- 1Department of PET/CT Center, Hwa Mei Hospital, University of Chinese Academy of Sciences, Ningbo, China
- 2Ningbo Institute of Life and Health Industry, University of Chinese Academy of Sciences, Ningbo, China
- 3Department of Nuclear Medicine, Hwa Mei Hospital, University of Chinese Academy of Sciences, Ningbo, China
- 4Department of Physical Examination Center, Ningbo First Hospital, Ningbo, China
- 5Department of Nephrology, Hwa Mei Hospital, University of Chinese Academy of Sciences, Ningbo, China
- 6Department of Education, Hwa Mei Hospital, University of Chinese Academy of Sciences, Ningbo, China
PET/CT with 18F-2-fluoro-2-deoxyglucose (18F-FDG) has been proposed as a promising modality for diagnosing and monitoring treatment response and evaluating prognosis for patients with non-small cell lung cancer (NSCLC). The status of epidermal growth factor receptor (EGFR) mutation is a critical signal for the treatment strategies of patients with NSCLC. Higher response rates and prolonged progression-free survival could be obtained in patients with NSCLC harboring EGFR mutations treated with tyrosine kinase inhibitors (TKIs) when compared with traditional cytotoxic chemotherapy. However, patients with EGFR mutation treated with TKIs inevitably develop drug resistance, so predicting the duration of resistance is of great importance for selecting individual treatment strategies. Several semiquantitative metabolic parameters, e.g., maximum standard uptake value (SUVmax), metabolic tumor volume (MTV), and total lesion glycolysis (TLG), measured by PET/CT to reflect 18F-FDG metabolic activity, have been demonstrated to be powerful in predicting the status of EGFR mutation, monitoring treatment response of TKIs, and assessing the outcome of patients with NSCLC. In this review, we summarize the biological and clinical correlations between EGFR mutation status and 18F-FDG metabolic activity in NSCLC. The metabolic activity of 18F-FDG, as an extrinsic manifestation of NSCLC, could reflect the mutation status of intrinsic factor EGFR. Both of them play a critical role in guiding the implementation of treatment modalities and evaluating therapy efficacy and outcome for patients with NSCLC.
Introduction
In 2020, it was estimated that there were approximately 228,820 newly diagnosed lung cancer cases and 135,720 deaths from lung cancer in the United States (1). Non-small cell lung cancer (NSCLC), a major phenotype of lung cancer, accounting for about 80%–85%, is one of the leading causes of cancer-related deaths worldwide despite improvements in diagnostic and therapeutic modalities (1, 2). Epidermal growth factor receptor (EGFR) mutations were found in about 35% of patients with NSCLC in East Asia and 10%–15% in the United States (3, 4). In addition, EGFR mutations were demonstrated to be significantly associated with adenocarcinoma, never smoking, and the female gender (5). Patients with EGFR mutations treated with tyrosine kinase inhibitors (TKIs) were linked to a higher response rate and longer progression-free survival (PFS) than those treated with conventional cytotoxic chemotherapy (6, 7). Eventually, however, resistance to TKIs inevitably occurred with a median PFS of 9 to 13 months (7–9). In this regard, accurate prediction of EGFR mutations and monitoring of TKI response rates and drug resistance will be of great value for clinicians to perform individual treatment strategies.
PET/CT with 18F-2-fluoro-2-deoxyglucose (18F-FDG) has been widely used for pretreatment staging and restaging, monitoring treatment response, and evaluating prognosis for patients with NSCLC (10–14). Several semiquantitative metabolic parameters, e.g., maximal standard uptake value (SUVmax), total lesion glycolysis (TLG), and metabolic tumor volume (MTV), have been demonstrated to be promising PET/CT indices to reflect the metabolic activity and/or tumor burden (15, 16). SUVmax, a parameter representing the maximum uptake value of 18F-FDG in a single-pixel adjusted for lean body mass, has been widely used as a marker for glucose metabolic activity, but it cannot clearly reflect tumor burden. TLG, a quantitative volume-based metabolic PET parameter, has been recognized as a promising index for its advantages to reflect the metabolic activity and tumor burden. Higher SUVmax, TLG, or MTV on 18F-FDG PET/CT scan usually revealed a short PFS or overall survival (OS) for patients with NSCLC (17–19). Consequently, a certain cross and overlap may have occurred between the roles of 18F-FDG PET/CT and EGFR in evaluating the efficacy and outcome of NSCLC patients.
Over the past two decades, a great number of studies have attempted to elucidate the relationship between the status of EGFR mutation and the metabolic activity of 18F-FDG in NSCLC (20–23). Obviously, EGFR mutation status represents an intrinsic factor of NSCLC, while 18F-FDG metabolic activity is an extrinsic manifestation of NSCLC. There is a close association between EGFR mutation status and 18F-FDG metabolic activity in NSCLC, but the relationship between them needs to be further clarified due to contradictory reports (24–26). A large sample study including 849 patients with NSCLC showed that low SUVmax of the primary tumor, lymph node, and distant metastasis were associated significantly with EGFR mutations (24), whereas another study presented opposite results that high SUVmax (≥6.0) of the primary tumor was more likely to have EGFR mutations in NSCLC (25). In addition, no significant difference in 18F-FDG uptake between mutant EGFR and wild-type EGFR was also observed in NSCLC patients (26). Accordingly, in this work, we aimed to comprehensively review the biological and clinical correlations between EGFR mutation status and 18F-FDG metabolic activity in NSCLC.
Biological Correlation Between Epidermal Growth Factor Receptor Mutation Status and 18F-FDG Metabolic Activity in Non-Small Cell Lung Cancer
Tumor cells utilize a variety of metabolic pathways, especially glucose, to meet the requirements of bioenergy and biosynthesis for growth and proliferation (27, 28). Oncogenic mutations are the driving force of high energetic metabolism that can be maintained persistently in cancer cells (29). In addition, glucose metabolism preferentially tends to aerobic glycolysis rather than mitochondrial oxidative phosphorylation, which is known as the Warburg effect (27). It has been reported that many oncogenic signaling pathways in cancer cells, particularly EGFR aberrant signaling, lead to the metabolic switch from mitochondrial oxidative phosphorylation to aerobic glycolysis (30, 31). Recently, EGFR has been identified as a driver of oncogenes in NSCLC, because the mutation of activating EGFR kinase domain enhances the activity of EGFR tyrosine kinase, leading to continuous activation of the downstream signal pathway, and then drives tumorigenesis and tumor progression (32). Targeted EGFR mutation therapies, such as EGFR-TKIs, including erlotinib and gefitinib, have shown to be highly effective in inhibiting glucose consumption in both in vitro and in vivo models of NSCLC (Figure 1) (33, 34).
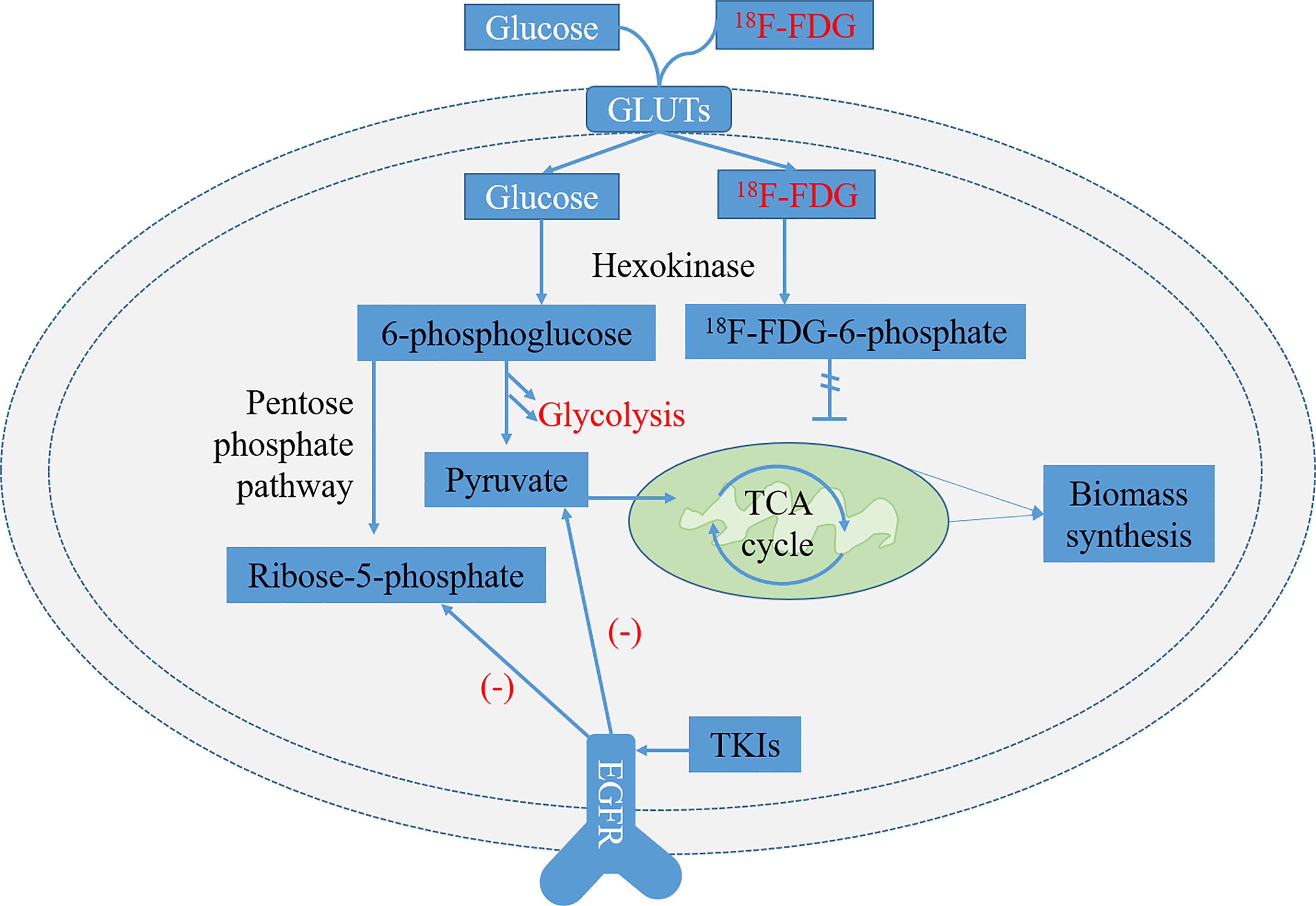
Figure 1 Glycolysis pathways of 18F-FDG and normal glucose and related metabolic pathways regulated by epidermal growth factor receptor (EGFR) signaling in EGFR-mutated non-small cell lung cancer (NSCLC). 18F-FDG is transported into cells by glucose transporters (GLUTs) and phosphorylated to 18F-FDG-6-phosphate by hexokinase (HK). It is trapped inside cells because 18F-FDG-6-phosphate is not a substrate of glycolysis or pentose phosphate pathway (PPP) and is unable to diffuse outside cells. The metabolites of pyruvate and ribose-5-phosphate in the glycolysis decreased significantly after treatment of lung adenocarcinoma cells with EGFR tyrosine kinase inhibitors (TKIs) (33).
18F-FDG, a glucose analog, is transported into cells by glucose transporters (GLUTs) and phosphorylated to 18F-FDG-6-phosphate by hexokinase (HK). It is trapped inside cells and dephosphorylated slowly because 18F-FDG-6-phosphate is not a substrate of glycolysis or pentose phosphate pathway (PPP) and is unable to diffuse outside cells (Figure 1) (35). Now, it has been widely used as a small molecule radiotracer for PET/CT imaging and has been applied extensively as a tracer to reflect glucose metabolic activity in diagnosing and evaluating treatment response of various malignant tumors, including NSCLC (36, 37). The overexpression of GLUT1 and HK-I is highly associated with the increased uptake of 18F-FDG in NSCLC, showing that the uptake of 18F-FDG seems to be regulated by glucose metabolism (38–40).
Several mutated oncogenes have been demonstrated to be associated with metabolic signaling pathways that affect tumor cell metabolism (41). In EGFR-mutated adenocarcinoma cells, lactate production, glucose-induced extracellular acidification rate, and glucose consumption were significantly decreased after treatment with TKIs, showing that EGFR signaling played a major role in aerobic glycolysis (33). In gefitinib-sensitive NSCLC cell lines with EGFR mutations, the uptake of 18F-FDG was also decreased significantly as early as 2 h after treatment, whereas no measurable changes in 18F-FDG uptake were observed in gefitinib-resistant cells, representing treatment response of gefitinib that could be closely reflected by glucose metabolic activity (34). Accordingly, to a certain extent, the metabolic activity of 18F-FDG in NSCLC cell lines is correlated with or may reflect the mutations of EGFR.
Clinical Correlation Between Epidermal Growth Factor Receptor Mutation Status and 18F-FDG Metabolic Activity in Non-Small Cell Lung Cancer
Predicting Epidermal Growth Factor Receptor Mutation Status With 18F-FDG PET/CT
A large number of studies reported that compared with traditional cytotoxic chemotherapy, NSCLC patients with EGFR mutation treated with TKIs had a higher response rate and prolonged PFS (6, 7). The presence of EGFR gene mutations in lung adenocarcinoma is a powerful predictor of better prognosis after gefitinib therapy (9). Accordingly, the status of EGFR mutations plays a critical role in selecting suitable treatment modalities for patients with NSCLC. However, in clinical practice, the status of EGFR mutation is usually determined by tissue-based analysis (42), which has a number of limitations, e.g., i) sampling bias due to tumor heterogeneous, ii) associated complications owing to invasive biopsies, iii) not rapid and expensive, and iv) failing to get reliable results due to the low quantity or quality of the tissue samples (43). In addition, the mutation status of EGFR may be changed in the course of chemotherapy or targeted therapy (44). Therefore, a non-invasive method is urgently needed to monitor EGFR mutation status in NSCLC.
PET/CT scan with 18F-FDG, a non-invasive and functional imaging method, has a powerful ability to predict the mutation status of EGFR in NSCLC (45–47). SUVmax is the most widely used index of 18F-FDG PET/CT in predicting EGFR mutations (24). Patients with NSCLC harboring EGFR mutations usually showed lower SUVmax than those with wild-type EGFR (Table 1) (21, 24, 48, 49). Normally, SUVmax was calculated only from the primary lesions of NSCLC, whereas the distant metastasis and/or metastatic lymph nodes were also monitored in some studies (24, 50). Low SUVmax of the distant metastasis was beneficial to the existence of EGFR mutations in advanced lung adenocarcinoma (50). Different cutoff values of SUVmax (range, 7.0–9.91) were determined to obtain a relatively high receiver operating characteristic (ROC) curve area (range, 0.557–0.75) (20, 24, 50). In addition to SUVmax, MTV was also used as a parameter to predict EGFR mutations in NSCLC. Patients with NSCLC harboring EGFR mutation had lower MTV than those with wild-type EGFR (57). Interestingly, the serum carcinoembryonic antigen (CEA) can increase during all adenocarcinomas not only in those EGFR mutated but also in wild type (58). The combination of serum CEA and SUVmax was also performed to predict EGFR mutations in patients with NSCLC, which demonstrated to have a moderate diagnostic accuracy (25, 51).
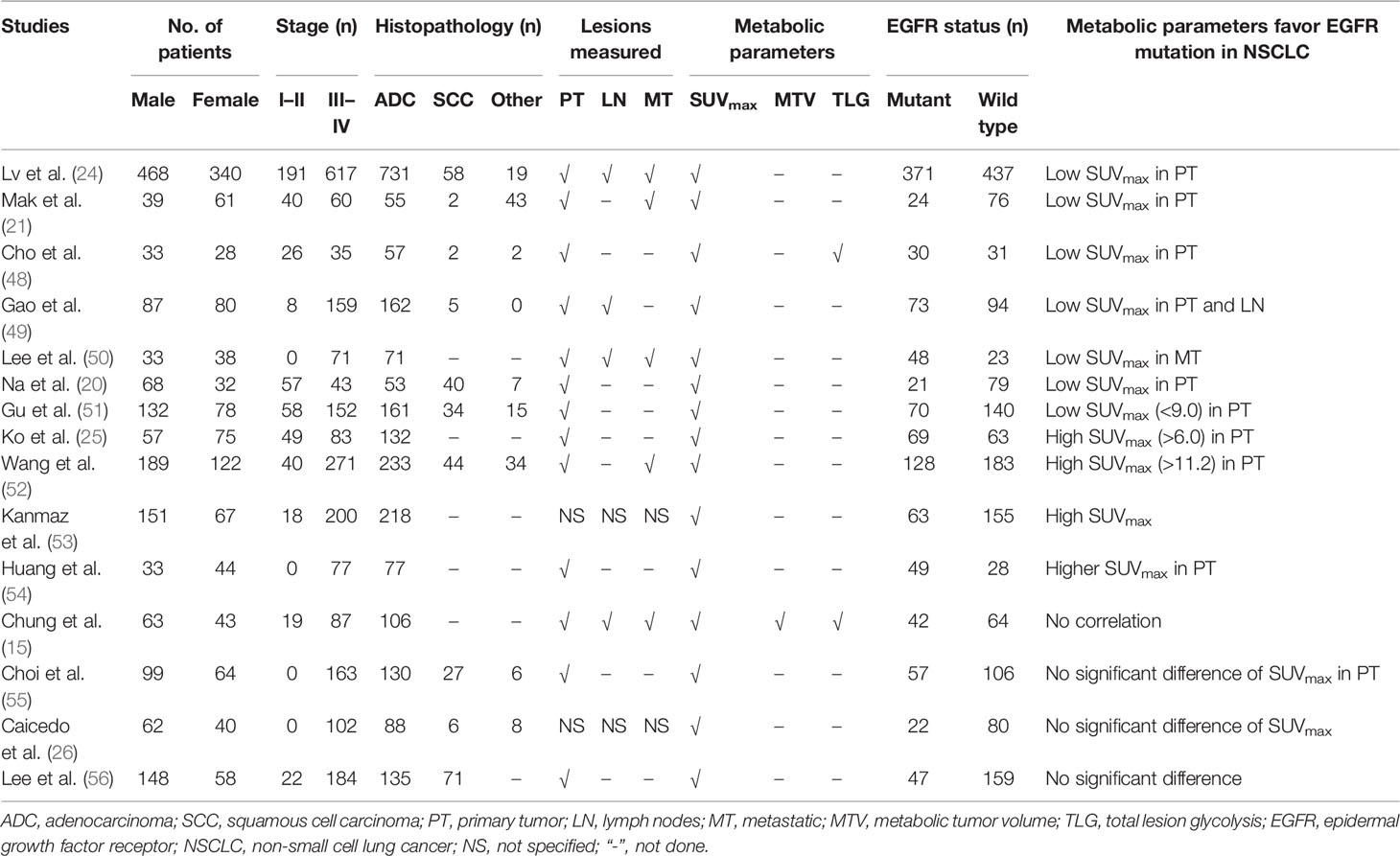
Table 1 The clinical and pathological features, glucose metabolic activity, and EGFR mutation status in NSCLC of previous studies.
However, opposite results could be observed that the metabolic activity of 18F-FDG (e.g., SUVmax) in NSCLC EGFR-mutant patients was significantly higher than that of wild-type patients (25, 52–54). The expression status of EGFR protein was also evaluated, and higher SUVmax was positively correlated with EGFR overexpression (59, 60). Furthermore, no significant difference in 18F-FDG uptake was observed between EGFR mutant and wild-type NSCLC patients in previous reports (Table 1) (26, 55, 56). Several reasons could lead to these conflicting results. First, the number of patients included in the studies varied widely, as low as only 61 patients and as high as up to 808 patients (24, 48, 57). Second, the rate of EGFR mutations varied greatly among NSCLC patients, from 21% to 68% (20, 50). Third, the proportion of histopathological subtypes of NSCLC (adenocarcinoma and squamous cell carcinoma) varied significantly, as EGFR mutations are difficult to detect in squamous cell carcinoma patients who smoke, while EGFR mutations are more common in adenocarcinoma (20, 21, 61). Fourth, the clinical stage (I–II vs. advanced stage) of patients with NSCLC was significantly different (25, 26). More importantly, multiple objective reasons, e.g., different PET/CT scanners, the plasma glucose level before PET/CT scan, fasting time, and region of interest parameters might result in contradictory results. Therefore, many novel techniques of PET/CT are performed to investigate the predictive efficacy of EGFR mutations in NSCLC.
Radiomics, an advanced mathematical model for quantifying the spatial relationships among image voxels, has become a growing research field in which a great number of imaging features are investigated in order to choose the most significantly relevant features with clinical, pathological, molecular, and genetic features, so as to improve the accuracy of diagnosis, prognosis, and curative effect evaluation (62, 63). Accordingly, the role of 18F-FDG PET/CT radiomics in predicting EGFR mutation status for patients with NSCLC has been evaluated (47, 64–67). The area under the ROC curve (AUC) was usually in the range of 0.57 to 0.86 when based on the radiomics features of PET/CT, whereas the performance would get a significantly higher efficacy when combined with clinical features and/or conventional PET/CT parameters, such as SUVmax, SUVmean, MTV, and TLG (47, 67, 68). In addition, four exons (18–21) of EGFR mutations have been observed in NSCLC patients (69), in which approximately 90% are exon 21 L858R substitutions and exon 19 deletions (70). Recently, research showed that two sets of prognostic radiomics features of 18F-FDG PET/CT could distinguish EGFR exon 19 deletions from EGFR exon 21 L858R missense, with an AUC of 0.87 in predicting EGFR mutation status (46).
In short, detection of EGFR mutation status in NSCLC plays a major role in the daily management of individual patients, especially in the selection of TKI targeted therapy. 18F-FDG PET/CT has been demonstrated to have a powerful efficacy to predict the EGFR mutation status in patients with NSCLC, not only based on conventional PET/CT parameters (e.g., SUVmax, MTV, and TLG) but also based on radiomics of PET/CT. The combination of clinical features, laboratory results, conventional PET/CT parameters, and PET/CT radiomics would provide higher accuracy in predicting EGFR mutation status. However, there are still many contradictory reports, so 18F-FDG PET/CT should be used with caution when predicting EGFR mutations in patients with NSCLC. More prospective cohort studies are needed to further verify the role of 18F-FDG PET/CT in predicting EGFR mutations.
Evaluating Treatment Response for Patients With Non-Small Cell Lung Cancer
Most patients with NSCLC develop late in the course of the disease, which is inoperable (12, 71). The standard treatment modality for those patients remains systematic chemotherapy (72). However, since not all patients with NSCLC respond well to chemotherapy and the treatment is toxic, it is important to identify those patients who are less or most likely to benefit from chemotherapy. Therefore, early prediction of treatment responses is particularly important, which can avoid the additional costs of unnecessary toxic and ineffective treatment or overtreatment, and possibly increase the chances of receiving other potentially effective therapy. Over the past two decades, EGFR TKIs, e.g., erlotinib and gefitinib, have been proposed to be effective treatment strategies for NSCLC patients with EGFR mutations (9, 73). The mutation status of EGFR is an optimal predictor of treatment response to TKIs for patients with NSCLC (3, 4). Nevertheless, only a small subset of patients with EGFR mutations respond well to TKIs, especially erlotinib, which have prolonged survival (74, 75). The response rate of EGFR mutations to TKIs in patients with NSCLC varied greatly. Accordingly, new approaches are obviously needed to determine which patients will benefit from TKI treatment.
Traditionally, response evaluation for NSCLC patients harboring EGFR mutations treated with TKIs is usually based on anatomic imaging features that mainly present with static, and calculating the change of tumor size on CT and using Response Evaluation Criteria in Solid Tumors (RECIST) for classification (76, 77). However, the differences between atelectasis or fibrosis and residual neoplasm cannot be distinguished significantly by conventional anatomic imaging modalities (78, 79). Accordingly, the detection of early treatment response using these anatomic imaging tools has limited value. 18F-FDG PET/CT, a molecular and functional imaging method, has emerged as a powerful ability in diagnosing, staging, and evaluating outcomes for patients with NSCLC (80). In addition, 18F-FDG PET/CT has been proposed to be of great value in predicting the efficacy of radiotherapy, chemoradiotherapy, neoadjuvant chemotherapy, and combined intercalated chemotherapy and erlotinib in patients with advanced NSCLC (81–85).
As for patients with TKI-treated NSCLC, 18F-FDG PET/CT could be used to monitor response (Table 2) as early as 2 days after therapy, and those patients who had a partial metabolic response and stable metabolic disease would have a significantly longer PFS than those with progressive metabolic disease (86). Moreover, patients with partial remission and stable disease showed a decreasing uptake of 18F-FDG, while patients with progressive disease presented an increasing 18F-FDG uptake, which was the early response on day 2 and week 4 after treatment with gefitinib (87). In reality, low SUVmax of the primary tumor on 18F-FDG PET/CT scan usually correlated with a higher response rate than high SUVmax (88). The subsequent tumor reduction could be predicted by the decreasing uptake of 18F-FDG on PET/CT scan as an early response to the initiation of TKI treatment for patients harboring EGFR-mutated NSCLC (89). The histopathologic response could also be monitored by 18F-FDG PET/CT using SUVmax changes, and it had an advantage over traditional CT to evaluate histopathologic response for patients with neoadjuvant erlotinib-treated NSCLC (90–92).
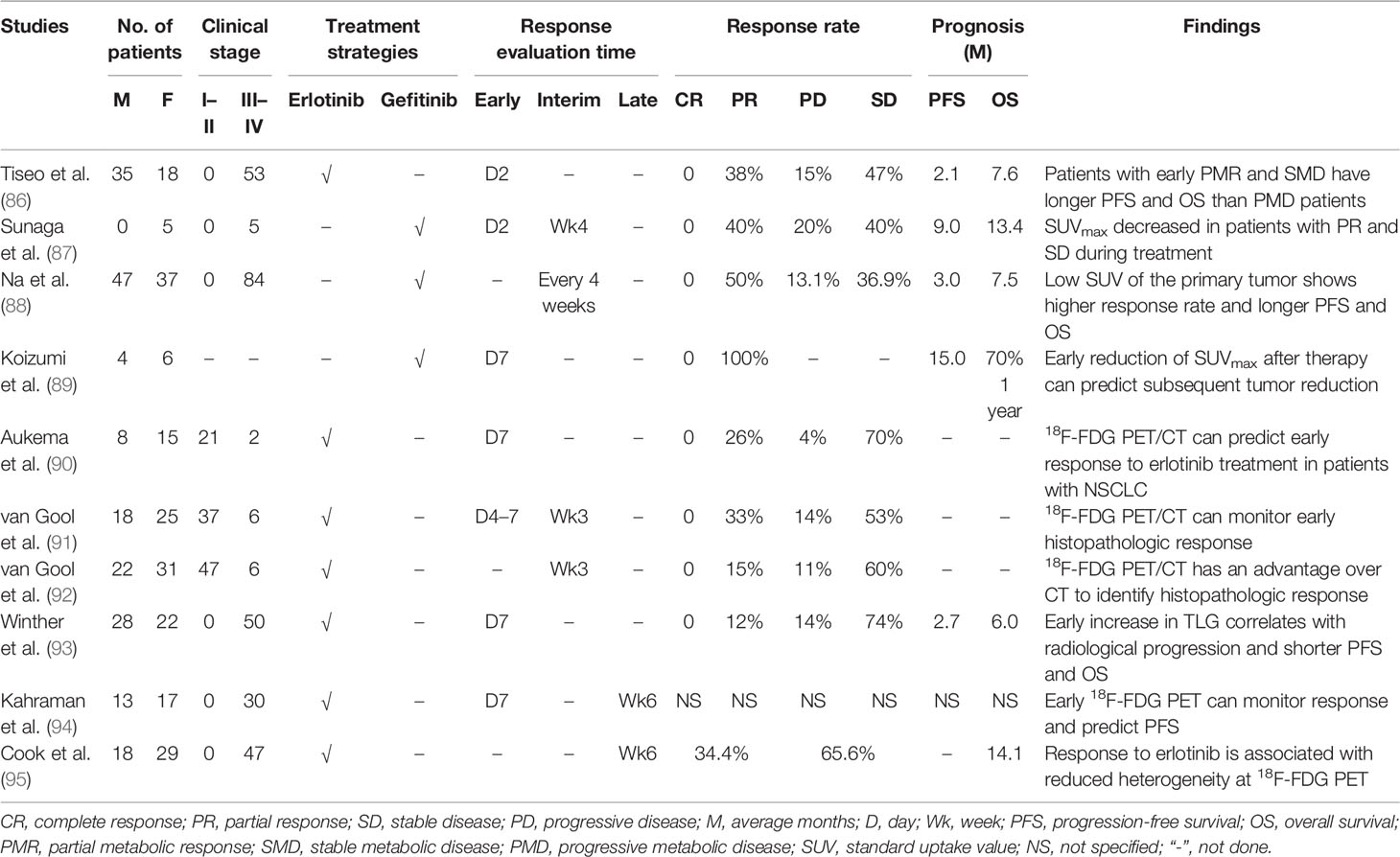
Table 2 The findings of 18F-FDG PET/CT in evaluating treatment response and outcome for TKIs treated patients with NSCLC.
The 18F-FDG metabolic activity of tumor on PET/CT scan can be revealed by several semiquantitative methods, e.g., SUVmax, SUV2Dpeak (2D peak SUV), SUV3Dpeak (3D peak SUV), SUVA50 (3D isocontour at 50% of the maximum pixel value adapted for background), SUVA41 (3D isocontour at 41% of the maximum pixel value adapted for background), SUV50 (3D isocontour at 50% of the maximum pixel value), MTV, and TLG; these parameters have been demonstrated to be useful in monitoring response for patients with TKI-treated NSCLC (93, 94). However, the best parameters for the early response monitoring might be the SUVmax, SUV50, SUVA50, and SUVA41 measured with 18F-FDG on PET/CT scan (94). Recently, tumor heterogeneity on 18F-FDG PET/CT has been evaluated for monitoring response in patients with erlotinib-treated NSCLC (95). The treatment response to erlotinib was related to the reduced heterogeneity of 18F-FDG PET. The change of first-order entropy was independently associated with treatment response and outcome (95). This study of NSCLC heterogeneity on 18F-FDG PET/CT opens a new window for monitoring therapy response.
As stated above, both EGFR and 18F-FDG PET/CT have potential value in monitoring TKI treatment response for NSCLC patients. Patients with mutant EGFR treated with TKIs benefit more than those with wild-type EGFR. 18F-FDG PET/CT demonstrates a high advantage in evaluating early treatment response. Several semiquantitative parameters of 18F-FDG metabolic activity present a significant role in assessing anatomical and histopathological responses for patients with NSCLC treated with TKIs. The heterogeneity of uptake of 18F-FDG on PET/CT may be a useful method to evaluate treatment response and prognosis for patients with NSCLC.
Predicting Prognosis for Patients With Non-Small Cell Lung Cancer
The prognosis of patients with NSCLC is heterogeneous and varies greatly. Tumor-node-metastasis (TNM) classification is a measure to specify the disease extent for patients with NSCLC and plays a vital role in choosing a treatment strategy (96). 18F-FDG PET/CT has been demonstrated to be powerful in staging procedures and is more accurate than conventional CT in mediastinal staging for patients with NSCLC (97). Patients with advanced stage are usually incurable with a short life expectancy. Accordingly, the choice of treatment methods must be discreetly balanced between the potential benefits and ineffective side, effects and a precise evaluation of the prognosis of patients with NSCLC is of great importance.
In the past two decades, EGFR is a well-known predictive marker of outcome for patients with NSCLC who were treated with TKIs (98). TKIs have become the first-line treatment strategy in standard therapy for advanced-stage NSCLC harboring EGFR mutations, e.g., deletion of exon 19 or exon 21 or the L858R point mutations (7, 8). The mutation in exon 19 of EGFR was a reliable predictor of favorable survival for patients with NSCLC (55). Patients with activated EGFR mutations treated with TKIs had a higher response rate and longer PFS than those treated with standard cytotoxic chemotherapy (6). However, resistance inevitably develops eventually for patients with NSCLC who are treated with EGFR TKIs, and it is difficult for clinicians to predict the time of recurrence or progression owing to the wide range of PFS in individual patients. Some patients progressed several years after starting TKI therapy, while others progressed rapidly and spread widely after just a few months, usually with a median of 9–13 months (7–9). To our knowledge, there is currently no reliable clinical tool to predict the prognosis of EGFR mutant NSCLC patients treated with TKIs. Meanwhile, only two clinical features, TNM staging and performance status, have been considered to be significantly associated with prognosis in patients with NSCLC, but they need to be further validated by prospective studies (99).
The prognosis of patients with NSCLC from early stage to advanced stage has been evaluated by several studies with numerous procedures (100, 101). The role of 18F-FDG PET and high-resolution CT in predicting the prognosis for patients with clinical stage-IA NSCLC has been assessed, which showed that SUVmax of the primary tumor and ground-glass opacity ratios on high-resolution CT images were significant prognosticators of these patients, which should be kept in mind before selecting therapeutic strategies (101). In patients with advanced NSCLC treated with erlotinib, 18F-FDG PET presented a predictive effect as early as 1 week after initiation of treatment, predicting PFS, OS, and non-progression after 6 weeks of treatment, and was independent of EGFR mutational status (100). Several different semiquantitative parameters, e.g., SUVmax, SUV2Dpeak, SUV3Dpeak, SUV50, SUVA50, and SUVA41, have been proved to be useful predictors of short-term prognosis in patients with advanced NSCLC in the early (1 week) and late (6 weeks) 18F-FDG PET/CT scans after initiation of erlotinib therapy (102). An updated systematic review and meta-analysis by the European lung cancer working party for the international association for the study of lung cancer staging project showed that SUV on 18F-FDG PET was potentially useful in predicting patient outcomes (13). Low SUVs of the primary tumor could predict favorable survival in NSCLC patients treated with TKIs (88, 102). Early evaluation of SUVmax changes on 18F-FDG PET at 2 days after initial treatment with gefitinib was of great significance to predict the clinical outcome of patients with lung adenocarcinoma (103). Moreover, early (day 14) partial metabolic response on 18F-FDG PET was independently associated with prolonged PFS and OS in patients with NSCLC treated with erlotinib (104).
In NSCLC patients with activating EGFR mutation, TLG has the potential role in predicting PFS and gefitinib resistance development on 18F-FDG PET (105). Measuring the baseline metabolic tumor burden with TLG before first-line TKIs will be very helpful to predict the time of acquired drug resistance (105). Intra-tumoral heterogeneity may be partially explained that not all patients with NSCLC harboring EGFR mutations will benefit from TKI therapy (106). Using an imaging tool may be a potentially simpler approach to assess tumor heterogeneity. Actually, heterogeneous textural parameters derived from baseline 18F-FDG PET/CT are demonstrated to be high predictors of clinical outcomes for NSCLC patients harboring EGFR mutations treated with TKIs (107). However, even though 18F-FDG PET/CT plays a vital role in predicting the prognosis for patients with NSCLC, contradictory results are also observed that SUVmax of the primary tumor cannot predict survival for patients with NSCLC (108, 109). Accordingly, furthermore, studies are needed to validate these findings to give clinicians an accurate recommendation.
Conclusion
In summary, the 18F-FDG metabolic activity of NSCLC, as an extrinsic manifestation, plays a critical role in monitoring treatment response and evaluating prognosis. Several semiquantitative parameters (e.g., SUVmax, MTV, and TLG) on 18F-FDG PET/CT can be used to reflect metabolic activity and tumor burden. EGFR mutation status, as an intrinsic factor, plays a vital role in guiding the implementation of treatment modalities (e.g., TKIs) and evaluating therapy efficacy and outcome for patients with NSCLC. Significant correlations are observed between 18F-FDG metabolic activity and EGFR mutation status, not only in biology but also in clinical practice. However, at present, there is still a lack of comprehensive evaluation of the association between 18F-FDG PET/CT and EGFR mutations in patients with NSCLC, e.g., using 18F-FDG PET/CT to predict EGFR mutation status and then monitor treatment response and evaluate the outcome, which needs to be carried out simultaneously in a large sample retrospective or prospective study.
Author Contributions
MJ and JJZ were responsible for the conception of this review. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Medical Scientific Research Foundation of Zhejiang Province, China (Grant no. 2021KY1014), Research Foundation of Hwa Mei Hospital, University of Chinese Academy of Sciences, China (Grant no. 2022HMKY27), Ningbo Public Service Technology Foundation, China (Grant No. 2021S176), and Medical Science and Technology Project of Ningbo, China (Grant no. 2020Y10), Ningbo Clinical Medical Research Center of Imaging Medicine (Grant No. 2021L003), and Provincial and Municipal Co-construction Key Discipline of Medical Imaging (Grant No. 2022-S02).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2020. CA Cancer J Clin (2020) 70:7–30. doi: 10.3322/caac.21590
2. Goldstraw P, Ball D, Jett JR, Le Chevalier T, Lim E, Nicholson AG, et al. Non-Small-Cell Lung Cancer. Lancet (2011) 378:1727–40. doi: 10.1016/S0140-6736(10)62101-0
3. Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating Mutations in the Epidermal Growth Factor Receptor Underlying Responsiveness of Non-Small-Cell Lung Cancer to Gefitinib. N Engl J Med (2004) 350:2129–39. doi: 10.1056/NEJMoa040938
4. Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR Mutations in Lung Cancer: Correlation With Clinical Response to Gefitinib Therapy. Science (2004) 304:1497–500. doi: 10.1126/science.1099314
5. Tokumo M, Toyooka S, Kiura K, Shigematsu H, Tomii K, Aoe M, et al. The Relationship Between Epidermal Growth Factor Receptor Mutations and Clinicopathologic Features in Non-Small Cell Lung Cancers. Clin Cancer Res (2005) 11:1167–73. doi: 10.1016/S0169-5002(05)80493-3
6. Sequist LV, Yang JC, Yamamoto N, O'Byrne K, Hirsh V, Mok T, et al. Phase III Study of Afatinib or Cisplatin Plus Pemetrexed in Patients With Metastatic Lung Adenocarcinoma With EGFR Mutations. J Clin Oncol (2013) 31:3327–34. doi: 10.1200/JCO.2012.44.2806
7. Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or Chemotherapy for Non-Small-Cell Lung Cancer With Mutated EGFR. N Engl J Med (2010) 362:2380–8. doi: 10.1056/NEJMoa0909530
8. Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. Gefitinib Versus Cisplatin Plus Docetaxel in Patients With Non-Small-Cell Lung Cancer Harbouring Mutations of the Epidermal Growth Factor Receptor (WJTOG3405): An Open Label, Randomised Phase 3 Trial. Lancet Oncol (2010) 11:121–8. doi: 10.1016/S1470-2045(09)70364-X
9. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or Carboplatin-Paclitaxel in Pulmonary Adenocarcinoma. N Engl J Med (2009) 361:947–57. doi: 10.1056/NEJMoa0810699
10. Hicks RJ. Role of 18F-FDG PET in Assessment of Response in Non-Small Cell Lung Cancer. J Nucl Med (2009) 50 Suppl 1:31S–42S. doi: 10.2967/jnumed.108.057216
11. Evangelista L, Cuppari L, Menis J, Bonanno L, Reccia P, Frega S, et al. 18f-FDG PET/CT in Non-Small-Cell Lung Cancer Patients: A Potential Predictive Biomarker of Response to Immunotherapy. Nucl Med Commun (2019) 40:802–7. doi: 10.1097/MNM.0000000000001025
12. Nahmias C, Hanna WT, Wahl LM, Long MJ, Hubner KF, Townsend DW. Time Course of Early Response to Chemotherapy in Non-Small Cell Lung Cancer Patients With 18F-FDG PET/Ct. J Nucl Med (2007) 48:744–51. doi: 10.2967/jnumed.106.038513
13. Berghmans T, Dusart M, Paesmans M, Hossein-Foucher C, Buvat I, Castaigne C, et al. Primary Tumor Standardized Uptake Value (SUVmax) Measured on Fluorodeoxyglucose Positron Emission Tomography (FDG-PET) is of Prognostic Value for Survival in Non-Small Cell Lung Cancer (NSCLC): A Systematic Review and Meta-Analysis (MA) by the European Lung Cancer Working Party for the IASLC Lung Cancer Staging Project. J Thorac Oncol (2008) 3:6–12. doi: 10.1097/JTO.0b013e31815e6d6b
14. Kirchner J, Sawicki LM, Nensa F, Schaarschmidt BM, Reis H, Ingenwerth M, et al. Prospective Comparison of (18)F-FDG PET/MRI and (18)F-FDG PET/CT for Thoracic Staging of Non-Small Cell Lung Cancer. Eur J Nucl Med Mol Imaging (2019) 46:437–45. doi: 10.1007/s00259-018-4109-x
15. Chung HW, Lee KY, Kim HJ, Kim WS, So Y. FDG PET/CT Metabolic Tumor Volume and Total Lesion Glycolysis Predict Prognosis in Patients With Advanced Lung Adenocarcinoma. J Cancer Res Clin Oncol (2014) 140:89–98. doi: 10.1007/s00432-013-1545-7
16. Moon SH, Cho SH, Park LC, Ji JH, Sun JM, Ahn JS, et al. Metabolic Response Evaluated by 18F-FDG PET/CT as a Potential Screening Tool in Identifying a Subgroup of Patients With Advanced Non-Small Cell Lung Cancer for Immediate Maintenance Therapy After First-Line Chemotherapy. Eur J Nucl Med Mol Imaging (2013) 40:1005–13. doi: 10.1007/s00259-013-2400-4
17. Nappi A, Gallicchio R, Simeon V, Nardelli A, Pelagalli A, Zupa A, et al. [F-18] FDG-PET/CT Parameters as Predictors of Outcome in Inoperable NSCLC Patients. Radiol Oncol (2015) 49:320–6. doi: 10.1515/raon-2015-0043
18. Seban RD, Mezquita L, Berenbaum A, Dercle L, Botticella A, Le Pechoux C, et al. Baseline Metabolic Tumor Burden on FDG PET/CT Scans Predicts Outcome in Advanced NSCLC Patients Treated With Immune Checkpoint Inhibitors. Eur J Nucl Med Mol Imaging (2020) 47:1147–57. doi: 10.1007/s00259-019-04615-x
19. Roengvoraphoj O, Kasmann L, Eze C, Taugner J, Gjika A, Tufman A, et al. Maximum Standardized Uptake Value of Primary Tumor (SUVmax_PT) and Horizontal Range Between Two Most Distant PET-Positive Lymph Nodes Predict Patient Outcome in Inoperable Stage III NSCLC Patients After Chemoradiotherapy. Transl Lung Cancer Res (2020) 9:541–8. doi: 10.21037/tlcr.2020.04.04
20. Na II, Byun BH, Kim KM, Cheon GJ, Choe du H, Koh JS, et al. 18f-FDG Uptake and EGFR Mutations in Patients With Non-Small Cell Lung Cancer: A Single-Institution Retrospective Analysis. Lung Cancer (2010) 67:76–80. doi: 10.1016/j.lungcan.2009.03.010
21. Mak RH, Digumarthy SR, Muzikansky A, Engelman JA, Shepard JA, Choi NC, et al. Role of 18F-Fluorodeoxyglucose Positron Emission Tomography in Predicting Epidermal Growth Factor Receptor Mutations in Non-Small Cell Lung Cancer. Oncologist (2011) 16:319–26. doi: 10.1634/theoncologist.2010-0300
22. Guan J, Xiao NJ, Chen M, Zhou WL, Zhang YW, Wang S, et al. 18f-FDG Uptake for Prediction EGFR Mutation Status in Non-Small Cell Lung Cancer. Med (Baltimore) (2016) 95:e4421. doi: 10.1097/MD.0000000000004421
23. Yoshida T, Tanaka H, Kuroda H, Shimizu J, Horio Y, Sakao Y, et al. Standardized Uptake Value on (18)F-FDG-PET/CT is a Predictor of EGFR T790M Mutation Status in Patients With Acquired Resistance to EGFR-TKIs. Lung Cancer (2016) 100:14–9. doi: 10.1016/j.lungcan.2016.07.022
24. Lv Z, Fan J, Xu J, Wu F, Huang Q, Guo M, et al. Value of (18)F-FDG PET/CT for Predicting EGFR Mutations and Positive ALK Expression in Patients With Non-Small Cell Lung Cancer: A Retrospective Analysis of 849 Chinese Patients. Eur J Nucl Med Mol Imaging (2018) 45:735–50. doi: 10.1007/s00259-017-3885-z
25. Ko KH, Hsu HH, Huang TW, Gao HW, Shen DH, Chang WC, et al. Value of (1)(8)F-FDG Uptake on PET/CT and CEA Level to Predict Epidermal Growth Factor Receptor Mutations in Pulmonary Adenocarcinoma. Eur J Nucl Med Mol Imaging (2014) 41:1889–97. doi: 10.1007/s00259-014-2802-y
26. Caicedo C, Garcia-Velloso MJ, Lozano MD, Labiano T, Vigil Diaz C, Lopez-Picazo JM, et al. Role of [(1)(8)F]FDG PET in Prediction of KRAS and EGFR Mutation Status in Patients With Advanced Non-Small-Cell Lung Cancer. Eur J Nucl Med Mol Imaging (2014) 41:2058–65. doi: 10.1007/s00259-014-2833-4
27. Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science (2009) 324:1029–33. doi: 10.1126/science.1160809
28. Koppenol WH, Bounds PL, Dang CV. Otto Warburg's Contributions to Current Concepts of Cancer Metabolism. Nat Rev Cancer (2011) 11:325–37. doi: 10.1038/nrc3038
29. Cantor JR, Sabatini DM. Cancer Cell Metabolism: One Hallmark, Many Faces. Cancer Discovery (2012) 2:881–98. doi: 10.1158/2159-8290.CD-12-0345
30. Levine AJ, Puzio-Kuter AM. The Control of the Metabolic Switch in Cancers by Oncogenes and Tumor Suppressor Genes. Science (2010) 330:1340–4. doi: 10.1126/science.1193494
31. Ward PS, Thompson CB. Metabolic Reprogramming: A Cancer Hallmark Even Warburg Did Not Anticipate. Cancer Cell (2012) 21:297–308. doi: 10.1016/j.ccr.2012.02.014
32. Pao W, Chmielecki J. Rational, Biologically Based Treatment of EGFR-Mutant Non-Small-Cell Lung Cancer. Nat Rev Cancer (2010) 10:760–74. doi: 10.1038/nrc2947
33. Makinoshima H, Takita M, Matsumoto S, Yagishita A, Owada S, Esumi H, et al. Epidermal Growth Factor Receptor (EGFR) Signaling Regulates Global Metabolic Pathways in EGFR-Mutated Lung Adenocarcinoma. J Biol Chem (2014) 289:20813–23. doi: 10.1074/jbc.M114.575464
34. Su H, Bodenstein C, Dumont RA, Seimbille Y, Dubinett S, Phelps ME, et al. Monitoring Tumor Glucose Utilization by Positron Emission Tomography for the Prediction of Treatment Response to Epidermal Growth Factor Receptor Kinase Inhibitors. Clin Cancer Res (2006) 12:5659–67. doi: 10.1158/1078-0432.CCR-06-0368
35. Kelloff GJ, Hoffman JM, Johnson B, Scher HI, Siegel BA, Cheng EY, et al. Progress and Promise of FDG-PET Imaging for Cancer Patient Management and Oncologic Drug Development. Clin Cancer Res (2005) 11:2785–808. doi: 10.1158/1078-0432.CCR-04-2626
36. Humbert O, Cadour N, Paquet M, Schiappa R, Poudenx M, Chardin D, et al. (18)FDG PET/CT in the Early Assessment of Non-Small Cell Lung Cancer Response to Immunotherapy: Frequency and Clinical Significance of Atypical Evolutive Patterns. Eur J Nucl Med Mol Imaging (2020) 47:1158–67. doi: 10.1007/s00259-019-04573-4
37. Dissaux G, Visvikis D, Da-Ano R, Pradier O, Chajon E, Barillot I, et al. Pretreatment (18)F-FDG PET/CT Radiomics Predict Local Recurrence in Patients Treated With Stereotactic Body Radiotherapy for Early-Stage Non-Small Cell Lung Cancer: A Multicentric Study. J Nucl Med (2020) 61:814–20. doi: 10.2967/jnumed.119.228106
38. Choi WH, Yoo I, O JH, Kim TJ, Lee KY, Kim YK. Is the Glut Expression Related to FDG Uptake in PET/CT of Non-Small Cell Lung Cancer Patients? Technol Health Care (2015) 23 Suppl 2:S311–8. doi: 10.3233/THC-150967
39. Higashi K, Ueda Y, Sakurai A, Wang XM, Xu L, Murakami M, et al. Correlation of Glut-1 Glucose Transporter Expression With. Eur J Nucl Med (2000) 27:1778–85. doi: 10.1007/s002590000367
40. Suzuki S, Okada M, Takeda H, Kuramoto K, Sanomachi T, Togashi K, et al. Involvement of GLUT1-Mediated Glucose Transport and Metabolism in Gefitinib Resistance of Non-Small-Cell Lung Cancer Cells. Oncotarget (2018) 9:32667–79. doi: 10.18632/oncotarget.25994
41. Cairns RA, Harris IS, Mak TW. Regulation of Cancer Cell Metabolism. Nat Rev Cancer (2011) 11:85–95. doi: 10.1038/nrc2981
42. Ellison G, Zhu G, Moulis A, Dearden S, Speake G, McCormack R. EGFR Mutation Testing in Lung Cancer: A Review of Available Methods and Their Use for Analysis of Tumour Tissue and Cytology Samples. J Clin Pathol (2013) 66:79–89. doi: 10.1136/jclinpath-2012-201194
43. Taniguchi K, Okami J, Kodama K, Higashiyama M, Kato K. Intratumor Heterogeneity of Epidermal Growth Factor Receptor Mutations in Lung Cancer and Its Correlation to the Response to Gefitinib. Cancer Sci (2008) 99:929–35. doi: 10.1111/j.1349-7006.2008.00782.x
44. Bai H, Wang Z, Chen K, Zhao J, Lee JJ, Wang S, et al. Influence of Chemotherapy on EGFR Mutation Status Among Patients With Non-Small-Cell Lung Cancer. J Clin Oncol (2012) 30:3077–83. doi: 10.1200/JCO.2011.39.3744
45. Yao G, Zhou Y, Gu Y, Wang Z, Yang M, Sun J, et al. Value of Combining PET/CT and Clinicopathological Features in Predicting EGFR Mutation in Lung Adenocarcinoma With Bone Metastasis. J Cancer (2020) 11:5511–7. doi: 10.7150/jca.46414
46. Liu Q, Sun D, Li N, Kim J, Feng D, Huang G, et al. Predicting EGFR Mutation Subtypes in Lung Adenocarcinoma Using (18)F-FDG PET/CT Radiomic Features. Transl Lung Cancer Res (2020) 9:549–62. doi: 10.21037/tlcr.2020.04.17
47. Zhang J, Zhao X, Zhao Y, Zhang J, Zhang Z, Wang J, et al. Value of Pre-Therapy (18)F-FDG PET/CT Radiomics in Predicting EGFR Mutation Status in Patients With Non-Small Cell Lung Cancer. Eur J Nucl Med Mol Imaging (2020) 47:1137–46. doi: 10.1007/s00259-019-04592-1
48. Cho A, Hur J, Moon YW, Hong SR, Suh YJ, Kim YJ, et al. Correlation Between EGFR Gene Mutation, Cytologic Tumor Markers, 18F-FDG Uptake in Non-Small Cell Lung Cancer. BMC Cancer (2016) 16:224. doi: 10.1186/s12885-016-2251-z
49. Gao XC, Wei CH, Zhang RG, Cai Q, He Y, Tong F, et al. (18)F-FDG PET/CT SUVmax and Serum CEA Levels as Predictors for EGFR Mutation State in Chinese Patients With Non-Small Cell Lung Cancer. Oncol Lett (2020) 20:61. doi: 10.3892/ol.2020.11922
50. Lee EY, Khong PL, Lee VH, Qian W, Yu X, Wong MP. Metabolic Phenotype of Stage IV Lung Adenocarcinoma: Relationship With Epidermal Growth Factor Receptor Mutation. Clin Nucl Med (2015) 40:e190-195. doi: 10.1097/RLU.0000000000000684
51. Gu J, Xu S, Huang L, Li S, Wu J, Xu J, et al. Value of Combining Serum Carcinoembryonic Antigen and PET/CT in Predicting EGFR Mutation in Non-Small Cell Lung Cancer. J Thorac Dis (2018) 10:723–31. doi: 10.21037/jtd.2017.12.143
52. Wang Y, Han R, Wang Q, Zheng J, Lin C, Lu C, et al. Biological Significance of (18)F-FDG PET/CT Maximum Standard Uptake Value for Predicting EGFR Mutation Status in Non-Small Cell Lung Cancer Patients. Int J Gen Med (2021) 14:347–56. doi: 10.2147/IJGM.S287506
53. Kanmaz ZD, Aras G, Tuncay E, Bahadir A, Kocaturk C, Yasar ZA, et al. Contribution of (1)(8)Fluorodeoxyglucose Positron Emission Tomography Uptake and TTF-1 Expression in the Evaluation of the EGFR Mutation in Patients With Lung Adenocarcinoma. Cancer biomark (2016) 16:489–98. doi: 10.3233/CBM-160588
54. Huang CT, Yen RF, Cheng MF, Hsu YC, Wei PF, Tsai YJ, et al. Correlation of F-18 Fluorodeoxyglucose-Positron Emission Tomography Maximal Standardized Uptake Value and EGFR Mutations in Advanced Lung Adenocarcinoma. Med Oncol (2010) 27:9–15. doi: 10.1007/s12032-008-9160-1
55. Choi YJ, Cho BC, Jeong YH, Seo HJ, Kim HJ, Cho A, et al. Correlation Between (18)F-Fluorodeoxyglucose Uptake and Epidermal Growth Factor Receptor Mutations in Advanced Lung Cancer. Nucl Med Mol Imaging (2012) 46:169–75. doi: 10.1007/s13139-012-0142-z
56. Lee SM, Bae SK, Jung SJ, Kim CK. FDG Uptake in Non-Small Cell Lung Cancer is Not an Independent Predictor of EGFR or KRAS Mutation Status: A Retrospective Analysis of 206 Patients. Clin Nucl Med (2015) 40:950–8. doi: 10.1097/RLU.0000000000000975
57. Liu A, Han A, Zhu H, Ma L, Huang Y, Li M, et al. The Role of Metabolic Tumor Volume (MTV) Measured by [18F] FDG PET/CT in Predicting EGFR Gene Mutation Status in Non-Small Cell Lung Cancer. Oncotarget (2017) 8:33736–44. doi: 10.18632/oncotarget.16806
58. Gao Y, Song P, Li H, Jia H, Zhang B. Elevated Serum CEA Levels are Associated With the Explosive Progression of Lung Adenocarcinoma Harboring EGFR Mutations. BMC Cancer (2017) 17:484. doi: 10.1186/s12885-017-3474-3
59. Lee Y, Lee HJ, Kim YT, Kang CH, Goo JM, Park CM, et al. Imaging Characteristics of Stage I Non-Small Cell Lung Cancer on CT and FDG-PET: Relationship With Epidermal Growth Factor Receptor Protein Expression Status and Survival. Korean J Radiol (2013) 14:375–83. doi: 10.3348/kjr.2013.14.2.375
60. Liu X, Zhang H, Yu X, Song T, Huang P, Wang H, et al. The Correlation of Expression of VEGF and EGFR With SUV of (18)FDG-PET-CT in Non-Small Cell Lung Cancer. Contemp Oncol (Pozn) (2014) 18:334–9. doi: 10.5114/wo.2014.45308
61. Toh CK, Gao F, Lim WT, Leong SS, Fong KW, Yap SP, et al. Never-Smokers With Lung Cancer: Epidemiologic Evidence of a Distinct Disease Entity. J Clin Oncol (2006) 24:2245–51. doi: 10.1200/JCO.2005.04.8033
62. Kuo MD, Jamshidi N. Behind the Numbers: Decoding Molecular Phenotypes With Radiogenomics–Guiding Principles and Technical Considerations. Radiology (2014) 270:320–5. doi: 10.1148/radiol.13132195
63. Wu J, Aguilera T, Shultz D, Gudur M, Rubin DL, Loo BW Jr., et al. Early-Stage Non-Small Cell Lung Cancer: Quantitative Imaging Characteristics of (18)F Fluorodeoxyglucose PET/CT Allow Prediction of Distant Metastasis. Radiology (2016) 281:270–8. doi: 10.1148/radiol.2016151829
64. Zhang M, Bao Y, Rui W, Shangguan C, Liu J, Xu J, et al. Performance of (18)F-FDG PET/CT Radiomics for Predicting EGFR Mutation Status in Patients With Non-Small Cell Lung Cancer. Front Oncol (2020) 10:568857. doi: 10.3389/fonc.2020.568857
65. Li X, Yin G, Zhang Y, Dai D, Liu J, Chen P, et al. Predictive Power of a Radiomic Signature Based on (18)F-FDG PET/CT Images for EGFR Mutational Status in NSCLC. Front Oncol (2019) 9:1062. doi: 10.3389/fonc.2019.01062
66. Nair JKR, Saeed UA, McDougall CC, Sabri A, Kovacina B, Raidu BVS, et al. Radiogenomic Models Using Machine Learning Techniques to Predict EGFR Mutations in Non-Small Cell Lung Cancer. Can Assoc Radiol J (2021) 72:109–19. doi: 10.1177/0846537119899526
67. Koyasu S, Nishio M, Isoda H, Nakamoto Y, Togashi K. Usefulness of Gradient Tree Boosting for Predicting Histological Subtype and EGFR Mutation Status of Non-Small Cell Lung Cancer on (18)F FDG-PET/Ct. Ann Nucl Med (2020) 34:49–57. doi: 10.1007/s12149-019-01414-0
68. Mu W, Jiang L, Zhang J, Shi Y, Gray JE, Tunali I, et al. Non-Invasive Decision Support for NSCLC Treatment Using PET/CT Radiomics. Nat Commun (2020) 11:5228. doi: 10.1038/s41467-020-19116-x
69. Gazdar AF, Minna JD. Inhibition of EGFR Signaling: All Mutations are Not Created Equal. PloS Med (2005) 2:e377. doi: 10.1371/journal.pmed.0020377
70. Shigematsu H, Lin L, Takahashi T, Nomura M, Suzuki M, Wistuba II, et al. Clinical and Biological Features Associated With Epidermal Growth Factor Receptor Gene Mutations in Lung Cancers. J Natl Cancer Inst (2005) 97:339–46. doi: 10.1093/jnci/dji055
71. Spiro SG, Silvestri GA. One Hundred Years of Lung Cancer. Am J Respir Crit Care Med (2005) 172:523–9. doi: 10.1164/rccm.200504-531OE
72. Pfister DG, Johnson DH, Azzoli CG, Sause W, Smith TJ, Baker S Jr., et al. American Society of Clinical Oncology Treatment of Unresectable Non-Small-Cell Lung Cancer Guideline: Update 2003. J Clin Oncol (2004) 22:330–53. doi: 10.1200/JCO.2004.09.053
73. Doroshow JH. Targeting EGFR in Non-Small-Cell Lung Cancer. N Engl J Med (2005) 353:200–2. doi: 10.1056/NEJMe058113
74. Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Douillard JY, et al. Multi-Institutional Randomized Phase II Trial of Gefitinib for Previously Treated Patients With Advanced Non-Small-Cell Lung Cancer (The IDEAL 1 Trial) [Corrected]. J Clin Oncol (2003) 21:2237–46. doi: 10.1200/JCO.2003.10.038
75. Tsao MS, Sakurada A, Cutz JC, Zhu CQ, Kamel-Reid S, Squire J, et al. Erlotinib in Lung Cancer - Molecular and Clinical Predictors of Outcome. N Engl J Med (2005) 353:133–44. doi: 10.1056/NEJMoa050736
76. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New Guidelines to Evaluate the Response to Treatment in Solid Tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst (2000) 92:205–16. doi: 10.1093/jnci/92.3.205
77. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur J Cancer (2009) 45:228–47. doi: 10.1016/j.ejca.2008.10.026
78. Mac Manus MP, Hicks RJ, Matthews JP, McKenzie A, Rischin D, Salminen EK, et al. Positron Emission Tomography is Superior to Computed Tomography Scanning for Response-Assessment After Radical Radiotherapy or Chemoradiotherapy in Patients With Non-Small-Cell Lung Cancer. J Clin Oncol (2003) 21:1285–92. doi: 10.1200/JCO.2003.07.054
79. Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving Considerations for PET Response Criteria in Solid Tumors. J Nucl Med (2009) 50 Suppl 1:122S–50S. doi: 10.2967/jnumed.108.057307
80. de Geus-Oei LF, van der Heijden HF, Corstens FH, Oyen WJ. Predictive and Prognostic Value of FDG-PET in Nonsmall-Cell Lung Cancer: A Systematic Review. Cancer (2007) 110:1654–64. doi: 10.1002/cncr.22979
81. van Baardwijk A, Bosmans G, Dekker A, van Kroonenburgh M, Boersma L, Wanders S, et al. Time Trends in the Maximal Uptake of FDG on PET Scan During Thoracic Radiotherapy. A Prospective Study in Locally Advanced Non-Small Cell Lung Cancer (NSCLC) Patients. Radiother Oncol (2007) 82:145–52. doi: 10.1016/j.radonc.2007.01.007
82. de Geus-Oei LF, van der Heijden HF, Visser EP, Hermsen R, van Hoorn BA, Timmer-Bonte JN, et al. Chemotherapy Response Evaluation With 18F-FDG PET in Patients With Non-Small Cell Lung Cancer. J Nucl Med (2007) 48:1592–8. doi: 10.2967/jnumed.107.043414
83. Lee DH, Kim SK, Lee HY, Lee SY, Park SH, Kim HY, et al. Early Prediction of Response to First-Line Therapy Using Integrated 18F-FDG PET/CT for Patients With Advanced/Metastatic Non-Small Cell Lung Cancer. J Thorac Oncol (2009) 4:816–21. doi: 10.1097/JTO.0b013e3181a99fde
84. Usmanij EA, de Geus-Oei LF, Troost EG, Peters-Bax L, van der Heijden EH, Kaanders JH, et al. 18f-FDG PET Early Response Evaluation of Locally Advanced Non-Small Cell Lung Cancer Treated With Concomitant Chemoradiotherapy. J Nucl Med (2013) 54:1528–34. doi: 10.2967/jnumed.112.116921
85. Zwitter M, Rajer M, Stanic K, Vrankar M, Doma A, Cuderman A, et al. Intercalated Chemotherapy and Erlotinib for Non-Small Cell Lung Cancer (NSCLC) With Activating Epidermal Growth Factor Receptor (EGFR) Mutations. Cancer Biol Ther (2016) 17:833–9. doi: 10.1080/15384047.2016.1195049
86. Tiseo M, Ippolito M, Scarlattei M, Spadaro P, Cosentino S, Latteri F, et al. Predictive and Prognostic Value of Early Response Assessment Using 18FDG-PET in Advanced Non-Small Cell Lung Cancer Patients Treated With Erlotinib. Cancer Chemother Pharmacol (2014) 73:299–307. doi: 10.1007/s00280-013-2356-x
87. Sunaga N, Oriuchi N, Kaira K, Yanagitani N, Tomizawa Y, Hisada T, et al. Usefulness of FDG-PET for Early Prediction of the Response to Gefitinib in Non-Small Cell Lung Cancer. Lung Cancer (2008) 59:203–10. doi: 10.1016/j.lungcan.2007.08.012
88. Na II, Byun BH, Kang HJ, Cheon GJ, Koh JS, Kim CH, et al. 18F-Fluoro-2-Deoxy-Glucose Uptake Predicts Clinical Outcome in Patients With Gefitinib-Treated Non-Small Cell Lung Cancer. Clin Cancer Res (2008) 14:2036–41. doi: 10.1158/1078-0432.CCR-07-4074
89. Koizumi T, Fukushima T, Gomi D, Kobayashi T, Sekiguchi N, Mamiya K, et al. Correlation of Early PET Findings With Tumor Response to Molecular Targeted Agents in Patients With Advanced Driver-Mutated Non-Small Cell Lung Cancer. Med Oncol (2017) 34:169. doi: 10.1007/s12032-017-1032-0
90. Aukema TS, Kappers I, Olmos RA, Codrington HE, van Tinteren H, van Pel R, et al. Is 18f-FDG PET/CT Useful for the Early Prediction of Histopathologic Response to Neoadjuvant Erlotinib in Patients With Non-Small Cell Lung Cancer? J Nucl Med (2010) 51:1344–8. doi: 10.2967/jnumed.110.076224
91. van Gool MH, Aukema TS, Schaake EE, Rijna H, Valdes Olmos RA, van Pel R, et al. Timing of Metabolic Response Monitoring During Erlotinib Treatment in Non-Small Cell Lung Cancer. J Nucl Med (2014) 55:1081–6. doi: 10.2967/jnumed.113.130674
92. van Gool MH, Aukema TS, Schaake EE, Rijna H, Codrington HE, Valdes Olmos RA, et al. (18)F-Fluorodeoxyglucose Positron Emission Tomography Versus Computed Tomography in Predicting Histopathological Response to Epidermal Growth Factor Receptor-Tyrosine Kinase Inhibitor Treatment in Resectable Non-Small Cell Lung Cancer. Ann Surg Oncol (2014) 21:2831–7. doi: 10.1245/s10434-014-3791-6
93. Winther-Larsen A, Fledelius J, Demuth C, Bylov CM, Meldgaard P, Sorensen BS. Early Change in FDG-PET Signal and Plasma Cell-Free DNA Level Predicts Erlotinib Response in EGFR Wild-Type NSCLC Patients. Transl Oncol (2016) 9:505–11. doi: 10.1016/j.tranon.2016.09.003
94. Kahraman D, Scheffler M, Zander T, Nogova L, Lammertsma AA, Boellaard R, et al. Quantitative Analysis of Response to Treatment With Erlotinib in Advanced Non-Small Cell Lung Cancer Using 18F-FDG and 3'-Deoxy-3'-18F-Fluorothymidine PET. J Nucl Med (2011) 52:1871–7. doi: 10.2967/jnumed.111.094458
95. Cook GJ, O'Brien ME, Siddique M, Chicklore S, Loi HY, Sharma B, et al. Non-Small Cell Lung Cancer Treated With Erlotinib: Heterogeneity of (18)F-FDG Uptake at PET-Association With Treatment Response and Prognosis. Radiology (2015) 276:883–93. doi: 10.1148/radiol.2015141309
96. Rami-Porta R, Crowley JJ, Goldstraw P. The Revised TNM Staging System for Lung Cancer. Ann Thorac Cardiovasc Surg (2009) 15:4–9.
97. Gould MK, Kuschner WG, Rydzak CE, Maclean CC, Demas AN, Shigemitsu H, et al. Test Performance of Positron Emission Tomography and Computed Tomography for Mediastinal Staging in Patients With Non-Small-Cell Lung Cancer: A Meta-Analysis. Ann Intern Med (2003) 139:879–92. doi: 10.7326/0003-4819-139-11-200311180-00013
98. Han SW, Kim TY, Hwang PG, Jeong S, Kim J, Choi IS, et al. Predictive and Prognostic Impact of Epidermal Growth Factor Receptor Mutation in Non-Small-Cell Lung Cancer Patients Treated With Gefitinib. J Clin Oncol (2005) 23:2493–501. doi: 10.1200/JCO.2005.01.388
99. Sculier JP, Chansky K, Crowley JJ, Van Meerbeeck J, Goldstraw P, International Staging C, et al. The Impact of Additional Prognostic Factors on Survival and Their Relationship With the Anatomical Extent of Disease Expressed by the 6th Edition of the TNM Classification of Malignant Tumors and the Proposals for the 7th Edition. J Thorac Oncol (2008) 3:457–66. doi: 10.1097/JTO.0b013e31816de2b8
100. Zander T, Scheffler M, Nogova L, Kobe C, Engel-Riedel W, Hellmich M, et al. Early Prediction of Nonprogression in Advanced Non-Small-Cell Lung Cancer Treated With Erlotinib by Using [(18)F]fluorodeoxyglucose and [(18)F]fluorothymidine Positron Emission Tomography. J Clin Oncol (2011) 29:1701–8. doi: 10.1200/JCO.2010.32.4939
101. Uehara H, Tsutani Y, Okumura S, Nakayama H, Adachi S, Yoshimura M, et al. Prognostic Role of Positron Emission Tomography and High-Resolution Computed Tomography in Clinical Stage IA Lung Adenocarcinoma. Ann Thorac Surg (2013) 96:1958–65. doi: 10.1016/j.athoracsur.2013.06.086
102. Kobe C, Scheffler M, Holstein A, Zander T, Nogova L, Lammertsma AA, et al. Predictive Value of Early and Late Residual 18F-Fluorodeoxyglucose and 18F-Fluorothymidine Uptake Using Different SUV Measurements in Patients With Non-Small-Cell Lung Cancer Treated With Erlotinib. Eur J Nucl Med Mol Imaging (2012) 39:1117–27. doi: 10.1007/s00259-012-2118-8
103. Takahashi R, Hirata H, Tachibana I, Shimosegawa E, Inoue A, Nagatomo I, et al. Early [18F]Fluorodeoxyglucose Positron Emission Tomography at Two Days of Gefitinib Treatment Predicts Clinical Outcome in Patients With Adenocarcinoma of the Lung. Clin Cancer Res (2012) 18:220–8. doi: 10.1158/1078-0432.CCR-11-0868
104. Mileshkin L, Hicks RJ, Hughes BG, Mitchell PL, Charu V, Gitlitz BJ, et al. Changes in 18F-Fluorodeoxyglucose and 18F-Fluorodeoxythymidine Positron Emission Tomography Imaging in Patients With Non-Small Cell Lung Cancer Treated With Erlotinib. Clin Cancer Res (2011) 17:3304–15. doi: 10.1158/1078-0432.CCR-10-2763
105. Keam B, Lee SJ, Kim TM, Paeng JC, Lee SH, Kim DW, et al. Total Lesion Glycolysis in Positron Emission Tomography Can Predict Gefitinib Outcomes in Non-Small-Cell Lung Cancer With Activating EGFR Mutation. J Thorac Oncol (2015) 10:1189–94. doi: 10.1097/JTO.0000000000000569
106. Gerlinger M, Rowan AJ, Horswell S, Math M, Larkin J, Endesfelder D, et al. Intratumor Heterogeneity and Branched Evolution Revealed by Multiregion Sequencing. N Engl J Med (2012) 366:883–92. doi: 10.1056/NEJMoa1113205
107. Park S, Ha S, Lee SH, Paeng JC, Keam B, Kim TM, et al. Intratumoral Heterogeneity Characterized by Pretreatment PET in Non-Small Cell Lung Cancer Patients Predicts Progression-Free Survival on EGFR Tyrosine Kinase Inhibitor. PloS One (2018) 13:e0189766. doi: 10.1371/journal.pone.0189766
108. Win T, Miles KA, Janes SM, Ganeshan B, Shastry M, Endozo R, et al. Tumor Heterogeneity and Permeability as Measured on the CT Component of PET/CT Predict Survival in Patients With Non-Small Cell Lung Cancer. Clin Cancer Res (2013) 19:3591–9. doi: 10.1158/1078-0432.CCR-12-1307
109. Agarwal M, Brahmanday G, Bajaj SK, Ravikrishnan KP, Wong CY. Revisiting the Prognostic Value of Preoperative (18)F-Fluoro-2-Deoxyglucose ( (18)F-FDG) Positron Emission Tomography (PET) in Early-Stage (I & II) Non-Small Cell Lung Cancers (NSCLC). Eur J Nucl Med Mol Imaging (2010) 37:691–8. doi: 10.1007/s00259-009-1291-x
Keywords: non-small cell lung cancer, epidermal growth factor receptor, tyrosine kinase inhibitors, positron emission tomography, 18F-FDG
Citation: Jiang M, Zhang X, Chen Y, Chen P, Guo X, Ma L, Gao Q, Mei W, Zhang J and Zheng J (2022) A Review of the Correlation Between Epidermal Growth Factor Receptor Mutation Status and 18F-FDG Metabolic Activity in Non-Small Cell Lung Cancer. Front. Oncol. 12:780186. doi: 10.3389/fonc.2022.780186
Received: 20 September 2021; Accepted: 25 March 2022;
Published: 20 April 2022.
Edited by:
Luciano Mutti, Temple University, United StatesReviewed by:
Vassilis Georgoulias, University of Crete, GreeceLetizia Gnetti, University Hospital of Parma, Italy
Copyright © 2022 Jiang, Zhang, Chen, Chen, Guo, Ma, Gao, Mei, Zhang and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianjun Zheng, emhlbmdqaWFuanVuQHVjYXMuYWMuY24=
 Maoqing Jiang
Maoqing Jiang Xiaohui Zhang1,2
Xiaohui Zhang1,2 Ping Chen
Ping Chen