- 1Department of Urology, Peking University First Hospital, Beijing, China
- 2Institute of Urology, Peking University, Beijing, China
- 3National Urological Cancer Center, Beijing, China
- 4Department of Nursing, Peking University First Hospital, Beijing, China
Objectives: To summarize the clinicopathological diagnostic features and evolutionary trends of upper tract urothelial carcinoma (UTUC) in China over the past 20 years.
Methods: All patients diagnosed with upper tract urothelial carcinoma in the Peking University First Hospital from 2001 to 2020 were retrospectively collected. Data were divided into two groups (2001-2010 and 2011-2020) according to the date of diagnosis. Statistical analysis was done with the SPSS V22.0. Chi-square analysis and t-test were adopted to analyze depending on the data type. Subgroup analysis based on 5 years was used for visualization to present trends. Both Kaplan-Meier curve and Cox regression were used for univariate and multivariate survival analysis.
Results: The study included 2561 cases diagnosed with upper tract urothelial carcinoma in total. Compared with the first decade (2001-2010), patients of the second decades (2011-2020) had elder mean age (66.65 versus 67.59, years, p=0.025), higher male proportion (43.5% versus 49.0%, p=0.034), lower incidence of renal pelvic tumors (53.4% versus 45.8%, p<0.001) and multifocality (18.6% versus 12.0%, p<0.001), higher incidence of ureteral tumors (52.2% versus 60.9%, p<0.001).In recent ten years, the incidence of muscle-invasive urothelial carcinoma (pT2+) decreased significantly (64.4% versus 54.9%, p<0.001),and the mean size of renal pelvic tumors increased(3.46 versus 3.73, cm, p=0.043). The size of the ureteral tumor, the histopathologic grade showed no significant change. The prognostic analysis based on 709 patients regularly followed at our center revealed that the male gender and G3 histopathological grade were independent risk factors for poorer prognosis in patients with UTUC.
Conclusion: In the past 20 years, the clinicopathological diagnostic features of upper tract urothelial carcinoma in the Chinese population has changed significantly, suggesting an increased risk of a poorer prognosis for UTUC. This trend may be related to updating diagnostic techniques and self-monitoring awareness. However, we need more high-grade, multicenter trials to verify it in the future.
Introduction
Upper tract urothelial carcinoma (UTUC) is an uncommon malignant disease that occurs in the pyelocaliceal cavity and ureter, accounting for only 5% to 10% of the urothelial carcinomas (UCs) (1). UTUCs usually were multicentric and prone to recurrence, and have a higher grade and stage at diagnosis (2). Therefore, the early diagnosis and management of UTUC have been a hot issue of clinical concern.
A recent study based on the SEER database showed that the incidence of UTUC in the United States gradually declined over the last 30 years and that the disease was significantly more prevalent in people over 70 years of age, in men, and renal pelvis (3). However, presumably due to the widespread application of aristolochic acid drugs, UTUC in the Chinese populations has different epidemiological characteristics from those of Western populations. Compared to Western people, the Chinese UTUC population has a higher tumor grade, relatively lower tumor stage, and lower malignancy in female patients compared to the males. And there were more female patients than their counterparts (4). To our knowledge, there was no study on the trend of evolution of the pathological characteristics of the UTUC of the Chinese population in recent years.
This study aimed to summarize the clinical and pathological characteristics of UTUC of the Chinese population based on large sample size and to explore the association between pathological features and clinical characteristics of the UTUC. We further analyzed and elucidated the evolution of the distribution of pathological and clinical features of UTUC in the Chinese population over the past 20 years.
Patients and Methods
Patient Selection
All consecutive UTUC patients who underwent radical nephroureterectomy or segmental ureterectomy or only ureteroscopy in Peking University First Hospital, one of the largest urological clinical centers in China with a large number of UTUC patients from all over China and the world each year, between January 2001 and December 2020, were included in the first screening process. The HIS (Hospital Information System) was the help in collecting basic patient information and pathology information. Cases with a pathological diagnosis of non-UTUC, cytological findings only, history of neoadjuvant chemotherapy, and duplicates were filtered and excluded by two researchers (CR Xu and CW Yuan). The protocol of this study was approved by the Ethics Committee of Peking University First Hospital. Approval reference number. 2021[130].
Pathological Diagnosis
Tumors were staged by the 2002 TNM classification system (5) and graded as G1, G2, and G3 according to the 1973 WHO classification system (6) (Considering that some pre-2004 cases have not yet adopted 2004 WHO updated grade criteria). Other pathological information such as tumor multifocality, primary location (renal pelvic or ureter), and the maximum diameter of the mass were analyzed as well. When masses are found in the renal pelvis and ureter separately at the same time, the primary sites were recorded separately. Each pathological diagnosis was made by two pathologists who independently reviewed and agreed on their conclusions, and in the event of disagreement between the two physicians, the diagnosis was reviewed by another higher-level pathologist.
Prognostic Information and Analysis
To further investigate the association of pathological features with patients’ prognosis and quality of survival, we collected a total of 709 unselected patients with UTUC who had previously received regular follow-up from 2001 to 2021 at our center and for whom complete prognostic survival data were available (all included in the overall sample of this study). Basic patient information and pathological characteristics such as gender, tumor location, multifocality or not, presence of muscle invasion, and pathological grade were included in the analysis. Cancer-specific survival (CSS) was adopted as the primary prognostic endpoint.
Statistical Analysis
All data were divided into two groups according to the time of diagnosis at every ten years (2001-2010 and 2011-2020). To better show the trends in pathological characteristics over time, subgroup analyses made senses according to 5 years (Shown in Tables 2-B, 3-B). For categorical variables, we applied the R*C columnar combined chi-square method to analyze the differences in the comparison rates. The t-test, on the other hand, was suitable for the comparison of sample means of continuous type variables. The results of Fisher’s exact test were adopted when the expectation value <5 appeared in the results of the R*C column table. Kaplan-Meier curve and Cox regression were used for prognostic analyses, and variables satisfying p < 0.2 after univariate analysis were selected for inclusion in the multivariate regression test. All statistical analysis processes were done with the help of SPSS V22.0 (IBM, Armonk, NY). P values are 2-sided, with statistical significance defined as P<0.05. Graphpad Prism version 8.0.1 (GraphPad, San Diego) was used to visualize data. Two authors (CR Xu and CW Yuan) separately completed the data input and statistical analysis, with the entire process supervised by a third author (XS Li).
Results
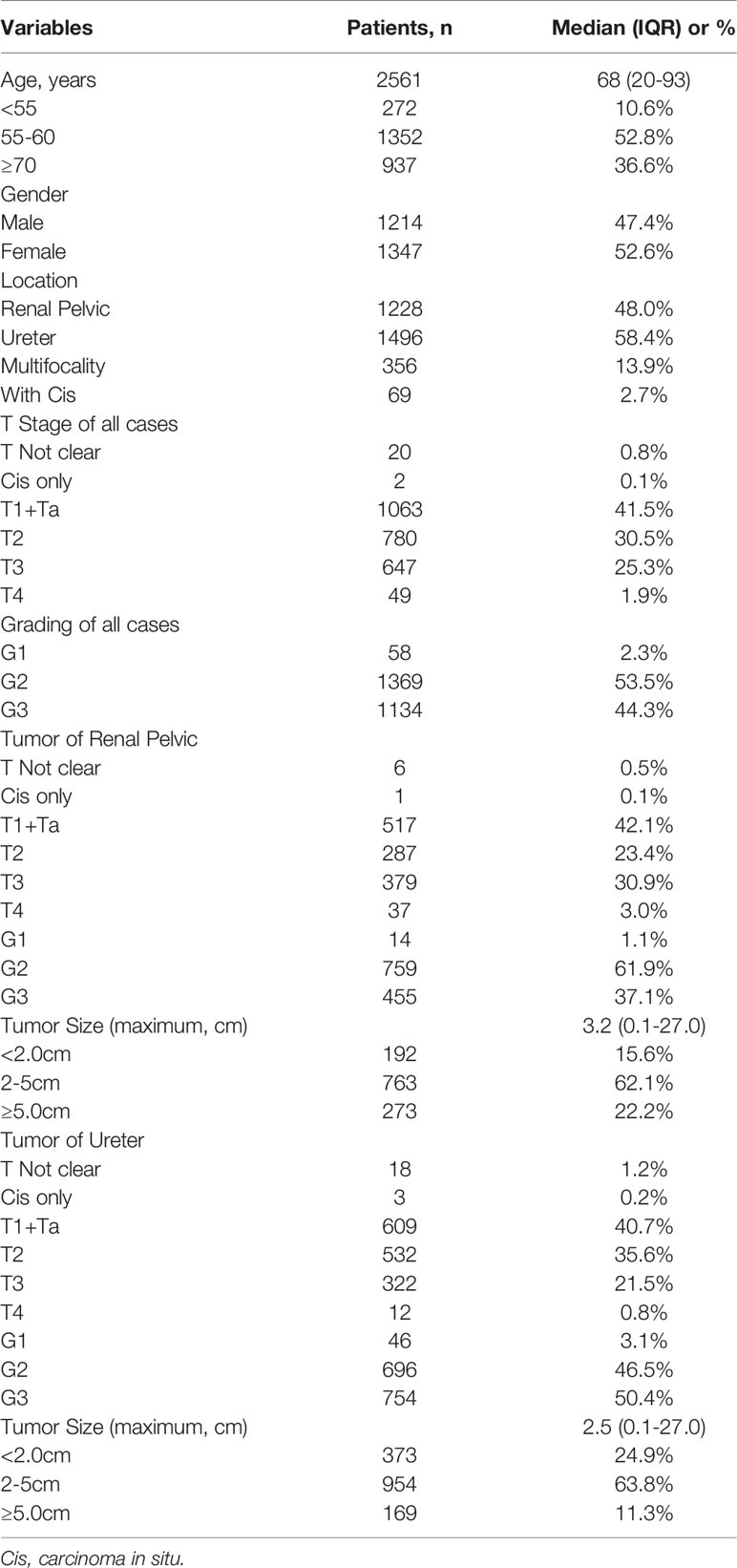
From 2001 to 2020, a total of 3269 patients recorded were found in the His system. After removing duplicate records, records of non-surgical treatments, and data with cytological pathology results only, 2561 cases of UTUC were finally recruited in this study. The clinicopathological characteristics of these patients, including 1214 (47.4%) males and 1347 (52.6%) females, with 1228 (48.0%) tumors of renal pelvic, 1496 (58.4%) ureteral tumors, and 356 (13.9%) multifocal tumors were summarized in Table 1.
Age, Gender and Primary Site of Tumors
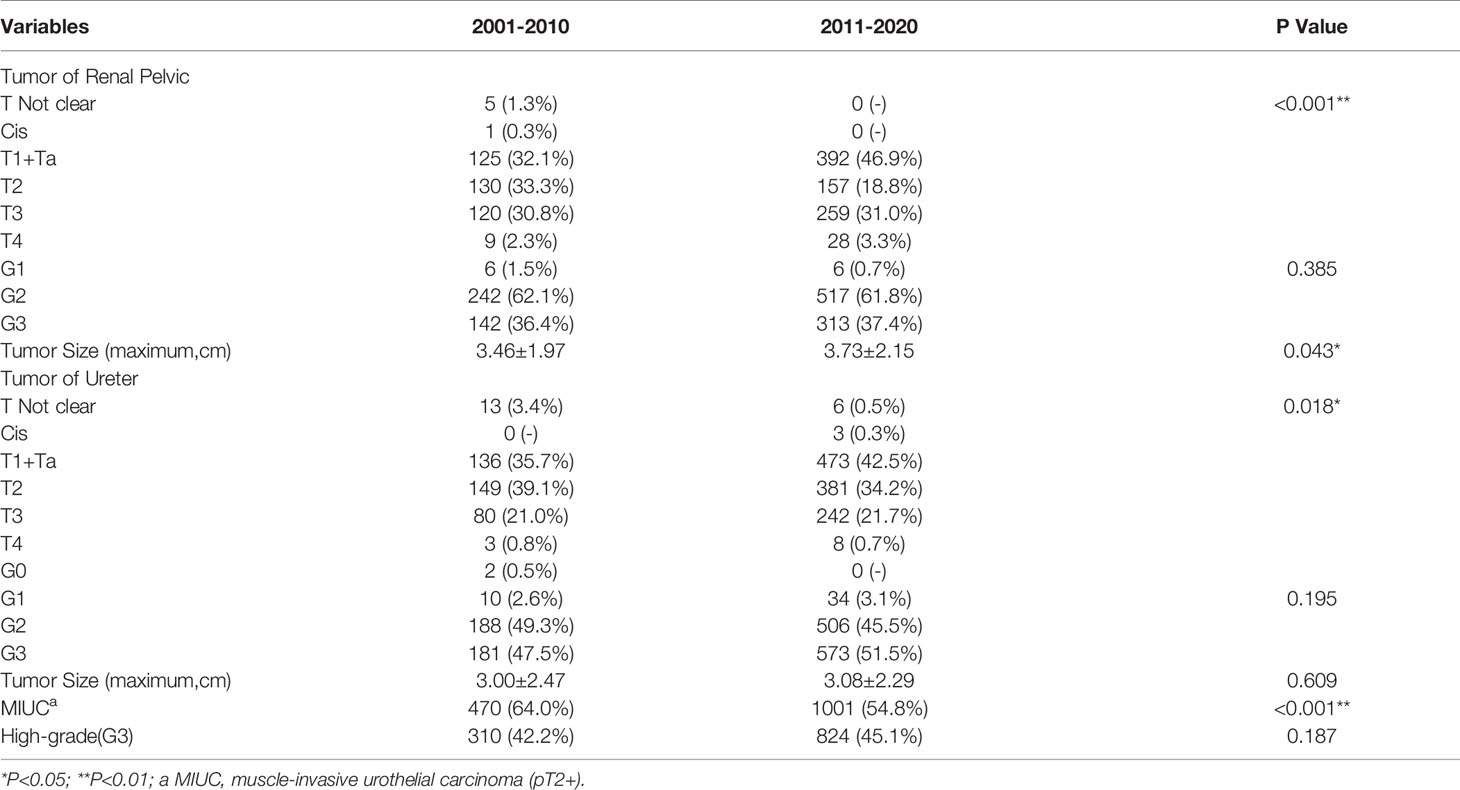
Table 2-A demonstrated the changes in age, gender composition, and primary site of the UTUC patients in the last 20 years. Comparing the 1st decade (2001 to 2010, abbreviated as 1stD) versus the 2nd decade (2011 to 2020, abbreviated as 2ndD), the age of UTUC patients showed a significant increasing trend (p=0.025), but both the proportion of the young patients (age<55 years old) and elderly patients group (age≥70 years old) did not change significantly (p=0.088; p=0.853). In terms of gender composition, there were more female patients than male patients in the last 20 years (1347 versus 1214, 1.11:1). The difference in sexual ratio decreased over the past ten years, while the proportion of male patients significantly rise (p=0.034).
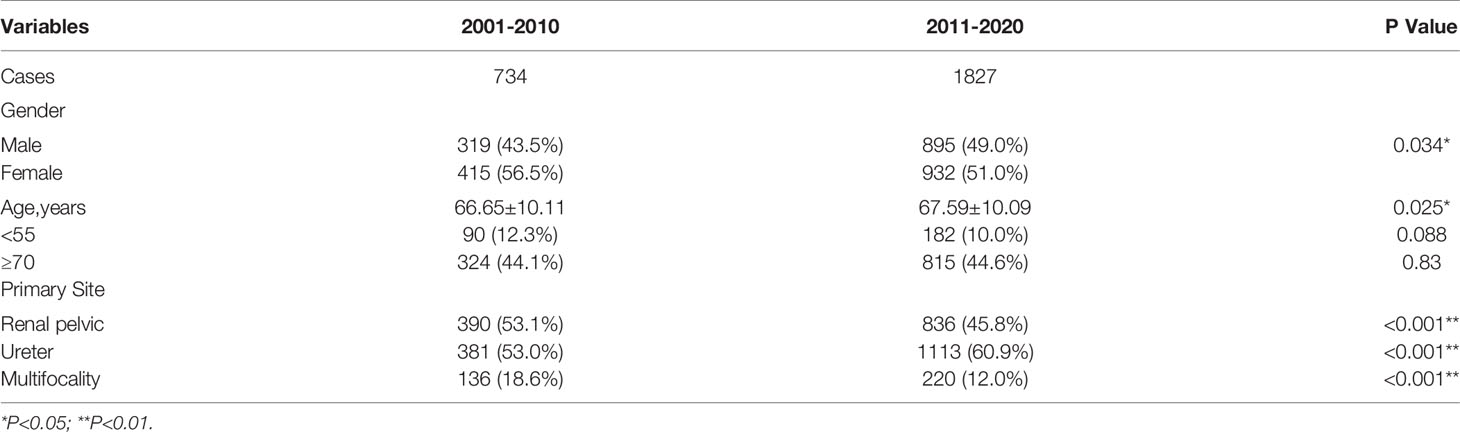
Table 2-A Changes in age, gender composition, and primary site of the UTUC patients in the last 20 years.
There was also a significant difference in the proportion of the primary site of tumors between 1stD and 2ndD. Renal pelvic tumors were found less than the last time (p<0.001), while the incidence of ureteral tumors has gradually increased (p<0.001). Specifically, the incidence of multifocal tumors decreased significantly in the recent ten years (p<0.001).
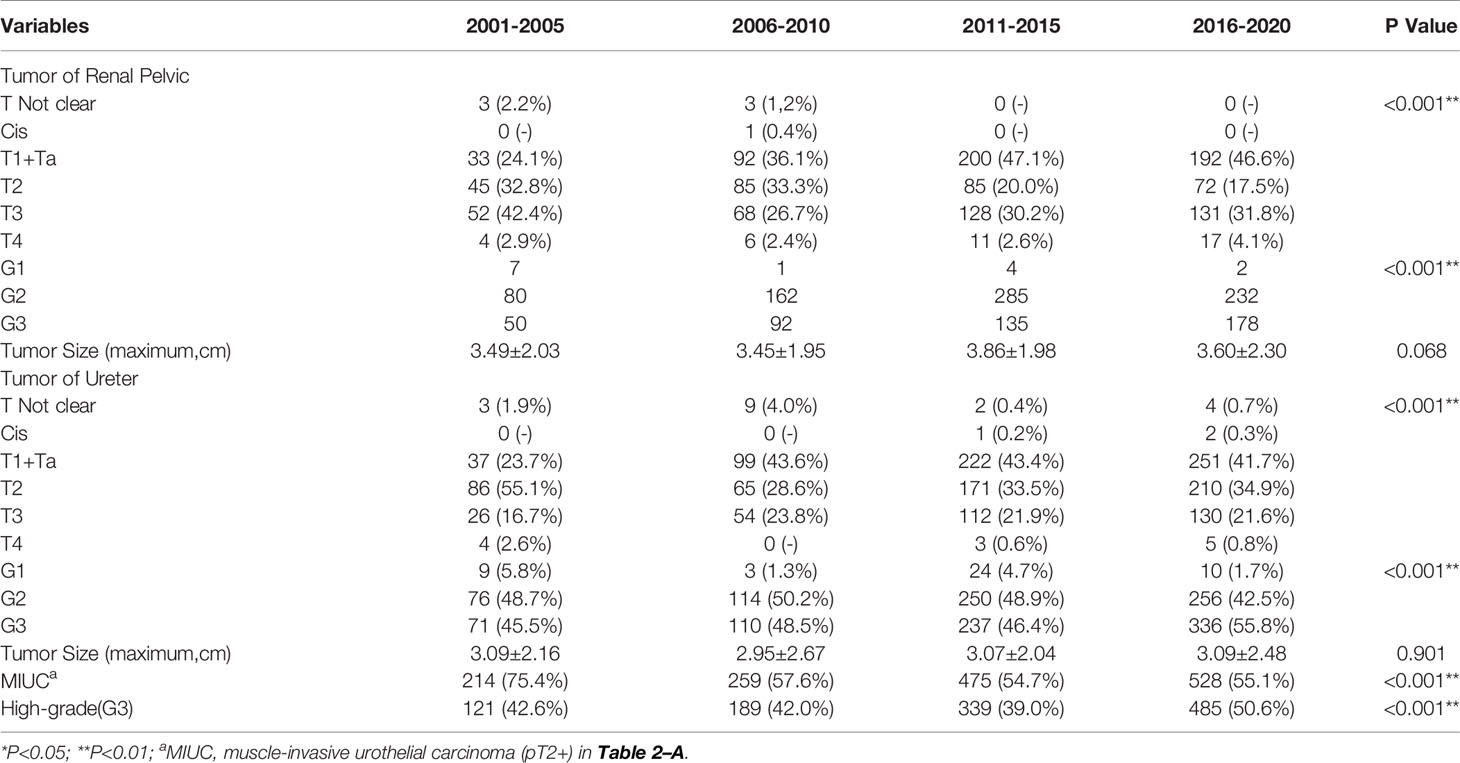
To better show trends in change, we further completed a five-year-based subgroup analysis. The results were broadly consistent with the ten-year-based results, with the difference that the incidence of elderly patients (≥70 years old) showed significant between-group variability (p=0.002), but the overall trend showed fluctuations. The results and trends were shown in Table 2-B and Figure 1.
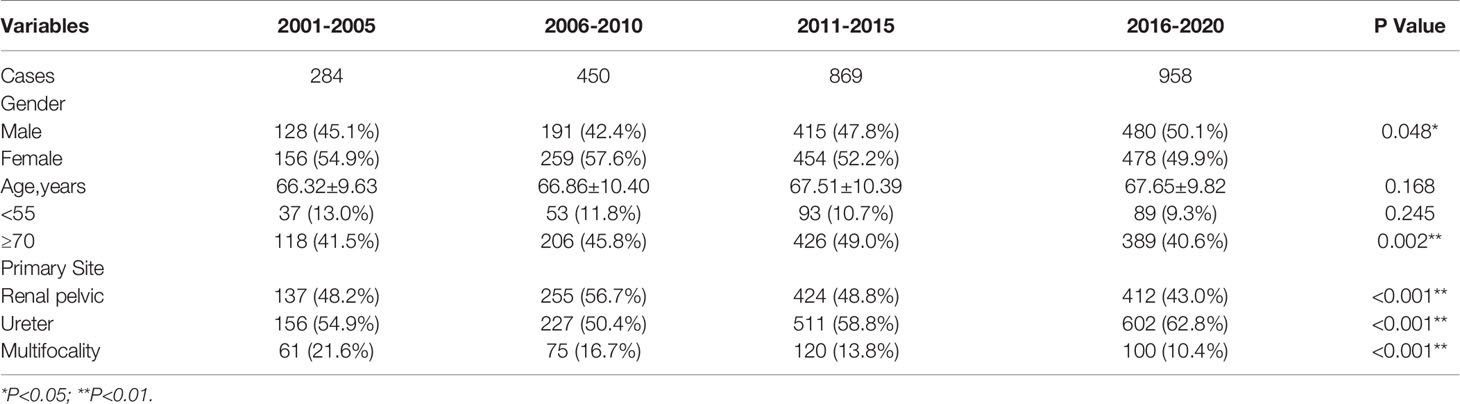
Table 2-B Five-year-based subgroup analysis of changes in age, gender composition, and primary site of the UTUC.
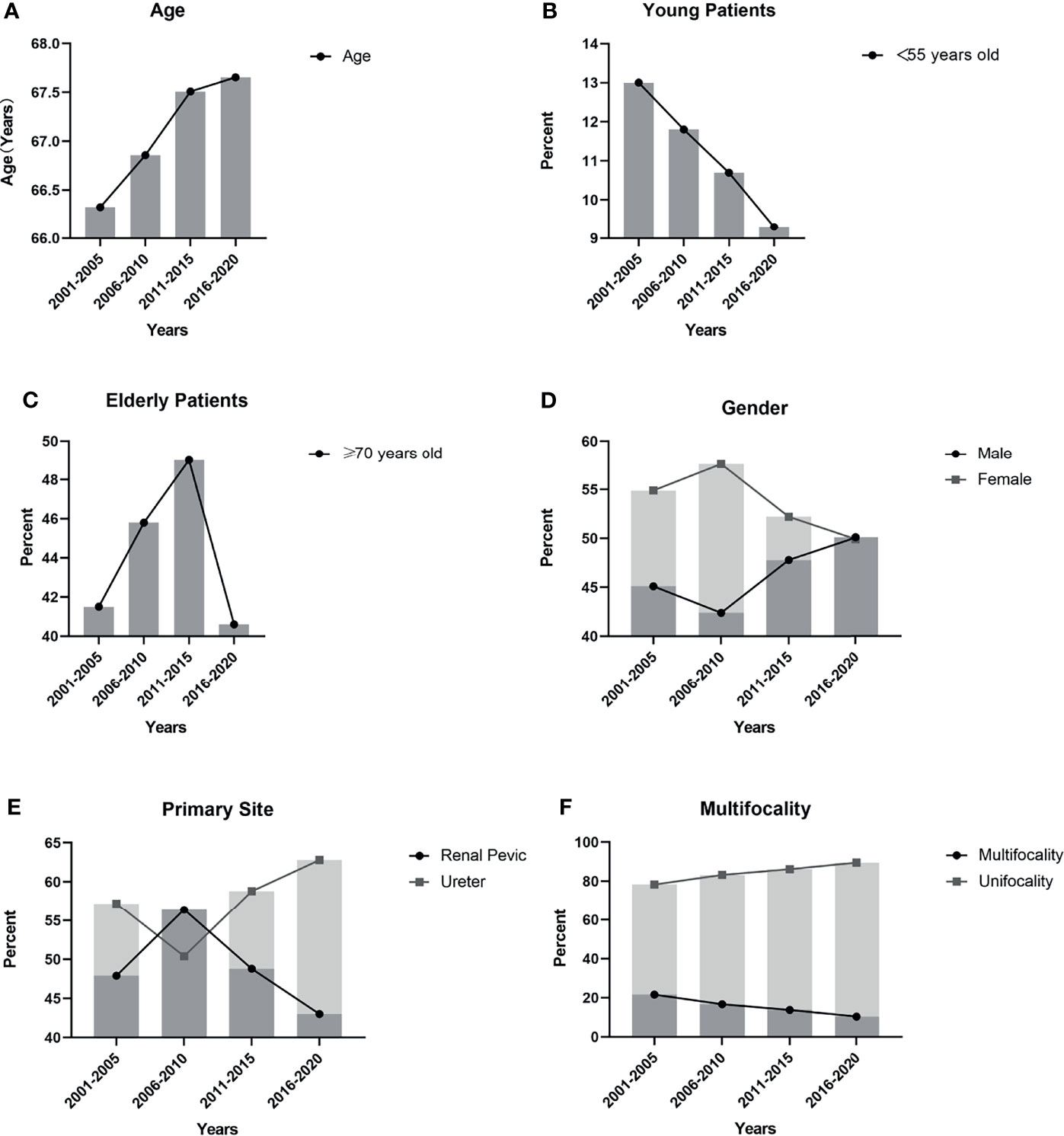
Figure 1 Trends in clinical characteristics based on 5 years: (A) The mean age of the UTUC patients gradually increased and the difference between two decades was significant (p=0.025); (B) The incidence of young patients (<55 years old) showed a gradual decrease, but there was no significant difference (p=0.245); (C) There was a significant difference (p=0.002) in the incidence rate of elderly patients (≥70 years old), but the trend fluctuated dramatically; (D) The proportion of male patients gradually declined, and the proportion of female patients gradually increased. The difference between two decades was significant (p=0.034); (E) The incidence of renal pelvic tumors and ureteral tumors and the difference between two decades was significant (p<0.001); (F) The trend showed the incidence of multifocal tumors gradually decreased and the difference between two decades was significant (p<0.001).
Tumor Stage and Histological Grading
We detailed the trends in tumor histologic T staging and grading in Tables 3-A, B.
First, we performed a subgroup analysis to investigate whether the pathological features of MICUs (muscle-invasive urothelial carcinomas, pT2+) and high-grade (G3) UTUC were associated with the clinical features of tumors. It was found that compared with the female, a higher proportion of male patients had MIUCs (54.5% versus 61.1%, p=0.001). Young patients (<55 years old, 37.9% vs 45.0%, p<0.001), patients with UTUC primarily sited in the renal pelvis (37.1% vs 50.9%, p<0.001) had a lower incidence of high-grade (G3) tumors compared with the elder patients (≥55 years old) and patients with no renal pelvic tumor. Patients with primary ureteral tumors had a higher incidence of high-grade tumors compared to non-ureteral tumors (50.7% versus 35.2%, p<0.001).
Comparing to the 1stD, significant variations in the composition of different pT-stages were seen in our study (p<0.001). To better understand the specific trends, we separately analyzed the incidence of MIUCs which has significantly decreased in the last 10 years (p<0.001). However, both the incidence of various histopathological grading of the renal pelvic tumors (p=0.385) and ureteral tumors (p=0.195) remained unchanged. Especially, the incidence of G3 tumors seemed not to change significantly between two decades (p=0.187). The evolutionary trend of pathological staging and grading depending on time were shown in Figure 2.
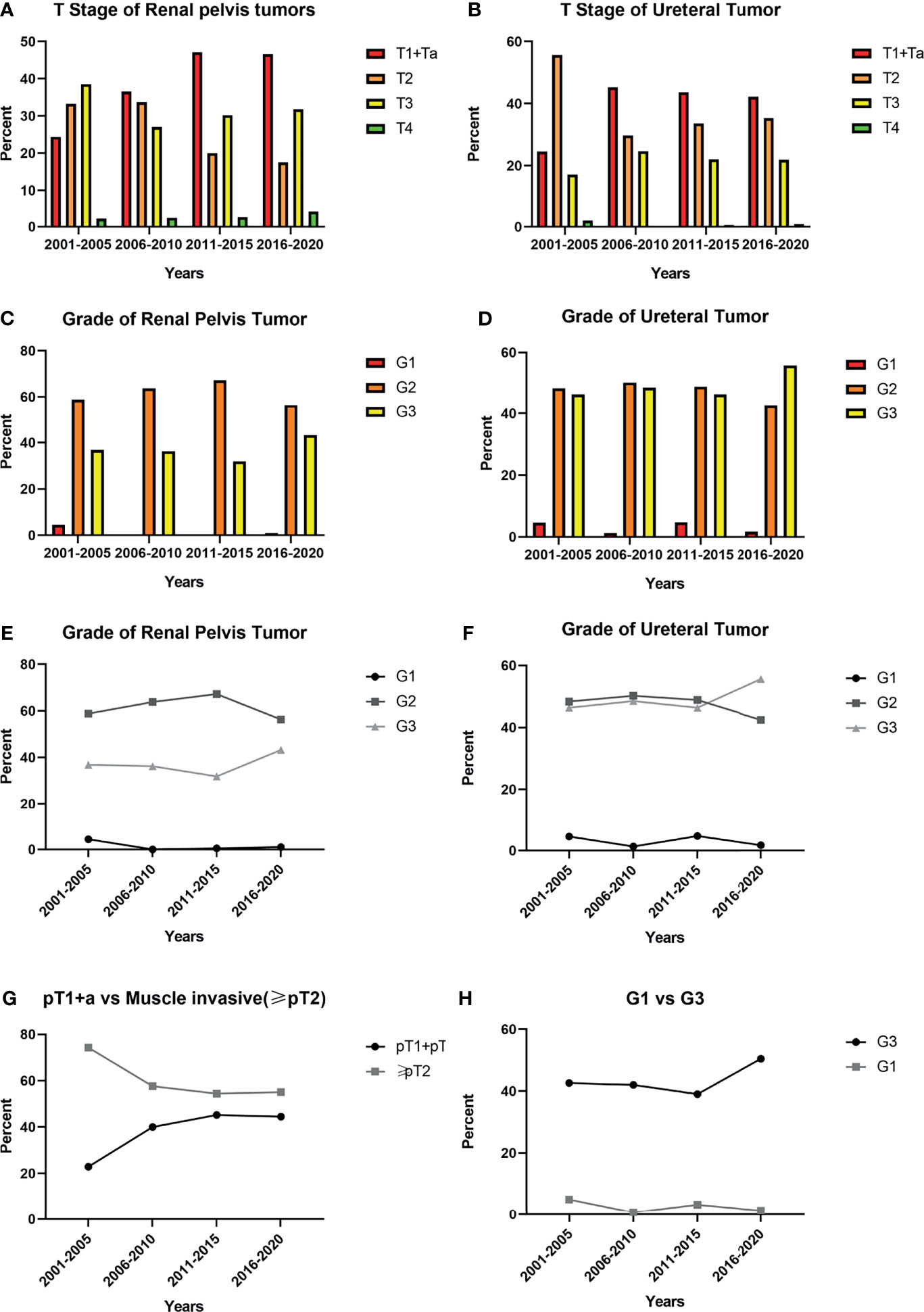
Figure 2 Trends in pathological characteristics based on 5 years: (A, B). The incidence of different pT stage of renal pelvic tumors and ureteral tumors in previous 20 years; (C, D): The incidence of different histological grading stage of renal pelvic tumors and ureteral tumors in previous 20 years; (E, F) The trend of incidence of different histological grading stage of renal pelvic tumors and ureteral tumors in previous 20 years; (G) The trend showed the incidence of muscle invasive urothelial carcinomas (MIUCs) gradually declined and the difference between two decades was significant (p<0.001); (H) The trend showed the incidence of high-grade(G3) UTUC gradually increased, but the difference between two decades was not significant (p=0.187).
Tumor Size
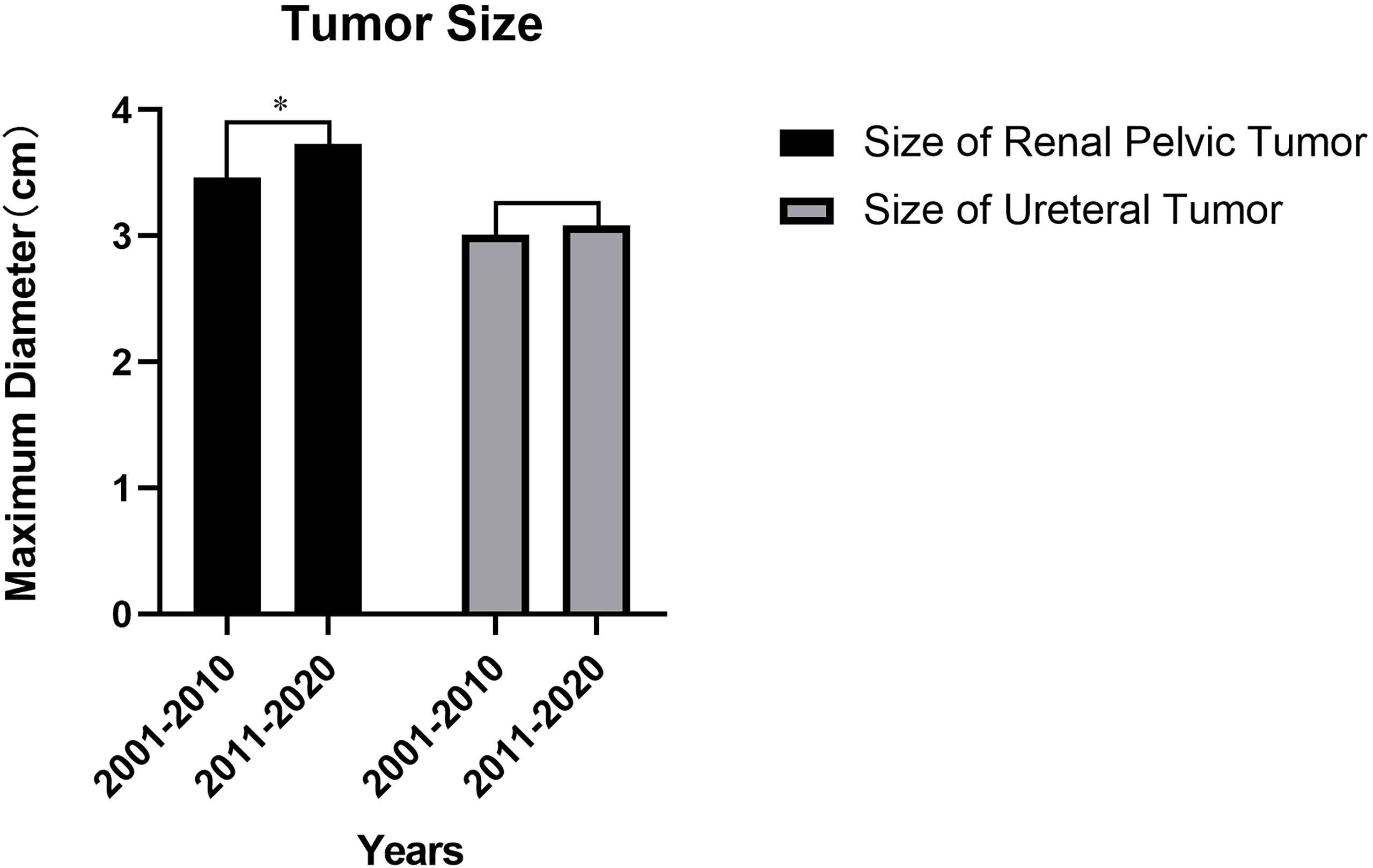
Compared to 1stD, all UTUC patients in 2ndD had an increase in the mean maximum tumor diameter before the surgery. There was a significant difference in the increase in size of renal pelvic tumors (3.46 versus 3.73, cm, p=0.043, shown in Figure 3), however, ureteral tumors remained almost unchanged (p=0.609) (Detailed change about the tumor size was shown in Tables 3-A, B).
Correlation Between Pathological Features and Prognosis
The outcome of the univariate regression analysis suggested that male gender (p<0.001), muscle invasion (pT2+, p=0.048), and G3 histopathological grade (p=0.030) significantly predicted poorer CSS. In contrast, the tumor location and multifocality did not seem to affect the UTUC patients’ survival outcome significantly. After multifactorial Cox regression analysis, both male gender (p<0.001, HR: 0.57, 95%CI: 0.43-0.76), G3 histopathological grade (p=0.013, HR:1.43, 95%CI:1.08-1.91) might be two independent risk factors for predicting worse CSS in UTUC patients (shown in Figure 4) This finding indicated that though the histological grade of tumor did not changed significantly between past two decades, the increased incidence in male patients would suggest a relatively worse survival in UTUC than before.
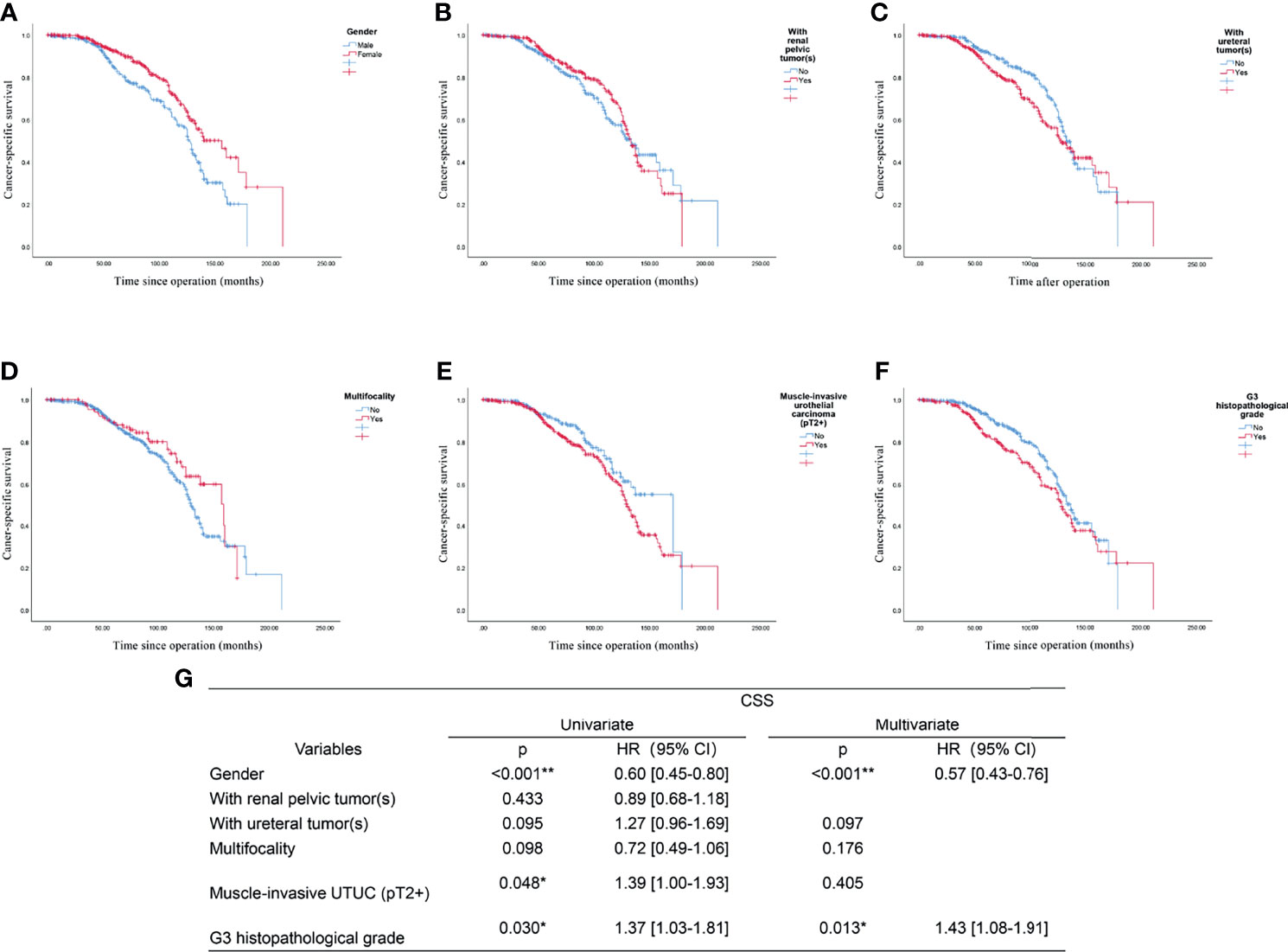
Figure 4 Prognostic analysis of the UTUC patients: the endpoint is cancer-specific survival, and the risk factor is: (A) Gender; (B) With renal pelvic tumor(s); (C) With ureteral tumor(s); (D) Multifocality; (E) Muscle-invasive urothelial carcinoma (pT2+); (F) G3 histopathological grade; (G) Univariate and multivariate analysis of all included factors. *p<0.05, **p<0.01.
Discussion
Upper tract urothelial carcinoma (UTUC) is a sort of urological malignant tumor which performs relatively low incidence but poor prognosis. Because UTUC is often insidious, multicentric, and aggressive in origin, there may be a high risk of recurrence even after timely radical surgical treatment (7). Among the many influencing factors associated with prognosis, high TNM stage of the tumor, high histological grade (G3), lymph node metastasis, multifocality, male patients, and renal pelvis tumor are notably associated with a high mortality or recurrence rate (4, 8). What is more, the pathological features of the tumors also make sense to the protocol of treatment of the patients. Although radical nephroureterectomy (RNU) remains the current gold standard procedure for localized UTUC, a proportion of patients will lose the opportunity to receive adjuvant chemotherapy due to postoperative renal insufficiency (9). Patients with low-risk UTUC (unifocal disease, non-muscle invasion, pathologic biopsy, and urine cytology suggesting low histologic grading, maximum diameter <2 cm, and with no evidence of metastasis) have the opportunity to undergo the kidney-sparing surgery, which means they could have a comparable prognosis and better quality of life (1, 10, 11). Therefore, understanding the characteristics of the natural history and pathology plays a crucial role in predicting the prognosis of patients with UTUC. Unlike the Western population, the Chinese patients of UTUC have distinctive characteristics (12). Thus, clinical treatment guidelines and assessment of prognosis for Chinese UTUC patients are always difficultly obtained by totally copy the experience of European and American countries, which deserves exploration in the future. After analysis, our study suggested that the morbidity characteristics of Chinese UTUC patients were predominantly in the middle to high age group (55-70 years old), female, with ureteral tumors, muscle invasion (pT2+), and high-grade pathology (G2, G3), which was consistent with the outcome of Singla’s (13). With the advancement of science and technology, the treatment paradigm of UTUC has been updated (14), but as far as we know, there were no studies have been conducted on the evolution of the natural history and pathological features of this disease over the past 20 years. Munoz (15) once analyzed the evolution of the incidence and survival of UTUC in the United States from 1973 to 1996, according to the National Cancer Institute Surveillance, Epidemiology and End Results (SEER) database, and concluded that the incidence of UTUC has shown a mild increase and a gradual increase in 5-disease specific survival, and hypothesized that this trend might be related to the widespread use of ureteroscopy. Raman et al. (16) similarly found a slow increase in the overall incidence of UTUC. Unlike the above two researchers, Wu (3) suggested a decreasing trend in the incidence of UTUC in the United States and a predominance of male patients, renal pelvic tumors, and patients older than 70 years. And this conclusion revealed a gradual change in the trend of UTUC pathogenesis.
In the present study, we found a significant decrease in the incidence of renal pelvic tumors, muscle-invasive and multifocal UTUC in the last 10 years, but the histological grading of the tumors remained unchanged, however, the incidence of the ureter and the size of the renal pelvic tumors showed an increasing trend. It was reported that patients with UTUC who were aware of active self-monitoring and intervene in the disease before the onset of symptoms tended to have relatively less malignant pathological features and better prognosis (17). Thus, we speculated that the increase in the proportion of early-stage tumors may be related to the increased use of imaging examination technology such as CTU and the increased awareness of the population for self-monitoring. The evolution of tumor unifocal onset, histological grade, and tumor size may be related to the daily lifestyle habits of the Chinese population such as the application of aristolochic acid drugs or the changing disease spectrum of some chronic diseases such as lithanguria and chronic kidney disease (18–20). Further trials are needed to validate the conjecture.
To evaluate whether there are any changes in the age and gender composition of current Chinese UTUC patients, we explored the trends in the proportion of different genders and the evolution of the patients into young patients group (<55 years old) and elderly patients (≥70 years old) group according to the previous studies (21). The result revealed that the proportion of male patients and the average age gradually increased over the past 20 years, but the proportion of young and elderly patients did not change significantly. Since previous studies have shown a female majority in the Chinese UTUC population, unlike in Western countries, this may be associated with the prevalence of aristolochic acid-based herbs. The current decrease in the proportion of female patients may be related to the reduction in the addition of aristolochic acid component drugs by Chinese pharmaceutical companies. At the same time, the risk factor of smoking makes the proportion of male patients appear relatively higher. Notably, we also found that male UTUC patients had significantly poorer cancer-specific survival females. This increasing-proportion trend of the male gender implies that the potential health and economic burden of UTUC on society are also increasing, which warrants early attention and preventive measures.
Based on the pathological features, the molecular characteristics of UTUC are a current research hotspot in the field of diagnosis and treatment. Despite sharing a similar histological type, it is still controversial whether the molecular features of UTUC are equivalent to bladder cancer due to the variability in clinical presentation and genomics (22). From an early understanding of the molecular mechanism of Lynch-related UTUC with mutations in MMR genes, the second-generation sequencing technology has immensely improved the efficiency of exploration now (22, 23). The discovery of molecules such as FGFR-3 has led to advances in molecular diagnosis and immunotherapy of UTUC (24). The maturation of urine-based methylation detection technology also marks the upcoming era of molecular non-invasive detection in UTUC (25). Recently, Fujii successfully classified UTUC into five subtypes based on mutation type (26). This subtype classification system may be combined with pathological diagnosis prospectively to better assist in foreseeing the prognosis of UTUC patients and selecting appropriate strategies of chemotherapy or immunotherapy. However, most of the conclusions of molecular studies still need to be validated by further clinical trials. We also expect a discovery of molecules which with higher selectivity for UTUC.
Our study still has some limits. It was a retrospective analysis, so the selective bias was relatively inevitable. And we could not reasonably infer the causes of this evolutionary trend based on the current conclusion. Secondly, the data analysis stemmed from a single-center database, one of the largest urologic oncology clinical centers in China, which meant the representativeness of the findings still needs to be validated by further refinement of the multicenter study. Finally, we selected all patients with well-established follow-up outcomes for prognostic analysis, which meant the representativeness would not be satisfied. However, the missed visits were because the patients treated were from various regions all over China, which makes it hard to return to our center and finish the follow-up. However, this situation also demonstrated that the selection bias of our study was relatively low.
Conclusion
In conclusion, UTUC in the Chinese population changed significantly in the last 20 years in patients’ age, gender composition, primary site, and multifocality. The proportion of high-T-stage UTUC was gradually decreasing and might imply an improved prognosis in general, but the size of the renal pelvic tumors seemed bigger, while there was no significant change in pathological grading. This trend may be related to the update of diagnostic techniques and self-monitoring awareness, which still needs multi-center trials to verify in the future.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of Peking University First Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
CX, CY, and CZ contributed equally to this article. CX was the first author and CY and CZ were the co-first author. JL, LQZ, and XL were the equally correspondent author of this article. All authors are accountable for all aspects of the work. Conceptualization: CX, CY, and DF. Formal analysis: CX, CY, and CZ. Investigation: CY, CX, ZL, and YW. Methodology: CX, CY, DF, XW, and XL. Project administration: LQZ, XL, CZ, and ZH. Resources: CY, YY, QT, GX, and LZ. Supervision: JL, XL, LQZ, and ZH. Visualization: CX, CY, and CZ. Writing – original draft: CX, CY, and CZ. Writing – review & editing: CX, CY, CZ, JL, LQZ, and XL. All authors approved the final article.
Funding
This study was funded by Wuxi “Taihu Talents Program” Medical and Health High-level Talents Project and Beijing Municipal Science and Technology Commission Projects (Nos. Z171100001017093).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors are grateful to all the colleagues who helped in the preparation of this article.
References
1. Roupret M, Babjuk M, Burger M, Capoun O, Cohen D, Comperat EM, et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2020 Update. Eur Urol (2021) 79:62–79. doi: 10.1016/j.eururo.2020.05.042
2. Yakoubi R, Colin P, Seisen T, Léon P, Nison L, Bozzini G, et al. Radical Nephroureterectomy Versus Endoscopic Procedures for the Treatment of Localised Upper Tract Urothelial Carcinoma: A Meta-Analysis and a Systematic Review of Current Evidence From Comparative Studies. Eur J Surg Oncol (2014) 40:1629–34. doi: 10.1016/j.ejso.2014.06.007
3. Wu J, Chen S, Wu X, Mao W, Wang Y, Xu B, et al. Trends of Incidence and Prognosis of Upper Tract Urothelial Carcinoma. Bosn J Basic Med Sci (2020) 21(5):607–19. doi: 10.17305/bjbms.2020.5345
4. Chen XP, Xiong GY, Li XS, Matin SF, Garcia M, Fang D, et al. Predictive Factors for Worse Pathological Outcomes of Upper Tract Urothelial Carcinoma: Experience From a Nationwide High-Volume Centre in China. BJU Int (2013) 112:917–24. doi: 10.1111/bju.12238
5. Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur Urol (2016) 70:93–105. doi: 10.1016/j.eururo.2016.02.029
6. Soukup V, Čapoun O, Cohen D, Hernández V, Babjuk M, Burger M, et al. Prognostic Performance and Reproducibility of the 1973 and 2004/2016 World Health Organization Grading Classification Systems in Non-Muscle-Invasive Bladder Cancer: A European Association of Urology Non-Muscle Invasive Bladder Cancer Guidelines Panel Systematic Review. Eur Urol (2017) 72:801–13. doi: 10.1016/j.eururo.2017.04.015
7. Krabbe LM, Eminaga O, Shariat SF, Hutchinson RC, Lotan Y, Sagalowsky AI, et al. Postoperative Nomogram for Relapse-Free Survival in Patients With High Grade Upper Tract Urothelial Carcinoma. J Urol (2017) 197:580–9. doi: 10.1016/j.juro.2016.09.078
8. Azémar MD, Comperat E, Richard F, Cussenot O, Rouprêt M. Bladder Recurrence After Surgery for Upper Urinary Tract Urothelial Cell Carcinoma: Frequency, Risk Factors, and Surveillance. Urol Oncol (2011) 29:130–6. doi: 10.1016/j.urolonc.2009.06.003
9. Fang D, Zhang Q, Li X, Qian C, Xiong G, Zhang L, et al. Nomogram Predicting Renal Insufficiency After Nephroureterectomy for Upper Tract Urothelial Carcinoma in the Chinese Population: Exclusion of Ineligible Candidates for Adjuvant Chemotherapy. BioMed Res Int (2014) 2014:1–10. doi: 10.1155/2014/529186
10. Seisen T, Peyronnet B, Dominguez-Escrig JL, Bruins HM, Yuan CY, Babjuk M, et al. Oncologic Outcomes of Kidney-Sparing Surgery Versus Radical Nephroureterectomy for Upper Tract Urothelial Carcinoma: A Systematic Review by the EAU Non-Muscle Invasive Bladder Cancer Guidelines Panel. Eur Urol (2016) 70:1052–68. doi: 10.1016/j.eururo.2016.07.014
11. Metcalf M, Pierorazio PM. Future Strategies to Enhance Kidney Preservation in Upper Urinary Tract Urothelial Carcinoma. Transl Androl Urol (2020) 9:1831–40. doi: 10.21037/tau.2019.11.09
12. Fang D, Singla N, Bao Z, Jafri SM, Su X, Cao Z, et al. The Significance of Preoperative Serum Sodium and Hemoglobin in Outcomes of Upper Tract Urothelial Carcinoma: Multi-Center Analysis Between China and the United States. Cancer Manag Res (2020) 12:9825–36. doi: 10.2147/CMAR.S267969
13. Singla N, Fang D, Su X, Bao Z, Cao Z, Jafri SM, et al. A Multi-Institutional Comparison of Clinicopathological Characteristics and Oncologic Outcomes of Upper Tract Urothelial Carcinoma in China and the United States. J Urol (2017) 197:1208–13. doi: 10.1016/j.juro.2016.11.094
14. Leow JJ, Liu Z, Tan TW, Lee YM, Yeo EK, Chong YL. Optimal Management of Upper Tract Urothelial Carcinoma: Current Perspectives. Onco Targets Ther (2020) 13:1–15. doi: 10.2147/OTT.S225301
15. Munoz JJ, Ellison LM. Upper Tract Urothelial Neoplasms: Incidence and Survival During the Last 2 Decades. J Urol (2000) 164:1523–5. doi: 10.1016/S0022-5347(05)67019-X
16. Raman JD, Messer J, Sielatycki JA, Hollenbeak CS. Incidence and Survival of Patients With Carcinoma of the Ureter and Renal Pelvis in the USA 1973-2005. BJU Int (2011) 107:1059–64. doi: 10.1111/j.1464-410X.2010.09675.x
17. Fang D, Gong YQ, Singla N, Yang KL, Xiong GY, Zhang L, et al. The Significance of the Initial Symptom in Chinese Patients With Upper Tract Urothelial Carcinoma: Regular Health Examination is Still Underutilized. Kaohsiung J Med Sci (2018) 34:511–21. doi: 10.1016/j.kjms.2018.01.003
18. Jeroen VDP, Piet V. Kidney Stones and the Risk of Renal Cell Carcinoma and Upper Tract Urothelial Carcinoma: The Netherlands Cohort Study. Br J Cancer (2019) 120(3):368–74. doi: 10.1038/s41416-018-0356-7
19. Bao Z, Du Y, Yuan Y, Zhu Y, Qian C, Zhan Y, et al. Prevalence, Clinicopathological Features, and Prognosis in Upper Tract Urinary Carcinoma Patients With Severe Preoperative Chronic Kidney Disease. Transl Androl Urol (2019) 8:641–50. doi: 10.21037/tau.2019.11.19
20. Han J, Xian Z, Zhang Y, Liu J, Liang A. Systematic Overview of Aristolochic Acids: Nephrotoxicity, Carcinogenicity, and Underlying Mechanisms. Front Pharmacol (2019) 10:648. doi: 10.3389/fphar.2019.00648
21. Cao Z. Clinicopathological Features and Prognosis of Young Patients With Upper Tract Urothelial Carcinoma. J Mod Urol (2017) 22:661–5. doi: 10.3969/j. issn. 1009-8291.2017.09.004
22. Hassler MR, Bray F, Catto JWF, Grollman AP, Hartmann A, Margulis V, et al. Molecular Characterization of Upper Tract Urothelial Carcinoma in the Era of Next-Generation Sequencing: A Systematic Review of the Current Literature. Eur Urol (2020) 78:209–20. doi: 10.1016/j.eururo.2020.05.039
23. Harper HL, Mckenney JK, Heald B, Stephenson A, Campbell SC, Plesec T, et al. Upper Tract Urothelial Carcinomas: Frequency of Association With Mismatch Repair Protein Loss and Lynch Syndrome. Mod Pathol (2017) 30:146–56. doi: 10.1038/modpathol.2016.171
24. Robinson BD, Vlachostergios PJ, Bhinder B, Liu W, Li K, Moss TJ, et al. Upper Tract Urothelial Carcinoma has a Luminal-Papillary T-Cell Depleted Contexture and Activated FGFR3 Signaling. Nat Commun (2019) 10:2977. doi: 10.1038/s41467-019-10873-y
25. Guo RQ, Xiong GY, Yang KW, Zhang L, He SM, Gong YQ, et al. Detection of Urothelial Carcinoma, Upper Tract Urothelial Carcinoma, Bladder Carcinoma, and Urothelial Carcinoma With Gross Hematuria Using Selected Urine-DNA Methylation Biomarkers: A Prospective, Single-Center Study. Urol Oncol (2018) 36:342.e315–42.e323. doi: 10.1016/j.urolonc.2018.04.001
Keywords: evolution, clinicopathological diagnostic features, upper tract urothelial carcinoma (UTUC), Chinese population, 20 years
Citation: Xu C, Yuan C, Zhang C, Fang D, Yu Y, Wang X, Li Z, Wang Y, Tang Q, Xiong G, Zhang L, He Z, Lin J, Zhou L and Li X (2022) The Evolution of Clinicopathological Diagnostic Features of Upper Tract Urothelial Carcinoma in China: A Summary of 2561 Cases in the Last 20 Years. Front. Oncol. 12:769252. doi: 10.3389/fonc.2022.769252
Received: 01 September 2021; Accepted: 07 February 2022;
Published: 09 March 2022.
Edited by:
Yige Bao, Sichuan University, ChinaReviewed by:
Jun Pang, Sun Yat-sen University, ChinaDi Gu, First Affiliated Hospital of Guangzhou Medical University, China
Copyright © 2022 Xu, Yuan, Zhang, Fang, Yu, Wang, Li, Wang, Tang, Xiong, Zhang, He, Lin, Zhou and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Lin, bGluamlhbmJqQDE2My5jb20=; Liqun Zhou, emhvdWxxbWFpbEBzaW5hLmNvbQ==; Xuesong Li, cGluZW5lZWRsZUBzaW5hLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Chunru Xu
Chunru Xu Changwei Yuan
Changwei Yuan Cuijian Zhang1,2,3†
Cuijian Zhang1,2,3† Dong Fang
Dong Fang Gengyan Xiong
Gengyan Xiong Jian Lin
Jian Lin Xuesong Li
Xuesong Li