
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 17 January 2023
Sec. Cancer Genetics
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.1101530
This article is part of the Research Topic Case Reports in Cancer Genetics : 2022 View all 16 articles
 Santiago Cadena-Ullauri1†
Santiago Cadena-Ullauri1† Elius Paz-Cruz1†
Elius Paz-Cruz1† Rafael Tamayo-Trujillo1†
Rafael Tamayo-Trujillo1† Patricia Guevara-Ramírez1
Patricia Guevara-Ramírez1 Viviana Ruiz-Pozo1
Viviana Ruiz-Pozo1 Paola Solis-Pazmino2,3
Paola Solis-Pazmino2,3 Cristhian Garcia3
Cristhian Garcia3 Richard Godoy3
Richard Godoy3 Eddy Lincango-Naranjo3,4,5
Eddy Lincango-Naranjo3,4,5 Ana Karina Zambrano1*
Ana Karina Zambrano1*Background: The incidence of thyroid cancer has increased worldwide. Ecuador presents the highest incidence among Latin American countries and the second around the world. Genetic alteration is the driving force for thyroid tumorigenesis and progression. The change from valine (V) to glutamic acid (E) at codon 600 of the BRAF gene (BRAFVal600Glu) is the most commonly reported mutation in thyroid cancer. Moreover, the BRAF mutation is not the only mutation that has been correlated with TC. For instance, mutations and overexpression of the KIT gene has been associated with different types of cancer, including lung and colon cancer, and neuroblastoma.
Case presentation: A woman in her early fifties, self-identified as mestizo, from Otavalo, Imbabura-Ecuador had no systemic diseases and denied allergies, but she had a family history of a benign thyroid nodule. Physical examination revealed a thyroid gland enlargement. The fine-needle aspiration biopsy indicated papillary thyroid cancer. The patient underwent a successful total thyroidectomy with an excellent recovery and no additional treatments after surgery. Using Next-Generation sequencing a heterozygous mutation in the BRAF gene, causing an amino acid change Val600Glu was identified. Similarly, in the KIT gene, a heterozygous mutation resulting in an amino acid change Leu678Phe was detected. Moreover, an ancestry analysis was performed, and the results showed 3.1% African, 20.9% European, and 76% Native American ancestry.
Conclusions: This report represents the genetic characteristics of papillary thyroid cancer in an Ecuadorian woman with a mainly Native American ethnic component. Further studies of pathological variants are needed to determine if the combined demographic and molecular profiles are useful to develop targeted treatments focused on the Ecuadorian population.
The incidence of thyroid cancer (TC) has increased worldwide (1). In the United States, thyroid cancer incidence has tripled over the last three decades from 5.5 to 14.0 per 100,000 people in 2019. TC ranks as the fifth most common cancer in Ecuadorian women (2, 3).
TC is classified into differentiated TC (DTC), poorly differentiated TC (PDTC), and anaplastic TC (ATC) (4). The most common type is differentiated TC (DTC) including papillary thyroid cancer (PTC), and particularly thyroid cancers of 1 cm or less in size, called papillary thyroid microcarcinoma (5). DTC, including PTC, is relatively indolent and highly curable; however, a significant recurrence rate, about 20% at 10 years and 30% at 30 years after initial treatment, is seen.
Genetic alteration is the driving force for thyroid tumorigenesis and progression. The main metabolic pathways associated with TC oncogenesis are the mitogen-activating protein kinase (MAPK) signaling pathway and the Phosphatidylinositol-3-kinase (PI3K)-AKT pathway (4). Mutations in protein components of these metabolic pathways lead to the translocation of transcription factors upregulating gene transcription and promoting oncogenesis 4). The change from valine (V) to glutamic acid (E) at codon 600 of the BRAF gene (BRAFVal600Glu) is the most commonly reported mutation in PTC (4, 6). The BRAF mutation leads to the activation of the BRAF kinase in the MAPK pathway; this is considered the initial event in the oncogenesis and progression of PTC (4). An association between the BRAFVal600Glu variant presence and worse prognosis, extrathyroidal extension, and lymph node metastasis has also been established (6, 7). Moreover, the BRAF mutation is not the only mutation that has been correlated with TC. For instance, mutations and overexpression of the KIT gene has been associated with different types of cancer, including lung cancer, neuroblastoma, and colon (8–10). Hence, it is important to identify mutations that along with the BRAFVal600Glu mutation are driving the tumorigenesis.
The present case report describes a woman in her early fifties who underwent total thyroidectomy with malignant histopathologic features and the presence of a BRAFVal600Glu and a KITLeu678Phe variants.
The present case report describes a woman in her early fifties, self-identified as mestizo, from Otavalo, Imbabura-Ecuador. In her familial history, the mother and father did not report any type of cancer, and only one of her brothers, among six, presented thyroid nodules; however, they were categorized as benign. The patient attended to the physician due to a lump in the neck that had been gradually enlarging for about 2 years. She had no symptoms of dysphagia and denied odynophagia or shortness of breath. The patient had no systemic diseases and denied allergies.
Physical examination revealed a thyroid gland enlargement. The ultrasound confirmed the thyroid growth (29 x 17 x 22 mm) and showed a solid nodule located in the left lobule with irregular borders, microcalcifications, highly vascularized. The fine-needle aspiration biopsy indicated a Bethesda VI consistent with papillary thyroid cancer. Based on the results, a total thyroidectomy was chosen as the best option (11). The subject’s blood tests were normal; therefore, the patient could undergo surgery. The procedure was successful with an excellent recovery. Posterior medical checkups did not show any complication.
Moreover, an ancestral composition analysis was performed, and the results showed 3.1% African, 20.9% European, and 76% Native American. A timeline of the relevant episodes of care is depicted in Figure 1.
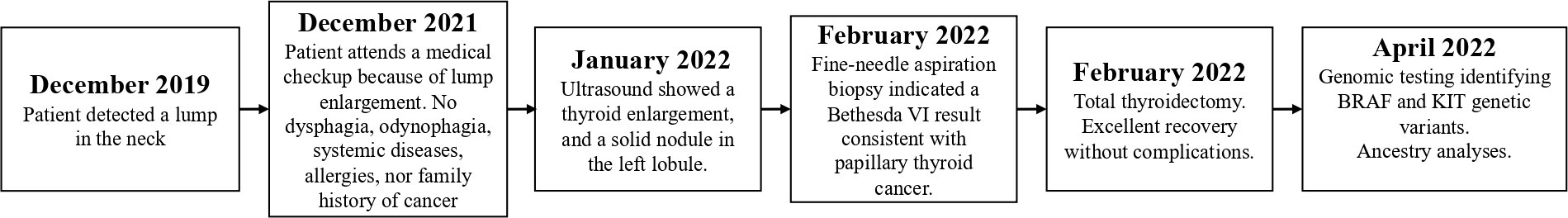
Figure 1 Subject’s relevant episodes of care. The disease-associated episodes of care are presented in the figure.
Next-generation sequencing was performed using the TruSight Tumor 15 kit from Illumina ®; this panel includes 15 genes commonly mutated in solid tumors. Among the mutated genes, two had an in silico pathogenic status: BRAF and KIT, as described in the Table 1. The BRAF gene suffered a heterozygous mutation, a change from adenine to thymine at position 1799; this caused an amino acid change from Valine to Glutamic acid at position 600 (Figure 2), confirmed with Sanger Sequencing. Similarly, in the KIT gene, a heterozygous mutation led to a thymine change to cytosine at position 2032, which resulted in an amino acid change from Leucine to Phenylalanine at position 678 (Figure 3).
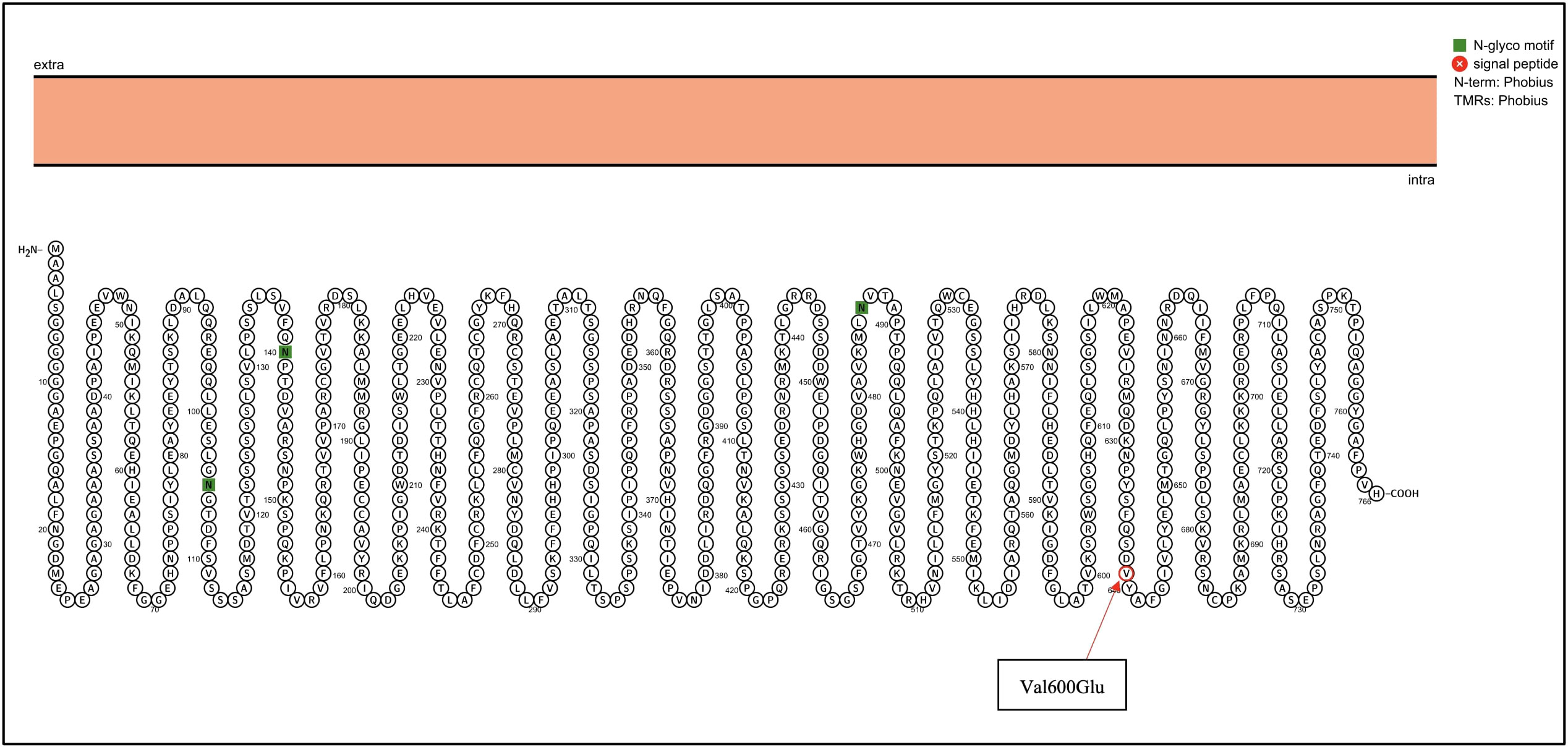
Figure 2 BRAF genetic mutation identified in the patient by NGS. Proteins were visualized with Protter (12). The red circle highlights the identified Val600Glu mutation in the BRAF gene.
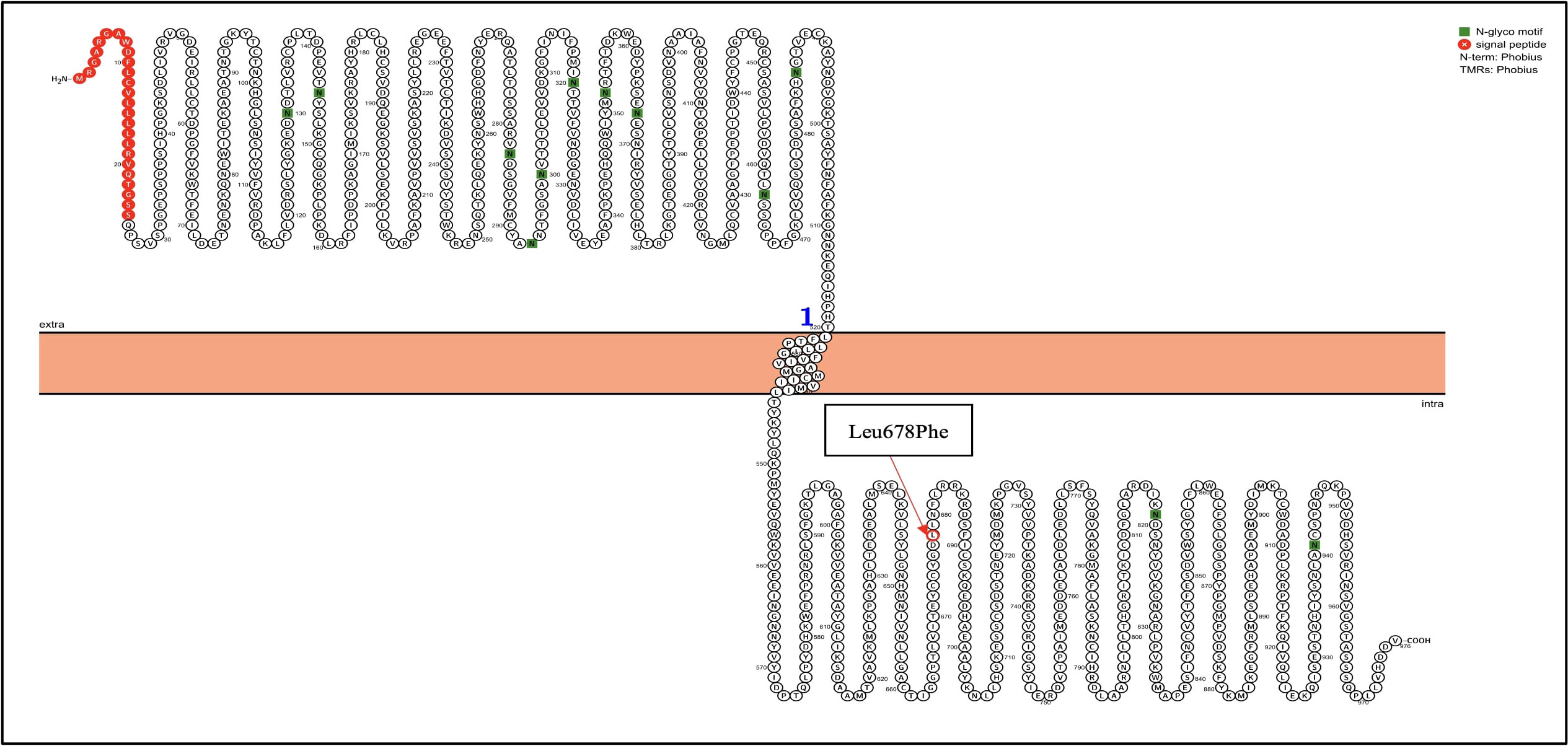
Figure 3 KIT genetic mutation identified in the patient by NGS. Proteins were visualized with Protter (12). The red circle highlights the identified Leu678Phe mutation in the KIT gene.
After the total thyroidectomy procedure, a tissue sample (21.3 mg) was sent to the Centro de Investigación Genética y Genómica for the genomic analyses. The patient’s informed consent was signed before the surgery. The DNA extraction was performed using the PureLink Genomic DNA Mini Kit, according to the manufacturer’s instructions (Invitrogen, USA), and was quantified using spectrophotometry.
A multiplex reaction was performed for the amplification of 46 ancestry-informative markers (AIMs)-InDels. The amplification procedure was based on the protocol by Pereira et al. with a 10ul final reaction volume, using a Qiagen Multiplex PCR Master Mix (Qiagen), 46 (AIMs)-InDels primers and genomic DNA (13). Positive DNA control 2800M was included in the reaction along with the patient sample. The PCR amplification program was 95°C for 15 min; 30 cycles at 94°C for 30 sec, 60°C for 90 sec, and 72°C for 45 sec; and a final extension at 72°C for 60 min (13). A 3500 Genetic Analyzer (Applied Biosystems) was used for fragment separation and detection. The results were collected in Data Collection v4.0 and Gene Mapper v5 (Applied Biosystems)
The statistical analyses were performed according to Zambrano, et al. (14) using the software STRUCTURE v2.3.4. The ancestry inferences compared the subject and each reference population from HGDP-CEPH (Africans, Europeans, and Native Americans) subset H952. The run consisted of a burn-in length of 10 000, and 10 000 Markov Chain Monte Carlo (MCMC) interactions.
The TruSight Tumor 15 kit from Illumina ® was used for sequencing on a MiSeq platform, according to the manufacturer’s instructions (Illumina, USA). Data analyses, including variant calling, were performed on the TruSight Tumor 15 v2.0.1 for mapping, Pisces 5.1.7.52 for variant caller, Sift and PolyPhen for in silico prediction and COSMIC and ClinVar for clinical association.
Thyroid cancer (TC) is a malignant tumor with the most rapid increase in the incidence rate in the last three decades (2, 15–20). The incidental finding is a worldwide phenomenon. However, few studies have reported that the prevalence of large and aggressive tumors is also increasing (21–23), suggesting that factors other than overdiagnosis might be affecting the increase of PTC incidence (24–28). Another factor that may be associated with a higher incidence of PTC is the use of radioactive drugs in hyperthyroid patients (29).
Several pieces of research suggest that thyroid cancer incidence is different among populations. In the USA, non-Hispanic Caucasians are less affected than the Hispanic and African-American populations (20, 30). Population-specific factors, such as American Indian ancestry, which influences other cancer patterns, may increase the risk of PTC in the Ecuadorian population (31–33). Moreover, Salazar-Vega et al. (2019) found that the mestizo population had a higher incidence of thyroid cancer in Ecuador (17). In the same study, they found that most of the thyroid cancer patients came from the highlands (2358masl), in comparison of those from the coast (93masl) and Amazon (731masl) regions (17); this is similar to what was found by Zeng, R. et al. (34) where high altitude was correlated with a higher thyroid cancer incidence (34). The subject comes from Otavalo a region located at 2532masl; hence the high altitude could be a risk factor for the presence of her thyroid cancer.
Furthermore, diet has been associated with an increased thyroid cancer predisposition (35). In Ecuador, grains are the most consumed food, especially in the highlands, and starch has been associated with an increased thyroid cancer predisposition (35); therefore, the Ecuadorian diet could also have a role in the increased thyroid cancer incidence.
In Ecuador, genetic studies are scarce. Solis et al. (21). found a BRAFVal600Glu mutation in 130 of 169 (76,9%) PTC patients from the northern Ecuadorian Andes treated at the Hospital Eugenio Espejo in Quito, Ecuador (21), similar to other populations around the world (22, 23, 32, 36–38). The BRAFVal600Glu mutation constitutively activates the MAPK pathway, promoting cell survival, proliferation, and growth, thus tumorigenesis (39, 40). The Supplementary Figure 1 represents the difference between the wild-type and mutant residues. There is a significant difference in size, hydrophobicity, and charge. Studies in vitro have identified a 500-fold greater activity of BRAFVal600Glu in comparison with the wild type (40). The BRAFVal600Glu mutation has been widely described, and nowadays, specific treatments have been designed for this mutation. For example, the FDA has approved a combination of dabrafenib and trametinib for the treatment of anaplastic thyroid cancer patients, carrying the BRAFVal600Glu mutation (41).
In silico modeling of the BRAF proteins was performed using the Swissmodel and Hope tools. It was observed that the overall structure of the protein (Supplementary Figure 1) was not altered (QMEAN score 0.57 ± 0.05 for both models); however, the mutation causes a change in residue Val600Glu. The mutated residue is located in a domain that is crucial for the activity of the protein, thus the mutation affects its function and interaction with other proteins.
A small number of studies have researched the role of KIT in PTC. Sanlorenzo et al. (42) mentioned that the proto-oncogene KIT encodes for the tyrosine kinase receptor and is involved in cell signal transduction with different downstream pathways: MAPK, phosphatidylinositol 3-kinases (PI3K), Janus kinase (JAK)/signal transducers and activators of transcription (STAT), SRC family kinases (SFK) and phospholipase Cγ (42). On the other hand, the mitogen-activated protein kinase (MAPK) pathway has been extensively researched, and the role of point mutations in the BRAF and RAS genes and RET/PTC rearrangements in PTC molecular pathogenesis has been described (43, 44).
In silico modeling of the KIT proteins was analyzed using the Swiss-Model and Hope tools. It was observed that the overall structure of the protein (Supplementary Figure 2) was not altered (QMEAN score 0.71 ± 0.05 for both models), but the mutation causes a change in residue Leu678Phe. The mutated residue is located in a domain that is important for binding of other molecules and also is in contact with residues in a domain that is important for the activity of the protein. Therefore, the mutation might disturb the interaction between protein domains and as such affect the function of the protein.
The 3D molecular structure obtained using the HOPE tool shows that the mutant residue is bigger than the wild-type residue (Supplementary Figures 1, 2). The wild-type residue was buried in the core of the protein, and the fact that it is bigger, probably causes that it does not fit.
The precise role of KIT in cancer is still unknown, and numerous investigations present discrepancies depending on the type of tumor. Studies show that it is highly expressed or mutated in small cell lung cancer, leukemia cells, colon cancer, and neuroblastoma. Other studies demonstrate that the KIT expression is lost in breast cancer and melanoma (8). Similarly, a low expression of the KIT gene has been reported during the transformation of normal thyroid epithelium to papillary carcinoma, suggesting a possible role of the gene in the differentiation of thyroid tissue (8, 45, 46).
The strength of the used approach relies on the high analytical sensitivity of NGS. NGS can efficiently detect variants even in tissue samples; moreover, it allows to analyze several genes at once, compared to Sanger (∼1000 bp at once) (47). On the other hand, NGS limitations include higher costs and the need of specialized bioinformatic tools (48).
To conclude, we believe that this report represents the genetic characteristics of PTC in an Ecuadorian woman with a mainly Native American ethnic component. Further studies of pathological variants are needed to determine if the combined demographic and molecular profiles are useful to develop targeted treatments focused on the Ecuadorian population.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by CEISH- UTE (CEISH-2021-014). The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the patient for publication of this case report.
Conceptualization, SC-U, EP-C, RT-T and AZ. Resources, PS-P, CG, RG, EL-N, AZ. Methodology, AZ, SC-U, EP-C, RT-T, PG-R and VR-P. Formal Analysis, SC-U, EP-C, RT-T and AZ. Writing – Review and Editing, SC-U, EP-C, RT-T and AZ, PS-P. Supervision, AZ. Project Administration, AZ. Funding Acquisition, AZ. All authors contributed to the article and approved the submitted version.
The experimentation and publication fee of this article are funded by Universidad UTE.
The authors are grateful to the patients for participating in the study, and to the Instituto de la Tiroides y Enfermedades de Cabeza y Cuello (ITECC) for their collaboration.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1101530/full#supplementary-material
Supplementary Figure 1 | Three-dimensional structures of wild and mutant proteins, obtained with the Swissmodel tool. (A) Wild Type BRAF Protein (P15056, extracted from UniProt). (B) Mutant protein (p.Val600Glu). (C) Close-up of the BRAF genetic mutation identified in the patient by NGS. The wild-type residue is in red, whereas the mutant residue is in green.
| Three-dimensional structures of wild and mutant proteins, obtained with the Swissmodel tool. (A) Wild Type KIT Protein (P10721-1, extracted from UniProt). (B) Mutant protein (p.Leu678Phe). (C) Close-up of the mutation. The KIT protein is colored grey, the side chains of both, the wild-type and the KITLeu678Phe variant residue are shown and colored green and red respectively.
TC, Thyroid cancer; DTC, Differentiated thyroid cancer; PTC, Papillary thyroid cancer; FTC, Follicular thyroid cancer; MAPK, Mitogen-activated protein kinase; (PI3K)-AKT, Phosphatidylinositol 3-kinase.
1. Khatami F, Mohammad Tavangar S, Tavangar SM. A review of driver genetic alterations in thyroid cancers. Iran J Pathol (2018) 13(2):125–35. doi: 10.30699/ijp.13.2.125
2. López Gavilanez E, Bautista Litardo N, Navarro Chávez M, Hernández Bonilla M, Segale Bajaña A. Thyroid cancer in Ecuador. BMC Cancer (2020) 20(1):1–3. doi: 10.1186/s12885-020-07137-0
3. Pacheco-Ojeda L, Martínez-Jaramillo A, Romo-Castillo H, Mario Montalvo-Burbano M. Differentiated thyroid cancer clinical trends in Quito, Ecuador. Int J Med Surg Sci (2021) 8(2):1–10. doi: 10.32457/ijmss.v8i2.1347
4. Nylén C, Mechera R, Maréchal-Ross I, Tsang V, Chou A, Gill AJ, et al. Molecular markers guiding thyroid cancer management. Cancers (Basel) (2020) 12(8):1–26. doi: 10.3390/cancers12082164
5. Brito JP, Hay ID. Management of papillary thyroid microcarcinoma. Endocrinol Metab Clin North Am (2019) 48(1):199–213. doi: 10.1016/j.ecl.2018.10.006
6. Silver JA, Bogatchenko M, Pusztaszeri M, Forest VI, Hier MP, Yang JW, et al. BRAF V600E mutation is associated with aggressive features in papillary thyroid carcinomas ≤ 1.5 cm. J Otolaryngol - Head Neck Surg (2021) 50(1):1–8. doi: 10.1186/s40463-021-00543-9
7. Al-Masri M, Al-Shobaki T, Al-Najjar H, Iskanderian R, Younis E, Abdallah N, et al. BRAF V600E mutation in papillary thyroid carcinoma: its relation to clinical features and oncologic outcomes in a single cancer centre experience. Endocr Connect (2021) 10(12):1531–7. doi: 10.1530/EC-21-0410
8. Franceschi S, Lessi F, Panebianco F, Tantillo E, La Ferla M, Menicagli M, et al. Loss of c-KIT expression in thyroid cancer cells. PloS One (2017) 12(3):1–15. doi: 10.1371/journal.pone.0173913
9. Chen EC, Karl TA, Kalisky T, Gupta SK, O’Brien CA, Longacre TA, et al. KIT signaling promotes growth of colon xenograft tumors in mice and is upregulated in a subset of human colon cancers. Gastroenterology (2015) 149(3):705–17. doi: 10.1053/j.gastro.2015.05.042
10. Yu G, Yin C, Jiang L, Zheng Z, Wang Z, Wang C, et al. Amyloid precursor protein cooperates with c-KIT mutation/overexpression to regulate cell apoptosis in AML1-ETO-positive leukemia via the PI3K/AKT signaling pathway. Oncol Rep (2016) 36(3):1626–32. doi: 10.3892/or.2016.4963
11. American Cancer Society. Treatment of thyroid cancer by type and stage. American Cancer Society (2021) Atlanta, Georgia. USA. p. 1–4. Available at: http://www.cancer.org/cancer/thyroidcancer/detailedguide/thyroid-cancer-treating-by-stage.
12. Omasits U, Ahrens CH, Müller S, Wollscheid B. Protter: Interactive protein feature visualization and integration with experimental proteomic data. Bioinformatics (2014) 30(6):884–6. doi: 10.1093/bioinformatics/btt607
13. Pereira R, Phillips C, Pinto N, Santos C, dos Santos SEB, Amorim A, et al. Straightforward inference of ancestry and admixture proportions through ancestry-informative insertion deletion multiplexing. PLoS One (2012) 7(1). doi: 10.1371/journal.pone.0029684
14. Zambrano AK, Gaviria A, Cobos-Navarrete S, Gruezo C, Rodríguez-Pollit C, Armendáriz-Castillo I, et al. The three-hybrid genetic composition of an Ecuadorian population using AIMs-InDels compared with autosomes, mitochondrial DNA and y chromosome data. Sci Rep (2019) 9(1):1–8. doi: 10.1038/s41598-019-45723-w
15. GLOBOCAN. Age-standardized rate (Ecuador) per 100 000, incidence, males and females thyroid (2022). Available at: https://gco.iarc.fr/overtime/en/dataviz/trends?populations=82610&sexes=1_2&types=0&multiple_populations=1&cancers=2.
16. Cordero FC, Ayala PC, Maldonado JY, Montenegro WT. Trends in cancer incidence and mortality over three decades in Quito-Ecuador. Colomb Med (2018) 49(1):35–41. doi: 10.25100/cm.v49i1.3785
17. Salazar-Vega J, Ortiz-Prado E, Solis-Pazmino P, Gómez-Barreno L, Simbaña-Rivera K, Henriquez-Trujillo AR, et al. Thyroid cancer in Ecuador, a 16 years population-based analysis (2001-2016). BMC Cancer (2019) 19(1):1–8. doi: 10.1186/s12885-019-5485-8
18. Xing MM, Alzahrani AS, Carson KA, Viola D, Elisei R, Bendlova B, et al. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA - J Am Med Assoc (2013) 309(14):1493–501. doi: 10.1001/jama.2013.3190
19. Solis-Pazmino P, Salazar-Vega J, Lincango-Naranjo E, Garcia C, Koupermann GJ, Ortiz-Prado E, et al. Thyroid cancer overdiagnosis and overtreatment: a cross- sectional study at a thyroid cancer referral center in Ecuador. BMC Cancer (2021) 21(1):1–10. doi: 10.1186/s12885-020-07735-y
20. Johnston LE, Tran Cao HS, Chang DC, Bouvet M. Sociodemographic predictors of survival in differentiated thyroid cancer: Results from the SEER database. ISRN Endocrinol (2012) 2012:1–8. doi: 10.5402/2012/384707
21. Solis-Pazmino P, Cucalon J, Jaramillo Koupermann G, Galvez G, Salazar-Vega J, Ortiz-Prado E, et al. High prevalence of BRAFV600E mutation in patients with papillary thyroid cancer from Northern Ecuadorian andes. Am Thyroid Assoc (2018) 28. doi: 10.1089/thy.2018.29065.abstracts
22. Pessôa-Pereira D, Medeiros MF da S, Lima VMS, da Silva JC, Cerqueira TL de O, da Silva IC, et al. Association between BRAF (V600E) mutation and clinicopathological features of papillary thyroid carcinoma: A brazilian single-centre case series. Arch Endocrinol Metab (2019) 63(2):97–106. doi: 10.20945/2359-3997000000120
23. Rashid FA, Munkhdelger J, Fukuoka J, Bychkov A. Prevalence of BRAFV600E mutation in Asian series of papillary thyroid carcinoma–a contemporary systematic review. Gland Surg (2020) 9(5):1878–900. doi: 10.21037/gs-20-430
24. Wang F, Zhao S, Shen X, Zhu G, Liu R, Viola D, et al. BRAF V600E confers male sex disease-specific mortality risk in patients with papillary thyroid cancer. J Clin Oncol (2018) 36(27):2787–95. doi: 10.1200/JCO.2018.78.5097
25. Pellegriti G, Frasca F, Regalbuto C, Squatrito S, Vigneri R. Worldwide increasing incidence of thyroid cancer: Update on epidemiology and risk factors. J Cancer Epidemiol (2013) 2013. doi: 10.1155/2013/965212
26. Liu R, Bishop J, Zhu G, Zhang T, Ladenson PW, Xing M. Mortality risk stratification by combining BRAF V600E and TERT promoter mutations in papillary thyroid cancer genetic duet of BRAF and TERT promoter mutations in thyroid cancer mortality. JAMA Oncol (2017) 3(2):202–8. doi: 10.1001/jamaoncol.2016.3288
27. Chakraborty A, Narkar A, Mukhopadhyaya R, Kane S, D’Cruz A, Rajan MGR. BRAFV600E mutation in papillary thyroid carcinoma: Significant association with node metastases and extra thyroidal invasion. Endocr Pathol (2012) 23(2):83–93. doi: 10.1007/s12022-011-9184-5
28. Vigneri R, Malandrino P, Vigneri P. The changing epidemiology of thyroid cancer: Why is incidence increasing? Curr Opin Oncol (2015) 27(1):1–7. doi: 10.1097/CCO.0000000000000148
29. Metso S, Auvinen A, Huhtala H, Salmi J, Oksala H, Jaatinen P. Increased cancer incidence after radioiodine treatment for hyperthyroidism. Cancer (2007) 109(10):1972–9. doi: 10.1002/cncr.22635
30. Aschebrook-Kilfoy B, Kaplan EL, Chiu BCH, Angelos P, Grogan RH. The acceleration in papillary thyroid cancer incidence rates is similar among racial and ethnic groups in the united states. Ann Surg Oncol (2013) 20(8):2746–53. doi: 10.1245/s10434-013-2892-y
31. Hoffman J, Fejerman L, Hu D, Huntsman S, Li M, John EM, et al. Identification of novel common breast cancer risk variants at the 6q25 locus among latinas 06 biological sciences. Breast Cancer Res (2019) 21(1):1–12. doi: 10.1186/s13058-018-1085-9
32. Estrada-Flórez AP, Bohórquez ME, Vélez A, Duque CS, Donado JH, Mateus G, et al. BRAF and TERT mutations in papillary thyroid cancer patients of Latino ancestry. Endocr Connect (2019) 8(9):1310–7. doi: 10.1530/EC-19-0376
33. Fejerman L, Ahmadiyeh N, Hu D, Huntsman S, Beckman KB, Caswell JL, et al. Genome-wide association study of breast cancer in latinas identifies novel protective variants on 6q25. Nat Commun (2014) 5(May). doi: 10.1038/ncomms6260
34. Zeng R, Shou T, Yang KX, Shen T, Zhang JP, Zuo RX, et al. Papillary thyroid carcinoma risk factors in the yunnan plateau of southwestern China. Ther Clin Risk Manage (2016) 12:1065–74. doi: 10.2147/TCRM.S105023
35. Fiore M, Cristaldi A, Okatyeva V, Lo Bianco S, Oliveri Conti G, Zuccarello P, et al. Dietary habits and thyroid cancer risk: A hospital-based case–control study in Sicily (South Italy). Food Chem Toxicol (2020) 146:111778. doi: 10.1016/j.fct.2020.111778
36. Cho U, Oh WJ, Bae JS, Lee S, Lee YS, Park GS, et al. Clinicopathological features of rare BRAF mutations in korean thyroid cancer patients. J Korean Med Sci (2014) 29(8):1054–60. doi: 10.3346/jkms.2014.29.8.1054
37. Ye Z, Xia X, Xu P, Liu W, Wang S, Fan Y, et al. The prognostic implication of the BRAF V600E mutation in papillary thyroid cancer in a Chinese population. Int J Endocrinol (2022) 2022:1–7. doi: 10.1155/2022/6562149
38. Frasca F, Nucera C, Pellegriti G, Gangemi P, Attard M, Stella M, et al. BRAF(V600E) mutation and the biology of papillary thyroid cancer. Endocr Relat Cancer (2008) 15(1):191–205. doi: 10.1677/ERC-07-0212
39. McCain J. The MAPK (ERK) pathway: Investigational combinations for the treatment of BRAF- mutated metastatic melanoma. P T (2013) 38(2):96-108.
40. Li C, Lee KC, Schneider EB, Zeiger MA. BRAF V600E mutation and its association with clinicopathological features of papillary thyroid cancer: A meta-analysis. J Clin Endocrinol Metab (2012) 97(12):4559–70. doi: 10.1210/jc.2012-2104
41. National Cancer Institute. Dabrafenib – trametinib braf combination approved for solid tumors with BRAF mutations. Cancer Currents Blog (2022) Bethesda, Maryland. USA. p. 1–6. Available at: https://www.cancer.gov/news-events/cancer-currents-blog/2022/fda-dabrafenib-trametinib-braf-solid-tumors#:~:text=FDA. cited 2022 Sep 28previously approved the dabrafenib,melanoma%2C and anaplastic thyroid cancer.
42. Sanlorenzo M, Vujic I, Posch C, Ma J, Lin K, Lai K, et al. Oncogenic KIT mutations in different exons lead to specific changes in melanocyte phospho-proteome. J Proteomics (2016) 144:140–7. doi: 10.1016/j.jprot.2016.05.019
43. Zou M, Baitei EY, Alzahrani AS, Binhumaid FS, Alkhafaji D, Al-Rijjal RA, et al. RET/PTC, or BRAF mutations in advanced stage of papillary thyroid carcinoma. Thyroid (2014) 24(8):1256–66. doi: 10.1089/thy.2013.0610
44. Guerra PTCA, Zeppa P, Bifulco M, Vitale M. Concomitant BRAF(V600E) mutation and RET/PTC rearrangement is a frequent occurrence in papillary thyroid carcinoma. Thyroid (2014) 24,2:1–20. doi: 10.1089/thy.2013.0235
45. Tanaka T, Umeki K, Yamamoto I, Kotani T, Sakamoto F, Tanaka T, et al. C-kit proto-oncogene is more likely to lose expression in differentiated thyroid carcinoma than three thyroid-specific genes: Thyroid peroxidase, thyroglobulin, and thyroid stimulating hormone receptor. Endocr J (1995) 42(5):723–8. doi: 10.1507/endocrj.42.723
46. Tomei S, Mazzanti C, Marchetti I, Rossi L, Zavaglia K, Lessi F, et al. C-KIT receptor expression is strictly associated with the biological behaviour of thyroid nodules. J Transl Med (2012) 10(1):1–9. doi: 10.1186/1479-5876-10-7
47. Chin ELH, da Silva C, Hegde M. Assessment of clinical analytical sensitivity and specificity of next-generation sequencing for detection of simple and complex mutations. BMC Genet (2013) 14(1):1. doi: 10.1186/1471-2156-14-6
Keywords: genomics, thyroid, cancer, mestizo, case report
Citation: Cadena-Ullauri S, Paz-Cruz E, Tamayo-Trujillo R, Guevara-Ramírez P, Ruiz-Pozo V, Solis-Pazmino P, Garcia C, Godoy R, Lincango-Naranjo E and Zambrano AK (2023) Identification of KIT and BRAF mutations in thyroid tissue using next-generation sequencing in an Ecuadorian patient: A case report. Front. Oncol. 12:1101530. doi: 10.3389/fonc.2022.1101530
Received: 17 November 2022; Accepted: 29 December 2022;
Published: 17 January 2023.
Edited by:
Athina Markou, National and Kapodistrian University of Athens, GreeceCopyright © 2023 Cadena-Ullauri, Paz-Cruz, Tamayo-Trujillo, Guevara-Ramírez, Ruiz-Pozo, Solis-Pazmino, Garcia, Godoy, Lincango-Naranjo and Zambrano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ana Karina Zambrano, YW5hemFtYnJhbm8xN0Bob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.