
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 11 April 2022
Sec. Gynecological Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.782030
This article is part of the Research Topic Women in Gynecological Oncology: 2021 View all 34 articles
Objective: To examine the effect of primary recurrence patterns on the prognosis of squamous cervical cancer after initial treatment.
Methods: Primary recurrence patterns and prognostic factors were examined in stage IB-IIA cervical cancer patients after initial treatment. Recurrence site (locoregional recurrence and distant metastasis or in-field and out-field recurrence for patients receiving adjuvant radiotherapy) and subtype (nodal and organ recurrence) were examined. Clinicopathological characteristics and survival rates were evaluated to generate a prognostic nomogram.
Results: A total of 472 patients were included. The median follow-up period, 5-year overall (OS) rate, and median OS were 59.1 months, 33.7%, and 24.0 months, respectively. Overall, 38.8% and 61.2% of the patients had locoregional recurrence and distant metastasis, respectively, and survival rates were comparable in these groups. Patients with nodal recurrence had better OS than those with organ recurrence (38.3% vs 30.7%, respectively; P = 0.001). Patients not receiving adjuvant radiotherapy had increased risk of pelvic recurrence [odds ratio (OR) = 0.148; 95% confidence interval[(CI): 0.075–0.291, P = 0.000]. Positive lymph-vascular space invasion (OR= 1.928; 95% CI: 1.151–3.229, P = 0.013) and no chemotherapy (OR = 0.521; 95% CI: 0.317–0.733, P = 0.040) increased the risk of distant metastasis. Positive lymph node status after initial treatment were associated with nodal recurrence (OR = 3.729; 95% CI: 1.838–7.563, P = 0.000), while elevated preoperative squamous cell carcinoma antigen (SCC-Ag) levels were associated with organ recurrence (OR = 1.642; 95% CI: 1.325–2.265, P = 0.002). Recurrence subtype, therapy for relapse, the International Federation of Gynecology and Obstetrics stage, adjuvant radiotherapy, preoperative SCC-Ag levels, and risk subgroup were independently associated with OS.
Conclusions: Primary recurrence patterns were associated with specific clinicopathological characteristics of cervical cancer. Recurrent cervical cancer prognosis was mainly affected by recurrence location and subtype.
Cervical cancer is the fourth most common gynecologic cancer worldwide and a leading cause of cancer-related deaths in developing countries (1). The incidence of cervical cancer has decreased owing to extensive screening and vaccination programs, with the latter targeting patients with high-risk human papilloma virus genotypes (2, 3). Standard treatment for early-stage cervical cancer [International Federation of Gynecology and Obstetrics (FIGO 2009) stage IB-IIA] includes radical surgery with or without adjuvant radiotherapy (RT) (4, 5). Consecutive editions of clinical guidelines have improved cervical cancer management, thus increasing remission rates and decreasing the risk of relapse; these changes have resulted in recently reported 5-year overall survival (OS) rates of early stage cervical cancer in the range of 70%–90% (6, 7). This progress notwithstanding, approximately 10%–15% of patients with early-stage disease experience recurrence (8).
Cervical cancer recurrence patterns may vary; however, they generally include locoregional recurrence (LR) and distant metastasis (DM). Recurrent disease management requires a personalized approach depending on the site of recurrence, which may determine prognosis. Previous studies have shown that patient prognosis after initial treatment was associated with clinicopathological characteristics, recurrence patterns, and relapse therapy type (9–11). However, few studies have examined risk factors for recurrence and the relationship between different recurrence patterns and prognosis. This study aimed to examine patients experiencing cervical cancer recurrence to identify their clinicopathological characteristics, recurrence patterns, and treatment type received, and to evaluate the relationship between different clinicopathological characteristics and recurrence patterns; this study aimed to examine the impact of recurrence patterns on prognosis.
We retrospectively extracted data of 472 patients with recurrent cervical cancer who underwent standard abdominal radical hysterectomy and pelvic lymph node (LN) dissection at the Department of Gynecologic Oncology, Fudan University Shanghai Cancer Center, China, between May 2006 and January 2014. All included patients had histologically confirmed squamous cell carcinoma and the 2009 FIGO stage IB–IIA disease after initial treatment. All patients provided consent for their data to be used for research purposes. Data on clinicopathological and prognostic characteristics, including age at diagnosis, the FIGO stage, postoperative pathological findings[histological type, tumor grade, lymph-vascular space invasion (LVSI) status, tumor volume, and LN status], treatment modalities, date and type of recurrence, and date of death or last follow-up, were collected. The study was approved by the Ethics Committee of Fudan University Shanghai Cancer Center, Shanghai, China.
Patients with intermediate-risk factors that met the Sedlis criteria, including tumor diameter, depth of stromal invasion (DSI), or LVSI status, and patients with more than one high-risk factor (including parametrial involvement, positive LN, or positive surgical margins) received adjuvant RT (ART) or concurrent chemoradiotherapy. Patients received pelvic intensity-modulated radiotherapy with computed tomography (CT) planned. Target delineation was based on the Radiation Therapy Oncology Group Consensus Guidelines (12, 13). Concurrent cisplatin was administered weekly at a dose of 40 mg/m2. Brachytherapy was administered among patients with vaginal margin invasion. Patients with one or more high-risk factors received 4–6 cycles of paclitaxel (135 mg/m2) and carboplatin (AUC = 5) on day 1.
Recurrence was confirmed based on findings from biopsy and/or imaging-based examinations such as CT, magnetic resonance imaging (MRI), or positron emission tomography (PET) scanning, obtained ahead of treatment planning. LR was defined as isolated pelvic recurrence, including vaginal recurrence with or without pelvic LN recurrence. DM was defined as distant site failure at either an organ or LN site. Multiple-site recurrence was defined as recurrence both inside and outside the pelvis; this type of recurrence was classified as DM. In-field and out-field recurrences were defined as recurrence inside or outside the pelvis for patients after ART. LN recurrence (LNR) included bilateral upper neck, supraclavicular, mediastinal, celiac, and pelvic and inguinal regions. Organ recurrence included recurrences in the vagina and other organs. Therapy for relapse included external beam radiation therapy and brachytherapy, surgery, and systemic chemotherapy. RT and surgery were classified as local therapy.
Patients’ demographic and clinicopathological characteristics and treatments for recurrent disease were reported as frequencies. Univariable survival curves were created using the Kaplan-Meier method. Between-group comparisons were performed using the log-rank test. Cox proportional hazards regression was used for univariate and multivariate analyses. Variables with p-values of <0.10 in univariate analysis were included in multivariate analyses. For all statistical tests, two-tailed p-values of <0.05 were considered significant. A prognostic nomogram was generated based on multivariable analysis results. Harrel’s concordance index (C-index) and a calibration curve were used to evaluate nomogram performance. The accuracy and reliability of the recurrence model were evaluated based on time-dependent receiver operating characteristic curves. All statistical analyses were performed in SPSS version 25.0 (SPSS, Chicago, IL) and R software ver. 4.0.5.
This study included 472 women who underwent pelvic LN dissection for cervical squamous cell carcinoma with FIGO stages IB-IIA. The patients’ median age was 47 (range: 22–77) years. All tumors were pathologically staged after radical surgery and included stages IB1 (n = 112, 23.7%), IB2 (n = 61, 12.9%), IIA1 (n = 162, 34.3%), and IIA2 (n = 137, 29.0%). Preoperative squamous cell carcinoma antigen (SCCA) levels of ≥2.55 ng/mL, bulky tumor size of ≥4 cm, DSI greater than 1/2 thickness, positive LVSI, and parametrial invasion were observed in 62.0% (n = 240), 56.8% (n = 268), 85.8% (n = 400), 63.0% (n = 289), and 14.4% (n = 64) of the patients, respectively. Thirty-eight (8.5%) patients had positive vaginal margins. Positive LN (PLN) was detected in 224 (50.3%) patients. The median number of harvested LNs was 23 (range: 7–77); a single (range: 0–37) PLN was harvested. A total of 356 (75.4%) patients underwent ART. Among 305 (85.0%) patients who underwent chemotherapy, 204 (64.2%) received concurrent chemotherapy (Table 1).
LR and DM accounted for 38.8% (183/472) and 61.2% (289/472) of recurrence cases, respectively. A total of 26.1% (n = 123) and 73.9% (n = 349) of failures were LNR and organ recurrence, respectively (Table 2).
RT, systemic chemotherapy, and surgery for relapse accounted for 44.0% (54/123), 41.5% (51/123), and 13.8% (17/123) of therapies for LNR, respectively. The corresponding rates for organ recurrence were 26.6% (93/349), 64.2% (224/349), and 9.2% (32/349), respectively (Table 1).
In univariate analysis, PLNs, SCCA levels of ≥2.55 ng/mL, tumor size of ≥4 cm, DSI, positive LVSI, parametrial invasion, and no ART were predictors of LR and DM (Table 2). In multivariate analysis, DSI (odds ratio [OR] = 1.494; 95% confidence interval [CI]: 1.286–2.853, P = 0.011) and ART (OR = 0.148; 95% CI: 0.075–0.291, P = 0.000) were independently associated with LR. LVSI (OR= 1.928; 95% CI: 1.151–3.229, P = 0.013) and adjuvant chemotherapy (OR = 0.521; 95% CI: 0.317–0.733, P = 0.040) were independently associated with DM. In addition, PLNs (OR = 3.729; 95% CI: 1.838–7.563, P = 0.000) and ART (OR = 0.470; 95% CI: 0.176–0.843, P = 0.003) were independently associated with LNR. SCCA levels of ≥2.55 ng/mL (OR = 1.642; 95% CI: 1.325–2.265, P = 0.002) and LVSI (OR = 1.462; 95% CI: 1.203–2.048, P = 0.005) were independently associated with organ recurrence (Table 3).
For cases of disease recurrence, the median follow-up time was 59.1 (range 1.9–146.6) months. The 5-year OS rate was 33.7%, and the median OS was 24.0 months. Patients with LR (5-year OS rate, 28.1%; median OS = 40 months) and those with DM (5-year OS rate, 38.9%; median OS = 37 months) (P = 0.755) had similar survival rates (Figure 1A). However, the prognosis was poorer for patients with LR with ART than for those without ART (Figure 1B). LNR (5-year OS rate, 38.3%; median OS = 51 months) had a better survival than organ recurrence (5-year OS rate, 30.7%; median OS = 34 months) (P = 0.001) (Figure 1C). Prognosis associated with pelvic LNR (5-year OS rate, 18.3%; median OS = 37 months) was poorer than that associated with LNR outside of the pelvis (5-OS rate, 36.9%; median OS = 50 months) (P = 0.019) (Figure 1D). Among patients with organ recurrence only, lung metastasis (5-year OS rate, 32.5%; median OS = 48 months) was associated with survival rates that were better than those associated with other organ recurrence sites (5-year OS rate, 19.7%; median OS = 34 months) (P = 0.039) (Figure 1E). Patients with vaginal recurrence (5-year OS rate, 31.7%; median OS = 34 months) had a worse prognosis than did those without vaginal recurrence (5-year OS rate, 46.0%; median OS = 51 months) (P = 0.001) (Figure 1F). According to ART stratification, in-field recurrence was associated with poorer prognosis (5-year OS rate, 24.9%; median OS = 29 months) than out-field recurrence (5-year OS rate, 30.8%; median OS = 42 months) (P = 0.024) (Figure 1B). For all recurrent cases, surgery was associated with 5-year OS rates that were better than those associated with RT and chemotherapy (5-year OS rate of surgery, RT and chemotherapy: 56.9%, 35.7% and 27.3%; median OS: NA, 46 months and 32 months, respectively, P < 0.001). For in-field recurrence only, surgery (5-year OS rate, 36.5%; median OS = 36 months) was associated with OS that was better than that associated with secondary RT (5-year OS rate, 12.2%; median OS = 24 months) (P = 0.047). In patients with LR, LNR, and organ recurrence, outcomes associated with surgery and RT were better than those associated with systemic chemotherapy (P = 0.011).
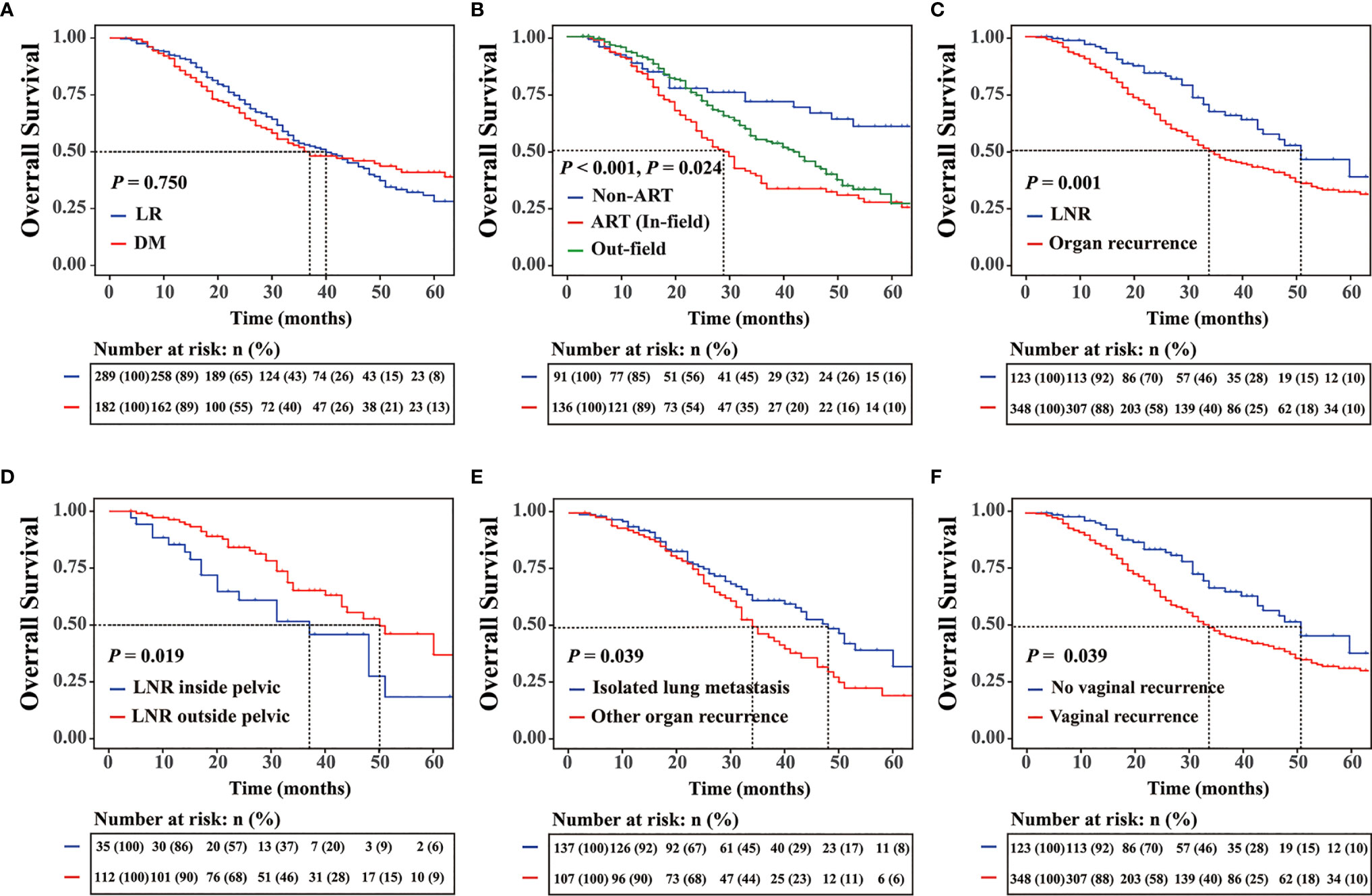
Figure 1 (A) Five-year overall survival (OS) rates for different recurrence sites. (B) OS rates for patients with locoregional recurrence (LR) with or without adjuvant radiotherapy (ART) and the OS for patients with the out-field recurrence. (C) Five-year OS rates for patients with different recurrence subtypes, (D) patients with lymph node recurrence, (E) patients with or without isolated lung metastasis, and (F) patients with or without vaginal stump.
In multivariate analysis, type of recurrence, therapy for relapse, the FIGO stage, ART, baseline serum SCCA levels, and risk factor subgroup were independently associated with prognosis after recurrence (Table 4). All relevant predictors were used to construct a prognostic nomogram, and points were assigned based on corresponding factor coefficients; the total score was used to predict 5-year OS rates. The C-index of the nomogram was 0.724 (95% CI, 0.679–0.769) in the internal validation set (Figure 2A). Calibration curves are presented in Figure 2B. The results indicated that the nomogram was well-calibrated. In addition, this nomogram was compared to commonly used risk prediction methods, including the FIGO stage and Sedlis criteria and other previously used models. The AUC of our nomogram was greater than those of models previously used (0.846 vs. 0.720 vs. 0.677, P < 0.001) (Figure 2C).
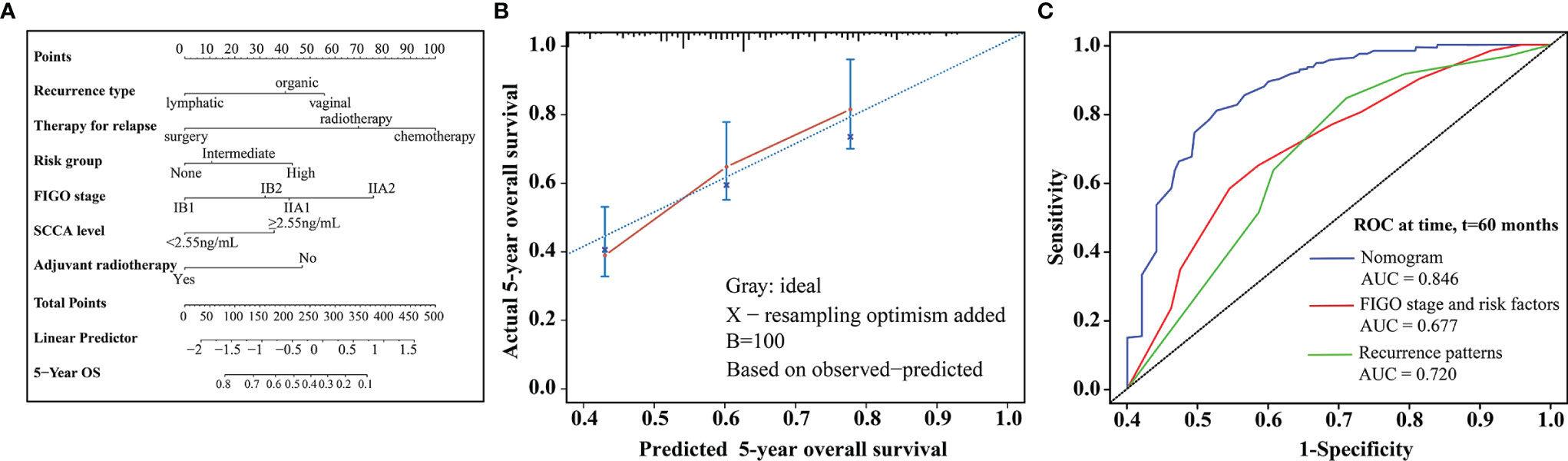
Figure 2 (A) Nomogram predicting 5-year OS rates for patients with relapse after initial treatment. The nomogram was based on scores corresponding to each independent variable. The total score (bottom of the scale) indicates the probabilities of 5-year OS rates. (B) The predicted and observed 5-year OS rates were used for model calibration. The x-axis displays nomogram-predicted probability, while the y-axis displays observed survival rates estimated using the Kaplan-Meier method. The dotted line indicates excellent model calibration, with good concordance between the predicted and observed survival rates. The vertical bars represent 95% confidence intervals. (C) Receiver operating characteristic curves with area under the curve values compare nomogram and traditional model discrimination. The blue lines represent survival rates predicted by the nomogram.
In the present study, primary recurrence patterns were associated with the prognosis of squamous cervical cancer after initial treatment. The patterns of recurrence in cervical cancer vary, and thus, different treatments for relapse may result in different prognoses. The National Comprehensive Cancer Network Guidelines recommend individualized therapy for relapse, including surgery, RT, and chemotherapy. For localized recurrence after initial treatment, radical retreatment, including RT and/or chemotherapy, and surgery may be administered. However, radical retreatment options may vary among recurrence sites; for example, patients with regional LN recurrence may be suitable candidates for radical surgery and RT. In such cases, patients eligible for radical treatment may achieve long-term disease-free survival rates of approximately 40% (14). Most previous studies evaluated prognosis based on patients’ clinicopathological features and recurrence sites. However, few studies have examined the relationship between specific parameters and recurrence patterns. The present study examined the prognostic relevance of recurrence patterns and subtypes, and that of clinicopathological factors.
In our cohort, there were more cases of DM than LR (61.2% vs. 38.8%). Furthermore, the incidence of organ recurrence was higher than that of LNR. Vaginal recurrence was the most common recurrence site, followed by the lung and bone. Previous population-based studies reported vaginal recurrence as the most common local recurrence site in patients with cervical cancer; meanwhile, the lung and bone were the common sites of distant metastases (15, 16). In our study, LNR was a particular kind of recurrence pattern; in addition, approximately 12.5% and 8.3% of recurrence cases were observed at para-aortic and supraclavicular LNs, respectively. The para-aortic lymphatic system is connected to the cervix and pelvic LNs, resulting in a high recurrence rate in para-aortic LNs (17, 18). Kim et al. reported rates of 59.5% and 40.5% for distant and pelvic recurrence, respectively (combined: 21.5%, central: 10.7%, pelvic 8.3%) (19). Pamela et al. used PET scans to detect recurrences in para-aortic LNs and reported a rate of 18.7%, which was consistent with the present study findings (20). Tae et al. reported a 5.4% recurrence rate in the supraclavicular region after radical hysterectomy in patients with early-stage cervical cancer (21).
Common prognostication methods include the FIGO staging system and the Peters and Sedlis criteria. However, these models fail to account for the effects of recurrence patterns. When patients experience disease recurrence, little attention is given to baseline clinical characteristics, which may help assess the risk of recurrence. Studies on the relevance of recurrence patterns and initial treatment types to the risk of relapse are rare. Nevertheless, baseline clinicopathological characteristics may help inform treatment as these factors may affect the risk of relapse. Consequently, we examined the associations among baseline clinicopathological characteristics, recurrence patterns, therapy for relapse, and prognosis to establish a predictive model to inform clinical practice.
In the present study, approximately half of the patients with recurrence had elevated serum SCCA levels at diagnosis and half had positive LN status after surgery. All cases with recurrence presented with intermediate- or high-risk factors or both at baseline. Different recurrence patterns may be associated with specific clinicopathological characteristics at initial treatment. According to univariate and multivariate analyses, DSI and the absence of ART were associated with a high risk of LR. Patients who did not receive adjuvant chemotherapy but had LVSI were more likely to experience DM than their counterparts. PLN status after initial treatment or lack of ART may result in LNR. Preoperative serum SCCA levels and positive LVSI findings were independent predictive factors for organ recurrence. Jeong et al. reported that approximately 59% of recurrence cases had elevated serum SCCA levels at diagnosis (22). Serum SCCA levels are biomarkers commonly used for auxiliary diagnosis and surveillance in cervical cancer; elevated serum SCCA levels are associated with the extent of the disease (23, 24). However, no previous study has examined the association between serum SCCA levels and specific recurrence patterns. According to the Sedlis criteria, DSI is an intermediate risk factor for recurrence in cervical cancer; we have previously shown that DSI may be independently associated with both DFS and OS (25). The effect of LVSI on early-stage cervical cancer remains controversial, and previous studies have yielded conflicting results. Balaya and Obrzut found that LVSI may be associated with decreased 5-year DFS and 10-year OS (26, 27). Meanwhile, Creasman et al. have suggested that LVSI may not be a prognostic factor (28). In the present study, patients with positive LVSI had increased risk of DM, specifically, at distant organs. The present study model was superior to previous models for disease prognostication (29–31).
The prognosis associated with LR was comparable to that associated with DM. More specifically, cases of LNR had better prognosis than cases of organ recurrence; however, pelvic LNR had poor overall prognosis. Isolated LNR may be radically treated with either surgery or RT; in contrast, organ recurrence is difficult to treat with any radical approach. For patients with organ recurrence, vaginal recurrence was associated with poor prognosis; meanwhile, isolated lung metastasis was associated with better OS rates compared to those associated with the other sites of organ recurrence. Isolated lung disease may be treatable with surgery or RT, which may improve prognosis. Previous studies have shown that patients who benefit from aggressive local therapy for oligometastatic disease include those with nodal or lung metastases (32). Nevertheless, pelvic recurrence was associated with poor prognosis in patients with LR after ART. The palliation of pelvic recurrences is difficult at previously irradiated sites that are not amenable to local pain control techniques or surgical resection. These sites are generally not responsive to chemotherapy; consequently, affected patients are often advised to undergo pelvic exenteration or receive systematic chemotherapy; however, pelvic exenteration is a complex procedure susceptible to complications, which may affect prognosis. Thus, the National Comprehensive Cancer Network guidelines recommend pelvic exenteration for very select patients. Moreover, secondary RT is not feasible due to the high risk of adverse effects and dose-limiting toxicity (33, 34). Chemotherapy is often recommended for patients with extra-pelvic metastases or recurrent disease who are not candidates for RT or exenterative surgery.
This study had some limitations that should be considered when interpreting its findings. First, it was a retrospective study, which makes it subject to the effects of selection bias and confounding factors. Second, nodal metastasis has been redefined in the 2018 FIGO staging system; however, this study dataset and the included citations refer mostly to the 2009 FIGO staging system. Finally, although local treatment status emerged as a prognostic factor for OS, the lack of precise information on recurrence treatment may impact the specificity of the present results.
Recurrent cervical cancer is associated with poor prognosis in cases of in-field recurrence. Different clinicopathological characteristics are associated with different recurrence sites and subtypes. The present findings suggest that patients with DSI and absence of ART are at a high risk of LR. Patients who did not receive adjuvant chemotherapy and presented with LVSI were more likely to experience DM than their counterparts. PLNs after initial treatment or lack of ART may increase the risk of LNR. Preoperative serum SCCA levels and positive LVSI status increased the risk of organ recurrence. Recurrent cervical cancer prognosis is associated with recurrence location and subtype.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Conception and design: ZZ, LJ, and JZ. Collection and assembly of data: ZZ and LJ. Data analysis and interpretation: ZZ, LJ, and JZ. Pathological slides reviewing: RB. Manuscript writing: ZZ and LJ. Final approval of manuscript: ZZ, LJ, RB, XW, GK, and JZ. All authors contributed to the article and approved the submitted version.
The present study was supported by the National Natural Science Foundation of China (Youth Fund no. 81902640).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank all the faculty members in our department and all the patients, and their families involved in the current study.
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
2. Lees BF, Erickson BK, Huh WK. Cervical Cancer Screening: Evidence Behind the Guidelines. Am J Obstet Gynecol (2016) 214(4):438–43. doi: 10.1016/j.ajog.2015.10.147
3. Lei T, Mao WM, Lei TH, Dai LQ, Fang L, Chen WQ, et al. Incidence and Mortality Trend of Cervical Cancer in 11 Cancer Registries of China. Chin J Cancer Res (2011) 23(1):10–4. doi: 10.1007/s11670-011-0010-x
4. American College of O, Gynecologists. ACOG Practice Bulletin. Diagnosis and Treatment of Cervical Carcinomas. Number 35, May 2002. American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet (2002) 78(1):79–91. doi: 10.1016/s0020-7292(02)90092-5
5. Gaffney DK, Erickson-Wittmann BA, Jhingran A, Mayr NA, Puthawala AA, Moore D, et al. ACR Appropriateness Criteria(R) on Advanced Cervical Cancer Expert Panel on Radiation Oncology-Gynecology. Int J Radiat Oncol Biol Phys (2011) 81(3):609–14. doi: 10.1016/j.ijrobp.2010.11.005
6. Peters WA 3rd, Liu PY, Barrett RJ 2nd, Stock RJ, Monk BJ, Berek JS, et al. Concurrent Chemotherapy and Pelvic Radiation Therapy Compared With Pelvic Radiation Therapy Alone as Adjuvant Therapy After Radical Surgery in High-Risk Early-Stage Cancer of the Cervix. J Clin Oncol (2000) 18(8):1606–13. doi: 10.1200/JCO.2000.18.8.1606
7. Wenzel HHB, Smolders RGV, Beltman JJ, Lambrechts S, Trum HW, Yigit R, et al. Survival of Patients With Early-Stage Cervical Cancer After Abdominal or Laparoscopic Radical Hysterectomy: A Nationwide Cohort Study and Literature Review. Eur J Cancer (2020) 133:14–21. doi: 10.1016/j.ejca.2020.04.006
8. van der Velden J, Mom CH, van Lonkhuijzen L, Tjiong MY, Westerveld H, Fons G. Analysis of Isolated Loco-Regional Recurrence Rate in Intermediate Risk Early Cervical Cancer After a Type C2 Radical Hysterectomy Without Adjuvant Radiotherapy. Int J Gynecol Cancer (2019) 29:874–8. doi: 10.1136/ijgc-2019-000445
9. Gee MS, Atri M, Bandos AI, Mannel RS, Gold MA, Lee SI. Identification of Distant Metastatic Disease in Uterine Cervical and Endometrial Cancers With FDG PET/CT: Analysis From the ACRIN 6671/GOG 0233 Multicenter Trial. Radiology (2018) 287(1):176–84. doi: 10.1148/radiol.2017170963
10. Salani R, Khanna N, Frimer M, Bristow RE, Chen LM. An Update on Post-Treatment Surveillance and Diagnosis of Recurrence in Women With Gynecologic Malignancies: Society of Gynecologic Oncology (SGO) Recommendations. Gynecol Oncol (2017) 146(1):3–10. doi: 10.1016/j.ygyno.2017.03.022
11. Yoneda JY, Braganca JF, Sarian LO, Borba PP, Conceicao JC, Zeferino LC. Surgical Treatment of Microinvasive Cervical Cancer: Analysis of Pathologic Features With Implications on Radicality. Int J Gynecol Cancer (2015) 25(4):694–8. doi: 10.1097/IGC.0000000000000416
12. Small W Jr, Bosch WR, Harkenrider MM, Strauss JB, Abu-Rustum N, Albuquerque KV, et al. NRG Oncology/RTOG Consensus Guidelines for Delineation of Clinical Target Volume for Intensity Modulated Pelvic Radiation Therapy in Postoperative Treatment of Endometrial and Cervical Cancer: An Update. Int J Radiat Oncol Biol Phys (2021) 109(2):413–24. doi: 10.1016/j.ijrobp.2020.08.061
13. Small W Jr, Mell LK, Anderson P, Creutzberg C, De Los Santos J, Gaffney D, et al. Consensus Guidelines for Delineation of Clinical Target Volume for Intensity-Modulated Pelvic Radiotherapy in Postoperative Treatment of Endometrial and Cervical Cancer. Int J Radiat Oncol Biol Phys (2008) 71(2):428–34. doi: 10.1016/j.ijrobp.2007.09.042
14. Thomas GM, Dembo AJ, Myhr T, Black B, Pringle JF, Rawlings G. Long-Term Results of Concurrent Radiation and Chemotherapy for Carcinoma of the Cervix Recurrent After Surgery. Int J Gynecol Cancer (1993) 3(4):193–8. doi: 10.1046/j.1525-1438.1993.03040193.x
15. Zhang Y, Guo Y, Zhou X, Wang X, Wang X. Prognosis for Different Patterns of Distant Metastases in Patients With Uterine Cervical Cancer: A Population-Based Analysis. J Cancer (2020) 11(6):1532–41. doi: 10.7150/jca.37390
16. Zhou S, Peng F. Patterns of Metastases in Cervical Cancer: A Population-Based Study. Int J Clin Exp Pathol (2020) 13(7):1615–23.
17. Bae BK, Park SH, Jeong SY, Chong GO, Kim MY, Kim JC. Mapping Patterns of Para-Aortic Lymph Node Recurrence in Cervical Cancer: A Retrospective Cohort Analysis. Radiat Oncol (2021) 16(1):128. doi: 10.1186/s13014-021-01856-9
18. Eifel PJ, Winter K, Morris M, Levenback C, Grigsby PW, Cooper J, et al. Pelvic Irradiation With Concurrent Chemotherapy Versus Pelvic and Para-Aortic Irradiation for High-Risk Cervical Cancer: An Update of Radiation Therapy Oncology Group Trial (RTOG) 90-01. J Clin Oncol (2004) 22(5):872–80. doi: 10.1200/JCO.2004.07.197
19. Kim TH, Kim MH, Kim BJ, Park SI, Ryu SY, Cho CK. Prognostic Importance of the Site of Recurrence in Patients With Metastatic Recurrent Cervical Cancer. Int J Radiat Oncol Biol Phys (2017) 98(5):1124–31. doi: 10.1016/j.ijrobp.2017.03.029
20. Peters PN, Pierson WE, Chen LM, Westphalen AC, Chapman JS, Hsu IC. PET-Detected Asymptomatic Recurrence Is Associated With Improved Survival in Recurrent Cervical Cancer. Abdom Radiol (NY) (2021) 46(1):341–50. doi: 10.1007/s00261-020-02633-0
21. Kong TW, Son JH, Paek J, Chang SJ, Ryu HS. Prognostic Factors Influencing Pelvic, Extra-Pelvic, and Intraperitoneal Recurrences in Lymph Node-Negative Early-Stage Cervical Cancer Patients Following Radical Hysterectomy. Eur J Obstet Gynecol Reprod Biol (2020) 252:94–9. doi: 10.1016/j.ejogrb.2020.06.030
22. Jeong BK, Huh SJ, Choi DH, Park W, Bae DS, Kim BG. Prognostic Value of Different Patterns of Squamous Cell Carcinoma Antigen Level for the Recurrent Cervical Cancer. Cancer Res Treat (2013) 45(1):48–54. doi: 10.4143/crt.2013.45.1.48
23. Fu J, Wang W, Wang Y, Liu C, Wang P. The Role of Squamous Cell Carcinoma Antigen (SCC Ag) in Outcome Prediction After Concurrent Chemoradiotherapy and Treatment Decisions for Patients With Cervical Cancer. Radiat Oncol (2019) 14(1):146. doi: 10.1186/s13014-019-1355-4
24. Salvatici M, Achilarre MT, Sandri MT, Boveri S, Vanna Z, Landoni F. Squamous Cell Carcinoma Antigen (SCC-Ag) During Follow-Up of Cervical Cancer Patients: Role in the Early Diagnosis of Recurrence. Gynecol Oncol (2016) 142(1):115–9. doi: 10.1016/j.ygyno.2016.04.029
25. Zhu J, Cao L, Wen H, Bi R, Wu X, Ke G. The Clinical and Prognostic Implication of Deep Stromal Invasion in Cervical Cancer Patients Undergoing Radical Hysterectomy. J Cancer (2020) 11(24):7368–77. doi: 10.7150/jca.50752
26. Balaya V, Guani B, Magaud L, Bonsang-Kitzis H, Ngo C, Mathevet P, et al. Validation of the 2018 FIGO Classification for Cervical Cancer: Lymphovascular Space Invasion Should Be Considered in IB1 Stage. Cancers (Basel) (2020) 12(12):3554. doi: 10.3390/cancers12123554
27. Obrzut B, Semczuk A, Narog M, Obrzut M, Krol P. Prognostic Parameters for Patients With Cervical Cancer FIGO Stages IA2-IIB: A Long-Term Follow-Up. Oncology (2017) 93(2):106–14. doi: 10.1159/000471766
28. Creasman WT, Kohler MF. Is Lymph Vascular Space Involvement an Independent Prognostic Factor in Early Cervical Cancer? Gynecol Oncol (2004) 92(2):525–9. doi: 10.1016/j.ygyno.2003.11.020
29. Ryu SY, Kim MH, Nam BH, Lee TS, Song ES, Park CY, et al. Intermediate-Risk Grouping of Cervical Cancer Patients Treated With Radical Hysterectomy: A Korean Gynecologic Oncology Group Study. Br J Cancer (2014) 110(2):278–85. doi: 10.1038/bjc.2013.716
30. Sedlis A, Bundy BN, Rotman MZ, Lentz SS, Muderspach LI, Zaino RJ. A Randomized Trial of Pelvic Radiation Therapy Versus No Further Therapy in Selected Patients With Stage IB Carcinoma of the Cervix After Radical Hysterectomy and Pelvic Lymphadenectomy: A Gynecologic Oncology Group Study. Gynecol Oncol (1999) 73(2):177–83. doi: 10.1006/gyno.1999.5387
31. Ramirez PT, Pareja R, Rendon GJ, Millan C, Frumovitz M, Schmeler KM. Management of Low-Risk Early-Stage Cervical Cancer: Should Conization, Simple Trachelectomy, or Simple Hysterectomy Replace Radical Surgery as the New Standard of Care? Gynecol Oncol (2014) 132(1):254–9. doi: 10.1016/j.ygyno.2013.09.004
32. Im JH, Yoon HI, Kim S, Nam EJ, Kim SW, Yim GW, et al. Tailored Radiotherapeutic Strategies for Disseminated Uterine Cervical Cancer Patients. Radiat Oncol (2015) 10:77. doi: 10.1186/s13014-015-0373-0
33. Sardain H, Lavoue V, Redpath M, Bertheuil N, Foucher F, Leveque J. Curative Pelvic Exenteration for Recurrent Cervical Carcinoma in the Era of Concurrent Chemotherapy and Radiation Therapy. A Systematic Review. Eur J Surg Oncol (2015) 41(8):975–85. doi: 10.1016/j.ejso.2015.03.235
Keywords: cervical cancer, recurrence patterns, therapy for relapse, locoregional recurrence, distant metastasis
Citation: Zhang Z, Jiang L, Bi R, Wu X, Ke G and Zhu J (2022) Prognostic Effect of Primary Recurrence Patterns in Squamous Cervical Carcinoma After Radical Surgery. Front. Oncol. 12:782030. doi: 10.3389/fonc.2022.782030
Received: 23 September 2021; Accepted: 16 March 2022;
Published: 11 April 2022.
Edited by:
Stefano Restaino, Ospedale Santa Maria della Misericordia di Udine, ItalyReviewed by:
Clarissa Polen-De, Mayo Clinic, United StatesCopyright © 2022 Zhang, Jiang, Bi, Wu, Ke and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Zhu, ZHJhZ29uemxkQDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.