- Department of Pharmacy, Third Affiliated Hospital of Naval Medical University, Shanghai, China
Gastrointestinal cancer is one of the most common malignancies with relatively high morbidity and mortality. Exosomes are nanosized extracellular vesicles derived from most cells and widely distributed in body fluids. They are natural endogenous nanocarriers with low immunogenicity, high biocompatibility, and natural targeting, and can transport lipids, proteins, DNA, and RNA. Exosomes contain DNA, RNA, proteins, lipids, and other bioactive components, which can play a role in information transmission and regulation of cellular physiological and pathological processes during the progression of gastrointestinal cancer. In this paper, the role of exosomes in gastrointestinal cancers is briefly reviewed, with emphasis on the application of exosomes as drug delivery systems for gastrointestinal cancers. Finally, the challenges faced by exosome-based drug delivery systems are discussed.
1 Introduction
Gastrointestinal cancer is one of the most common and aggressive malignancies with relatively high morbidity and mortality in the world (1). According to the statistics of the International Agency for Research on Cancer (IARC), gastrointestinal cancer accounts for 45% of cancer-related deaths in China. At present, the therapeutic strategies for gastrointestinal cancer mainly include surgery, endoscopy, radiotherapy, chemotherapy, targeted therapy, and immunotherapy (2, 3). However, the prognosis of gastrointestinal cancer is still poor because early symptoms are occultic or asymptomatic and are detected at an advanced stage. Therefore, promising therapeutic strategies are needed to reduce the mortality of gastrointestinal cancer.
Recently, numerous studies have demonstrated that exosomes can be used as drug delivery systems and have the potential to improve the therapeutic effect on tumors (4–7). Exosomes are nanosized extracellular vesicles with a diameter of 40 - 100 nm, composed mainly of lipids, proteins, and genetic material. They are secreted by a variety of cells and widely distributed in body fluids, so they are biosafe, stable, and have good targeting specificity (8). In addition, exosomes as nanocarriers have the advantages of small size, negative charge, immune escape, and deep tissue penetration (9). Therefore, exosomes can be used as ideal natural nanocarriers for drug delivery. In this review, we briefly outline the role of exosomes in gastrointestinal cancers, focusing on the potential and challenges of exosomes as drug delivery systems for gastrointestinal cancers.
2 The role of exosomes in gastrointestinal cancers
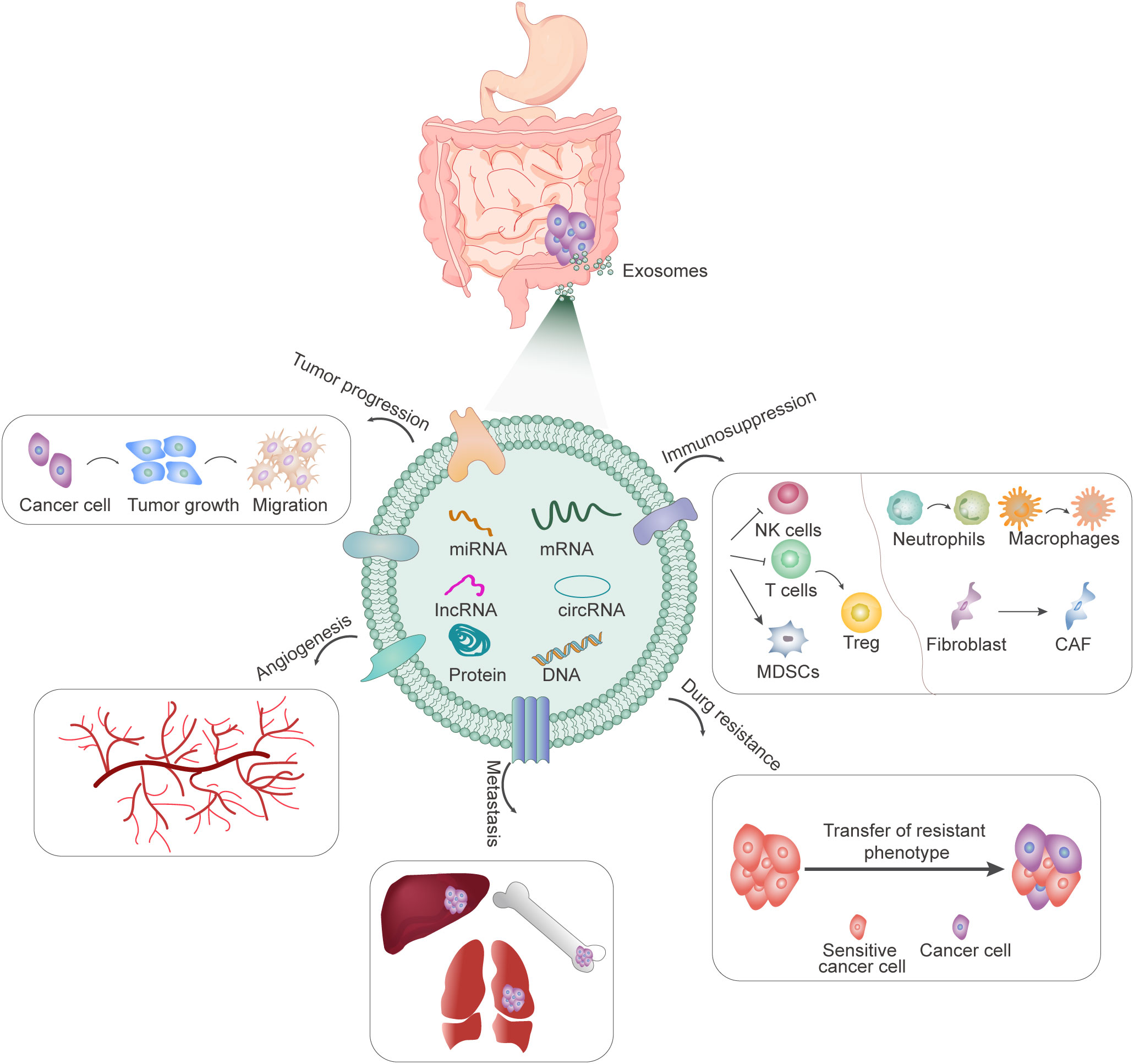
Exosomes may be involved in multiple processes of gastrointestinal progression, including proliferation, invasion and metastasis, angiogenesis, drug resistance, and immune escape (Figure 1). In 2009, it was first reported that gastric cancer cell-derived exosomes can promote the proliferation of SGC7901 and BGC823 cells through PI3K/AKT and MAPK/ERK activation (10). Subsequently, numerous studies have found that not only exosomes produced by tumor cells, but also exosomes secreted by mesenchymal stem cells (MSCs), fibroblasts, and other cells can also release contents to regulate the proliferation and metastasis of gastrointestinal cancers (11, 12). Exosomes can promote epithelial-mesenchymal transformation (EMT), improve the invasion and metastasis ability of receptor cells, and participate in matrix remodeling and metastasis formation. The important role of exosomes in gastrointestinal cancer metastasis is also manifested by their involvement in angiogenesis. Tumor cell-derived exosomes can promote angiogenesis and tumor progression in many ways (13, 14). Tumor-induced increased vascular permeability and angiogenesis are also important features of the formation of pre-metastatic niches, which in turn are closely related to distant metastasis of tumors. In addition, exosomes are involved in drug resistance, mediating drug resistance transfer between resistant and sensitive cells, as well as between tumor and stromal cells (15–18). Another important feature of gastrointestinal cancer-derived exosomes is their ability to modulate tumor immunity. Exosomes from different tumors carry different substances and information. They are specific to tumor cells and contain a variety of immunosuppressive molecules, which can inhibit the activity of NK cells and T cells, transform T cells into Treg-like cells, transform the phenotype of neutrophils and macrophages, promote the transformation of fibroblasts into cancer-associated fibroblasts (CAFs), and induce the proliferation of myeloid-derived suppressor cells (MDSCs), and play an important role in the suppression of tumor immune response (19, 20). In conclusion, tumor-derived exosomes contain a variety of proteins and miRNA, which bind to different targets to induce immunosuppression, forming a pre-metastasis microenvironment, and promoting tumor growth, differentiation, invasion, and metastasis.
3 Exosomes as drug delivery systems for the treatment of gastrointestinal cancers
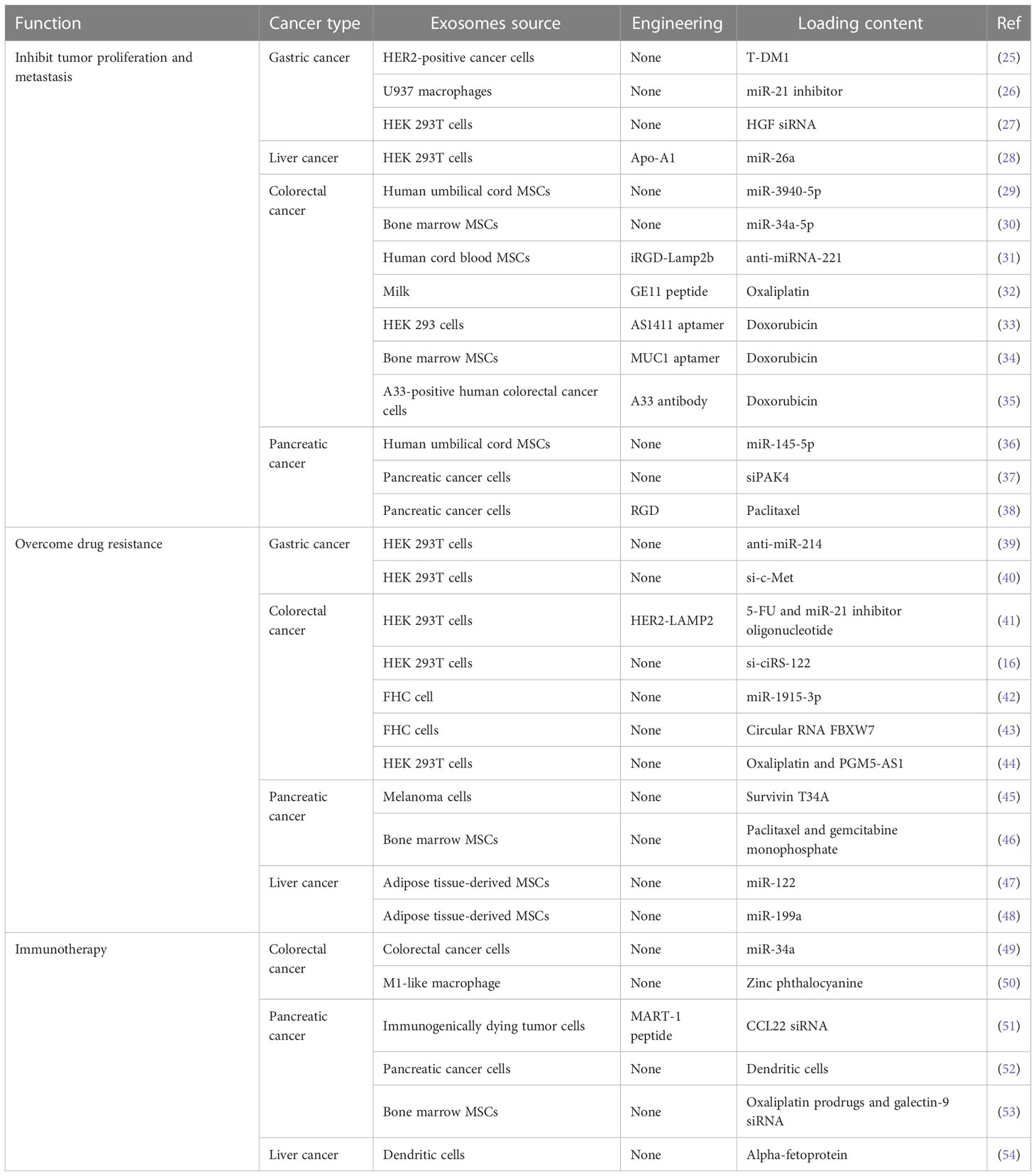
Exosomes, as natural drug carriers, have been widely used and studied. It has many advantages over traditional nanocarriers in terms of drug and gene delivery. First, exosome delivery can improve the stability of drugs. For example, exosomes can protect nucleic acids from degradation during transport (21, 22). At the same time, exosomes can directly enter the cell fluid to avoid metabolic elimination, thus extending the drug circulation time. Second, exosomes have natural targeting capabilities based on parental cells. As drug delivery carriers, exosomes can target specific cell types and are suitable for targeted therapy. Moreover, exosomes from different cell sources express different molecules on the surface, so they have certain selectivity to the recipient cells, and thus are more advantageous in therapy (23). In addition, exosomes are nanoscale molecules that carry cell surface substances, so they have a strong ability to penetrate various biological barriers (24). Therefore, exosomes have a good application prospect in the field of drug carriers. Next, we will focus on the application of exosomes as drug delivery systems in gastrointestinal cancers (Table 1).
3.1 Exosomes-based drug delivery systems inhibit tumor proliferation and metastasis in gastrointestinal cancers
Exosomes are natural nanocarriers containing many active components. Therefore, they can be used to deliver a variety of components, including proteins, nucleic acids, and small molecules drugs. Trastuzumab emtansine (T-DM1) is an antibody-drug-conjugates (ADC) that binds the tubulin inhibitor emtansine to trastuzumab. The drug targets HER2-positive tumor cells and induces mitotic arrest and apoptosis through the intracellular release of the cytotoxic drug emtansine. It was found that exosomes derived from HER2+ cancer cells delivered T-DM1 to cancer cells and induce apoptosis (25).
miRNA is a class of highly conserved endogenous non-coding single-stranded small RNA that plays a vital role in gene regulation and tumor development. However, the successful delivery of miRNA is hampered by the difficulty of developing sustainable and efficient delivery systems. miRNA-21, one of the earliest miRNAs found in human cells, is highly expressed in a variety of cancers such as gastric cancer, and is closely related to the incidence of cancer. It has shown that exosomes derived from macrophages can be used as vectors to deliver exogenous miR-21 inhibitors into BGC-823 gastric cancer cells and regulate their proliferation (26). Moreover, exosome-mediated miR-21 inhibitor delivery has less cytotoxicity and more effective inhibition than conventional transfection methods. Liang et al. used engineered exosomes to target miR-26a to liver cancer cells expressing scavenger receptor class B type 1, down-regulating Cyclin D2, Cyclin E2, and CDK6 levels, inducing cell cycle arrest and inhibiting cell proliferation and metastasis in hepatocellular carcinoma (28). MSCs are ideal sources of exosomes for drug delivery because they are easy to isolate, have self-repair and multidirectional differentiation capabilities, as well as immune and specific homing properties. miR-3940-5p was significantly down-regulated in colorectal cancer. When it was loaded into MSCs derived exosomes and transfected into colorectal cancer cells, it inhibited EMT and invasion in vitro and inhibited tumor growth and metastasis in vivo (29). Similarly, MSCs-derived exosomes transfected with miR-34a-5p suppressed the growth of colorectal cancer cells and the tumorigenesis of colorectal cancer (30). In addition, functionalizing exosomes with targeting molecules can effectively enhance tumor-targeting ability. The anti-miRNA-221 oligonucleotide was delivered by human cord blood MSCs-derived exosomes, which were modified by the fusion gene iRGD-Lamp2b and were specifically taken up by tumor cells through their interaction with NRP-1 protein. The modified exosomes were significantly enriched at tumor sites and could significantly inhibit tumor growth both in vitro and in vivo (31). Human umbilical cord MSCs-derived exosomes can also effectively deliver miR-145-5p to pancreatic ductal adenocarcinoma cells, inhibits cell proliferation and invasion, and reduce tumor growth (36).
RNA interference has emerged as a promising clinical therapeutic tool that can lead to specific gene silencing. However, there are some limitations in the application of siRNA, including its poor cellular uptake and degradation by nucleases. Many vectors, such as viral vectors and cationic liposomes, have been used to deliver siRNA, but all have some limitations. Studies have shown that cell-derived exosomes are effective carriers of siRNA and can effectively inhibit tumor growth and angiogenesis in gastric cancer by delivering HGF siRNA (27). SiRNA (siPAK4) is encapsulated into pancreatic cancer-derived exosomes by electroporation for pancreatic ductal adenocarcinoma therapy. It can induce obvious tissue apoptosis and prolong the survival time of tumor-bearing mice (37). Pancreatic cancer-derived exosomes as an in vivo RNAi transfection agent showed efficacy comparable to that of polyethyleneimine (PEI), a commercial transfection agent, but was safer.
In addition to proteins and nucleic acids, exosomes can also be used to deliver small-molecule drugs, increase the stability and prolong the circulation time, thus improving the efficacy of drugs. To enhance the therapeutic effect, reduce the toxicity to normal cells, and expand the targeted drug delivery ability of exosomes, targeted modifications were made to endow them with cell and tissue specificity. Extracellular vesicles containing oxaliplatin bound to the GE11 peptide inhibit EGFR-expressing cancers through GE11 peptide-mediated EGFR targeting anticancer drug delivery. It showed that the engineered extracellular vesicles have the greatest therapeutic effect on tumor progression in colorectal cancer (32). Moreover, RGD and magnetic nanoparticles were conjugated to the surface of extracellular vesicles derived from human pancreatic cancer cells and loaded with paclitaxel for pancreatic cancer therapy. It can effectively penetrate and internalize tumor cells, and eventually cause tumor regression (38). Aptamer is an oligonucleotide sequence that can bind to target molecules with high affinity and specificity. It has the advantages of a wide range of target molecules, high stability, safety and economy, and simple preparation methods. Doxorubicin-loaded exosomes derived from HEK293 can target colorectal cancer by modifying with AS1411 aptamer (33). MSCs-derived exosomes loaded with doxorubicin can effectively target colorectal cancer and significantly inhibit tumor growth by covalently modifying carboxylic acid-end MUC1 aptamers (34). In recent years, the application of superparamagnetic nanoparticles in tumor therapy has attracted extensive attention. Exosomes were isolated from A33-positive human colorectal cancer cells and loaded with doxorubicin. Then, surface carboxylated superparamagnetic iron oxide nanoparticles coated with A33 antibodies bind to A33-positive exosomes and target A33-positive colorectal cancer cells. The results showed that A33 antibody-functionalized exosomes had the obvious tumor-targeting ability and have been confirmed to inhibit tumor growth (35). In conclusion, target-modified functional exosomes have proved to be novel and effective targeted drug delivery systems for gastrointestinal cancer therapy.
3.2 Exosomes-based drug delivery systems overcome drug resistance in gastrointestinal cancers
Drug resistance in tumor cells is usually identified as intrinsic (or innate) and extrinsic (or acquired) resistance. The former refers to cancer cells that are not sensitive to drugs at the beginning of treatment, while the latter refers to cancer cells that are originally sensitive to drugs, which developed drug resistance after repeated treatment and exposure to drugs. Once the tumor develops drug resistance, the drug cannot play an anti-cancer role. Even if the majority of the tumor is killed, the small number of drug-resistant cancer cells will continue to grow, causing cancer recurrence and rendering future anti-cancer chemotherapy ineffective. Therefore, drug resistance of cancer cells is one of the major challenges in cancer therapy. There are many factors leading to drug resistance, including abnormal gene expression, overexpression of transporters such as P-glycoprotein, and metabolic detoxification (55). However, the use of nanocarriers (such as exosomes) to deliver drugs can effectively overcome these factors, reverse drug resistance, and then exert good antitumor activity.
Cisplatin is an anti-cancer drug, which is widely used to treat a variety of cancers, including gastric cancer, colorectal cancer, and lung cancer. It inhibits tumor cell proliferation and induces apoptosis mainly by targeting DNA replication (56, 57). Although cisplatin has extensive anticancer activity, its use is limited due to its drug resistance and toxicity to untargeted tissues. The molecular mechanism of cisplatin resistance is complex and is mainly related to the abnormal expression of transporters, blocked apoptosis, enhanced intracellular detoxification, and enhanced DNA damage repair ability, as well as genetic and epigenetic changes (58, 59). miR-214 is an essential molecule in the process of drug resistance and is overexpressed in many malignant tumors. Exosomes containing anti-miR-214 can reverse cisplatin resistance and inhibit tumor growth in gastric cancer (39). In addition, c-MET, also known as hepatocyte growth factor receptor (HGFR), a protein with tyrosine kinase activity, is abnormally expressed or mutated in a variety of solid tumors and plays an important role in tumor proliferation, invasion, and metastasis. Transfecting HEK293T cells with si-c-Met and isolating exosomes can reverse the cisplatin resistance in gastric cancer, and inhibit the invasion and migration of gastric cancer cells and tumor growth (40).
5-fluorouracil (5-FU) is a first-line standardized chemotherapeutic drug for colorectal cancer, and the acquisition of 5-FU resistance often affects the therapeutic efficiency (60, 61). Exosome-delivered circ_0000338 enhances 5-FU resistance in colorectal cancer by negatively regulating miR-217 and miR-485-3p (62). Functional exosomes have been used to overcome drug resistance. Her2, a specific tumor-homing polypeptide, fuses with LAMP2, a protein found abundantly in exosome membranes. The HER2-LAMP2 fusion protein is expressed on the exosome surface and promotes the uptake of targeted cells through EGFR receptor-mediated endocytosis, effectively targeting colon cancer-resistant cells. The results showed that engineered exosomes loaded with 5-FU and miR-21 inhibitor oligonucleotides could effectively reverse colorectal cancer resistance and improve cancer therapeutic efficacy (41).
In addition, oxaliplatin-based chemotherapy is also one of the effective strategies for the therapy of colorectal cancer. Similarly, oxaliplatin resistance appears significantly in colorectal cancer, leading to treatment failure (63). Oxaliplatin-resistant colorectal cancer cells transfer ciRS-122 to oxaliplatin-sensitive cells via exosomes, thereby enhancing glycolysis and drug resistance. Exosome delivery of ciRS-122 siRNA enhances drug response (16). EMT refers to the transformation of epithelial cells into mesenchymal cells. The series of changes that occur after EMT activation contributes to the spread, invasion of surrounding tissues, and distant metastasis of tumor cells. EMT plays a key role in tumor invasion, metastasis, and drug resistance (64–66). More and more studies have shown that EMT markers can be used as prognostic indicators and potential therapeutic targets for colorectal cancer (67, 68). Exosome delivery of miR-1915-3p can downregulate the EMT-promoting oncogenes PFKFB3 and USP2, thereby improving the chemotherapeutic efficacy of oxaliplatin in colorectal cancer cells (42). Similarly, exosome delivery of circ-FBXW7 can inhibit EMT and oxaliplatin efflux by directly binding to miR-128-3p, increase oxaliplatin-induced apoptosis, and improve the sensitivity to oxaliplatin in colorectal cancer (43). Recently, the role of lncRNA in chemical resistance has been extensively studied. lncRNA PGM5 antisense RNA 1 (PGM5‐AS1) inhibits proliferation, migration, and acquired oxaliplatin tolerance in colon cancer cells. Exosomes co‐delivery of oxaliplatin and PGM5‐AS1 reverse drug resistance (44).
Gemcitabine is the current first-line treatment for pancreatic cancer. However, although gemcitabine has shown significant benefits in clinical application, its drug resistance severely limits its use. The transport, activation, and metabolism of gemcitabine are regulated by a variety of enzymes, and thus the development of resistance is regulated by a variety of factors (69). To overcome gemcitabine resistance in pancreatic cancer, survivin T34A was delivered by melanoma-cell-derived exosomes to restore the sensitivity of gemcitabine to pancreatic cancer cell lines. Compared with gemcitabine alone, apoptotic cell death is significantly increased (45). Moreover, exosomes from bone marrow-MSCs were used as homing carriers of pancreatic ductal adenocarcinoma to deliver paclitaxel and gemcitabine monophosphate as intermediates of gemcitabine metabolism. The results showed good penetration, anti-matrix, and anti-chemoresistance (46).
Exosome-based drug delivery systems have also shown promising therapeutic effects against drug resistance of other drugs in gastrointestinal cancers. miR-122 can promote the chemosensitivity of hepatocellular carcinoma cells. Delivery of miR-122 through adipose tissue-derived MSC exosomes can significantly improve the efficacy of sorafenib against hepatocellular carcinoma (47). Moreover, adipose tissue-derived MSC exosomes can effectively mediate the transfer of miR-199a to hepatocellular carcinoma cells and improve the sensitivity of hepatocellular carcinoma to doxorubicin (48). Taken together, this ability to deliver drugs and nucleic acids, along with other advantages such as low immunogenicity, biocompatibility, and natural targeting, make exosomes a promising and effective strategy for overcoming drug resistance in cancer therapy.
3.3 Exosomes-based drug delivery systems for immunotherapy in gastrointestinal cancers
Cancer immunotherapy is a therapeutic approach to control and eliminate tumors by modulating the immune system to activate anti-tumor immune responses or overcome tumor immune escape (70, 71). In recent years, the application of exosomes in cancer immunotherapy has been revealed. Numerous studies have shown that tumor and dendritic cells (DCs)-derived exosomes can express abundant tumor markers such as heat shock protein (HSP) and major histocompatibility complex (MHC). These molecules play a key role in antigen presentation and activation of T cells and have been demonstrated to provoke CD8+ T cell-mediated anti-tumor responses (72–75). Therefore, the application of exosomes in immunotherapy is of great significance to the progression of tumors, as the carrier of stimulating anti-tumor immune responses.
Tumor-derived exosomes are ideal antigen carriers, carrying many molecules and factors from tumor cells, and therefore easy to be recognized and taken up by immune cells. miR-34a is a major tumor suppressor that interferes with various colorectal cancer processes, including tumor proliferation, migration, and angiogenesis. Exosomes isolated from colorectal cancer cells and loaded with miR-34a mimic can reduce the expression of immune-evasion-related genes and induce cytotoxic T cells, significantly reducing the tumor size and prolonging the survival time of colorectal cancer mice (49). In addition, exosomes from immunogenically dying tumor cells modified with MART-1 peptide showed immunogenicity and were able to amplify CD8+ T cells for adoptive T cell therapy. The modified exosomes can enhance the anti-tumor immune response, and loaded with CCL22 siRNA can inhibit the expansion of Treg. The results showed that it was an effective preventive vaccine to delay tumor growth and a good adjuvant for pancreatic ductal adenocarcinoma chemotherapeutic drugs (51).
In addition to tumor-derived exosomes, DC-derived exosomes also have a promising application in tumor immunotherapy. DCs have unique antigen-presentation and activation properties of acquired and innate immune responses. DC-derived exosomes appear to act as antigen carriers, revealing their potential as cancer immunotherapy. Studies have shown that alpha-fetoprotein-rich DC-derived exosomes can induce effective antigen-specific anti-tumor immune responses and reshape the tumor microenvironment of hepatocellular carcinoma (54). Although DC-derived exosomes have a promising application prospect in tumor immunotherapy. However, the production of enough DC-derived exosomes remains a barrier to its widespread application in immunotherapy. Genetic engineering K562 has been used to produce artificial antigen-presenting cells, which secrete exosomes expressing HLA-A2 and costimulatory molecules that can enhance the anti-tumor immune effect of CD8+T cells, and these exosomes have a similar stimulative capacity as DC-derived exosomes (76).
Immunogenic cell death (ICD) is a kind of regulatory cell death, which can stimulate the immune system to produce immune responses through the release of tumor-associated antigens and tumor-specific antigens. It can be driven by different pressures, including intracellular pathogens, traditional chemotherapy drugs, targeted anti-cancer drugs, and a variety of physical therapies, such as radiotherapy and photodynamic therapy (77–79). The photosensitizer zinc phthalocyanine was added to exosomes from multiple cellular sources, such as immune cells, cancer cells, and external sources, to compare the antitumor effects of exosome-mediated photodynamic therapy. The results showed that M1-like macrophage-derived exosomes loaded with zinc phthalocyanine could initiate ICD, induce DC maturation, effectively inhibit colon cancer, and induce immune memory (50). These results indicate that the cell type and immune status from which exosomes are derived have a great influence on the therapeutic efficacy.
Cancer vaccines aim to stimulate the release and presentation of cancer antigens, immune cell initiation, and immune cell activation in the anti-cancer immune cycle. Once the immune cells are activated, they still need to complete the remaining steps: peripheral mobilization, infiltration to the tumor site, recognition of cancer cells, and activation of cytotoxicity against the cancer cell. Therefore, resistance mechanisms of anti-cancer immunity, especially in the tumor microenvironment, still reduce the efficiency of cancer vaccines, and to enhance cancer vaccines are being explored (80, 81). The combination of pancreatic cancer-derived exosome-loaded DCs vaccination with drugs that inhibit MDSCs, such as gemcitabine, sunitinib, and all-trans retinoic acid, can significantly inhibit the spread of metastasis, and prolong the survival time of mice due to the presence of more activated T cells in the tumor (52). Zhou et al. constructed a dual-delivery biosystem based on exosomes, which could significantly improve the tumor-targeting effect and thus increase the accumulation of drugs at the tumor site. This delivery system is composed of bone marrow MSC (BM-MSC) exosomes, surficially modified with oxaliplatin prodrugs and electroporation-loaded galectin-9 siRNA. The combination therapy can induce ICD in tumor cells, initiate DC maturation and antigen presentation, reverse immunosuppression, recruit anti-tumor cytotoxic T lymphocytes, and activate innate and adaptive anti-pancreatic ductal adenocarcinoma immunity (53).
4 Challenges for exosome-based drug delivery systems
As natural intercellular information carriers, exosomes have become one of the ideal drug delivery systems due to their nanoscale size, biocompatibility, permeability, and low immunogenicity. Although exosome-based drug delivery systems have been extensively studied, there still exist limitations in clinical application. First of all, it is difficult to obtain natural pure exosomes. Currently, exosomes are separated by a variety of methods, including ultracentrifugation, ultrafiltration, size-exclusion chromatography, polymer precipitation, immunoaffinity capture, and microfluidics-based techniques. Each method has advantages and limitations (8, 82). Ultracentrifugation is the current gold standard and the most commonly used exosome isolation approach. The resulting exosomes have a large volume but insufficient purity and the exosomes can be found to aggregate into blocks during electron microscopy identification, which is not conducive to subsequent experiments. Ultrafiltration is relatively simple and time-saving and is mostly used for the separation of exosomal RNA. However, exosomes may block the filtration pore, resulting in a shortened membrane life and low separation efficiency. Size-exclusion chromatography can obtain exosomes with high purity to ensure their integrity and activity. However, it is not suitable for amplification and only suitable for medium sample processing capacity. Polymer precipitation is simple and fast, but there are false positives (impurity protein or polymer), and mechanical forces or chemical additions will destroy exosomes. The immunoaffinity capture has the advantages of high specificity, simple operation, and no influence on the morphological integrity of exosomes. However, it has low efficiency and the biological activity of exosomes is easily affected by pH and salt concentration, which is not conducive to downstream experiments and difficult to be widely popularized. Microfluidics-based techniques are easy to automate but lack standardized and large-scale clinical sample testing and methodological validation. At present, no method can fully meet the demand, not only to maintain the integrity, high yield, and purity of exosomes but also to control the quality of exosomes. Therefore, the production of GMP-grade medicinal exosomes remains a major challenge, and the continuous optimization of the process will take a long time. Secondly, some researchers believe that exosomes are vesicles secreted by autologous cells and have the natural targeting ability based on donor cells, which can avoid phagocytosis by the mononuclear phagocyte system (MPS). Moreover, the expression of CD47 on the surface of exosomes is also conducive to avoiding clearance of MPS and prolonging blood circulation time (83, 84). However, some studies have shown that exosomes have a certain natural targeting ability but are not strong, and are easily recognized and quickly absorbed by MPS after systematic administration (85–87). Therefore, further research should reveal whether the clearance of exosomes by MPS is actually due to their intrinsic properties or exogenous characteristics acquired from in vitro culture. At present, to avoid rapid clearance and improve the targeting of exosomes, researchers have made various modifications to the exosome surface. However, although targeted modification methods have made progress in experiments, the in vivo environment is complex, and it is uncertain whether the modified exosome still has the expected targeting ability after entering the body. Therefore, targeted modification of exosomes is still the key point to be overcome. In addition, whether the modified exosomes induce immune responses should be further investigated in the future. Next, compared with conventional nanocarriers, common exosome loading strategies (such as passive mixing, electroporation, and exogenous loading) usually have low loading efficiency (<30%). These and other factors present significant challenges to the large-scale manufacturing of exosomes for drug delivery that, if overcome, could be translated into nanomedicine. Finally, the selection of the cellular sources of exosomes is also very important. Exosomes from different cellular sources have different components, and their potential biological functions are also significantly different. For example, exosomes derived from tumor cells as drug delivery systems can be well-homed to the tumor site, but at the same time have the risk of promoting tumor growth and immunosuppression (88). Exosomes derived from macrophages have a good inflammatory tendency and can cross the blood-brain barrier for the therapy of brain diseases (89). Exosomes derived from DCs have good immune effects (90). Therefore, selecting the cellular sources of exosomes is the premise of achieving the best therapeutic effect.
5 Conclusions
As extracellular nanovesicles, exosomes can deliver bioactive substances such as miRNA, lncRNA, and protein between cells, playing an extremely important role in the occurrence and development of gastrointestinal cancers. In addition, it can be used as a drug delivery system for the transportation of tumor therapeutic agents to inhibit tumor proliferation and metastasis, reverse drug resistance, and induce an anti-tumor immune response in gastrointestinal cancers. Although exosomes as drug delivery systems have potential advantages such as biocompatibility, stability, and intrinsic targeting, the research on exosomes as drug delivery systems is still insufficient, and many problems remain to be solved. The research aimed at clinical transformation should make continuous efforts to improve the production, purity, targeting, and bioactivity of exosomes. In conclusion, the clinical successful application of exosomes as drug delivery systems will take some time, but it is believed that it will benefit the majority of patients soon.
Author contributions
FX and LB provided the direction and guidance for this manuscript. FX wrote the whole manuscript. YH, YZ, and LB revised this manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewers WL and JH declared a shared parent affiliation, with no collaboration, with the authors to the handling editor at the time of the review.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin (2022) 72(1):7–33. doi: 10.3322/caac.21708
2. Moehler M, Delic M, Goepfert K, Aust D, Grabsch HI, Halama N, et al. Immunotherapy in gastrointestinal cancer: Recent results, current studies and future perspectives. Eur J Cancer (2016) 59:160–70. doi: 10.1016/j.ejca.2016.02.020
3. Abdul-Latif M, Townsend K, Dearman C, Shiu KK, Khan K. Immunotherapy in gastrointestinal cancer: The current scenario and future perspectives. Cancer Treat Rev (2020) 88:102030. doi: 10.1016/j.ctrv.2020.102030
4. Elsharkasy OM, Nordin JZ, Hagey DW, de Jong OG, Schiffelers RM, Andaloussi SE, et al. Extracellular vesicles as drug delivery systems: Why and how? Adv Drug Del Rev (2020) 159:332–43. doi: 10.1016/j.addr.2020.04.004
5. Zhao X, Wu D, Ma X, Wang J, Hou W, Zhang W. Exosomes as drug carriers for cancer therapy and challenges regarding exosome uptake. BioMed Pharmacother (2020) 128:110237. doi: 10.1016/j.biopha.2020.110237
6. Patil SM, Sawant SS, Kunda NK. Exosomes as drug delivery systems: A brief overview and progress update. Eur J pharmaceutics biopharmaceutics: Off J Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV (2020) 154:259–69. doi: 10.1016/j.ejpb.2020.07.026
7. Zheng Y, Hasan A, Nejadi Babadaei MM, Behzadi E, Nouri M, Sharifi M, et al. Exosomes: Multiple-targeted multifunctional biological nanoparticles in the diagnosis, drug delivery, and imaging of cancer cells. BioMed Pharmacother (2020) 129:110442. doi: 10.1016/j.biopha.2020.110442
8. Zhang Y, Bi J, Huang J, Tang Y, Du S, Li P. Exosome: A review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int J nanomed (2020) 15:6917–34. doi: 10.2147/IJN.S264498
9. Liang Y, Duan L, Lu J, Xia J. Engineering exosomes for targeted drug delivery. Theranostics (2021) 11(7):3183–95. doi: 10.7150/thno.52570
10. Qu JL, Qu XJ, Zhao MF, Teng YE, Zhang Y, Hou KZ, et al. Gastric cancer exosomes promote tumour cell proliferation through PI3K/Akt and MAPK/ERK activation. Dig Liver Dis (2009) 41(12):875–80. doi: 10.1016/j.dld.2009.04.006
11. Zhao LX, Zhang K, Shen BB, Li JN. Mesenchymal stem cell-derived exosomes for gastrointestinal cancer. World J Gastrointest Oncol (2021) 13(12):1981–96. doi: 10.4251/wjgo.v13.i12.1981
12. Zhou L, Li J, Tang Y, Yang M. Exosomal LncRNA LINC00659 transferred from cancer-associated fibroblasts promotes colorectal cancer cell progression via miR-342-3p/ANXA2 axis. J Transl Med (2021) 19(1):8. doi: 10.1186/s12967-020-02648-7
13. Huang XY, Huang ZL, Huang J, Xu B, Huang XY, Xu YH, et al. Exosomal circRNA-100338 promotes hepatocellular carcinoma metastasis via enhancing invasiveness and angiogenesis. J Exp Clin Cancer Res (2020) 39(1):20. doi: 10.1186/s13046-020-1529-9
14. Zeng Z, Li Y, Pan Y, Lan X, Song F, Sun J, et al. Cancer-derived exosomal miR-25-3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nat Commun (2018) 9(1):5395. doi: 10.1038/s41467-018-07810-w
15. Lin H, Zhang L, Zhang C, Liu P. Exosomal MiR-500a-3p promotes cisplatin resistance and stemness via negatively regulating FBXW7 in gastric cancer. J Cell Mol Med (2020) 24(16):8930–41. doi: 10.1111/jcmm.15524
16. Wang X, Zhang H, Yang H, Bai M, Ning T, Deng T, et al. Exosome-delivered circRNA promotes glycolysis to induce chemoresistance through the miR-122-PKM2 axis in colorectal cancer. Mol Oncol (2020) 14(3):539–55. doi: 10.1002/1878-0261.12629
17. Hu YB, Yan C, Mu L, Mi YL, Zhao H, Hu H, et al. Exosomal wnt-induced dedifferentiation of colorectal cancer cells contributes to chemotherapy resistance. Oncogene (2019) 38(11):1951–65. doi: 10.1038/s41388-018-0557-9
18. Zheng P, Chen L, Yuan X, Luo Q, Liu Y, Xie G, et al. Exosomal transfer of tumor-associated macrophage-derived miR-21 confers cisplatin resistance in gastric cancer cells. J Exp Clin Cancer Res (2017) 36(1):53. doi: 10.1186/s13046-017-0528-y
19. Yamada N, Kuranaga Y, Kumazaki M, Shinohara H, Taniguchi K, Akao Y. Colorectal cancer cell-derived extracellular vesicles induce phenotypic alteration of T cells into tumor-growth supporting cells with transforming growth factor-β1-mediated suppression. Oncotarget (2016) 7(19):27033–43. doi: 10.18632/oncotarget.7041
20. Wang X, Luo G, Zhang K, Cao J, Huang C, Jiang T, et al. Hypoxic tumor-derived exosomal miR-301a mediates M2 macrophage polarization via PTEN/PI3Kgamma to promote pancreatic cancer metastasis. Cancer Res (2018) 78(16):4586–98. doi: 10.1158/0008-5472.CAN-17-3841
21. Ingato D, Lee JU, Sim SJ, Kwon YJ. Good things come in small packages: Overcoming challenges to harness extracellular vesicles for therapeutic delivery. J Controlled release: Off J Controlled Release Society. (2016) 241:174–85. doi: 10.1016/j.jconrel.2016.09.016
22. Duan L, Xu L, Xu X, Qin Z, Zhou X, Xiao Y, et al. Exosome-mediated delivery of gene vectors for gene therapy. Nanoscale (2021) 13(3):1387–97. doi: 10.1039/D0NR07622H
23. Kotmakçı M, Bozok Çetintaş V. Extracellular vesicles as natural nanosized delivery systems for small-molecule drugs and genetic material: Steps towards the future nanomedicines. J Pharm Pharm Sci (2015) 18(3):396–413. doi: 10.18433/J36W3X
24. Jiang XC, Gao JQ. Exosomes as novel bio-carriers for gene and drug delivery. Int J pharmaceutics (2017) 521(1-2):167–75. doi: 10.1016/j.ijpharm.2017.02.038
25. Barok M, Puhka M, Vereb G, Szollosi J, Isola J, Joensuu H. Cancer-derived exosomes from HER2-positive cancer cells carry trastuzumab-emtansine into cancer cells leading to growth inhibition and caspase activation. BMC Cancer. (2018) 18(1):504. doi: 10.1186/s12885-018-4418-2
26. Wang JJ, Wang ZY, Chen R, Xiong J, Yao YL, Wu JH, et al. Macrophage-secreted exosomes delivering miRNA-21 inhibitor can regulate BGC-823 cell proliferation. Asian Pacific J Cancer prevention: APJCP. (2015) 16(10):4203–9. doi: 10.7314/APJCP.2015.16.10.4203
27. Zhang H, Wang Y, Bai M, Wang J, Zhu K, Liu R, et al. Exosomes serve as nanoparticles to suppress tumor growth and angiogenesis in gastric cancer by delivering hepatocyte growth factor siRNA. Cancer Sci (2018) 109(3):629–41. doi: 10.1111/cas.13488
28. Liang G, Kan S, Zhu Y, Feng S, Feng W, Gao S. Engineered exosome-mediated delivery of functionally active miR-26a and its enhanced suppression effect in HepG2 cells. Int J nanomed (2018) 13:585–99. doi: 10.2147/IJN.S154458
29. Li T, Wan Y, Su Z, Li J, Han M, Zhou C. Mesenchymal stem cell-derived exosomal microRNA-3940-5p inhibits colorectal cancer metastasis by targeting integrin α6. Digest Dis Sci (2021) 66(6):1916–27. doi: 10.1007/s10620-020-06458-1
30. Zhao J, Lin H, Huang K. Mesenchymal stem cell-derived extracellular vesicles transmitting MicroRNA-34a-5p suppress tumorigenesis of colorectal cancer through c-MYC/DNMT3a/PTEN axis. Mol Neurobiol (2022) 59(1):47–60. doi: 10.1007/s12035-021-02431-9
31. Han S, Li G, Jia M, Zhao Y, He C, Huang M, et al. Delivery of anti-miRNA-221 for colorectal carcinoma therapy using modified cord blood mesenchymal stem cells-derived exosomes. Front Mol Biosci (2021) 8. doi: 10.3389/fmolb.2021.743013
32. Go G, Park HJ, Lee JH, Yun CW, Lee SH. Inhibitory effect of oxaliplatin-loaded engineered milk extracellular vesicles on tumor progression. Anticancer Res (2022) 42(2):857–66. doi: 10.21873/anticanres.15543
33. Hosseini NF, Amini R, Ramezani M, Saidijam M, Hashemi SM, Najafi R. AS1411 aptamer-functionalized exosomes in the targeted delivery of doxorubicin in fighting colorectal cancer. Biomed Pharmacother (2022) 155:113690. doi: 10.1016/j.biopha.2022.113690
34. Bagheri E, Abnous K, Farzad SA, Taghdisi SM, Ramezani M, Alibolandi M. Targeted doxorubicin-loaded mesenchymal stem cells-derived exosomes as a versatile platform for fighting against colorectal cancer. Life Sci (2020) 261:118369. doi: 10.1016/j.lfs.2020.118369
35. Li Y, Gao Y, Gong C, Wang Z, Xia Q, Gu F, et al. A33 antibody-functionalized exosomes for targeted delivery of doxorubicin against colorectal cancer. Nanomed: Nanotechnol Biology Med (2018) 14(7):1973–85. doi: 10.1016/j.nano.2018.05.020
36. Ding Y, Cao F, Sun H, Wang Y, Liu S, Wu Y, et al. Exosomes derived from human umbilical cord mesenchymal stromal cells deliver exogenous miR-145-5p to inhibit pancreatic ductal adenocarcinoma progression. Cancer Lett (2019) 442:351–61. doi: 10.1016/j.canlet.2018.10.039
37. Xu L, Faruqu FN, Lim YM, Lim KY, Liam-Or R, Walters AA, et al. Exosome-mediated RNAi of PAK4 prolongs survival of pancreatic cancer mouse model after loco-regional treatment. Biomaterials (2021) 264:120369. doi: 10.1016/j.biomaterials.2020.120369
38. Al Faruque H, Choi ES, Kim JH, Kim E. Enhanced effect of autologous EVs delivering paclitaxel in pancreatic cancer. J Controlled release: Off J Controlled Release Soc. (2022) 347:330–46. doi: 10.1016/j.jconrel.2022.05.012
39. Wang X, Zhang H, Bai M, Ning T, Ge S, Deng T, et al. Exosomes serve as nanoparticles to deliver anti-miR-214 to reverse chemoresistance to cisplatin in gastric cancer. Mol therapy: J Am Soc Gene Ther (2018) 26(3):774–83. doi: 10.1016/j.ymthe.2018.01.001
40. Zhang Q, Zhang H, Ning T, Liu D, Deng T, Liu R, et al. Exosome-delivered c-met siRNA could reverse chemoresistance to cisplatin in gastric cancer. Int J Nanomed (2020) 15:2323–35. doi: 10.2147/IJN.S231214
41. Liang G, Zhu Y, Ali DJ, Tian T, Xu H, Si K, et al. Engineered exosomes for targeted co-delivery of miR-21 inhibitor and chemotherapeutics to reverse drug resistance in colon cancer. J nanobiotechnol (2020) 18(1):10. doi: 10.1186/s12951-019-0563-2
42. Xiao Z, Liu Y, Li Q, Liu Q, Liu Y, Luo Y, et al. EVs delivery of miR-1915-3p improves the chemotherapeutic efficacy of oxaliplatin in colorectal cancer. Cancer chemother Pharmacol (2021) 88(6):1021–31. doi: 10.1007/s00280-021-04348-5
43. Xu Y, Qiu A, Peng F, Tan X, Wang J, Gong X. Exosomal transfer of circular RNA FBXW7 ameliorates the chemoresistance to oxaliplatin in colorectal cancer by sponging miR-18b-5p. Neoplasma (2021) 68(1):108–18. doi: 10.4149/neo_2020_200417N414
44. Hui B, Lu C, Wang J, Xu Y, Yang Y, Ji H, et al. Engineered exosomes for co-delivery of PGM5-AS1 and oxaliplatin to reverse drug resistance in colon cancer. J Cell Physiol (2022) 237(1):911–33. doi: 10.1002/jcp.30566
45. Aspe JR, Diaz Osterman CJ, Jutzy JM, Deshields S, Whang S, Wall NR. Enhancement of gemcitabine sensitivity in pancreatic adenocarcinoma by novel exosome-mediated delivery of the survivin-T34A mutant. J Extracellular Vesicles (2014) 3. doi: 10.3402/jev.v3.23244
46. Zhou Y, Zhou W, Chen X, Wang Q, Li C, Chen Q, et al. Bone marrow mesenchymal stem cells-derived exosomes for penetrating and targeted chemotherapy of pancreatic cancer. Acta Pharm Sin B (2020) 10(8):1563–75. doi: 10.1016/j.apsb.2019.11.013
47. Lou G, Song X, Yang F, Wu S, Wang J, Chen Z, et al. Exosomes derived from miR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J Hematol Oncol (2015) 8:122. doi: 10.1186/s13045-015-0220-7
48. Lou G, Chen L, Xia C, Wang W, Qi J, Li A, et al. MiR-199a-modified exosomes from adipose tissue-derived mesenchymal stem cells improve hepatocellular carcinoma chemosensitivity through mTOR pathway. J Exp Clin Cancer Res (2020) 39(1):4. doi: 10.1186/s13046-019-1512-5
49. Hosseini M, Baghaei K, Hajivalili M, Zali MR, Ebtekar M, Amani D. The anti-tumor effects of CT-26 derived exosomes enriched by MicroRNA-34a on murine model of colorectal cancer. Life Sci (2022) 290:120234. doi: 10.1016/j.lfs.2021.120234
50. Huis In ‘t Veld RV, Lara P, Jager MJ, Koning RI, Ossendorp F, Cruz LJ. M1-derived extracellular vesicles enhance photodynamic therapy and promote immunological memory in preclinical models of colon cancer. J Nanobiotechnol (2022) 20(1):252. doi: 10.1186/s12951-022-01448-z
51. Zhou W, Chen X, Zhou Y, Shi S, Liang C, Yu X, et al. Exosomes derived from immunogenically dying tumor cells as a versatile tool for vaccination against pancreatic cancer. Biomaterials (2022) 280:121306. doi: 10.1016/j.biomaterials.2021.121306
52. Xiao L, Erb U, Zhao K, Hackert T, Zöller M. Efficacy of vaccination with tumor-exosome loaded dendritic cells combined with cytotoxic drug treatment in pancreatic cancer. OncoImmunology (2017) 6(6):e1319044. doi: 10.1080/2162402X.2017.1319044
53. Zhou W, Zhou Y, Chen X, Ning T, Chen H, Guo Q, et al. Pancreatic cancer-targeting exosomes for enhancing immunotherapy and reprogramming tumor microenvironment. Biomaterials (2021) 268:120546. doi: 10.1016/j.biomaterials.2020.120546
54. Lu Z, Zuo B, Jing R, Gao X, Rao Q, Liu Z, et al. Dendritic cell-derived exosomes elicit tumor regression in autochthonous hepatocellular carcinoma mouse models. J Hepatology. (2017) 67(4):739–48. doi: 10.1016/j.jhep.2017.05.019
55. Wu Q, Yang Z, Nie Y, Shi Y, Fan D. Multi-drug resistance in cancer chemotherapeutics: Mechanisms and lab approaches. Cancer letters. (2014) 347(2):159–66. doi: 10.1016/j.canlet.2014.03.013
56. Ghosh S. Cisplatin: The first metal based anticancer drug. Bioorganic Chem (2019) 88:102925. doi: 10.1016/j.bioorg.2019.102925
57. Dasari S, Bernard Tchounwou P. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur J Pharmacol (2014) 740:364–78. doi: 10.1016/j.ejphar.2014.07.025
58. Galluzzi L, Senovilla L, Vitale I, Michels J, Martins I, Kepp O, et al. Molecular mechanisms of cisplatin resistance. Oncogene (2012) 31(15):1869–83. doi: 10.1038/onc.2011.384
59. Shen DW, Pouliot LM, Hall MD, Gottesman MM. Cisplatin resistance: a cellular self-defense mechanism resulting from multiple epigenetic and genetic changes. Pharmacol Rev (2012) 64(3):706–21. doi: 10.1124/pr.111.005637
60. Vodenkova S, Buchler T, Cervena K, Veskrnova V, Vodicka P, Vymetalkova V. 5-fluorouracil and other fluoropyrimidines in colorectal cancer: Past, present and future. Pharmacol Ther (2020) 206:107447. doi: 10.1016/j.pharmthera.2019.107447
61. Blondy S, David V, Verdier M, Mathonnet M, Perraud A, Christou N. 5-fluorouracil resistance mechanisms in colorectal cancer: From classical pathways to promising processes. Cancer Sci (2020) 111(9):3142–54. doi: 10.1111/cas.14532
62. Zhao K, Cheng X, Ye Z, Li Y, Peng W, Wu Y, et al. Exosome-mediated transfer of circ_0000338 enhances 5-fluorouracil resistance in colorectal cancer through regulating MicroRNA 217 (miR-217) and miR-485-3p. Mol Cell Biol (2021) 41(5):e00517–20. doi: 10.1128/MCB.00517-20
63. Hsu HH, Chen MC, Baskaran R, Lin YM, Day CH, Lin YJ, et al. Oxaliplatin resistance in colorectal cancer cells is mediated via activation of ABCG2 to alleviate ER stress induced apoptosis. J Cell Physiol (2018) 233(7):5458–67. doi: 10.1002/jcp.26406
64. Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol (2014) 15(3):178–96. doi: 10.1038/nrm3758
65. Lu W, Kang Y. Epithelial-mesenchymal plasticity in cancer progression and metastasis. Dev Cell (2019) 49(3):361–74. doi: 10.1016/j.devcel.2019.04.010
66. Du B, Shim JS. Targeting epithelial-mesenchymal transition (EMT) to overcome drug resistance in cancer. Molecules (2016) 21(7). doi: 10.3390/molecules21070965
67. Zhang N, Ng AS, Cai S, Li Q, Yang L, Kerr D. Novel therapeutic strategies: targeting epithelial-mesenchymal transition in colorectal cancer. Lancet Oncol (2021) 22(8):e358–e68. doi: 10.1016/S1470-2045(21)00343-0
68. Cao H, Xu E, Liu H, Wan L, Lai M. Epithelial-mesenchymal transition in colorectal cancer metastasis: A system review. Pathol Res Pract (2015) 211(8):557–69. doi: 10.1016/j.prp.2015.05.010
69. Gebregiworgis T, Bhinderwala F, Purohit V, Chaika NV, Singh PK, Powers R. Insights into gemcitabine resistance and the potential for therapeutic monitoring. Metabolomics (2018) 14(12):156. doi: 10.1007/s11306-018-1452-7
70. Zhang Y, Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol (2020) 17(8):807–21. doi: 10.1038/s41423-020-0488-6
71. Velcheti V, Schalper K. Basic overview of current immunotherapy approaches in cancer. Am Soc Clin Oncol Educ Book. (2016) 35:298–308. doi: 10.1200/EDBK_156572
72. Pitt JM, Andre F, Amigorena S, Soria JC, Eggermont A, Kroemer G, et al. Dendritic cell-derived exosomes for cancer therapy. J Clin Invest. (2016) 126(4):1224–32. doi: 10.1172/JCI81137
73. Pitt JM, Charrier M, Viaud S, Andre F, Besse B, Chaput N, et al. Dendritic cell-derived exosomes as immunotherapies in the fight against cancer. J Immunol (2014) 193(3):1006–11. doi: 10.4049/jimmunol.1400703
74. Naseri M, Bozorgmehr M, Zoller M, Ranaei Pirmardan E, Madjd Z. Tumor-derived exosomes: the next generation of promising cell-free vaccines in cancer immunotherapy. Oncoimmunology (2020) 9(1):1779991. doi: 10.1080/2162402X.2020.1779991
75. Tang Q, Yang S, He G, Zheng H, Zhang S, Liu J, et al. Tumor-derived exosomes in the cancer immune microenvironment and cancer immunotherapy. Cancer letters. (2022) 548:215823. doi: 10.1016/j.canlet.2022.215823
76. Kim S, Sohn HJ, Lee HJ, Sohn DH, Hyun SJ, Cho HI, et al. Use of engineered exosomes expressing HLA and costimulatory molecules to generate antigen-specific CD8+ T cells for adoptive cell therapy. J Immunother. (2017) 40(3):83–93. doi: 10.1097/CJI.0000000000000151
77. Zhou J, Wang G, Chen Y, Wang H, Hua Y, Cai Z. Immunogenic cell death in cancer therapy: Present and emerging inducers. J Cell Mol Med (2019) 23(8):4854–65. doi: 10.1111/jcmm.14356
78. Li Y, Liu X, Zhang X, Pan W, Li N, Tang B. Immunogenic cell death inducers for enhanced cancer immunotherapy. Chem Commun (2021) 57(91):12087–97. doi: 10.1039/D1CC04604G
79. Jin MZ, Wang XP. Immunogenic cell death-based cancer vaccines. Front Immunol (2021) 12:697964. doi: 10.3389/fimmu.2021.697964
80. Saxena M, van der Burg SH, Melief CJM, Bhardwaj N. Therapeutic cancer vaccines. Nat Rev Cancer. (2021) 21(6):360–78. doi: 10.1038/s41568-021-00346-0
81. Zhu S, Zhang T, Zheng L, Liu H, Song W, Liu D, et al. Combination strategies to maximize the benefits of cancer immunotherapy. J Hematol Oncol (2021) 14(1):156. doi: 10.1186/s13045-021-01164-5
82. Yang D, Zhang W, Zhang H, Zhang F, Chen L, Ma L, et al. Progress, opportunity, and perspective on exosome isolation - efforts for efficient exosome-based theranostics. Theranostics (2020) 10(8):3684–707. doi: 10.7150/thno.41580
83. Zeng W, Wen Z, Chen H, Duan Y. Exosomes as carriers for drug delivery in cancer therapy. Pharm Res (2022). doi: 10.1007/s11095-022-03224-y
84. Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA, et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature (2017) 546(7659):498–503. doi: 10.1038/nature22341
85. Yin Y, Han X, Li C, Sun T, Li K, Liu X, et al. The status of industrialization and development of exosomes as a drug delivery system: A review. Front Pharmacol (2022) 13:961127. doi: 10.3389/fphar.2022.961127
86. Ferreira D, Moreira JN, Rodrigues LR. New advances in exosome-based targeted drug delivery systems. Crit Rev Oncol Hematol (2022) 172:103628. doi: 10.1016/j.critrevonc.2022.103628
87. Parada N, Romero-Trujillo A, Georges N, Alcayaga-Miranda F. Camouflage strategies for therapeutic exosomes evasion from phagocytosis. J Adv Res (2021) 31:61–74. doi: 10.1016/j.jare.2021.01.001
88. Whiteside TL. Tumor-derived exosomes and their role in cancer progression. Adv Clin Chem (2016) 74:103–41. doi: 10.1016/bs.acc.2015.12.005
89. Liu J, Wu F, Zhou H. Macrophage-derived exosomes in cancers: Biogenesis, functions and therapeutic applications. Immunol Lett (2020) 227:102–8. doi: 10.1016/j.imlet.2020.08.003
Keywords: gastrointestinal cancer, exosomes, extracellular vesicles, drug delivery system, cancer therapy
Citation: Xie F, Huang Y, Zhan Y and Bao L (2023) Exosomes as drug delivery system in gastrointestinal cancer. Front. Oncol. 12:1101823. doi: 10.3389/fonc.2022.1101823
Received: 18 November 2022; Accepted: 29 December 2022;
Published: 25 January 2023.
Edited by:
Li Liang, National Center for Liver Cancer, ChinaReviewed by:
Jin Hou, Second Military Medical University, ChinaWei Li, Second Military Medical University, China
Wan Zhuo, Fourth Military Medical University, China
Copyright © 2023 Xie, Huang, Zhan and Bao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Leilei Bao, YW5uYWJhbzIxMkAxMjYuY29t
†These authors have contributed equally to this work
 Fangyuan Xie
Fangyuan Xie Yueying Huang†
Yueying Huang† Leilei Bao
Leilei Bao