- 1Medical School of the Chinese People’s Liberation Army (PLA), Beijing, China
- 2Department of Radiation Oncology, First Medical Center of Chinese PLA General Hospital, Beijing, China
- 3Department of Radiation Oncology, China-Japan Friendship Hospital, Beijing, China
- 4Department of Radiation Oncology, Beijing Luhe Hospital, Affiliated to Capital Medical University, Beijing, China
- 5Department of Radiation Oncology, Hainan Hospital of the Chinese PLA General Hospital, Sanya, China
- 6Department of Otorhinolaryngology, First Medical Center of Chinese PLA General Hospital, Beijing, China
Background: To evaluate the long-term survival and treatment-related toxicities of helical tomotherapy (HT) in nasopharyngeal carcinoma (NPC) patients.
Methods: One hundred and ninety newly diagnosed non-metastatic NPC patients treated with HT from September 2007 to August 2012 were analyzed retrospectively. The dose at D95 prescribed was 70-74Gy, 60-62.7Gy and 52-56Gy delivered in 33 fractions to the primary gross tumor volume (pGTVnx) and positive lymph nodes (pGTVnd), the high risk planning target volume (PTV1), and the low risk planning target volume (PTV2), respectively, using simultaneous integrated boost technique. The statistical analyses were performed and late toxicities were evaluated and scored according to the Common Terminology Criteria for Adverse Events (version 3.0).
Results: The median follow-up time was 145 months. The 10-year local relapse-free survival (LRFS), nodal relapse-free survival (NRFS), distant metastasis-free survival (DMFS) and overall survival (OS) were 94%, 95%, 86%, and 77.8%; respectively. Fifty (26.3%) patients had treatment-related failures at the last follow-up visit. Distant metastasis, occurred in 25 patients, was the major failure pattern. Multivariate analysis showed that age and T stage were independent predictors of DMFS and OS, Concomitant chemotherapy improved overall survival, but anti-EGFR monoclonal antibody therapy failed. The most common late toxicities were mainly graded as 1 or 2.
Conclusions: Helical tomotherapy with simultaneous integrated boost technique offered excellent long-term outcomes for NPC patients, with mild late treatment-related toxicities. Age and clinical stage were independent predictors of DMFS and OS. And, concurrent chemotherapy means better OS. Further prospective study is needed to confirm the superiority of this technology and to evaluate the roles of anti-EGFR monoclonal antibody treatment.
Introduction
Nasopharyngeal carcinoma (NPC) is the most common malignant tumor of the head and neck in China, about 70% ~ 80% of patients are combined with the neck lymph node metastasis, and 10% ~ 15% combined with distant metastases at the first diagnosis (1). Radiation therapy is the only curative treatment method for non-metastatic NPC, and intensity-modulated radiation therapy (IMRT) has been accepted as the standard radiation technique (2). The addition of chemotherapy and anti-EGFR monoclonal antibody (Mab) treatment improves the local control rate (LCR) and the overall survival rate (OS) for advanced NPC patients (3, 4).
Helical tomotherapy (HT) is an emerging IMRT technique that mounts a 6-MV linear accelerator on a ring frame around the accelerator bed (5). The couch passes axially through the center of the stand as it rotates to irradiate the target area. With the capacity to deliver highly conformal dose distribution and pretreatment setup verification (6), HT has achieved better results in the treatment of head and neck cancer (7). In September 2007, our center installed the first HT device in China and the initial observation showed that 3-year local relapse-free survival, nodal relapse-free survival and distant metastasis-free survival were more than 90%, and 3-year overall survival was more than 85% for NPC patients (8). Here, we present a retrospective analysis of long-term (10-year) outcomes and late toxicities of HT in 190 patients with NPC.
Materials and methods
Patient’s characteristics
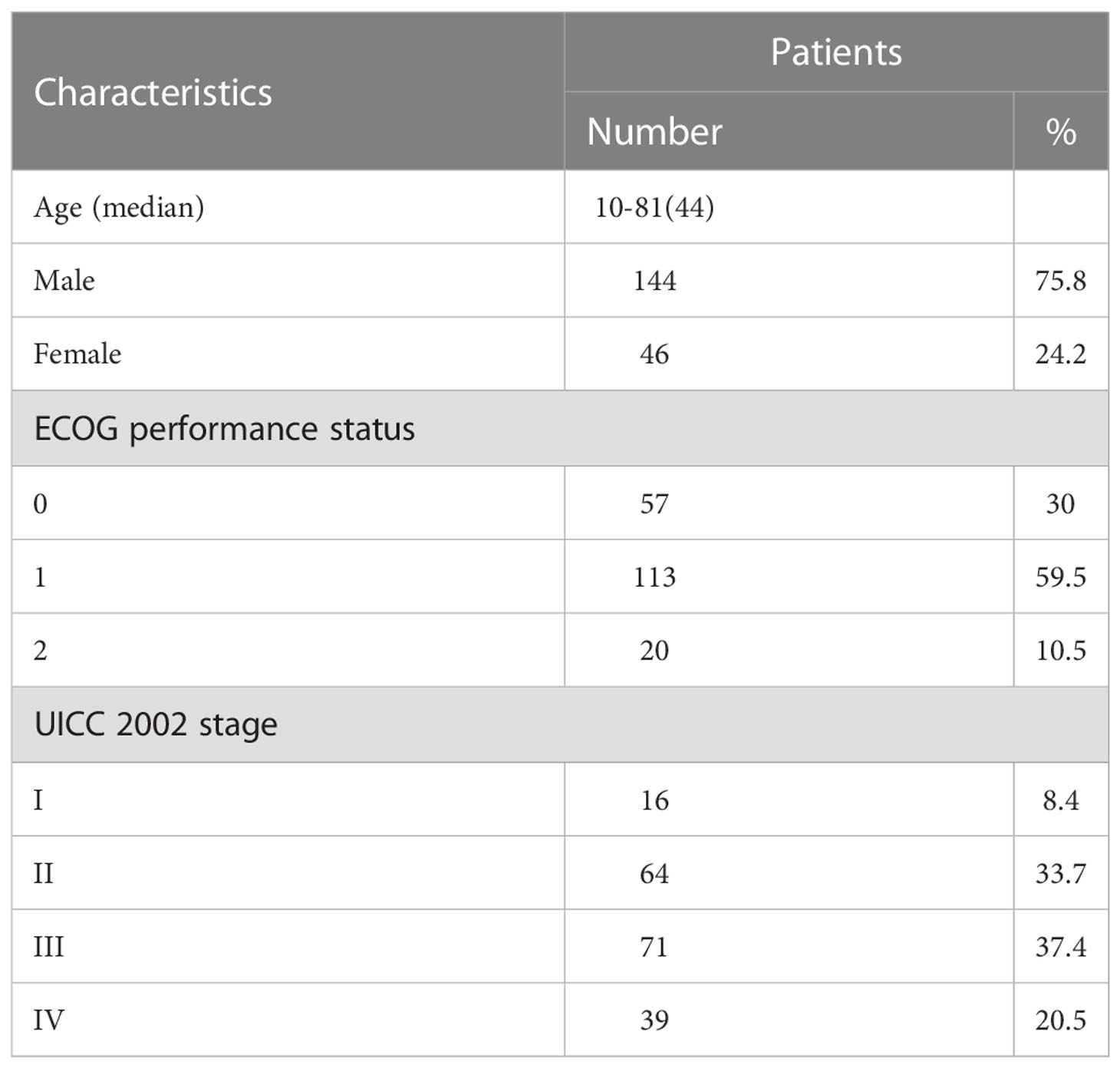
One hundred and ninety NPC patients, treated with Hi Art TomoTherapy system (Accuray, America) at our center between September 2007 and August 2012, were analyzed. All patients underwent nasopharyngeal and skull base computed tomography (CT) or magnetic resonance imaging (MRI), endoscopic evaluation, complete blood count, liver and kidney function tests, neck and abdomen ultrasound, and bone scan. Positron emission tomography (PET) is optional. The clinical stage was determined according to the UICC 2002 staging system. Table 1 summarizes patient characteristics. This study was conducted in accordance with the declaration of Helsinki. This study was conducted with approval from the Ethics Committee of the Chinese PLA General Hospital. Written informed consent was obtained from all participants.
Treatment
The definition of the targets, techniques of planning and the HT delivery were described previously (2). Briefly, the planning dose at D95 was prescribed to the gross tumor volume (pGTVnx) and positive lymph nodes (pGTVnd) at 70–74Gy, the high risk planning target volume (PTV1) at 60–62.7 Gy and the low risk planning target volume (PTV2) at 52–56 Gy, respectively, in 33 fractions. The irradiation was delivered once daily, 5 days per week. MVCT imaging was performed prior to each part of HT treatment to validate patient settings during HT treatment. MVCT image-guidance is the automatic and manual registration of MVCT images and planned CT images, based on bone and tissue anatomy.
Cisplatin-based chemotherapy with or without concomitant anti-EGFR Mab treatment was given to 129 patients, concomitant anti-EGFR Mab treatment without chemotherapy was given to 30 patients, and radiotherapy alone was given to 31 patients. Neoadjuvant chemotherapy includes 1–2 cycles of DP (docetaxel 75 mg/m2, d1, cisplatin 80 mg/m2, d1 and every 3 weeks) or a single DDP regimen. According to clinical stages, tolerance and economic status, concurrent chemotherapy and/or anti-EGFR Mab therapy were performed in one of the four modes: 1) cisplatin 80 mg/m2, d1, every 3 weeks; 2) docetaxel 60 mg/m2, d1 and cisplatin 60 mg/m2, every 3 weeks; 3) cetuximab 250 mg/m2 or nimotuzumab 200 mg, d1, every week; 4) cetuximab 250 mg/m2 or nimotuzumab 200 mg, d1, every week and cisplatin 80mg/m2, d1, every 3 weeks. Adjuvant chemotherapy consisted of 4~6 cycles of DP program. Each patient received no more than 6 cycles of chemotherapy (including neoadjuvant, concurrent and adjuvant modes).
Follow-up and evaluation of outcomes
Acute side-effects were investigated weekly and peak toxicities were recorded and graded according to the established RTOG/EORTC criteria and the Common Terminology Criteria for Adverse Events (Version 3.0) as described previously (8). Patients’ follow-up examinations were conducted 1 month after the completion of radiotherapy, and then every 3 months for the first year, every 6 months in the second year, and annually thereafter. Late toxicity were evaluated and scored according to the Common Terminology Criteria for Adverse Events version 3.0 (9) (CTCAE 3.0).
Statistical analysis
The Kaplan-Meier method was used to analyze local recurrence-free survival (LRFS), lymph node recurrence-free survival (NRFS), distant metastasis-free survival (DMFS) and overall survival (OS). Different prognostic factors were analyzed by log-rank test and multivariate analysis was performed using Cox proportional hazards model. P<0.05 was considered significant. Statistical analyzes were performed using the SPSS software package (Version 22.0, SPSS Inc., an IBM Company; Chicago, IL, USA).
Results
Treatment outcomes
Case selection, follow-up and statistical analysis were done by two different researchers to ensure the accuracy of the data. The median follow-up was 145 months, ranging from 141 to 148 months from the start of radiation therapy. The median age of the patients was 44 years (range 10-81 years), and the ratio of females (n=46) to males (n=144) was 1:3. The details were shown in Table 1.
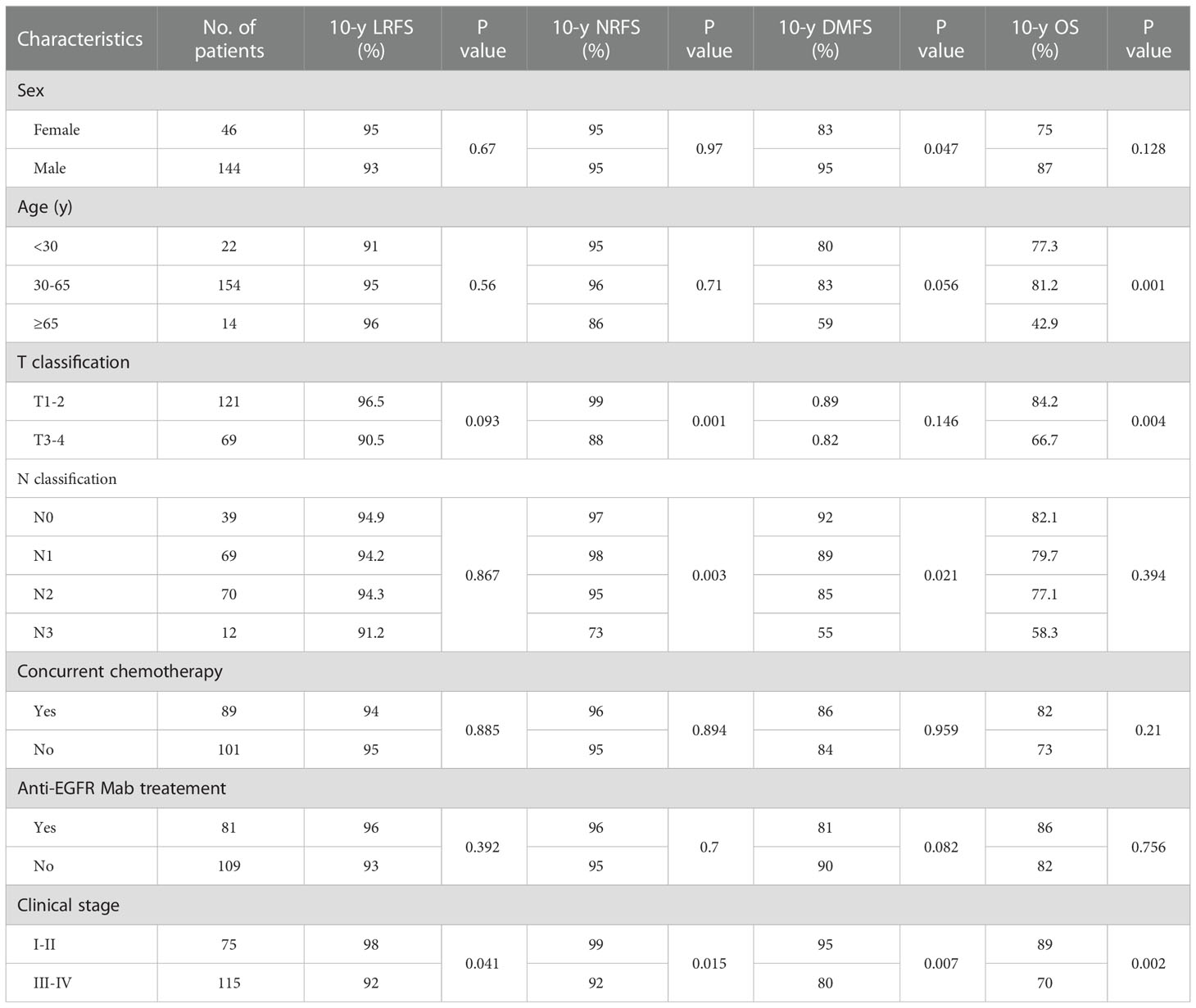
The 10-year LRFS, NRFS, DMFS, and OS were 94.0%, 95%, 86% and 77.8%, respectively. The 10-year OS and NRFS for patients with stage T1/2 and stage T3/4 were 81% vs.67% and 99%vs. 88%, respectively (c= 3.9, p= 0.01 and c= 10, p= 0.01). The 10-year LRFS, NRFS, DMFS and OS for patients with stage I/II were better than those with stage III/IV(p< 0.05). The 10-year NRFS and DMFS for patients with N0-1 were better than those with N2-3 (p< 0.05). No significant differences were observed in OS and LRFS, between the patients with N0-1 and N2-3 (p> 0.05) (Table 2; Figure 1).
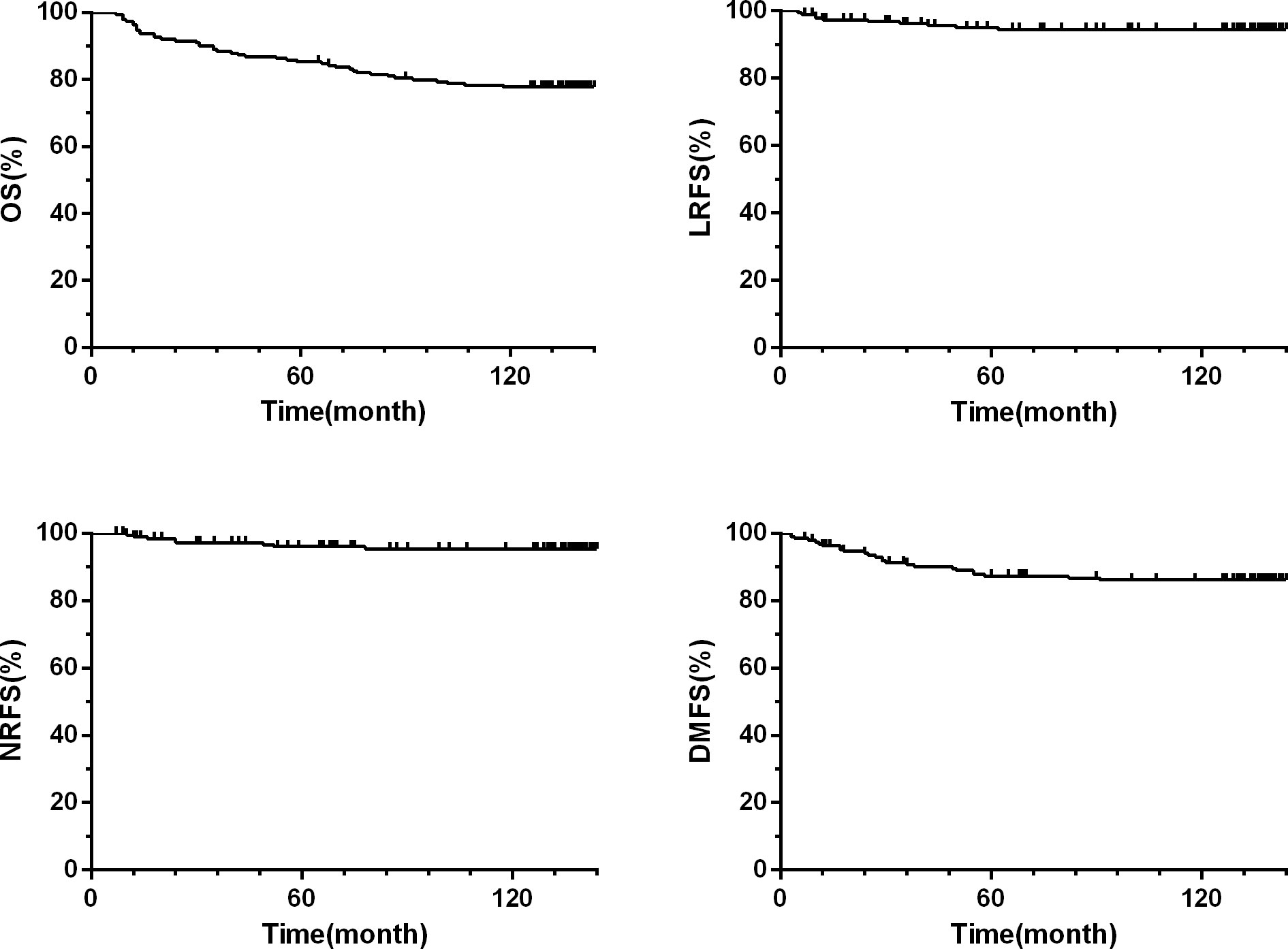
Figure 1 Kaplan-Meier estimate of local relapse-free survival (LRFS), nodal relapse-free survival (NRFS), distant metastases-free survival (DMFS), and overall survival (OS) rates.
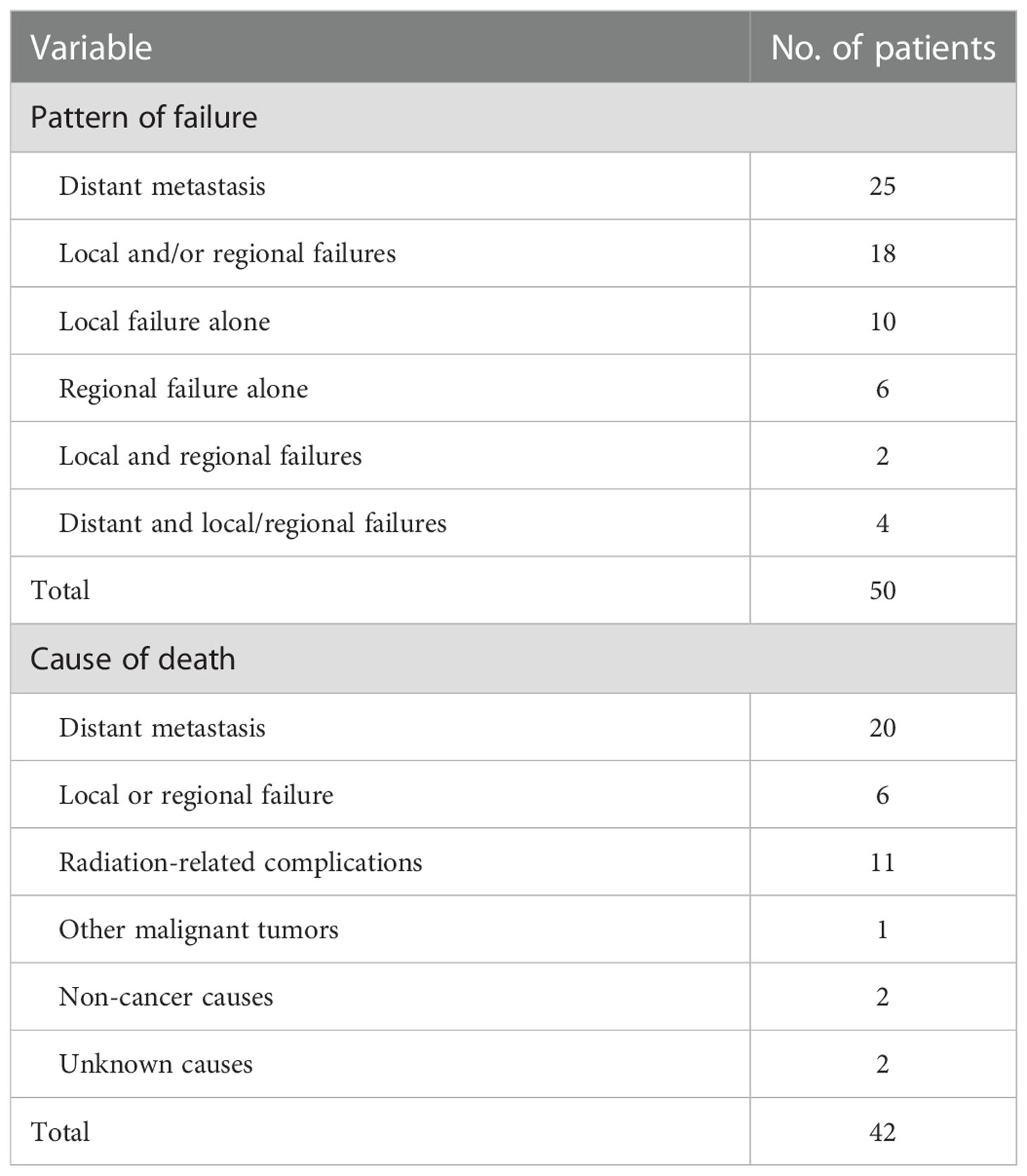
Until the last follow-up, treatment failure was observed in 50 patients. Among these patients, 12 and 8 patients developed local and nodal failure, respectively, and distant metastasis occurred in 25 patients was the major failure pattern after treatment. Lung, bone and liver were the most common metastatic organs. Among the 50 failed patients, 6 patients died of local failure or regional failure and 20 patients died of multiple organ failure. In addition, Five patients (stage T3-4) died of primary pharyngeal vessel bleeding after the completion of radiotherapy, and 6 died of massive hemorrhage 1 year after radiotherapy. One patient died of cerebral hernia 9 months after the completion of radiotherapy. Otherwise, 2 patients died of other causes, one from systemic lupus erythematosus and one from gas poisoning. (Table 3).
Prognostic factors
The potential prognostic factors for OS, LRFS, NRFS, and DMFS included gender, age, T stage, N stage, and various chemotherapy and anti-EGFR Mab treatment patterns. Univariate analysis by log-rank test showed that age and N stage were significantly associated with DMFS, age and T stage were significantly associated with OS, T stage and N stage were significantly associated with NRFS, and clinical stage was significantly associated with LRFS, NRFS, DMFS and OS. (Table 2).
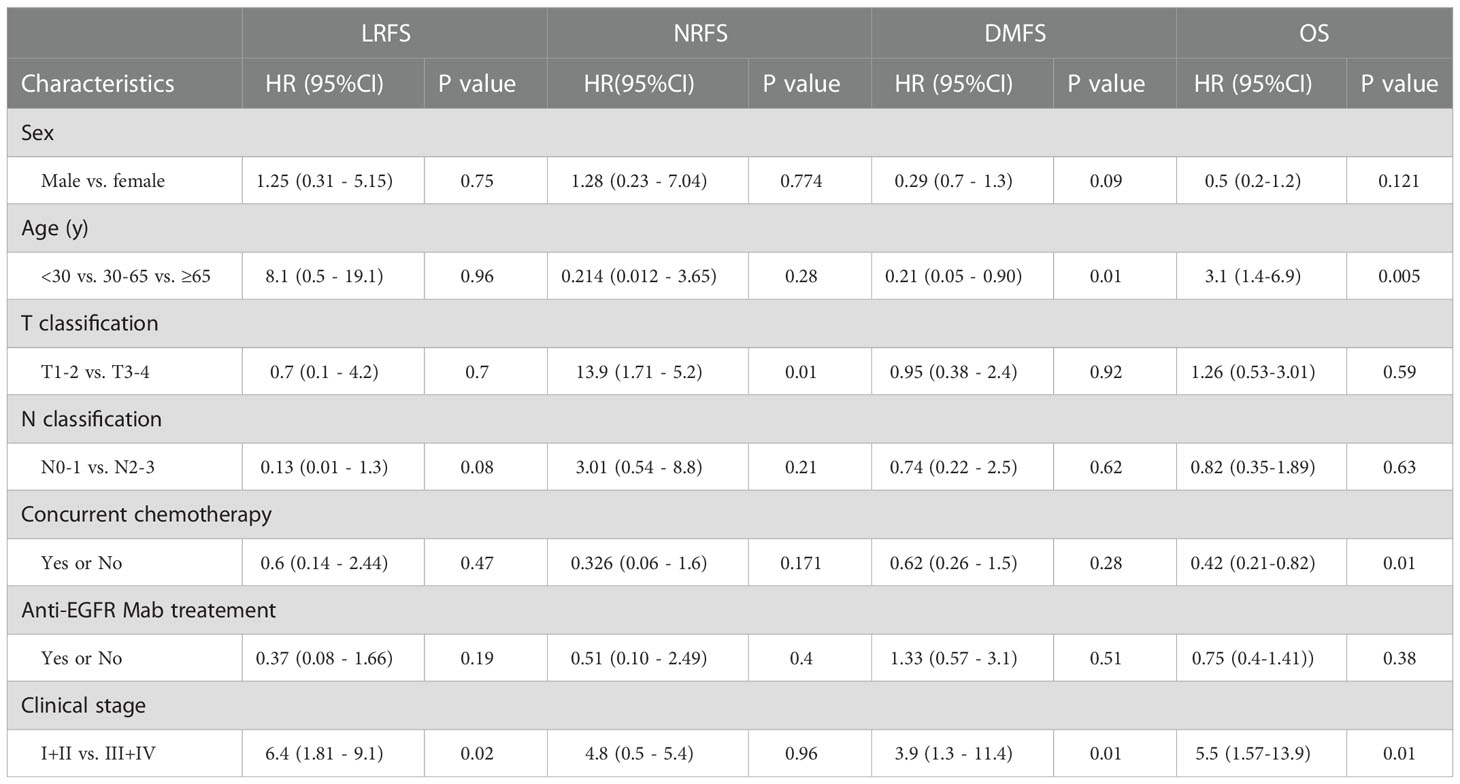
Multivariate analysis by Cox proportional hazards model showed that only the T stage was an independent predictor for NRFS (HR=13.9, 95%CI 1.72–5.2, p= 0.01).The clinical stage was an independent predictor for LRFS, DMFS and OS. And, age and concurrent chemotherapy were independent predictors for OS. (Table 4; Figure 2).
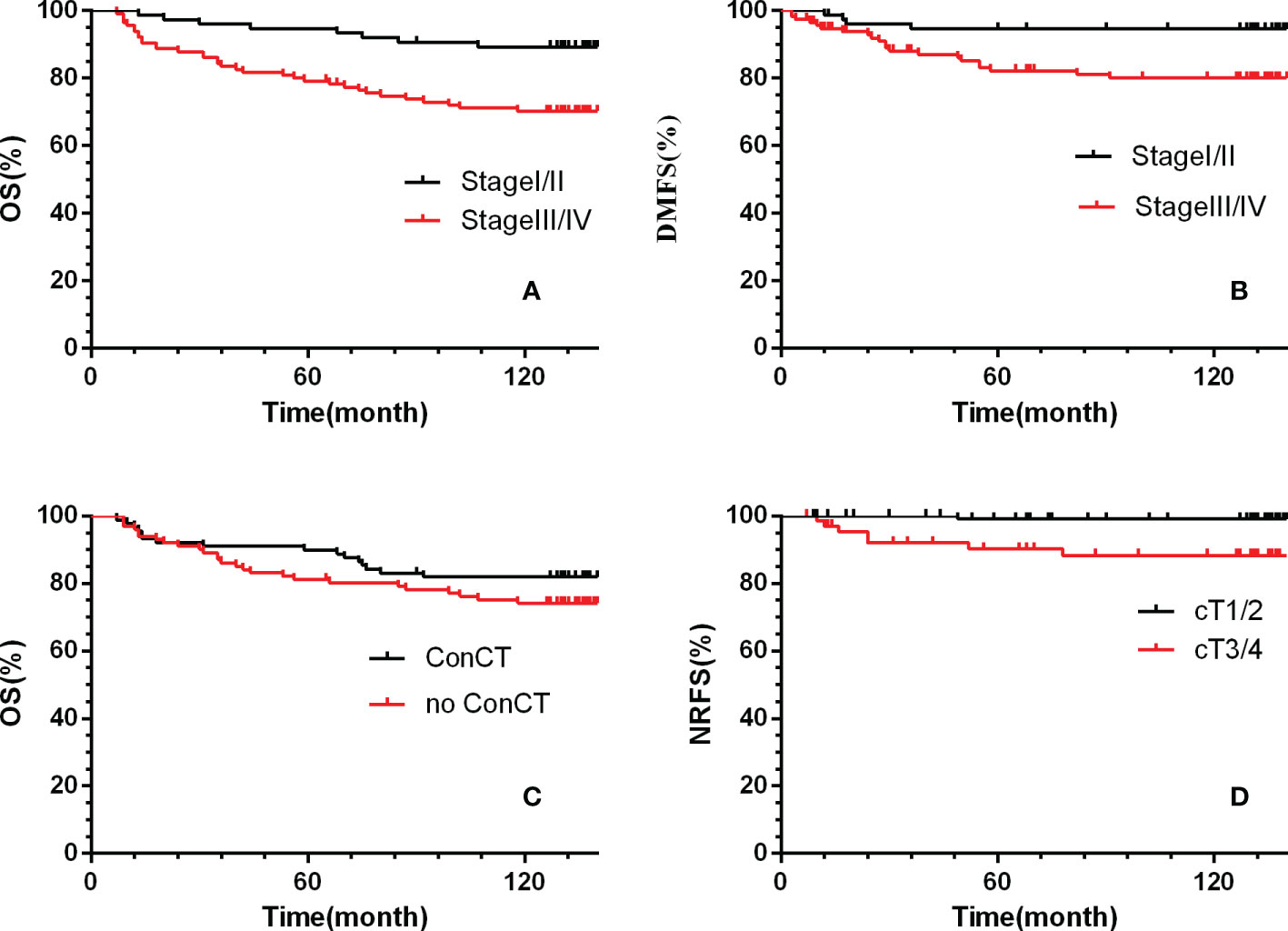
Figure 2 Kaplan-Meier survival curves for the two treatment groups. (A, C) overall survival, (B) distant metastasis-free survival, (D) nodal relapse-free survival. ConCT=concurrent chemotherapy.
Late toxicities
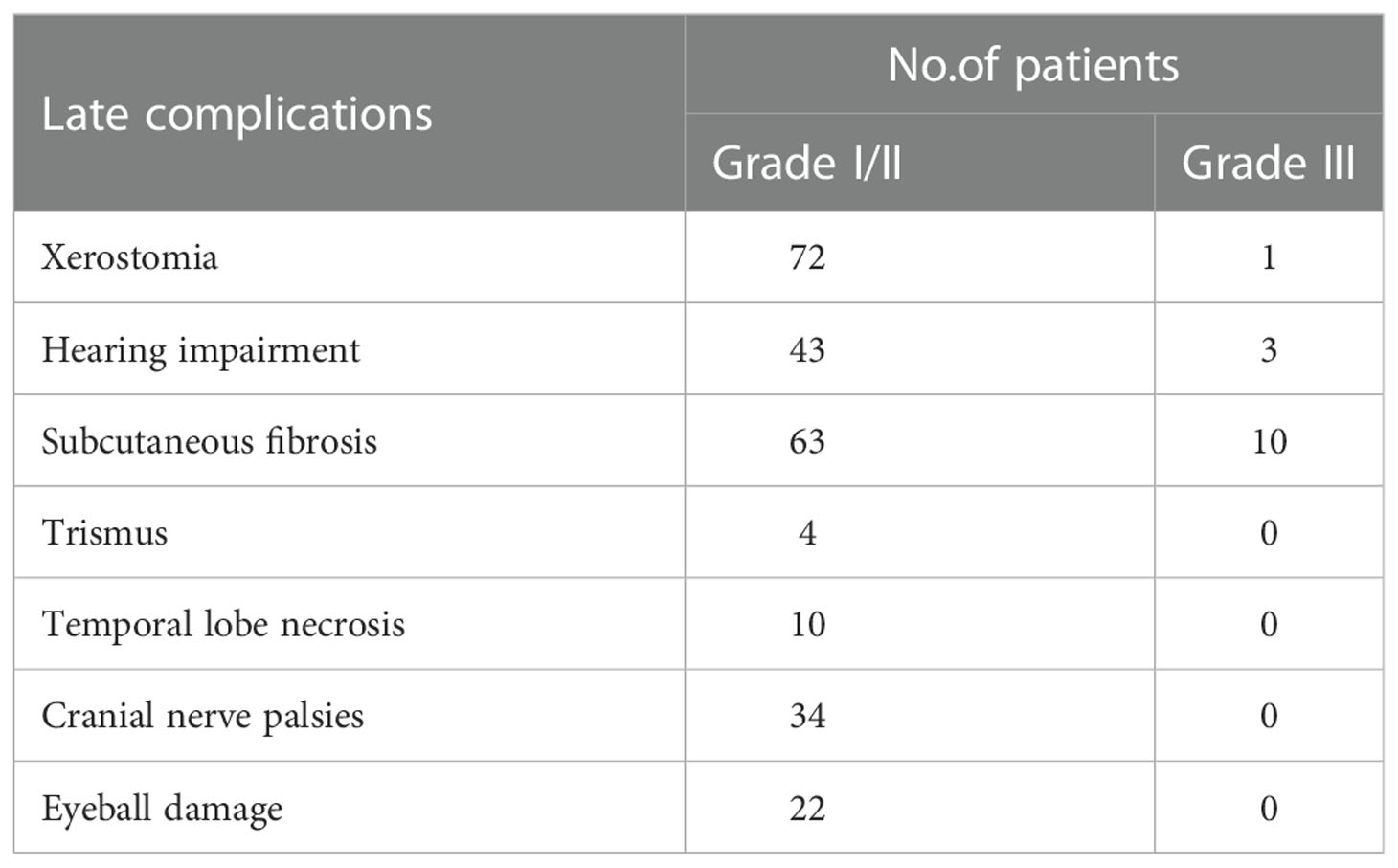
The acute treatment-related toxicities were described previously (8). At the last follow-up visit, 139 patients could be evaluated for the late treatment-related toxicities, with 51 patients lost or died. Grade1~2 xerostomia, subcutaneous tissue fibrosis, hearing loss, and tooth sensitivity were the most common late toxicities. Two patients had loss of tooth, and 2 others had pulpitis and treated with surgical operation. No toxicity and side effects of grade 3 or above trismus, temporal lobe necrosis, cranial nerve palsy, and eyeball injury were found. The data of late treatment-related toxicities were listed in Table 5.
Discussion
Intensity-modulated radiotherapy (IMRT) has been demonstrated to be potentially less toxic and more effective than conventional radiation techniques, even achieved a survival benefit compared with three-dimensional conformal radiotherapy 3DCRT (10). The advent of helical tomotherapy (HT) as image-guided radiotherapy (IGRT) has offered the potential of improved target conformation and sparing of critical structures (11). HT is particularly suitable for NPC due to the irregular target volume and proximity of critical structures (12). In the previous study, we observed satisfactory short-term efficacy, with 3-year loco-regional control rate and DMFS of more than 95%, and 3-year OS was more than 85% in 190 NPC patients treated with HT (13), this study with long-term follow-up showed that 10-year local and regional control remained at about 95% at 10 years, while DMFS reduced to 86%, and OS to 77.8%.
In a phase III trial including 408 patients with stage III/IV NPC, were treated with conventional 2-dimensional radiotherapy, the 10-year DFS, LRFS, and OS were 66.9%, 80.8% and 49.5%, respectively (8). Han et al. reported that the 10-year LRFS, DFS and disease-specific survival (DSS) for NPC patients with stage II-III, treated with IMRT technique, were 92%, 83.4% and 78.6% respectively (14). In the present study, the 10-year LRFS, NRFS, DMFS and OS were 94%, 95%, 86%, and 77.8%, respectively, which were significantly higher than those reported in the era of conventional RT. The reason of our better results may be related to the inclusion of stage I-II patients, with stage I accounting for 8%. In our study, the 10-year DMFS and OS of stage III-IV patients were 80% and 76%; respectively. The 10-year LRFS for patients with stage I-II was better than those with stage III-IV (98% vs. 92%, p= 0.04). In a phase III trial, 230 NPC patients with stage II achieved excellent results, with the 10-year DFS, PFS and OS of 94%, 76.7% and 83.6%; respectively (15). HT provides a technical platform to increase the dose in tumor target volume, and represents better local control (16). Belgioia et al. reported that 2 and 4-year loco-regional control rates were 92.9% and 88.2%, respectively, and 4-year OS was 93.9% in advanced NPC patients treated by HT with simultaneous integrated boost (SIB) technique, and with a dose of 66Gy/30F to the primary tumor (17).
In our research, although the 10-year NRFS and OS in patients with stage T3-4 were worse than those with stage T1-2 (p< 0.05), No statistical difference was detected in multivariate analysis (p>0.05). The 10-year LRFS and OS in patients with negative node were better than those with metastatic nodes, although without statistical significance (p>0.05). The 10-year NRFS and DMFS in patients with stage N2-3 were worse than those with stage N0-1 (p< 0.05), but no statistical difference was detected in multivariate analysis (p>0.05). A review showed, in 610 NPC patients with stage N0 undergoing definitive radiotherapy to their primary lesion and prophylactic radiation to upper neck, the 5-year and 10-year regional failure-free survival could be 95.8% and 91.8%; respectively (17). Han et al. demonstrated that omitting bilateral or contralateral lower neck radiotherapy would be safe and feasible for NPC patients with stage N0-1, with the potential to reduce late toxicities, is a direction of reducing toxicity and increasing efficiency (18).
Distant metastasis has become the most common mode of treatment failure. According to previous literature reports (14), the 10-year distant metastasis rate was 16.6% (144/865). In our study, there were 25 cases of distant metastases, and the rate of distant metastases was 13.2%, which was consistent with previous research results and even lower. An advanced N stage has been shown to be one of the risk factors to predicting the occurrence of metastasis (19). This raises the question of how to select patients at high risk of distant metastases who would benefit from individual therapy to improve their OS. The results of the this study indicated that the 10-year DMFS for patients with negative node were better than those with positive node. The factor of the clinical stage was also associated with the risk of distant metastasis. Patients with a more advanced stage, especially those with T4N+ disease with intracranial or neural invasion, have a higher risk of lymph node metastasis and distant metastasis. T and N stage factors were associated with distant metastasis, although without statistical significance (p>0.05) in multivariate analysis. This may mean that the difference between the treatment and the patient itself has become a confounding factor in the analysis.
In this study, 12, 8 and 25 patients had developed local failure, lymph nodal failure and distant metastasis; respectively. Lung, bone and live were the most common metastatic organs. In addition, Five patients (stage T3-4) died of primary pharyngeal vascular hemorrhage within one year after radiotherapy. Six patients still died of massive nasal and laryngopharyngeal hemorrhage in long-term follow-up after radiotherapy, and one patients died of cerebral hernia 9 months after the completion of radiotherapy, meaning that pharyngeal vessel bleeding (primary or secondary) was one of the leading cause of death. On the premise of ensuring the effect of tumor control, the radiation dose protection, nutrition, flushing and closer follow-up may reduce the occurrence of such events.
At present, radical radiation therapy alone is recommended to stage I NPC, additional chemotherapy with neoadjuvant, concomitant or adjuvant forms is recommended to loco-regionally advanced NPC (14). Meta-analyses showed that concomitant chemo-radiotherapy improves survival in patients with loco-regionally advanced NPC, but the specific benefits of adjuvant chemotherapy after concomitant chemo-radiotherapy needs further study (20). Blanchard et al. analyzed data from 19 trials and 4806 patients and found that chemotherapy improved absolute overall survival by 6.3% over 5 years, and also improved progression-free survival, loco-regional control, distant control and reduced cancer mortality significantly (p <0.0001). The benefit of concomitant with or without adjuvant chemotherapy to radiation therapy was significantly (p=0.01), but not adjuvant chemotherapy alone or neoadjuvant chemotherapy alone. In this study, the 10-year OS in patients with concomitant chemotherapy group was better than that in the non-chemotherapy group in multivariate analysis (p<0.05).
n recent years, anti-EGFR Mabs such as cetuximab and nimotuzumab were applied as a concomitant therapy with radiation therapy for head and neck cancer (21). Bonner et al. (22)reported significant improvement in survival in patients with non-NPC head and neck cancer when cetuximab was added to radiation therapy in a phase III trial. In the meta-analysis of Yuan et al. (23), anti-EGFR Mab combined with radiation therapy and/or chemotherapy improved the short-term therapeutic effect in NPC, but this benefit disappeared 1 year later. In this study, 81 patients (42.6%) had anti-EGFR Mab with HT and 30 (15.8%) with HT and chemotherapy, Unfortunately, only benefit in DMFS was detected, and without statistical significance (p>0.05). So the effect of anti-EGFR Mab combined with chemo-radiotherapy in NPC needs further investigations.
Huang et al. (24) reported that age and N stage were independent prognostic factors for OS, radiation dose was an independent prognostic factor for loco-regional control, and N stage was an independent prognostic factor for distant metastasis. In our update, age, concurrent chemotherapy and stage were independent prognostic factors for OS, which was consistent with the report.
Since the era of IMRT, the incidence of late grade 3 to 4 toxicities of xerostomia and neck fibrosis after radiotherapy was reduced to 16.7% and 8.3% (24). HT is a unique IMRT modality that can provide better conformity index, steeper dose gradient, shorter treatment time, and better protection of many organs at risk (OARs) especially for parotids (25).The acute treatment-related toxicities of HT in NPC treatment, which were relatively mild, In addition, the incidence of acute grade- 2 xerostomia was only 7.3% were described in our previous report (8). Belgioia et al. reported that the most significant acute toxicities were grade 2 or 3 mucositis (43%); grade 2 xerostomia was reported in 11 patients after 6 months from the end of treatment and downgrade to level 1 in 55% (6/11) patients within 12 months (6). At the last follow-up visit in our study, 139 out of 190 patients could be evaluated for the late treatment-related toxicities, xerostomia, subcutaneous tissue fibrosis, hearing loss, and tooth sensitivity were the most common late toxicities which were mainly scored as grade1 or 2. Two patients had tooth looseness, and 2 others pulpitis which needed surgical operation. It is worth noting that although the response to radiotherapy and chemotherapy is not significant, the incidence of massive bleeding complications of nasopharyngeal carcinoma in the follow-up study was 5.8%. Five cases occurred within one year after radiotherapy, and six cases occurred in long-term follow-up after radiotherapy. It suggests that the risk of major bleeding is still high for patients with late T stage and neck metastatic lymph node fusion surrounding blood vessels. During follow-up, attention should be paid to nasal cavity flushing and prevention.
There are several limitations of this study. First, the retrospective study affect the outcomes; second, patient distributions in our cohort were complex, I-IV, T1-4, N0-3 stages, which could affect the outcomes with confounding factors; third, synchronous and/or adjuvant chemotherapy, the nonuniformity pattern of combined chemotherapy could affect the outcomes of the study.
Conclusions
Helical tomotherapy with simultaneous integrated boost technique offered excellent long-term outcomes for NPC patients, with mild late treatment-related toxicities. Age and clinical stage was independent predictor of DMFS and OS. Further prospective study is needed to confirm the superiority of this technology and to evaluate the roles of anti-EGFR monoclonal antibody treatment.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
This study was conducted with approval from the Ethics Committee of the Chinese PLA General Hospital. Written informed consent was obtained from all participants.
Author contributions
LMe, FT, and QL made equal contributions to this work, participated in the design of the research, carried out research, made statistical analysis and drafted the manuscript. LMa conceived and designed the study and reviewed the manuscript. All authors read and approved the final manuscript.
Acknowledgments
The authors thank the staff of this study and all patients associated with this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Liu Z, Xu C, Jiang R, Liu G, Liu Q, Zhou J, et al. Treatment of locally advanced nasopharyngeal carcinoma by helical tomotherapy: An observational, prospective analysis. Transl Oncol (2019) 12(5):757–63. doi: 10.1016/j.tranon.2019.02.011
2. Chen JL, Huang YS, Kuo SH, Hong RL, Ko JY, Lou PJ, et al. Intensity-modulated radiation therapy achieves better local control compared to three-dimensional conformal radiation therapy for T4-stage nasopharyngeal carcinoma. Oncotarget (2017) 8(8):14068–77. doi: 10.18632/oncotarget.12736
3. Blanchard P, Lee A, Marguet S, Leclercq J, Ng WT, Ma J, et al. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC meta-analysis. Lancet Oncol (2015) 16(6):645–55. doi: 10.1016/S1470-2045(15)70126-9
4. Liang R, Yang L, Zhu X. Nimotuzumab, an anti-EGFR monoclonal antibody, in the treatment of nasopharyngeal carcinoma. Cancer Control (2021) 28:1073274821989301. doi: 10.1177/1073274821989301
5. Van Gestel D, Van den Weyngaert D, De Kerf G, De Ost B, Vanderveken O, Van Laer C, et al. Helical tomotherapy in head and neck cancer: a European single-center experience. Oncologist (2015) 20(3):279–90. doi: 10.1634/theoncologist.2014-0337
6. Lee FK, Yip CW, Cheung FC, Leung AK, Chau RM, Ngan RK. Dosimetric difference amongst 3 techniques: TomoTherapy, sliding-window intensity-modulated radiotherapy (IMRT), and RapidArc radiotherapy in the treatment of late-stage nasopharyngeal carcinoma (NPC). Med Dosim (2014) 39(1):44–9. doi: 10.1016/j.meddos.2013.09.004
7. Thariat J, Bolle S, Demizu Y, Marcy PY, Hu Y, Santini J, et al. New techniques in radiation therapy for head and neck cancer: IMRT, CyberKnife, protons, and carbon ions. improved effectiveness and safety? impact on survival? Anticancer Drugs (2011) 22(7):596–606. doi: 10.1097/CAD.0b013e328340fd2b
8. Du L, Zhang XX, Ma L, Feng LC, Li F, Zhou GX, et al. Clinical study of nasopharyngeal carcinoma treated by helical tomotherapy in China: 5-year outcomes. BioMed Res Int (2014) 2014:980767. doi: 10.1155/2014/980767
9. Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol (2003) 13(3):176–81. doi: 10.1016/s1053-4296(03)00031-6
10. Peng G, Wang T, Yang KY, Zhang S, Zhang T, Li Q, et al. A prospective, randomized study comparing outcomes and toxicities of intensity-modulated radiotherapy vs. conventional two-dimensional radiotherapy for the treatment of nasopharyngeal carcinoma. Radiother Oncol (2012) 104(3):286–93. doi: 10.1016/j.radonc.2012.08.013
11. Puebla F, Lopez Guerra JL, Garcia Ramirez JM, Matute R, Marrone I, Miguez C, et al. Effectiveness and toxicity of helical tomotherapy for patients with locally recurrent nasopharyngeal carcinoma. Clin Transl Oncol (2015) 17(11):925–31. doi: 10.1007/s12094-015-1328-5
12. Jeong S, Yoo EJ, Kim JY, Han CW, Kim KJ, Kay CS. Re-irradiation of unresectable recurrent head and neck cancer: using helical tomotherapy as image-guided intensity-modulated radiotherapy. Radiat Oncol J (2013) 31(4):206–15. doi: 10.3857/roj.2013.31.4.206
13. Huang PY, Zeng Q, Cao KJ, Guo X, Guo L, Mo HY, et al. Ten-year outcomes of a randomised trial for locoregionally advanced nasopharyngeal carcinoma: A single-institution experience from an endemic area. Eur J Cancer (2015) 51(13):1760–70. doi: 10.1016/j.ejca.2015.05.025
14. Tian Y-M, Liu M-Z, Zeng L, Bai L, Lin C-G, Huang S-M, et al. Long-term outcome and pattern of failure for patients with nasopharyngeal carcinoma treated with intensity-modulated radiotherapy. Head neck. (2019) 41(5):1246–52. doi: 10.1002/hed.25545
15. Li XY, Chen QY, Sun XS, Liu SL, Yan JJ, Guo SS, et al. Ten-year outcomes of survival and toxicity for a phase III randomised trial of concurrent chemoradiotherapy versus radiotherapy alone in stage II nasopharyngeal carcinoma. Eur J Cancer (2019) 110:24–31. doi: 10.1016/j.ejca.2018.10.020
16. Belgioia L, Bacigalupo A, Vecchio S, Marcenaro M, Pupillo F, Agostinelli S, et al. Excellent survival regardless of disease stage in patients with advanced nasopharyngeal cancer. Tumori (2016) 102(4):381–6. doi: 10.5301/tj.5000483
17. Sun J-D, Chen C-Z, Chen J-Z, Li D-S, Chen Z-J, Zhou M-Z, et al. Long term outcomes and prognostic factors of n0 stage nasopharyngeal carcinoma: a single institutional experience with 610 patients. Asian Pacific J Cancer Prev APJCP. (2012) 13(5):2101–7. doi: 10.7314/APJCP.2012.13.5.2101
18. Sun Z, Wang J, Huang R, Wang X, Chen C, Deng M, et al. Contralateral lower neck sparing radiotherapy in stage N1 nasopharyngeal carcinoma: Long-term survival outcomes and late toxicities. Front Oncol (2021) 11:628919. doi: 10.3389/fonc.2021.628919
19. Jiang C, Gao H, Zhang L, Li H, Zhang T, Ma J, et al. Distribution pattern and prognosis of metastatic lymph nodes in cervical posterior to level V in nasopharyngeal carcinoma patients. BMC Cancer. (2020) 20(1):667. doi: 10.1186/s12885-020-07146-z
20. Baujat B, Audry H, Bourhis J, Chan AT, Onat H, Chua DT, et al. Chemotherapy in locally advanced nasopharyngeal carcinoma: an individual patient data meta-analysis of eight randomized trials and 1753 patients. Int J Radiat Oncol Biol Phys (2006) 64(1):47–56. doi: 10.1016/j.ijrobp.2005.06.037
21. Hsu HW, Wall NR, Hsueh CT, Kim S, Ferris RL, Chen CS, et al. Combination antiangiogenic therapy and radiation in head and neck cancers. Oral Oncol (2014) 50(1):19–26. doi: 10.1016/j.oraloncology.2013.10.003
22. Bonner JA, Harari PM, Giralt J, Cohen RB, Jones CU, Sur RK, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol (2010) 11(1):21–8. doi: 10.1016/S1470-2045(09)70311-0
23. Yuan C, Xu XH, Chen Z. Combination treatment with antiEGFR monoclonal antibodies in advanced nasopharyngeal carcinoma : a meta-analysis. J Buon (2015) 20(6):1510–7.
24. Chen B-B, Lu S-Y, Peng H, Sun F-F, Zhu J, Wang J, et al. Comparison of long-term outcomes and sequelae between children and adult nasopharyngeal carcinoma treated with intensity modulated radiation therapy. Int J Radiat Oncol Biol Physics (2020) 106(4):848–56. doi: 10.1016/j.ijrobp.2019.11.035
Keywords: nasopharyngeal carcinoma, helical tomotherapy (HT), survival, chemotherapy, radiotherapy
Citation: Meng L, Teng F, Liu Q, Du L, Cai B, Xie C, Gong H, Zhang X and Ma L (2023) Long-term outcomes of nasopharyngeal carcinoma treated with helical tomotherapy using simultaneous integrated boost technique: A 10-year result. Front. Oncol. 12:1083440. doi: 10.3389/fonc.2022.1083440
Received: 29 October 2022; Accepted: 28 December 2022;
Published: 20 January 2023.
Edited by:
Lorenz Kadletz-Wanke, Medical University of Vienna, AustriaReviewed by:
Jia-Ming Wu, Wuwei Cancer Hospital of Gansu Province, ChinaHans Christiansen, Hannover Medical School, Germany
Copyright © 2023 Meng, Teng, Liu, Du, Cai, Xie, Gong, Zhang and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lin Ma, bWFsaW5waGFybUBzaW5hLmNvbQ==
†These authors have contributed equally to this work
 Lingling Meng
Lingling Meng Feng Teng
Feng Teng Qiteng Liu
Qiteng Liu Lei Du5
Lei Du5 Boning Cai
Boning Cai Hanshun Gong
Hanshun Gong Xinxin Zhang
Xinxin Zhang Lin Ma
Lin Ma