- 1Clinical Laboratory, Huzhou Central Hospital, Affiliated Central Hospital of Huzhou University, Huzhou, Zhejiang, China
- 2Department of Urology, Huzhou Central Hospital, Affiliated Central Hospital of Huzhou University, Huzhou, Zhejiang, China
Background: Despite previous research examining the predictive value of the geriatric nutritional risk index (GNRI) in individuals with urological cancers (UCs), results have been conflicting. This study aimed to comprehensively explore the potential link between GNRI and the prognosis of UCs using a meta-analysis.
Methods: The Cochrane Library, PubMed, Embase, and Web of Science databases were systematically and exhaustively searched. We estimated the prognostic importance of the GNRI in patients with UCs by calculating the pooled hazard ratios (HRs) and 95% confidence intervals (CIs) on survival outcomes. Publication bias was identified using Egger’s test and Begg’s funnel plot.
Results: Eight trials with 6,792 patients were included in our meta-analysis. Patients with UCs who had a lower GNRI before treatment had a higher risk of experiencing worse overall survival (HR = 2.62, 95% CI = 1.69–4.09, p < 0.001), recurrence-free survival/progression-free survival (HR = 1.77, 95% CI = 1.51–2.08, p < 0.001), and cancer-specific survival (HR = 2.32, 95% CI = 1.28–4.20, p = 0.006). Moreover, the subgroup analysis did not change the predictive significance of the GNRI in individuals with UCs. Neither Egger’s nor Begg’s test indicated substantial bias in this analysis.
Conclusion: As a result of our meta-analysis, we found that a low GNRI strongly predicts poor prognosis for patients with UCs. A lower pretreatment GNRI indicates poor survival outcomes in UCs.
1 Introduction
Urological cancers (UCs), including urothelial carcinoma (UC), renal cell carcinoma (RCC), and prostate cancer (PCa), are the primary causes of public health issues globally (1). UCs account for 380,480 new cases and 46,620 cancer-related deaths in men in the United States by 2022 (2). The incidence and mortality of UCs have been increasing in recent years, and UCs are more prevalent in Western countries than in Eastern regions (3, 4). Personalized medicine plays an important role in the treatment of UCs. The foundation of medical care includes androgen deprivation therapy (ADT) for PCa, tyrosine kinase inhibitors for RCC, and cytotoxic chemotherapy for UC (5). Patients undergoing urological oncology surgeries, such as radical prostatectomy, radical cystectomy, and radical nephroureterectomy, show a particular community at risk of poor prognosis (6). For example, for patients with bladder who underwent radical cystectomy (RC), the overall 3, 5 and 10-year survival after RC was 62%, 52% and 37%, respectively (6). However, finding new prognostic markers for patients with UCs is crucial for the design of therapeutic approaches.
Numerous studies have demonstrated a robust association between malnutrition and poor prognosis in patients with cancer. Nutritional evaluations, such as the prognostic nutritional index (7), controlling nutritional status score (8), and geriatric nutritional risk index (GNRI) (9), are commonly used to evaluate malnutrition in patients (7–9). In 2005, Bouillanne et al. (10) initially suggested the GNRI to evaluate the likelihood of death or disability in medically stable older adult individuals. The ideal weight, current weight, and serum albumin level (10) were used to determine GNRI. GNRI was calculated as GNRI = 14.89 * albumin (mg/dl) + 41.7 * (current/ideal body) weight. Nutritional status in patients with cancer may be evaluated using the GNRI because it is a straightforward method. Previous research has revealed the predictive usefulness of the GNRI in many different forms of cancer, including gastric cancer (11), hepatocellular carcinoma (9), pancreatic cancer (12), and oral squamous cell carcinoma (13). The prognostic factor of GNRI in patients with UC has been the subject of several studies with varying results (14–21). We collated relevant literature and conducted this study to evaluate the correlation between prognosis and GNRI in patients.
2 Materials and methods
2.1 Ethics statement
This meta-analysis did not require the use of an institutional review board or ethical committee. Additionally, the primary data were obtained from previously published research; therefore, there was no direct effect on the participants.
2.2 Study guideline
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines were used to compile the data for this meta-analysis (22).
2.3 Literature search
We systematically and extensively searched the Cochrane Library, Embase, PubMed, and Web of Science databases. Our exhaustive and targeted search methodology consisted of the following steps: (geriatric nutritional risk index OR GNRI) AND (bladder cancer OR renal cell cancer OR prostate cancer OR urothelial cancer OR urological cancer OR urinary cancer). A new search update was implemented on September 10, 2022. Articles written in languages other than English were also excluded. Furthermore, we also analyzed all the cited sources of the reviews and studies to find other papers that were relevant to our topic.
2.4 Inclusion and exclusion criteria
The inclusion criteria were as follows: (i) patients with upper tract urothelial cancer, bladder cancer, PCa, RCC, and UC were pathologically diagnosed; (ii) patients were divided into subgroups based on their GNRI; (iii) a GNRI cut-off value was determined; (iv) the GNRI was calculated as 14.89 × albumin (mg/dl) + 41.7 × (present/ideal body) weight (kg) before treatment; (v) hazard ratios (HRs) and 95% confidence intervals (CIs) were reported or adequate data were provided to compute them; and (vi) recurrence-free survival (RFS), cancer-specific survival (CSS), overall survival (OS), and progression-free survival (PFS) were reported. The following studies were excluded: animal studies, studies that did not provide enough data for analysis, studies that were duplicated and featured the same patients, reviews and conference abstracts, letters and case reports, and comments.
2.5 Data extraction and quality assessment
The literature review was conducted by two scholars working separately (QW and FY). All disagreements were discussed and resolved verbally until agreement was reached. Data from relevant studies included the first author’s name, year of publication, sample size, country, sex, time period, type of cancer, study design, study center (multicenter or single-center), treatment, tumor-node-metastasis (TNM) stage, duration of follow-up, GNRI cut-off value, type of survival analysis, survival outcomes, and HRs and 95% CIs. When both multivariate and univariate HRs and 95% CIs were used, the results of the multivariate analysis (MVA) were employed. In cases where only UVA was available, the HRs and 95% CIs were used instead. Each study included in the list was scored on the Newcastle-Ottawa scale (NOS) (23) to evaluate the research design quality. The final NOS score may range from 0 to 9, with points awarded for comparability (1–2), patient selection (0–4), and outcome (0–3). A high-quality study received a score of ≥ 6.
2.6 Statistical analysis
The predictive significance of the GNRI in patients with UCs was evaluated by calculating the 95% CI and HR for survival outcomes. The I2 statistic and Cochrane Q statistic were used to assess statistical heterogeneity between studies. Owing to the low levels of heterogeneity, indicated by an I2 value below 50% and a Q-test significance level above 0.10, a fixed-effects model (FEM) was used. Without this information, a random-effects model (REM) was utilized. To determine the origin of the observed variation, a subgroup analysis was performed, stratified by several clinicopathological characteristics. Publication bias was determined using Egger’s test and Begg’s funnel plot. The Stata version 12.0 was used for all statistical analysis (Stata Corporation, College Station, TX, USA) was used for all statistical analyses. Statistical significance was set at p < 0.05.
3 Results
3.1 Study selection
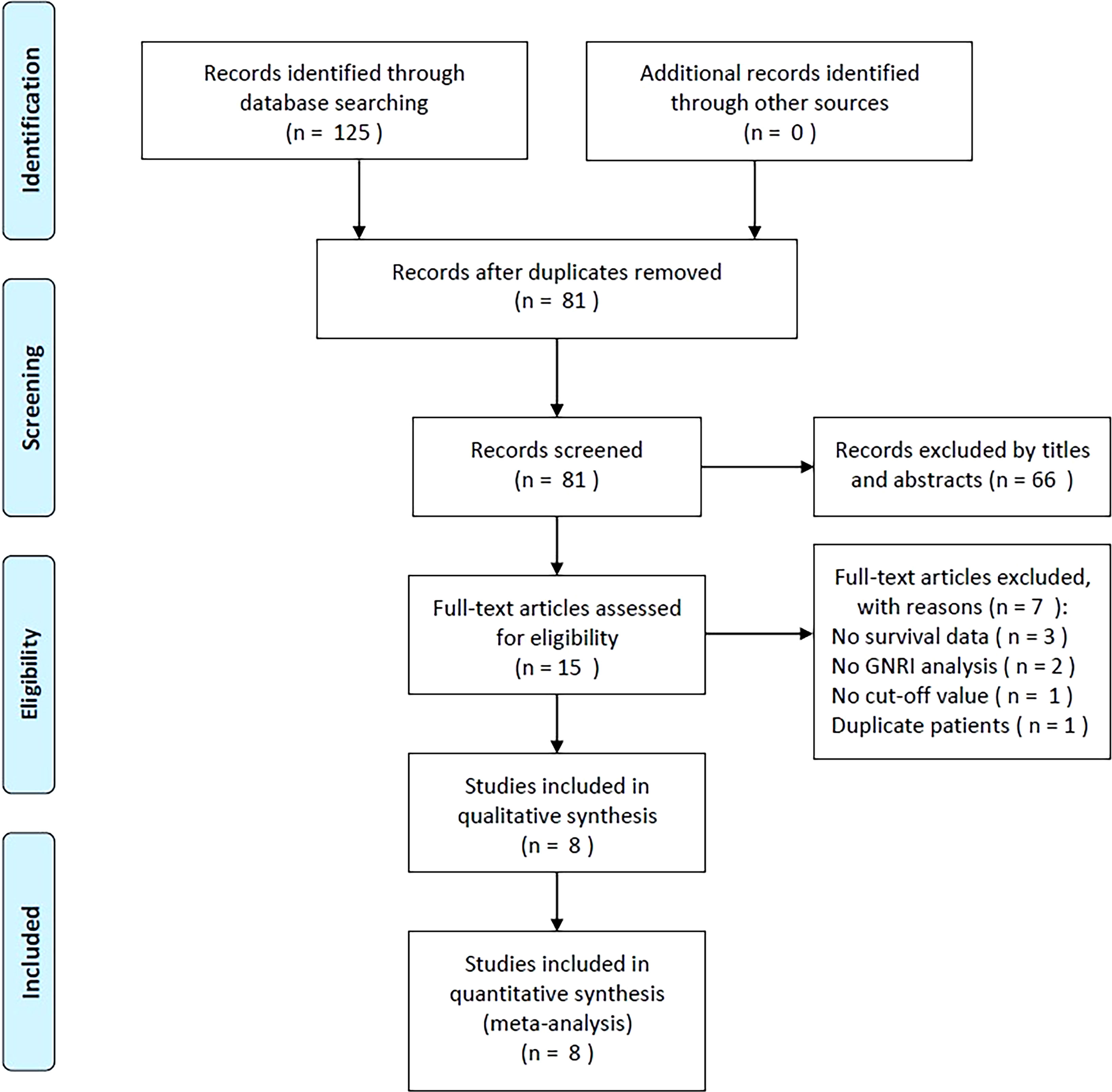
As shown in Figure 1, the initial literature search generated a total of 125 items. After filtering out 44 duplicates, the abstracts and titles of 81 papers were read. Thereafter, 66 papers were discarded, leaving only 15 for the full-text analysis. Seven studies were excluded for the following reasons: (1) they did not provide survival data (n = 3), (2) they did not perform a GNRI analysis (n = 2), (3) they did not determine a GNRI cut-off value (n = 1), and (4) they included patients who had already been studied (n = 1). Eight studies with 6,792 patients (14–21) were included in the final meta-analysis (Figure 1).
3.2 Features of the included research
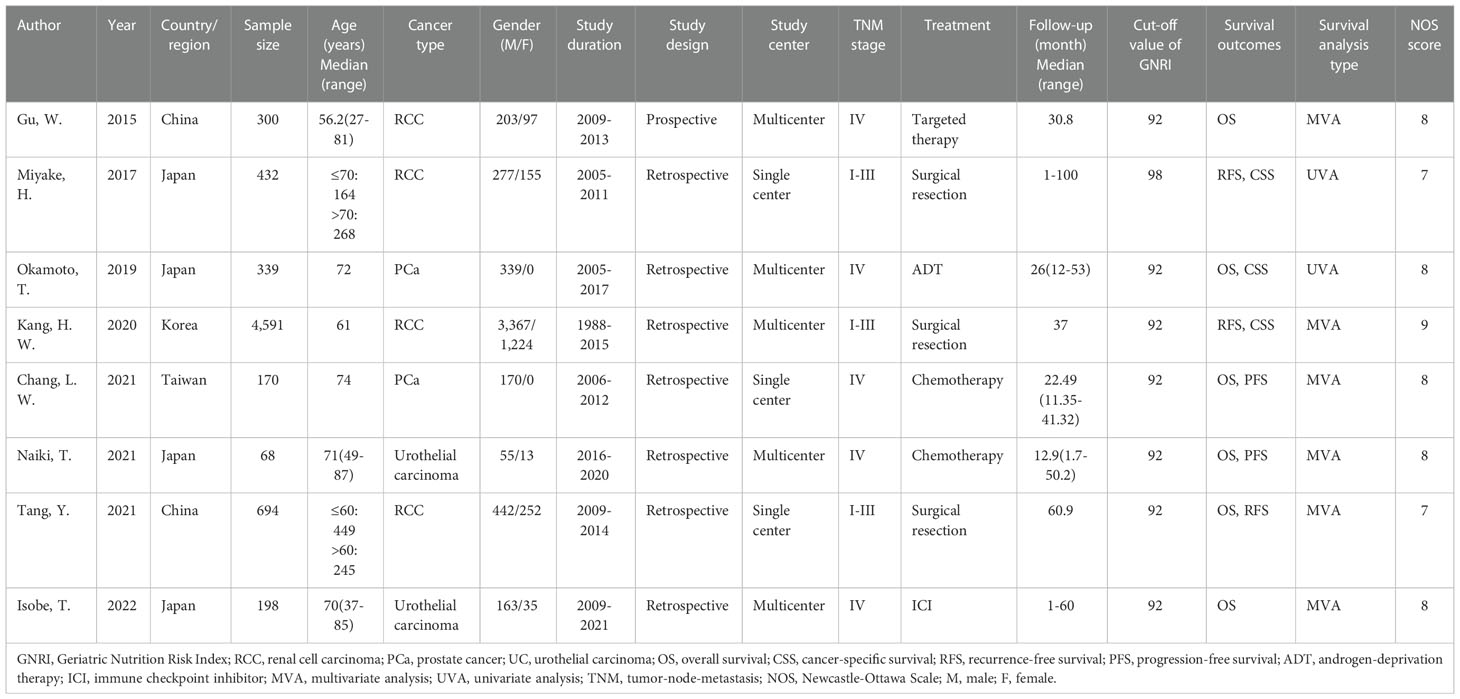
Table 1 shows the typical characteristics of the included studies. The articles considered were published in full-text format in the English language between 2015 and 2022 (14–21). Four studies were performed in Japan (15, 16, 19, 21), two in China (14, 20), and one each in Korea (17) and Taiwan (18). The sample sizes ranged from 68 to 4,591, with a median of 319.5. Four studies recruited patients with RCC (14, 15, 17, 20), two studies enrolled patients with PCa (16, 18), and two studies included patients with UC (19, 21). Seven studies were retrospective studies (15–21) and one was a prospective trial (14). Five studies recruited patients with TNM stage IV (14, 16, 18, 19, 21) and three studies enrolled patients with TNM stages I–III (15, 17, 20). Three studies included patients receiving surgery (15, 17, 20), two studies recruited patients undergoing chemotherapy (18, 19), and one study used ADT (16), immune checkpoint inhibitor (21), and targeted therapy (14). Seven studies adopted 92 as the cut-off value for the GNRI (14, 16–21) and one study adopted 98 (15). The significance of the GNRI as an OS prognostic factor was revealed in six studies (14, 16, 18–21), three studies presented the association between the GNRI and RFS (15, 17, 20), two studies reported the HR and 95%CI for PFS (18, 19), and three studies demonstrated a correlation between the GNRI and CSS (15–17). Six studies described the HRs and 95% CIs from the MVA (14, 17–21), and two studies reported data from the UVA (15, 16). Five studies were multicenter (14, 16, 17, 19, 21) and three were single-center (15, 18, 20). The NOS score of the considered studies varied from 7 to 9, with a median of 8, showing that the methodology of all considered studies was of a high standard.
3.3 GNRI and OS in UCs
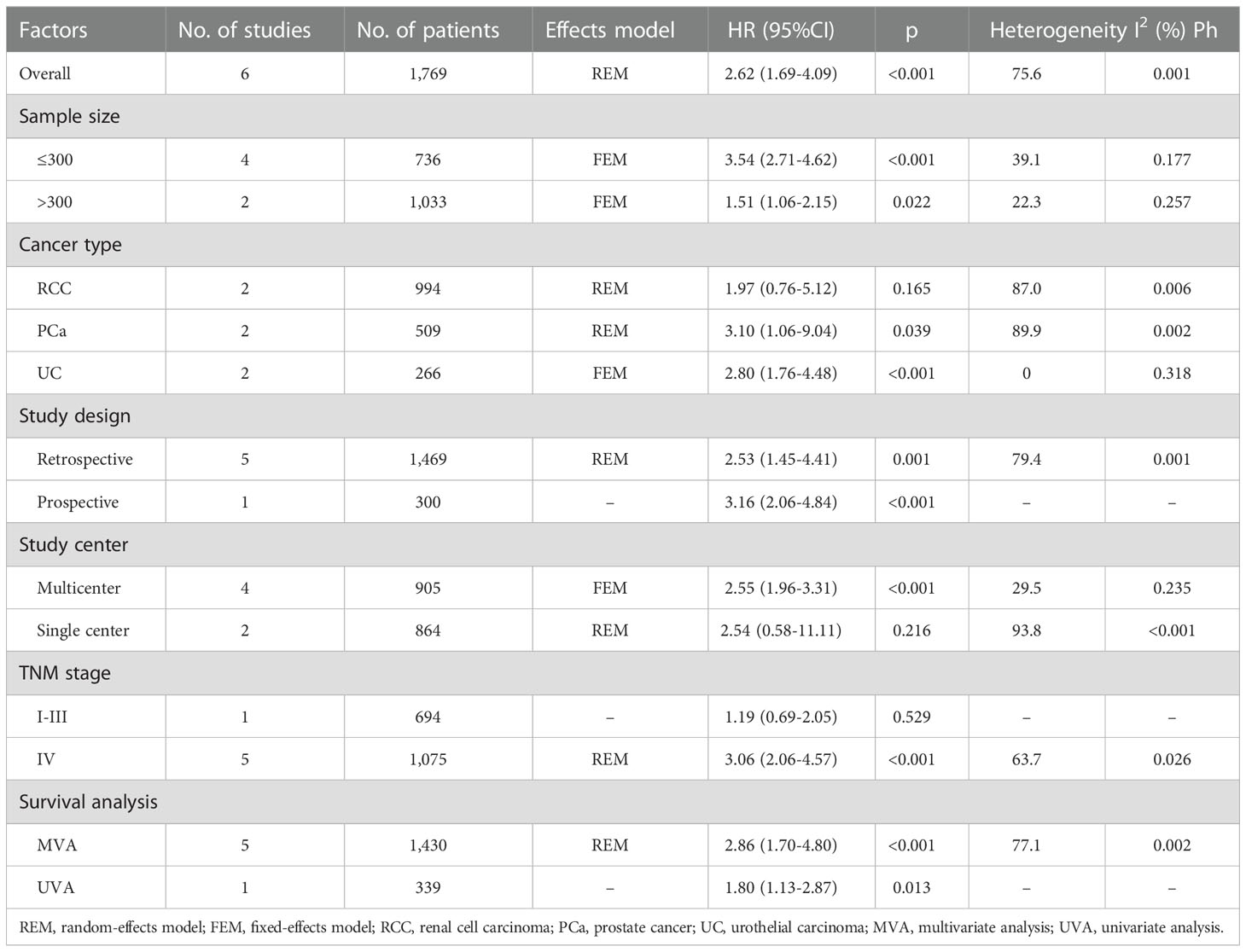
The predictive importance of the GNRI for OS in patients with UCs was revealed in six investigations, including a total of 1,769 participants (14, 16, 18–21). In this case, substantial heterogeneity (I2=75.6%, Ph=0.001) necessitated REM deployment. As shown in Table 2 and Figure 2, the combined results indicated that a low GNRI was significantly associated with poor OS in patients with UCs (HR = 2.62, 95% CI = 1.69–4.09, p < 0.001). The subgroup analysis revealed that regardless of study design, type of survival analysis, or sample size, a low GNRI was a clear indication of worse OS (Table 2). Patients with UC and PCa, but not RCC, had a low GNRI and poor OS (Table 2).
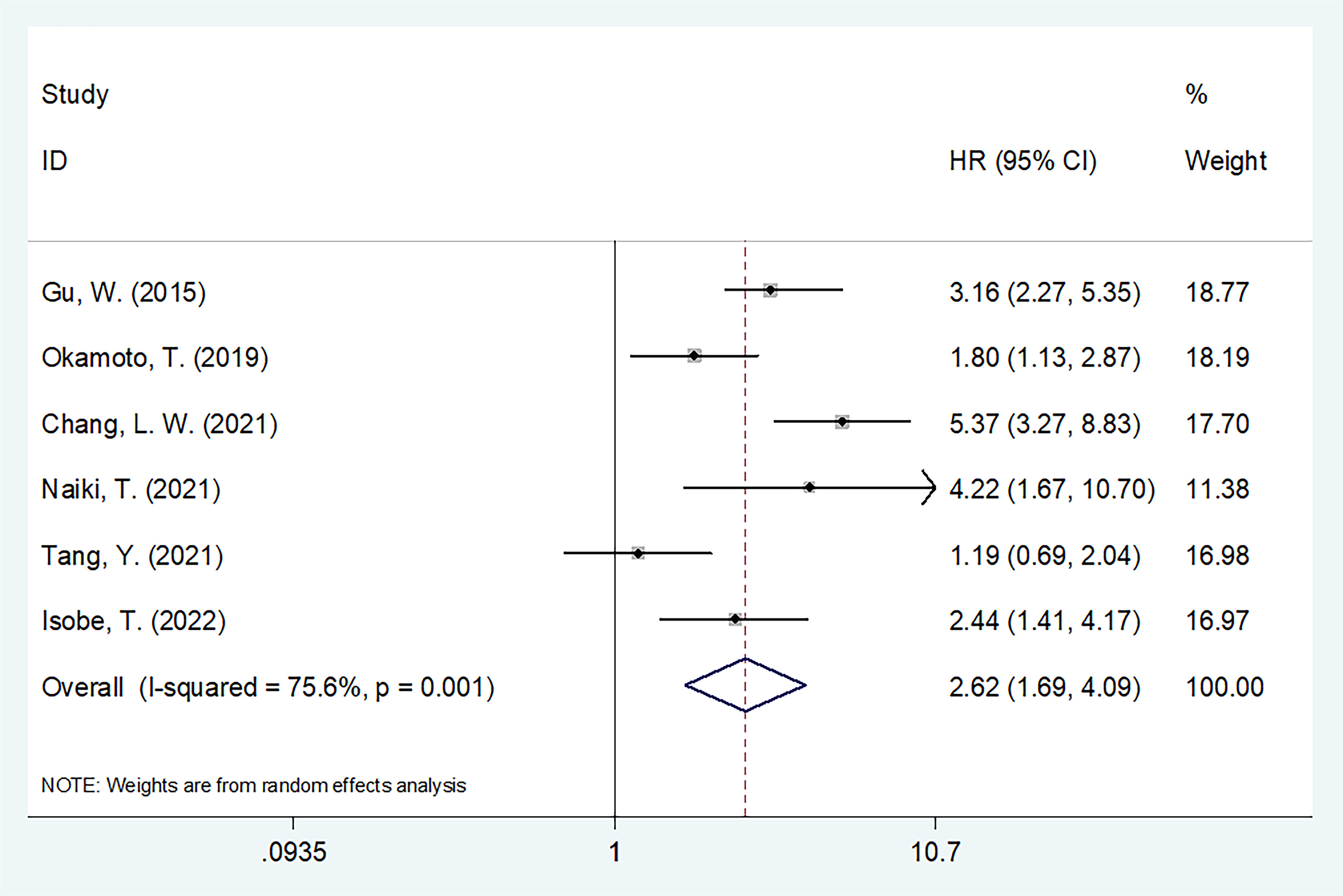
Figure 2 The forest plot of the association of pretreatment GNRI with overall survival (OS) of patients with UCs.
3.4 GNRI and RFS/PFS in UCs
We merged RFS and PFS into the RFS/PFS groups because they were both event-free survival endpoints. Five studies comprising 5,955 patients (15, 17–20) reported the relationship between RFS/PFS and GNRI. The pooled HR and 95% CI were as follows: p < 0.001, HR = 1.77, 95% CI = 1.51–2.08 in the FEM (Figure 3, Table 3), which suggested that patients with UCs with low GNRI had poor RFS/PFS. The prognostic significance of GNRI for RFS/PFS remained significant in various subgroups of sample size, cancer type, study center, TNM stage, and cut-off value, as shown in Table 3 from the subgroup analysis.
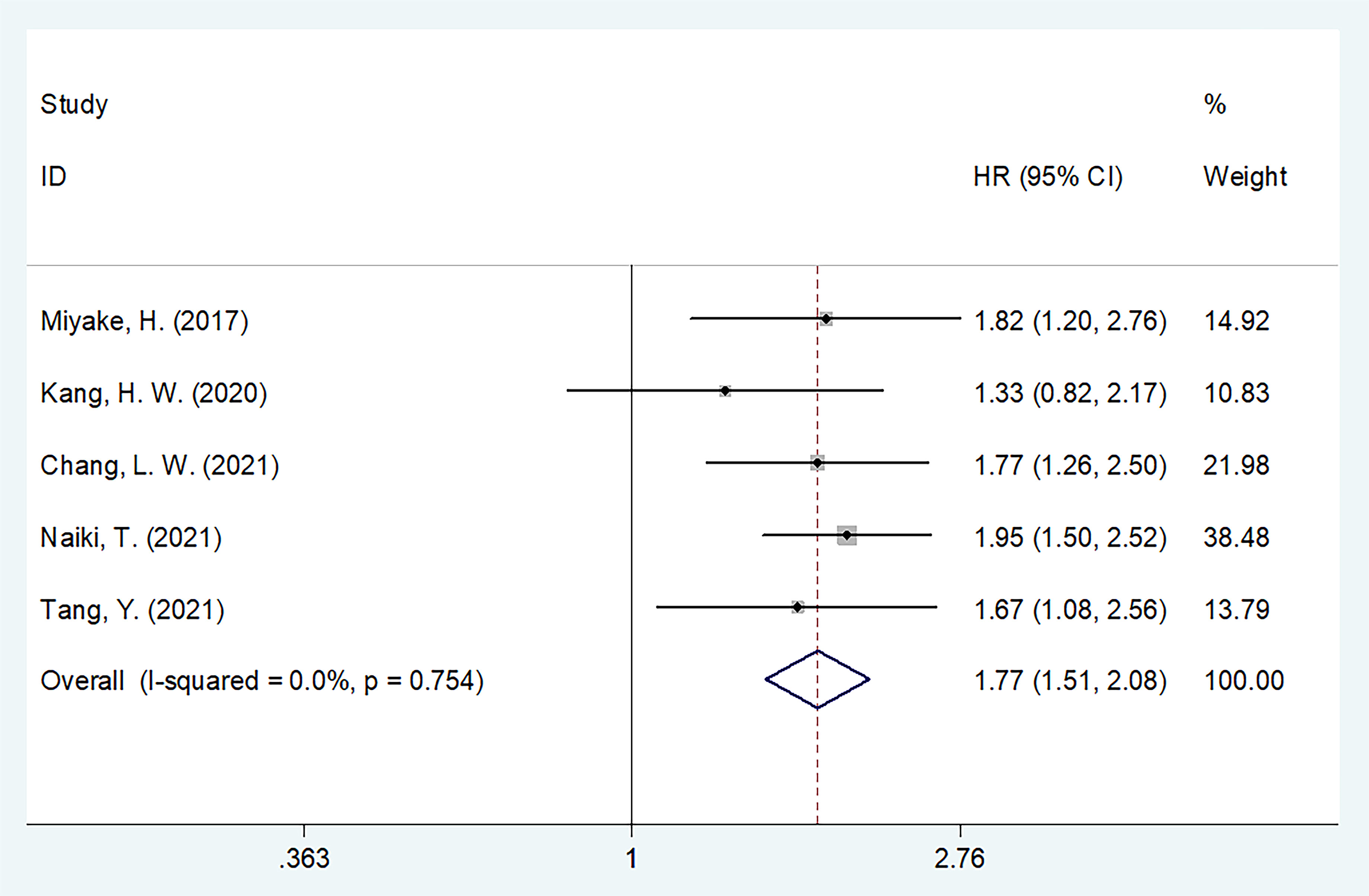
Figure 3 The forest plot of the association of pretreatment GNRI with recurrence-free survival/progression-free survival (RFS/PFS) of patients with UCs.
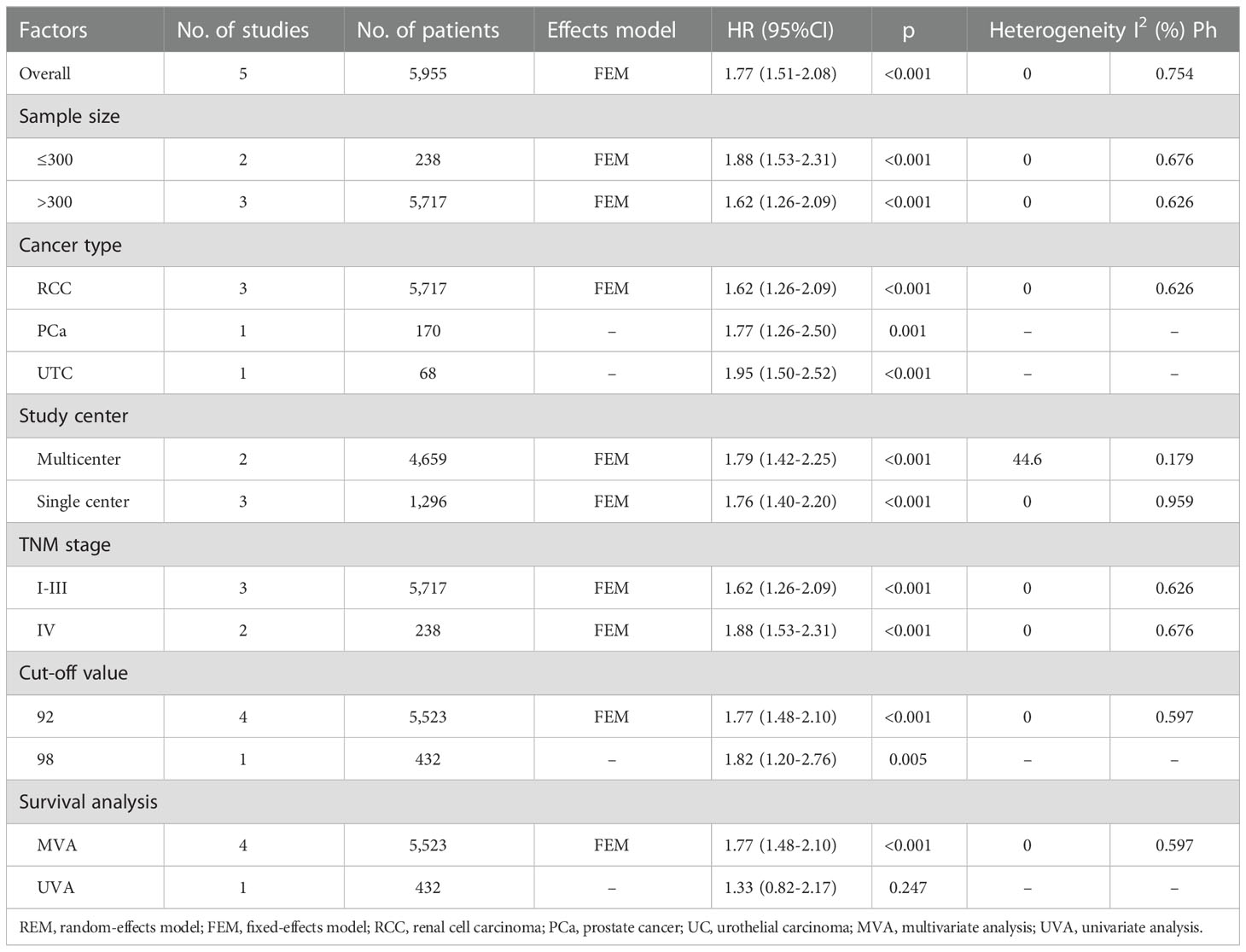
Table 3 Subgroup analysis of the prognostic value of GNRI for RFS/PFS in patients with urologic cancers.
3.5 GNRI and CSS in UCs
Three studies, consisting of 5,362 patients (15–17) described the HRs and 95% CIs for CSS. REM was used, and the combined outcomes were as follows: HR = 2.32, 95% CI = 1.28–4.20, p = 0.006 (Figure 4). As shown in Table 4, subgroup analysis revealed that decreased GNRI was an important prognostic marker for poor CSS, regardless of the study center and cut-off value in patients with UCs.
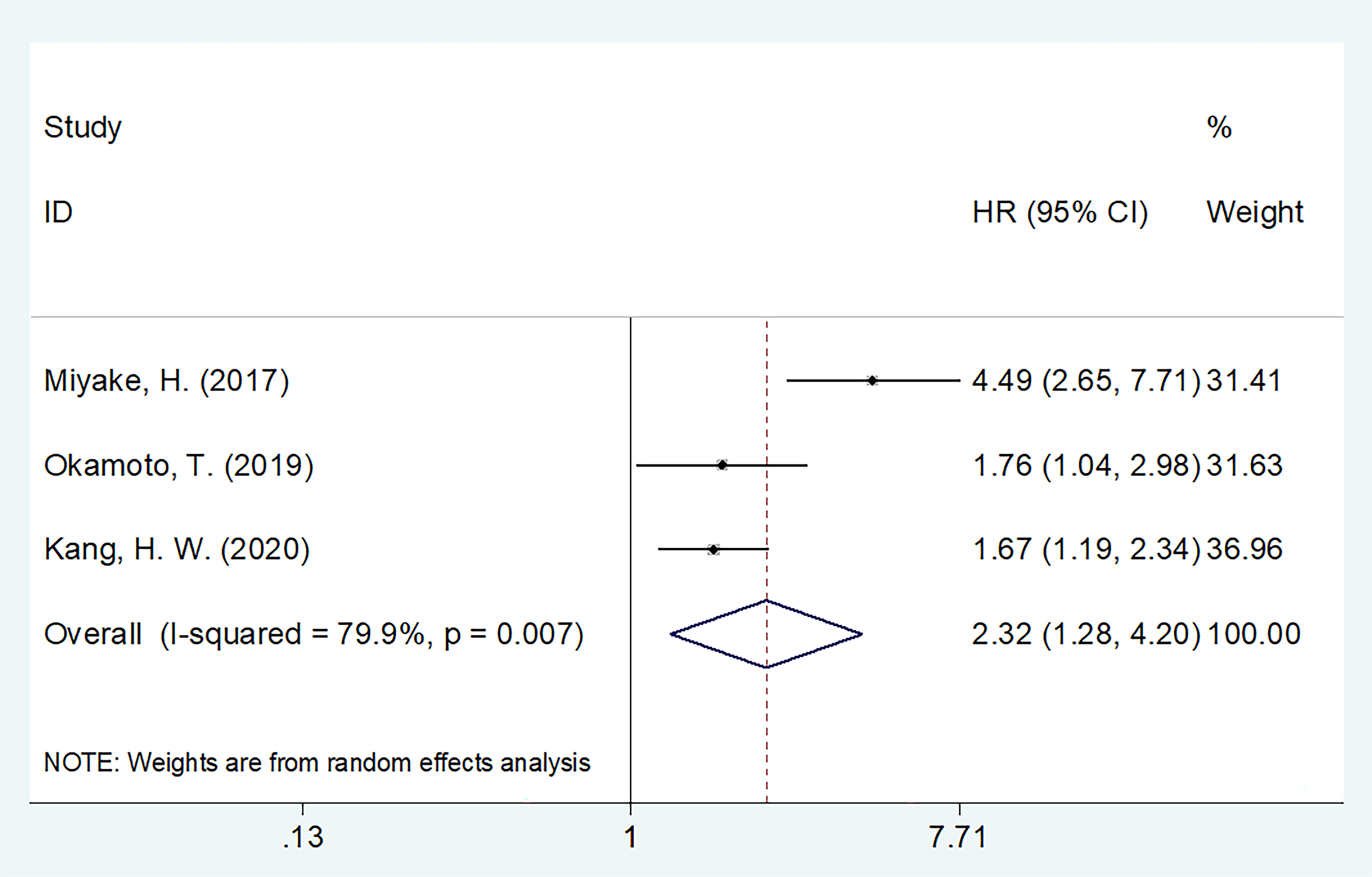
Figure 4 The forest plot of the association of pretreatment GNRI with cancer-specific survival (CSS) of patients with UCs.
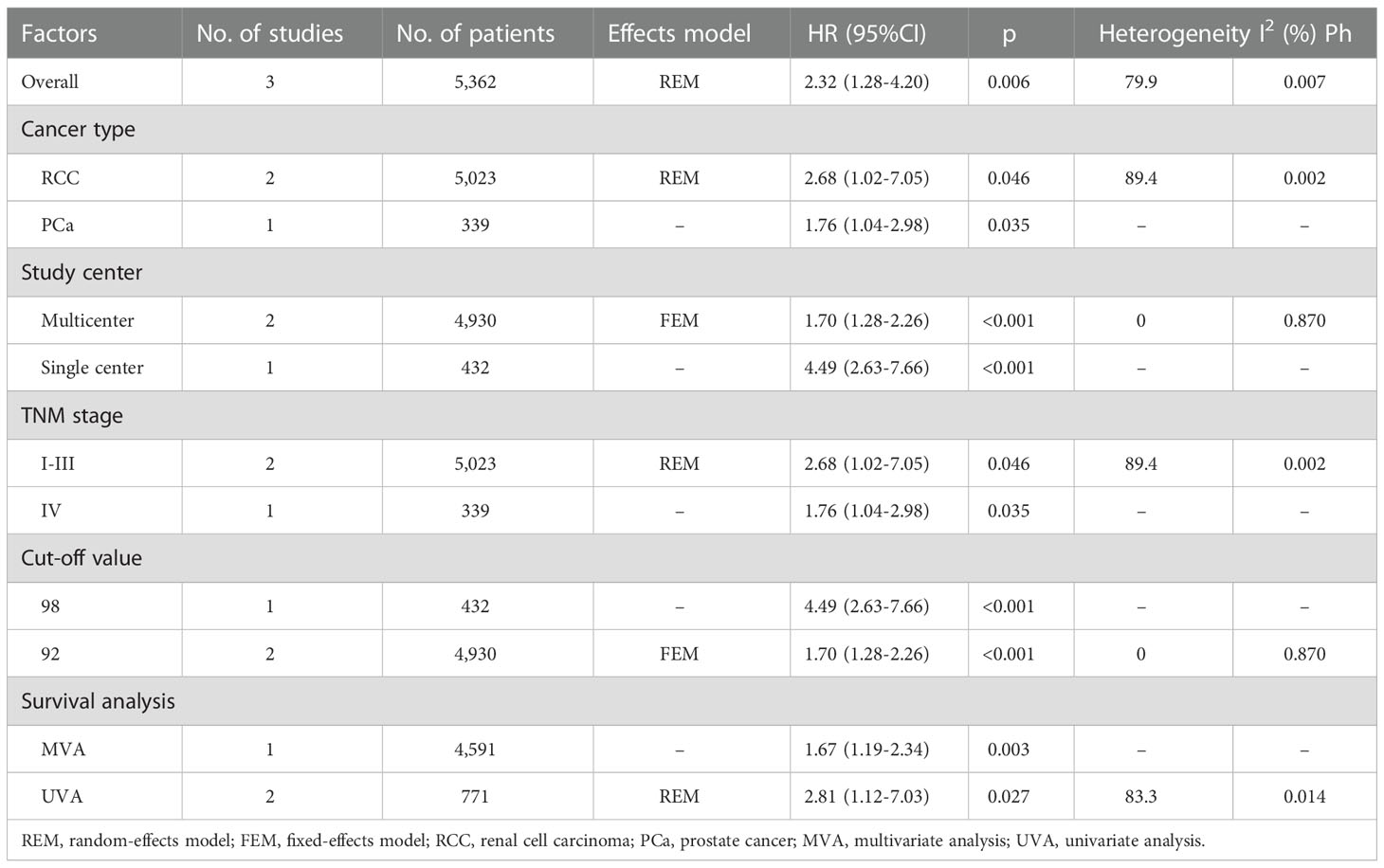
Table 4 Subgroup analysis of the prognostic value of GNRI for CSS in patients with urologic cancers.
3.6 Publication bias
This meta-analysis did not exhibit any significant publication bias according to Egger’s test and Begg’s test (Figure 5).
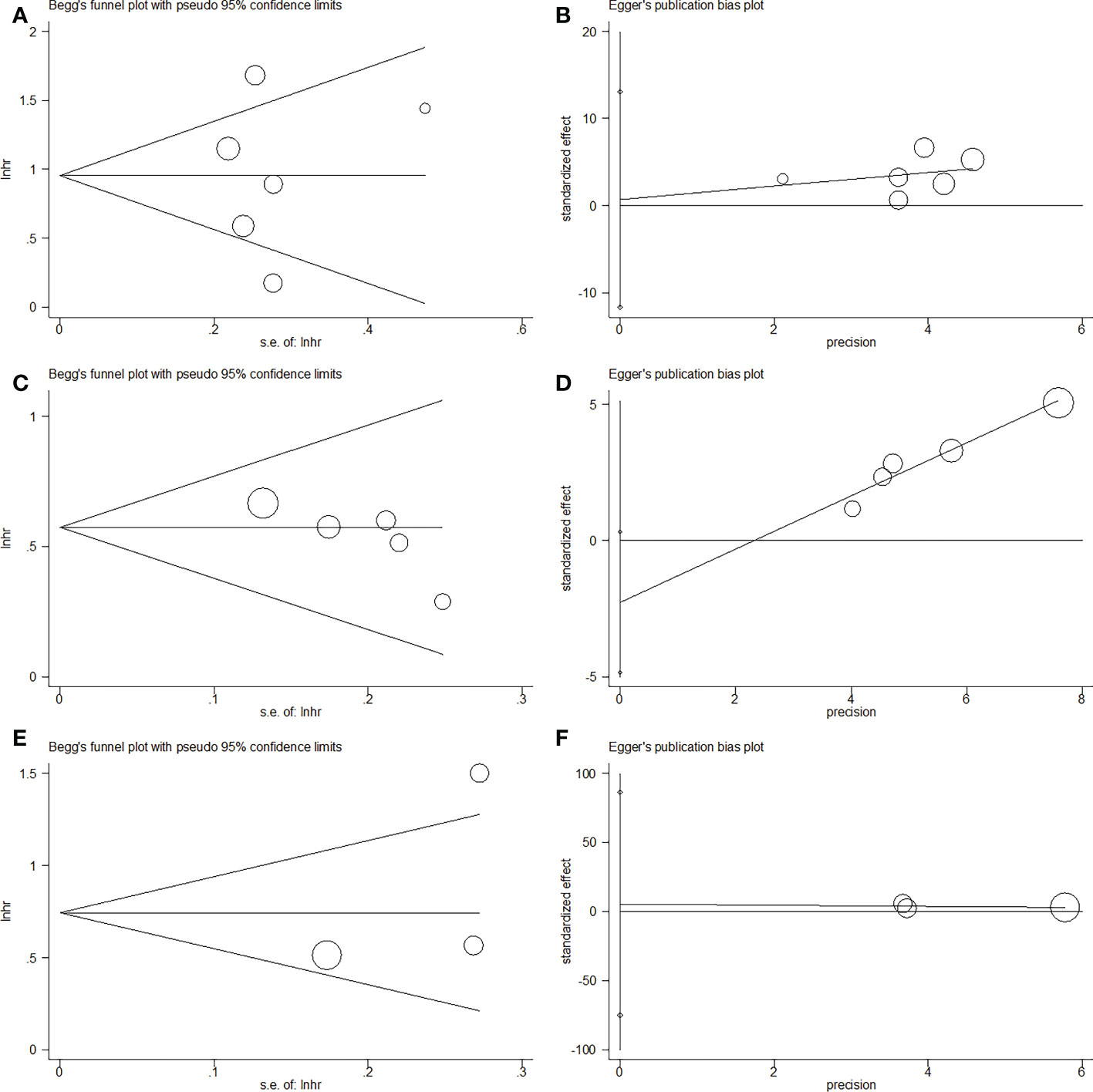
Figure 5 Publication bias by Begg’s test and Egger’s test in this meta-analysis. (A) Begg’s test for OS, p=0.851; (B) Egger’s test for OS, p=0.883; (C) Begg’s test for RFS/PFS, p=0.086; (D) Egger’s test for RFS/PFS, p=0.068; (E) Begg’s test for CSS, p=0.296; (F) Egger’s test for CSS, p=0.548.
4 Discussion
Prior research has shown conflicting results regarding the prognostic efficacy of GNRI in patients with UCs. In the present meta-analysis, we included eight studies with a total of 6,792 patients and found that low GNRI predicted poor RFS/PFS, CSS, and OS in patients with UCs. In addition, the prognostic impact of the GNRI in these patients remained stable in diverse subgroups. The publication bias test identified non-significant publication bias and validated the accuracy of our findings. To our knowledge, this is the first meta-analysis to explore the association between pre-treatment survival outcomes and GNRI in UCs. Based on our meta-analysis, we know that a low GNRI is an easy and reliable prognostic indicator for patients with UCs in clinical practice.
The GNRI is a nutritional index based on body weight and albumin level. Therefore, the roles of these two components in cancer can provide insights into the processes underlying the association between GNRI and prognosis in UCs. Albumin levels are often used to assess patients’ nutritional and inflammatory health when dealing with UCs. There was a correlation between low albumin levels and increased fetoprotein levels, portal vein thrombosis, larger maximal tumor diameters, increased tumor multifocality, and shorter overall survival time (24). Therefore, a lower serum albumin level directly indicates the malnutrition status of patients with cancer. Moreover, current evidence shows that malnutrition is a common issue among patients with cancer, with an incidence of 39–71% (25, 26). Researchers have found that low albumin levels are a strong predictor of poor health outcomes in patients with advanced cancer (27). In contrast, weight is a proxy for the extent of a systemic ailment and reserves of protein and calories. To calculate the GNRI, we must first calculate the body mass index by comparing an individual’s actual weight to their ideal weight. It is well established that low body mass index is associated with poor prognosis in patients with cancer (28).
Some recent studies have provided pivotal evidence for the clinical use of nutritional indices for the prognosis of patients with urological cancers (29, 30). A recent single-center retrospective study including 510 cases showed that the fibrinogen-to-albumin ratio (FAR) in patients with bladder cancer who had elevated preoperative FAR might be more likely to have advanced-stage cancer and malignancy (29). Another recent study proposed that the lymphocyte-to-monocyte ratio could be a promising prognostic indicator for tumor progression in patients with bladder cancer (30).
Several recent meta-analyses have documented the prognostic importance of GNRI (31–34). In a meta-analysis of 11 trials, Zhou et al. demonstrated that a low GNRI was associated with poor CSS and OS in patients with esophageal cancer (31). In a meta-analysis of 3,239 patients, Xu et al. found that a low GNRI score was associated with a higher risk of death and postoperative complications in Asian patients with colon cancer (35). The authors of a recent meta-analysis of 8 studies conducted by Wang et al. (36) found that low GNRI levels were associated with shorter RFS, CSS, and OS in patients with lung cancer. Consistent with earlier findings in other cancer types, our meta-analysis showed that a lower GNRI was an effective prognostic predictor of RFS/PFS, CSS, and OS in patients with UC.
This meta-analysis has some limitations. First, all included studies were conducted in East Asia. Therefore, it is important to confirm our meta-analysis results in locations other than Asia. Second, because many studies in this meta-analysis were retrospective, there is a possibility of intrinsic selection bias and heterogeneity. Third, there was no consistent GNRI cut-off value across studies that were considered; hence, an ideal cut-off value should be determined. It is important to conduct multinational large-scale prospective trials across nations to corroborate our findings.
In summary, our meta-analysis concluded that a low GNRI significantly predicts worse outcomes for patients with UC. A lower pretreatment GNRI indicates poor survival outcomes in UCs. The GNRI may be a potential parameter for evaluating prognosis and developing appropriate treatment approaches for patients with UC.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
QW and FY designed the study. QW and FY established the process of literature selection and screened the abstracts and articles. QW analyzed data and wrote the main manuscript. All authors reviewed and approved the final manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
- GNRI, geriatric nutritional risk index; UCs, urological cancers; HR, hazard ratio; CI, confidence interval; RCC, renal cell carcinoma; PCa, prostate cancer; UC, urothelial carcinoma; ADT, androgen deprivation therapy; OS, overall survival; RFS, recurrence-free survival; PFS, progression-free survival; CSS, cancer-specific survival; TNM, tumor-node-metastasis; MVA, multivariate analysis; UVA, univariate analysis; NOS, Newcastle-Ottawa scale; FEM, fixed-effects model; REM, random-effects model; BMI, body mass index.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA: Cancer J Clin (2022) 72(1):7–33. doi: 10.3322/caac.21708
3. Zhou CK, Check DP, Lortet-Tieulent J, Laversanne M, Jemal A, Ferlay J, et al. Prostate cancer incidence in 43 populations worldwide: An analysis of time trends overall and by age group. Int J Cancer (2016) 138(6):1388–400. doi: 10.1002/ijc.29894
4. Znaor A, Lortet-Tieulent J, Laversanne M, Jemal A, Bray F. International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol (2015) 67(3):519–30. doi: 10.1016/j.eururo.2014.10.002
5. Yuasa T, Urasaki T, Oki R. Recent advances in medical therapy for urological cancers. Front Oncol (2022) 12:746922. doi: 10.3389/fonc.2022.746922
6. Boorjian SA, Kim SP, Tollefson MK, Carrasco A, Cheville JC, Thompson RH, et al. Comparative performance of comorbidity indices for estimating perioperative and 5-year all cause mortality following radical cystectomy for bladder cancer. J Urol (2013) 190(1):55–60. doi: 10.1016/j.juro.2013.01.010
7. Liu J, Wu P, Lai S, Song X, Wang M, Wang X, et al. Clinicopathological and prognostic significance of preoperative prognostic nutritional index in patients with upper urinary tract urothelial carcinoma. Nutr Cancer (2022) 74(8):2964–74. doi: 10.1080/01635581.2022.2049829
8. Ishihara H, Kondo T, Yoshida K, Omae K, Takagi T, Iizuka J, et al. Preoperative controlling nutritional status (CONUT) score as a novel predictive biomarker of survival in patients with localized urothelial carcinoma of the upper urinary tract treated with radical nephroureterectomy. Urologic Oncol (2017) 35(9):539.e9–.e16. doi: 10.1016/j.urolonc.2017.04.012
9. Kinoshita A, Hagiwara N, Osawa A, Akasu T, Matsumoto Y, Ueda K, et al. The geriatric nutritional risk index predicts tolerability of lenvatinib in patients with hepatocellular carcinoma. In Vivo (2022) 36(2):865–73. doi: 10.21873/invivo.12775
10. Bouillanne O, Morineau G, Dupont C, Coulombel I, Vincent JP, Nicolis I, et al. Geriatric nutritional risk index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr (2005) 82(4):777–83. doi: 10.1093/ajcn/82.4.777
11. Furuke H, Matsubara D, Kubota T, Kiuchi J, Kubo H, Ohashi T, et al. Geriatric nutritional risk index predicts poor prognosis of patients after curative surgery for gastric cancer. Cancer Diagn Progn (2021) 1(2):43–52. doi: 10.21873/cdp.10007
12. Grinstead C, George T, Han B, Yoon SL. Associations of overall survival with geriatric nutritional risk index in patients with advanced pancreatic cancer. Nutrients (2022) 14(18):3800. doi: 10.3390/nu14183800
13. Ito Y, Abe A, Hayashi H, Momokita M, Furuta H. Prognostic impact of preoperative geriatric nutritional risk index in oral squamous cell carcinoma. Oral Dis (2022). doi: 10.1111/odi.14255
14. Gu W, Zhang G, Sun L, Ma Q, Cheng Y, Zhang H, et al. Nutritional screening is strongly associated with overall survival in patients treated with targeted agents for metastatic renal cell carcinoma. J cachexia sarcopenia Muscle (2015) 6(3):222–30. doi: 10.1002/jcsm.12025
15. Miyake H, Tei H, Fujisawa M. Geriatric nutrition risk index is an important predictor of cancer-specific survival, but not recurrence-free survival, in patients undergoing surgical resection for non-metastatic renal cell carcinoma. Curr Urol (2017) 10(1):26–31. doi: 10.1159/000447147
16. Okamoto T, Hatakeyama S, Narita S, Takahashi M, Sakurai T, Kawamura S, et al. Impact of nutritional status on the prognosis of patients with metastatic hormone-naïve prostate cancer: a multicenter retrospective cohort study in Japan. World J Urol (2019) 37(9):1827–35. doi: 10.1007/s00345-018-2590-2
17. Kang HW, Seo SP, Kim WT, Yun SJ, Lee SC, Kim WJ, et al. A low geriatric nutritional risk index is associated with aggressive pathologic characteristics and poor survival after nephrectomy in clear renal cell carcinoma: A multicenter retrospective study. Nutr Cancer (2020) 72(1):88–97. doi: 10.1080/01635581.2019.1621357
18. Chang LW, Hung SC, Li JR, Chiu KY, Yang CK, Chen CS, et al. Geriatric nutritional risk index as a prognostic marker for patients with metastatic castration-resistant prostate cancer receiving docetaxel. Front Pharmacol (2021) 11:601513. doi: 10.3389/fphar.2020.601513
19. Naiki T, Nagai T, Sugiyama Y, Etani T, Nozaki S, Iida K, et al. First report of oncological outcome and prognostic analysis in a first-line setting of short hydration gemcitabine and cisplatin chemotherapy for patients with metastatic urothelial carcinoma. Oncology (2021) 99(10):622–31. doi: 10.1159/000517326
20. Tang Y, Liang J, Liu Z, Zhang R, Zou Z, Wu K, et al. Clinical significance of prognostic nutritional index in renal cell carcinomas. Med (Baltimore) (2021) 100(10):e25127. doi: 10.1097/md.0000000000025127
21. Isobe T, Naiki T, Sugiyama Y, Naiki-Ito A, Nagai T, Etani T, et al. Chronological transition in outcome of second-line treatment in patients with metastatic urothelial cancer after pembrolizumab approval: a multicenter retrospective analysis. Int J Clin Oncol (2022) 27(1):165–74. doi: 10.1007/s10147-021-02046-z
22. Moher D, Liberati A, Tetzlaff J, Altman DG, Grp P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann Internal Med (2009) 151(4):264–W64. doi: 10.7326/0003-4819-151-4-200908180-00135
23. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses . Available at: http://wwwohrica/programs/clinical_epidemiology/oxfordasp.
24. Bağırsakçı E, Şahin E, Atabey N, Erdal E, Guerra V, Carr BI. Role of albumin in growth inhibition in hepatocellular carcinoma. Oncology (2017) 93(2):136–42. doi: 10.1159/000471807
25. Silva FR, de Oliveira MG, Souza AS, Figueroa JN, Santos CS. Factors associated with malnutrition in hospitalized cancer patients: a croos-sectional study. Nutr J (2015) 14:123. doi: 10.1186/s12937-015-0113-1
26. Gyan E, Raynard B, Durand JP, Lacau Saint Guily J, Gouy S, Movschin ML, et al. Malnutrition in patients with cancer: Comparison of perceptions by patients, relatives, and physicians-results of the NutriCancer2012 study. JPEN J Parenteral Enteral Nutr (2018) 42(1):255–60. doi: 10.1177/0148607116688881
27. Conrad LB, Awdeh H, Acosta-Torres S, Conrad SA, Bailey AA, Miller DS, et al. Pre-operative core muscle index in combination with hypoalbuminemia is associated with poor prognosis in advanced ovarian cancer. J Surg Oncol (2018) 117(5):1020–8. doi: 10.1002/jso.24990
28. Doleman B, Mills KT, Lim S, Zelhart MD, Gagliardi G. Body mass index and colorectal cancer prognosis: A systematic review and meta-analysis. Tech Coloproctol (2016) 20(8):517–35. doi: 10.1007/s10151-016-1498-3
29. Barone B, Napolitano L, Reccia P, De Luca L, Morra S, Turco C, et al. Preoperative fibrinogen-to-Albumin ratio as potential predictor of bladder cancer: A monocentric retrospective study. Medicina (Kaunas Lithuania) (2022) 58(10):1490. doi: 10.3390/medicina58101490
30. Ferro M, Caputo VF, Barone B, Imbimbo C, de Cobelli O, Crocetto F. Lymphocyte to monocyte ratio: A new independent prognostic factor in bladder cancer progression? Front Oncol (2021) 11:754649. doi: 10.3389/fonc.2021.754649
31. Zhou J, Fang P, Li X, Luan S, Xiao X, Gu Y, et al. Prognostic value of geriatric nutritional risk index in esophageal carcinoma: A systematic review and meta-analysis. Front Nutr (2022) 9:831283. doi: 10.3389/fnut.2022.831283
32. Yuan F, Yuan Q, Hu J, An J. Prognostic role of pretreatment geriatric nutritional risk index in colorectal cancer patients: A meta-analysis. Nutr Cancer (2023) 75(1):276–85. doi: 10.1080/01635581.2022.2109692
33. Yang M, Liu Z, Li G, Li B, Li C, Xiao L, et al. Geriatric nutritional risk index as a prognostic factor of patients with non-small cell lung cancer: A meta-analysis. Horm Metab Res (2022) 54(9):604–12. doi: 10.1055/a-1903-1943
34. Lidoriki I, Schizas D, Frountzas M, Machairas N, Prodromidou A, Kapelouzou A, et al. GNRI as a prognostic factor for outcomes in cancer patients: A systematic review of the literature. Nutr Cancer (2021) 73(3):391–403. doi: 10.1080/01635581.2020.1756350
35. Xu J, Sun Y, Gong D, Fan Y. Predictive value of geriatric nutritional risk index in patients with colorectal cancer: A meta-analysis. Nutr Cancer (2022) 75(1):24–32. doi: 10.1080/01635581.2022.2115521
Keywords: GNRI, urological cancers, meta-analysis, survival, clinical use
Citation: Wu Q and Ye F (2023) Prognostic impact of geriatric nutritional risk index on patients with urological cancers: A meta-analysis. Front. Oncol. 12:1077792. doi: 10.3389/fonc.2022.1077792
Received: 23 October 2022; Accepted: 14 December 2022;
Published: 11 January 2023.
Edited by:
Yafeng Ma, Ingham Institute of Applied Medical Research, AustraliaReviewed by:
Biagio Barone, University of Naples Federico II, ItalyEleonora Lai, University Hospital and University of Cagliari, Italy
Copyright © 2023 Wu and Ye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fagen Ye, eWZnY2wyMDIyQDE2My5jb20=
 Quan Wu1
Quan Wu1 Fagen Ye
Fagen Ye