoneal Cisplatin Versus No Further Treatment: 8-Year Results of EORTC 55875, A Randomized Phase III Study in Ovarian Cancer Patients With a Pathologically Complete Remission After Platinum-Based Intravenous Chemotherapy. Int J Gynecol Cancer (2003) 13(Suppl 2):196–203. doi: 10.1136/ijgc-00009577-200311001-00012
17. du Bois A, Weber B, Rochon J, Meier W, Goupil A, Olbricht S, et al. Addition of Epirubicin as a Third Drug to Carboplatin-Paclitaxel in First-Line Treatment of Advanced Ovarian Cancer: A Prospectively Randomized Gynecologic Cancer Intergroup Trial by the Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Cancer Study Group and the Groupe D'investigateurs Nationaux Pour l'Etude Des Cancers Ovariens. J Clin Oncol (2006) 24(7):1127–35. doi: 10.1200/JCO.2005.03.2938
18. Pfisterer J, Weber B, Reuss A, Kimmig R, du Bois A, Wagner U, et al. Randomized Phase III Trial of Topotecan Following Carboplatin and Paclitaxel in First-Line Treatment of Advanced Ovarian Cancer: A Gynecologic Cancer Intergroup Trial of the AGO-OVAR and GINECO. J Natl Cancer Inst (2006) 98(15):1036–45. doi: 10.1093/jnci/djj296
19. Lai CH, Vallikad E, Lin H, Yang LY, Jung SM, Liu HE, et al. Maintenance of Pegylated Liposomal Doxorubicin/Carboplatin in Patients With Advanced Ovarian Cancer: Randomized Study of an Asian Gynecologic Oncology Group. J Gynecol Oncol (2020) 31(1):e5. doi: 10.3802/jgo.2020.31.e5
20. Hrobjartsson A, Gotzsche PC. Is the Placebo Powerless? An Analysis of Clinical Trials Comparing Placebo With No Treatment. N Engl J Med (2001) 344(21):1594–602. doi: 10.1056/NEJM200105243442106
21. Sheldon R, Opie-Moran M. The Placebo Effect in Cardiology: Understanding and Using it. Can J Cardiol (2017) 33(12):1535–42. doi: 10.1016/j.cjca.2017.09.017
22. Brody H. Meaning and an Overview of the Placebo Effect. Perspect Biol Med (2018) 61(3):353–60. doi: 10.1353/pbm.2018.0048
23. Jonas WB, Crawford C, Colloca L, Kaptchuk TJ, Moseley B, Miller FG, et al. To What Extent are Surgery and Invasive Procedures Effective Beyond a Placebo Response? A Systematic Review With Meta-Analysis of Randomised, Sham Controlled Trials. BMJ Open (2015) 5(12):e009655. doi: 10.1136/bmjopen-2015-009655
24. Požgain I, Požgain Z, Degmečić D. Placebo and Nocebo Effect: A Mini-Review. Psychiatr Danub (2014) 26(2):100–7.
25. Haas JW, Ongaro G, Jacobson E, Conboy LA, Nee J, Iturrino J, et al. Patients' Experiences Treated With Open-Label Placebo Versus Double-Blind Placebo: A Mixed Methods Qualitative Study. BMC Psychol (2022) 10(1):20. doi: 10.1186/s40359-022-00731-w
26. Shepherd M. The Placebo: From Specificity to the non-Specific and Back. Psychol Med (1993) 23(3):569–78. doi: 10.1017/S0033291700025356
27. Rosenzweig P, Brohier S, Zipfel A. The Placebo Effect in Healthy Volunteers: Influence of Experimental Conditions on the Adverse Events Profile During Phase I Studies. Clin Pharmacol Ther (1993) 54(5):578–83. doi: 10.1038/clpt.1993.190
28. de la Cruz M, Hui D, Parsons HA, Bruera E. Placebo and Nocebo Effects in Randomized Double-Blind Clinical Trials of Agents for the Therapy for Fatigue in Patients With Advanced Cancer. Cancer (2010) 116(3):766–74. doi: 10.1002/cncr.24751
29. Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015: Elaboration and Explanation. BMJ (2015) 350:g7647. doi: 10.1136/bmj.g7647
30. Ma J, Deng H, Li J, Hu S, Yang Y, Liu S, et al. Efficacy and Safety of Olaparib Maintenance Therapy in Platinum-Sensitive Ovarian Cancer Patients With BRCA Mutations: A Meta-Analysis on Randomized Controlled Trials. Cancer Manag Res (2019) 11:3061–78. doi: 10.2147/CMAR.S191107
31. Tomao F, Bardhi E, Di Pinto A, Sassu CM, Biagioli E, Petrella MC, et al. Parp Inhibitors as Maintenance Treatment in Platinum Sensitive Recurrent Ovarian Cancer: An Updated Meta-Analysis of Randomized Clinical Trials According to BRCA Mutational Status. Cancer Treat Rev (2019) 80:101909. doi: 10.1016/j.ctrv.2019.101909
32. Oza AM, Estevez-Diz M, Grischke EM, Hall M, Marmé F, Provencher D, et al. A Biomarker-Enriched, Randomized Phase II Trial of Adavosertib (AZD1775) Plus Paclitaxel and Carboplatin for Women With Platinum-Sensitive TP53-Mutant Ovarian Cancer. Clin Cancer Res (2020) 26(18):4767–76. doi: 10.1158/1078-0432.CCR-20-0219
33. Ledermann JA, Embleton AC, Raja F, Perren TJ, Jayson GC, Rustin GJS, et al. Cediranib in Patients With Relapsed Platinum-Sensitive Ovarian Cancer (ICON6): A Randomised, Double-Blind, Placebo-Controlled Phase 3 Trial. Lancet (2016) 387(10023):1066–74. doi: 10.1016/S0140-6736(15)01167-8
34. Aghajanian C, Goff B, Nycum LR, Wang YV, Husain A, Blank SV. Final Overall Survival and Safety Analysis of OCEANS, A Phase 3 Trial of Chemotherapy With or Without Bevacizumab in Patients With Platinum-Sensitive Recurrent Ovarian Cancer. Gynecol Oncol (2015) 139(1):10–6. doi: 10.1016/j.ygyno.2015.08.004
35. Tewari KS, Burger RA, Enserro D, Norquist BM, Swisher EM, Brady MF, et al. Final Overall Survival of a Randomized Trial of Bevacizumab for Primary Treatment of Ovarian Cancer. J Clin Oncol (2019) 37(26):2317–28. doi: 10.1200/JCO.19.01009
36. Ray-Coquard I, Cibula D, Mirza MR, Reuss A, Ricci C, Colombo N, et al. Final Results From GCIG/ENGOT/AGO-OVAR 12, A Randomised Placebo-Controlled Phase III Trial of Nintedanib Combined With Chemotherapy for Newly Diagnosed Advanced Ovarian Cancer. Int J Cancer (2020) 146(2):439–48. doi: 10.1002/ijc.32606
37. Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, et al. Incorporation of Bevacizumab in the Primary Treatment of Ovarian Cancer. N Engl J Med (2011) 365(26):2473–83. doi: 10.1056/NEJMoa1104390
38. Du Bois A, Floquet A, Kim JW, Rau J, del Campo JM, Friedlander M, et al. Incorporation of Pazopanib in Maintenance Therapy of Ovarian Cancer. J Clin Oncol (2014) 32(30):3374–82. doi: 10.1200/JCO.2014.55.7348
39. Friedlander M, Matulonis U, Gourley C, du Bois A, Vergote I, Rustin G, et al. Long-Term Efficacy, Tolerability and Overall Survival in Patients With Platinum-Sensitive, Recurrent High-Grade Serous Ovarian Cancer Treated With Maintenance Olaparib Capsules Following Response to Chemotherapy. Br J Cancer (2018) 119(9):1075–85. doi: 10.1038/s41416-018-0271-y
40. Mirza MR, Monk BJ, Herrstedt J, Oza AM, Mahner S, Redondo A, et al. Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. N Engl J Med (2016) 375(22):2154–64. doi: 10.1056/NEJMoa1611310
41. Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Olaparib Maintenance Therapy in Platinum-Sensitive Relapsed Ovarian Cancer. N Engl J Med (2012) 366(15):1382–92. doi: 10.1056/NEJMoa1105535
42. Pujade-Lauraine E, Ledermann JA, Selle F, Gebski V, Penson RT, Oza AM, et al. Olaparib Tablets as Maintenance Therapy in Patients With Platinum-Sensitive, Relapsed Ovarian Cancer and a BRCA1/2 Mutation (SOLO2/ENGOT-Ov21): A Double-Blind, Randomised, Placebo-Controlled, Phase 3 Trial. Lancet Oncol (2017) 18(9):1274–84. doi: 10.1016/S1470-2045(17)30469-2
43. Vergote I, Du Bois A, Floquet A, Rau J, Kim JW, Del Campo JM, et al. Overall Survival Results of AGO-OVAR16: A Phase 3 Study of Maintenance Pazopanib Versus Placebo in Women Who Have Not Progressed After First-Line Chemotherapy for Advanced Ovarian Cancer. Gynecol Oncol (2019) 155(2):186–91. doi: 10.1016/j.ygyno.2019.08.024
44. Cognetti F, Bagnato A, Colombo N, Savarese A, Scambia G, Sehouli J, et al. A Phase II, Randomized, Double-Blind Study of Zibotentan (ZD4054) in Combination With Carboplatin/Paclitaxel Versus Placebo in Combination With Carboplatin/Paclitaxel in Patients With Advanced Ovarian Cancer Sensitive to Platinum-Based Chemotherapy (AGO-OVAR 2.14). Gynecol Oncol (2013) 130(1):31–7. doi: 10.1016/j.ygyno.2012.12.004
45. Ledermann JA, Hackshaw A, Kaye S, Jayson G, Gabra H, McNeish I, et al. Randomized Phase II Placebo-Controlled Trial of Maintenance Therapy Using the Oral Triple Angiokinase Inhibitor BIBF 1120 After Chemotherapy for Relapsed Ovarian Cancer. J Clin Oncol (2011) 29(28):3798–804. doi: 10.1200/JCO.2010.33.5208
46. Coleman RL, Oza AM, Lorusso D, Aghajanian C, Oaknin A, Dean A, et al. Rucaparib Maintenance Treatment for Recurrent Ovarian Carcinoma After Response to Platinum Therapy (ARIEL3): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet (2017) 390(10106):1949–61. doi: 10.1016/S0140-6736(17)32440-6
47. Du Bois A, Kristensen G, Ray-Coquard I, Reuss A, Pignata S, Colombo N, et al. Standard First-Line Chemotherapy With or Without Nintedanib for Advanced Ovarian Cancer (AGO-OVAR 12): A Randomised, Double-Blind, Placebo-Controlled Phase 3 Trial. Lancet Oncol (2016) 17(1):78–89. doi: 10.1016/S1470-2045(15)00366-6
48. Vergote I, Scambia G, O'Malley DM, Van Calster B, Park SY, Del Campo JM, et al. Trebananib or Placebo Plus Carboplatin and Paclitaxel as First-Line Treatment for Advanced Ovarian Cancer (TRINOVA-3/ENGOT-Ov2/GOG-3001): A Randomised, Double-Blind, Phase 3 Trial. Lancet Oncol (2019) 20(6):862–76. doi: 10.1016/S1470-2045(19)30178-0
49. Coleman RL, Fleming GF, Brady MF, Swisher EM, Steffensen KD, Friedlander M, et al. Veliparib With First-Line Chemotherapy and as Maintenance Therapy in Ovarian Cancer. N Engl J Med (2019) 381(25):2403–15. doi: 10.1056/NEJMoa1909707
50. Liu J, Li H, Cao S, Zhang X, Yu J, Qi J, et al. Maintenance Therapy With Autologous Cytokine-Induced Killer Cells in Patients With Advanced Epithelial Ovarian Cancer After First-Line Treatment. J Immunother (2014) 37(2):115–22. doi: 10.1097/CJI.0000000000000021
51. Meier W, du Bois A, Rau J, Gropp-Meier M, Baumann K, Huober J, et al. Randomized Phase II Trial of Carboplatin and Paclitaxel With or Without Lonafarnib in First-Line Treatment of Epithelial Ovarian Cancer Stage IIB-Iv. Gynecol Oncol (2012) 126(2):236–40. doi: 10.1016/j.ygyno.2012.04.050
52. Oza AM, Cook AD, Pfisterer J, Embleton A, Ledermann JA, Pujade-Lauraine E, et al. Standard Chemotherapy With or Without Bevacizumab for Women With Newly Diagnosed Ovarian Cancer (ICON7): Overall Survival Results of a Phase 3 Randomised Trial. Lancet Oncol (2015) 16(8):928–36. doi: 10.1016/S1470-2045(15)00086-8
53. Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G, et al. A Phase 3 Trial of Bevacizumab in Ovarian Cancer. N Engl J Med (2011) 365(26):2484–96. doi: 10.1056/NEJMoa1103799
54. Sorbe B. Consolidation Treatment of Advanced (FIGO Stage III) Ovarian Carcinoma in Complete Surgical Remission After Induction Chemotherapy: A Randomized, Controlled, Clinical Trial Comparing Whole Abdominal Radiotherapy, Chemotherapy, and No Further Treatment. Int J Gynecol Cancer (2003) 13(3):278–86. doi: 10.1046/j.1525-1438.2003.13193.x
55. Vergote IB, Jimeno A, Joly F, Katsaros D, Coens C, Despierre E, et al. Randomized Phase III Study of Erlotinib Versus Observation in Patients With No Evidence of Disease Progression After First-Line Platin-Based Chemotherapy for Ovarian Carcinoma: A European Organisation for Research and Treatment of Cancer-Gynaecological Cancer Group, and Gynecologic Cancer Intergroup Study. J Clin Oncol (2014) 32(4):320–6. doi: 10.1200/JCO.2013.50.5669
56. Oza AM, Cibula D, Benzaquen AO, Poole C, Mathijssen RH, Sonke GS, et al. Olaparib Combined With Chemotherapy for Recurrent Platinum-Sensitive Ovarian Cancer: A Randomised Phase 2 Trial. Lancet Oncol (2015) 16(1):87–97. doi: 10.1016/S1470-2045(14)71135-0
57. Coleman RL, Brady MF, Herzog TJ, Sabbatini P, Armstrong DK, Walker JL, et al. Bevacizumab and Paclitaxel-Carboplatin Chemotherapy and Secondary Cytoreduction in Recurrent, Platinum-Sensitive Ovarian Cancer (NRG Oncology/Gynecologic Oncology Group Study GOG-0213): A Multicentre, Open-Label, Randomised, Phase 3 Trial. Lancet Oncol (2017) 18(6):779–91. doi: 10.1016/S1470-2045(17)30279-6
58. Mannel RS, Brady MF, Kohn EC, Hanjani P, Hiura M, Lee R, et al. A Randomized Phase III Trial of IV Carboplatin and Paclitaxel × 3 Courses Followed by Observation Versus Weekly Maintenance Low-Dose Paclitaxel in Patients With Early-Stage Ovarian Carcinoma: A Gynecologic Oncology Group Study. Gynecol Oncol (2011) 122(1):89–94. doi: 10.1016/j.ygyno.2011.03.013
59. Chacon MR, Enrico DH, Burton J, Waisberg FD, Videla VM. Incidence of Placebo Adverse Events in Randomized Clinical Trials of Targeted and Immunotherapy Cancer Drugs in the Adjuvant Setting: A Systematic Review and Meta-Analysis. JAMA Netw Open (2018) 1(8):e185617. doi: 10.1001/jamanetworkopen.2018.5617
Keywords: ovarian cancer, maintenance therapy, efficacy, safety, placebo, randomized controlled trials
Citation: Wang J-f, Zhao L-b, Bin Y-d, Zhang K-l, Sun C, Wang Y-r, Feng X, Ji J, He L-s, Chen F-y and Li Q-l (2022) Efficacy and Safety of Placebo During the Maintenance Therapy of Ovarian Cancer in Randomized Controlled Trials: A Systematic Review and Meta-analysis. Front. Oncol. 12:796983. doi: 10.3389/fonc.2022.796983
Received: 18 October 2021; Accepted: 22 April 2022;
Published: 25 May 2022.
Edited by:
Cara Mathews, Women and Infants Hospital of Rhode Island, United StatesReviewed by:
Luigi Turco, Mater Olbia Hospital, ItalyRichard Penson, Harvard Medical School, United States
Copyright © 2022 Wang, Zhao, Bin, Zhang, Sun, Wang, Feng, Ji, He, Chen and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qi-ling Li, bGlxaWxpbmdAbWFpbC54anR1LmVkdS5jbg==; Fang-yao Chen, Y2hlbmZ5QG1haWwueGp0dS5lZHUuY24=
 Jin-feng Wang
Jin-feng Wang Lan-bo Zhao
Lan-bo Zhao Ya-di Bin
Ya-di Bin Kai-lu Zhang
Kai-lu Zhang Chao Sun1
Chao Sun1 Xue Feng
Xue Feng Jing Ji
Jing Ji Li-song He
Li-song He Qi-ling Li
Qi-ling Li