- 1Department of Respiratory Medicine, Affiliated Nanjing Brain Hospital, Nanjing Medical University, Nanjing, Jiangsu, China
- 2Clinical Center of Nanjing Respiratory Diseases and Imaging, Nanjing chest hospital, Jiangsu, China
Objective: Invasive lung adenocarcinoma is composed of five different histological subgroups with diverse biological behavior and heterogeneous morphology, the acinar/papillary-predominant lung adenocarcinomas are the most common subgroups and recognized as an intermediate-grade group. In the real world, clinicians primarily consider predominant patterns and ignore the impact of minor components in the prognosis of lung adenocarcinoma. The study evaluated the clinicopathologic characteristics of the lepidic, solid, and micropapillary patterns as non-predominant components and whether the minimal patterns had prognostic value on acinar/papillary-predominant lung adenocarcinomas.
Methods: A total of 153 acinar/papillary-predominant lung adenocarcinoma patients with tumor size ≤4 cm were classified into four risk subgroups based on the presence of lepidic and micropapillary/solid components: MP/S−Lep+, MP/S+Lep+, MP/S−Lep−, and MP/S+Lep− groups. The Cox-proportional hazard regression model was used to assess disease-free survival (DFS).
Results: The risk subgroups based on the non-predominant patterns were associated with differentiation (P = 0.001), lymphovascular invasion (P = 0.001), and recurrence (P = 0.003). In univariate analysis, DFS was correlated with non-predominant components (P = 0.014), lymphovascular invasion (P = 0.001), carcinoembryonic antigen (CEA) (P = 0.001), and platelet-to-lymphocyte ratio (PLR) (P = 0.012). In the multivariate analysis, non-predominant components (P = 0.043) and PLR (P = 0.032) were independent prognostic factors for DFS. The 5-year survival rates of MP/S−Lep+, MP/S+Lep+, MP/S−Lep− and MP/S+Lep− subgroups were 93.1%,92.9%,73.1%,61.9%, respectively. The MP/S−Lep+ subgroup had the favorable prognosis than MP/S+Lep− subgroup with a statistically significant difference (P = 0.002). As minor components, the lepidic patterns were a protective factor, and the solid and micropapillary components were poor factors. The recurrence was related to the presence of non-predominant patterns rather than their proportion. Adjuvant chemotherapy did not significantly improve the prognosis of the MP/S+Lep- subgroup (P = 0.839).
Conclusions: Regardless of the proportion, the presence of micropapillary/solid components and the absence of lepidic patterns are aggressive factors of DFS in patients with resected stage I acinar- or papillary-predominant lung adenocarcinoma.
1. Introduction
Non-small cell lung accounts for 85% of lung cancer and the most common histological type of which is lung adenocarcinoma. Histological classification of lung adenocarcinoma was established by the International Association for the Study of Lung Cancer, American Thoracic Society and European Respiratory Society (IASLC/ATS/ERS), which is an international standard for histologic subclassification of lung adenocarcinoma (1). Lung adenocarcinoma is comprised of five pathological subtypes including lepidic, acinar, papillary, micropapillary, and solid, of which the acinar-predominant component is the most common subtype accounting for about 40-50%. The lepidic pattern is categorized as low grade, acinar and papillary patterns as an intermediate grade, and micropapillary and solid patterns as high grade (2, 3). Many studies have reported a negative prognosis for solid- and micropapillary-predominant adenocarcinoma and a beneficial prognosis for patients with lepidic-predominant adenocarcinoma (4, 5). The DFS rate of lepidic predominant adenocarcinomas and the acinar/papillary predominant adenocarcinomas was 99% and 80.8-82.4% respectively, micropapillary and solid predominant lung adenocarcinomas have a poor prognosis with the 5-year DFS rate of approximately 33.3-73.6% (6).
Interestingly, recent studies have shown the minimal presence of lepidic components plays a protective role and micropapillary (7, 8) and solid components are risk factors in early-stage lung adenocarcinoma (9, 10), even if not as predominant patterns. Studies have shown that solid and micropapillary components account for 41.8% and 60.4% of early stage lung adenocarcinoma, and even in stage IA, solid and micropapillary components are still unfavorable factors even though they are not the predominant components (11–13). When the most predominant histologic pattern was intermediate-grade, the second most predominant pattern was high-grade, and recurrence risk increased by 4.2-fold compared with the low-grade group (14). However, in most cases, the composition of lung adenocarcinoma is complex and heterogeneous, with few lung adenocarcinomas having a pure component and most adenocarcinomas (80-90%) having a mixture of two or more growth patterns. In the real world, clinicians primarily consider the predominant pattern and pay little attention to the effect of minimal components in the prognosis of lung adenocarcinoma. Liu. et al. found that the recurrence hazard curve in early stage adenocarcinoma patients showed a typical “double-peaked” pattern. The first recurrence peak occurred 20–22 months after surgery and the second peak occurred 5–6 years after surgery (15). The DFS is more informative than over survival (OS) in predicting the biological behavior in early stage tumors. We explored the clinicopathological features of the non-predominant components and focused on the impact of lepidic, micropapillary, and solid as minimal components in the recurrence of intermediate-grade adenocarcinoma.
2. Patients and methods
2.1. Patients
We enrolled 153 patients with clinical stage T1-2aN0M0 stage I lung adenocarcinoma who underwent complete resection at Nanjing Chest Hospital from May 2014 to August 2017, and the follow-up deadline was August 2022. The inclusion criteria were as follows: 1. anatomical resection with standard mediastinal Lymph node dissection; 2. The pathological stages were determined according to the WHO eighth edition classification criteria; 3. The predominant subtype is acinar/papillary; 4. The minimal patterns include lepidic, solid, and micropapillary; Exclusion criteria were variants adenocarcinomas, adenocarcinomas in situ and minimally invasive adenocarcinomas, preoperative neoadjuvant chemotherapy or radiotherapy, positive surgical margin, a history of infection or other malignant tumors.
This study was approved by the Ethics Committee of Nanjing Brain Hospital. Informed consent was obtained from all participants in the study.
2.2. Histopathological evaluation
All resected specimens were formalin-fixed and stained with hematoxylin and eosin. The histologic classification of all slides was evaluated by two experienced pathologists according to IASLC/ATS/ERS classification criteria, respectively. The discrepancies were discussed to reach a consensus. Each histologic component present was recorded semiquantitatively in 5% increments. The non-predominant pattern was greater than or equal to 5% of the tumor. When the percentage of patterns was less than 5%, which was considered absent (16, 17). The predominant histological subtype was the highest proportion of the tumor, not necessarily 50% or greater (18, 19).
A total of 153 lung adenocarcinoma patients were classified into four groups based on the presence or absence of minimal patterns: MP/S−Lep+ group (micropapillary and solid components were absent, and lepidic components were present), MP/S+Lep+ group (either micropapillary or solid components were present, and lepidic components were present), MP/S−Lep− group (both micropapillary, solid and lepidic components were absent) MP/S+Lep− group (either micropapillary or solid components were present, and lepidic components were absent).
2.3. Methods
The histopathologic and clinicopathologic features of clinical data are as follows: gender, age, smoking history, surgical resection, predominant subtype, the proportion of micropapillary/solid components, the presence of lepidic and micropapillary/solid components, stage, differentiation, chemotherapy, laterality, visceral pleural invasion, lymphovascular invasion, DFS, OS, CEA, and PLR. The medical records and telephone were used for follow-up. A total of 153 patients were evaluated every 6 months for the first 2 years after surgery, and then once a year for the next 3-5 years. The last follow-up was in August 2022. The primary outcome was disease‐free survival (DFS). DFS was calculated from the date of surgery to the date of first recurrence or death from any cause, or last contact. The definition of overall survival (OS) was the time interval from surgery to the date of death or last contact. The normal value for CEA was < 5ug/l.
2.4. Statistical analysis
The categorical variables were summarized as frequencies and percentages, and the difference was analyzed by the chi-square test. The optimal cut-off value of PLR was determined by Youden’s Index and implemented through receiver operating characteristic (ROC). DFS was estimated by the Kaplan–Meier method and compared using the log-rank test. The Cox proportional hazards model was used to perform univariate and multivariate analyses. P < 0.05 values were considered statistically significant. The data were performed with SPSS 25.0 statistical software.
3. Results
3.1. The basic characteristics
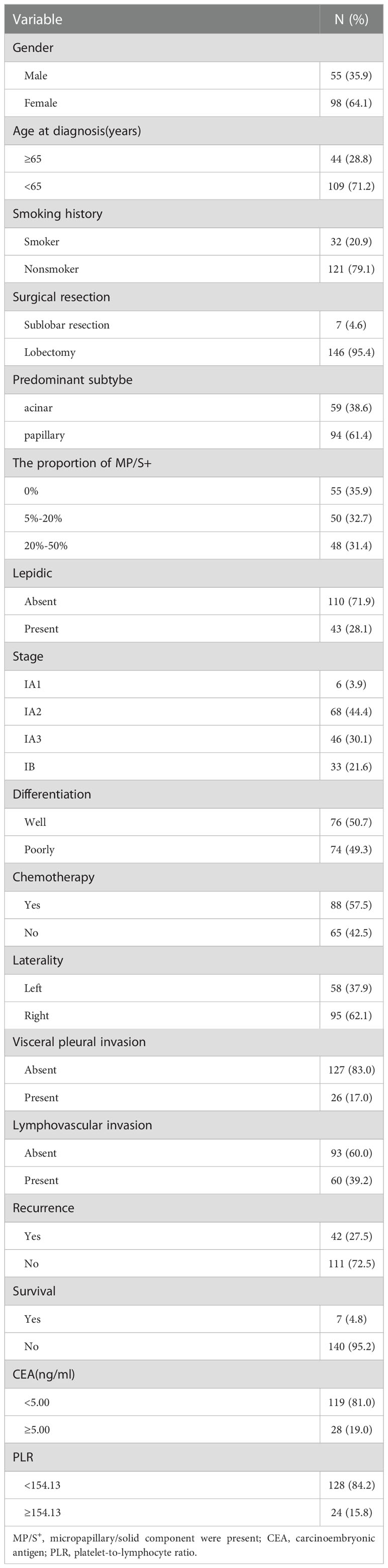
The basic characteristics of the included patients were presented in Table 1. There were 55 females (35.9%) and 98 males (64.1%) with a median age of 60 years (31–84 years). Smokers were observed in 32 of 153 cases (20.9%). 146 patients (95.4%) underwent lobectomy and the remaining (n=7) underwent sublobar resection. Out of 153 patients, 88(57.5%) received postoperative adjuvant chemotherapy. Chemotherapy comprised platinum-based regimens, the platinum double‐drug chemotherapy programs were: pemetrexed in 73 cases (83.0%), docetaxel in 5 cases (5.7%), gemcitabine in 2 cases (2.3%), paclitaxel in 2 cases (2.3%), and unknown regimens in 6 cases (6.7%). The percentage of stage IA1, IA2, IA3 and IB patients was 3.9%, 44.4%, 30.1%, and 21.6%, respectively. 58 tumors (37.9%) were located in the left lung. The visceral pleural invasion was observed in 26 cases (17.0%) and lymphovascular invasion was seen in 60 cases (39.2%). Poor differentiation was observed in 74 cases (49.3%). CEA ≥5 ng/ml was found in 28 cases (19.0%).
For the predominant subtype, the frequency of papillary predominant adenocarcinoma was 61.4%, followed by acinar predominant adenocarcinoma (38.6%). As a non-predominant pattern, lepidic components were displayed in 43 cases (28.1%), solid and micropapillary patterns in 98 tumors (64.1%) according to the new IASLC histologic grading system. The proportion of high-grade patterns greater than 5% or equal to 5% and less than 20% was observed in 50 cases (32.7%). The proportion of high-grade patterns greater than 20% or equal to 20% and less than 50% was observed in 48 cases (31.4%). The percentage of MP/S−Lep+, MP/S+Lep+, MP/S−Lep− and MP/S+Lep− group was 19.0%, 9.2%, 17.0%, and 54.9%, respectively. The median time to follow up was 55.3 months (interquartile range, 56.5 to 67 months). In the follow-up period, 27.5% of patients experienced recurrence and 4.8% of patients occurred death.
3.2. Optimal cut-off value for ROC curve
A cut-off value of 154.1 was used to discriminate between patients with high and low preoperative PLR, with AUC of 0.561 (sensitivity: 0.286, specificity: 0.892). Patients were distributed into PLR high groups (n = 24) and low PLR groups (n =128). PLR and CEA were combined for diagnosis, and the cut off value was 0.298 (sensitivity: 0.488; specificity: 0.783; AUC: 0.612), which can slightly enhance the diagnostic performance (Figure 1).
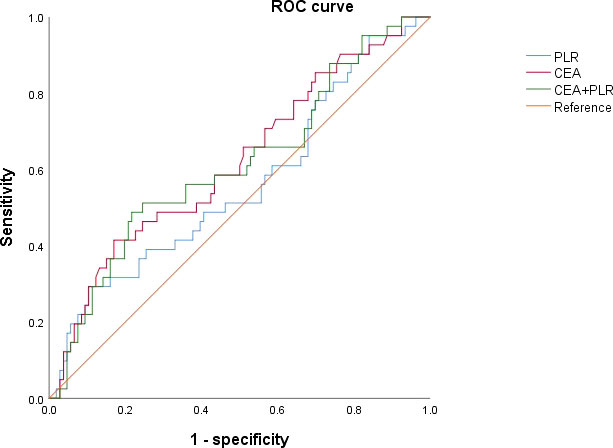
Figure 1 Receiver operating characteristic curves for analysis of using CEA and PLR in predicting disease-free survival.
3.3. Relationships between clinicopathological characteristics and the risk subgroups based on non-predominant components
The relationship between the risk subgroup divided according to the minor components and clinicopathological features was shown in Table 2. The risk groups based on non-predominant components were associated with differentiation (P = 0.001). The MP/S+Lep- group was vulnerable to poorly differentiation. The minor pattern was related to Lymphovascular invasion (P = 0.001). The MP/S+Lep- group was more susceptible to Lymphovascular invasion. The minor pattern was significantly correlated with postoperative recurrence (P = 0.003). The 5-year DFS rates of MP/S−Lep+, MP/S+Lep+, MP/S−Lep− and MP/S+Lep− group were 93.1%, 92.9%, 73.1%, 61.9%, respectively. The survival time of MP/S−Lep+ group was longer than MP/S+Lep− group with a statistically significant difference (P = 0.002) (Figure 2). It indicated the presence of micropapillary/solid components and absence of lepidic patterns are poor prognostic factors of DFS in patients with resected stage I acinar- or papillary-predominant lung adenocarcinoma.
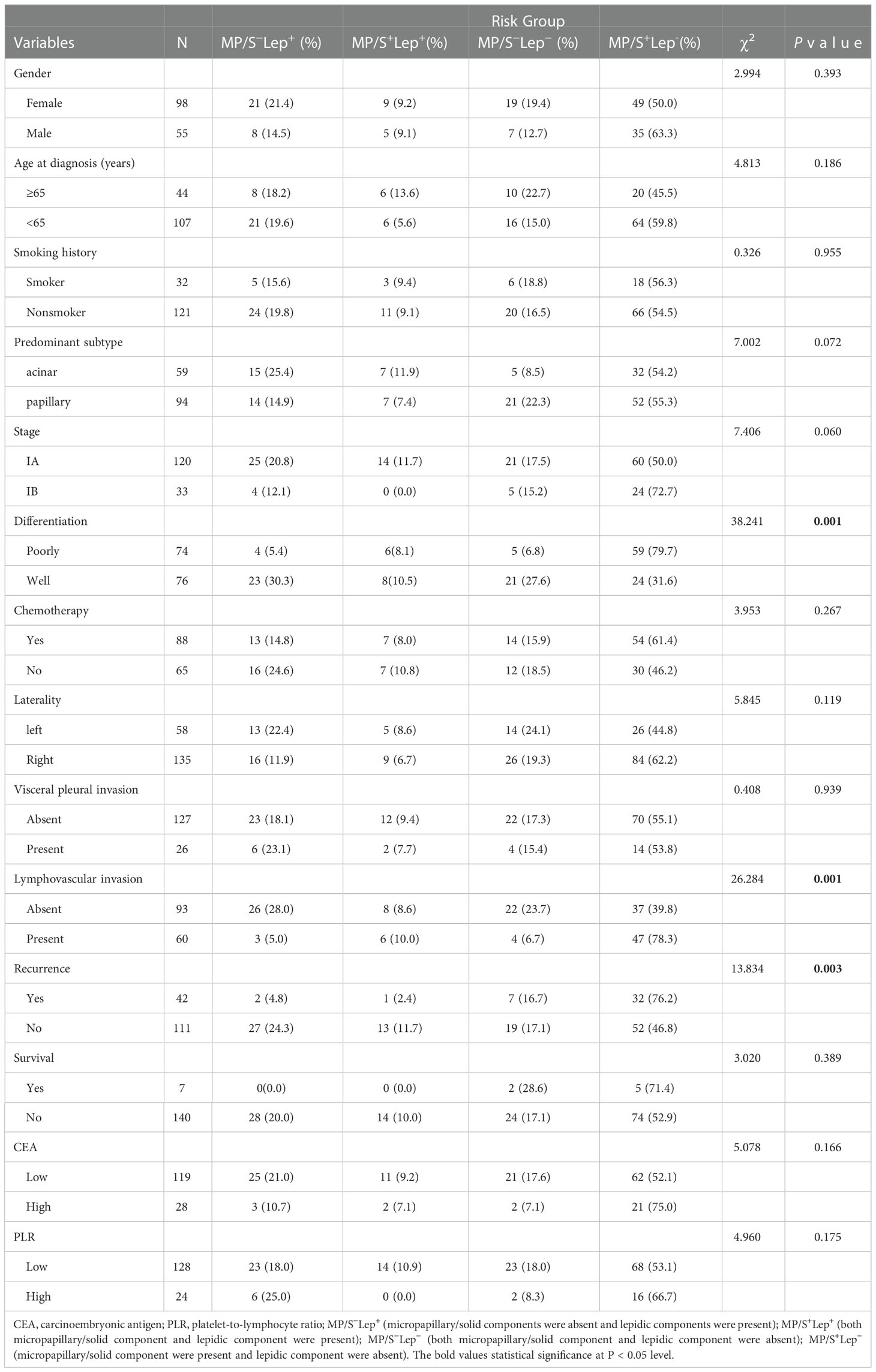
Table 2 Relationships between clinicopathological characteristics and the presence of lepidic and micropapillary/solid pathological patterns as minor components in patients with stage I lung adenocarcinoma.
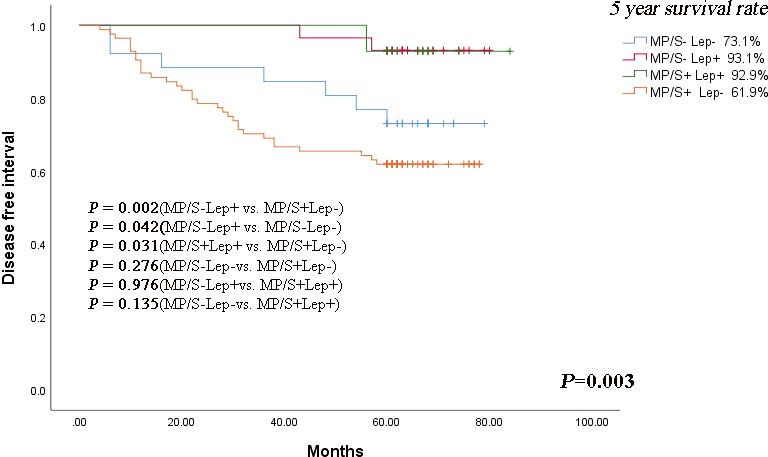
Figure 2 Kaplan-Meier curve of DFS according to lepidic, micropapillary/solid minor components in stage I lung adenocarcinoma patients. The comparison of DFS between 2 groups: MP/S−Lep+ vs. MP/S+Lep− group (P = 0.002), MP/S−Lep+ vs. MP/S−Lep− group (P=0.042), MP/S+Lep+ vs. MP/S+Lep− group (P = 0.031), MP/S−Lep− vs. MP/S+Lep− group (P = 0.276), MP/S−Lep+ vs. MP/S+Lep+ group (P = 0.976), MP/S-Lep- vs. MP/S+Lep+ group (P = 0.135).
In the patients without micropapillary/solid patterns, the lepidic components predicted a better prognosis compared with the non-lepidic components (P=0.042). The same result was seen in the patients with micropapillary/solid patterns (P = 0.031). The results suggested that lepidic components played a protective role in the early stage acinar- or papillary-predominant lung adenocarcinoma. In the patients with or without the lepidic components, the solid and micropapillary components showed no beneficial effect on prognosis (P = 0.976 and P = 0.276, respectively).
Nevertheless, we found the MP/S-Lep- group had shorter DFS than MP/S+Lep+ group, but there was no statistically significant difference in the 5-year DFS rate between the two groups (P = 0.135), indicating there was no statistically significant difference in the rate of relapse when the high grade and low grade were both present or absent together. The minor components were related to aggressive factors such as poorly differentiation and lymphovascular invasion. The MP/S+Lep- group was more vulnerable to recurrence compared with other subgroups.
3.4. The prognostic factors of disease-free survival in univariate analysis and multivariate analysis
Univariate analysis indicated that non-predominant patterns (P = 0.014), lymphovascular invasion (P = 0.001), elevated CEA level (P = 0.001), and elevated PLR level (P = 0.012) were the potential predictive factors for the stage I acinar- or papillary- predominant adenocarcinoma.
Multivariate Cox regression analysis further revealed that MP/S+Lep- group (P = 0.043) and elevated PLR level (P = 0.032) were independent risk factors of DFS (Table 3).
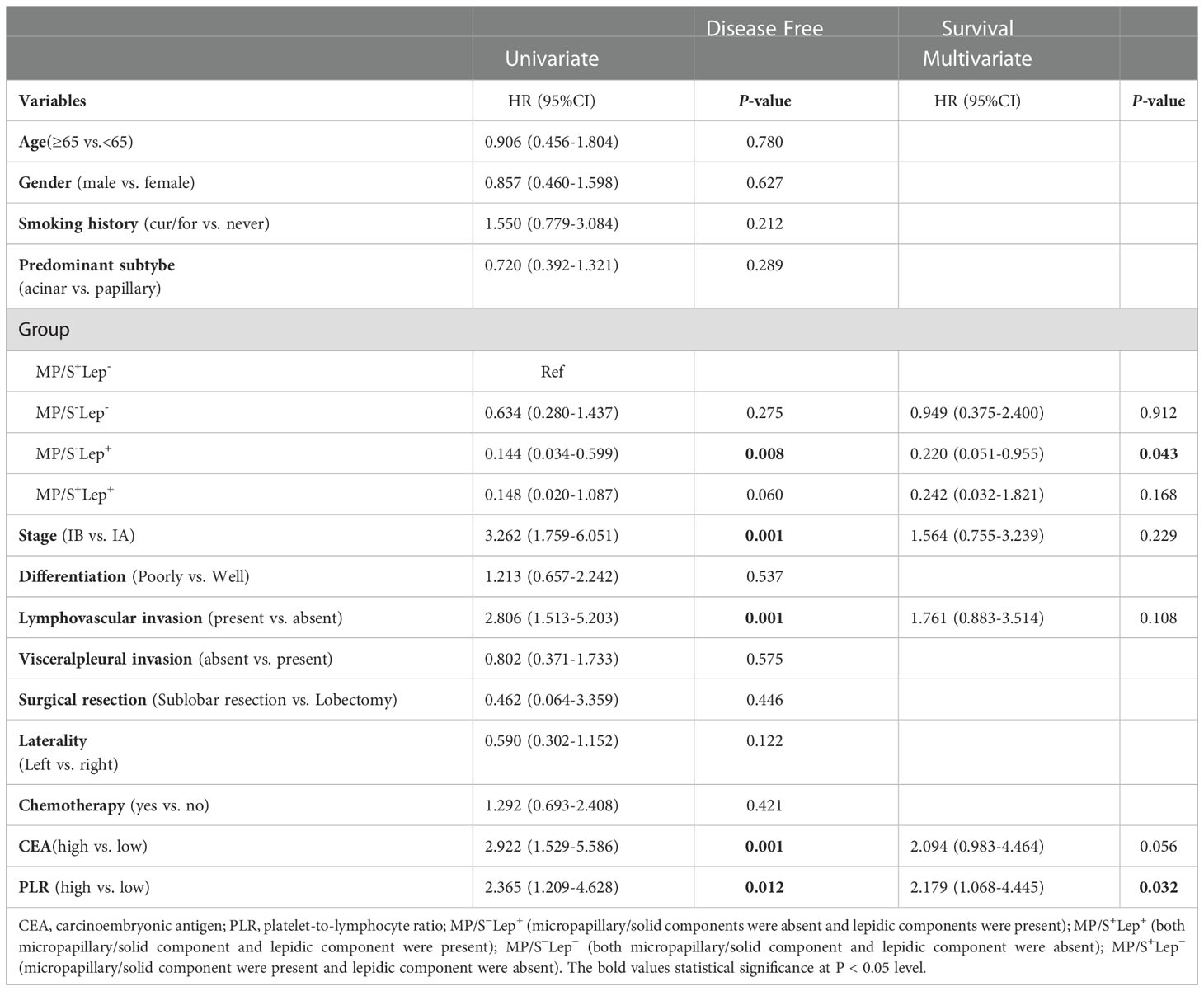
Table 3 Univariate and multivariate COX regression analysis for disease-free survival in stage I lung adenocarcinoma.
3.5. Subgroup analysis of minimal components
We categorized acinar/papillary-predominant lung adenocarcinoma according to the presence of lepidic patterns. There were 28.1% (n = 43) patients in the group with the presence of lepidic patterns, the remaining was 71.9% (n =110) patients. The 5-year DFS rates of the group with the presence of lepidic patterns were 93.0% and the group with the absence of lepidic patterns was 64.5%. Therefore the lepidic pattern was a positive factor in stage I lung adenocarcinoma (Figure 3A). The non-predominant components were divided into two groups according to the presence of solid and micropapillary components: MP+/S+ subtype (either micropapillary or solid component was present) and MP-&S- subtype (both micropapillary and solid components were absent). The MP+/S+ subtype was present in 63.3% (n = 97) of patients and MP-&S- subtype was observed in 36.7% (n = 56) of patients. The 5-year DFS rates of MP+/S+ subtype and MP-&S- subtype were 66.0% and 83.9%, respectively, and the difference was significant (P = 0.014). The solid and micropapillary patterns were associated with poor outcomes (Figure 3B).
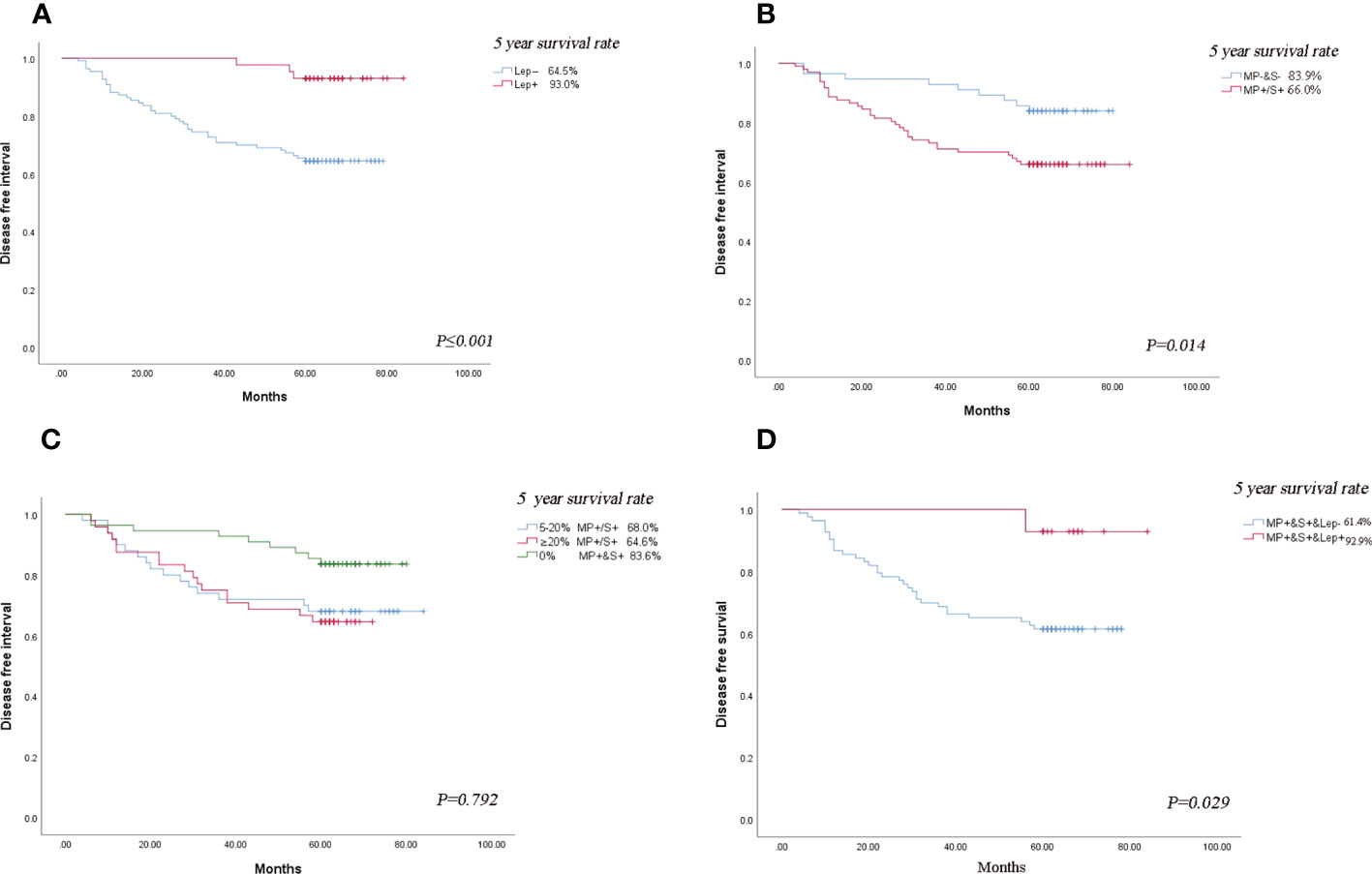
Figure 3 Subgroup analysis. (A) Comparison of DFS in patients with lung adenocarcinoma based on presence or absence of lepidic minor component. (B) Comparison of DFS in patients with lung adenocarcinoma based on the presence or absence of micropapillary/solid minor components component. (C) Comparison of DFS in patients with lung adenocarcinoma based on the proportion of micropapillary/solid minor components component. (D) Comparison of the effect of the presence or absence of lepidic component on DFS in patients with the micropapillary/solid minor component. The symbol of “&” means and.
The 2020 IASLC/ATS/ERS grading system proposed a cut-off of 20% for high-grade patterns as a risk factor (2). According to the proposal of the new IASA system, we classified the total proportion of solid and micropapillary components (TPSM) in tumors into two groups in our study: patients with 5-20% proportion of solid and micropapillary were defined as TPSM-low (n=50) and patients with 20%-50% proportion of solid and micropapillary were defined as TPSM-high (n = 48). The 5-year DFS rates in TPSM-low and TPSM-high were 68.0% and 64.6%, respectively. The result showed there was no significant difference between the two groups (P = 0.792) (Figure 3C). The presence of solid/micropapillary patterns rather than the proportion of solid/micropapillary patterns affected the DFS in lung adenocarcinoma.
For Lung adenocarcinoma with MP+/S+ subtype, the DFS rates in patients with the presence of lepidic patterns and absence of those were 92.9% and 61.4%, respectively. The results demonstrated that even in patients with poor prognosis, the presence of lepidic patterns could improve survival significantly (P = 0.029) (Figure 3D). The published literature only took the solid and micropapillary components into account, or just considered the lepidic component alone. In the future, we should emphasize the inclusion of all three components simultaneously when assessing the prognosis of lung adenocarcinoma.
The MPS-/Lep+ group had the adverse outcome among those with acinar- or papillary-predominant lung adenocarcinoma, hence we explored whether these patients could benefit from adjuvant chemotherapy. In terms of the prognosis of patients in MPS-/Lep+ group, the DFS rate was no significant difference between postoperative chemotherapy and non-postoperative chemotherapy (P = 0.839) (Figure 4).
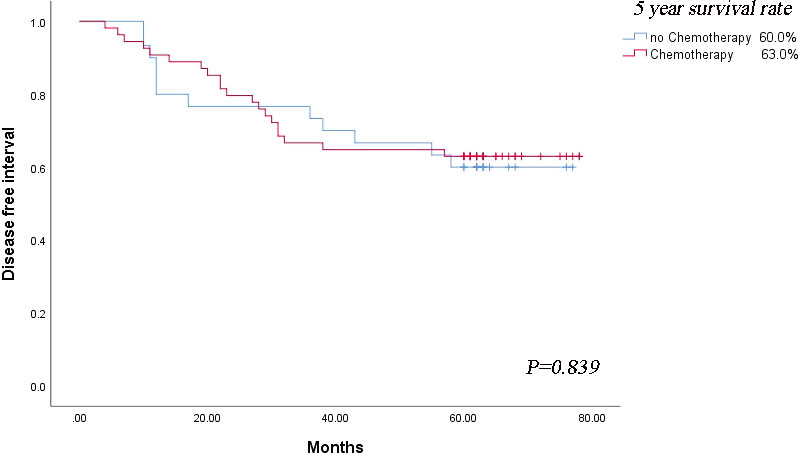
Figure 4 Survival outcomes regarding disease-free survival based on the strategy of adjuvant chemotherapy.
4. Discussion
The MP/S+Lep- and high PLR level were determined as poor risks in acinar/papillary-predominant lung adenocarcinoma patients with tumor size ≤4 cm. The MP/S+Lep- group was prone to recurrence, which may be strongly associated with poorly differentiation and lymphovascular invasion. Adjuvant chemotherapy did not significantly improve DFS in the MP/S+Lep- subgroup. The above results reflected that even for the same predominant histopathological subtype, the prognosis varied with the diverse composition of the minimal patterns. It is advised to concentrate on all growth patterns observed in tumors beyond predominant components. Zhao et al. also concluded not only predominant subtypes but also minor components had an important value in clinical outcomes (20). Mäkinen et al. reported that the non-predominant lepidic components were related to a favorable outcome in invasive adenocarcinoma (21). A study indicated solid minor components and solid predominant subtypes both predicted a worse prognosis compared with the absent solid pattern. Chemotherapy was beneficial for solid predominant components rather than solid as minor patterns (22). The MP/S+Lep- was an independent factor for DFS and OS, and stage IA patients in the MP/S+Lep- subgroup did not benefit from chemotherapy. Those findings are supported by our results (23).
We found that patients in the MP/S+Lep- subgroup were closely connected with poorly differentiation and lymphovascular invasion. Perhaps this can be explained by the following theoretical mechanism. The solid/micropapillary patterns include increased laminin-5 expression levels, which is an extracellular matrix protein that is crucial in cell migration, intercellular adhesion, and tumor invasion reflecting the biologically aggressive nature of tumors (24). The presence of lepidic patterns indicated lower cancer cell-specific expression levels of hypoxia markers and a smaller number of tumor-promoting stromal cells (25, 26). The presence of solid and micropapillary patterns were related to metastatic lymph nodes, inversely, the presence of lepidic patterns predicted nonmetastatic lymph nodes (27). In conclusion, the lung adenocarcinoma with the presence of solid/micropapillary components and the absence of lepidic pattern presented the worse DFS. To the best of our knowledge, this is the first study investigating the relationship between PLR and non-predominant components on survival.
We noticed that the presence of non-predominant patterns rather than their proportion was associated with recurrence in stage I acinar/papillary-predominant lung adenocarcinomas. Chen et al. reported the proportion of solid or micropapillary patterns was not related to recurrence in IA lung adenocarcinoma (28). As a result, identifying the presence of a solid or micropapillary component is more valuable than determining the percentage of either of these components. A prognostic nomogram according to a new classification of combined micropapillary and solid components revealed that patients with a total proportion of solid and micropapillary components ≥40% in stage IA patients had shorter DFS and OS compared with less than the total proportion (29). Our conclusions contradict the above findings, maybe due to our small sample size.
In our study, the MP/S+Lep- group could not benefit from adjuvant chemotherapy. Cao et al. also reported that adjuvant chemotherapy is beneficial for solid predominant patterns of stage IB lung adenocarcinoma, while those with solid minor patterns will not (30). The low relapse rates in the non‐predominant population especially of stage I tumors and limited sample size may explain the failure of adjuvant chemotherapy as a prognostic factor.
There are some limitations in this study. Firstly, owing to the short follow-up period plus the small number of participants, it was difficult to perform the analysis of OS, and we are working to enlarge the sample size. Secondly, the data collection of this study is retrospective, there is an imbalance in the distribution of histological grades, so it is urgent to conduct multi-institutional randomized clinical trials to verify the results. Additionally, we did not evaluate the status of EGFR and KRAS mutation and the PD-L1 expression to explore whether the immunotherapy or targeted therapy could benefit the MP/S+Lep- group. The results show the emphasis should be placed on the non-predominant histological subtype and the necessity of classifying acinar/papillary-predominant adenocarcinomas into different risk groups so as to select high-risk subgroups of patients to administer more intensive surveillance.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study was approved by the Ethics Committee of Nanjing Brain Hospital. Informed consent was obtained from all participants in the study. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
WL and QZ searched the database, judged study eligibility, and extracted data. WL and TZ analyzed data and wrote the paper. CX and LLdesigned the study and revised this paper. All authors contributed to the article and approved the submitted version.
Funding
The study was supported by the Major Program of Nanjing Medical Science and Technique Development Foundation (ZKX17044). The funders had no role in the study design, data collection, analysis, or preparation of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor HL declared a shared parent affiliation with the authors at the time of review.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Travis WD, Brambilla E, Noguchi M, Nicholson A, Geisinger K, Yatabe Y, et al. International association for the study of lung Cancer/American thoracic Society/European respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol (2011) 6:244–85. doi: 10.1097/JTO.0b013e318206a221
2. Moreira AL, Ocampo PS, Xia Y, Zhong H, Russell PA, Minami Y, et al. A grading system for invasive pulmonary adenocarcinoma: a proposal from the IASLC pathology committee. J Thorac Oncol (2020) 15(10):1599–610. doi: 10.1016/j.jtho.2020.06.001
3. Kim M, Chung YS, Kim KA, Shim HS. Prognostic factors of acinar-or papillary-predominant adenocarcinoma of the lung. Lung Cancer (2019) 137:129–35. doi: 10.1016/j.lungcan.2019.09.026
4. Watanabe K, Sakamaki K, Ito H, Yokose T, Yamada K, Nakayama H, et al. Impact of the micropapillary component on the timing of recurrence in patients with resected lung adenocarcinoma. Eur J Cardiothorac Surg (2020) 58(5):1010–8. doi: 10.1093/ejcts/ezaa138
5. Ma M, She Y, Ren Y, Dai C, Zhang L, Xie H, et al. Micropapillary or solid pattern predicts recurrence free survival benefit from adjuvant chemotherapy in patients with stage IB lung adenocarcinoma. J Thorac Dis (2018) 10(9):5384–93. doi: 10.21037/jtd.2018.08.64
6. Murakami S, Ito H, Tsubokawa N, Mimae T, Sasada S, Yoshiya T, et al. Prognostic value of the new IASLC/ATS/ERS classification of clinical stage IA lung adenocarcinoma. Lung Cancer (2015) 90(2):199–204. doi: 10.1016/j.lungcan.2015.06.022
7. Zhu E, Dai C, Xie H, Su H, Hu X, Li M, et al. Lepidic component identifies a subgroup of lung adenocarcinoma with a distinctive prognosis: a multicenter propensity-matched analysis. Ther Adv Med Oncol (2020) 12:1758835920982845. doi: 10.1177/1758835920982845
8. Okubo Y, Kashima J, Teishikata T, Muraoka Y, Yotsukura M, Yoshidav Y, et al. Prognostic impact of the histological lepidic component in pStage IA adenocarcinoma. J Thorac Oncol (2022) 17(1):67–75. doi: 10.1016/j.jtho.2021.09.006
9. Wang W, Hu Z, Zhao J, Huang Y, Rao S, Yang J, et al. Both the presence of a micropapillary component and the micropapillary predominant subtype predict poor prognosis after lung adenocarcinoma resection: a meta-analysis. J Cardiothorac Surg (2020) 15(1):154. doi: 10.1186/s13019-020-01199-8
10. Ninomiya H, Inamura K, Mun M, Nishio M, Ishikawa Y. Relationship between PathologicT1 categories and pathologic factors affecting prognosis in pulmonary adenocarcinoma. JTO Clin Res Rep (2022) 3:100293. doi: 10.1016/j.jtocrr.2022.100293
11. Peng B, Li G, Guo Y. Prognostic significance of micropapillary and solid patterns in stage IA lung adenocarcinoma. Am J Transl Res (2021) 13(9):10562–9.
12. Zhao Z-R, To KF, Mok TSK, Ng C. Is there significance in identification of non-predominant micropapillary or solid components in early-stage lung adenocarcinoma? Interact Cardiovasc Thorac Surg (2017) 24(1):121–5. doi: 10.1093/icvts/ivw283
13. Choi SH, Jeong JY, Lee SY, Shin KM, Jeong SY, Park TI, et al. Clinical implication of minimal presence of solid or micropapillary subtype in early-stage lung adenocarcinoma. Thorac Cancer (2021) 12(2):235–44. doi: 10.1111/1759-7714.13754
14. Choi Y, Kim J, Park H, Kim H, Kim J, Jeong J, et al. Rethinking a non-predominant pattern in invasive lung adenocarcinoma: Prognostic dissection focusing on a high-grade pattern. Cancers (Basel) (2021) 13(11):2785. doi: 10.3390/cancers13112785
15. Liu X, Sun K, Yang F, et al. Different pathologic types of early stage lung adenocarcinoma have different post-operative recurrence patterns. Thorac Cancer (2021) 12:2205–13. doi: 10.1111/1759-7714.14049
16. Qu Y, Lin H, Zhang C, Sui X, Jiang G, Wang J, et al. Cribriform pattern in lung invasive adenocarcinoma correlates with poor prognosis in a Chinese cohort. Pathol Res Pract (2019) 215(2):347–53. doi: 10.1016/j.prp.2018.12.014
17. Yanagawa N, Shiono S, Abiko M, Katahira M, Osakabe M, Ogata S, et al. The clinical impact of solid and micropapillary patterns in resected lung adenocarcinoma. J Thorac Oncol (2016) 11(11):1976–83. doi: 10.1016/j.jtho.2016.06.014
18. Yoshizawa A, Motoi N, Riely GJ, Sima CS, Gerald WL, Kris MG, et al. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol (2011) 24(5):653–64. doi: 10.1038/modpathol.2010.232
19. Yao J, Zhu E, Li M, Liu J, Zhang L, Ke H, et al. Prognostic impact of micropapillary component in patients with node-negative subcentimeter lung adenocarcinoma: A Chinese cohort study. Thorac Cancer (2020) 11(12):3566–75. doi: 10.1111/1759-7714.13702
20. Zhao Y, Wang R, Shen X, Pan Y, Cheng C, Li Y, et al. Minor components of micropapillary and solid subtypes in lung adenocarcinoma are predictors of lymph node metastasis and poor prognosis. Ann Surg Oncol (2016) 23(6):2099–105. doi: 10.1245/s10434-015-5043-9
21. Mäkinen JM, Laitakari K, Johnson S, Mäkitaro R, Bloigu R, Lappi-Blanco E, et al. Nonpredominant lepidic pattern correlates with better outcome in invasive lung adenocarcinoma. Lung Cancer (2015) 90(3):568–74. doi: 10.1016/j.lungcan.2015.10.014
22. Chen T, Luo J, Gu H, Gu Y, Huang Q, Wang Y, et al. Impact of solid minor histologic subtype in postsurgical prognosis of stage I lung adenocarcinoma. Ann Thorac Surg (2018) 105(1):302–8. doi: 10.1016/j.athoracsur.2017.08.018
23. Hou Y, Song W, Chen M, Zhang J, Luo Q, Um S, et al. The presence of lepidic and micropapillary/solid pathological patterns as minor components has prognostic value in patients with intermediate-grade invasive lung adenocarcinoma. Transl Lung Cancer Res (2022) 11(1):64–74. doi: 10.21037/tlcr-21-934
24. Takuwa T, Ishii G, Nagai K, Yoshida J, Nishimura M, Hishida T, et al. Characteristic immunophenotype of solid subtype component in lung adenocarcinoma. Ann Surg Oncol (2012) 19(12):3943–52. doi: 10.1245/s10434-012-2428-x
25. Chong Y, Wu Y, Liu J, Han C, Gong L, Liu X, et al. Clinicopathological models for predicting lymph node metastasis in patients with early-stage lung adenocarcinoma: the application of machine learning algorithms. J Thorac Dis (2021) 13(7):4033–42. doi: 10.21037/jtd-21-98
26. Katsumata S, Aokage K, Miyoshi T, Tane K, Nakamura H, Sugano M, et al. Differences of tumor microenvironment between stage I lepidic-positive and lepidic-negative lung adenocarcinomas. J Thorac Cardiovasc Surg (2018) 156(4):1679–1688.e2. doi: 10.1016/j.jtcvs.2018.05.053
27. Mäkinen JM, Laitakari K, Johnson S, Mäkitaro R, Bloigu R, Pääkkö P, et al. Histological features of malignancy correlate with growth patterns and patient outcome in lung adenocarcinoma. Histopathology (2017) 71(3):425–36. doi: 10.1111/his.13236
28. Chen C, Chen Z-J, Li W-J, Pan X-F, Wen Y-Y, Deng T, et al. Impact of minimal solid and micropapillary components on invasive lung adenocarcinoma recurrence. Ann Diagn Pathol (2022) 59:151945. doi: 10.1016/j.anndiagpath.2022.151945
29. Huang W, Zhang H, Zhang Z, Zhang B, Sun X, Huo Y, et al. A prognostic nomogram based on a new classification of combined micropapillary and solid components for stage IA invasive lung adenocarcinoma. J Surg Oncol (2022) 125(4):796–808. doi: 10.1002/jso.26760
Keywords: lung adenocarcinoma, prognosis, stage I, non-predominant patterns, histological subtype
Citation: Liu W, Zhang Q, Zhang T, Li L and Xu C (2022) Minor histological components predict the recurrence of patients with resected stage I acinar- or papillary-predominant lung adenocarcinoma. Front. Oncol. 12:1090544. doi: 10.3389/fonc.2022.1090544
Received: 05 November 2022; Accepted: 07 December 2022;
Published: 23 December 2022.
Edited by:
Hongda Liu, Nanjing Medical University, ChinaReviewed by:
Jing Yang, Chongqing Technology and Business University, ChinaXiaoliang Shao, First People’s Hospital of Changzhou, China
Copyright © 2022 Liu, Zhang, Zhang, Li and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunhua Xu, eHVjaDIxODhAMTYzLmNvbQ==; Li Li, bGlsaV9uajg2QDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Wei Liu1,2†
Wei Liu1,2† Chunhua Xu
Chunhua Xu