- 1Oncology Department, Luzerner Kantonsspital, Luzerne, Switzerland
- 2Bone Tumour Reference Center, Institute of Medical Genetics and Pathology, University Hospital Basel, Basel, Switzerland
- 3Neurology Department, University Hospital and University of Zurich, Zurich, Switzerland
- 4Pathology Institute Enge, University of Zurich, Zurich, Switzerland
Mesenchymal chondrosarcoma is a rare and aggressive sarcoma subtype with high risk for distant metastases and poor prognosis. Currently NCCN- and ESMO-Guidelines recommend using Ewing sarcoma protocols as standard treatment. Nevertheless, in localized disease overall 5-year survival rates are below 50% whereas in metastatic spread median progression-free survival rates of only 5 months can be expected. Here we present a patient with metastatic osseous spread of mesenchymal chondrosarcoma that showed a sustained clinical improvement and a good partial response on imaging over a period of one year when treated with the multi-tyrosine kinase inhibitor cabozantinib. Although we cannot explain the exact mechanism underlying this treatment effect, tumors with similar genetic patterns might respond to the same therapy as well.
Introduction
Mesenchymal chondrosarcoma (MCS) is a rare sarcoma subtype accounting for less than 5% of all chondrosarcomas. The HEY1::NCOA2 gene fusion is present in virtually all MCS and is considered the main driver of tumorigenesis through different mechanisms including direct DNA-binding, protein-protein-interaction and epigenetic modification (1, 2). Recently, it was shown that the main downstream targets of HEY1::NCOA2 are PDGFRA, PDGFRB and BCL2 (3). Optimal treatment for patients with localized MCS remains controversial. Due to an aggressive behavior and high risk for distant metastases, complete resection together with (neo-)adjuvant treatment according to Ewing sarcoma (ES) protocols is recommended according to the NCCN- and ESMO-Guidelines (4). Nonetheless, the prognosis for MCS remains poor with 5-year survival rates not exceeding 40-50% (5, 6). In metastatic disease, response rates to chemotherapy are generally below 30% and median progression-free survival rates of five months have been reported (7).
Cabozantinib is a broad acting multi-kinase-inhibitor which targets MET, VEGFR-1, VEGFR-2, VEGFR-3, KIT, NTRK2, FLT3, AXL, RET, TEK, ROS1, TYRO3, and MER. Efficacy of cabozantinib has recently been described in recurrent ES and osteosarcoma as well as in selected soft tissue sarcomas (8–11). To the best of our knowledge, no patients with metastatic MCS treated with cabozantinib have been reported so far.
Case report
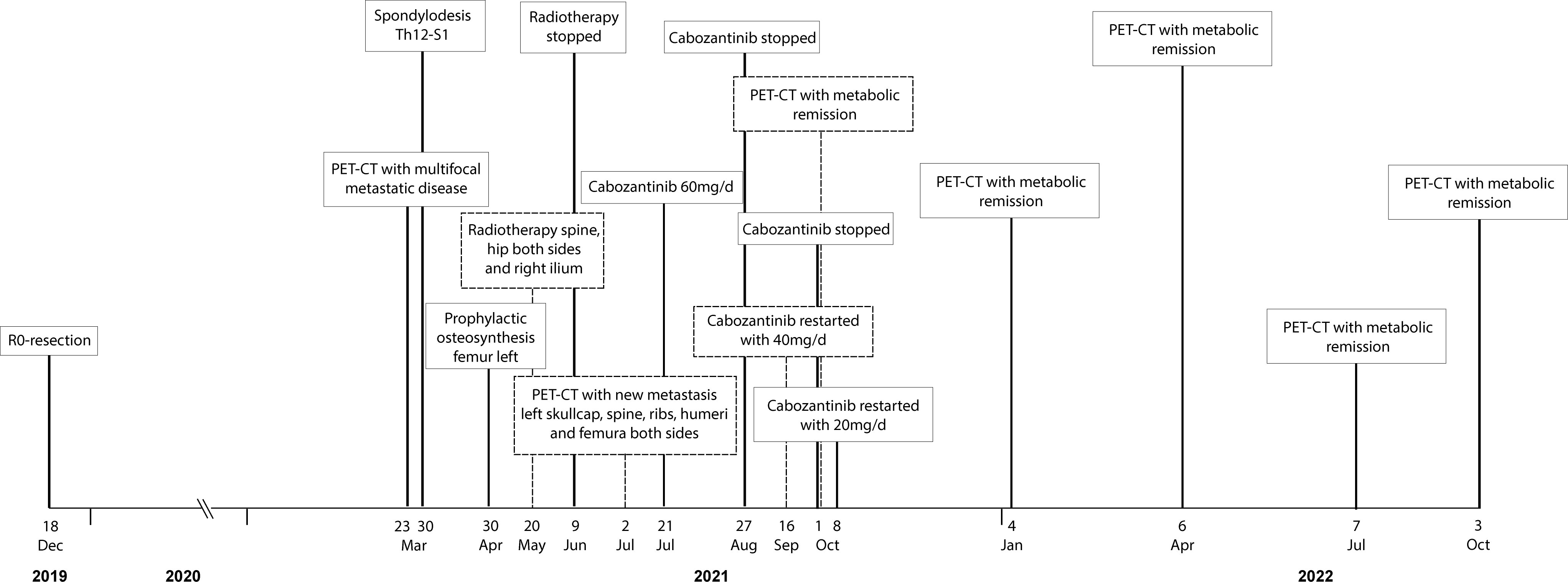
A 47-year-old female with no relevant medical history was diagnosed with localized MCS of the right tibia harboring the disease defining HEY1::NCOA2 fusion. The biopsy showed a biphasic tumor consisting of solid sheets of small round to spindle cells admixed with islands of hyaline cartilage (Figure 1). The patient declined neoadjuvant chemotherapy and a resection with clear margins was performed. One and a half years later, she presented with pain in her lower spine. A FDG-PET-CT scan revealed multiple osseous lesions in the spine, pelvis, both proximal femurs, and several ribs, which were confirmed to represent metastatic disease on biopsy.
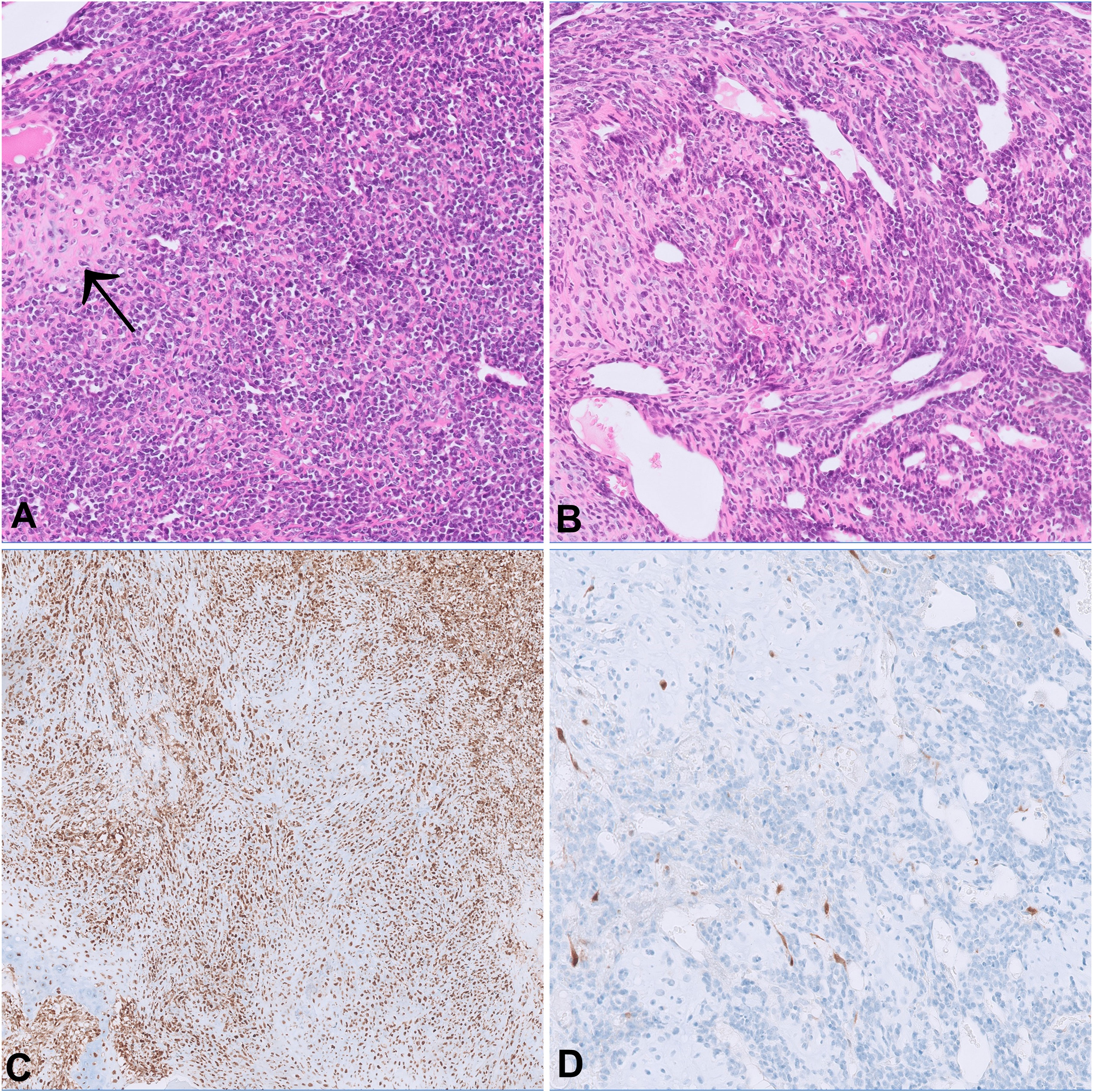
Figure 1 The morphology of both the primary tumor (A) and the metastasis from the femoral head (B) show cellular sheets of small round to spindle cells merging with foci of cartilaginous differentiation (arrow). On immunohistochemistry, p16 expression is retained in the primary tumor (C) and lost in the metastasis (D).
Due to myelocompression and a high risk of fracture, the metastasis in the third lumbar vertebrae was treated by debulking and spondylodesis from T12 to S1. The left proximal femur was also prophylactically stabilized by osteosynthesis after curetting the metastasis. Postoperative radiotherapy was performed in the region of L3-5, both hips and the right ilium, each with 13 x 3 Gy. Subsequently, the irradiated areas showed no FDG-uptake in the PET-CT anymore, but new and partly progressive osteolytic lesions on the left skullcap, spine, ribs, both femurs, and humerus were detected (Figure 2A).
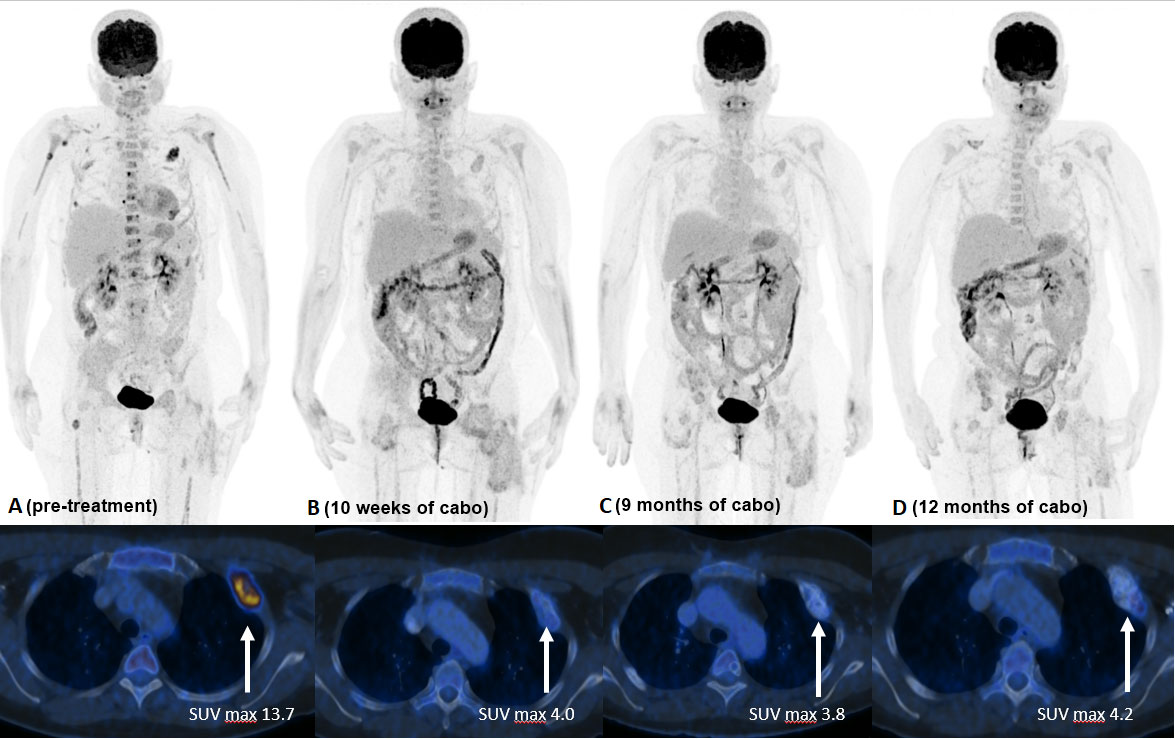
Figure 2 FDG PET/CT [(A) 7/21] with multiple FDG avid bone metastases (ribs, spine, femurs, humerus, skullcap). SUV max, in the large left sided rib metastasis 13.7. FDG PET/CT [(B) 10/21] 10 weeks after start of TKI treatment with reduction of uptake in all bone metastases (rib lesion reduction to SUV max. 4.0). No new FDG avid lesions visible. FDG PET/CT [(C) 4/22] 9 months and [(D) 7/22] 12 months after TKI start with ongoing good response.
Despite severe bone pain, the patient still refused to undergo conventional chemotherapy. Encouraged by the recently published data on the efficacy of cabozantinib in patients with recurrent ES and osteosarcoma (9), the drug was offered as an alternative approach, which she agreed to. Cabozantinib was started at a dosage of 60mg, administered once daily. Bone pain increased during the first two weeks of treatment, but steadily improved thereafter. Due to a palmar-plantar erythrodysesthesia, dosage had to be reduced to 40mg/d after the first cycle and to 20mg/d after 6 weeks of treatment. The patient tolerated Cabozantinib 20 mg/d without further adverse events. A FDG PET-CT scan performed 10 weeks after the beginning of treatment showed a partial metabolic response in all metastasis: as an example SUV max of a rib metastasis decreased from 13.7 to 4.0 and no new manifestations developed. Additional FDG PET-CT scans carried out after 27, 37, 48, and 60 weeks confirmed a sustained partial response (Figure 2). A detailed timeline with relevant data from the episode of care is presented in Figure 3. Clinically, the patient described a significant reduction of pain requiring only occasional oral analgesics, improvement in general condition and mobility.
Materials and methods
Formalin-fixed paraffin-embedded tissue samples of the primary tumor and metastases from three different sites (lumbar spine, femur and ilium) were subjected to copy number and methylome analysis. Copy number variations were inferred from the Infinium Human Methylation Epic Array platform using the R-package conumee after pre-processing of raw data using the R-package minfi as described before (12). A tissue sample from the primary tumor was additionally investigated by Oncomine Comprehensive Panel version 3 and Archer FusionPlex Custom V2 Panel (Thermo Fisher Scientific, USA) according to routine protocols.
Results
Both the primary tumor and the metastases histologically showed a consistent biphasic pattern of undifferentiated small round to spindle cells with intermingled islands of well-differentiated hyaline cartilage, typical for MSC (Figure 1). The tumor defining HEY1-NCOA2 gene fusion was identified in the primary tumor using the Archer FusionPlex Custom V2 Panel (Thermo Fisher Scientific, USA). DNA methylation profiling revealed high similarity with the established methylation class MSC using unsupervised clustering approaches following dimension reduction (data not shown) (13, 14). The copy number profiles of both the primary tumor and all three metastases showed aneuploidy of chromosome 12 as well as copy number variations of well-established cancer drivers (Figure 4). Among them, a low-level copy number gain of MYC was noted, which was confirmed by FISH analysis in a subset of tumor cells, supporting the interpretation of a subclonal event. Of note, all metastases showed a homozygous loss at 9p21.3 harboring CDKN2A (p16), that was absent in the primary tumor. This finding was confirmed by immunohistochemistry, with consistent and strong positivity of p16 in the primary tumor and loss of expression in the metastases (Figure 1). All three metastases shared identical DNA methylation and copy number profiles suggesting that they developed from an identical clone. An Oncomine Comprehensive Panel v3 (Thermo Fisher Scientific, USA) was performed in one of the metastases and did not reveal any point mutations within 135 cancer genes (including RB1).
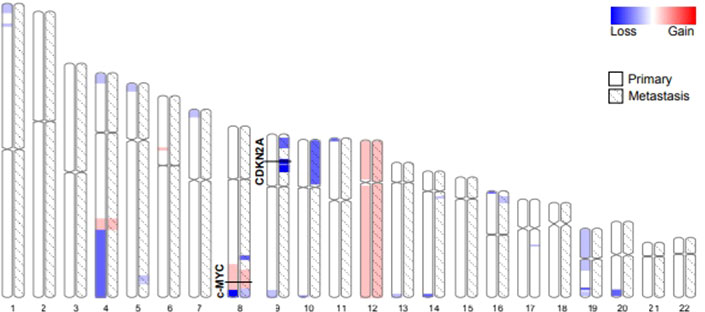
Figure 4 Autosome ideograms comparing copy number variations of the primary tumor (blank) and a metastasis (hatched). Both copy number profiles have been derived from DNA methylation arrays. The magnitude of gains (red) and losses (blue) are represented by a color gradient. The two loci specifically addressed in this study (c-Myc and CDKN2A) are highlighted.
Discussion
The use of TKI in patients with sarcoma in advanced stages not responding to conventional treatment strategies are currently being intensively studied. The use of levantinib, a different TKI, is currently under evaluation in the treatment of selected sarcomas including osteosarcoma and chondrosarcoma (NCT04784247). This drug acts through the inhibition of VEGFR and impairs the activity of tyrosine kinase receptors, such as RET, similar to cabozantinib (15). Moreover, recent findings highlight the efficacy of Lenvatinib alone or in combination with denosumab compared to denosumab alone in treating bone sarcoma cell models, which is in line with the involvement of VEGFR in osteolytic lesions (16, 17). Additionally, several case reports of chondrosarcoma presenting prolonged disease control when treated with the TKI pazopanib were reported (18, 19).
Predictive biomarkers to identify patients that might benefit from these innovative approaches are urgently needed. Potential therapeutic targets have been described in various sequencing studies but usually using retrospective case series in which the efficacy of a targeted approach can no longer be validated (20, 21). Here, we present a patient with widespread metastatic MCS that showed an impressive and long lasting response to cabozantinib. The primary tumor as well as three metastases were comprehensively investigated for molecular alterations.
In a subset of tumor cells, we found a low level amplification of MYC. Despite being a well known stimulator of VEGFA, which is a direct target of cabozantinib, subclonal MYC amplification is unlikely to have caused the clinical response to treatment. As a strong inhibitor of MET, cabozantinib might have acted through downstream targets of the MCS-related chimeric protein itself. Indeed, HEY1 is a mediator and effector of the activated Notch developmental and stemness pathway (1) and crosstalks between Notch and MET signaling have been described (22). Recently it has furthermore been shown that the fusion protein also activates PDGFRA and PDGFRB and dramatically increases phospho-AKT, thus stimulating the PDGF-PI3K-AKT axis (3) which is another target of cabozantinib.
In contrast to the primary tumor, the bone metastases showed a consistent and homozygous loss of the tumor suppressor gene CDKN2A (p16). Loss of p16 results in increased Cyclin D1 and CDK4/6 activity promoting the partial phosphorylation of the C-terminal region of RB1. As a consequence, pRB1 can release E2F transcription factors which initiate the mitotic G1-S transition. This cascade is known to stimulate additional pathways, such as PDGF-PI3K-AKT and RAS-RAF-ERK MAPK, thereby promoting uncontrolled cellular proliferation, neoangiogenesis and tumor microenvironment remodeling. Loss of p16 has been associated with tumor progression and a poor prognosis in various tumors, including MCS (20). However, a similar dramatic response to the kinase inhibitor Palbociclib has been reported in a 62-year-old patient with advanced chordoma which was also deficient of p16 (21).
Increased cellular proliferation induces hypoxia and thereby triggers a shift to anaerobic metabolism in the neoplastic cells through the HIF-1 pathway which stimulates VEGF expression and neoangiogenesis. Additionally, in a hypoxic state, activated VEGF can lead to imbalances in the ANG-TEK system, which further dysregulates the angiogenic signaling network. The TEK ligands Angiopoietin 1 and 2 promote angiogenesis in the presence of VEGF-A, whereas TEK-expressing macrophages support neovascularization by recruiting endothelial cells, and also promote a dissemination of tumor cells. Inhibiting the tyrosine kinase activity of TEK, MET, RET, FLT3, NTRK2, and AXL, cabozantinib blocks these disrupted angiogenic pathways. Moreover, targeting the tumor microenvironment through VEGF and TEK receptors directly downregulates tumor-induced angiogenesis which sustains tumor cell migration, extracellular matrix invasion and tumor related macrophages recruitment.
The activity of cabozantinib in the patient presented here is therefore likely explained by inhibition of a complex interplay between different signaling cascades, addressing both downstream targets of the HEY1::NCOA2 fusion, as well as tumor microenvironment. The limitation of this study is our inability to elucidate the exact pathway underlying the response to the treatment with only one case.
Conclusion
Finding the optimal treatment for patients with rare sarcomas is challenging, particularly because systematic clinical studies are usually not feasible. Our case illustrates an unexpected sustained clinical response to the multi-kinase-inhibitor Cabozantinib in a patient with metastatic mesenchymal chondrosarcoma with comprehensive molecular work-up. Although we cannot explain the exact mechanism underlying this treatment effect, this being the most important limitation of this study, tumors with similar genetic patterns might respond to the same therapy as well. Identifying genetic patterns requires more tumors to undergo systematic sequencing that should include copy number alterations as panel sequencing of 135 cancer genes did not reveal any pathogenic mutations in the case presented here. Taken together, our case illustrates that even in advanced sarcomas, targeted treatment approaches can show impressive clinical responses that should whenever possible be correlated with sequencing data.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Ethikkommission beider Basel (reference 274/12). The patients/participants provided their written informed consent to participate in this study.
Author contributions
DB conceived and designed the study. BA analysed and interpreted the methylation data. DB, VB, and VA prepared the manuscript with contributions from all other authors. All authors contributed to the article and approved the submitted version.
Funding
The authors were supported by the Foundation of the Basel Bone Tumour Reference Centre, the Gertrude von Meissner Stiftung, The Stiftung für krebskranke KinderßRegio Basiliensis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. El Beaino M, Roszik J, Livingston JA, Wang W-L, Lazar AJ, Amini B, et al. Mesenchymal chondrosarcoma: A review with emphasis on its fusion-driven biology. Curr Oncol Rep (2018) 20:37. doi: 10.1007/s11912-018-0668-z
2. Wang L, Motoi T, Khanin R, Olshen A, Mertens F, Bridge J, et al. Identification of a novel, recurrent HEY1-NCOA2 fusion in mesenchymal chondrosarcoma based on a genome-wide screen of exon-level expression data. Genes Chromosomes Cancer (2012) 51:127–39. doi: 10.1002/gcc.20937
3. Qi W, Rosikiewicz W, Yin Z, Xu B, Jiang H, Wan S, et al. Genomic profiling identifies genes and pathways dysregulated by HEY1-NCOA2 fusion and shines a light on mesenchymal chondrosarcoma tumorigenesis. J Pathol (2022) 257:579–92. doi: 10.1002/path.5899
4. Dantonello TM, Int-Veen C, Leuschner I, Schuck A, Furtwaengler R, Claviez A, et al. Mesenchymal chondrosarcoma of soft tissues and bone in children, adolescents, and young adults: Experiences of the CWS and COSS study groups. Cancer (2008) 112:2424–31. doi: 10.1002/cncr.23457
5. Amer KM, Munn M, Congiusta D, Abraham JA, Basu Mallick A. Survival and prognosis of chondrosarcoma subtypes: SEER database analysis. J Orthop Res Off Publ Orthop Res Soc (2020) 38:311–9. doi: 10.1002/jor.24463
6. Schneiderman BA, Kliethermes SA, Nystrom LM. Survival in mesenchymal chondrosarcoma varies based on age and tumor location: A survival analysis of the SEER database. Clin Orthop (2017) 475:799–805. doi: 10.1007/s11999-016-4779-2
7. Italiano A, Mir O, Cioffi A, Palmerini E, Piperno-Neumann S, Perrin C, et al. Advanced chondrosarcomas: Role of chemotherapy and survival. Ann Oncol Off J Eur Soc Med Oncol (2013) 24:2916–22. doi: 10.1093/annonc/mdt374
8. Chuk MK, Widemann BC, Minard CG, Liu X, Kim A, Bernhardt MB, et al. A phase 1 study of cabozantinib in children and adolescents with recurrent or refractory solid tumors, including CNS tumors: Trial ADVL1211, a report from the children’s oncology group. Pediatr Blood Cancer (2018) 65:e27077. doi: 10.1002/pbc.27077
9. Italiano A, Mir O, Mathoulin-Pelissier S, Penel N, Piperno-Neumann S, Bompas E, et al. Cabozantinib in patients with advanced Ewing sarcoma or osteosarcoma (CABONE): A multicentre, single-arm, phase 2 trial. Lancet Oncol (2020) 21:446–55. doi: 10.1016/S1470-2045(19)30825-3
10. Mukaihara K, Tanabe Y, Kubota D, Akaike K, Hayashi T, Mogushi K, et al. Cabozantinib and dastinib exert anti-tumor activity in alveolar soft part sarcoma. PloS One (2017) 12:e0185321. doi: 10.1371/journal.pone.0185321
11. O’Sullivan Coyne G, Kummar S, Hu J, Ganjoo K, Chow WA, Do KT, et al. Clinical activity of single-agent cabozantinib (XL184), a multi-receptor tyrosine kinase inhibitor, in patients with refractory soft-tissue sarcomas. Clin Cancer Res Off J Am Assoc Cancer Res (2022) 28:279–88. doi: 10.1158/1078-0432.CCR-21-2480
12. Ameline B, Nathrath M, Nord KH, Flon FH, Bovée JVMG, Krieg AH, et al. Methylation and copy number profiling: Emerging tools to differentiate osteoblastoma from malignant mimics? Mod Pathol Off J U. S. Can Acad Pathol Inc (2022) 35:1204–11. doi: 10.1038/s41379-022-01071-1
13. Haefliger S, Tzankov A, Frank S, Bihl M, Vallejo A, Stebler J, et al. NUT midline carcinomas and their differentials by a single molecular profiling method: A new promising diagnostic strategy illustrated by a case report. Virchows Arch Int J Pathol (2021) 478:1007–12. doi: 10.1007/s00428-020-02869-7
14. Koelsche C, Schrimpf D, Stichel D, Sill M, Sahm F, Reus DE, et al. Sarcoma classification by DNA methylation profiling. Nat Commun (2021) 12:498. doi: 10.1038/s41467-020-20603-4
15. Hao Z, Wang P. Lenvatinib in management of solid tumors. Oncologist (2020) 25:e302–10. doi: 10.1634/theoncologist.2019-0407
16. De Vita A, Vanni S, Miserocchi G, Fausti V, Pieri F, Spadazz C, et al. A rationale for the activity of bone target therapy and tyrosine kinase inhibitor combination in giant cell tumor of bone and desmoplastic fibroma: Translational evidences. Biomedicines (2022) 10:372. doi: 10.3390/biomedicines10020372
17. Kumta SM, Huang L, Cheng YY, Chow LTC, Lee KM, Zheng MH, et al. Expression of VEGF and MMP-9 in giant cell tumor of bone and other osteolytic lesions. Life Sci (2003) 73:1427–36. doi: 10.1016/S0024-3205(03)00434-X
18. Tsavaris O, Economopoulou P, Kotsantis I, Reppas L, Avgerinou C, Spathas N, et al. Clinical benefit of pazopanib in a patient with metastatic chondrosarcoma: A case report and review of the literature. Front Oncol (2018) 8:45. doi: 10.3389/fonc.2018.00045
19. Paoluzzi L, Ghesani M. Extraskeletal myxoid chondrosarcoma with massive pulmonary metastases. Clin Sarcoma Res (2018) 8:20. doi: 10.1186/s13569-018-0108-8
20. Meijer D, Jong D, Pansuriya TC, Akker den van BE, Picci P, Szuhai K, et al. Genetic characterization of mesenchymal, clear cell, and dedifferentiated chondrosarcoma. Genes Chromosomes Cancer (2012) 51:899–909. doi: 10.1002/gcc.21974
21. van Oosterwijk JG, Meijer D, Ruler van MAJH, Akker den van BEWM, Oosting J, Krenács T, et al. Screening for potential targets for therapy in mesenchymal, clear cell, and dedifferentiated chondrosarcoma reveals bcl-2 family members and TGFβ as potential targets. Am J Pathol (2013) 182:1347–56. doi: 10.1016/j.ajpath.2012.12.036
Keywords: mesenchymal chondrosarcoma, metastatic disease, p16 loss, tyrosine kinase inhibitors, case report, cabozantinib
Citation: Blum V, Andrei V, Ameline B, Hofer S, Fuchs B, Strobel K, Allemann A, Bode B and Baumhoer D (2022) Metastatic mesenchymal chondrosarcoma showing a sustained response to cabozantinib: A case report. Front. Oncol. 12:1086677. doi: 10.3389/fonc.2022.1086677
Received: 01 November 2022; Accepted: 25 November 2022;
Published: 12 December 2022.
Edited by:
Fabrizio Martelli, National Institute of Health (ISS), ItalyReviewed by:
Alessandro De Vita, Scientific Institute of Romagna for the Study and Treatment of Tumors (IRCCS), ItalyMarielle Elizabeth Yohe, National Institutes of Health (NIH), United States
Copyright © 2022 Blum, Andrei, Ameline, Hofer, Fuchs, Strobel, Allemann, Bode and Baumhoer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniel Baumhoer, RGFuaWVsLmJhdW1ob2VyQHVzYi5jaA==
†These authors have contributed equally to this work and share first authorship
 Veronika Blum1†
Veronika Blum1† Vanghelita Andrei
Vanghelita Andrei Silvia Hofer
Silvia Hofer Klaus Strobel
Klaus Strobel Daniel Baumhoer
Daniel Baumhoer