- Department of Urology, Peking University Third Hospital, Beijing, China
Objectives: To demonstrate the progression-free survival (PFS) of nonmetastatic renal cell carcinoma (RCC) patients with venous thrombus after radical nephrectomy and venous thrombectomy (RN-VT) and to develop and validate a nomogram to predict the PFS of patients after RN-VT.
Materials and Methods: We reported our prospective follow-up data of RCC patients with venous thrombus from January 2014 to September 2020 (n = 199). We used the Kaplan–Meier method to assess the PFS. The Cox proportional hazards regression model was used to determine the predictors. Nomograms predicting the PFS was established, and external validation was performed. Calibration curves and decision curves were generated to assess the predictive efficacy and clinical benefit.
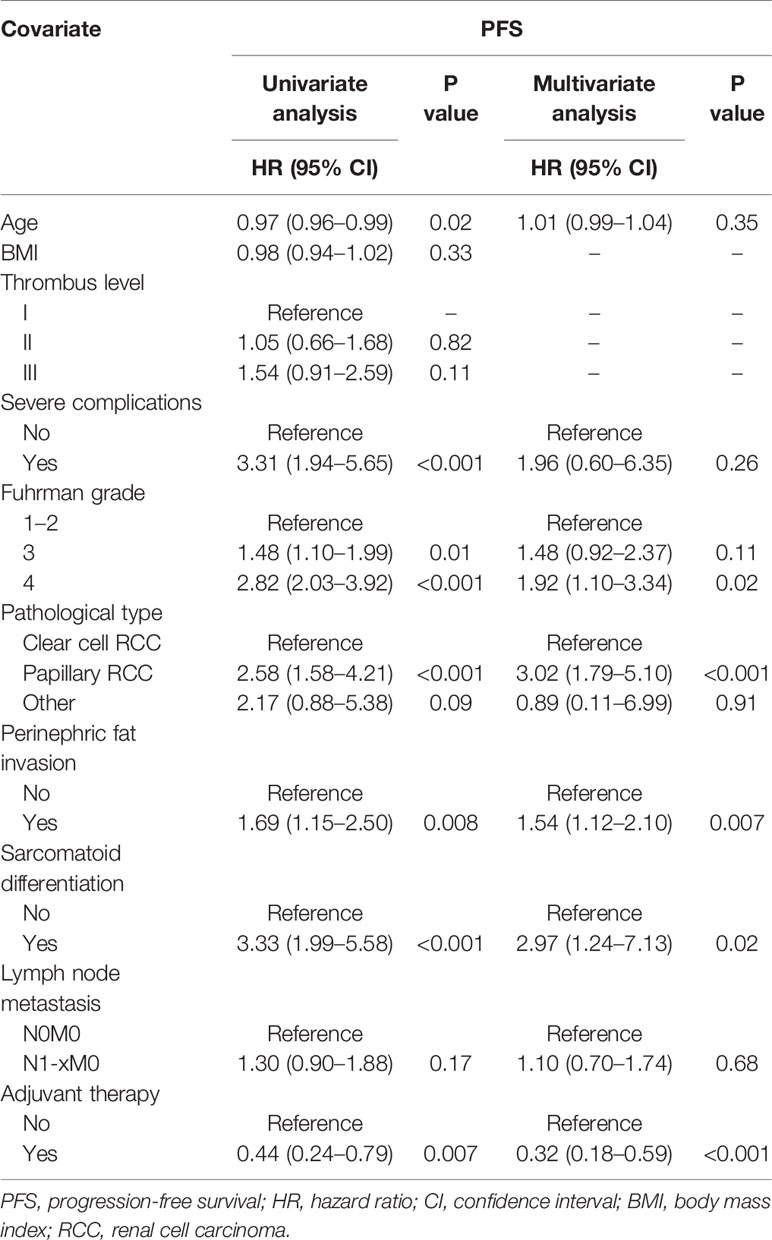
Results: After a median follow-up of 32 months, 79 patients (39.7%) had disease progression and the median PFS was 41.0 months (95% CI 34.8–53.2 months). The 1-year, 3-year, and 5-year PFS rates were 78.4%, 45.4%, and 30.0%, respectively. Multivariate analysis showed that Fuhrman grade [grade 4: hazard ratio (HR) 1.92, 95% CI 1.10–3.34, P = 0.02], pathological type (papillary RCC: HR 3.02, 95% CI 1.79–5.10, P < 0.001), perinephric fat invasion (HR 1.54, 95% CI 1.12–2.10, P = 0.007), sarcomatoid differentiation (HR 2.97, 95% CI 1.24–7.13, P = 0.02) were associated with a worse PFS, and adjuvant therapy (HR 0.32, 95% CI 0.18–0.59, P < 0.001) could lead to a better PFS. A nomogram based on the predictors was externally validated to have good discrimination and calibration, and it could improve PFS prediction to obtain a clinical benefit.
Conclusions: We constructed and validated a nomogram to predict the 1-year, 3-year, and 5-year PFS of M0 RCC patients with venous thrombus after surgery. The model can help identify patients who can benefit the most from surgery and develop the criteria for clinical trial enrollment.
Introduction
Radical nephrectomy and venous thrombectomy (RN-VT) has been the curative option for renal cell carcinoma (RCC) patients with venous thrombus (1–3). Previous studies focusing on the survival of such patients reported that the 5-year cancer-specific survival could be 46.0%–53.4%, and the 5-year overall survival could be 39%–42.2% after RN-VT (2, 4–6). These findings supported the benefit of RN-VT in this patient group. However, the 3-year recurrence-free survival was reported to be only 35.9% for M0 patients with thrombus extending above the hepatic veins (6). This highlighted the high recurrence risk nature in such patients and reminded us of the disease progression during follow-up.
To date, no systematic effort has been made to assess the progression-free survival (PFS) of M0 RCC patients with venous thrombus after surgery. In the current study, we aimed to report the PFS based on the prospective follow-up data and to assess the prognostic factors in M0 RCC patients with venous thrombus. Besides that, we endeavored to develop and validate a model to better predict the PFS. We hypothesized that our nomogram could help predict individual PFS probability with good discrimination and calibration and lead to a clinical benefit.
Materials and Methods
Study Cohort and Design
We have been building the Peking University Third Hospital Thrombus Database (PUTH-TD) since January 2014 after obtaining the institutional review board approval. Our study cohort contained RCC patients with venous thrombus from January 2014 to September 2020, and all patients were prospectively followed up to March 2021. The inclusive criteria were as follows: (1) patients with pathologically confirmed RCC; (2) patients treated with surgical procedures. The patients with a minimum follow-up of less than 6 months and distant metastasis were excluded. A total of 199 patients were enrolled in the study. All procedures were performed by experienced surgeons. Adjuvant therapy is one of the comprehensive therapeutic methods of cancer, including cytokine therapy, radiotherapy, or targeted therapy. All the patients at our institution were recommended to receive adjuvant therapy unless the patients could not tolerate the toxicity of adjuvant therapy due to severe complications or poor postoperative physical condition.
Outcomes and Definitions
The primary endpoint was the PFS after surgery. Progression was defined as local recurrence (tumor recurrence in or abutting the previous surgical bed), distant metastasis progression (new lesions in other organs, brain, lung, liver, bone, et al.), or death from tumor after surgery. PFS was defined as the time from surgery to the progression event. The local recurrence and distant metastasis were evaluated based on computed tomography (CT) or magnetic resonance imaging (MRI).
Local symptoms were defined as a palpable mass, pain, and gross hematuria. Patients with edema, fever, swelling, fatigue, and weight loss were thought to have systemic symptoms. The American Society of Anesthesiologists Physical Status classification system (ASA level) (7) was introduced to estimate the operative risk. Complications were graded according to the Clavien–Dindo grading system (8). Severe complication was defined as Clavien–Dindo grade above II.
We classified the thrombus into three levels: 1) level I, renal vein thrombus (Mayo 0); 2) level II, thrombus extending into the renal vein but below the intrahepatic vena cava (Mayo I and II); and 3) level III, thrombus extending into the intrahepatic vena cava or even into the right atrium (Mayo III and IV) (1, 9). The histological diagnosis of renal tumors was based on the World Health Organization (WHO) classification (2004 and 2016 version) (10, 11). The Fuhrman system was applied to RCC nuclear grading (12). A sarcomatoid differentiation was defined as RCC accompanied by histological appearance of spindle-cell sarcoma. The 2017 version of the tumor-node-metastasis (TNM) classification was used for clinical staging based on postoperative pathological specimens.
Follow-Up Protocol
Two full-time clinical data managers had all access to the database and performed the follow-up. We provided the same follow-up plan to all patients, and follow-up data were prospectively collected (symptoms and signs, laboratory tests, imaging examination of the chest, abdomen, and pelvis). The laboratory tests included routine blood test and blood biochemical test. The imaging examination included CT, MRI, and X-ray. Patients were followed up every 3 months after surgery in the first year, then 6 months to the third year, then annually thereafter. Except for the routine review after surgery, the data managers conducted telephone interviews every 3–6 months and collected the follow-up information to reduce the withdraw bias.
Nomogram Construction and External Validation
Nomograms were built based on the predictors determined by Cox proportional hazards regression analysis, and the C-index was calculated to assess the discrimination of the model. We calculated the total points of each patient in the validation cohort according to the nomogram established based on the training cohort. The total point in the validation cohort was used as a factor in the Cox regression analysis, and the C-index and calibration curve were derived according to the regression analysis. We randomly grouped patients in a ratio of 3:1 to determine the training cohort and validation cohort.
Statistical Analysis
Baseline characteristics were shown for categorical variables and continuous variables. Non-normally distributed continuous variables were reported as medians and interquartile ranges, and normally distributed continuous variables were reported as means and standard deviations. We reported the categorical variables as frequencies and proportions. The chained multiple imputation was used to resolve the missing data.
We used the Kaplan–Meier method to perform PFS analysis. Cox proportional hazards regression analysis was used to estimate the predictors of PFS, which should satisfy the proportional hazards assumptions first. Calibration curves and decision curves were generated to assess the calibration and clinical benefit.
All statistical tests were performed by SPSS version 25.0 (IBM, Armonk, NY, USA) and the R statistics package version 4.1.0 (R Project for Statistical Computing, www.r-project.org). All tests were two-sided, and the significance level was set at P < 0.05.
Results
Baseline Characteristics and Outcomes of Study Cohort
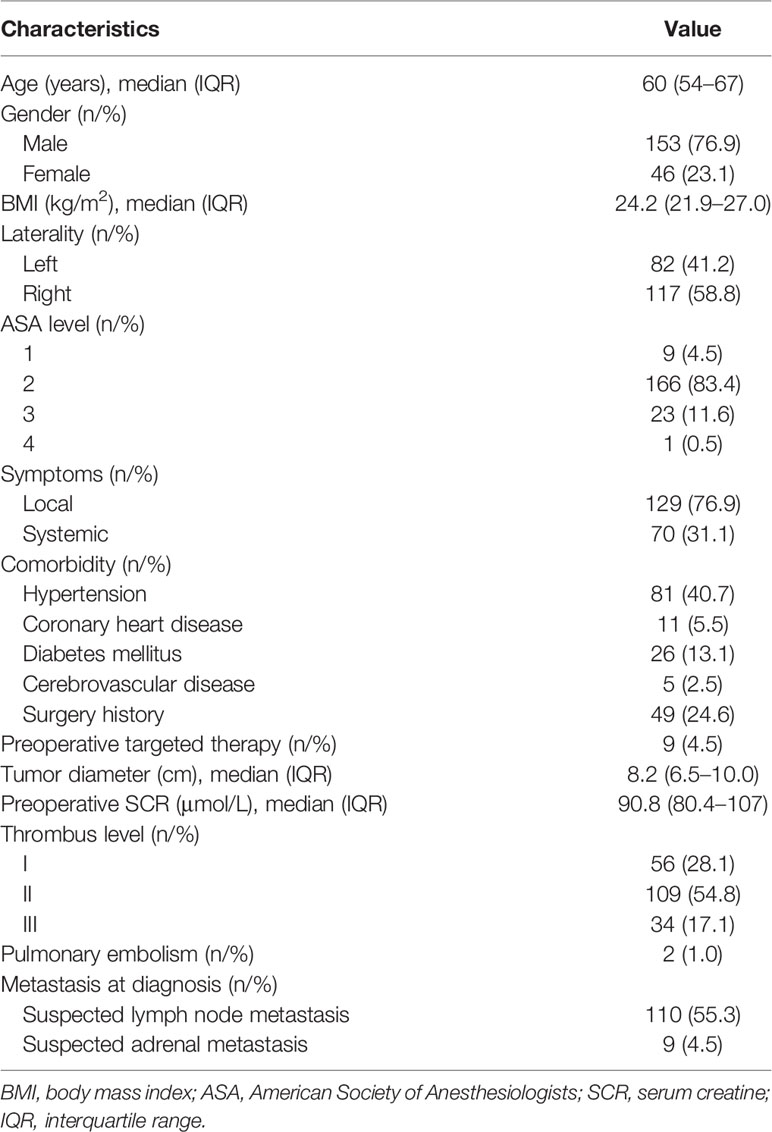
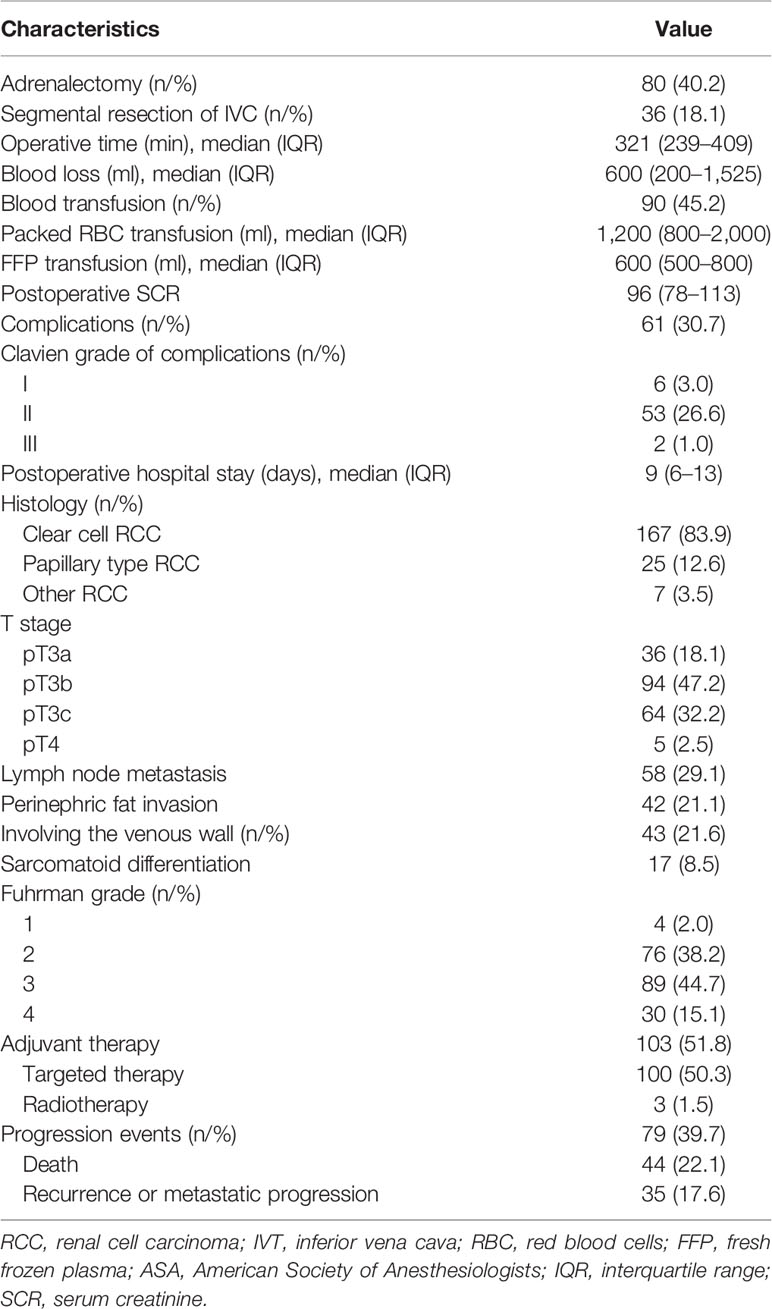
Patient characteristics are listed in Table 1. A total of 199 patients with RCC and venous thrombus formed the study cohort, including 56 patients (28.1%) with level I thrombus, 109 patients (54.8%) with level II thrombus, and 34 patients (17.1%) with level III thrombus. Among them, 79 patients (39.7%) had progression events, including death (n = 44), local recurrence or distant metastasis progression (n = 35). Of the 199 patients, 167 patients (83.9%) had clear cell RCC, 25 patients (12.6%) had papillary RCC, and 7 patients (3.5%) had other RCC types. Lymph node metastasis was confirmed in 58 patients (29.1%), and perinephric fat invasion was found in 42 patients (21.1%). The venous wall was involved in 43 patients (21.6%), and 36 patients (18.1%) received segmental resection of Inferior vena cava (IVC). The number of Fuhrman grade I, II, III, and IV RCC patients was 4 (2.0%), 76 (38.2%), 89 (44.7%), and 30 (15.1%), respectively. A total of 103 patients (51.8%) received adjuvant therapy, 39 patients (19.6%) did not receive adjuvant therapy due to poor postoperative physical condition or severe complications, and 57 patients (28.6%) refused it due to high cost. The surgical, pathological, and oncologic outcomes were shown in Table 2.
Progression-Free Survival
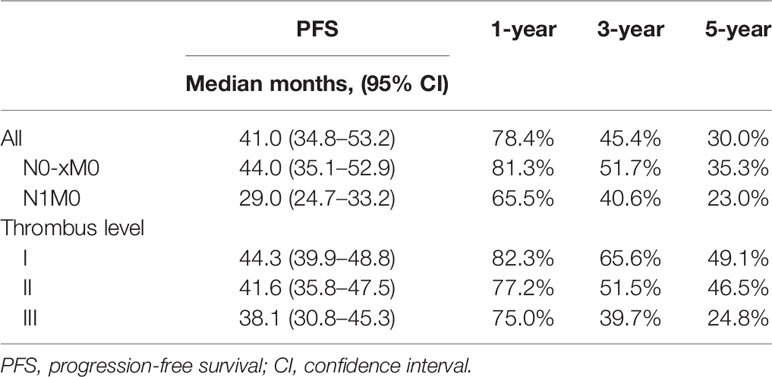
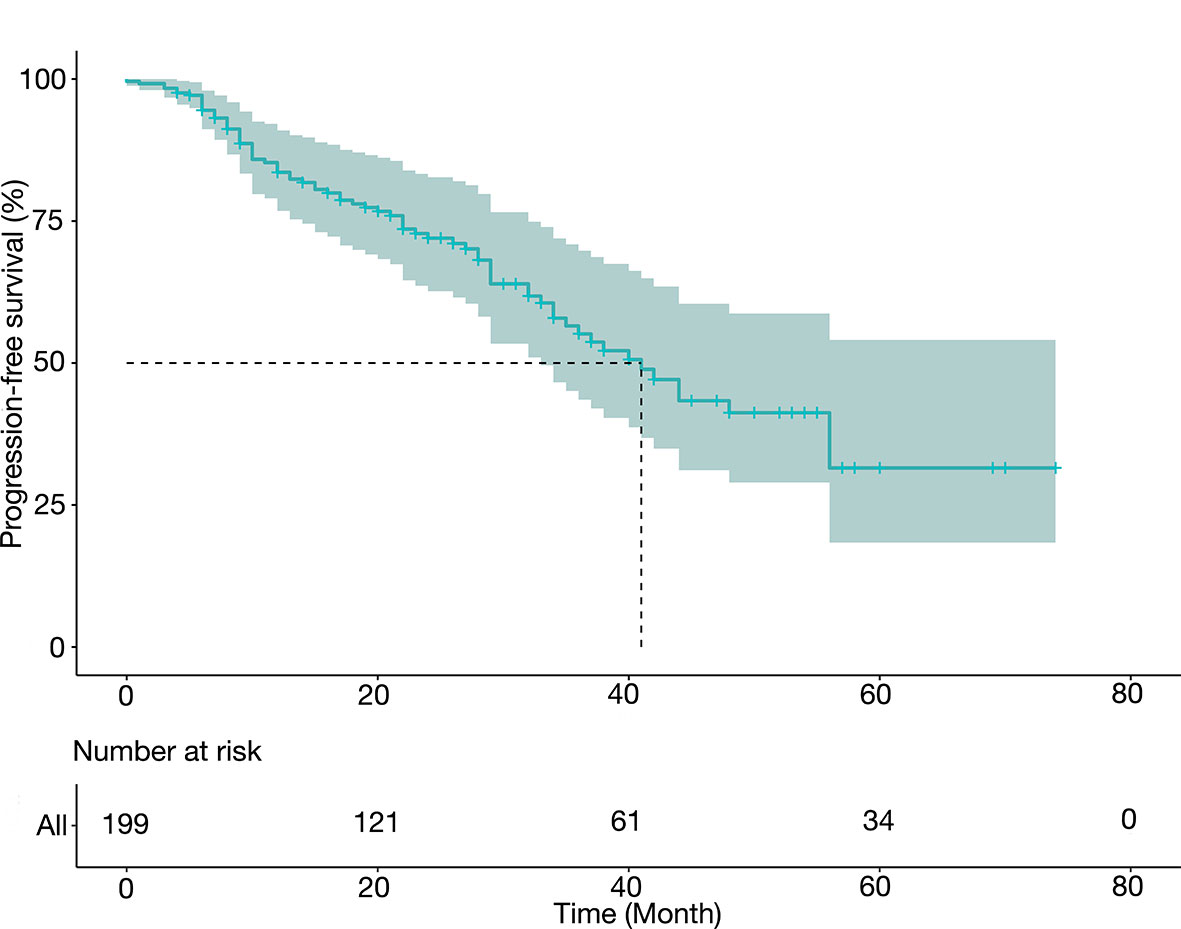
Table 3 summarized the PFS data and presented them in groups. After a median follow-up of 32 months, the median PFS was 41.0 months (34.8–53.2 months), and the 1-year, 3-year, and 5-year PFS rates were 78.4%, 45.4%, and 30.0%, respectively. The median PFS was 44.3 months (95% CI 39.9–48.8 months) for level I thrombus, 41.6 months (95% CI 35.8–47.5 mon) for level II thrombus, and 38.1 months (95% CI 30.8–45.3 months) for level III thrombus. Figure 1 depicted the PFS curve.
Univariate and Multivariate Analyses of Progression-Free Survival
Though some predictors were found in univariate analysis, multivariate analysis showed that only Fuhrman grade (grade 4, HR 1.92, 95% CI 1.10–3.34, P = 0.02), pathological type (papillary RCC, HR 3.02, 95% CI 1.79–5.10, P < 0.001), perinephric fat invasion (HR 1.54, 95% CI 1.12–2.10, P = 0.007), sarcomatoid differentiation (HR 2.97, 95% CI 1.24–7.13, P = 0.02), and adjuvant therapy were associated with PFS (Table 4).
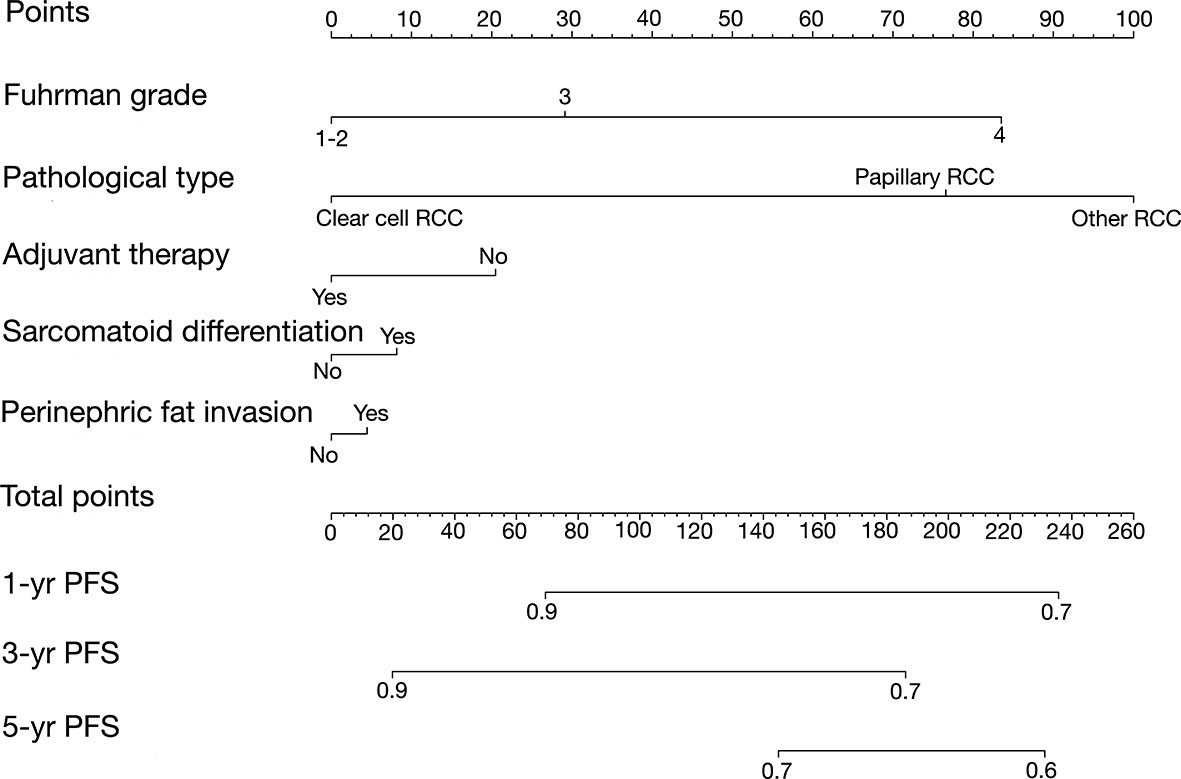
Development and External Validation of Nomogram Predicting Progression-Free Survival
For the construction of the PFS model, patients were split randomly into the training cohort (n = 148) and validation cohort (n = 51). We developed the Peking University nomogram (PKUN) based on the predictors identified in the multivariate analysis to predict the 1-year, 3-year, and 5-year PFS after RN-VT. The predictive C-indexes for 1-year, 3-year, and 5-year PFS were 0.83, 0.77, and 0.78 (Figure 2).
On each calibration plot, the predicted PFS probability is represented on the x-axis, and the actual risk of progression is represented on the y-axis. The 45° line indicates perfect agreement between predicted probability and observed risk. The externally validated calibration plots demonstrated virtually overlapping the 45° line of progression (Figures 3A–C). The decision curve analysis demonstrated the net clinical benefit originating from applying this model with probabilities >1% for 1-year PFS, >25% for 3-year PFS, and >40% for 5-year PFS (Figure 4).
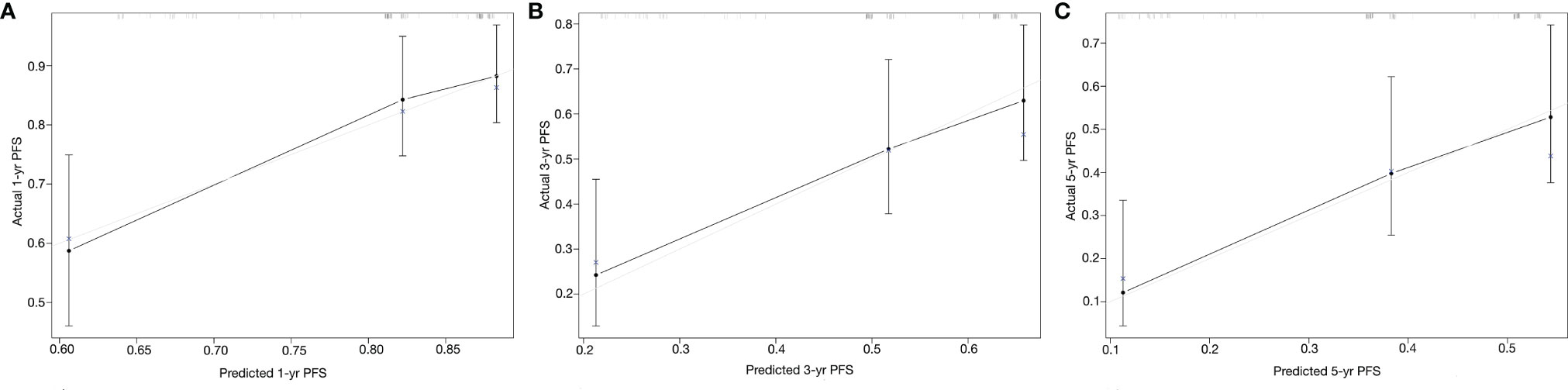
Figure 3 Calibration curves of the Peking University nomogram (PKUN) model. (A) for 1-year PFS, (B) for 3-year PFS, and (C) for 5-year PFS.

Figure 4 Decision curves of the Peking University nomogram (PKUN) model. (A) for 1-year PFS, (B) for 3-year PFS, and (C) for 5-year PFS.
Discussion
Progression of RCC patients with venous thrombus has been rarely evaluated, though the long-term survival was confirmed to be acceptable (2, 4, 6). Here, we reported that such M0 patients had a median PFS of 41 months, and that the 5-year PFS was only 30.0%. To provide an individualized PFS prediction, we developed the PKUN model and validated that the PKUN model had good discrimination and calibration. We anticipated that the PKUN model would be useful for patient counseling, treatment decision-making, and enrollment of clinical trials in the RCC and venous thrombus population.
Several studies evaluated the overall survival or cancer-specific survival of RCC patients with venous thrombus (4, 6, 13). However, the PFS was less reported. Reese et al. (14) once reported that the median PFS was only 5 months and the 1-year PFS was 29.0% in untreated RCC patients with venous thrombus. Rigaud et al. (2) reported that the 5-year PFS was 8.9% in 40 patients receiving surgery after a median follow-up of 28.5 months. In our study, the 5-year PFS was 30.0% for the entire cohort. The following reasons could explain the higher 5-year PFS at our institution. Firstly, the proportion of nuclear grade III and IV was much higher in their cohort (90% vs. 69.8%). Next, 47.5% of the patients in their study had perinephric fat invasion, higher than 21.1% of our cohort. Lastly, the proportion of patients receiving adjuvant therapy was 51.8% in our cohort. That might help reduce the risk of disease progression.
Individual prediction of PFS of patients after surgery is important to perform precise adjuvant therapy. To better evaluate the risk of progression, we developed and validated the PKUN model. Our nomogram could be used to inform RCC patients with venous thrombus the necessity of adjuvant therapy. Several aspects of our nomogram were noteworthy. First, our result showed that thrombus level was not a prognostic factor of PFS. Actually, the predictive value of thrombus level on long-term survival was still controversial (9, 15, 16). However, Abel et al. (17) reported that thrombus level was an independent predictor of recurrence-free survival in M0 patients. We thought that the exact association between thrombus level and survival should be further studied. Second, we demonstrated that patients with higher Fuhrman grade, perinephric fat invasion, sarcomatoid differentiation or non-clear cell RCC had higher risk of PFS. These variables were also validated to be associated with overall survival or cancer-specific survival in previous studies (4, 5). Last, we confirmed the prognostic value of adjuvant therapy in PFS and the exact effect of adjuvant therapy on PFS need be evaluated in the future.
To the best of our knowledge, The PKUN model was the first nomogram predicting PFS in M0 RCC patients with venous thrombus. Abel et al. (18) once developed a nomogram predicting recurrence following surgery in M0 RCC patients with venous thrombus. Compared to their model, the PKUN model predicted not only the recurrence risk of M0 patients but also the risk of death. When recurrence or metastatic progression occurred, adjuvant therapy or salvage therapy was usually necessary. We believed that our nomogram could be used for enrollment assessment in future adjuvant therapy clinical trials. Actually, whether the application of PKUN model could lead to clinical benefit was a major concern. We performed the decision curve analysis and confirmed that PKUN could result in net benefit when the cutoff was 1% for 1-year PFS, 25% for 3-year PFS, and 40% for 5-year PFS.
From a clinical standpoint, the PKUN model improves the ability to identify patients with a higher risk of progression after surgery. Its implementation could help determine which kind of patients need active intervention and closer follow-up. However, the consistency between the predicted risk and the actual risk was another concern. In our study, we drew the calibration plots to assess the extent of overestimation or underestimation associated with PKUN use. The externally validated calibration curves of predicted probability against the observed PFS indicated excellent concordance. Besides that, the C-indexes showed that PKUN had good discrimination in clinical practice.
Distinct facets of our results deserve attention. First, our results showed that variables such as Fuhrman grade or pathological type were predictors of PFS, but not the thrombus level. These observations indicated that the inherent cancer characteristics were associated with disease progression. Second, we confirmed that patients receiving adjuvant therapy had a better PFS, though Gu et al. (19) reported in a prospective cohort study that adjuvant therapy showed no benefit in PFS for M0 RCC patients with venous thrombus. Last, although lymph node metastasis was not a predictive variable, we found that patients with lymph node metastasis had a worse PFS than N0M0 patients. The worse PFS for N1 patients indicated that rigorous follow-up and adjuvant therapy were necessary to improve the survival.
Our study has strengths in the prospective follow-up design and prospective data collection. In addition, the study period is from January 2014 to March 2021, and it can represent the current clinical practice, especially the comprehensive therapy based on surgical treatment. Despite several strengths, our study is not devoid of limitations. The first is its single-center experience nature, and it contains a relatively small number of patients. A multicenter prospective follow-up covering sufficient data is needed to better evaluate the PFS. Furthermore, a relatively shorter follow-up time limited the observation of progression events. This study would definitely benefit from a longer follow-up.
Conclusion
We constructed and validated the PKUN model to predict the 1-year, 3-year, and 5-year PFS of M0 RCC patients with venous thrombus after surgery. The model can help counsel patients and identify patients who can benefit the most from surgery and develop the criteria for clinical trial enrollment.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Ethics Statement
This study receives ethics approval from Peking University Third Hospital Ethics Committee.
Author Contributions
YZ: Data collection/project development/data analysis/article writing, XJT Project development/critical revision, HB Project development/data analysis/article writing, YY Project development, ZL Data collection/project development, SDZ Project development/critical revision, CL Project development/critical revision, LLM Project development/critical revision. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (81972381).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank all the staff of the Department of Urology in the Peking University Third Hospital. We also thank Mr. JiaJu Huang, who was the founder of BEYOND band. His music has inspired us to pursue the truth, freedom, and essential nature of science in the past 20 years.
References
1. Blute ML, Leibovich BC, Lohse CM, Cheville JC, Zincke H. The Mayo Clinic Experience With Surgical Management, Complications and Outcome for Patients With Renal Cell Carcinoma and Venous Tumour Thrombus. BJU Int (2004) 94(1):33–41. doi: 10.1111/j.1464-410X.2004.04897.x
2. Rigaud J, Hetet JF, Braud G, Battisti S, Le Normand L, Glemain P, et al. Surgical Care, Morbidity, Mortality and Follow-Up After Nephrectomy for Renal Cancer With Extension of Tumor Thrombus Into the Inferior Vena Cava: Retrospective Study Since 1990s. Eur Urol (2006) 50(2):302–10. doi: 10.1016/j.eururo.2006.02.065
3. Lardas M, Stewart F, Scrimgeour D, Hofmann F, Marconi L, Dabestani S, et al. Systematic Review of Surgical Management of Nonmetastatic Renal Cell Carcinoma With Vena Caval Thrombus. Eur Urol (2016) 70(2):265–80. doi: 10.1016/j.eururo.2015.11.034
4. Ciancio G, Manoharan M, Katkoori D, De Los Santos R, Soloway MS. Long-Term Survival in Patients Undergoing Radical Nephrectomy and Inferior Vena Cava Thrombectomy: Single-Center Experience. Eur Urol (2010) 57(4):667–72. doi: 10.1016/j.eururo.2009.06.009
5. Tilki D, Nguyen HG, Dall'Era MA, Bertini R, Carballido JA, Chromecki T, et al. Impact of Histologic Subtype on Cancer-Specific Survival in Patients With Renal Cell Carcinoma and Tumor Thrombus. Eur Urol (2014) 66(3):577–83. doi: 10.1016/j.eururo.2013.06.048
6. Haddad AQ, Wood CG, Abel EJ, Krabbe LM, Darwish OM, Thompson RH, et al. Oncologic Outcomes Following Surgical Resection of Renal Cell Carcinoma With Inferior Vena Caval Thrombus Extending Above the Hepatic Veins: A Contemporary Multicenter Cohort. J Urol (2014) 192(4):1050–6. doi: 10.1016/j.juro.2014.03.111
7. Hackett NJ, De Oliveira GS, Jain UK, Kim JY. ASA Class Is a Reliable Independent Predictor of Medical Complications and Mortality Following Surgery. Int J Surg (2015) 18:184–90. doi: 10.1016/j.ijsu.2015.04.079
8. Mitropoulos D, Artibani W, Graefen M, Remzi M, Roupret M, Truss M, et al. Reporting and Grading of Complications After Urologic Surgical Procedures: An Ad Hoc EAU Guidelines Panel Assessment and Recommendations. Eur Urol (2012) 61(2):341–9. doi: 10.1016/j.eururo.2011.10.033
9. Haferkamp A, Bastian PJ, Jakobi H, Pritsch M, Pfitzenmaier J, Albers P, et al. Renal Cell Carcinoma With Tumor Thrombus Extension Into the Vena Cava: Prospective Long-Term Followup. J Urol (2007) 177(5):1703–8. doi: 10.1016/j.juro.2007.01.039
10. Ljungberg B, Bensalah K, Canfield S, Dabestani S, Hofmann F, Hora M, et al. EAU Guidelines on Renal Cell Carcinoma: 2014 Update. Eur Urol (2015) 67(5):913–24. doi: 10.1016/j.eururo.2015.01.005
11. Ljungberg B, Albiges L, Abu-Ghanem Y, Bensalah K, Dabestani S, Fernandez-Pello S, et al. European Association of Urology Guidelines on Renal Cell Carcinoma: The 2019 Update. Eur Urol (2019) 75(5):799–810. doi: 10.1016/j.eururo.2019.02.011
12. Ficarra V, Novara G, Martignoni G. The Use of Simplified Versions of the Fuhrman Nuclear Grading System in Clinical Practice Requires the Agreement of a Multidisciplinary Panel of Experts. Eur Urol (2009) 56(5):782–4; discussion 784-5. doi: 10.1016/j.eururo.2009.07.024
13. Xie L, Hong G, Nabavizadeh R, Patil D, Ethun CG, Ogan K, et al. Outcomes in Patients With Renal Cell Carcinoma Undergoing Inferior Vena Cava Ligation Without Reconstruction Versus Thrombectomy: A Retrospective, Case Controlled Study. J Urol (2021) 205(2):383–91. doi: 10.1097/JU.0000000000001354
14. Reese AC, Whitson JM, Meng MV. Natural History of Untreated Renal Cell Carcinoma With Venous Tumor Thrombus. Urol Oncol (2013) 31(7):1305–9. doi: 10.1016/j.urolonc.2011.12.006
15. Wagner B, Patard JJ, Mejean A, Bensalah K, Verhoest G, Zigeuner R, et al. Prognostic Value of Renal Vein and Inferior Vena Cava Involvement in Renal Cell Carcinoma. Eur Urol (2009) 55(2):452–9. doi: 10.1016/j.eururo.2008.07.053
16. Kaushik D, Linder BJ, Thompson RH, Eisenberg MS, Lohse CM, Cheville JC, et al. The Impact of Histology on Clinicopathologic Outcomes for Patients With Renal Cell Carcinoma and Venous Tumor Thrombus: A Matched Cohort Analysis. Urology (2013) 82(1):136–41. doi: 10.1016/j.urology.2013.02.034
17. Abel EJ, Margulis V, Bauman TM, Karam JA, Christensen WP, Krabbe LM, et al. Risk Factors for Recurrence After Surgery in non-Metastatic RCC With Thrombus: A Contemporary Multicentre Analysis. BJU Int (2016) 117(6B):E87–94. doi: 10.1111/bju.13268
18. Abel EJ, Masterson TA, Karam JA, Master VA, Margulis V, Hutchinson R, et al. Predictive Nomogram for Recurrence Following Surgery for Nonmetastatic Renal Cell Cancer With Tumor Thrombus. J Urol (2017) 198(4):810–6. doi: 10.1016/j.juro.2017.04.066
Keywords: RCC, venous thrombus, nomogram, PFS, RN-VT
Citation: Zhang Y, Tian X, Bi H, Yan Y, Liu Z, Liu C, Zhang S and Ma L (2022) A Nomogram Predicting the Progression-Free Survival of Nonmetastatic Renal Cell Carcinoma Patients With Venous Thrombus After Surgery. Front. Oncol. 12:765092. doi: 10.3389/fonc.2022.765092
Received: 26 August 2021; Accepted: 24 February 2022;
Published: 24 March 2022.
Edited by:
Sanja Štifter, Skejby Sygehus, DenmarkCopyright © 2022 Zhang, Tian, Bi, Yan, Liu, Liu, Zhang and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: LuLin Ma, bWFsdWxpbnBrdUAxNjMuY29t; ShuDong Zhang, emhhbmdzaHVkb25nQGJqbXUuZWR1LmNu
†These authors have contributed equally to this work
 Yu Zhang†
Yu Zhang† Hai Bi
Hai Bi Ye Yan
Ye Yan LuLin Ma
LuLin Ma