- 1Hematopathology Laboratory, Advanced Centre for Treatment, Research and Education in Cancer (ACTREC), Tata Memorial Center, Homi Bhabha National Institute (HBNI) University, Mumbai, India
- 2Department of Medical Oncology, Tata Memorial Center, HBNI University, Mumbai, India
- 3Department of Cancer Cytogenetics, ACTREC, Tata Memorial Center, HBNI University, Mumbai, India
- 4Department of Pathology, Tata Memorial Center, HBNI University, Mumbai, India
Background: T-cell/NK-cell non-Hodgkin’s lymphoma (T/NK-NHL) is an uncommon heterogeneous group of diseases. The current classification of T/NK-NHL is mainly based on histopathology and immunohistochemistry. In practice, however, the lack of unique histopathological patterns, overlapping cytomorphology, immunophenotypic complexity, inadequate panels, and diverse clinical presentations pose a great challenge. Flow cytometric immunophenotyping (FCI) is a gold standard for the diagnosis, subtyping, and monitoring of many hematological neoplasms. However, studies emphasizing the role of FCI in the diagnosis and staging of T/NK-NHL in real-world practice are scarce.
Methods: We included T-cell non-Hodgkin’s lymphoma (T-NHL) patients evaluated for the diagnosis and/or staging of T/NK-NHL using FCI between 2014 and 2020. We studied the utility of FCI in the diagnosis and subtyping of T/NK-NHL and correlated the FCI findings with the results of histopathology/immunohistochemistry. For correlation purposes, patients were categorized under definitive diagnosis and subtyping, inadequate subtyping, inadequate diagnosis, and misdiagnosis based on the findings of each technique.
Results: A total of 232 patients were diagnosed with T/NK-NHL. FCI findings provided definitive diagnoses in 198 patients and subtyping in 187/198 (95.45%) patients. The correlation between FCI and histopathological/immunohistochemistry results (n = 150) demonstrated an agreement on the diagnosis and subtyping in 69/150 (46%) patients. Of the remaining cases, the diagnosis and subtyping were inadequate in 64/150 (42.7%), and 14/150 (9.33%) were misdiagnosed on histopathology/immunohistochemistry results. FCI provided definitive diagnosis and subtyping in 51/64 (79.7%) patients. Among these, 13 patients diagnosed with peripheral T-cell lymphoma not-otherwise-specified were reclassified (angioimmunoblastic T-cell lymphoma (AITL)-11 and prolymphocytic leukemia-2) on FCI. It corrected the diagnosis in 14 patients that were misdiagnosed (6 B-cell NHL (B-NHL), 3 Hodgkin’s lymphoma, 1 acute leukemia, and 1 subcutaneous panniculitis-like T-cell lymphoma) and misclassified (3 T-NHL) on histopathological results. AITL was the commonest T-NHL misclassified on histopathological results. FCI also confirmed the definite involvement in 7/83 (8.4%) and 27/83 (32.5%) bone marrow (BM) samples reported as suspicious and uninvolved, respectively, on histopathological evaluation.
Conclusion: AITL was the most frequently diagnosed T/NK-NHL in this study. FCI provided a distinct advantage in detecting BM involvement by T/NK-NHL, especially in patients with low-level involvement. Overall, our study concluded that FCI plays a critical role in the diagnosis, subtyping, and staging of T/NK-NHL in real-world practice.
1 Introduction
T-cell non-Hodgkin’s lymphoma (T-NHL) is a heterogeneous group of aggressive NHL arising from T-cell and NK-cell subsets accounting for approximately 10%–15% of all NHLs (1–3). The prevalence of T-NHL is slightly higher in Asia and Central-South America than in Western countries (2). The current WHO classification of hematopoietic neoplasms has enlisted more than 30 definite or provisional entities under the heading of mature T- and NK-cell neoplasms (3). The diagnosis and subtyping of T-cell/NK-cell NHL (T/NK-NHL) heavily rely on a multifactorial approach that includes clinical presentation, morphology, immunophenotype, and chromosomal abnormalities (4). In recent years, the treatment regimens for T-NHL has seen great improvements with the availability of newer agents such as denileukin diftitox, brentuximab vedotin, pralatrexate, alemtuzumab, vorinostat, and romidepsin (5–8). The selection of newer therapeutic agents including targeted therapy (e.g., brentuximab vedotin and alemtuzumab) may be subtype specific (7, 9, 10). Further, in middle- or low-income countries with limited resources, the prognosis of lymphoma, which can vary between the subtypes of T/NK-NHL, can help in prioritizing the available resources (11, 12). Thus, accurate diagnosis and subtyping of T/NK-NHL has become a basic requirement for the clinical management of patients.
In practice, however, lack of unique histopathological patterns, overlapping cytomorphological features, immunophenotypic complexity, paucity of specific genetic abnormalities, and diverse clinical presentations pose a great challenge to the diagnosis and subtyping in the majority of mature T/NK-NHLs (12–17). Despite recent technical advances, approximately 30% of peripheral T-NHL cases remain unclassifiable and categorized as “not otherwise specified” (4, 15). Additionally, limited resources, inadequate tissue, lack of expertise, and financial constraints add up to the variability in the diagnostic workup, accounting for relatively poor reproducibility of the diagnoses in T/NK-NHL (18–22).
In real-world practice, most of the centers rely on histopathological findings and minimal immunohistochemistry (IHC)-based immunophenotype for the lymphoma diagnosis and classification (18, 22–24). For the definitive diagnosis, an optimal IHC-based immunophenotyping workup is required, which depends upon the tissue adequacy and the availability of a comprehensive antibody panel. Furthermore, obtaining surgical excisional biopsies (SEBs) in patients with the involvement of deep-seated lymph nodes and extranodal sites is practically improbable in many instances. In such a scenario, the diagnosis solely depends on the core-needle biopsies (CNBs) and radiological findings (25, 26). Several reports have highlighted the limitations of CNB-based diagnosis of NHL due to inadequate tissue as being responsible for the inability to evaluate histopathological patterns and immunophenotype by IHC (27–32). Thus, lower incidence, inadequate immunophenotyping workup, limited tissue, and lack of expertise collectively contribute to inadequate opinion, incorrect subtyping, or sometimes even misdiagnosis such as Hodgkin’s lymphoma, B-cell NHL (B-NHL), inflammatory process, or reactive proliferation in a significant percentage of T-NHL, skewing the true incidence (12, 14, 25, 33–51).
Flow cytometric immunophenotyping (FCI) is a powerful tool for single-cell analysis that allows the study of multiple protein expressions simultaneously in thousands to millions of cells in a short duration of time (52). It is widely available and routinely used for the diagnosis, subtyping, staging, and monitoring of hematological neoplasms like acute leukemia (AL), myelodysplastic syndrome, B-NHL, and multiple myeloma (53). The unique ability of advanced flow cytometry instruments in simultaneous detection ≥8 proteins on a single cell and the availability of an expanding list of new antibodies and fluorochromes have made it possible to trace the cells of tumor origin easily (52, 54, 55). Many studies have documented the role of FCI in the diagnosis or exclusion of T-NHL describing immunophenotypic profile and clonality assessment (14, 26, 44, 52, 56–65). However, studies emphasizing the role of FCI in the diagnosis and staging of T/NK-NHL in real-world practice are scarce. This study highlights the critical contribution of FCI-based immunophenotyping and clonality assessment in diagnosing and staging T/NK-NHL in routine practice.
2 Materials and Methods
This study was approved by the Institutional Ethical Committee. Patients diagnosed with mature T-/NK-cell neoplasms were identified from the electronic medical records (EMRs) of Tata Memorial Center (TMC), India. We included the patients evaluated for FCI in Hematopathology Laboratory for either diagnosis or staging of the T/NK-NHL (Figure 1). This retrospective study included patients investigated for the last 7 years (2014–2020). The clinical details, laboratory findings, and treatment history were recorded from the EMR. The final diagnosis and subtyping were made in accordance with WHO 2008 and 2016 hematolymphoid classification based on the available details on clinical presentation, cytomorphological and histopathological features, immunophenotypic (using both IHC and FCI) data, radiological features, and genetic findings (66, 67).
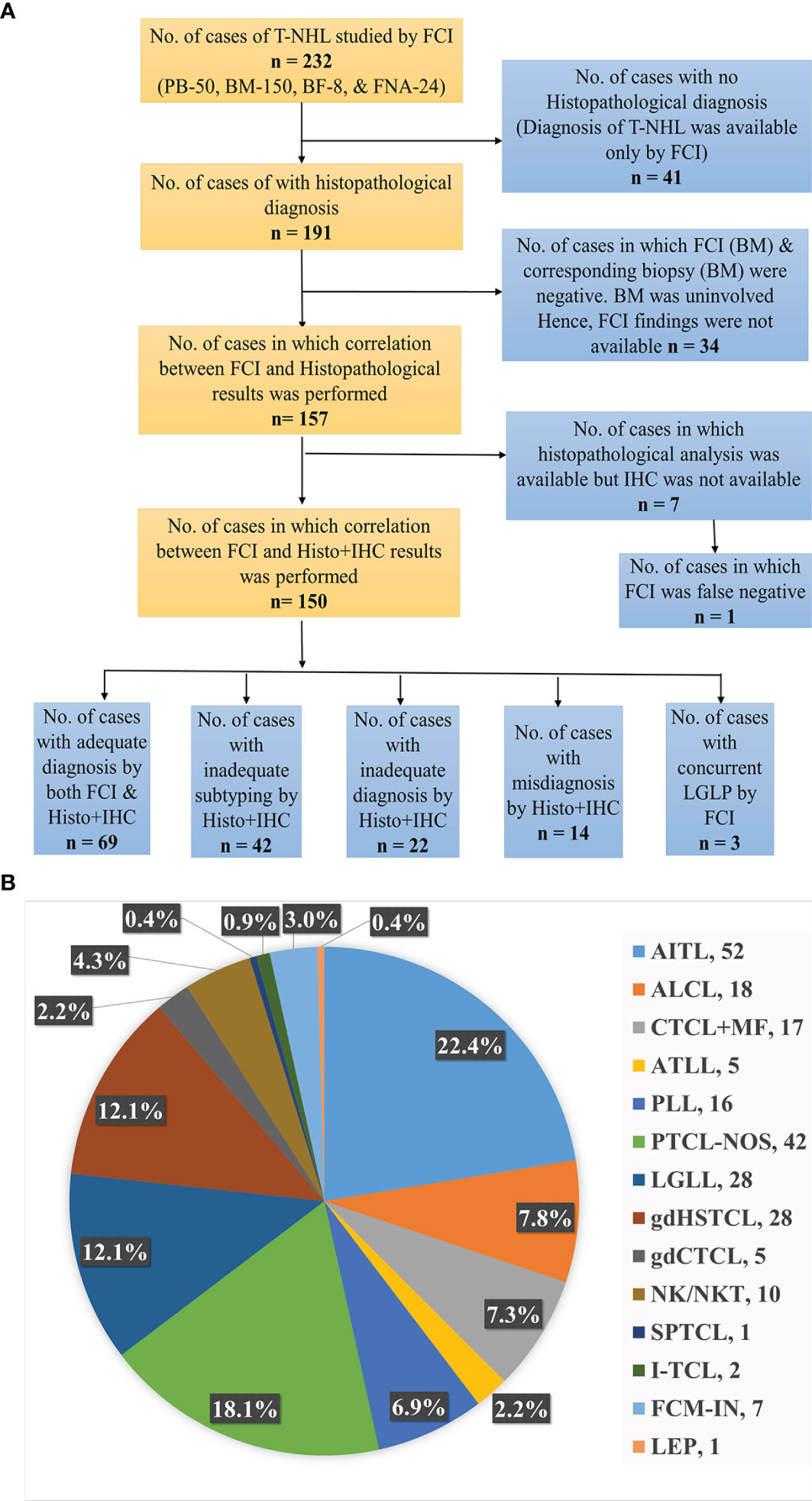
Figure 1 (A) Flowchart representing the distribution of T-cell/NK-cell NHL patients studied. BM, bone marrow; BF, body fluids; FCI, flow cytometric immunophenotyping; FNA, fine-needle aspiration; Histo, histopathology; IHC, immunohistochemistry; PB, peripheral blood; NHL, non-Hodgkin’s lymphoma. (B) Distribution of subtypes of T-cell/NK-cell NHL patients. AITL, angioimmunoblastic T-cell lymphoma; ALCL, anaplastic large T-cell lymphoma; ATLL, adult T-cell leukemia/lymphoma; CTCL, cutaneous T-cell lymphoma; FCM, flow cytometry; FHTCL, follicular helper type T-cell lymphoma; HSTCL, hepatosplenic T-cell NHL; I, intestinal; IN, inadequate; LEP, lupus erythematosus panniculitis; LGLL, large granular lymphocytic leukemia; M, male; MF, mycosis fungoides; NK/NKTCL, NK-/T-cell lymphoma; PTCL-NOS, peripheral T-cell lymphoma not otherwise specified; PLT, platelets; PLL, prolymphocytic leukemia; SPTCL, subcutaneous panniculitis-like T-cell lymphoma.
2.1 Cytomorphology
Peripheral blood (PB), bone marrow (BM) aspiration, body fluid (BF), and fine-needle aspiration (FNA) smears were stained with Wright’s stain. Morphological details, including the adequacy of cellularity, differential count, and nuclear and cytoplasmic details of lymphocytes, were studied. Tissue biopsy (TB) examination was conducted on H&E-stained sections in conjunction with IHC studies. TB sections were performed using 5-µm-thick, formalin-fixed, paraffin-embedded tissue, and IHC was performed after heat-induced epitope retrieval as described below.
2.2 Immunohistochemistry Studies
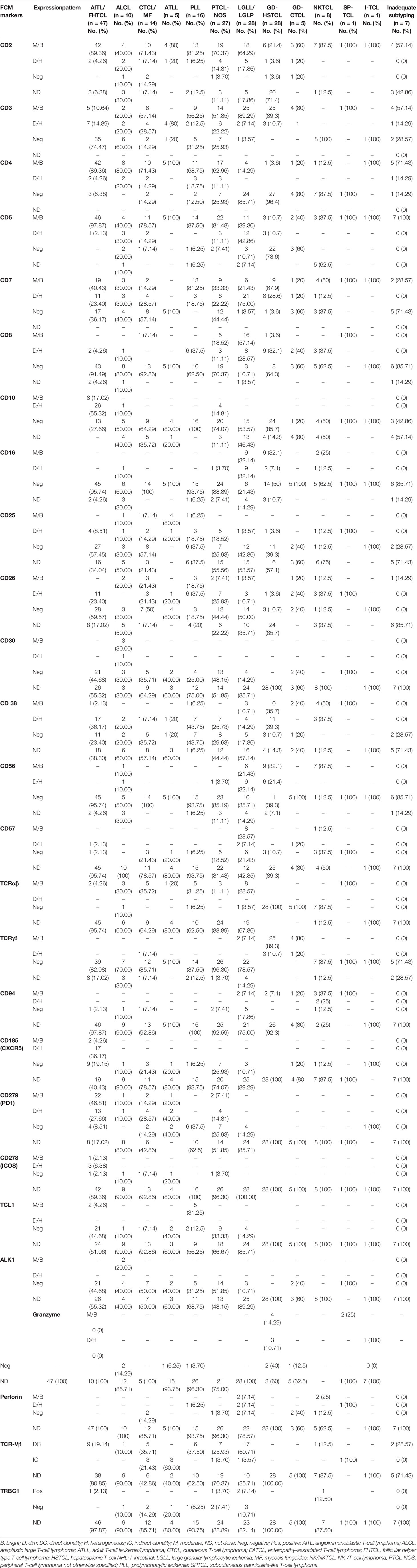
IHC was performed using the avidin–biotin complex method on formalin-fixed paraffin sections using automated immunostainers (Roche Diagnostics, Skopje, North Macedonia: Ventana BenchMark XT). The panel of antibodies studied included LCA, CD1a, CD3, CD5, CD4, CD7, CD8, CD10, CD15, CD20, CD23, CD30, CD31, CD34, CD43, CD56, CD57, CD138, CD163, AE1/AE3, ALK-1, BCL2, BCL6, BOB1, c-Kit, C-myc, CK7, CK20, EMA, Granzyme-B, MIB1, myeloperoxidase (MPO), MUM-1, OCT2, PAX-5, terminal deoxynucleotidyl transferase (TdT), and EBVLMP1. The details of antibody dilution, clone, and company are given in Supplementary Table S1. EBER-ISH was done with an EBV probe in situ hybridization (ISH) kit (Novocastra Laboratories Ltd., Newcastle upon Tyne, UK). The IHC panels performed were mainly based on the tissue adequacy, availability of reagents at the time of evaluation, and judgment of the reporting pathologist. It was categorized into inadequate and adequate IHC panels. Based on the “International Peripheral T-Cell and Natural Killer/T-Cell Lymphoma Study 2008,” the IHC panel with ≤5 markers including only basic markers such as LCA, CD3, CD5, CD20, and PAX-5 were categorized as inadequate IHC panel and the IHC panel with >5 markers including additional markers useful for T-NHL diagnosis and classification such as CD4, CD7, CD8, CD10, CD23, CD30, CD34, CD56, CD57, ALK-1, BCL6, C-myc, Granzyme-B, MIB1, TdT, and EBVLMP1 were categorized as adequate IHC panel (2). However, IHC for PD1, CXCL13, ICOS, βF1, and TCRδ1 was not available in most of the samples.
2.3 Flow Cytometric Immunophenotyping
FCI was performed using the bulk–lyse–wash technique as described elsewhere (68–70). In brief, the cell suspension was prepared by erythrocyte lysing with ammonium chloride-based lysing reagent (100 µl to 2 ml of sample in 15 to 48 ml of lysing reagent). The cells were stained a 10- to 13-color comprehensive antibody panel (Supplementary Table S2) and acquired on Navios and CytoFlex flow cytometry instruments (Beckman Coulter, Miami, FL, USA). For each panel, the following were acquired: in samples with ≥10% atypical lymphocyte on microscopic examination, a minimum of 100,000 events; in samples with <10% atypical lymphocyte, a minimum of 500,000 events. Initially, we studied a primary antibody panel (Supplementary Table S2A) that included antibodies against CD2, CD3, CD4, CD5, CD7, CD8, CD10, CD11c, CD16, CD19, CD20, CD23, CD26, CD38, CD45, CD49d, CD56, CD200, kappa/lambda, and γδT-cell receptor (TCRγδ). Based on the expressions of CD4 and CD8 in tumor cells from the results of the primary antibody panel, additional antibody panels (Supplementary Table S2B) were performed (Figure 2). T-cell clonality was studied using TCR-Vβ staining using IOTest Beta Mark TCR Repertoire Kit (Beckman Coulter, Marseille, France) that includes monoclonal antibodies (mAbs) against 24 distinct TCR-Vβ families (Supplementary Table S2C) (62). T-cell clonality was also studied by TRBC1 (Supplementary Table S2D) expression in a few recent cases (71). It was assessed in those samples only where suspicious T cells were surface CD3 positive and TCRγδ negative. A cutoff of the cluster of a minimum of 50 events of abnormal cells was used to define the abnormal T-cell population. Data were analyzed with KaluzaV2.1 software (Beckman Coulter, Miami, FL, USA) using predesigned templates.
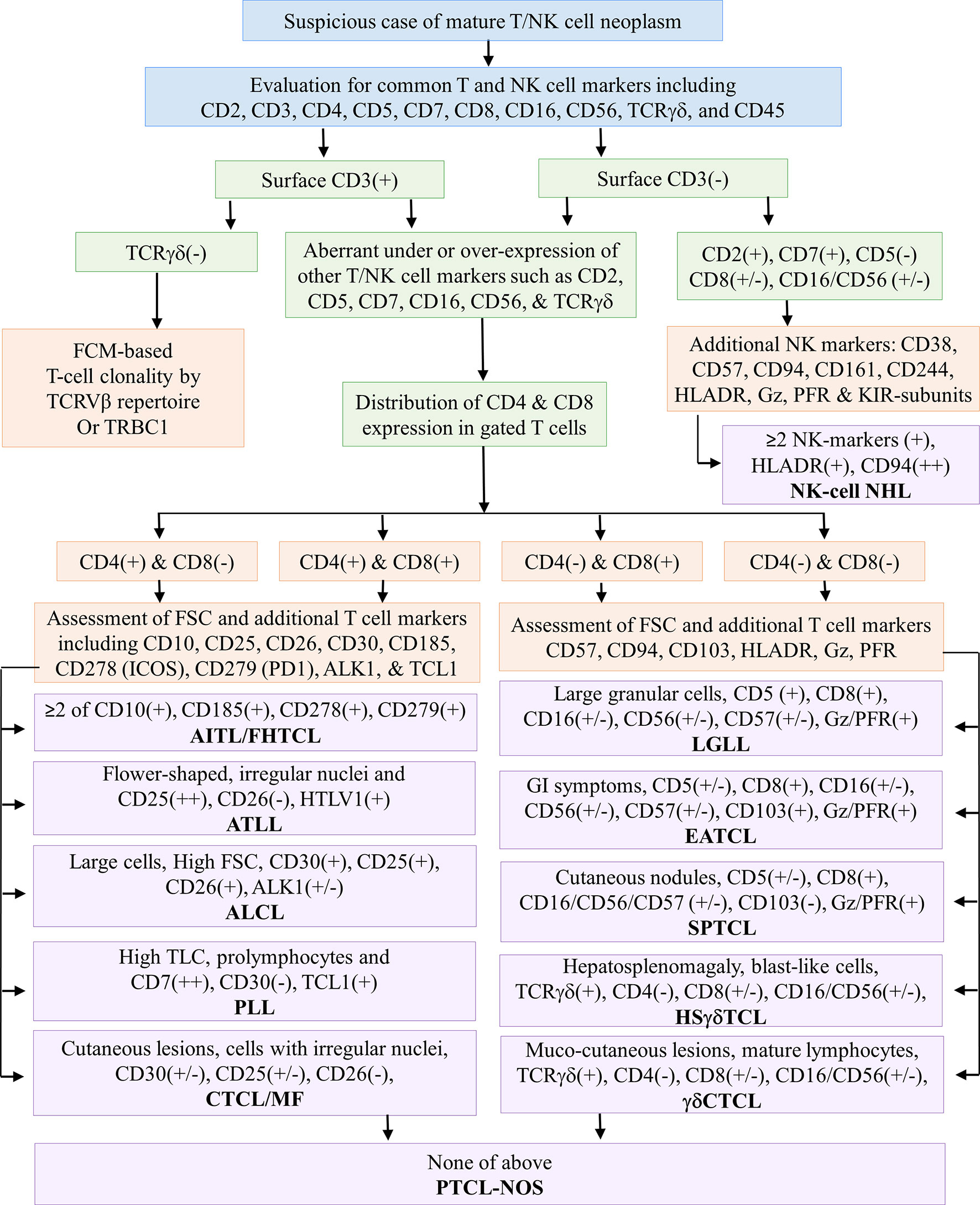
Figure 2 Flowchart demonstrates an immunophenotypic approach for assessing common T-cell markers using a primary antibody panel followed by the selection of an additional antibody panel based on the CD4 and/or CD8 distribution. The latter half of the flowchart demonstrates the utility of selective markers for the subtyping of T-cell NHL. AITL, angioimmunoblastic T-cell lymphoma; ALCL, anaplastic large T-cell lymphoma; ATLL, adult T-cell leukemia/lymphoma; CTCL, cutaneous T-cell lymphoma; EATCL, enteropathy-associated T-cell lymphoma; FCM, flow cytometry; FHTCL, follicular helper type T-cell lymphoma; Gz, granzyme; HSTCL, hepatosplenic T-cell NHL; I, intestinal; IN, inadequate; KIR, killer immunoglobulin-like receptors; LEP, lupus erythematosus panniculitis; LGLL, large granular lymphocytic leukemia; M, male; MF, mycosis fungoides; NK/NKTCL, NK-/T-cell lymphoma; PFR, perforin; PTCL-NOS, peripheral T-cell lymphoma not otherwise specified; PLT, platelets; PLL, prolymphocytic leukemia; SPTCL, subcutaneous panniculitis-like T-cell lymphoma; (+) positive expression; (++) strong positive expression; (+/−) positive or negative or heterogeneous expression; (−) negative expression.
2.3.1 Gating Strategy
The gating strategy is shown in Supplementary Figure S1. Initial gating was based on CD45 versus side scatter (SSC) characteristics in which lymphoid cells were gated using strong CD45 and low SSC followed by an evaluation of T cells using CD2, CD3, CD4, CD5, CD7, and CD8 expressions. In some cases, an alternative gating strategy was also implemented using CD45 or SSC versus pan-T-cell markers such as CD2, CD3, CD5, and CD7. T cells were studied for expression patterns (under or over or asynchronous expression) of pan-T-cell markers such as CD2, CD3, CD5, and CD7 as well as NK-cell markers such as CD16 and CD56 followed by CD4 and CD8 restriction. Based on the CD4 or CD8 expression, additional markers were studied. As demonstrated in Figure 2, in samples with predominantly CD4-expressing abnormal T cells, the gated T-cell population was further studied for CD10, CD25, CD26, CD30, CD185 (CXCR5), CD278 (ICOS), CD279 (PD1), ALK-1, and TCL1. In samples with predominantly CD8-expressing abnormal T cells, the gated T-cell population was further studied for CD16, CD25, CD26, CD30, CD56, CD57, CD94, CD161, CD244, granzyme, and perforin. The immunophenotypic approach adapted to diagnose and subclassify T-NHL or NK-NHL is shown in Figure 2.
For T-cell clonality assessment, the initial gating strategy was based on CD45 vs. CD3 expression followed by CD4+CD3+ T and CD8+CD3+ T-cell population. The suspicious T-cell population was further isolated using the altered expressions in CD4, CD3, CD5, CD7, CD16, and CD56. The clonality was defined as direct or indirect as described earlier (62, 71–73).
2.4 Cytogenetic Studies
The cytogenetic evaluation was performed using conventional karyotyping and fluorescence ISH (FISH) in BM samples. The samples were cultured as direct and overnight cultures, followed by harvesting of lymphocytes, fixation, and FISH. The FISH panel included LSI 7q31 probe, LSI IGH (14q32), LSI BCL6 (3q27), LSI c-MYC (8q24), LSI ALK (2p23), and CEP 7 and CEP 8 probes from Abbott Molecular (Abbott Park, IL, USA). It also included LSP TCRα (14q11) probe (CytoTest, Rockville, MD, USA) and LSP TCRβ (7q34) probe (Cytocell, Begbroke Science Park, Oxfordshire, UK). Two hundred interphase cells were analyzed on the Applied Spectral Imaging GENASIS software platform (Netzer Sereni, Israel) and reported according to the International System for Human Cytogenomic Nomenclature (ISCN) 2016.
3 Statistical Analysis
The median and range of the various parameters were evaluated using Microsoft Office Excel version 16. The relation between the type of biopsy (SEB vs. CNB) and inadequacy of diagnosis/subtyping of T-NHL was studied using Fisher’s exact test. Similarly, the relation between the IHC panel (adequate vs. inadequate) and inadequacy of diagnosis/subtyping of T-NHL was also studied using Fisher’s exact test. p-Value <0.05 was considered statistically significant.
4 Results
4.1 Patient Characteristics
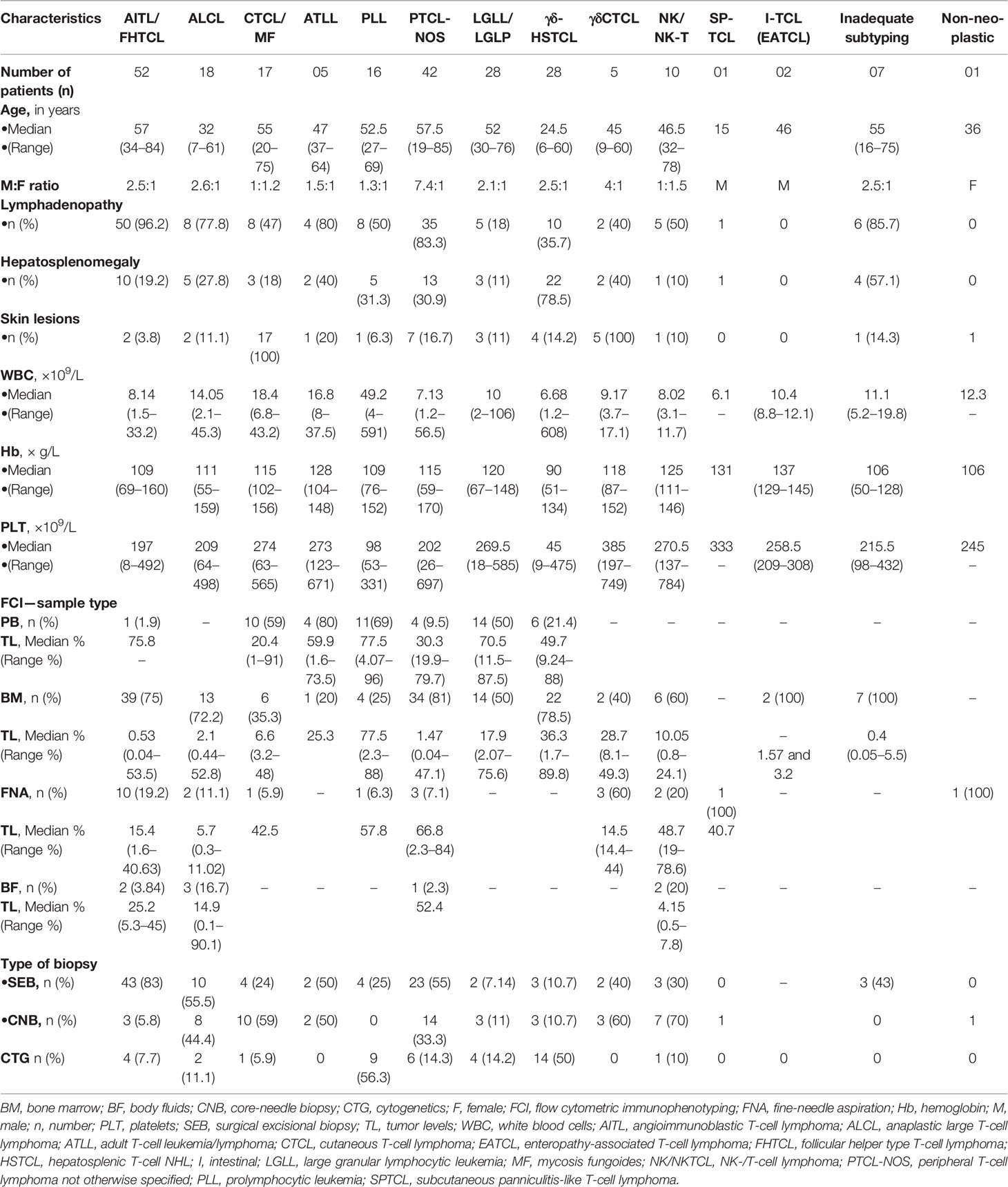
The study included 232 patients diagnosed with mature T/NK-NHLs with FCI data out of 4,862 patients evaluated using FCI for lymphoma diagnosis and/or staging between 2014 and 2020 (Figure 1). Clinical presentation and baseline characteristics at diagnosis are given in Table 1. The median age of the patients was 51 years (range, 6–85 years), and M:F ratio was 2.3:1. Our data included 16 patients younger than 18 years (M:F, 13:3), including eight patients with hepatosplenic γδT-NHL (γδHSTCL), six with anaplastic large T-cell lymphoma (ALCL), one with cutaneous γδT-NHL (γδCTCL), and one with subcutaneous panniculitis-like T-cell lymphoma (SPTCL). The detailed clinical findings were available in 91% of patients. The radiological details were available in 173/232 (PET scan, 135/232; and MRI/CT/USG scan, 76/232) patients. Figures 1A, B describe the distribution of patients.
4.2 Flow Cytometric Immunophenotypic Findings
A total of 255 samples were received for FCI at diagnosis and/or staging from 232 patients. The samples submitted included 50 PB, 173 BM, 24 FNA, and 8 BF samples. In 23 patients, a BM sample was received in addition to PB, FNA, and BF. The frequency distribution of these patients is shown in Figure 1B. The angioimmunoblastic T-cell lymphoma (AITL)/follicular helper type T-cell lymphoma (FHTCL) was the commonest T-NHL (52/232, 22.4%) in our cohort. BF samples (n = 8) included 3 ALCL, 2 NK-cell lymphomas (NKCLs), 2 AITL/FHTCL, and a case of PTCL-NOS. The FCI findings were negative in 34 patients due to uninvolved BM (by FCI as well as biopsy). Of the 198 samples with FCI results, a comprehensive (primary and additional) antibody panel was performed in 165 (83.3%) samples, and only a primary antibody panel was performed in 33 (16.7%) samples due to either inadequate sample or paucicellularity. The histopathological and IHC findings were not available in 41 patients. The histopathological findings were available in a total of 157 patients, but IHC was available in 150 patients. So the results of both FCI and histopathological/IHC findings were available in a total of 150 patients (Figure 1).
The FCI findings are described in Table 2. Briefly, tumor cells in AITL/FHTCL demonstrated a very characteristic immunophenotypic signature with positive expressions of CD2, CD4, CD5, CD45, and heterogeneous CD7 but negative expressions of surface CD3, CD8, CD16, CD56, CD25, CD26, and CD30. The tumor cells also expressed one or more of CD10, CXCR5, ICOS, and PD1 wherever available. Abnormal lymphocytes in cutaneous T-cell lymphoma [CTCL; including mycosis fungoides (MF)] were characterized by CD4 restriction, variable loss of CD7 and CD26, moderate CD2, moderate-to-dim surface CD3 and CD5, and heterogeneous CD279 (PD1) but negative expressions of CD8, CD10, CD16, CD56, CD57, CD185 (CXCR5), and TCRγδ. T-cell prolymphocytic leukemia (PLL) cells were characterized by homogenously moderate CD2, CD5, and CD7, variable loss of surface CD3, moderate-to-dim CD26, heterogeneous CD25, and moderate TCL1. However, they were characterized by negative expressions of CD10, CD16, CD56, CD57, CD185 (CXCR5), CD279 (PD1), and TCRγδ as described by Matutues et al. (57, 74). All cases of PLL were positive for TCL1 expression, and a subset showed heterogeneous co-expression of CD8. Tumor cells in ALCL were large with higher forward scatter (FSC)/SSC and revealed variable loss of pan-T-cell markers (i.e., CD2, CD3, CD5, CD7, and CD4 restriction) and co-expressions of CD25 and CD26 but were negative for CD10, CD16, CD56, CD57, CD185 (CXCR5), CD279 (PD1), TCL1, and TCRγδ expressions. A subset of ALCL was positive for CD30 and ALK-1. Adult T-cell leukemia/lymphoma (ATLL) cells showed typical irregular nuclear contours and were characterized by moderate CD2, CD4, and CD5, loss of surface CD3, CD7, and CD26, and dim-to-negative CD279. However, they were characterized by negative expressions of CD10, CD16, CD56, CD57, CD279 (PD1), and TCRγδ. All cases of ATLL showed homogenous CD25 expression.
Lymphoma cells in γδHSTCL showed blast-like morphology and were typically positive for moderate CD2, surface CD3, CD7, and TCRγδ, and weak-to-negative CD5. However, they were negative for CD4, CD10, CD16, and CD26. Approximately half of the γδHSTCL was positive for CD56 and showed heterogeneous expression of CD8. Contrary to γδHSTCL, tumor cells of γδCTCL were small to intermediate in size with mature nuclear chromatin. Immunophenotype of γδCTCL was similar to that of γδHSTCL except for the negative expressions of CD16 and CD56. Typical immunophenotype of large granular lymphocytic leukemia (LGLL) included positive expressions of CD2, surface CD3 and CD8 (occasionally CD4), heterogeneous CD7, and over-/under-expression of CD5 but negative for CD25, CD26, and TCRγδ. The LGLL cells demonstrated positive expressions for one or more of CD16, CD56, CD57, CD94, granzyme, and perforin. Of note, out of 28 patients categorized under LGLL/LGLP category, a concurrently existing small clonal large granular lymphocytic population (LGLP) (CD3+CD8+CD56+CD57+ T cells) was detected in patients diagnosed with primary mediastinal B-cell lymphoma (PMBCL), T-cell rich B-cell lymphoma (TCRBCL), and cutaneous ALCL. These patients were included in the LGLL/LGLP category (Table 2). Tumor cells in NKCL were consistently negative for CD3, CD4, and CD5 and showed moderate CD2, CD38, CD56, and CD94 and heterogeneous expressions of CD7, CD8, CD16, and CD26.
T-cell clonality by TCR-Vβ repertoire was performed in 57 patients and using TRBC1 antibody in 15 patients (Table 2). Among them, 47 cases showed direct clonality with a single TCR-Vβ protein restriction, and 9 showed indirect evidence of T-cell clonality as described previously (62). In one case, there was no evidence of clonality. On clonality assessment by TRBC1 expression, ten samples showed negative expression, and five showed positive expression in the abnormal T-cell population.
Thus, FCI allowed the definitive diagnosis and subtyping in 190/198 (95.45%) patients. Of the remaining eight patients, the diagnosis of T-NHL was suggested, but subtyping was not possible in seven patients (Table 1). FCI was falsely negative in a patient of ALCL (due to marked hemodilution of BM sample and focal tumor involvement).
4.3 Histopathology and Immunohistochemistry Findings
The histopathological (tissue and/or BM biopsy) findings were available in 191/232 patients. Among 191 patients, TB and BM biopsy findings were available in 138, with only TB findings in 18 and only BM biopsy findings in 35 patients. Out of a total of 156 TB samples, 100 (64.1%) were SEB samples and 56/191 (35.9%) were CNB samples. The IHC results were available in 184/191 (SEB, 100/100; CNB, 55/56; and BM, 29/35) patients. The IHC panel was adequate in 105/184 (57.1%) TB (SEB, 62/100, 62%; and CNB, 36/55, 65.5%) and 7/29 (24.1%) BM biopsy samples. The results of IHC are given in Table 3. Among these 184 patients with IHC results, the diagnosis and subtyping on histopathology/IHC evaluation were made in 125/184 (67.9%; 115/155 in patients with tissue biopsies and 10/29 BM biopsies) patients. These included 42 (22.8%) PTCL, 16 (8.7%) AITL/FHTCL, 15 (8.2%) ALCL, 14 (7.6%) CTCL, 12 (6.5%) γδHSTCL, 7 (3.8%) NKCL, 3 (1.6%) SPTCL, 2 (1.1%) ATLL, 2 (1.1%) LGLL, 1 (0.5%) γδCTCL, and 1 (0.5%) enteropathy-associated T-cell lymphoma (EATCL). In addition, 6/184 (3.2%) cases were diagnosed as B-NHL (diffuse large B-cell lymphoma (DLBCL), 3; low-grade B-NHL, 3), 3 (1.6%) as Hodgkin’s lymphoma (classical HL, 2; and nodular lymphocyte-predominant Hodgkin’s lymphoma (NLPHL), 1), and 1 (0.5%) as AL. Of the remaining 59/184 patients, the diagnosis of T-NHL was made, but further subtyping was not possible (i.e., inadequate subtyping) in 29/184 (15.7%), and the suspicion of T-NHL/atypical T-cell proliferation (i.e., inadequate diagnosis) was suggested in 22/184 (12%). The 8/184 (4.3%) cases were reported negative for T-cell NHL due to either scanty/non-representative tissue or low-level involvement (tumor cells <5% of all cells).
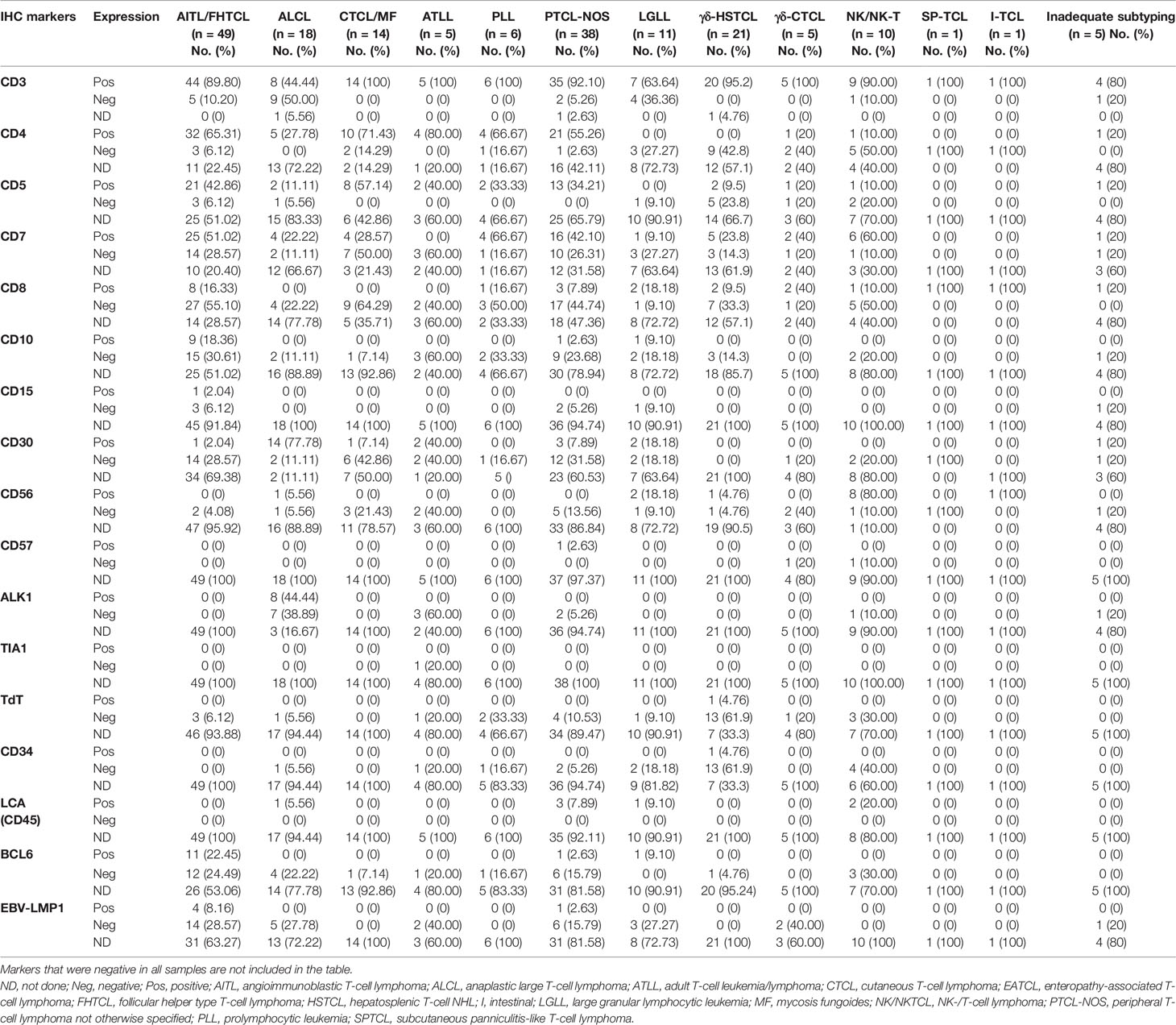
Table 3 Results of immunohistochemistry (IHC) after final diagnosis and subtyping of T-NHL (n = 184).
Further, we investigated the effect of the type of biopsy on the inadequacy of the histological diagnosis. Interestingly, the proportion of inadequate subtyping/diagnosis was higher in SEB compared to CNB (29.9% vs. 12.7%, p = 0.017). We also asked if the adequacy of the IHC panel affected the final diagnosis. As expected, the proportion of inadequate subtyping/diagnosis was relatively higher in samples with inadequate IHC (50% vs. 18.2%, p < 0.001). Thus, the inadequate IHC panel was likely to be one of the reasons for inadequate diagnosis/subtyping of T-NHL on histopathological evaluation.
4.4 Cytogenetic Findings
The relevant cytogenetic studies were available only in 40 (17.2%) patients, which included 16/28 (57.14%) patients with γδHSTCL revealing isochromosome 7q or del7 in 10/14, trisomy 8 in 4/14, and both in two patients. Additional 6/40 patients showed other structural abnormalities in non-γδHSTCL T-NHLs. The rest of the patients did not show any significant cytogenetic abnormality.
4.5 Correlation and Discrepancies Between Flow Cytometric Immunophenotyping Findings and Histopathology/Immunohistochemistry Results
As shown in Figure 1A, correlation and discrepancies between the diagnosis using FCI and histopathological/IHC results were assessed in 150 patients. The correlation details are given in Table 4. A complete agreement of diagnosis and subtyping between FCI and histopathological/IHC results was found in 69/150 (46%) patients. However, of the remaining patients, 13 (of 42) patients diagnosed with PTCL-NOS on histopathology were further reclassified as AITL/FHTCL (n = 11) and T-PLL (n = 2) on FCI. These 13 patients were included in the inadequate subtyping category (Table 4). Additionally, FCI provided definitive diagnosis and subtyping in 25/29 patients categorized under inadequate subtyping and 21/22 patients categorized under inadequate diagnosis on histopathological results (Table 4). Moreover, FCI provided the correct diagnosis and subtyping in 14 patients who were misdiagnosed (6 B-NHL, 3 HL, 1 AL, and 1 SPTCL) or misclassified T-NHL (3 patients) as shown in Table 4. Six of nine patients misdiagnosed with B-NHL or HL were found to be involved by AITL on FCI in BM aspiration samples. Of these, 4/6 (1 DLBCL and 3 low-grade B-NHL) cases were further confirmed as AITL by additional FCI on FNA from enlarged lymph nodes, which showed abnormal T cells and polyclonal B-cell lymphocytosis (Figure 3). Similarly, one classical HL and one NLPHL were also re-confirmed as AITL by additional FCI on FNA from enlarged lymph nodes (Figure 4). Two DLBCL cases were reviewed along with follow-up biopsies and confirmed as ALCL and PTCL-NOS. One case each of classical HL, AL, PTCL-NOS, and γδHSTCL (based on sinusoidal pattern) were reclassified as PTCL-NOS, γδHSTCL, γδCTCL (Figure 5), and PTCL-NOS, respectively, after reviewing histopathological results along with findings of FCI. Out of the 3 SPTCL, one was reclassified as NK-/T-cell lymphoma (NKTCL), and the other case had polyclonal mixed CD4 and CD8 T-cell population on FCI in FNA from the subcutaneous nodule. The latter case was finally diagnosed as lupus erythematosus panniculitis (LEP).
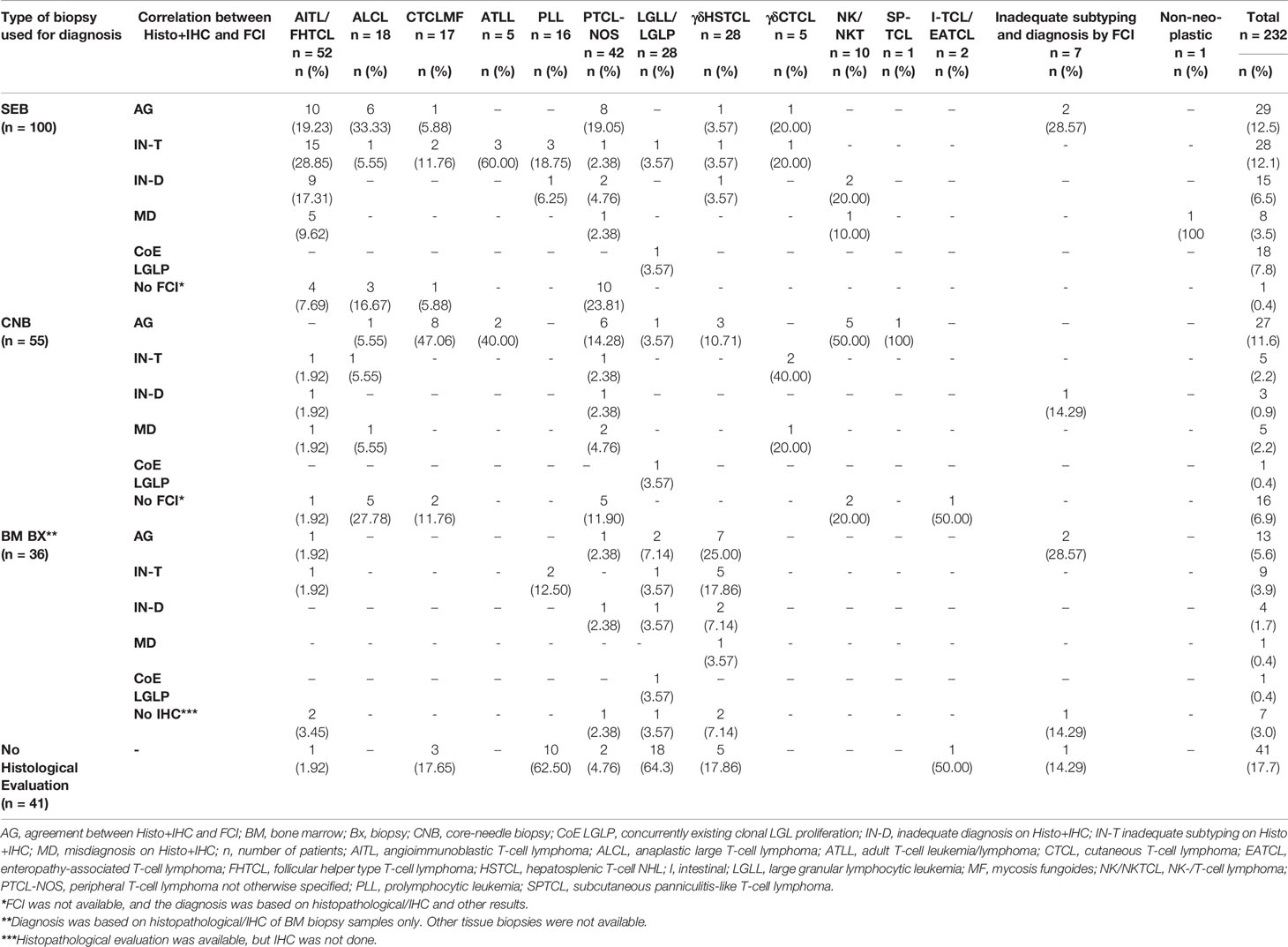
Table 4 Correlation and discrepancies between the flow cytometric immunophenotyping (FCI) and histopathological/IHC findings. .
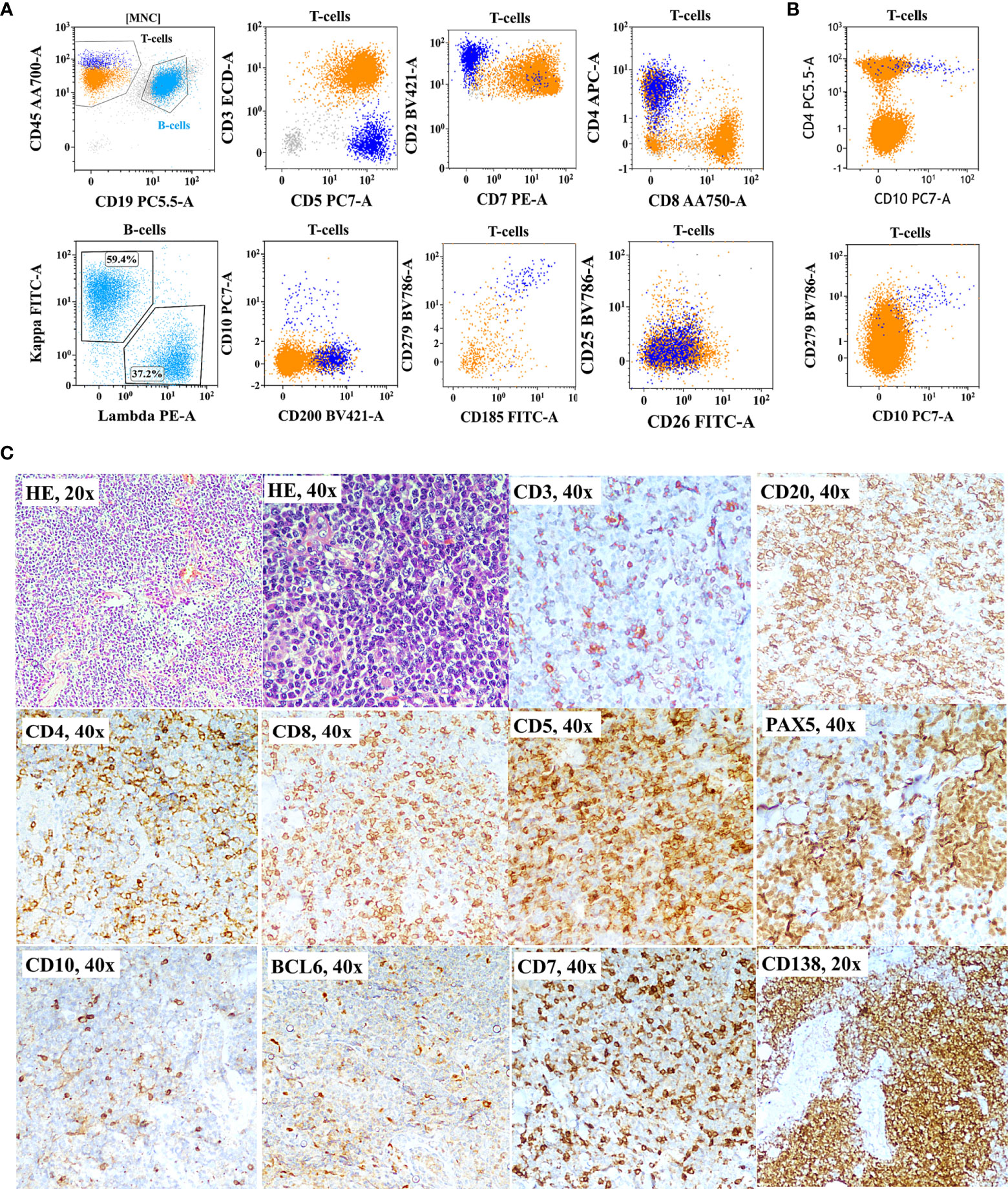
Figure 3 Flow cytometric immunophenotyping (FCI) findings (A, B) and lymph node histopathology and immunohistochemistry results (C) from a representative case of angioimmunoblastic T-cell-lymphoma (AITL) that was misdiagnosed as B-cell non-Hodgkin’s lymphoma (B-NHL). (A) The dot-plots of FCI from the lymph node fine-needle aspiration sample. (B) The dot-plots of FCI from the bone marrow aspiration sample from the same patient. In these dot-plots, the abnormal T cells (dark blue dots) show bright CD45, moderate CD5, bright CD2, moderate CD4, partial CD10, bright CD279 (PD1), and moderate CD185 (CXCR5) expressions but aberrant loss of surface CD3 and CD7 expressions. The orange dots represent normal T cells, and light blue dots show polyclonal B cells. (C) Microscopic pictures of histological (H&E staining) and immunohistochemistry results from the lymph node biopsy demonstrating B cell [highlighted by CD20 and PAX5 immunohistochemistry (IHC)] and plasma cell hyperplasia (highlighted by CD138 IHC). T cells were highlighted by CD5, CD7, CD3, CD4, and CD8 IHC.
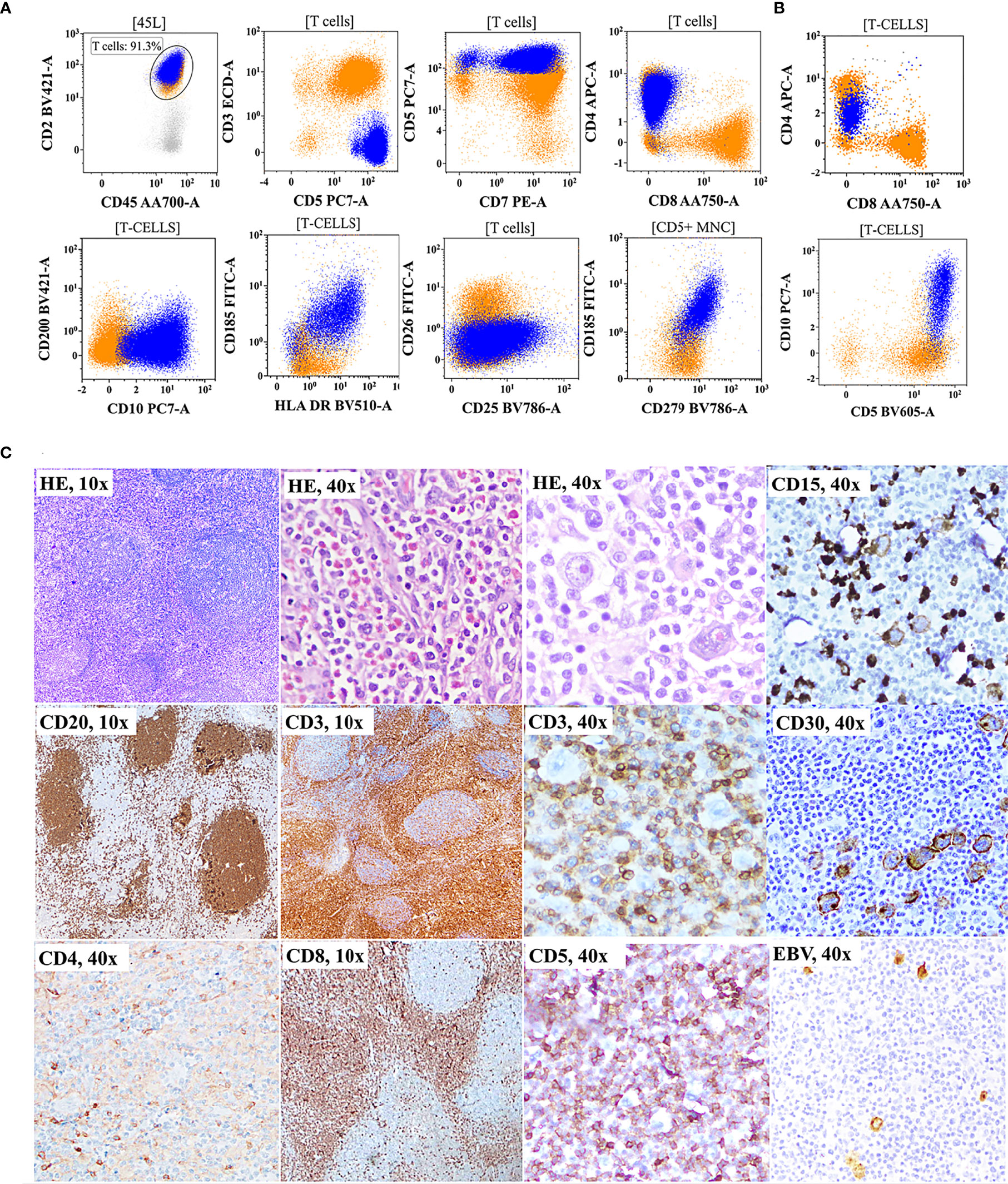
Figure 4 Flow cytometric immunophenotyping (FCI) findings (A, B) and lymph node histopathology and immunohistochemistry results (C) from a representative case of angioimmunoblastic T-cell-lymphoma (AITL) that was misdiagnosed as classical Hodgkin’s lymphoma (cHL). (A) the dot-plots of FCI from the lymph node fine-needle aspiration sample. (B) The dot-plots of FCI from the bone marrow aspiration sample from the same patient. In these dot-plots, the abnormal T cells (dark blue dots) show bright CD45, bright CD2, bright CD5, moderate-to-dim CD4, moderate-to-dim CD10, moderate CD279 (PD1), moderate CD185 (CXCR5), and moderate HLA-DR expressions but abnormal loss of surface CD3 expression. The orange dots represent normal T cells. (C) Microscopic pictures of histological (H&E staining) and immunohistochemistry results from the lymph node biopsy showing follicles highlighted by CD20 and expanded paracortex in the CD3 immunohistochemistry (IHC). Scattered Reed–Sternberg (RS)-like cells are indicated with an arrow.
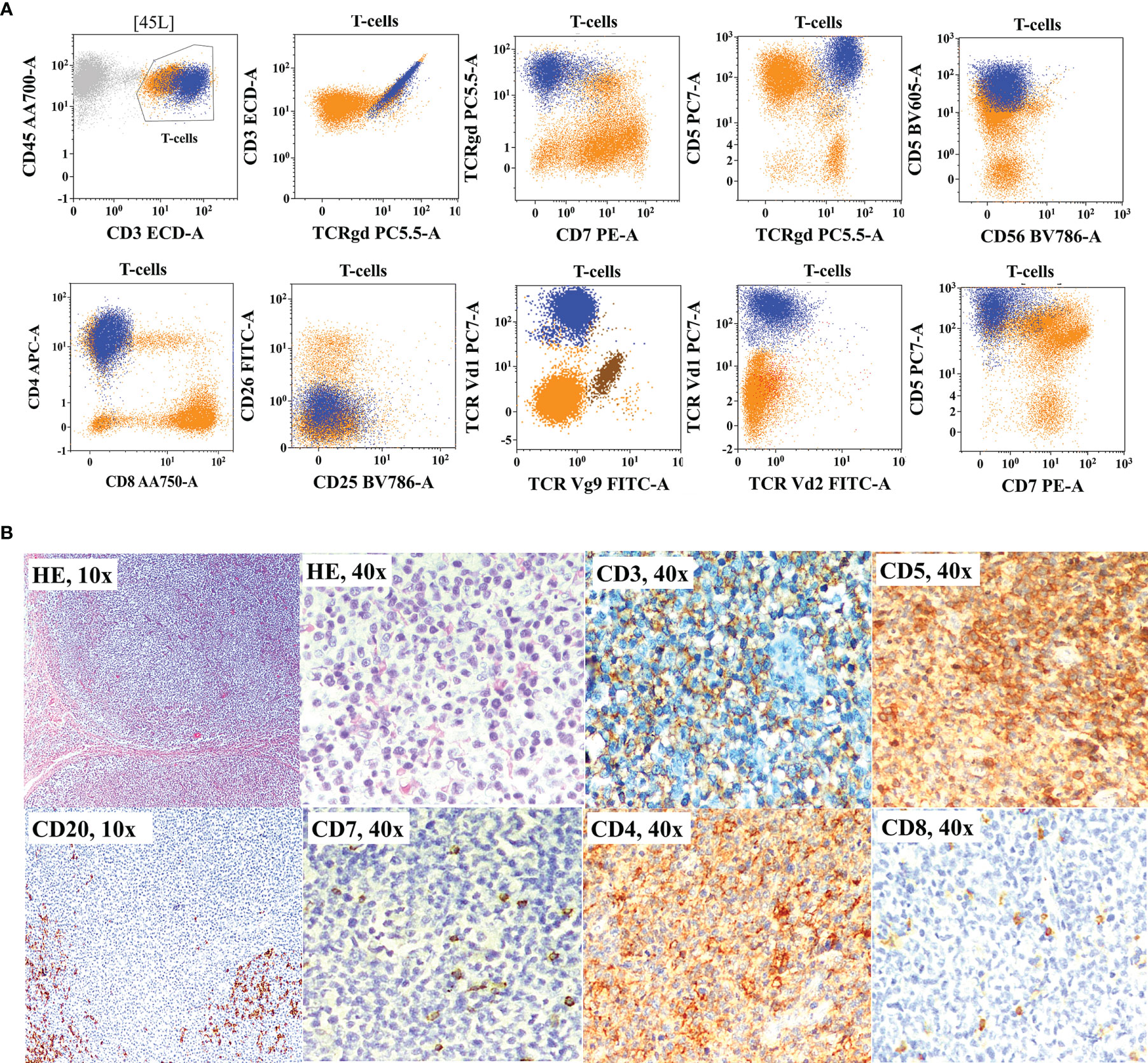
Figure 5 Flow cytometric immunophenotyping (FCI) findings (A) and histopathology and immunohistochemistry results (B) from a representative case of primary cutaneous γδT-cell lymphoma (γδCTCL) that was misclassified as peripheral T-cell lymphoma not otherwise classified (PTCL-NOS). (A) The dot-plots of FCI of fine-needle aspiration sample from the subcutaneous nodule. In these dot-plots, the abnormal γδT cells (dark blue dots) shows bright CD45, bright CD3 bright CD5, moderate CD4, and bright γδT-cell receptor (TCRγδ) expressions but abnormal loss of CD7 expression. On additional immunophenotyping, these cells also show TCRVδ1 restriction but negative TCRVδ2 and TCRVγ9 expressions. The orange dots represent normal T cells. (B) Microscopic pictures of histological (H&E staining) and immunohistochemistry results from the surgical biopsy from the same nodule show significantly increased proportion of CD4-positive T cells with decreased expressions of CD3 and CD7.
4.6 Correlation and Discrepancies Between Flow Cytometric Immunophenotyping Findings and Histopathology/Immunohistochemistry Results in Bone Marrows of T-Cell Non-Hodgkin’s Lymphoma
Of the 232 patients, BM aspiration and BM biopsy samples were available in 173 patients, but IHC was done in only 83 samples. So we correlated FCI findings on BM aspiration with corresponding BM biopsy results in these 83 samples. We observed an agreement on the BM involvement by T-NHL in 50/83 (60.2%) between FCI on BM aspiration and corresponding BM biopsy findings. Among these 50, there was also an agreement on the further subtyping in 36 samples. However, histopathological/IHC evaluation of BM biopsy findings was reported suspicious for involvement by T-NHL in 7/83 (8.4%) samples and no T-NHL involvement in 27/83 (32.5%) samples, highlighting the critical value of FCI for assessment of BM samples and hence staging. Notably, in 9 of these 27 samples (33.3%), the level of tumor burden was lower than 5%, indicating the utility of high-sensitivity FCI in detecting the low-level involvement in T-NHL. FCI has also missed the BM involvement in one sample (ALCL) due to marked hemodilution and focal tumor involvement. Thus, FCI shows a distinct advantage in detecting BM involvement by T-NHLs, especially in patients with AITL and patients with low-level involvement not easily discernable by morphology.
5 Discussion
In the present study, we investigated the contribution of FCI in the diagnosis, classification, and staging of T- and NK-cell NHL in routine clinical practice. We retrospectively analyzed the data from 232 patients diagnosed with T/NK-NHL and correlated the results of FCI with histopathological/IHC findings in 150 patients. FCI had identified the tumor cells in 198 patients (34 were uninvolved). Although a majority of the samples for FCI were primarily submitted for staging, it successfully provided the diagnosis and subtyping of T-NHL and NK-NHL in 190/198 (96%) patients. These data suggested an invaluable role of FCI in the diagnosis and subtyping of T-NHL, especially in BM samples primarily submitted for staging purposes.
The correlation between FCI and histopathological/IHC findings (n = 150) revealed an agreement for diagnosing and subtyping in 46% of patients. However, our data showed that in more than one-third of cases (13/42) diagnosed as PTCL-NOS on histopathology, they could be further subclassified as AITL and PLL on FCI. Additionally, FCI provided the diagnosis and subclassification in those cases where adequate subtyping was not possible, and the diagnosis of T-NHL was difficult on histopathological/IHC analysis. We also studied the effect of factors such as the limited tissue availability due to CNB or limited IHC panel on the final impression of histopathological examination. As expected, the frequency of inadequate subtyping and diagnosis was relatively higher in samples where the IHC panel was limited. However, our data did not show an effect of CNB on inadequate histopathological impression. There are controversial reports on the advantages or limitations of CNB in lymphoma diagnosis. Although these studies emphasize the advantages of SEB over CNB, these reports are predominantly focused on B-NHL with a limited cohort of T-NHL (29, 30, 32, 75).
AITL was one of the commonest T-NHL (22.4%) in our cohort, which is in line with the earlier data published from India (11). It is also known for its usual presentation with advanced clinical stages involving BM and other extranodal sites (76). We diagnosed AITL in the BM samples from 41/49 (83.7%) patients studied for FCI. AITL is a well-established and relatively common subtype among the group of T-NHLs (26, 76–81). However, it is challenging to diagnose due to the lack of a unique histopathological pattern and its relatively low tumor burden in the background of abundant inflammatory cells (26, 77, 79, 81, 82). Additionally, it is characterized by reactive B-cell/plasma cell proliferations obscuring tumor cells on histopathological evaluation, which can sometimes lead to misdiagnosis of B-NHL (26, 45, 47, 76, 77, 79, 83, 84). Our data included four cases that mimicked B-NHL due to florid B-cell proliferation, which were later confirmed as AITL after demonstrating polyclonal B-cell proliferation and the presence of abnormal clonal T cells with follicular-helper T-cell immunophenotype on FCI. Occasionally, large-size immunoblasts with Reed–Sternberg (RS) cell-like appearance are seen in AITL. The presence of background cells with an admixture of reactive inflammatory cells such as eosinophils and plasma cells along with RS-like cells can mimic the morphology of HL (47, 63). We also had two cases of AITL, which were initially diagnosed as classical HL and NLPHL on histopathological evaluation and FCI on FNA, and follow-up repeat biopsy corrected the diagnosis. A reliable diagnosis of AITL requires a higher degree of suspicion and a large (and multicolor) IHC panel inclusive of immune makers specific to recognize its follicular helper T-cell origins such as CD10, CXCR5, PD1, ICOS, and CXCL13 (77, 79, 81, 82). Unfortunately, a large IHC panel in real-world practice may not be possible, especially in cases with a low degree of suspicion of T-NHL due to misleading morphology. It is also challenging to identify a small population of tumor cells in the background of reactive T cells based on the single-marker IHC. In contrast, FCI has the unique ability to identify rare tumor cells and simultaneously detect many tumor-associated molecules. An additional advantage of FCI is that it can confidently recognize even a minor alteration in the expression levels of T-cell markers such as CD3, CD5, and CD7. AITL cells are characterized by downregulation of surface CD3 and CD7 expressions, homogenous CD5, and heterogeneous CD10 expression. These markers are commonly included in the FCI panel. Hence, FCI can easily distinguish AITL tumor cells in the reactive lymphoid proliferations and helps in diagnosing AITL correctly (26, 85–87).
As shown in Table 4, AITL was the most common type of T-NHL inadequately subclassified on histopathological examination. Furthermore, our study included 11 cases misdiagnosed (6 B-NHL, 3 HL, 1 AL, and 1 SPTCL), and 3 cases were misclassified. Most of the cases misdiagnosed as B-NHL and HL were again confirmed as AITL on FCI in FNA and BM samples. The misclassified cases include SPTCL, γδ-TCL, and NK-TCL. Thus, FCI has a distinct role in picking up as well as preventing diagnosis in AITL, the commonest T-cell NHL in this series.
Another diagnostically challenging T-NHL on histopathological examination is γδ-TCL (γδHSTCL and γδ-CTCL) (88–91). Our study included 28 cases of γδHSTCL and 5 cases of γδ-CTCL. The γδHSTCL often presents with extranodal involvement such as in the liver, spleen, and BM (92). Hence, the diagnostic tissue is usually in the form of BM biopsy or liver/spleen CNB (88, 90). Although it is characterized by typical sinusoidal involvement, a limited tissue from BM or CNB makes it challenging to identify the scanty tumor cells and characterize them further using a limited IHC panel. Moreover, the mAb against TCRγδ for IHC was not easily available until recently. Even if available, it has limited reproducibility due to technical issues (91, 93). Furthermore, it is difficult to differentiate reactive versus abnormal γδT cells in tissues with scant involvement. In our study, histopathological findings resulted in inadequate diagnosis/subtyping of 9/28 (32%) cases of γδHSTCL, and one case each was misclassified as AL and PTCL-NOS. Alternatively, mAb against TCRγδ used in FCI is readily available and consistently reproducible. Thus, blastic morphology and FCI provide an accurate and fast diagnosis in γδHSTCL (90). Similarly, FCI in FNA samples from subcutaneous/submucosal lesions and BM samples helped in the correct diagnosis of 4 out 5 cases of γδCTCL.
Our data revealed that patients with PLL and ATLL also faced similar diagnostic dilemmas on histopathological assessment. PLL and ATLL are often diagnosed on PB smear examination and FCI, but nodal and extranodal (skin) involvement is also seen in a significant proportion of patients (14, 94). These can be easily misclassified as PTCL-NOS in the absence of IHC markers such as TCL-1 and CD25 (74). Both diseases usually present with specific immunophenotypic signatures on FCI and typical morphology on PB/BM smears. We also found 2 cases misdiagnosed as SPTCL. One was corrected as NKTCL, and the other showed normal polyclonal T cells FCI on FNA samples. The latter case was further confirmed as LEP. SPTCL is also an extremely rare T-NHL and is traditionally diagnosed on histopathological/IHC findings (95). LEP has histologically and NKTCL immunophenotypically (cytotoxic T-cell phenotype) overlapping features with SPTCL creating diagnostic dilemmas (95, 96).
Besides the availability of clinical information, histopathological findings, and immunophenotypic profile, T-cell clonality assessment is essential for diagnosing T-NHL in a substantial number of cases. T-cell clonality assessment through molecular studies is time-consuming, is less sensitive, needs additional tissue, and does not provide the immunophenotype of clonal T cells (62). In contrast, FCI allows simultaneous assessment of abnormal immunophenotype and T-cell clonality in the immunophenotypically selected suspicious population using TCR-Vβ repertoire and recently introduced a single TRBC1 antibody (71, 73, 97–99). FCI-based T-cell clonality assessment allows the detection of even a small population of clonal T cells in the background of normal T cells, thus providing a highly sensitive tool (62, 72). We studied TCR-Vβ repertoire-based T-cell clonality in 57 samples. Among them, 47 samples showed direct clonality through a single Vβ protein restriction, and nine samples showed indirect evidence of clonality through markedly reduced usage of all 24 Vβ proteins. We also used a single-marker, TRBC1, in 15 samples and found complete positive restriction in five and negative expression in ten cases, confirming the clonal proliferation of T cells. TRBC1 being a single antibody is cost-effective as compared to the TCR-Vβ repertoire. Thus, FCI provided an additional advantage of T-cell clonality assessment wherever required.
Next, we studied the correlation between FCI in BM aspiration and BM biopsy samples for the T-NHL involvement investigated as a part of clinical staging. FCI confirmed the BM involvement by T-NHL in samples with suspicion of involvement in 8.4% and no involvement in 32.5% of samples. In approximately one-third of samples where BM biopsy findings could not detect T-NHL involvement, the tumor burden was less than 5%. On the contrary, FCI missed the involvement in only one sample due to hemodilution and focal involvement. Earlier studies have also reported similar findings (87, 100). These observations highlighted the critical value of FCI for the assessment of BM involvement for correct clinical staging in T-NHL.
Overall, these data highlighted the limitations of purely histopathology-based diagnosis and subclassification. In a significant proportion of patients, the initial diagnosis and subtyping were corrected after FCI findings were incorporated as part of staging or follow-up evaluation. These results strongly argue for the simultaneous workup for histopathological evaluation, IHC, and FCI at diagnosis only. Thus, in addition to adequate clinical data, histopathological/IHC results and FCI findings play a vital complementary role for accurate diagnosis, classification, and staging in T/NK-NHL in real-world practice.
The present study included a cohort of consecutive patients in which FCI was performed as a part of routine diagnosis and staging. Our samples included mainly PB and BM samples but did not include many lymph node tissue or aspirates. Hence, these data do not represent all cases of T/NK-NHL diagnosed in our institution, as FCI was unavailable in all patients. Also, a proportion of T/NK-NHLs does not usually show BM involvement. Hence, the frequencies of T-NHL subtypes and other demographic parameters documented in these data may not be entirely representative. Nevertheless, our data provide a strong rationale for a prospective study with simultaneous assessment of nodal/extranodal tissues and BM samples using FCI and histopathological/IHC assessments. In conclusion, this retrospective study of the clinical impact of FCI in the correct diagnosis and subtyping of T- and NK-NHL in real-world practice demonstrates that a comprehensive FCI is a robust tool for identifying and immunophenotypic characterization of the abnormal T-cell population, even in samples with a low disease burden. We showed that FCI improves the subtyping and provides confirmatory evidence for T-NHL diagnosis. Our data also demonstrate that AITL is most frequently misdiagnosed on conventional histopathology/IHC evaluation and is the commonest T-NHL involving BM at a low level, not discernable by morphology but detectable by FCI. Thus, FCI plays a critical role in the T- and NK-cell NHL diagnosis, subtyping, and staging in real-world practice.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by Institutional Ethical Committee, Tata Memorial Centre. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author Contributions
PT designed and performed the study, performed the data analysis, interpreted the data, performed the statistical analysis, and wrote the paper. GC performed the study, performed the data analysis, and helped with additional experiments and manuscript writing. PT, AC, PS, GC, NP, and SR performed the flow cytometric immunophenotyping and analyzed the data. SGG, KG, and NDe performed the quality control of the study and processed the samples for flow cytometry. AC, NDa, TK, and SV collected the data and performed the data analysis. DS performed the cytogenetic study. TA, SG, TS, and SE performed the histopathological evaluation. MS, BB, and HJ treated the patients and provided the clinical data. All authors contributed to the manuscript writing and approved the final version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.779230/full#supplementary-material
References
1. Rizvi MA, Evens AM, Tallman MS, Nelson BP, Rosen ST. T-Cell Non-Hodgkin Lymphoma. Blood (2006) 107:1255–64. doi: 10.1182/blood-2005-03-1306
2. Vose J, Armitage J, Weisenburger D, International T-Cell Lymphoma Project. International Peripheral T-Cell and Natural Killer/T-Cell Lymphoma study: Pathology Findings and Clinical Outcomes. J Clin Oncol (2008) 26(25):4124–30. doi: 10.1200/JCO.2008.16.4558
3. Satou A, Bennani NN, Feldman AL. Update on the Classification of T-Cell Lymphomas, Hodgkin Lymphomas, and Histiocytic/Dendritic Cell Neoplasms. Expert Rev Hematol (2019) 12:833–43. doi: 10.1080/17474086.2019.1647777
4. De Leval L. Approach to Nodal-Based T-Cell Lymphomas. Pathology (2020) 52:78–99. doi: 10.1016/j.pathol.2019.09.012
5. Foss FM, Zinzani PL, Vose JM, Gascoyne RD, Rosen ST, Tobinai K. Peripheral T-Cell Lymphoma. Blood (2011) 117:6756–67. doi: 10.1182/blood-2010-05-231548
6. Rogers AM, Brammer JE. Hematopoietic Cell Transplantation and Adoptive Cell Therapy in Peripheral T Cell Lymphoma. Curr Hematol Malig Rep (2020) 15:316–32. doi: 10.1007/s11899-020-00590-5
7. Nizamuddin I, Galvez C, Pro B. Management of ALCL and Other CD30+ Peripheral T-Cell Lymphomas With a Focus on Brentuximab Vedotin. Semin Hematol (2021) 58:85–94. doi: 10.1053/j.seminhematol.2021.02.006
8. Saleh K, Michot JM, Ribrag V. Updates in the Treatment of Peripheral T-Cell Lymphomas. J Exp Pharmacol (2021) 13:577–91. doi: 10.2147/JEP.S262344
9. Jiang L, Yuan CM, Hubacheck J, Janik JE, Wilson W, Morris JC, et al. Variable CD52 Expression in Mature T Cell and NK Cell Malignancies: Implications for Alemtuzumab Therapy. Br J Haematol (2009) 145:173–9. doi: 10.1111/j.1365-2141.2009.07606.x
10. Xue W, Zhang M. Updating Targets for Natural Killer/T-Cell Lymphoma Immunotherapy. Cancer Biol Med (2021) 18:52–62. doi: 10.20892/j.issn.2095-3941.2020.0400
11. Naresh KN, Agarwal B, Sangal BC, Basu DD, Kothari AS, Soman CS. Regional Variation in the Distribution of Subtypes of Lymphoid Neoplasms in India. Leuk Lymphoma (2002) 43:1939–43. doi: 10.1080/1042819021000016069
12. Hsi ED, Said J, Macon WR, Rodig SJ, Ondrejka SL, Gascoyne RD, et al. Diagnostic Accuracy of a Defined Immunophenotypic and Molecular Genetic Approach for Peripheral T/NK-Cell Lymphomas. A North American PTCL Study Group Project. Am J Surg Pathol (2014) 38:768–75. doi: 10.1097/PAS.0000000000000188
13. Sandell RF, Boddicker RL, Feldman AL. Genetic Landscape and Classification of Peripheral T Cell Lymphomas. Curr Oncol Rep (2017) 19:28. doi: 10.1007/s11912-017-0582-9
14. Licata MJ, Janakiram M, Tan S, Fang Y, Shah UA, Verma AK, et al. Diagnostic Challenges of Adult T-Cell Leukemia/Lymphoma in North America – a Clinical, Histological, and Immunophenotypic Correlation With a Workflow Proposal. Leukemia Lymphoma (2018) 59:1188–94. doi: 10.1080/10428194.2017.1365862
15. Drieux F, Ruminy P, Abdel-Sater A, Lemonnier F, Viailly PJ, Fataccioli V, et al. Defining Signatures of Peripheral T-Cell Lymphoma With a Targeted 20-Marker Gene Expression Profiling Assay. Haematologica (2020) 105:1582–92. doi: 10.3324/haematol.2019.226647
16. Miguel AP. Peripheral T-Cell Lymphoma Diagnosis: Building a Molecular Tool. Haematologica (2020) 105:1472–4. doi: 10.3324/haematol.2020.249052
17. Nnachi OC, Ezenwenyi IP, Okoye AE, Akpa CO, Uzoigwe CJ. And Ugwu, G.C). Diagnostic Challenges of Anaplastic Large Cell Lymphoma in a Resource-Limited Setting: A Case Report and Literature Review. Case Rep Hematol (2021) 6677638. doi: 10.1155/2021/6677638
18. Naresh KN, Srinivas V, Soman CS. Distribution of Various Subtypes of non-Hodgkin's Lymphoma in India: A Study of 2773 Lymphomas Using R.E.A.L. And WHO Classifications. Ann Oncol (2000) 11(Suppl. 1):63–7. doi: 10.1093/annonc/11.suppl_1.S63
19. Naresh KN, Advani S, Adde M, Aziz Z, Banavali S, Bhatia K, et al. Report of an International Network of Cancer Treatment and Research Workshop on Non-Hodgkin's Lymphoma in Developing Countries. Blood Cells Mol Dis (2004) 33:330–7. doi: 10.1016/j.bcmd.2004.08.001
20. Michenet P, Bouchy C, Bonneau C, Kerdraon R, Heitzmann A, Blechet C, et al. Second Expert Review on Lymphoma: Assessment of a One Year Activity in a General Hospital. Ann Pathol (2012) 32:248–53. doi: 10.1016/j.annpat.2012.02.014
21. Nair R, Arora N, Mallath MK. Epidemiology of Non-Hodgkin's Lymphoma in India. Oncology (2016) 91(suppl 1):18–25. doi: 10.1159/000447577
22. Ghesquières H, Rossi C, Cherblanc F, Le Guyader-Peyrou S, Bijou F, Sujobert P, et al. A French Multicentric Prospective Prognostic Cohort With Epidemiological, Clinical, Biological and Treatment Information to Improve Knowledge on Lymphoma Patients: Study Protocol of the "REal World Data in LYmphoma and Survival in Adults" (REALYSA) Cohort. BMC Public Health (2021) 21:432. doi: 10.1186/s12889-021-10433-4
23. Economopoulos T, Papageorgiou S, Dimopoulos MA, Pavlidis N, Tsatalas C, Symeonidis A, et al. Non-Hodgkin's Lymphomas in Greece According to the WHO Classification of Lymphoid Neoplasms. A Retrospective Analysis of 810 Cases. Acta Haematol (2005) 113:97–103. doi: 10.1159/000083446
24. Nemani S, Korula A, Agrawal B, Kavitha ML, Manipadam MT, Sigamani E, et al. Peripheral T Cell Lymphoma: Clinico-Pathological Characteristics & Outcome From a Tertiary Care Centre in South India. Indian J Med Res (2018) 147:464–70. doi: 10.4103/ijmr.IJMR_1108_16
25. Demurtas A, Stacchini A, Aliberti S, Chiusa L, Chiarle R, Novero D. Tissue Flow Cytometry Immunophenotyping in the Diagnosis and Classification of Non-Hodgkin's Lymphomas: A Retrospective Evaluation of 1,792 Cases. Cytometry Part B: Clin Cytometry (2013) 84B:82–95. doi: 10.1002/cyto.b.21065
26. Loghavi S, Wang SA, Medeiros LJ, Jorgensen JL, Li X, Xu-Monette ZY, et al. Immunophenotypic and Diagnostic Characterization of Angioimmunoblastic T-Cell Lymphoma by Advanced Flow Cytometric Technology. Leuk Lymphoma (2016) 57:2804–12. doi: 10.3109/10428194.2016.1170827
27. Meda BA, Buss DH, Woodruff RD, Cappellari JO, Rainer RO, Powell BL, et al. Diagnosis and Subclassification of Primary and Recurrent Lymphoma. The Usefulness and Limitations of Combined Fine-Needle Aspiration Cytomorphology and Flow Cytometry. Am J Clin Pathol (2000) 113:688–99. doi: 10.1309/0Q7F-QTGM-6DPD-TLGY
28. Metzgeroth G, Schneider S, Walz C, Reiter S, Hofmann WK, Marx A, et al. Fine Needle Aspiration and Core Needle Biopsy in the Diagnosis of Lymphadenopathy of Unknown Aetiology. Ann Hematol (2012) 91:1477–84. doi: 10.1007/s00277-012-1476-4
29. Frederiksen JK, Sharma M, Casulo C, Burack WR. Systematic Review of the Effectiveness of Fine-Needle Aspiration and/or Core Needle Biopsy for Subclassifying Lymphoma. Arch Pathol Lab Med (2015) 139:245–51. doi: 10.5858/arpa.2013-0674-RA
30. Johl A, Lengfelder E, Hiddemann W, Klapper W. Core Needle Biopsies and Surgical Excision Biopsies in the Diagnosis of Lymphoma-Experience at the Lymph Node Registry Kiel. Ann Hematol (2016) 95:1281–6. doi: 10.1007/s00277-016-2704-0
31. Amini RM, Sundström C. Core Needle Biopsies for Lymphoma Diagnosis Seriously Affect Diagnostics, Treatment Development and Research. Lakartidningen (2017) 114:EMDH.
32. Li J, Han J, Wang Y, Mo Y, Li J, Xiang J, et al. Core Needle Biopsy Targeting the Viable Area of Deep-Sited Dominant Lesion Verified by Color Doppler and/or Contrast-Enhanced Ultrasound Contribute to the Actionable Diagnosis of the Patients Suspicious of Lymphoma. Front Oncol (2020) 10:500153. doi: 10.3389/fonc.2020.500153
33. Matutes E, Robinson D, O'brien M, Haynes BF, Zola H, Catovsky D. Candidate Counterparts of Sézary Cells and Adult T-Cell Lymphoma-Leukaemia Cells in Normal Peripheral Blood: An Ultrastructural Study With the Immunogold Method and Monoclonal Antibodies. Leuk Res (1983) 7:787–801. doi: 10.1016/0145-2126(83)90073-5
34. Uherova P, Ross CW, Finn WG, Singleton TP, Kansal R, Schnitzer B. Peripheral T-Cell Lymphoma Mimicking Marginal Zone B-Cell Lymphoma. Mod Pathol (2002) 15:420–5. doi: 10.1038/modpathol.3880541
35. Jayaraman AG, Cassarino D, Advani R, Kim YH, Tsai E, Kohler S. Cutaneous Involvement by Angioimmunoblastic T-Cell Lymphoma: A Unique Histologic Presentation, Mimicking an Infectious Etiology. J Cutan Pathol (2006) 33(Suppl. 2):6–11. doi: 10.1111/j.1600-0560.2006.00489.x
36. Warnke RA, Jones D, Hsi ED. Morphologic and Immunophenotypic Variants of Nodal T-Cell Lymphomas and T-Cell Lymphoma Mimics. Am J Clin Pathol (2007) 127:511–27. doi: 10.1309/QBLAMA321K9AD2XK
37. Ahsanuddin AN, Brynes RK, Li S. Peripheral Blood Polyclonal Plasmacytosis Mimicking Plasma Cell Leukemia in Patients With Angioimmunoblastic T-Cell Lymphoma: Report of 3 Cases and Review of the Literature. Int J Clin Exp Pathol (2011) 4:416–20.
38. Goto N, Tsurumi H, Sawada M, Kanemura N, Hara T, Kasahara S, et al. Follicular Variant of Peripheral T-Cell Lymphoma Mimicking Follicular Lymphoma: A Case Report With a Review of the Literature. Pathol Int (2011) 61:326–30. doi: 10.1111/j.1440-1827.2011.02656.x
39. Sohani AR, Jaffe ES, Harris NL, Ferry JA, Pittaluga S, Hasserjian RP. Nodular Lymphocyte-Predominant Hodgkin Lymphoma With Atypical T Cells: A Morphologic Variant Mimicking Peripheral T-Cell Lymphoma. Am J Surg Pathol (2011) 35:1666–78. doi: 10.1097/PAS.0b013e31822832de
40. Moroch J, Copie-Bergman C, De Leval L, Plonquet A, Martin-Garcia N, Delfau-Larue MH, et al. Follicular Peripheral T-Cell Lymphoma Expands the Spectrum of Classical Hodgkin Lymphoma Mimics. Am J Surg Pathol (2012) 36:1636–46. doi: 10.1097/PAS.0b013e318268d9ff
41. Tidwell WJ, Malone J, Callen JP. Cutaneous T-Cell Lymphoma Misdiagnosed as Lipodermatosclerosis. JAMA Dermatol (2016) 152:487–8. doi: 10.1001/jamadermatol.2015.6106
42. Lee J, Park KS, Kang MH, Kim Y, Son SM, Choi H, et al. Primary Hepatic Peripheral T-Cell Lymphoma Mimicking Hepatocellular Carcinoma: A Case Report. Ann Surg Treat Res (2017) 93:110–4. doi: 10.4174/astr.2017.93.2.110
43. Mangana J, Guenova E, Kerl K, Urosevic-Maiwald M, Amann VC, Bayard C, et al. Angioimmunoblastic T-Cell Lymphoma Mimicking Drug Reaction With Eosinophilia and Systemic Symptoms (DRESS Syndrome). Case Rep Dermatol (2017) 9:74–9. doi: 10.1159/000458752
44. De Mel S, Li JB, Abid MB, Tang T, Tay HM, Ting WC, et al. The Utility of Flow Cytometry in Differentiating NK/T Cell Lymphoma From Indolent and Reactive NK Cell Proliferations. Cytometry Part B: Clin Cytometry (2018) 94:159–68. doi: 10.1002/cyto.b.21529
45. Ellis C, Ramirez J, Lafond AA. Angioimmunoblastic T-Cell Lymphoma Mimicking Diffuse Large B-Cell Lymphoma. Cutis (2018) 102:179–82.
46. Sokol K, Kartan S, Johnson WT, Alpdogan O, Nikbakht N, Haverkos BM, et al. Extreme Peripheral Blood Plasmacytosis Mimicking Plasma Cell Leukemia as a Presenting Feature of Angioimmunoblastic T-Cell Lymphoma (AITL). Front Oncol (2019) 9:509. doi: 10.3389/fonc.2019.00509
47. Szablewski V, Dereure O, René C, Tempier A, Durand L, Alame M, et al. Cutaneous Localization of Angioimmunoblastic T-Cell Lymphoma may Masquerade as B-Cell Lymphoma or Classical Hodgkin Lymphoma: A Histologic Diagnostic Pitfall. J Cutan Pathol (2019) 46:102–10.
48. Vroobel KM, O'connor S, Cunningham D, Wren D, Sharma B, Wotherspoon A, et al. Florid T Follicular Helper Cell Hyperplasia Associated With Extranodal Marginal Zone Lymphoma: A Diagnostic Pitfall Which may Mimic T Cell Lymphoma. Histopathology (2019) 75:287–90. doi: 10.1111/his.13858
49. Sakakibara A, Suzuki Y, Kato H, Yamamoto K, Sakata-Yanagimoto M, Ishikawa Y, et al. Follicular T-Cell Lymphoma Mimicking Lymphocyte-Rich Classic Hodgkin Lymphoma: A Case Report of a Diagnostic Pitfall. J Clin Exp Hematop (2021) 61:97–101. doi: 10.3960/jslrt.20052
50. Wang C, Mao X, Liu S, He C, Wang Y, Zhu L, et al. The Early Diagnostic Dilemma in Angioimmunoblastic T Cell Lymphoma With Excessive Plasma Cells Proliferation. Case Rep Med (2021) 2021:9951122. doi: 10.1155/2021/9951122
51. Younes S, Rojansky RB, Menke JR, Gratzinger D, Natkunam Y. Pitfalls in the Diagnosis of Nodular Lymphocyte Predominant Hodgkin Lymphoma: Variant Patterns, Borderlines and Mimics. Cancers (Basel) (2021) 13(12):3021. doi: 10.3390/cancers13123021
52. Tembhare PR, Gujral S, Krishnamurthy H. Measurement of Predictive Cancer Biomarkers by Flow Cytometry. In: Badve S, Kumar GL, editors. Predictive Biomarkers in Oncology: Applications in Precision Medicine. Cham: Springer International Publishing (2019). p. 119–29.
53. Gujral S, Subramanian P, Patkar N, Badrinath Y, Kumar A, Tembhare P, et al. Report of Proceedings of the National Meeting on "Guidelines for Immunophenotyping of Hematolymphoid Neoplasms by Flow Cytometry". Indian J Pathol Microbiol (2008) 51:161–6. doi: 10.4103/0377-4929.41602
54. Nettey L, Giles AJ, Chattopadhyay PK. OMIP-050: A 28-Color/30-Parameter Fluorescence Flow Cytometry Panel to Enumerate and Characterize Cells Expressing a Wide Array of Immune Checkpoint Molecules. Cytometry A (2018) 93:1094–6. doi: 10.1002/cyto.a.23608
55. Liechti T, Roederer M. OMIP-060: 30-Parameter Flow Cytometry Panel to Assess T Cell Effector Functions and Regulatory T Cells. Cytometry Part A (2019) 95:1129–34. doi: 10.1002/cyto.a.23853
56. Matutes E, Brito-Babapulle V, Worner I, Sainati L, Foroni L, Catovsky D. T-Cell Chronic Lymphocytic Leukaemia: The Spectrum of Mature T-Cell Disorders. Nouv Rev Fr Hematol (1988) 30:347–51.
57. Matutes E, Catovsky D. Mature T-Cell Leukemias and Leukemia/Lymphoma Syndromes: Review of Our Experience in 175 Cases. Leuk Lymphoma (1991) 4:81–91. doi: 10.3109/10428199109068049
58. Jamal S, Picker LJ, Aquino DB, Mckenna RW, Dawson DB, Kroft SH. Immunophenotypic Analysis of Peripheral T-Cell Neoplasms. A Multiparameter Flow Cytometric Approach. Am J Clin Pathol (2001) 116:512–26. doi: 10.1309/QF6N-VAQW-N74H-4JE2
59. Gorczyca W, Weisberger J, Liu Z, Tsang P, Hossein M, Wu CD, et al. An Approach to Diagnosis of T-Cell Lymphoproliferative Disorders by Flow Cytometry. Cytometry (2002) 50:177–90. doi: 10.1002/cyto.10003
60. Matutes E. Chronic T-Cell Lymphoproliferative Disorders. Rev Clin Exp Hematol (2002) 6:401–20; discussion 449-450. doi: 10.1046/j.1468-0734.2002.00306.x
61. Stacchini A, Demurtas A, Aliberti S, Francia Di Celle P, Godio L, Palestro G, et al. The Usefulness of Flow Cytometric CD10 Detection in the Differential Diagnosis of Peripheral T-Cell Lymphomas. Am J Clin Pathol (2007) 128:854–64. doi: 10.1309/MC7QRGPTV0LRR98X
62. Tembhare P, Yuan CM, Xi L, Morris JC, Liewehr D, Venzon D, et al. Flow Cytometric Immunophenotypic Assessment of T-Cell Clonality by Vβ Repertoire Analysis: Detection of T-Cell Clonality at Diagnosis and Monitoring of Minimal Residual Disease Following Therapy. Am J Clin Pathol (2011) 135:890–900. doi: 10.1309/AJCPV2D1DDSGJDBW
63. Alikhan M, Song JY, Sohani AR, Moroch J, Plonquet A, Duffield AS, et al. Peripheral T-Cell Lymphomas of Follicular Helper T-Cell Type Frequently Display an Aberrant CD3(-/Dim)CD4(+) Population by Flow Cytometry: An Important Clue to the Diagnosis of a Hodgkin Lymphoma Mimic. Mod Pathol (2016) 29:1173–82. doi: 10.1038/modpathol.2016.113
64. Kumar S, Dhamija B, Marathe S, Ghosh S, Dwivedi A, Karulkar A, et al. The Th9 Axis Reduces the Oxidative Stress and Promotes the Survival of Malignant T Cells in Cutaneous T-Cell Lymphoma Patients. Mol Cancer Res (2020) 18:657–68. doi: 10.1158/1541-7786.MCR-19-0894
65. Statuto T, D'auria F, Del Vecchio L, Mansueto GR, Villani O, Lalinga AV, et al. Atypical Mature T-Cell Neoplasms: The Relevance of the Role of Flow Cytometry. Onco Targets Ther (2020) 13:7605–14. doi: 10.2147/OTT.S258512
66. Swerdlow Sh CE, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th. Lyon, France: IARC Press (2008).
67. Swerdlow Sh CE, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. Mature T and NK Cell Neoplasms. Chapter 14. In: Editors are the Three Former Authors WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th Vol. 346-. . Lyon, France: IARC Press. (2017). p. 422.
68. Kalina T, Flores-Montero J, van der Velden VHJ, Martin-Ayuso M, Böttcher S, Ritgen M, et al. EuroFlow Standardization of Flow Cytometer Instrument Settings and Immunophenotyping Protocols Leukemia 26(9):1986–2010.
69. Tembhare PR, Narula G, Khanka T, Ghogale S, Chatterjee G, Patkar NV, et al. Post-Induction Measurable Residual Disease Using Multicolor Flow Cytometry Is Strongly Predictive of Inferior Clinical Outcome in the Real-Life Management of Childhood T-Cell Acute Lymphoblastic Leukemia: A Study of 256 Patients. Front Oncol (2020) 10:577. doi: 10.3389/fonc.2020.00577
70. Tembhare PR, Chatterjee G, Khanka T, Ghogale S, Badrinath Y, Deshpande N, et al. Eleven-Marker 10-Color Flow Cytometric Assessment of Measurable Residual Disease for T-Cell Acute Lymphoblastic Leukemia Using an Approach of Exclusion. Cytometry B Clin Cytom (2021) 100:421–33. doi: 10.1002/cyto.b.21939
71. Novikov ND, Griffin GK, Dudley G, Drew M, Rojas-Rudilla V, Lindeman NI, et al. Utility of a Simple and Robust Flow Cytometry Assay for Rapid Clonality Testing in Mature Peripheral T-Cell Lymphomas. Am J Clin Pathol (2019) 151:494–503. doi: 10.1093/ajcp/aqy173
72. Tembhare P, Yuan CM, Morris JC, Janik JE, Filie AC, Stetler-Stevenson M. Flow Cytometric Immunophenotypic Assessment of T-Cell Clonality by Vβ Repertoire Analysis in Fine-Needle Aspirates and Cerebrospinal Fluid. Am J Clin Pathol (2012) 137:220–6. doi: 10.1309/AJCPPT93VZMAREHK
73. Muñoz-García N, Lima M, Villamor N, Morán-Plata FJ, Barrena S, Mateos S, et al. Anti-TRBC1 Antibody-Based Flow Cytometric Detection of T-Cell Clonality: Standardization of Sample Preparation and Diagnostic Implementation. Cancers (Basel) (2021) 13(17):4379. doi: 10.3390/cancers13174379
74. Herling M, Khoury JD, Washington LT, Duvic M, Keating MJ, Jones D. A Systematic Approach to Diagnosis of Mature T-Cell Leukemias Reveals Heterogeneity Among WHO Categories. Blood (2004) 104:328–35. doi: 10.1182/blood-2004-01-0002
75. Cohen OC, Brodermann MH, Dervin A, Raja N, Marafioti T, Otero S, et al. Lymph Node Core Biopsies Reliably Permit Diagnosis of Lymphoproliferative Diseases. Real-World Experience From 554 Sequential Core Biopsies From a Single Centre. Eur J Haematology (2021) 106:267–72. doi: 10.1111/ejh.13545
76. Yabe M, Dogan A, Horwitz SM, Moskowitz AJ. Angioimmunoblastic T-Cell Lymphoma. Cancer Treat Res (2019) 176:99–126. doi: 10.1007/978-3-319-99716-2_5
77. Ocampo-Garza J, Herz-Ruelas ME, González-Lopez EE, Mendoza-Oviedo EE, Garza-Chapa JI, Ocampo-Garza SS, et al. Angioimmunoblastic T-Cell Lymphoma: A Diagnostic Challenge. Case Rep Dermatol (2014) 6:291–5. doi: 10.1159/000370302
78. Fujisawa M, Chiba S, Sakata-Yanagimoto M. Recent Progress in the Understanding of Angioimmunoblastic T-Cell Lymphoma. J Clin Exp Hematop (2017) 57:109–19. doi: 10.3960/jslrt.17019
79. Lunning MA, Vose JM. Angioimmunoblastic T-Cell Lymphoma: The Many-Faced Lymphoma. Blood (2017) 129:1095–102. doi: 10.1182/blood-2016-09-692541
80. Tari G, Lemonnier F, Morschhauser F. Epigenetic Focus on Angioimmunoblastic T-Cell Lymphoma: Pathogenesis and Treatment. Curr Opin Oncol (2021) 33(5):400–5. doi: 10.1097/CCO.0000000000000773
81. Xie Y, Jaffe ES. How I Diagnose Angioimmunoblastic T-Cell Lymphoma. Am J Clin Pathol (2021) 156:1–14. doi: 10.1093/ajcp/aqab090
82. Chiba S, Sakata-Yanagimoto M. Advances in Understanding of Angioimmunoblastic T-Cell Lymphoma. Leukemia (2020) 34:2592–606. doi: 10.1038/s41375-020-0990-y
83. Sachdev R, Goel S, Gautam D, Sood N. Angioimmunoblastic T-Cell Lymphoma Presenting With Extensive Marrow Plasmacytosis and Hypergammaglobulinaemia: A Diagnostic Challenge. Pathology (2018) 50:665–8. doi: 10.1016/j.pathol.2018.03.014
84. Lee PH, Weng SW, Liu TT, You HL, Liao CK, Wang MC, et al. RHOA G17V Mutation in Angioimmunoblastic T-Cell Lymphoma: A Potential Biomarker for Cytological Assessment. Exp Mol Pathol (2019) 110:104294. doi: 10.1016/j.yexmp.2019.104294
85. Lee PS, Lin CN, Chuang SS. Immunophenotyping of Angioimmunoblastic T-Cell Lymphomas by Multiparameter Flow Cytometry. Pathol Res Pract (2003) 199:539–45. doi: 10.1078/0344-0338-00459
86. Chen W, Kesler MV, Karandikar NJ, Mckenna RW, Kroft SH. Flow Cytometric Features of Angioimmunoblastic T-Cell Lymphoma. Cytometry B Clin Cytom (2006) 70:142–8. doi: 10.1002/cyto.b.20107
87. Yabe M, Gao Q, Ozkaya N, Huet S, Lewis N, Pichardo JD, et al. Bright PD-1 Expression by Flow Cytometry is a Powerful Tool for Diagnosis and Monitoring of Angioimmunoblastic T-Cell Lymphoma. Blood Cancer J (2020) 10:32. doi: 10.1038/s41408-020-0301-x
88. Calvaruso M, Gulino A, Buffa S, Guarnotta C, Franco G, Cacciatore M, et al. Challenges and New Prospects in Hepatosplenic γδ T-Cell Lymphoma. Leuk Lymphoma (2014) 55:2457–65. doi: 10.3109/10428194.2014.889821
89. Daniels J, Doukas PG, Escala MEM, Ringbloom KG, Shih DJH, Yang J, et al. Cellular Origins and Genetic Landscape of Cutaneous Gamma Delta T Cell Lymphomas. Nat Commun (2020) 11:1806. doi: 10.1038/s41467-020-15572-7
90. Pro B, Allen P, Behdad A. Hepatosplenic T-Cell Lymphoma: A Rare But Challenging Entity. Blood (2020) 136:2018–26. doi: 10.1182/blood.2019004118
91. Torres-Cabala CA, Huen A, Iyer SP, Miranda RN. Gamma/Delta Phenotype in Primary Cutaneous T-Cell Lymphomas and Lymphoid Proliferations: Challenges for Diagnosis and Classification. Surg Pathol Clin (2021) 14:177–94. doi: 10.1016/j.path.2021.03.001
92. Foss FM, Horwitz SM, Civallero M, Bellei M, Marcheselli L, Kim WS, et al. Incidence and Outcomes of Rare T Cell Lymphomas From the T Cell Project: Hepatosplenic, Enteropathy Associated and Peripheral Gamma Delta T Cell Lymphomas. Am J Hematol (2020) 95:151–5. doi: 10.1002/ajh.25674
93. Jungbluth AA, Frosina D, Fayad M, Pulitzer MP, Dogan A, Busam KJ, et al. Immunohistochemical Detection of γ/δ T Lymphocytes in Formalin-Fixed Paraffin-Embedded Tissues. Appl Immunohistochem Mol Morphol (2019) 27:581–3. doi: 10.1097/PAI.0000000000000650
94. Staber PB, Herling M, Bellido M, Jacobsen ED, Davids MS, Kadia TM, et al. Consensus Criteria for Diagnosis, Staging, and Treatment Response Assessment of T-Cell Prolymphocytic Leukemia. Blood (2019) 134:1132–43. doi: 10.1182/blood.2019000402
95. Leblanc RE, Tavallaee M, Kim YH, Kim J. Useful Parameters for Distinguishing Subcutaneous Panniculitis-Like T-Cell Lymphoma From Lupus Erythematosus Panniculitis. Am J Surg Pathol (2016) 40:745–54. doi: 10.1097/PAS.0000000000000596
96. Alsomali DY, Bakshi N, Kharfan-Dabaja M, El Fakih R, Aljurf M. Diagnosis and Treatment of Subcutaneous Panniculitis-Like T-Cell Lymphoma: A Systematic Literature Review. Hematology/Oncology Stem Cell Ther (2021) S1658-3876(21)00051-0. doi: 10.1016/j.hemonc.2021.04.001
97. Salameire D, Solly F, Fabre B, Lefebvre C, Chauvet M, Gressin R, et al. Accurate Detection of the Tumor Clone in Peripheral T-Cell Lymphoma Biopsies by Flow Cytometric Analysis of TCR-Vβ Repertoire. Mod Pathol (2012) 25:1246–57. doi: 10.1038/modpathol.2012.74
98. Wu D, Anderson MM, Othus M, Wood BL. Clinical Experience With Modified, Single-Tube T-Cell Receptor Vβ Flow Cytometry Analysis for T-Cell Clonality. Am J Clin Pathol (2016) 145:467–85. doi: 10.1093/ajcp/aqw015
99. Shi M, Jevremovic D, Otteson GE, Timm MM, Olteanu H, Horna P. Single Antibody Detection of T-Cell Receptor αβ Clonality by Flow Cytometry Rapidly Identifies Mature T-Cell Neoplasms and Monotypic Small CD8-Positive Subsets of Uncertain Significance. Cytometry B Clin Cytom (2020) 98:99–107. doi: 10.1002/cyto.b.21782
Keywords: immunophenotyping, T cell, non-Hodgkin’s lymphoma, real-world practice, flow cytometry
Citation: Tembhare PR, Chatterjee G, Chaturvedi A, Dasgupta N, Khanka T, Verma S, Ghogale SG, Deshpande N, Girase K, Sengar M, Bagal B, Jain H, Shetty D, Rajpal S, Patkar N, Agrawal T, Epari S, Shet T, Subramanian PG and Gujral S (2022) Critical Role of Flow Cytometric Immunophenotyping in the Diagnosis, Subtyping, and Staging of T-Cell/NK-Cell Non-Hodgkin’s Lymphoma in Real-World Practice: A Study of 232 Cases From a Tertiary Cancer Center in India. Front. Oncol. 12:779230. doi: 10.3389/fonc.2022.779230
Received: 18 September 2021; Accepted: 26 January 2022;
Published: 01 March 2022.
Edited by:
Onder Alpdogan, Thomas Jefferson University, United StatesReviewed by:
Daisy Alapat, University of Arkansas for Medical Sciences, United StatesIole Cordone, Hospital Physiotherapy Institutes (IRCCS), Italy
Copyright © 2022 Tembhare, Chatterjee, Chaturvedi, Dasgupta, Khanka, Verma, Ghogale, Deshpande, Girase, Sengar, Bagal, Jain, Shetty, Rajpal, Patkar, Agrawal, Epari, Shet, Subramanian and Gujral. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Prashant R. Tembhare, ZG9jcHJ0QGdtYWlsLmNvbQ==; Sumeet Gujral, c19ndWpyYWxAb3V0bG9vay5jb20=
†These authors share first authorship
 Prashant R. Tembhare
Prashant R. Tembhare Gaurav Chatterjee
Gaurav Chatterjee Anumeha Chaturvedi
Anumeha Chaturvedi Niharika Dasgupta
Niharika Dasgupta Twinkle Khanka
Twinkle Khanka Shefali Verma
Shefali Verma Sitaram G. Ghogale1
Sitaram G. Ghogale1 Nilesh Deshpande
Nilesh Deshpande Karishma Girase
Karishma Girase Manju Sengar
Manju Sengar Hasmukh Jain
Hasmukh Jain Sweta Rajpal
Sweta Rajpal Nikhil Patkar
Nikhil Patkar Tushar Agrawal
Tushar Agrawal Sridhar Epari
Sridhar Epari Tanuja Shet
Tanuja Shet Papagudi G. Subramanian
Papagudi G. Subramanian Sumeet Gujral
Sumeet Gujral