- 1Department of Biochemistry and Molecular Biology, Shantou University Medical College, Shantou, China
- 2Department of Clinical Laboratory Medicine, the Cancer Hospital of Shantou University Medical College, Shantou, China
- 3Guangdong Esophageal Cancer Research Institute, Shantou University Medical College, Shantou, China
Esophageal carcinoma is one of the most aggressive malignant diseases. At present, neoadjuvant chemotherapy and neoadjuvant chemoradiotherapy are regarded as the standard modalities for the treatments of locally advanced esophageal cancers based on several landmark trials. However, the optimal regimen, radiation dose, and surgical intervals are uncertain and the rate of recurrence after neoadjuvant therapy is high. Patients receiving neoadjuvant therapy and reaching a pathological complete response have been reported to have a better survival benefit and a fewer recurrence risk than those non-pathological complete responses. Nevertheless, less than half of patients will reach a pathological complete response after neoadjuvant therapy, and the methods to evaluate the efficacy after neoadjuvant therapy accurately are limited. Immune checkpoint inhibitors have been recommended for the treatments of advanced esophageal cancers. Recently, research has been beginning to evaluate the safety and efficacy of immunotherapy combined with neoadjuvant therapy. Here, we will review and discuss the development of the neoadjuvant therapy of locally advanced esophageal cancers and unsolved clinical problems.
Introduction
Esophageal carcinoma has been regarded as the seventh common cancer and the sixth leading cause of cancer death (1). Histologically, esophageal cancers include squamous cancer, which is common in Asian countries, and adenocarcinoma, which is common in western countries. To date, the treatment for esophageal cancers is still a tough clinical problem. For locally advanced esophageal cancers (LAECs), neoadjuvant chemotherapy (nCT) and neoadjuvant chemoradiotherapy (nCRT) have been recommended as standard treatments. Many studies have proved their anti-tumor efficacy, while some unsolved clinical problems also exist (2–4). Immune checkpoint inhibitors (ICIs) are being widely researched in various tumors. For esophageal cancers, ICIs have been recommended for the second/first-line treatment of advanced esophageal cancers. Recently, research regarding immunotherapy combined with conventionally neoadjuvant chemo(radio)therapy is ongoing. Hence, here we review and discuss the development of neoadjuvant therapy for LAECs and potential clinical problems.
Neoadjuvant Chemotherapy
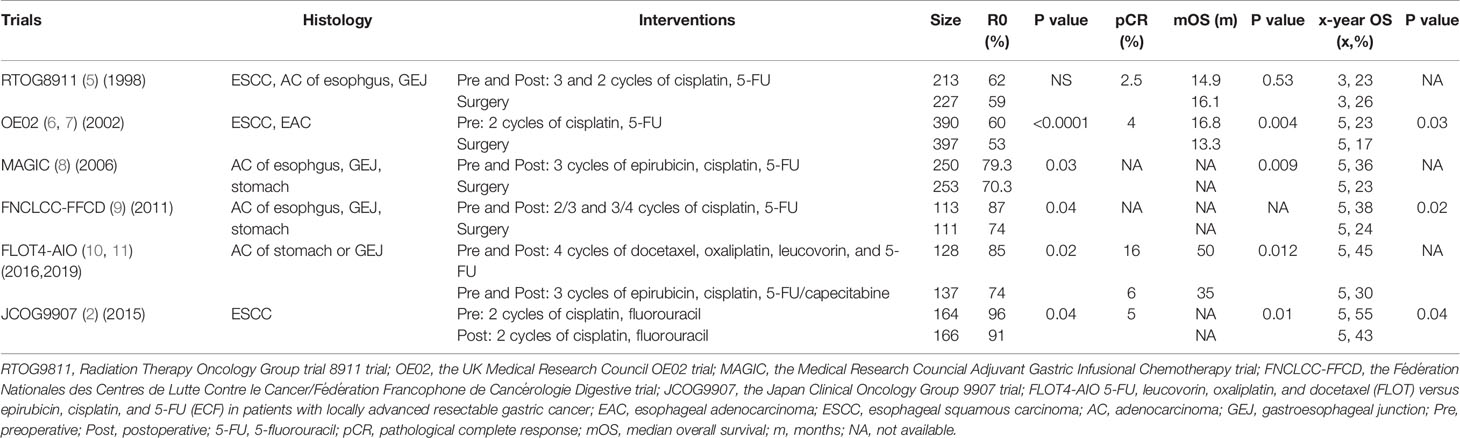
The research regarding the efficacy of nCT for LAECs has been conducted since the 1980s. As we summarized in Table 1, for adenocarcinoma, the utilization status of neoadjuvant treatments is owed to three randomized controlled trials. The UK Medical Research Council (OE02) trial was the first large-sized study to demonstrate a survival benefit of nCT for patients with esophageal cancer, in which patients were randomly assigned to receive preoperative chemotherapy or surgery alone, showing a 5% increase in 5-year survival for patients with adenocarcinoma (6, 7). Thus, nCT became a standard treatment for local esophageal adenocarcinoma (EAC). Besides, the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) and Fédération Nationales des Centres de Lutte Contre le Cancer/Fédération Francophone de Cancérologie Digestive trials (FNCLCC-FFCD), which included 26% and 75% LAEC in the group receiving perioperative epirubicin, cisplatin, fluorouracil and cisplatin, fluorouracil respectively, showed that the perioperative chemotherapy group had a higher overall survival as shown by a hazard ratio (HR) reduction from 25% to 31% and a 5-year survival increase from 13% to 14% (8, 9). Since the benefit of survival, perioperative chemotherapy has become a standard treatment for locally gastroesophageal carcinoma. Recently, results from the trial 5-FU, Leucovorin, Oxaliplatin and Docetaxel (FLOT) Versus Epirubicin, Cisplatin and 5-FU (ECF) in Patients With Locally Advanced Resectable Gastric Cancer (FLOT4-AIO) revealed that the regimen with docetaxel, oxaliplatin, leucovorin, and fluorouracil showed better survival and disease control. Compared with perioperative epirubicin, cisplatin, and fluorouracil/capecitabine, the median overall survival (mOS) in patients administered with the FLOT regimen increased by 15 months (P=0.012) and the median disease-free survival (mDFS) increased by 12 months (P=0.0036) (10, 11). Thus, the FLOT has been one of the standard regimens for perioperative chemotherapy.
For esophageal squamous carcinoma (ESCC), in the OE02 trial, 247 patients with ESCC were randomly assigned to receive nCT or surgery alone (6). Long-term results showed a benefit survival outcome (25.5% vs. 17.0% in 5-year OS) (7). The standard preoperative chemotherapy for locally advanced ESCC is cisplatin-fluorouracil based, which improved the R0 resection and overall survival (OS) in the Japan Clinical Oncology Group (JCOG) 9907 trial showing a 5-year OS of 55% in the nCT group while 43% in the postoperative treatment group (P=0.04). Based on this trial, the nCT based on cisplatin–fluorouracil (CF) has become the standard treatment for locally ESCC in Japan (2).
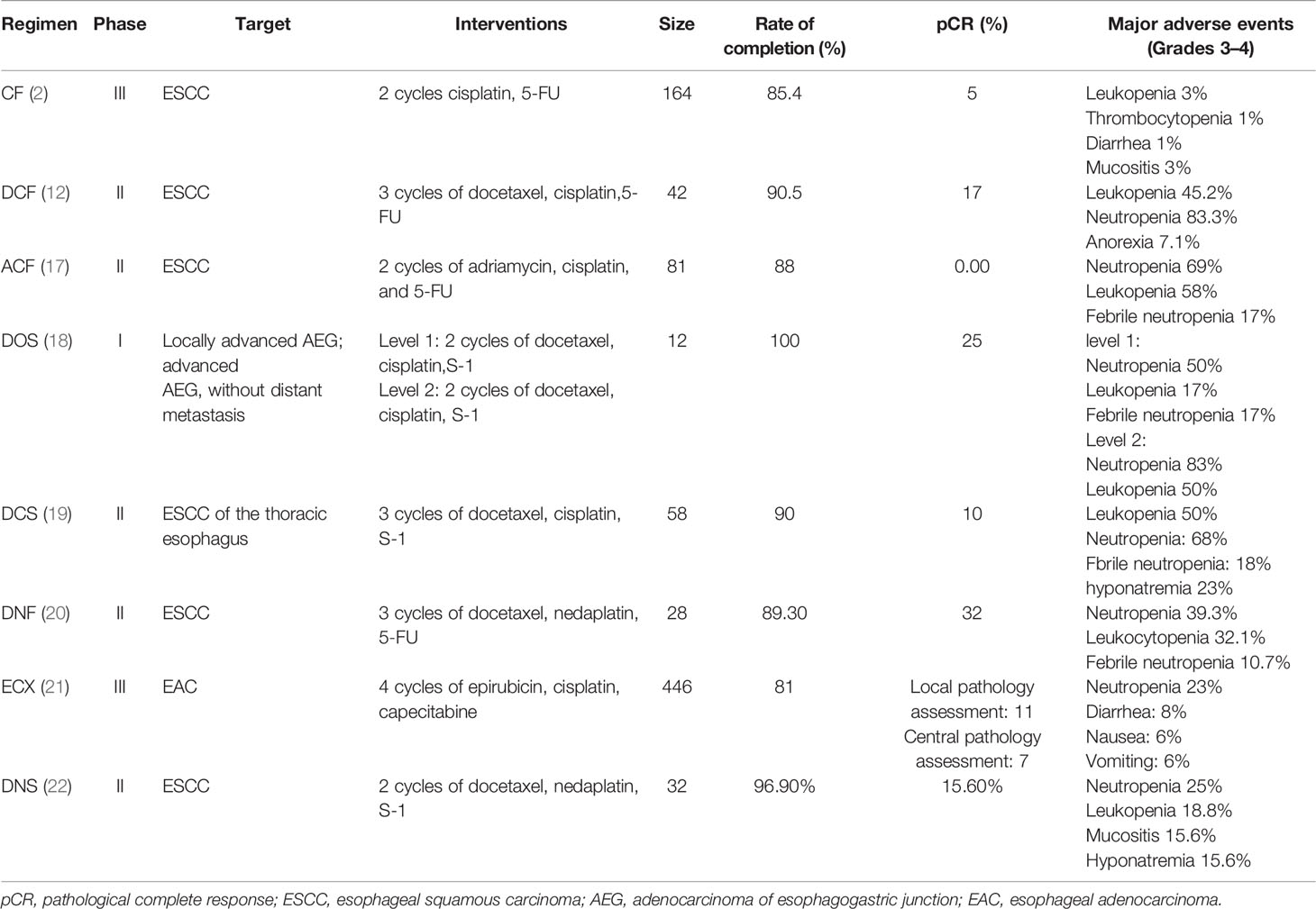
In summary, compared with surgery alone, adding chemotherapy before surgery has been proved to improve R0 resection rate and survival for patients with LAEC. For adenocarcinoma, the 5-year OS increased by 13%–15% by adding nCT. For ESCC, evidence from large-scale clinical trials is limited. The efficacy of nCT was confirmed in the JCOG9907 trial by comparing it with postoperative therapy (2). Nevertheless, the efficacy of nCT remains unsatisfactory as shown by a low pathological complete response (pCR) rate after nCT. For adenocarcinoma, thanks to the FLOT regimen, the pCR rate has been significantly improved, showing an increase of up to 16% (10). For ESCC, the pCR rate after a preoperative CF-based treatment was only 5%. Furthermore, although the JCOG9907 trial showed an OS benefit after nCT compared with postoperative therapy, there were no differences in progression-free survival (PFS; HR 0.84, 95%CI 0.63–1.11) indicating no improvement in the quality of life. Subgroup analysis reported that patients at stage III had less benefit than those at stage II. Thus, a more effective regimen is needed (2). Recent research has shown the promising efficacy of the regimen with docetaxel, cisplatin, and 5-FU (DCF). Results from a phase II trial reported a 90.5% completion rate and 17% pCR rate suggesting the feasibility of the DCF regimen (12). For disease control, the DCF regimen showed a higher 5-year PFS than that in the CF regimen (38.2% vs. 58.3%, P=0.006) (13). For security, the DCF regimen had no effects on surgical outcomes (14). However, the incidence of blood adverse events (AEs) was high (grade ≥3 neutropenia: 83%) (12). A propensity score-matched analysis reported that the DCF showed better efficacy for patients with locally advanced squamous carcinoma at stage III [objective response rate (ORR): 61.0% vs. 43.2%, P=0.021; HR for death 0.49, 95%CI 0.24–0.999, P=0.050] (15). Most recently, the JCOG1109 NExT trial (UMIN000009482), aiming to compare the efficacy between the CF regimen, the DCF regimen, and CF combined with radiotherapy in patients with locally advanced ESCC, reported its results in the 2022 American Society of Clinical Oncology Gastrointestinal Cancers Symposium (ASCO-GI) meeting, demonstrating that the DCF regimen was superior to the CF regimen in both OS and PFS with a high pCR rate of 19.8% and a manageable toxicity profile (mOS: 4.6 years versus not reach; median progression-free survival (mPFS): 2.7 years versus not reach). Based on this result, the DCF might be a new standard neoadjuvant treatment for locally advanced ESCC (16). In addition, there are other regimens for nCT (Table 2). However, it should be pointed out that there is a lack of large size, head-to-head clinical trials to compare the efficacy between nCT regimens. Besides exploring new regimens, the exploration of new modalities has also attracted much attention, like the combination of chemotherapy and immunotherapy, which will be shown below.
Neoadjuvant Chemoradiotherapy
Preoperative chemoradiotherapy plus surgery has been widely used for LAECs (Table 3). At the early stage, the results from clinical research were controversial since the heterogeneity between the studies included chemoradiotherapy regimens, surgical technique, tumor histology, sample size, and less advanced diagnostic methods. Until the ChemoRadiotherapy for Oesophageal cancer followed by Surgery Study (CROSS) trial and the Phase III Study of Neo-adjuvant Chemoradiotherapy Followed by Surgery for Squamous Cell Esophageal Cancer (NEOCRTEC5010) trial, the standard treatment status of nCRT for LAEC was established.
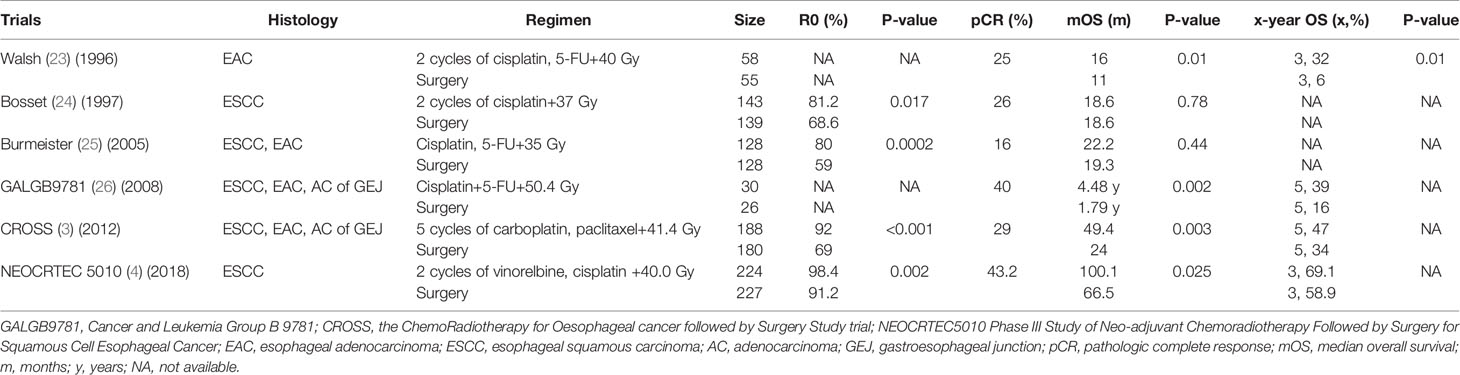
Table 3 Characteristics and main outcomes of clinical trials regarding neoadjuvant chemoradiotherapy versus surgery.
For adenocarcinoma, in the CROSS trial, 336 patients, in which EAC accounted for 75%, were enrolled to receive nCRT or surgery alone. Initial results showed that after nCRT, the resection specimen demonstrated that a pCR was 23% and there were no differences in postoperative complications and in-hospital mortality between the nCRT group and the surgery group, which meant the feasibility and acceptable toxicity of the CROSS regimen (3). Long-term results revealed a benefit of OS [mOS: 43.2 vs. 21.7 months (m)] and PFS (mPFS: 29.9 vs. 17.7 m), and the effect of OS was up to 10 years of follow-up, indicating that compared with surgery, the nCRT based on the CROSS regimen significantly prolonged lifespans and improved disease control (27, 28). Thus, nCRT was regarded as the standard treatment for locally EAC and the regimen; 5 cycles of paclitaxel (50 mg/m2) and 3 cycles of carboplatin (2 mg/AUC) concurrent with 41.4Gy became the standard regimen for nCRT.
For ESCC, however, although the CROSS trial reported a much better efficacy of nCRT (pCR rate: 49%; mOS: 81.6 m:21.1 m; mPFS: 74.7 m: 11.6 m; 10-year OS rate: 46% vs. 23%), it should be noted that only 23% tumor types were ESCC, which might make it hard to convince the benefit of ESCC (3, 27, 28). Most recently, the results from the NEOCRTEC5010 trial demonstrated the better efficacy of nCRT (vinorelbine, cisplatin, 40.0 Gy) versus surgery alone. The R0 rate was significantly higher in the nCRT group than that in the surgery group. Resection specimens showed that the pCR rate was 43.2%. With a median follow-up of 41.0 months, significant differences in mOS and 3-year OS were found in favor of nCRT (mOS: 100.1 vs. 66.5 m, P=0.025; 3-year OS: 69.1% vs. 58.9%). Furthermore, disease-free survival (DFS) was also significantly improved in the nCRT group compared with the surgery group (100.1 vs. 41.7 m, P<0.001) (4). Based on these two trials, nCRT also became the standard treatment modality for locally ESC and the regimen, 2 cycles of vinorelbine (25 mg/m2) and cisplatin (75 mg/m2) concurrent with 40.0 Gy became the standard regimen for nCRT in China.
In summary, most of the studies comparing the efficacy of nCRT to surgery alone obtained negative results before the 2000s. After the 2000s, many studies showed that nCRT was better than surgery alone. Based on the results from the landmark trials, CROSS and NEOCRTEC5010, nCRT has become the standard treatment for LAECs. The CROSS regimen has been widely used around the world. Current evidence shows that compared with surgery alone treatment, nCRT can ameliorate R0, pCR, OS, and recurrence. After nCRT, R0, pCR, and OS range from 81% to 98%, 25% to 43%, and 16 to 100.1 months, respectively.
Neoadjuvant Chemoradiotherapy vs. Neoadjuvant Chemotherapy
As mentioned above, nCT and nCRT are two main modalities for the treatment of LAECs. In Japan, based on its own research, nCT was the standard modality for LAECs. In some western countries, nCRT was the preferred treatment modality based on the results of the CROSS trial. Based on the NEOCRTEC5010, nCRT also has become the standard treatment for locally ESCC in China. Whether nCRT or nCT brings better efficacy for patients with LAECs is still uncertain so far. Research regarding the comparison between nCT versus nCRT directly is limited and the current evidence is inconclusive. Current research demonstrated that whether in ESCC or EAC, patients receiving nCRT are more likely to reach pCR. The pCR rate in nCRT is higher than that in nCT. However, there are no differences between these two modalities in survival outcomes.
For EAC, only three prospective studies directly compared the advantages and disadvantages of the two modalities. In the PreOperative therapy in Esophagogastric Adenocarcinoma (POET) trial, patients after nCRT showed a significantly higher pCR rate of 14.3% compared with 1.9% after nCT. For OS, there was a trend in favor of nCRT as shown by a longer mOS and higher rate of long-term survival (29, 30). In the Neoadjuvant Chemotherapy Versus Radiochemotherapy for Cancer of the Esophagus or Cardia (NeoRes) trial, which enrolled over 70% of patients with EAC, the authors demonstrated a significant increase of 19% of pCR in the nCRT setting but no differences for OS compared with nCT (31, 32). Also, Burmeister et al. reported similar results that the pCR rate in nCRT significantly increased, while mOS and the long-term survival rate were only higher numerically (33).
The research about the efficacy of nCRT versus nCT in ESCC is limited (Table 4). The results from the NeoRes trial containing less than 30% of patients with ESSC could not find benefit in long-term survival although the pCR rate after nCRT was significantly higher than after nCT. The trial, Comparison Between NCRT and NCT Followed by MIE for Treatment of Locally Advanced Resectable ESCC (CMISG1701), reported its initial results, which were to compare the efficacy of four cycles of paclitaxel (50 mg/m2)/cisplatin(25 mg/m2) as nCRT regimen, followed by minimally invasive esophagectomy (MIE) versus two cycles of paclitaxel (135 mg/m2)/cisplatin(75 mg/m2) as nCT regimen followed MIE in ESCC. The study showed that patients undergoing nCRT had better pathologic outcomes including higher pCR (35.7% vs. 3.8% P<0.01) and less lymph nodes involved (66.1% vs. 46.2%, P = 0.03). However, 1-year OS was not different between the two groups (34).
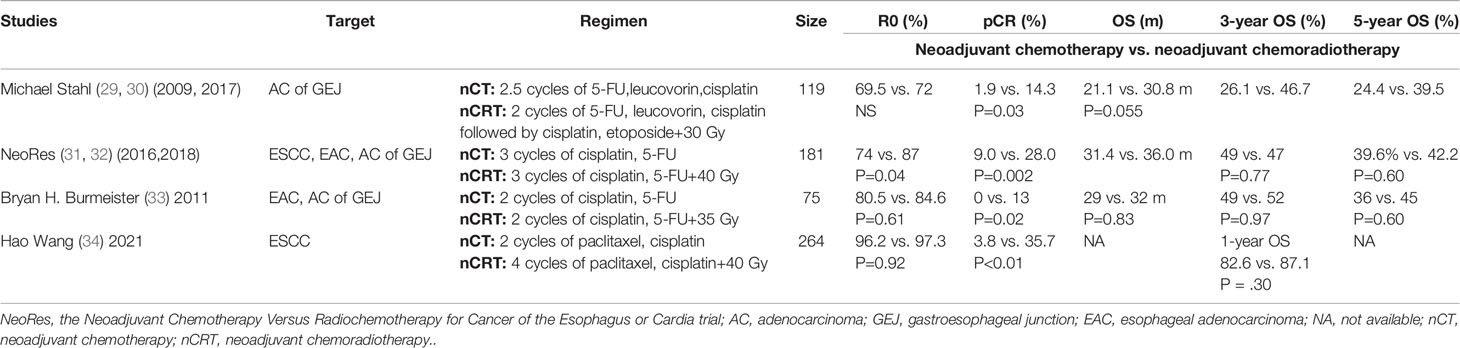
Table 4 Characteristics and outcomes of studies regarding neoadjuvant chemotherapy vs. neoadjuvant chemoradiotherapy.
As the lack of evidence of direct comparison, meta-analyses were conducted. A network meta-analysis including 26 studies compared the efficacy of surgery alone, nCT, neoadjuvant radiotherapy, nCRT, surgery followed by adjuvant chemotherapy, adjuvant radiotherapy, or adjuvant chemoradiotherapy. A ranking analysis reported that nCRT might be the best option for patients diagnosed with LAECs. When compared to surgery alone, in all the treatments, nCRT yielded the best benefit in terms of OS and PFS/DFS (HR = 0.76, 95%CI 0.67–0.85; HR = 0.8, 95%CI 0.68–0.94, respectively). Also, only nCRT associated with a statistically confident decrease in locoregional recurrence or distant metastasis [odd ratios (OR)=0.48, 95%CI 0.30–0.77; OR = 0.67, 95%CI 0.49–0.93, respectively] (35).
To sum up, currently, it is still unable to define which modality is better for LAECs. Current evidence suggests that despite the histological type, patients with LAECs are more likely to develop pCR after nCRT, whereas in the OS of nCRT, there was no statistical improvement compared to that of nCT. Reasons why the higher rate of pCR after nCRT fails to translate into the benefit of survival, are still a primary concern. The toxicities of treatments and perioperative complications may contribute to the problem. The POET trial showed that the grade 3/4 toxicities were 5% in the nCT group, while grade 3/4 leukocytopenia and thrombocytopenia were 12% and 5%, respectively, in the nCRT group (29). The NeoRes trial demonstrated that the severity of postoperative complications in the nCRT group was significantly higher than that in the nCT group (P=0.001) (31). Long-term results showed that the patients after nCRT had higher risks of postoperative complications (9% vs. 1% P=0.02) (32). Meta-analyses also reported a significantly higher postoperative mortality in nCRT (RR 1.58, 95% CR 1.00– 2.49) (36). We hypothesize that in looking for new drugs or treatment modalities that can lead to fewer toxicities, and the improvement of surgery technology, the introduction of early interdisciplinary supportive care (ESC) may help solve the problem. Most recently, a phase III clinical trial explored whether ESC combined with the standard first-line treatment for patients with metastatic esophageal cancers could improve the prognosis. Results showed the mOS in the ESC group was significantly higher than those in the standard care group (14.8 vs. 11.9 m, HR 0.68, 95%CI 0.51–0.9, P=0.021) (37). Another study demonstrated that a multidisciplinary team approach started before neoadjuvant therapy would decrease the risk of AE rate during chemotherapy (P=0.007) and provide safe perioperative conditions(P=0.003) (38). MIE is becoming more and more common in surgical treatments. A new meta-analysis has reported that MIE decreased 18% risk of all-cause 5-year mortality for patients with esophageal cancers compared with open surgery (39). Nowadays, the regimens of neoadjuvant therapy are various. CROSS, MAGIC, and FLOT regimens have been widely used. Here, we summarize the ongoing clinical trials comparing the efficacy of nCRT versus nCT based on these regimens, expecting that more valid and powerful evidence can be provided by these trials (Table 5).
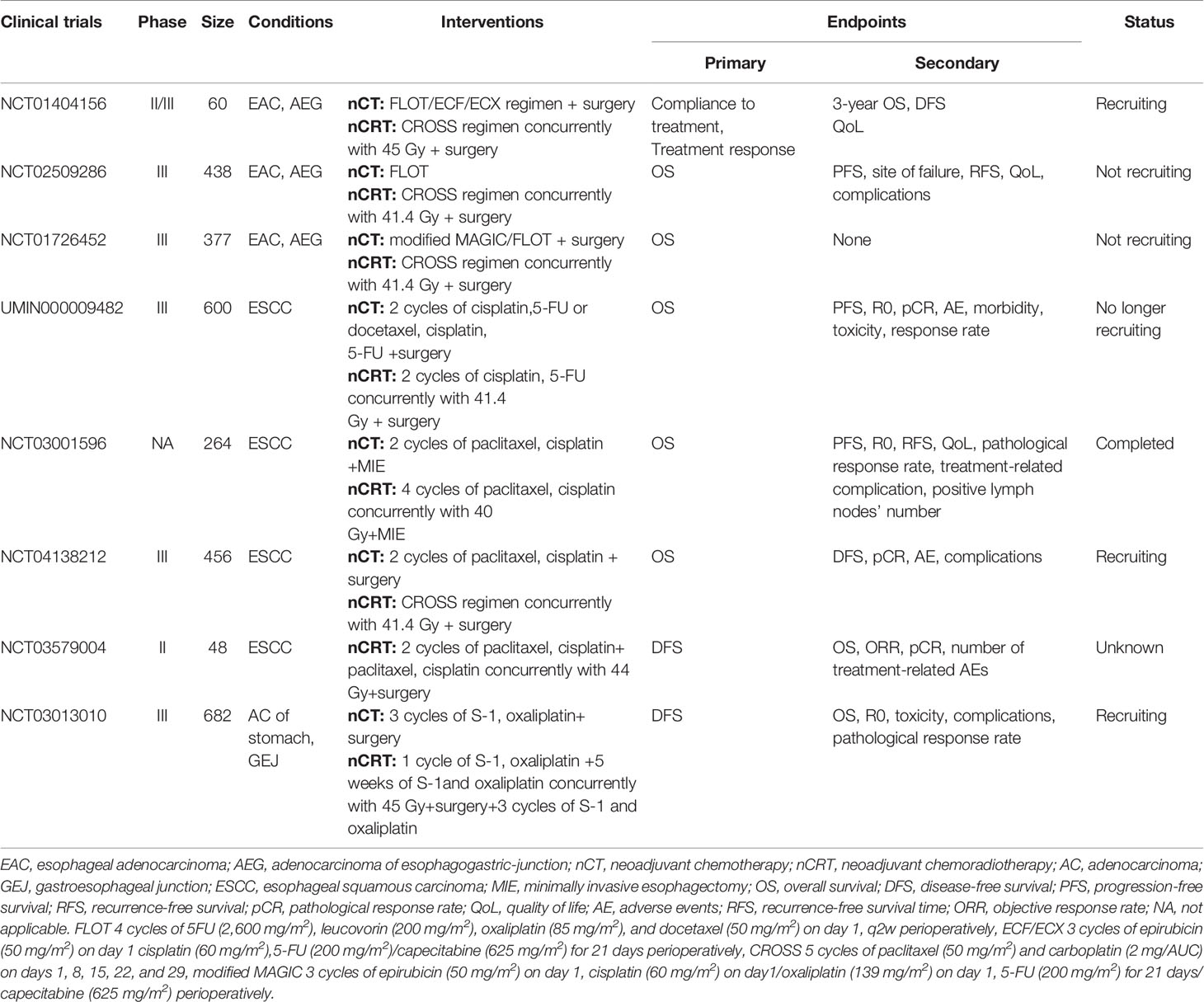
Table 5 Characteristics clinical trials regarding neoadjuvant chemotherapy vs. neoadjuvant chemoradiotherapy.
Problems in Neoadjuvant Chemoradiotherapy
For nCRT, however, there are some problems to be solved, which are shown as follows.
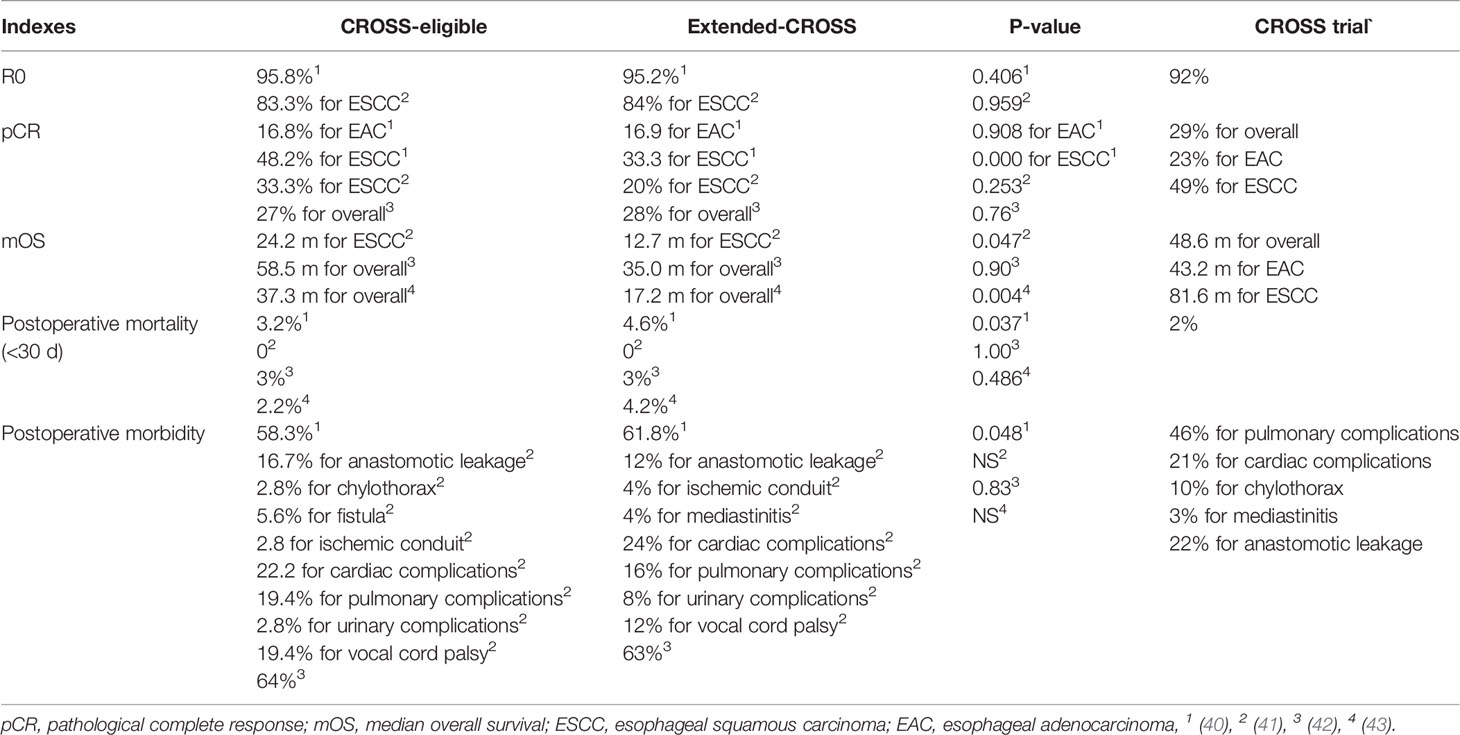
Nowadays, the optimal regimen for nCRT is still inconclusive. The excellent efficacy of nCRT showed in clinical trials cannot be reproduced completely in a real-world scenario (Table 6). Four studies that evaluated the efficacy of the CROSS regimen in the real-world scenario demonstrated that patients who did not fully meet the CROSS criteria had a lower efficacy than those who fully met them, showing a lower pCR and mOS and possibly higher postoperative morbidity and mortality (40–43). Moreover, even patients eligible for the criteria could not obtain the efficacy as well as that in the CROSS trial. Based on the results of the CROSS trial, the CROSS regimen has replaced the CF-based regimen that was used widely before. However, no prospective head-to-head comparative study was conducted. A propensity score-matched study, comparing the efficacy of the cisplatin/fluorouracil regimen versus the CROSS regimen in patients with locally advanced ESCC, recently reported that there were no differences in the pathological or survival outcome between the two regimens but the study showed a trend in favor of the cisplatin/fluorouracil regimen (44). For adenocarcinoma, one retrospective study (adenocarcinoma: 86%) showed that the CF regimen could increase the pCR rate (P=0.032), improve the recurrence-free survival (HR 0.39, 95%CI 0.21–0.73, P=0.003) and OS (HR 0.46, 95%CI 0.24–0.87, P=0.016) (45). In addition, retrospective research, comparing the NEOCRTECT5010 regimen to the CF regimen, demonstrated the former increased pCR rate (47.4% vs. 28.1%, P=0.034) and contributed to better OS (52.8 vs. 25.2 m, P=0.001) while leading to increasing hematologic toxicities (P=0.03) (46).
The radiation dose is various in nCRT ranging from 37 to 50.4 Gy. A retrospective study evaluated the efficacy of high dose (>45Gy) versus low dose (≤45 Gy) in ESCC, showing no differences in pCR rate and survival (47). Recently, a systematic review incorporated 110 studies, involving ESCC/EAC, where patients receiving nCRT up to a dose of 50.4 Gy, to evaluate the efficacy and safety of different radiation doses and try to find an optimal dose. Results demonstrated that 40.0–41.4 Gy might be a rational dose for nCRT (48). Prospective controlled studies are needed to confirm the optimal dose.
So far, the surgical interval for nCRT is 4–6 weeks. The optimal surgical interval for nCRT is still inconclusive. The proper extension of the surgical interval may increase the pCR rate because of the shrinkage of tumors under the effects of nCRT. Also, it gives patients more time to recover from the preoperative treatments, which may reduce the risk of surgical-related AEs. All these may bring a benefit of survival. However, some research reported the increase in pCR rate profiting from the extending surgical interval failed to translate into a benefit of survival (49, 50). There may be several reasons. First, these studies are retrospective studies where patients delayed surgeries because of their poor body conditions or the AEs from nCRT rather than their preferences. Second, although extending surgical interval increases the pCR rate, its contribution is not enough to reflect on a statistically significant benefit in survival. Subgroup analysis, comparing the efficacy of extension of surgical interval between patients reaching pCR versus non-pCR, showing a significantly better survival benefit supported this point (8.7 years for patients with pCR vs. 2.0 years) (49). Recently, one prospective research, avoiding the gap of the first reason, reported that the extension of the surgical interval had no effects on short-term operative outcomes (overall postoperative complication: 63.2% vs. 72.6%, P=0,134; severe postoperative complications: 31.6% vs. 34.9%; median length of hospital stays: 15 vs. 17 d, P=0.234) (51). More prospective studies are needed to focus on the effect of surgical interval on survival.
Although nCRT improves the recurrences of patients compared with surgery, the recurrent rate after nCRT is still high. The ten-year outcome of the CROSS trial revealed that recurrences occurred in 48% of patients, and 33.7% of patients reported in the NEOCRTEC5010 trial (4, 28). Most recurrences occurred in the first 3 years after surgery. Compared with distant metastases, nCRT mainly improved local or regional recurrences. Data from the ten-year outcome of the CROSS trial demonstrated that the overall local-regional recurrence rate after nCRT reduced significantly from 40% to 21% compared with surgery alone (28). Similarly, in the NEOCRTEC5010 trial, nCRT significantly improved the overall local–regional recurrence rate (14.1% vs. 22.5%, P=0.031) while the overall distant metastasis rate had no statistical differences (23.9% vs. 31.7%, P=0.08) (52). Thus, distant metastasis is the main failed mode after nCRT. Subgroup analysis showed that patients who reached pCR had a lower recurrence rate than those with non-pCR. Histologically, the patients with ESCC or EAC who did not reach pCR after nCRT showed a different recurrent pattern. The patients with EAC showed a likelihood of recurrence compared with ESCC (43.2% vs. 34.3%, P=0.023). The patients with ESCC had a higher risk of regional and supraclavicular recurrences while a lower hematogenous metastasis compared with EAC. In addition, it was reported compared with EAC, patients with ESCC had a significantly higher rate of failure to receive salvage treatments (P=0.005) mainly because of poor performance status (53).
The high recurrence rate after nCRT demands a close monitor. Besides, salvage measures are essential when recurrence occurs. The differences in recurrence patterns between ESCC and EAC require different strategies based on histological tumor types. For EAC, distant metastasis was the main recurrence pattern, which means the necessity of the systematic treatment after nCRT. The Checkmate577 trial had reported that the addition of nivolumab as an adjuvant therapy could significantly reduce the risk of recurrences for patients with residual disease after nCRT (HR 0.74, 95%CI 0.60–0.92). The DFS in the nivolumab group was twice as long as that in the nCRT alone group (22.4 vs. 11.0 m, P<0.001) (54). For ESCC, local–regional recurrence was the main failed mode, indicating the need for the enhancement of local treatment. Systematic lymph node dissection has been recommended in the surgery alone for patients with esophageal cancers. However, whether patients with nCRT followed by systematic lymph node dissection can obtain a survival benefit is debatable. Most recently, a second analysis from the result of the NEOCRTEC5010 trial revealed that systematic lymph node dissection did not increase the surgical risk and could improve the survival and control of disease for patients with nCRT (mOS: 100.0 vs. 85.5 m, P=0.01; 3-year OS: 75.2% vs. 61.5%; 3-year DFS: 70.2% vs. 55.5%, P<0.001). Compared with the dissection of lymph node <20, the dissection of lymph node ≥20 brought a lower recurrence rate (25.8% vs. 41.2%, P=0.027) and better control of disease (5.2% vs. 18.8%, P=0.004) (55). Thus, systematic lymph node dissection should be recommended in nCRT for ESCC.
Evaluation for the Efficacy of Neoadjuvant Setting
pCR is a strong predictor of a good prognosis after nCRT. Many studies have shown that patients reaching pCR after nCRT would obtain a longer survival and a lower risk of recurrences compared with those with non-pCR. Current evidence shows that after nCRT, the rate of pCR ranges from 20% to 43%, which means that a lot of patients still cannot benefit from nCRT. Furthermore, treatment-related toxicities and the extension of operation may lead to a poorer physical condition and tumor progression. Thus, the development of methods to evaluate a pathological response after nCRT is essential to improve the efficacy of nCRT and avoid unnecessary treatments.
The accuracy of using a single imaging method to find residual disease after nCRT is limited. Recently, a meta-analysis reported the limited accuracy of endoscopic biopsies, endoscopic ultrasound, and positron emission tomography with 2-deoxy-2-[fluorine-18]fluoro-D-glucose integrated (with computed tomography) (18F-FDG PET(-CT)) as single modalities to detect residual disease after nCRT for patients with LAECs (56).
Magnetic resonance imaging (MRI) has been reported to have a promising accuracy in the evaluation of efficacy after nCRT. A prospective study showed the relative increase of the parameter of diffusion-weighted magnetic resonance imaging (DW-MRI), ΔADCduring-pre(median apparent diffusion coefficient; during 2–3 weeks during nCRT) was positively correlatively with pCR. A cut-off value of 29% yielded a sensitivity of 100%, specificity of 75%, accuracy of 95%, positive predictive value of 94%, and negative predictive value of 100% (57). The parameters of diffusion contrast-enhanced magnetic resonance imaging (DCE-MRI), ΔAUC (area under the concentration–time curve), were reported to predict pCR. At a cut-off of 24.6%, ΔAUCpost-pre mostly predicted a pCR, yielding a sensitivity of 83%, specificity of 88%, positive predictive value of 71%, and negative predictive value of 93% (58). Based on these two studies, another study evaluated the accuracy of the combination of DW-MRI and DCE-MRI and reported their complementary value (59). The addition of MRI into gastroscopy with biopsies and endosonographic ultrasound with fine-needle aspiration has been reported to improve the detection of residual tumor after nCRT, as shown by an increased sensitivity from 47% to 89% (60). A prospective study evaluated the combined value of DW-MRI and 18F-FDG PET/CT to predict pCR in patients after nCRT. Results showed that early changes of the parameter on DW-MRI during nCRT and changes on 18F-FDG PET/CT after nCRT might yield a complementary value in the assessment of pCR (61). The Surgery AS Needed for Oesophageal Cancer (preSANO) trial evaluated the efficiency of the combination of different methods to detect residual disease after nCRT and tried to propose an optimal modality. Results showed that endoscopic ultrasonography, bite-on-bite biopsies, and a fine-needle aspiration of suspicious lymph nodes to detect locoregional residual disease combined with PET-CT for the detection of interval metastases were an effective modality for clinical evaluation (62). Now, a phase III trial (NTR6803) has been conducted to compare the outcome of active surveillance with standard resection in patients who reached pCR by using this strategy. Based on the preSANO trial, Chinese scholars are evaluating this strategy in patients with locally advanced ESCC(NCT03937362). Another ongoing trial is evaluating the combined value of DW-MRI, DCE-MRI, 18F-FDG PET/CT, and circulating tumor DNA (ctDNA) to predict the pathological response (NCT03474341).
Compared with multi-imaging methods, a single-imaging method provides limited information. Moreover, usually, imaging methods like CT and endoscopic ultrasound mainly provide anatomical information. However, there are lots of biological parameters like tumor metabolism, structure, and function of blood vessels in tumor tissues, which are sensitive and change early when the tumor tissues react to clinical interventions. This may explain that the combination of multiple imaging methods and addition of MRI can improve the evaluation of pathological response after nCRT. In the future, more attention should be paid to the application of radiomics in the efficacy evaluation after nCRT.
Biomarkers, related to tumor growth, DNA repair, cell cycle, etc., have been investigated to see the predictive value in histology response after nCRT in LAECs. ERCC1 and p53 were probably studied widely. As results regarding the predictive value of p53 to pCR were debatable, Zhang et al. assembled 28 studies in their meta-analysis and reported that the wild-type form of p53 status was probably a predictive biomarker for pCR after nCRT (63). Other molecular markers like cyclinD, p53R2, COX-2, Gli-1, and miRNA also have been explored. Recently, one meta-analysis analyzed that 56 biomarkersm except for p53 from 46 articles, demonstrated that the low expression of COX2, miR-200c, ERCC1, and TS, or a higher expression of CDC258 and p16, were associated with the prediction of response for patients receiving neoadjuvant chemo(radio)therapy (64). However, there are still no effective biomarkers to predict whether someone will respond to chemoradiotherapy or not. The joining of imaging techniques and specimen detection (tissue and/or liquid) has rarely been reported yet, which may have synergistic effects on the evaluation or prediction of response to the neoadjuvant setting.
Immunotherapy in Locally Advanced Esophageal Cancer
With the insight of the molecular mechanisms of tumors and highlight of individualized treatment, targeted therapy has emerged as a hot direction. The overexpression of Epidermal Growth Factor Receptor (EGFR), Erb-B2 Receptor Tyrosine Kinase 2 (HER2), and Vascular Endothelial Growth Factor Receptor (VEGFR) has been reported in esophageal cancer, which enhances tumor occurrence, progression, and drug resistance. Adding drugs targeting these molecules to the current treatment may bring a synergistic effect. However, so far, most studies failed to show a satisfactory efficacy (65, 66). In recent years, ICIs have been a new modality used in tumor treatments like none small cell lung cancer and melanoma because of their promising efficacy. For esophageal cancer, some research has reported the anti-tumor activity of ICIs. Three landmark clinical trials, Study of Pembrolizumab (MK-3475) Versus Investigator’s Choice Standard Therapy for Participants With Advanced Esophageal/Esophagogastric Junction Carcinoma That Progressed After First-Line Therapy (KEYNOTE181), Study of Nivolumab in Unresectable Advanced or Recurrent Esophageal Cancer (ATTRACTION-3), and Study of SHR-1210 Versus Investigator’s Choice Standard Therapy for Participants With Advanced Esophageal Cancer (ESCORT) have confirmed the anti-tumor activity of pembrolizumab, nivolumab, and camrelizumab in second-line treatment for advanced/metastatic esophageal cancer by demonstrating that these drugs improved survival, the ORR, and duration of response (DoR) compared with conventional chemotherapy (67–69). With the success in second-line treatments, the usage as first-line treatment in advanced/metastatic esophageal cancer is already being developed. A KEYNOTE-590 trial reported that pembrolizumab combined with a platinum-based regimen (cisplatin/5-FU) as the first-line treatment for advanced/metastatic esophageal cancers showed better survival outcomes compared with a platinum-based regimen alone. For safety, the two regimens were similar (70). A Checkmate-649 trial firstly compared the efficacy of nivolumab combined with capecitabine/oxaliplatin or leucovorin/fluorouracil/oxaliplatin to chemotherapy alone. Data showed that the ORR in patients receiving the new regimen was more than 50%. Compared with chemotherapy alone, the new regimen prolonged the DoR by 2.5 months, reduced the risk of death by 23%, increased the median OS by 2.2 months (71). The Study of SHR-1210 in Combination With Chemotherapy in Advanced Esophageal Cancer (ESCORT-1st) trial evaluated the efficacy of camrelizumab combined with paclitaxel/cisplatin as the first-line regimen for advanced/metastatic ESCC. Data demonstrated that compared with chemotherapy alone, camrelizumab combined with chemotherapy reduced the risk of death by 30% and 44% in the risk of progression. The median OS and PFS were prolonged by 2.1 and 1.3 months, respectively. The ORR rate was 72.1% in the new regimen group, and the DoR increased by 2.4 months. Moreover, the addition of camrelizumab did not increase the rate of AEs (72).
All these results revealed that immunotherapy combined with chemotherapy could improve survival with no unacceptable AEs for advanced/metastatic esophageal cancers. Notably, the higher ORR after immunotherapy combined with chemotherapy means more patients can reduce their tumor volume by 30% after the treatment. Tumor shrinking may lead to downstage, which may lay a foundation for the addition of ICIs into neoadjuvant therapy for LAECs (Supplementary Table 1).
For EAC, one trial (NCT03044613) evaluated the safety and efficacy of the induction therapy of nivolumab, followed by mivolumab concurrently with nCRT. Data suggested acceptable toxicities without the delay of surgery and a high pCR of 40% (73).
For ESCC, one research reported a promising efficacy with acceptable toxicity of the addition of pembrolizumab to paclitaxel–carboplatin-based perioperative therapy, showing a high pCR rate of 46.1% and a rate of 82.1% in 1-year OS (74). The trial, Preoperative Anti-PD-1 Antibody Combined With Chemoradiotherapy for Locally Advanced Squamous Cell Carcinoma of Esophageus, was conducted to evaluate the safety and efficacy of a combination of pembrolizumab and the CROSS regimen. Of 65% grade ≥3 AEs, the most common were leukopenia, lymphopenia, anemia, esophagitis, alopecia, and fatigue, which were all acceptable in clinical practices. The addition of pembrolizumab did not lead to the delay of surgery. After the treatment, the pCR reached 55.6%, which was higher than 49% in the CROSS trial and 43.2% in the NEOCRTEC5010 trial (75). The trial, PDL-1 Targeting in Resectable esophageal Cancer (PERFECT), reported the feasibility of atezolizumab combined with the CROSS regimen for locally advanced ESCC. The rate of completion was 83% with no effects on operation interval. 40% of grade ≥3 AEs were observed, of which the most common AEs were anorexia, nausea, and syncope. The rate of immune-related AEs was 16% including 2 for grade 3 rash, 2 for grade 2 colitis/proctitis, and 2 for grade 2 thyroiditis. However, PCR, mOS, and mPFS had no statistical differences compared with a CROSS regimen cohort (114 patients) (76).
As shown above, current studies suggested neoadjuvant immunotherapy combined with chemo(radio)therapy was feasible. Patients who received such modality had a comparable or even higher pCR compared with conventionally neoadjuvant therapy. Notability, most current results are from phase I/II trials. Whether neoadjuvant immunotherapy combined with chemo(radio)therapy can bring a long survival benefit for patients with LAEC requires adequately valid evidence from phase III trials. The synergistic effects between immunotherapy, chemotherapy, and radiation have been reported. Chemotherapy can either lead to immunosuppression or immune activation, which is related to the change of the composition of the tumor microenvironment like priming or inhibiting the expression of immunosuppressor genes (77). Radiation can help expose the tumor antigen to enhance immune response, while immunotherapy can increase the sensitivity of tumors to radiation. These interactive actions should inspire scientists to focus on the sequence between immunotherapy and conventional chemo(radio)therapy. So far, a trial (NCT03985670) is exploring the effect of the sequence of toripalimab and chemotherapy (paclitaxel/cisplatin, sequential or concurrent) on pCR. Initial results showed a fivefold discrepancy in DFS between the two settings (78). Moreover, as the synergistic effects between immunotherapy and radiation, the modality of radiation including dose and fraction may be changed since its role is no longer to lead to cytotoxicity alone but also to assist the immune system. The interactive actions between radiation and tumor microenvironment should be paid attention to. In addition, the KEYNOTE181 trial showed different efficacy in different populations. Also, some research demonstrated that patients with a higher expression of PD-L1 obtain more benefits. All these raise another question about how to screen the benefit population.
Summary
At present, neoadjuvant therapy is the mainstay for patients with locally esophageal carcinoma. Based on several landmark trials, nCRT has been confirmed to be superior to surgery alone in R0 resection, survival outcomes, and recurrence. So far, the standard regimens for nCRT are the paclitaxel–carboplatin-based regimen for EAC or ESCC from the CROSS trial and vinorelbine–cisplatin-based regimen for ESCC from the NEOCRTEC5010 trial. nCT is also a kind of strategy for LAECs. Especially in Japan, based on its own studies, nCT with the CF-based regimen is the standard modality for locally advanced ESCC. The DCF regimen may replace the CF regimen as a new standard regimen for nCT based on the results from the JCOG1109 NExT trial recently. For locally advanced EAC, nCT based on the MAGIC regimen or perioperative chemotherapy based on the FLOT regimen are the main strategies. As the evidence from randomized clinical trials is limited, it is not yet clear which of these two treatment modalities is better. Histologically, the OE02 trial demonstrated that the efficacy of nCT based on the CF regimen is irrespective of the histological type as no heterogeneity of treatment effect (P=0.81). However, the results from meta-analyses demonstrated that nCT did not improve the survival of patients with ESCC(P=0.18) but increased the survival of those with EAC (P=0.01) (79). Besides, as mentioned above, the recurrence pattern between these two pathologic tumor types is different. Patients with EAC are more likely to have distant metastasis, while those with ESCC are more prone to local recurrence. Furthermore, compared with EAC, ESCC is more sensitive to radiation. Considering these facts, patients with locally advanced EAC might be prone to receive preoperative or perioperative chemotherapy, while those with locally advanced ESCC might be prone to choose nCRT. However, it is more essential to depend on individual characteristics and the building of hospital technology such as physical conditions, individual tumor characteristics, the prediction of pCR or recurrence, and a multidisciplinary cancer treatment team. Although nCRT has been regarded as the standard treatment for LAECs, some unsolved clinical problems exist like the optimal regimen, radiation dose, surgical intervals, and a high risk of recurrences. pCR is an important predictor of a good prognosis of patients. However, currently, accurate methods to evaluate pathological response after nCRT are limited. Future studies should focus on the research regarding multiple parameters to predict pCR. Immunotherapy combined with neoadjuvant therapy has shown promising anti-efficacy. For a better synergistic effect, future research should focus on the sequence of immunotherapy and chemo(radio)therapy and biomarkers to the recognized beneficiary population.
Author Contributions
Conceptualization: RH, ZQ, and EL. Writing – Original Draft Preparation: RH and ZQ. Writing – Review and Editing: YX and EL. Funding Acquisition: YX and EL. All authors read and approved the final manuscript.
Funding
This work was supported by funding from the Guangdong College Students’ Scientific and Technological Innovation cultivation special fund subsidy project(pdjhb0195; pdjh2020a0218), Cultivation of Guangdong College Students’ Scientific and Technological Innovation (‘Climbing Program’ Special Funds, pdjh2019a0182), the National Undergraduate Training Program for Innovation and Entrepreneurship (201810560037), ‘Young Physician Scientist Cultivation’ Program of Shantou University Medical College-Li Ka Shing Foundation, 2017-2020 (SMLYPSC-01), and the National Natural Science Foundation of China (21907063).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.734581/full#supplementary-material
Glossary
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Ando N, Kato H, Igaki H, Shinoda M, Ozawa S, Shimizu H, et al. A Randomized Trial Comparing Postoperative Adjuvant Chemotherapy With Cisplatin and 5-Fluorouracil Versus Preoperative Chemotherapy for Localized Advanced Squamous Cell Carcinoma of the Thoracic Esophagus (JCOG9907). Ann Surg Oncol (2012) 19(1):68–74. doi: 10.1245/s10434-011-2049-9
3. van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, et al. Preoperative Chemoradiotherapy for Esophageal or Junctional Cancer. N Engl J Med (2012) 366(22):2074–84. doi: 10.1056/NEJMoa1112088
4. Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, et al. Neoadjuvant Chemoradiotherapy Followed by Surgery Versus Surgery Alone for Locally Advanced Squamous Cell Carcinoma of the Esophagus (NEOCRTEC5010): A Phase III Multicenter, Randomized, Open-Label Clinical Trial. J Clin Oncol (2018) 36(27):2796–803. doi: 10.1200/JCO.2018.79.1483
5. Kelsen DP, Ginsberg R, Pajak TF, Sheahan DG, Gunderson L, Mortimer J, et al. Chemotherapy Followed by Surgery Compared With Surgery Alone for Localized Esophageal Cancer. N Engl J Med (1998) 339(27):1979–84. doi: 10.1056/NEJM199812313392704
6. Medical Research Council Oesophageal Cancer Working G. Surgical Resection With or Without Preoperative Chemotherapy in Oesophageal Cancer: A Randomised Controlled Trial. Lancet (2002) 359(9319):1727–33. doi: 10.1016/S0140-6736(02)08651-8
7. Allum WH, Stenning SP, Bancewicz J, Clark PI, Langley RE. Long-Term Results of a Randomized Trial of Surgery With or Without Preoperative Chemotherapy in Esophageal Cancer. J Clin Oncol (2009) 27(30):5062–7. doi: 10.1200/JCO.2009.22.2083
8. Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative Chemotherapy Versus Surgery Alone for Resectable Gastroesophageal Cancer. N Engl J Med (2006) 355(1):11–20. doi: 10.1056/NEJMoa055531
9. Ychou M, Boige V, Pignon JP, Conroy T, Bouche O, Lebreton G, et al. Perioperative Chemotherapy Compared With Surgery Alone for Resectable Gastroesophageal Adenocarcinoma: An FNCLCC and FFCD Multicenter Phase III Trial. J Clin Oncol (2011) 29(13):1715–21. doi: 10.1200/JCO.2010.33.0597
10. Al-Batran SE, Hofheinz RD, Pauligk C, Kopp HG, Haag GM, Luley KB, et al. Histopathological Regression After Neoadjuvant Docetaxel, Oxaliplatin, Fluorouracil, and Leucovorin Versus Epirubicin, Cisplatin, and Fluorouracil or Capecitabine in Patients With Resectable Gastric or Gastro-Oesophageal Junction Adenocarcinoma (FLOT4-AIO): Results From the Phase 2 Part of a Multicentre, Open-Label, Randomised Phase 2/3 Trial. Lancet Oncol (2016) 17(12):1697–708. doi: 10.1016/S1470-2045(16)30531-9
11. Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, et al. Perioperative Chemotherapy With Fluorouracil Plus Leucovorin, Oxaliplatin, and Docetaxel Versus Fluorouracil or Capecitabine Plus Cisplatin and Epirubicin for Locally Advanced, Resectable Gastric or Gastro-Oesophageal Junction Adenocarcinoma (FLOT4): A Randomised, Phase 2/3 Trial. Lancet (2019) 393(10184):1948–57. doi: 10.1016/S0140-6736(18)32557-1
12. Hara H, Tahara M, Daiko H, Kato K, Igaki H, Kadowaki S, et al. Phase II Feasibility Study of Preoperative Chemotherapy With Docetaxel, Cisplatin, and Fluorouracil for Esophageal Squamous Cell Carcinoma. Cancer Sci (2013) 104(11):1455–60. doi: 10.1111/cas.12274
13. Yamashita K, Hosoda K, Moriya H, Katada C, Sugawara M, Mieno H, et al. Prognostic Advantage of Docetaxel/Cisplatin/ 5-Fluorouracil Neoadjuvant Chemotherapy in Clinical Stage II/III Esophageal Squamous Cell Carcinoma Due to Excellent Control of Preoperative Disease and Postoperative Lymph Node Recurrence. Oncology (2017) 92(4):221–8. doi: 10.1159/000455128
14. Akiyama Y, Iwaya T, Endo F, Chiba T, Takahara T, Otsuka K, et al. Investigation of Operative Outcomes of Thoracoscopic Esophagectomy After Triplet Chemotherapy With Docetaxel, Cisplatin, and 5-Fluorouracil for Advanced Esophageal Squamous Cell Carcinoma. Surg Endosc (2018) 32(1):391–9. doi: 10.1007/s00464-017-5688-5
15. Nomura M, Oze I, Abe T, Komori A, Narita Y, Masuishi T, et al. Impact of Docetaxel in Addition to Cisplatin and Fluorouracil as Neoadjuvant Treatment for Resectable Stage III or T3 Esophageal Cancer: A Propensity Score-Matched Analysis. Cancer Chemother Pharmacol (2015) 76(2):357–63. doi: 10.1007/s00280-015-2806-8
16. Kato K, Ito Y, Daiko H, Ozawa S, Ogata T, Hara H, et al. A Randomized Controlled Phase III Trial Comparing Two Chemotherapy Regimen and Chemoradiotherapy Regimen as Neoadjuvant Treatment for Locally Advanced Esophageal Cancer, JCOG1109 NExT Study. J Clin Oncol (2022) 40(4_suppl):238–. doi: 10.1200/JCO.2022.40.4_suppl.238
17. Shiraishi O, Yamasaki M, Makino T, Motoori M, Miyata H, Shinkai M, et al. Feasibility of Preoperative Chemotherapy With Docetaxel, Cisplatin, and 5-Fluorouracil Versus Adriamycin, Cisplatin, and 5-Fluorouracil for Resectable Advanced Esophageal Cancer. Oncology (2017) 92(2):101–8. doi: 10.1159/000452765
18. Hosoda K, Azuma M, Katada C, Ishido K, Niihara M, Ushiku H, et al. A Phase I Study of Docetaxel/Oxaliplatin/S-1 (DOS) Combination Neoadjuvant Chemotherapy for Patients With Locally Advanced Adenocarcinoma of the Esophagogastric Junction. Int J Clin Oncol (2020) 25(6):1090–7. doi: 10.1007/s10147-020-01638-5
19. Hayata K, Ojima T, Nakamori M, Nakamura M, Katsuda M, Kitadani J, et al. Neoadjuvant Chemotherapy With Docetaxel, Cisplatin and S-1 for Resectable Advanced Esophageal Cancer. Anticancer Res (2018) 38(9):5267–73. doi: 10.21873/anticanres.12852
20. Ohnuma H, Sato Y, Hayasaka N, Matsuno T, Fujita C, Sato M, et al. Neoadjuvant Chemotherapy With Docetaxel, Nedaplatin, and Fluorouracil for Resectable Esophageal Cancer: A Phase II Study. Cancer Sci (2018) 109(11):3554–63. doi: 10.1111/cas.13772
21. Alderson D, Cunningham D, Nankivell M, Blazeby JM, Griffin SM, Crellin A, et al. Neoadjuvant Cisplatin and Fluorouracil Versus Epirubicin, Cisplatin, and Capecitabine Followed by Resection in Patients With Oesophageal Adenocarcinoma (UK MRC OE05): An Open-Label, Randomised Phase 3 Trial. Lancet Oncol (2017) 18(9):1249–60. doi: 10.1016/S1470-2045(17)30447-3
22. Tanaka Y, Yoshida K, Tanahashi T, Okumura N, Matsuhashi N, Yamaguchi K. Phase II Trial of Neoadjuvant Chemotherapy With Docetaxel, Nedaplatin, and S1 for Advanced Esophageal Squamous Cell Carcinoma. Cancer Sci (2016) 107(6):764–72. doi: 10.1111/cas.12943
23. Walsh TN, Noonan N, Hollywood D, Kelly A, Keeling N, Hennessy TP. A Comparison of Multimodal Therapy and Surgery for Esophageal Adenocarcinoma. N Engl J Med (1996) 335(7):462–7. doi: 10.1056/NEJM199608153350702
24. Bosset JF, Gignoux M, Triboulet JP, Tiret E, Mantion G, Elias D, et al. Chemoradiotherapy Followed by Surgery Compared With Surgery Alone in Squamous-Cell Cancer of the Esophagus. N Engl J Med (1997) 337(3):161–7. doi: 10.1056/NEJM199707173370304
25. Burmeister BH, Smithers BM, Gebski V, Fitzgerald L, Simes RJ, Devitt P, et al. Surgery Alone Versus Chemoradiotherapy Followed by Surgery for Resectable Cancer of the Oesophagus: A Randomised Controlled Phase III Trial. Lancet Oncol (2005) 6(9):659–68. doi: 10.1016/S1470-2045(05)70288-6
26. Tepper J, Krasna MJ, Niedzwiecki D, Hollis D, Reed CE, Goldberg R, et al. Phase III Trial of Trimodality Therapy With Cisplatin, Fluorouracil, Radiotherapy, and Surgery Compared With Surgery Alone for Esophageal Cancer: CALGB 9781. J Clin Oncol (2008) 26(7):1086–92. doi: 10.1200/JCO.2007.12.9593
27. Shapiro J, van Lanschot JJB, Hulshof M, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, et al. Neoadjuvant Chemoradiotherapy Plus Surgery Versus Surgery Alone for Oesophageal or Junctional Cancer (CROSS): Long-Term Results of a Randomised Controlled Trial. Lancet Oncol (2015) 16(9):1090–8. doi: 10.1016/S1470-2045(15)00040-6
28. Eyck BM, van Lanschot JJB, Hulshof M, van der Wilk BJ, Shapiro J, van Hagen P, et al. Ten-Year Outcome of Neoadjuvant Chemoradiotherapy Plus Surgery for Esophageal Cancer: The Randomized Controlled CROSS Trial. J Clin Oncol (2021) 39(18):1995–2004. doi: 10.1200/JCO.20.03614
29. Stahl M, Walz MK, Stuschke M, Lehmann N, Meyer HJ, Riera-Knorrenschild J, et al. Phase III Comparison of Preoperative Chemotherapy Compared With Chemoradiotherapy in Patients With Locally Advanced Adenocarcinoma of the Esophagogastric Junction. J Clin Oncol (2009) 27(6):851–6. doi: 10.1200/JCO.2008.17.0506
30. Stahl M, Walz MK, Riera-Knorrenschild J, Stuschke M, Sandermann A, Bitzer M, et al. Preoperative Chemotherapy Versus Chemoradiotherapy in Locally Advanced Adenocarcinomas of the Oesophagogastric Junction (POET): Long-Term Results of a Controlled Randomised Trial. Eur J Cancer (2017) 81:183–90. doi: 10.1016/j.ejca.2017.04.027
31. Klevebro F, Alexandersson von Dobeln G, Wang N, Johnsen G, Jacobsen AB, Friesland S, et al. A Randomized Clinical Trial of Neoadjuvant Chemotherapy Versus Neoadjuvant Chemoradiotherapy for Cancer of the Oesophagus or Gastro-Oesophageal Junction. Ann Oncol (2016) 27(4):660–7. doi: 10.1093/annonc/mdw010
32. von Dobeln GA, Klevebro F, Jacobsen AB, Johannessen HO, Nielsen NH, Johnsen G, et al. Neoadjuvant Chemotherapy Versus Neoadjuvant Chemoradiotherapy for Cancer of the Esophagus or Gastroesophageal Junction: Long-Term Results of a Randomized Clinical Trial. Dis Esophagus (2019) 32(2):1–11. doi: 10.1093/dote/doy078
33. Burmeister BH, Thomas JM, Burmeister EA, Walpole ET, Harvey JA, Thomson DB, et al. Is Concurrent Radiation Therapy Required in Patients Receiving Preoperative Chemotherapy for Adenocarcinoma of the Oesophagus? A Randomised Phase II Trial. Eur J Cancer (2011) 47(3):354–60. doi: 10.1016/j.ejca.2010.09.009
34. Wang H, Tang H, Fang Y, Tan L, Yin J, Shen Y, et al. Morbidity and Mortality of Patients Who Underwent Minimally Invasive Esophagectomy After Neoadjuvant Chemoradiotherapy vs Neoadjuvant Chemotherapy for Locally Advanced Esophageal Squamous Cell Carcinoma: A Randomized Clinical Trial. JAMA Surg (2021) 156(5):444–51. doi: 10.1001/jamasurg.2021.0133
35. Yuan M, Bao Y, Ma Z, Men Y, Wang Y, Hui Z. The Optimal Treatment for Resectable Esophageal Cancer: A Network Meta-Analysis of 6168 Patients. Front Oncol (2021) 11:628706. doi: 10.3389/fonc.2021.628706
36. Chan KKW, Saluja R, Delos Santos K, Lien K, Shah K, Cramarossa G, et al. Neoadjuvant Treatments for Locally Advanced, Resectable Esophageal Cancer: A Network Meta-Analysis. Int J Cancer (2018) 143(2):430–7. doi: 10.1002/ijc.31312
37. Lu Z, Fang Y, Liu C, Zhang X, Xin X, He Y, et al. Early Interdisciplinary Supportive Care in Patients With Previously Untreated Metastatic Esophagogastric Cancer: A Phase III Randomized Controlled Trial. J Clin Oncol (2021) 39(7):748–56. doi: 10.1200/JCO.20.01254
38. Shirakawa Y, Noma K, Maeda N, Tanabe S, Sakurama K, Sonoyama-Hanaoka A, et al. Early Intervention of the Perioperative Multidisciplinary Team Approach Decreases the Adverse Events During Neoadjuvant Chemotherapy for Esophageal Cancer Patients. Esophagus (2021) 18(4):797–805. doi: 10.1007/s10388-021-00844-y
39. Gottlieb-Vedi E, Kauppila JH, Mattsson F, Lindblad M, Nilsson M, Lagergren P, et al. Long-Term Survival in Esophageal Cancer After Minimally Invasive Esophagectomy Compared to Open Esophagectomy. Ann Surg (2021). doi: 10.1097/SLA.0000000000004645
40. Wang HH, de Heer EC, Hulshoff JB, Kats-Ugurlu G, Burgerhof JGM, van Etten B, et al. Effect of Extending the Original CROSS Criteria on Tumor Response to Neoadjuvant Chemoradiotherapy in Esophageal Cancer Patients: A National Multicenter Cohort Analysis. Ann Surg Oncol (2021) 28(7):3951–60. doi: 10.1245/s10434-020-09372-y
41. Wong IYH, Lam KO, Chan W, Wong C, So TH, Chan KK, et al. Real-World Scenario: CROSS Regimen as Preoperative Therapy for Oesophageal Squamous Cell Carcinoma. J Gastrointest Surg (2020) 24(9):1937–47. doi: 10.1007/s11605-020-04704-5
42. Toxopeus E, van der Schaaf M, van Lanschot J, Lagergren J, Lagergren P, van der Gaast A, et al. Outcome of Patients Treated Within and Outside a Randomized Clinical Trial on Neoadjuvant Chemoradiotherapy Plus Surgery for Esophageal Cancer: Extrapolation of a Randomized Clinical Trial (CROSS). Ann Surg Oncol (2018) 25(8):2441–8. doi: 10.1245/s10434-018-6554-y
43. de Heer EC, Hulshoff JB, Klerk D, Burgerhof JGM, de Groot DJA, Plukker JTM, et al. Effect of Extending the Original Eligibility Criteria for the CROSS Neoadjuvant Chemoradiotherapy on Toxicity and Survival in Esophageal Cancer. Ann Surg Oncol (2017) 24(7):1811–20. doi: 10.1245/s10434-017-5797-3
44. Wong IYH, Lam KO, Zhang RQ, Chan WWL, Wong CLY, Chan FSY, et al. Neoadjuvant Chemoradiotherapy Using Cisplatin and 5-Fluorouracil (PF) Versus Carboplatin and Paclitaxel (CROSS Regimen) for Esophageal Squamous Cell Carcinoma (ESCC): A Propensity Score-Matched Study. Ann Surg (2020) 272(5):779–85. doi: 10.1097/SLA.0000000000004329
45. Haisley KR, Hart KD, Nabavizadeh N, Bensch KG, Vaccaro GM, Thomas CR Jr., et al. Neoadjuvant Chemoradiotherapy With Concurrent Cisplatin/5-Fluorouracil is Associated With Increased Pathologic Complete Response and Improved Survival Compared to Carboplatin/Paclitaxel in Patients With Locally Advanced Esophageal Cancer. Dis Esophagus (2017) 30(7):1–7. doi: 10.1093/dote/dox015
46. Liu SL, Yang H, Zhang P, Zhang L, Zhao L, Luo LL, et al. Neoadjuvant Chemoradiotherapy With Cisplatin Plus Vinorelbine Versus Cisplatin Plus Fluorouracil for Esophageal Squamous Cell Carcinoma: A Matched Case-Control Study. Radiother Oncol (2015) 116(2):262–8. doi: 10.1016/j.radonc.2015.07.020
47. Yang Y, Xu X, Zhou X, Bao W, Zhang D, Gu F, et al. Impact of Radiation Dose on Survival for Esophageal Squamous Cell Carcinoma Treated With Neoadjuvant Chemoradiotherapy. Front Oncol (2020) 10:1431. doi: 10.3389/fonc.2020.01431
48. Li Y, Liu H, Sun C, Yin X, Tong J, Zhang X, et al. Comparison of Clinical Efficacy of Neoadjuvant Chemoradiation Therapy Between Lower and Higher Radiation Doses for Carcinoma of the Esophagus and Gastroesophageal Junction: A Systematic Review. Int J Radiat Oncol Biol Phys (2021) 111(2):405–16. doi: 10.1016/j.ijrobp.2021.04.031
49. Haisley KR, Laird AE, Nabavizadeh N, Gatter KM, Holland JM, Vaccaro GM, et al. Association of Intervals Between Neoadjuvant Chemoradiation and Surgical Resection With Pathologic Complete Response and Survival in Patients With Esophageal Cancer. JAMA Surg (2016) 151(11):e162743. doi: 10.1001/jamasurg.2016.2743
50. Shapiro J, van Hagen P, Lingsma HF, Wijnhoven BP, Biermann K, ten Kate FJ, et al. Prolonged Time to Surgery After Neoadjuvant Chemoradiotherapy Increases Histopathological Response Without Affecting Survival in Patients With Esophageal or Junctional Cancer. Ann Surg (2014) 260(5):807–13; discussion 13-4. doi: 10.1097/SLA.0000000000000966
51. Nilsson K, Klevebro F, Rouvelas I, Lindblad M, Szabo E, Halldestam I, et al. Surgical Morbidity and Mortality From the Multicenter Randomized Controlled NeoRes II Trial: Standard Versus Prolonged Time to Surgery After Neoadjuvant Chemoradiotherapy for Esophageal Cancer. Ann Surg (2020) 272(5):684–9. doi: 10.1097/SLA.0000000000004340
52. Liu S, Wen J, Yang H, Li Q, Chen Y, Zhu C, et al. Recurrence Patterns After Neoadjuvant Chemoradiotherapy Compared With Surgery Alone in Oesophageal Squamous Cell Carcinoma: Results From the Multicenter Phase III Trial NEOCRTEC5010. Eur J Cancer (2020) 138:113–21. doi: 10.1016/j.ejca.2020.08.002
53. Xi M, Yang Y, Zhang L, Yang H, Merrell KW, Hallemeier CL, et al. Multi-Institutional Analysis of Recurrence and Survival After Neoadjuvant Chemoradiotherapy of Esophageal Cancer: Impact of Histology on Recurrence Patterns and Outcomes. Ann Surg (2019) 269(4):663–70. doi: 10.1097/SLA.0000000000002670
54. Kelly RJ, Ajani JA, Kuzdzal J, Zander T, Van Cutsem E, Piessen G, et al. Adjuvant Nivolumab in Resected Esophageal or Gastroesophageal Junction Cancer. N Engl J Med (2021) 384(13):1191–203. doi: 10.1056/NEJMoa2032125
55. Guo X, Wang Z, Yang H, Mao T, Chen Y, Zhu C, et al. Impact of Lymph Node Disscinoma: From the Results of NEOCRTEC5010, a Randomized Multicenter Study. Ann Surg (2021). doi: 10.1097/SLA.0000000000004798
56. Eyck BM, Onstenk BD, Noordman BJ, Nieboer D, Spaander MCW, Valkema R, et al. Accuracy of Detecting Residual Disease After Neoadjuvant Chemoradiotherapy for Esophageal Cancer: A Systematic Review and Meta-Analysis. Ann Surg (2020) 271(2):245–56. doi: 10.1097/SLA.0000000000003397
57. van Rossum PS, van Lier AL, van Vulpen M, Reerink O, Lagendijk JJ, Lin SH, et al. Diffusion-Weighted Magnetic Resonance Imaging for the Prediction of Pathologic Response to Neoadjuvant Chemoradiotherapy in Esophageal Cancer. Radiother Oncol (2015) 115(2):163–70. doi: 10.1016/j.radonc.2015.04.027
58. Heethuis SE, van Rossum PS, Lips IM, Goense L, Voncken FE, Reerink O, et al. Dynamic Contrast-Enhanced MRI for Treatment Response Assessment in Patients With Oesophageal Cancer Receiving Neoadjuvant Chemoradiotherapy. Radiother Oncol (2016) 120(1):128–35. doi: 10.1016/j.radonc.2016.05.009
59. Heethuis SE, Goense L, van Rossum PSN, Borggreve AS, Mook S, Voncken FEM, et al. DW-MRI and DCE-MRI are of Complementary Value in Predicting Pathologic Response to Neoadjuvant Chemoradiotherapy for Esophageal Cancer. Acta Oncol (2018) 57(9):1201–8. doi: 10.1080/0284186X.2018.1473637
60. Vollenbrock SE, van Dieren JM, Voncken FEM, van Turenhout ST, Kodach LL, Hartemink KJ, et al. Added Value of MRI to Endoscopic and Endosonographic Response Assessment After Neoadjuvant Chemoradiotherapy in Oesophageal Cancer. Eur Radiol (2020) 30(5):2425–34. doi: 10.1007/s00330-019-06605-x
61. Borggreve AS, Goense L, van Rossum PSN, Heethuis SE, van Hillegersberg R, Lagendijk JJW, et al. Preoperative Prediction of Pathologic Response to Neoadjuvant Chemoradiotherapy in Patients With Esophageal Cancer Using (18)F-FDG PET/CT and DW-MRI: A Prospective Multicenter Study. Int J Radiat Oncol Biol Phys (2020) 106(5):998–1009. doi: 10.1016/j.ijrobp.2019.12.038
62. Noordman BJ, Spaander MCW, Valkema R, Wijnhoven BPL, van Berge Henegouwen MI, Shapiro J, et al. Detection of Residual Disease After Neoadjuvant Chemoradiotherapy for Oesophageal Cancer (preSANO): A Prospective Multicentre, Diagnostic Cohort Study. Lancet Oncol (2018) 19(7):965–74. doi: 10.1016/S1470-2045(18)30201-8
63. Zhang SS, Huang QY, Yang H, Xie X, Luo KJ, Wen J, et al. Correlation of P53 Status With the Response to Chemotherapy-Based Treatment in Esophageal Cancer: A Meta-Analysis. Ann Surg Oncol (2013) 20(7):2419–27. doi: 10.1245/s10434-012-2859-4
64. Li Y, Huang HC, Chen LQ, Xu LY, Li EM, Zhang JJ. Predictive Biomarkers for Response of Esophageal Cancer to Chemo(Radio)Therapy: A Systematic Review and Meta-Analysis. Surg Oncol (2017) 26(4):460–72. doi: 10.1016/j.suronc.2017.09.003
65. Ruhstaller T, Thuss-Patience P, Hayoz S, Schacher S, Knorrenschild JR, Schnider A, et al. Neoadjuvant Chemotherapy Followed by Chemoradiation and Surgery With and Without Cetuximab in Patients With Resectable Esophageal Cancer: A Randomized, Open-Label, Phase III Trial (SAKK 75/08). Ann Oncol (2018) 29(6):1386–93. doi: 10.1093/annonc/mdy105
66. Cunningham D, Stenning SP, Smyth EC, Okines AF, Allum WH, Rowley S, et al. Peri-Operative Chemotherapy With or Without Bevacizumab in Operable Oesophagogastric Adenocarcinoma (UK Medical Research Council ST03): Primary Analysis Results of a Multicentre, Open-Label, Randomised Phase 2-3 Trial. Lancet Oncol (2017) 18(3):357–70. doi: 10.1016/S1470-2045(17)30043-8
67. Kojima T, Shah MA, Muro K, Francois E, Adenis A, Hsu CH, et al. Randomized Phase III KEYNOTE-181 Study of Pembrolizumab Versus Chemotherapy in Advanced Esophageal Cancer. J Clin Oncol (2020) 38(35):4138–48. doi: 10.1200/JCO.20.01888
68. Kato K, Cho BC, Takahashi M, Okada M, Lin CY, Chin K, et al. Nivolumab Versus Chemotherapy in Patients With Advanced Oesophageal Squamous Cell Carcinoma Refractory or Intolerant to Previous Chemotherapy (ATTRACTION-3): A Multicentre, Randomised, Open-Label, Phase 3 Trial. Lancet Oncol (2019) 20(11):1506–17. doi: 10.1016/S1470-2045(19)30626-6
69. Huang J, Xu J, Chen Y, Zhuang W, Zhang Y, Chen Z, et al. Camrelizumab Versus Investigator's Choice of Chemotherapy as Second-Line Therapy for Advanced or Metastatic Oesophageal Squamous Cell Carcinoma (ESCORT): A Multicentre, Randomised, Open-Label, Phase 3 Study. Lancet Oncol (2020) 21(6):832–42. doi: 10.1016/S1470-2045(20)30110-8
70. Kato K, Sun JM, Shah MA, Enzinger PC, Adenis A, Doi T, et al. LBA8_PR Pembrolizumab Plus Chemotherapy Versus Chemotherapy as First-Line Therapy in Patients With Advanced Esophageal Cancer: The Phase 3 KEYNOTE-590 Study. Ann Oncol (2020) 31:S1192–S3. doi: 10.1016/j.annonc.2020.08.2298
71. Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. First-Line Nivolumab Plus Chemotherapy Versus Chemotherapy Alone for Advanced Gastric, Gastro-Oesophageal Junction, and Oesophageal Adenocarcinoma (CheckMate 649): A Randomised, Open-Label, Phase 3 Trial. Lancet (2021) 398(10294):27–40. doi: 10.1016/S0140-6736(21)00797-2
72. Xu R-h, Luo H, Lu J, Bai Y, Mao T, Wang J, et al. ESCORT-1st: A Randomized, Double-Blind, Placebo-Controlled, Phase 3 Trial of Camrelizumab Plus Chemotherapy Versus Chemotherapy in Patients With Untreated Advanced or Metastatic Esophageal Squamous Cell Carcinoma (ESCC). J Clin Oncol (2021) 39(15_suppl):4000–. doi: 10.1200/JCO.2021.39.15_suppl.4000
73. Kelly RJ, Smith KN, Anagnostou V, Thompson E, Hales RK, Battafarano RJJ, et al. Neoadjuvant Nivolumab Plus Concurrent Chemoradiation in Stage II/III Esophageal/Gastroesophageal Junction Cancer. J Clin Oncol (2019) 37(4_suppl):142–. doi: 10.1200/JCO.2019.37.4_suppl.142
74. Hong MH, Kim H, Park SY, Kim DJ, Lee CG, Cho J, et al. A Phase II Trial of Preoperative Chemoradiotherapy and Pembrolizumab for Locally Advanced Esophageal Squamous Cell Carcinoma (ESCC). J Clin Oncol (2019) 37(15_suppl):4027–. doi: 10.1200/JCO.2019.37.15_suppl.4027
75. Li C, Zhao S, Zheng Y, Han Y, Chen X, Cheng Z, et al. Preoperative Pembrolizumab Combined With Chemoradiotherapy for Oesophageal Squamous Cell Carcinoma (PALACE-1). Eur J Cancer (2021) 144:232–41. doi: 10.1016/j.ejca.2020.11.039
76. tEnde T, Clercq N, Henegouwen M, Gisbertz SS, Meijer SL, Schokker S, et al. A Phase II Feasibility Trial of Neoadjuvant Chemoradiotherapy Combined With Atezolizumab for Resectable Esophageal Adenocarcinoma: The PERFECT Trial. J Clin Oncol (2019) 37(15_suppl):4045–. doi: 10.1200/JCO.2019.37.15_suppl.4045
77. Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunological Effects of Conventional Chemotherapy and Targeted Anticancer Agents. Cancer Cell (2015) 28(6):690–714. doi: 10.1016/j.ccell.2015.10.012
78. Zhao L, Xing W, Yang Y, Zhang Y, Ma B, Fu X, et al. The Sequence of Chemotherapy and Anti-PD-1 Antibody Influence the Efficacy of Neoadjuvant Immunochemotherapy in Locally Advanced Esophageal Squamous Cell Cancer: A Phase II Study. J Clin Oncol (2021) 39(15_suppl):4051–. doi: 10.1200/JCO.2021.39.15_suppl.4051
Keywords: locally advanced esophageal cancers, neoadjuvant, chemoradiotherapy, chemotherapy, immunotherapy
Citation: Huang R, Qiu Z, Zheng C, Zeng R, Chen W, Wang S, Li E and Xu Y (2022) Neoadjuvant Therapy for Locally Advanced Esophageal Cancers. Front. Oncol. 12:734581. doi: 10.3389/fonc.2022.734581
Received: 01 July 2021; Accepted: 09 March 2022;
Published: 07 April 2022.
Edited by:
Sripathi Sureban, University of Oklahoma Health Sciences Center, United StatesReviewed by:
Mohamed Rahouma, NewYork-Presbyterian, United StatesDongryul Oh, Sungkyunkwan University, South Korea
Copyright © 2022 Huang, Qiu, Zheng, Zeng, Chen, Wang, Li and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yiwei Xu, eWl3ZWk1MTJAMTI2LmNvbQ==; Enmin Li, bm1saUBzdHUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Runkai Huang
Runkai Huang Zhenbin Qiu
Zhenbin Qiu Chunwen Zheng1
Chunwen Zheng1 Ruijie Zeng
Ruijie Zeng Wanxian Chen
Wanxian Chen Simeng Wang
Simeng Wang Yiwei Xu
Yiwei Xu