
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Oncol., 13 January 2023
Sec. Gastrointestinal Cancers: Gastric and Esophageal Cancers
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.1106762
This article is part of the Research TopicBiomarkers, Functional Mechanisms, and Therapeutic Potentials in Gastrointestinal CancersView all 40 articles
Pancreatic cancer (PC) is a malignant tumor with high malignancy that is difficult to diagnose and treat. PC is a major medical problem because of its low early diagnosis rate, high surgical mortality rate, low cure rate, and expensive related testing cost. Therefore, the significance of finding new markers for PC is self-evident. Semaphorins (Semas) have been shown to affect angiogenesis and lymphangiogenesis and can also directly affect the behavior of tumor cells. The expression and related action targets of its family members on PC are summarized in this review.
Pancreatic cancer (PC) is an extremely aggressive, fatal disease caused by early metastatic transmission without curative surgical resection. Most PCs (~90%) are pancreatic ductal adenocarcinoma (PDA) (1). Although the prognosis has improved slightly in recent years, the pancreatic cancer-related mortality rate has gradually increased over the last decade, and the 5-year survival rate is still less than 8% (1, 2). Despite the increasing number of surgical treatments, about 70% of patients will still develop early recurrent (3) within 6–12 months after surgery.
Semaphorins (Semas) have been recognized as critical contributors to neural development (4), the immune response (5, 6), and tumor progression (7). Semaphorins consists of a large family of secreted and membrane-associated proteins with a highly conserved, about 500 amino acids, semaphorin domain. Semaphorins were first mentioned in early 1990s. There are more than 20 types of these proteins have been described as guidance factors assisted axon pathfinding during neuronal development (8, 9). These proteins are divided into eight classes based on structural elements and distribution among different phyla (10). Class 1 and 2 Semas are found only in invertebrates, while class 3–7 are found only in vertebrates. Class 8 members are found in viruses. Class 1, 4, 5, and 6 members are transmembrane Semas. Class 2, 3, and 8 Semas are secreted, and class 7 members are glycosylphosphatidylinositol (GPI)-linked (11).
It was reported that cancer mortality declined by 32% between 1991 and 2019 in the United States. Notably, the risk of cancer death decreased by about 2% per year between 2015 and 2019. The remarkable effects of cancer prevention and control, diagnosis, and treatment in recent years are strongly illustrated by this data (1). The improvement in diagnosis levels has enabled the timely diagnosis of some cancers. Advances in adjuvant chemotherapy, targeted therapy, and combination therapy have significantly improved patient outcomes. Nevertheless, the survival rate of PC is still low. Therefore, we summarized the current relevant mechanisms of Semas in PC and their potential for PC treatment in this review.
Sema3A has received less focus than its receptors. The study of Sema3A and its receptors, such as plexinsA1-A4 (PLXNA1-A4) and neuropilin-1 (NRP1) in PC, demonstrates that SEMA3A had no impact on the growth or survival of pancreatic tumor cells and was upregulated in PC. Its signaling might partially mediated by Rac1 (12). Interestingly, one research study composed a novel type of hybrid peptide, Sema3A-lytic, which is be formed of two functional amino acid domains: a sequence that binds to NRP1 from Sema3A and a cytotoxic lytic peptide. In addition, this hybrid peptide shows that the Sema3A-lytic hybrid peptide would be a possible anti-cancer agent for treating human PC (13). This may provide a new idea for subsequent studies.
Sema3B, as a potential tumor suppressor gene, gene loss or down-regulation of its function can promote tumor progressions, such as in lung cancer, breast cancer, ovarian cancer, liver cancer, and cholangiocarcinoma (14–17). Plexins are essential receptors for Sema3B activation of downstream signaling pathways, and Sema3B function depends on the NRP receptor to provide binding sites for both receptors. This can exert anti-tumor angiogenesis and promote tumor cell apoptosis through specific binding to the high affinity of both receptors. Sema3B can compete with VEGF for NRP receptors, hindering the promoting effect of VEGF on vascular endothelial cell division, and partly inhibiting angiogenesis. Thus, it indirectly blocks the nutrient supply of tumor cells and slows down cell division (18). Gao (18) et al. shows that lentiviral vector transfection can construct a stable Sema3B upregulated model in PC CFPAC-1 and PANC-1 cell lines. Overexpression of Sema3B could significantly inhibit the proliferation, invasion, and migration of CFPAC-1 and PANC-1 cells, which means that Sema3B may have an essential role in inhibiting tumor genesis and development in PC. The expression level of Sema3B in PC tissues is closely related to the PC tumor stage, local invasion, lymphatic metastasis, and distant metastasis, and the expression level of Sema3B in PC tissues is closely related to the degree of malignancy of PC. The lower the Sema3B expression level, the higher the pathological malignancy of PC. However, due to the few studies on Sema3B in PC, its specific molecular mechanism still needs to be elucidated.
Sema3C has a role in promoting tumor development (19), and it is hypomethylated, and its expression increases with tumor progression (20) in PC. Xu (21) et al. suggested that abnormal expression of Sema3C is associated with poor prognosis in patients with PDAC, and high Sema3C expression in PC was associated with a shorter survival time (22). Sema3C may promote PC cell migration and invasion by inducing the epithelial-mesenchymal transition (EMT) and may contribute to cancer therapy. The extracellular signal-regulated kinase (ERK)1/2 is the most critical pathway for promoting cell proliferation, metastasis, and EMT during tumorigenesis. Sema3C promoted the phosphorylation of ERK in PC and confirmed that Sema3C mediated cell proliferation in PC both in vitro and in vivo. Furthermore, Proliferation, migration, invasion, and EMT in a PC cell line attenuated and PDAC cell tumorigenesis upon after xenotransplantation into nude mice reduced since Sema3C was knockdown. Overexpression of Sema3C had the contrary effects and boosted the ERK1/2 signaling pathway (21). Furthermore, the combination of trametinib (MEK inhibitor, downstream of KRAS) and Sema3C inhibitor can induce a synergistic effect in KRAS G12D cells, which shows Sema3C might be a potentially prospects and attractive target for PC therapy, especially in patients with G12D mutation in KRAS (22). These features suggest the strong potential of Sema3C in the treatment of PC.
Sema3D and its receptor PlexinD1 (PlxnD1) are particularly interesting, as they are the part that is frequently amplified and mutated in human PDA. Jurcak (23) et al. found that the invasive and metastatic capacity of PDA cells is increased due to the interaction of Sema3D with its co-receptors PlxnD1 and Neuropilin-1. It is supported in another study (24) that tumor cells expressing Sema3D can travel toward PlxnD1-expressing neurons, according to their Diagnosis Related Groups (DRG) in vitro data. However, the molecular mechanism of Sema3D in assisting increased PDA cell invasion and metastasis is unknown. Interestingly, this increase of glycolytic gene expression was inhibited with the neutralizing antibody PlexinD1. They also suggested that Sema3D signaling pathway could offer another selectivity in mutant Kras cells.
Moreover, Sema3D in PC is interesting in the conduction relationship between Sema3D, Annexin A2 (AnxA2), and PlexinD1. Foley (25) et al. elucidated one mechanism of PDA metastasis formation: Sema3D can bind to PlxnD1 on the surface of PDA tumor cells in the extracellular space by the regulation of its autocrine function control of AnxA2. This mechanism has also been proven to promote the invasion and metastasis of PDA cells (26) and the tumor. Mice carrying PDA prolong survival and reduces metastasis due the knockdown of Sema3D (24). These studies increase therapeutic options in PDA and provide a strong rationale for the development of adjuvant therapies targeting AnxA2 and Sema3D for PDA after local resection (26).
Unlike the other three classes of signaling elements, Sema3E can directly bind to its receptor, PlxnD1, which activates cellular signaling. This combination has been shown to contribute to the aggressive and metastatic spread of cancer cells. Lin et al. have shown that Sema3E was significantly expressed in the nuclei of PC cells and overexpressed in human PC which was associated with tumor progression and poor survival (27, 28). Also, they have shown that overexpression of Sema3E in PC cells promoted cell proliferation and migration in vitro, while cancer cell proliferation and migration in vitro had the opposite function (27). In other words, higher incidence and growth of tumors were exhibited by Sema3E overexpressed cells (28), whereas they were reduced by Sema3E knockout cells. Moreover, Sema3E induces cell proliferation through MAPK/ERK pathway (28) (Figure 1). Therefore, Sema3E may be a suitable prognostic marker and an attractive new therapeutic target. It has been confirmed in a study (29) that Sema3E is significantly upregulated after therapeutic expression of panc28 cell lines and is one of the common biomarkers.
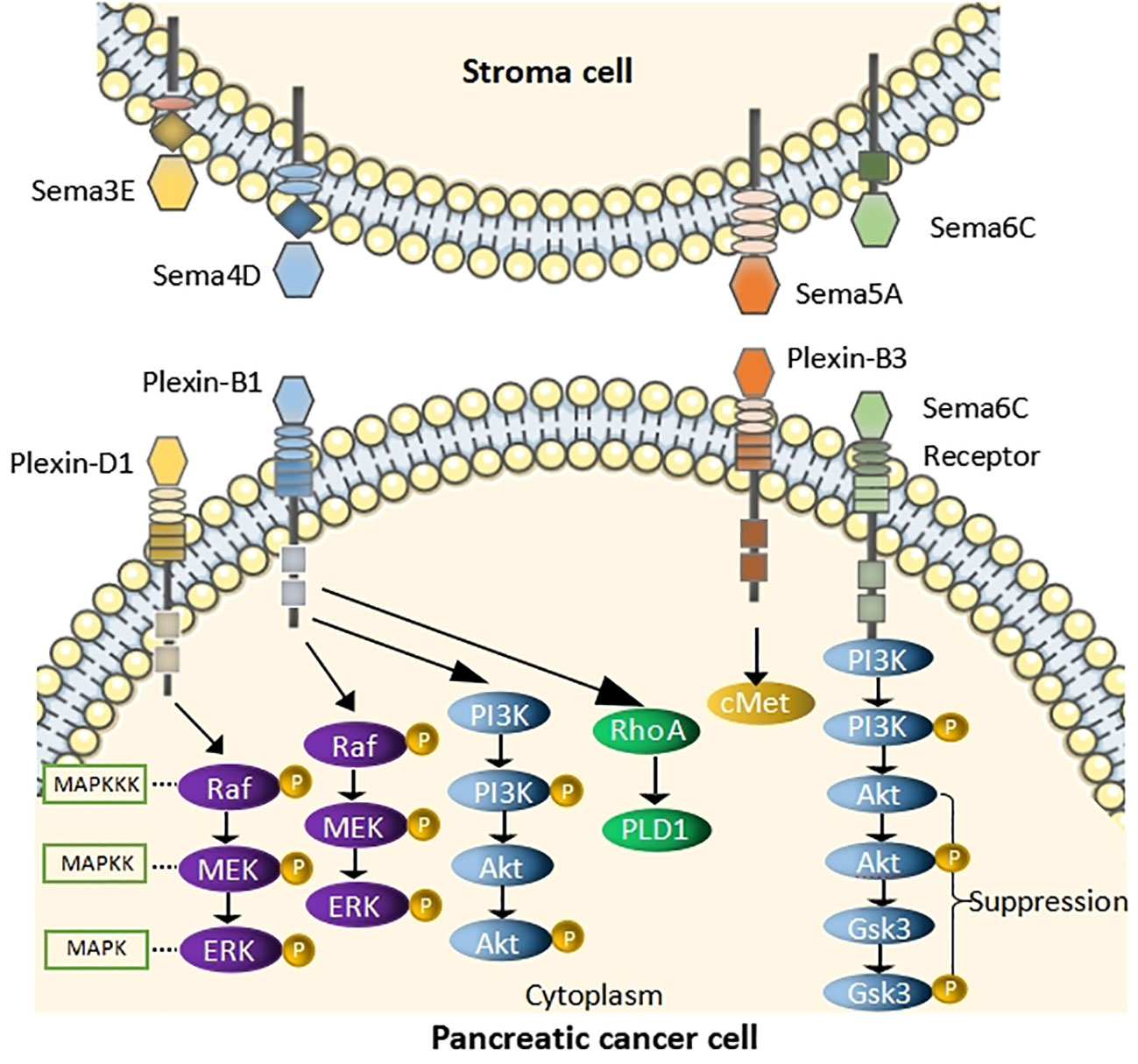
Figure 1 Schematic of pathway depicting the role of Semas in PC Sema3E binds to Plexin-D1and induces cell proliferation by acting through the MAPK/ERK pathway. Sema4D binds to Plexin-B1, activates RhoA, causes the phosphorylation of MAPK and Akt. Sema5A binding to Plexin-B3 activates cMet signaling to enhance migration of PC cells. Sema6C binds to its receptor and suppress AKT/GSK3 signaling pathway.
Interestingly, the ADAMTS9-AS1-SEMA3G pathway is predicted to play an essential role in immune cell regulation by database screening and validation (30). TCGA and GEO databases were analyzed to screen differential lncRNA ADAMTS9-AS1 associated with prognosis. GO and KEGG enrichment analyses were performed, and the results showed that the gene was significantly associated with immunity. The infiltration, significant positive correlation with B cells, CD4+ T cells, CD8+ T cells, neutrophils, macrophages, and dendritic cells (DCs) was verified using the TIMER database, and a more significant correlation with CD8+ T cells and macrophages in PC. Negative correlation between the lncRNA and tumor grade was shown in a clinical correlation analysis. Thus, it shows that the higher the lncRNA expression in PC, the more immune infiltration in the tumor, the lower the tumor grade, and the better the patient prognosis (30). Therefore, the ADAMTS9-AS1-SEMA3G pathway is predicted to play an essential role in immune cell regulation.
Additionally, Gao (31) et al. have proven the role of Sema3G in proliferative capacity and cell migration in PC cells. Human PC PANC-1 cells were transfected with the Sema3G lentivirus interference vector, whose proliferation, invasion, and migration capacity were significantly decreased, indicating that Sema3G could significantly inhibit the proliferation, invasion, and migration of PC. The mechanism of Sema3G inhibiting PC invasion and metastasis and the signaling pathways it works with to inhibit PC progression still needs further intensive investigation.
Sema4D has been one of the research hotspots in recent years. It has been deemed to promote the progress of malignancies by affecting cell proliferation, apoptosis, and immigration (32). Sema4D can bind to the ligand Plexin-B1 and activate small GTPase Ras homolog gene family, member A (RhoA), causing the phosphorylation of MAPK and Akt, thereby enhancing the invasive energy of PC cells (33, 34) (Figure 1). It was reported in the relevant literature that inhibition of Sema4D in tumor cells not only inhibits tumor angiogenesis but also may restore T-cell immune activity specific to antigens (34, 35). Notably, Sema4D-siRNA downregulates the expression of Sema4D in pancreatic cells, inhibiting the proliferation of PC cells and reducing their invasive ability and apoptosis (36). Therefore, a team proposed immunotherapy strategies to block Sema4D, which could improve the sensitivity of PDAC to immune checkpoint blockade (ICB) (37), and treatment with Sema4D blocking antibody improved response to ICB in combination by using the standard of care FOLFIRINOX in preclinical murine studies (38). Moreover, the considerable progress of Pepinemab, the Sema 4D antibody, is well tolerated and has potent anti-tumor activity (39), but resistance to immunotherapy often limits patient efficacy. It has been suggested in studies that when Sema4D antibodies are combined with immune checkpoint inhibitors, such as the anti-Sema4D antibody Pepinemab, and combined with the immune checkpoint inhibitor PD-L1 mAb Avelumab, they can enhance T-cell activity, thus persistently inhibiting tumor growth (40).
Sema5A is tumor-promoting in PC. Moreover, Sema5A is expressed in most PC tissues and is not or low expressed in normal pancreatic tissues, which elucidates that Sema5A could be a marker for PC (41, 42). Furthermore, upregulation of mouse Sema5A in Panc1 enhanced tumor cell proliferation and the incidence of distant metastasis to the lymph nodes, liver, spleen, and peritoneal cavity after in situ injection in mice. It was suggested by these results that Sema5A was associated with tumor growth, metastatic potential, and invasiveness (43). It is noteworthy that PC cell migration is enhanced by Sema5A by activating cMet signaling in a PlexinB3-dependent manner (44) (Figure 1). Additionally, proliferation and angiogenesis are promoted by SEMA5A in the primary tumor setting, thereby enhancing metastasis (44, 45). Thus, Sema5A/PlexinB3 may represent an attractive targetable axis in PC.
Sema6C might be a potential tumor suppressor in PC and serve as a poor prognostic biomarker in PC (45). Sema6C, a tumor suppressor in PC, causes reduction of cyclin D1 expression and cell proliferation which includes cyclin-dependent kinase 4/6(CDK4/6) (46) by inhibiting the AKT/GSK3 signaling axis (47) (Figure 1). Moreover, they demonstrated a brand new regulatory role of miR-124-3p in suppressing Sema6C and suggested the treatment of Sema6C-downregulated cancer by CDK4/6 inhibitors (48).
Recently, Semas have been aberrantly expressed in many tumors and have a regulatory role in tumor development, represented by Sema3 and Sema4. An anti-Sema3A antibody has been patented to reduce immunosuppression caused by tumor-secreted Sema3A, and is available to the treatment of Alzheimer disease and immune dysfunction; Sema4D inhibitors are also patented to increase the frequency of tumor-infiltrating leukocytes by blocking the combination of Sema4D with its receptor (49), and is suitable for the treatment of human head and neck cancer, colon cancer, breast cancer, etc (50). Moreover, in Phase I prospective multiple escalating dose trials completed in patients with advanced refractory solid tumors, treatment with anti-Sema4D antibodies remains well tolerated (51) and still be safe, such as the combination of Avelumab and the Sema4D inhibitor Pepinemab (52). Semas, which may be used as prognostic markers, can be further investigated to select molecular inhibitors with high selectivity and establish PDX models for drug selection evaluation. In addition, signaling pathways that have not yet been identified can be screened and validated against databases to obtain relevant signaling pathways, on which further studies can be conducted.The current research on Semaphorins in PC is only singularly focused on one or two class, and it is hoped that some investigators will keep further research on the above viable and relevant targets to enhance pre-prognostic and prognostic diagnosis, the addition of synthetic emerging drugs and novel combinations of existing drugs.
The targets and treatment of Semaphorins in PC are shown in Table 1. Further research is needed to investigate the mechanism of Semas in PC, with the most promising potential being Sema3C and Sema4D, which are already research hotspots. Among them, the Sema5A/plexinB1 axis and the 6CSEMA6C AKT/GSK3 axis provide us with brand-new ideas, but related research still needs to elucidate its practical feasibility further. The treatment related to Sema4D shows that the multicombination of drug treatment can solve the problem of drug resistance, improve the durability of the treatment, and make noteworthy progress in treating PC.
HY designed the study and contributed to manuscript revision. DL, JL and FQ collected the data, interpreted the data, and wrote the draft of the manuscript. All authors contributed to the article and approved the submitted version.
The work in the Liu Lab is supported by The National Natural Science Foundation of China (Grant No. 82270413, 81870307), The Natural Science Foundation of Guangdong Province of China (Grant No. 2022A1515011368), and The Key Projects of Department of Education of Guangdong Province of China (Grant No. 2022ZDZX2057, 2022ZXKC474). The work in the You Lab is supported by The National Natural Science Foundation of China (Grant No. 81911530169).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Liu MQ, Ji SR, Xu XW, Yu XJ. Advances in the research and treatment of pancreatic cancer in 2019. Chin J Cancer (2020) 30(01):1–10. doi: 10.12688/f1000research.21981.1
2. Siegel Rebecca L, Miller Kimberly D, Fuchs Hannah E, Jemal A. Cancer statistics, 2019. CA Cancer J Clin (2022) 72:7–33. doi: 10.3322/caac.21551
3. Cui K, Jin S, Du Y, Yu J, Feng H, Fan Q, et al. Long noncoding RNA DIO3OS interacts with miR-122 to promote proliferation and invasion of pancreatic cancer cells through upregulating ALDOA. Cancer Cell Int (2019) 19(1):1–10. doi: 10.1186/s12935-019-0922-y
4. Pasterkamp RJ, Peschon JJ, Spriggs MK, Kolodkin AL. Semaphorin 7A promotes axon outgrowth through integrins and MAPKs. Nature (2003) 424:398–405. doi: 10.1038/nature01790
5. Kikutani H, Suzuki K, Kumanogoh A. Immune semaphorins: increasing members and their diverse roles. Adv Immunol (2007) 93:121–43. doi: 10.1016/S0065-2776(06)93003-X
6. Feinstein J, Ramkhelawon B. Netrins & semaphorins: Novel regulators of the immune response. Biochim Biophys Acta (BBA) - Mol Basis Dis (2017) 1863(12):3183–9. doi: 10.1016/j.bbadis.2017.09.010
7. Neufeld G, Sabag AD, Rabinovicz N, Kessler O. Semaphorins in angiogenesis and tumor progression. Cold Spring Harbor Perspect Med (2012) 2(1):a006718. doi: 10.1101/cshperspect.a006718
8. Kolodkin AL, Matthes DJ, Goodman CS. The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules. Cell (1993) 75(7):1389–99. doi: 10.1016/0092-8674(93)90625-Z
9. Pasterkamp RJ, Kolodkin AL. Semaphorin junction: making tracks toward neural connectivity. Curr Opin Neurobiol (2003) 13(1):79–89. doi: 10.1016/S0959-4388(03)00003-5
10. Fard D, Tamagnone L. Semaphorins in health and disease. Cytokine Growth factor Rev (2021) 57:55–63. doi: 10.1016/j.cytogfr.2020.05.006
11. Alto LT, Terman JR. Semaphorins and their signaling mechanisms. Methods Mol Biol (2017) 1493:1. doi: 10.1007/978-1-4939-6448-2_1
12. Müller MW, Giese NA, Swiercz JM, Ceyhan GO, Esposito I, Hinz U, et al. Association of axon guidance factor semaphorin 3A with poor outcome in pancreatic cancer. Int J Cancer (2007) 121(11):2421–33. doi: 10.1002/ijc.22949
13. Ueyama H, Horibe T, Nakajima O, Ohara K, Kohno M, Kawakami K, et al. Semaphorin 3A lytic hybrid peptide binding to neuropilin-1 as a novel anti-cancer agent in pancreatic cancer. Biochem Biophys Res Commun (2011) 414(1):60–6. doi: 10.1016/j.bbrc.2011.09.021
14. Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell (1998) 92(6):735–45. doi: 10.1016/S0092-8674(00)81402-6
15. Varshavsky A, Kessler O, Abramovitch S, Kigel B, Zaffryar S, Akiri G, et al. Semaphorin-3B is an angiogenesis inhibitor that is inactivated by furin-like pro-protein convertases. Cancer Res (2008) 68(17):e6922–31. doi: 10.1158/0008-5472.CAN-07-5408
16. Trusolino L, Comoglio PM. Scatter-factor and semaphorin receptors: cell signalling for invasive growth. Nat Rev Cancer (2002) 2(4):289–300. doi: 10.1038/nrc779
17. Ochi K, Mori T, Toyama Y, Nakamura Y, Arakawa H. Identification of semaphorin3B as a direct target of p53. Neoplasia (2002) 4(1):e82–7. doi: 10.1038/sj.neo.7900211
18. Gao X. The biological pattern of SEMA3B and its clinical value in pancreatic carcinoma. [master’s thesis]. (Gansu Lanzhou: Lanzhou University) (2017).
19. Miyato H, Tsuno NH, Kitayama J. Semaphorin 3C is involved in the progression of gastric cancer. Cancer Sci (2012) 103(11):1961–6. doi: 10.1111/cas.12003
20. Tan AC, Jimeno A, Lin SH, Wheelhouse J, Chan F, Solomon A, et al. Characterizing DNA methylation patterns in pancreatic cancer genome. Mol Oncol (2009) 3(5-6):425–38. doi: 10.1016/j.molonc.2009.03.004
21. Xu X, Zhao Z, Guo S, Li J, Liu S, You Y, et al. Increased semaphorin 3c expression promotes tumor growth and metastasis in pancreatic ductal adenocarcinoma by activating the ERK1/2 signaling pathway. Cancer Lett (2017) 397:12–22. doi: 10.1016/j.canlet.2017.03.014
22. Zhang DL, Lindstrom A, Kim EJ, Hwang CI, Hall ML, Lin TY, et al. SEMA3C supports pancreatic cancer progression by regulating the autophagy process and tumor immune microenvironment. Front Oncol (2022) 2686:890154. doi: 10.3389/fonc.2022.890154
23. Jurcak NR, Rucki AA, Muth S, Thompson E, Sharma R, Ding D, et al. Axon guidance molecules promote perineural invasion and metastasis of orthotopic pancreatic tumors in mice. Gastroenterology (2019) 157(3):838–50. doi: 10.1053/j.gastro.2019.05.065
24. Jurcak NR, Muth S, Fujiwara K, Rucki A, Foley K, Murphy A, et al. Semaphorin3D signaling in the invasion and metastasis of pancreatic ductal adenocarcinoma. Cancer Res (2019) 79(13):167. doi: 10.1158/1538-7445.AM2019-167
25. Foley K, Rucki A A, Xiao Q, Zhou D, Leubner A, Mo G, et al. Semaphorin 3D autocrine signaling mediates the metastatic role of annexin A2 in pancreatic cancer. Sci Signaling (2015) 8(388):77. doi: 10.1126/scisignal.aaa5823
26. Murphy A, Kleponis J, Rucki A, Jaffee EM, Zheng L, Foley K, et al. Targeting Sema3D in pancreatic cancer: A novel therapeutic strategy. J Clin Oncol (2015) 33(15):4129. doi: 10.1200/jco.2015.33.15_suppl.4129
27. Yong LK, Lai S, Liang Z, Poteet E, Chen F, Van BG, et al. Overexpression of semaphorin-3E enhances pancreatic cancer cell proliferation ad is associated with patient poor survival. Oncotarget (2016) 7(52):8743. doi: 10.18632/oncotarget.13704
28. Liang Z, Yong LK, Van BG, Fisher W, Hwang R, Chen C, et al. Abstract B031: Semaphorin 3E promotes pancreatic cancer metastasis through activating stromal cell. Mol Cancer Ther (2018) 17(1):31. doi: 10.1158/1535-7163
29. Youns M, Abdel Halim Hegazy W. The natural flavonoid fisetin inhibits cellular proliferation of hepatic, colorectal, and pancreatic cancer cells through modulation of multiple signaling pathways. PloS One (2017) 12(1):e0169335. doi: 10.1371/journal.pone.0169335
30. Ren KK, Lin XY, Hou MY, Zhou B, Ma J. ADAMTS9-AS1-SEMA3G affects the infiltration of immune cells in pancreatic cancer. J Med Postgraduates (2020) 12:169–73. doi: 10.16571/j.cnki.1008-8199.2020.02.012
31. Gao ZF, Gao X, Wu YN, Bai ZT, Zhang L, Li X, et al. Effects of SEMA3G overexpression mediated by lentivirus on human pancreatic cancer cell line PANC-1. Basic Clin Med (2016) 36(7):891. doi: 10.16352/j.issn.1001-6325.2016.07.003
32. Liu Y, Zhou H, Ma L, Hou Y, Pan J, Sun C, et al. MiR-214 suppressed ovarian cancer and negatively regulated semaphorin 4D. Tumour Biol (2016) 37(6):8239–48. doi: 10.1007/s13277-015-4708-0
33. Kato S, Kubota K, Shimamura T, Shinohara Y, Kobayashi N, Watanabe S, et al. Semaphorin 4D, a lymphocyte semaphorin, enhances tumor cell motility through binding its receptor, plexinB1, in pancreatic cancer. Cancer Sci (2011) 102(11):2029–37. doi: 10.1111/j.1349-7006.2011.02053.x
34. Xu XD, Yang L, Zheng LY, Pan YY, Cao ZF, Zhang ZQ, et al. Suberoylanilide hydroxamic acid, an inhibitor of histone deacetylase, suppresses vasculogenic mimicry and proliferation of highly aggressive pancreatic cancer PaTu8988 cells. BMC Cancer (2014) 14(1):1–13. doi: 10.1186/1471-2407-14-373
35. Younis RH, Han KL, Webb TJ. Human head and neck squamous cell carcinoma–associated semaphorin 4D induces expansion of myeloid-derived suppressor cells. J Immunol (2016) 196(3):1419–29. doi: 10.4049/jimmunol.1501293
36. Ru JJ, Li BR, Geng WY. Effect of interference of Sema4D expression on cell biological characteristics in pancreatic cancer. Chin J Clin Hepatol (2018) 34(2):350–3. doi: 10.3969/j.issn.1001-5256.2018.02.026
37. Ruffolo LI, Ullman NA, Jackson KM, Burchard PR, Fields RC, Yeh JJ, et al. Semaphorin 4D blockade enhances T-cell penetration and potentiates response to immune checkpoint blockade in a murine model of pancreatic cancer. J Am Coll Surgeons (2021) 233(5):S252–3. doi: 10.1016/j.jamcollsurg.2021.07.522
38. Ruffolo LI, Ullman NA, Dale B, Jackson KM, Burchard P, Georger M, et al. Antibody blockade of semaphorin 4D to sensitize pancreatic cancer to immune checkpoint blockade. J Clin Oncol (2020) 38(5):26. doi: 10.1200/JCO.2020.38.5_suppl.26
39. Tao ZG, Du JH, Tian K, Wang DH, Gong W, Chen MY, et al. Effects of silencing Sema4D on the growth, autophagy and epithelial-mesenchymal transformation of gastric cancer cells SGC-7901. Med J Chin People’s Liberation Army (2021) 46(1):11–7. doi: 10.11855/j.issn.0577-7402.2021.01.03
40. Shafique MR, Fisher TL, Evans EE, Leonard JE, Pastore DRE, Mallow CL, et al. A phase Ib/II study of pepinemab in combination with avelumab in advanced non–small cell lung CancerPepinemab in combination with avelumab in NSCLC. Clin Cancer Res (2021) 27(13):3630–40. doi: 10.1158/1078-0432.CCR-20-4792
41. Sadanandam A, Varney ML, Singh S, Ashour AE, Moniaux N, Deb S, et al. High gene expression of semaphorin 5A in pancreatic cancer is associated with tumor growth, invasion and metastasis. Int J Cancer (2010) 127(6):1373–83. doi: 10.1002/ijc.25166
42. Sadanandam A, Sidhu SS, Wullschleger S, Singh S, Varney ML, Yang CS, et al. Secreted semaphorin 5A suppressed pancreatic tumour burden but increased metastasis and endothelial cell proliferation. Br J Cancer (2012) 107(3):501–7. doi: 10.1038/bjc.2012.298
43. Saxena S, Hayashi Y, Wu L, Awaji M, Atri P, Varney ML, et al. Pathological and functional significance of semaphorin-5A in pancreatic cancer progression and metastasis. Oncotarget (2018) 9(5):5931. doi: 10.18632/oncotarget.23644
44. Saxena S, Purohit A, Varney ML, Hayashi Y, Singh RK. Semaphorin-5A maintains epithelial phenotype of malignant pancreatic cancer cells. BMC Cancer (2018) 18(1):1–15. doi: 10.1186/s12885-018-5204-x
45. Saxena S, Prajapati DR, Goel P, Tomar B, Hayashi Y, Atri P, et al. Plexin-B3 regulates cellular motility, invasiveness, and metastasis in pancreatic cancer. Cancers (2021) 13(4):818. doi: 10.3390/cancers13040818
46. Hung YH, Hsu SH, Hou YC, Wang J, Xu B, Zeng J, et al. Semaphorin 6C suppresses proliferation of pancreatic cancer cells via inhibition of the AKT/GSK3/β-Catenin/Cyclin D1 pathway. Int J Mol Sci (2022) 23(5):2608. doi: 10.3390/ijms23052608
47. Wu G, Deng Z, Jin Z, Chu PY, Su YY, Shan YS, et al. Identification of prognostic immune-related genes in pancreatic adenocarcinoma and establishment of a prognostic nomogram: A bioinformatic study. BioMed Res Int (2020) 2020:1–15. doi: 10.1155/2020/1346045
48. Qie S, Diehl JA. Cyclin D1, cancer progression, and opportunities in cancer treatment. J Mol Med (2016) 94(12):1313–26. doi: 10.1007/s00109-016-1475-3
49. Chen LH, Cuang EY. Importance of semaphorins in cancer immunity. Trans Lung Cancer Res (2019) 8(4):S468–70. doi: 10.21037/tlcr.2019.12.22
50. Evans EE, Jonason AS, Bussler H, Torno S, Veeraraghavan J, Reilly C, et al. Antibody blockade of semaphorin 4D promotes immune infiltration into tumor and enhances response to other immunomodulatory TherapiesImmunomodulatory anti-SEMA4D promotes tumor rejection. Cancer Immunol Res (2015) 3(6):689–701. doi: 10.1158/2326-6066.CIR-14-0171
51. Evans EE, Hu-Lieskovan S, Bussler H, Torno S, Mallow C, Reilly C, et al. Antibody blockade of semaphorin 4D breaks down barriers to enhance tumoricidal immune infiltration and supports rational immunotherapy combinations. J ImmunoTherapy Cancer (2015) 3(2):1–2. doi: 10.1186/2051-1426-3-S2-P220
Keywords: pancreatic cancer, semaphorins, mechanisms, Sema3, Sema4D
Citation: Liu D, Li J, Qi F and You H (2023) Semaphorins and their receptors in pancreatic cancer: Mechanisms and therapeutic opportunities. Front. Oncol. 12:1106762. doi: 10.3389/fonc.2022.1106762
Received: 24 November 2022; Accepted: 23 December 2022;
Published: 13 January 2023.
Edited by:
Qun Zhang, Nanjing Medical University, ChinaReviewed by:
Guojun Qian, Guangzhou Medical University, ChinaCopyright © 2023 Liu, Li, Qi and You. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hua You, eW91aHVhMzA3QDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.