- 1Severance Biomedical Science Institute, Yonsei University College of Medicine, Seoul, South Korea
- 2Yonsei New Il Han Institute for Integrative Lung Cancer Research, Yonsei University College of Medicine, Seoul, South Korea
- 3International Centre for Diarrhoeal Disease Research (iccdr,b), Dhaka, Bangladesh
- 4Shenzhen Institute of Advanced Technology (SIAT), Chinese Academy of Sciences (CAS), Shenzhen, China
- 5Institute of Biochemistry and Cell Biology, National Research Council (CNR), Napoli, Italy
- 6Stazione Zoologica Anton Dohrn (SZN), Napoli, Italy
- 7China-Italy Joint Laboratory of Pharmacobiotechnology for Medical Immunomodulation, Shenzhen, China
Editorial on the Research Topic
Development of small molecule inhibitors and antibodies targeting AXL for tumor therapy and infectious disease control
For a long time, anti-cancer therapeutic strategies have mainly addressed the tumor, in the attempt to subvert the altered signaling pathways leading to oncogenesis (1, 2). It is however evident that the tumor microenvironment plays a major role in determining tumor survival and growth. In particular, the role of immunity in eliminating or promoting tumor growth has been now clearly recognized (3–5).
The complex nature of tumorigenesis requires the identification of different therapeutic targets and the implementation of different therapeutic approaches, including drugs/strategies that could overcome resistance after treatment. The immunotherapeutic approaches with immune checkpoint inhibitors are an example of treatment that does not induce resistance, as its target is not the tumor but the re-activation of specific tumoricidal immune cells (6, 7). Thus, the development of anti-PD-1 and anti-PD-L1 immune checkpoint inhibitors has opened the way to novel anti-tumor approaches that go beyond inhibiting oncogenic signals and address the tumor microenvironment as a whole. In this context, AXL is an attractive target for designing future treatment targets.
AXL is a receptor tyrosine kinase of the TAM (TYRO3-AXL-MER) family (8–10). High AXL expression in tumor cells promotes aggressiveness, metastatic capacity and refractoriness to drug treatment (11, 12). In parallel, it was observed that the expression of AXL and other TAM receptors is higher in macrophages polarized towards the M2 anti-inflammatory pro-tumoral functional phenotype and that activation of AXL can induce M2 macrophages polarization (13–16). Since the M2 phenotype is typical of pro-tumoral tumor-associated macrophages, this points to AXL inhibition as a double target to reduce the aggressiveness of tumor cells and the tumor-promoting activity of the innate immune cells (macrophages). Importantly, AXL has been identified as a receptor used by several viruses (Zika, Dengue, RSV, JEV, SARS-CoV-2) for entry into host cells and spreading infections (17–20). Thus, inhibition of AXL in infectious diseases could have the double advantage of blocking virus entry and allowing protective macrophage activation (M1 polarization), by inhibiting M2 development. For these reasons, new drugs targeting AXL are being developed. An overview of AXL role in tumor and immune cells and the potential benefits of its inhibition are presented in Figure 1.
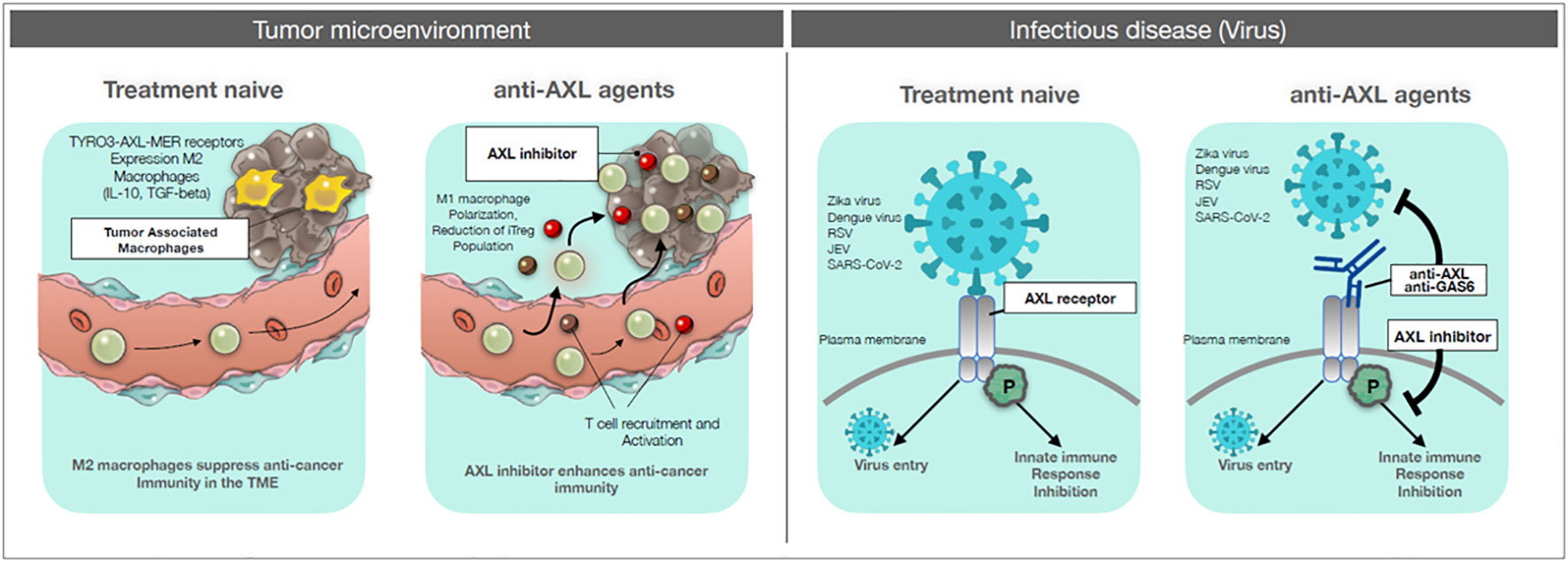
Figure 1 AXL targeting drugs on cancer and infectious diseases. Left panels: Tumor microenvironment. Left: treatment-naïve: circulating monocytes and tissue-resident macrophages are recruited to the tumor site. These tumor-associated macrophages are polarized towards an M2 functional phenotype, which can suppress anti-tumor response and promote tumor growth. Right: anti-AXL agents: by blocking the signaling pathway of AXL with anti-AXL agents, recruited monocytes and macrophages can be polarized towards the inflammatory, immunostimulatory and cytocidal M1 phenotype, thereby allowing the development of anti-tumor immune responses and also acting as anti-tumor killer cells. Right panels: Infectious diseases. Left: treatment-naïve: viruses (Zika, Dengue, RSV, JEV, SARS-CoV-2) can gain entry into the host cell through interaction with an AXL receptor, thereby efficiently infecting the host. In addition, viral binding can induce an AXL-mediated inhibitory signaling for innate immune responses. Right: anti-AXL agents: anti-AXL agents that bind to the extracellular domain of AXL can block virus binding and subsequent infection. Also, anti-AXL agents that block the virus-induced AXL inhibitory signaling can restore efficient innate immunity against the viral infection.
New technologies to suppress AXL functions include inhibition of downstream signaling with antibodies or blockade of GAS6, the ligand of AXL, and the use of small molecules that can inhibit AXL phosphorylation. The state-of-the-art of the use of AXL inhibitors in cancer therapy is reported by Tanaka et al., who have extensively described the AXL signaling pathway and listed the most promising AXL inhibitors that have been developed clinically or preclinically (21). The review presents the anti-tumor mechanisms of AXL inhibitors, and the i privement of therapeutic efficacy and immunoactivity of chemotherapy currently use in clinical practice (21).
The contribution of AXL to immune evasion underlines the importance of its inhibition in anti-tumor therapies. GAS6/AXL signaling can participate to evasion from anti-tumor immune response within the tumor microenvironment in multiple ways (Son and Jeong). These include modulation of MHC-1 and PD-L1 expression, production of immunosuppressive cytokines, increased recruitment of myeloid derived suppressor cells and decreased infiltration of T cells, inhibition of apoptosis, promotion of CTLA4 and Foxp3 expression in Treg cells and of their immunosuppressive activity, and regulation of tumor associated innate immune cells. The use of AXL inhibitors, both in preclinical studies and in clinical trials, provides valuable information on the modulation of GAS6/AXL-mediated immune evasion (Son and Jeong). Not only AXL but also other receptors of the family, such as TYRO3, are strongly involve in the regulation of tumor cell apoptosis, as demonstrated in colon carcinoma in vitro and in vivo models by using a specific inhibitor (Kim et al.).
The potential application of AXL inhibitor as a single agent or in combination with immunotherapy in patients with lung cancer has been investigated based on clinical treatment results and treatment advantages (Sang et al.). AXL inhibitors have shown therapeutic benefits in combination with chemotherapy to increase survival rate through enhanced activation of immune cells (Lee et al.). However, detailed anti-tumor effects of AXL signaling pathway is yet to be understood.
While the current development of AXL inhibitors offers promising opportunities for effective therapeutic intervention against cancer, future research should aim at fully clarifying the effects of AXL and, more in general, TAM inhibitors. This should start from a complete understanding of the physiological role of these receptors and the assessment of their pathology-linked molecular and functional variations. On these grounds, AXL inhibition can be precisely targeted, to attain high therapeutic efficacy and limited side effects. The possibility of modulating AXL functions in macrophages and in virus-host interactions opens a large spectrum of therapeutic/preventive possibilities beyond cancer.
Author contributions
KP wrote the editorial, RM and DB contributed to writing the manuscript and critically revised it. All authors approved the submitted version.
Funding
This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MSIT) (No. 2022M3E5F3081138). KHP was supported by a Dongin Sports research grant of Yonsei University College of Medicine (6-2020-0172). DB was supported by the EU H2020 project PANDORA (GA 671881) and by the Presidential International Fellowship Initiative (PIFI) of the Chinese Academy of Sciences (2020VBA0028).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Falzone L, Salomone S, Libra M. Evolution of cancer pharmacological treatments at the turn of the third millennium. Front Pharmacol (2018) 9:1300.
2. Huang L, Jiang S, Shi Y. Tyrosine kinase inhibitors for solid tumors in the past 20 years (2001-2020). J Hematol Oncol (2020) 13:143.
3. Galli F, Aguilera JV, Palermo B, Markovic SN, Nistico P, Signore A. Relevance of immune cell and tumor microenvironment imaging in the new era of immunotherapy. J Exp Clin Cancer Res (2020) 39:89.
4. Lei X, Lei Y, Li JK, Du WX, Li RG, Yang J, et al. Immune cells within the tumor microenvironment: Biological functions and roles in cancer immunotherapy. Cancer Lett (2020) 470:126–33.
5. Mao X, Xu J, Wang W, Liang C, Hua J, Liu J, et al. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives. Mol Cancer (2021) 20:131.
6. Zhang Y, Zhang Z. The history and advances in cancer immunotherapy: Understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol (2020) 17:807–21.
7. Jacob JB, Jacob MK, Parajuli P. Review of immune checkpoint inhibitors in immuno-oncology. Adv Pharmacol (2021) 91:111–39.
8. Wu G, Ma Z, Cheng Y, Hu W, Deng C, Jiang S, et al. Targeting Gas6/TAM in cancer cells and tumor microenvironment. Mol Cancer (2018) 17:20.
9. Burstyn-Cohen T, Maimon A. TAM receptors, phosphatidylserine, inflammation, and cancer. Cell Commun Signal (2019) 17:156.
10. Ghosh Roy S. TAM receptors: a phosphatidylserine receptor family and its implications in viral infections. Int Rev Cell Mol Biol (2020) 357:81–122.
11. Shieh YS, Lai CY, Kao YR, Shiah SG, Chu YW, Lee HS, et al. Expression of axl in lung adenocarcinoma and correlation with tumor progression. Neoplasia (2005) 7:1058–64.
12. Chai ZT, Zhang XP, Ao JY, Zhu XD, Wu MC, Lau WY, et al. AXL overexpression in tumor-derived endothelial cells promotes vessel metastasis in patients with hepatocellular carcinoma. Front Oncol (2021) 11:650963.
13. Fujimori T, Grabiec AM, Kaur M, Bell TJ, Fujino N, Cook PC, et al. The axl receptor tyrosine kinase is a discriminator of macrophage function in the inflamed lung. Mucosal Immunol (2015) 8:1021–30.
14. Chiu KC, Lee CH, Liu SY, Chou YT, Huang RY, Huang SM, et al. Polarization of tumor-associated macrophages and Gas6/Axl signaling in oral squamous cell carcinoma. Oral Oncol (2015) 51:683–9.
15. Chen SY, Chiang CF, Chiu KC, Cheng CW, Huang SM, Chen PH, et al. Macrophage phenotypes and Gas6/Axl signaling in apical lesions. J Dent Sci (2019) 14:281–7.
16. Jimenez-Garcia L, Mayer C, Burrola PG, Huang Y, Shokhirev MN, Lemke G. The TAM receptor tyrosine kinases axl and mer drive the maintenance of highly phagocytic macrophages. Front Immunol (2022) 13:960401.
17. Shibata T, Makino A, Ogata R, Nakamura S, Ito T, Nagata K, et al. Respiratory syncytial virus infection exacerbates pneumococcal pneumonia via Gas6/Axl-mediated macrophage polarization. J Clin Invest (2020) 130:3021–37.
18. Wang S, Qiu Z, Hou Y, Deng X, Xu W, Zheng T, et al. AXL is a candidate receptor for SARS-CoV-2 that promotes infection of pulmonary and bronchial epithelial cells. Cell Res (2021) 31:126–40.
19. Wang ZY, Zhen ZD, Fan DY, Qin CF, Han DS, Zhou HN, et al. Axl deficiency promotes the neuroinvasion of Japanese encephalitis virus by enhancing IL-1α production from pyroptotic macrophages. J Virol (2020) 94:e00606–20.
20. Xie S, Zhang H, Liang Z, Yang X, Cao R. AXL, an important host factor for DENV and ZIKV replication. Front Cell Infect Microbiol (2021) 11:575346.
Keywords: AXL, small molecule inhibitors, antibodies, tumor therapy, therapy of infections
Citation: Pyo K-H, Rahman SMM and Boraschi D (2023) Editorial: Development of small molecule inhibitors and antibodies targeting AXL for tumor therapy and infectious disease control. Front. Oncol. 12:1121120. doi: 10.3389/fonc.2022.1121120
Received: 11 December 2022; Accepted: 28 December 2022;
Published: 10 January 2023.
Edited and Reviewed by:
Olivier Feron, Université catholique de Louvain, BelgiumCopyright © 2023 Pyo, Rahman and Boraschi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kyoung-Ho Pyo, cGtocHNoQGdtYWlsLmNvbQ==
†These authors have contributed equally to this work and share senior authorship
 Kyoung-Ho Pyo
Kyoung-Ho Pyo S. M. Mazidur Rahman
S. M. Mazidur Rahman Diana Boraschi
Diana Boraschi