- 1Division of Gastrointestinal Surgery, Department of General Surgery, West China Hospital, Sichuan University, Chengdu, China
- 2Gastric Cancer Center, Department of General Surgery, West China Hospital, Sichuan University, Chengdu, China
- 3Department of Gastrointestinal Surgery, The Third People’s Hospital of Chengdu, Chengdu, China
- 4Department of Anesthesiology, West China Hospital, Sichuan University, Chengdu, China
Introduction: Solitary metachronous small bowel metastasis from renal cell carcinoma (RCC) is rare. In contrast to idiopathic intussusception frequently occurring in children, adult intussusception is fairly uncommon and usually indicates a malignancy.
Case presentation: We presented an 84-year-old man with small bowel intussusception and obstruction due to a solitary metachronous metastasis from RCC. Computed tomography with intravenous contrast revealed small bowel obstruction and a 4 × 4 cm intraluminal soft-tissue mass with moderate enhancement. During urgent exploratory laparotomy, a pedunculated tumor of the distal ileum was found to be the lead point of intussusception. Hence, reduction of the intestinal invagination and segmental resection of the ileum with functional end-to-end anastomosis were performed. Histological examination finally confirmed the diagnosis. The postoperative recovery was uneventful. The patient was discharged without any complications on postoperative day 6.
Conclusion: The case report highlights the rarity of solitary metachronous small bowel metastases from RCC and suggests that life-long follow-up of RCC patients is critical due to its unpredictable behavior and the possibility of a long period of dormancy. Complete surgical resection remains the mainstay treatment for such patients.
Introduction
Renal cell carcinoma (RCC) is the third most frequently diagnosed cancer among urological tumors, with an estimated annual incidence of 0.4 million cases worldwide (1). RCC is a male-predominant (2:1 ratio) malignancy. It appears between 60 and 80 years old, with a median age of approximately 64 years and a near-normal distribution (2). Clear cell RCC (ccRCC) represents the most prevalent histological subtype of RCC (70-80%) and accounts for most RCC-related deaths (3). Partial or radical nephrectomy remains the gold standard in localized ccRCC therapy (4).
Nearly one-third of localized ccRCC patients will eventually develop metastases even after curative nephrectomy (5). Distant metastases in metastatic RCC (mRCC) patients are most frequently found in the lung (45.2%), followed by the bone (29.5%), lymph node (21.8%), liver (20.3%), and adrenal gland (8.9%), whereas the proportion of small bowel metastasis is extremely low (1.1%) (6). Portocaval venous shunts may facilitate small bowel involvement. Metachronous metastasis of RCC may occur more than a decade after the initial presentation and diagnosis. Despite significant progress in systemic treatment over the past two decades, en bloc resection of the oligometastatic disease is still a curative option for late RCC metastasis (≥ 2 years) (7).
Adults have intussusception less frequently than children, accounting for less than 5% of all intussusception and 1% of bowel obstruction cases (8). The most frequent pathologic condition that causes adult intussusception is malignancy, and the small bowel is the most affected site (9, 10). Nevertheless, gastrointestinal tract metastasis usually develops from breast cancer, melanoma, lung cancer, and esophageal squamous cell carcinoma, with the small bowel being the least involved site (2%) (11, 12). As a result, initial reduction combined with surgical resection remains the mainstay treatment in adult small bowel intussusception (13).
Following the principles of the CAse REport (CARE) guidelines (14), we reported a small bowel intussusception ascribed to an intramural cauliflower-like mass that proved to be a metastasis from a ccRCC 5 years after radical nephrectomy. In contrast to our case, most adult small bowel intussusceptions are benign illnesses, while large bowel involvement is likely to be malignant. The present case highlights the need for clinicians to maintain a high index of suspicion of metastasis when assessing the occurrence of new symptoms in these patients with a distant history of presumably curative cancer treatment.
Case presentation
In September 2021, an 83-year-old male patient presented to the emergency department with intermittent abdominal pain and distention accompanied by cessation of passage of flatus and stool for 3 days. The concurrent medical problems included type 2 diabetes mellitus and pulmonary emphysema for more than 30 years. More significantly, he underwent left radical nephrectomy for ccRCC (pT3aN0M0, Fuhrman Grade 3) 5 years previously. The scheduled four cycles of adjuvant therapy with tyrosine kinase inhibitor sunitinib were discontinued because of patient intolerance. Computed tomography (CT) and serum tumor marker levels showed favorable local control and no signs of distant progression during intensive surveillance. The last follow-up visit was just 6 months before this admission.
On admission, he was afebrile with negative result of coronavirus disease 2019 testing. The blood pressure was 123/84 mmHg, and the heart rate was 105 beats per minute. Physical examination revealed pallor, mild dehydration, abdominal distention, and metallic bowel sounds, without rebound tenderness and palpable abdominal mass. Blood tests indicated iron deficiency anemia (hemoglobin, 9.8 g/dL; reference range, 13.0 – 17.5 g/dL) and an elevated percentage of neutrophils (87%; reference range, 40 – 75%). CT of the chest and abdomen with intravenous contrast demonstrated small bowel obstruction due to intussusception of the distal ileum containing an approximately 4 × 4 cm intraluminal soft-tissue mass with moderate enhancement (Figures 1A, B). No lung, bone, or liver metastasis or local relapse of RCC was visible on radiologic examination.
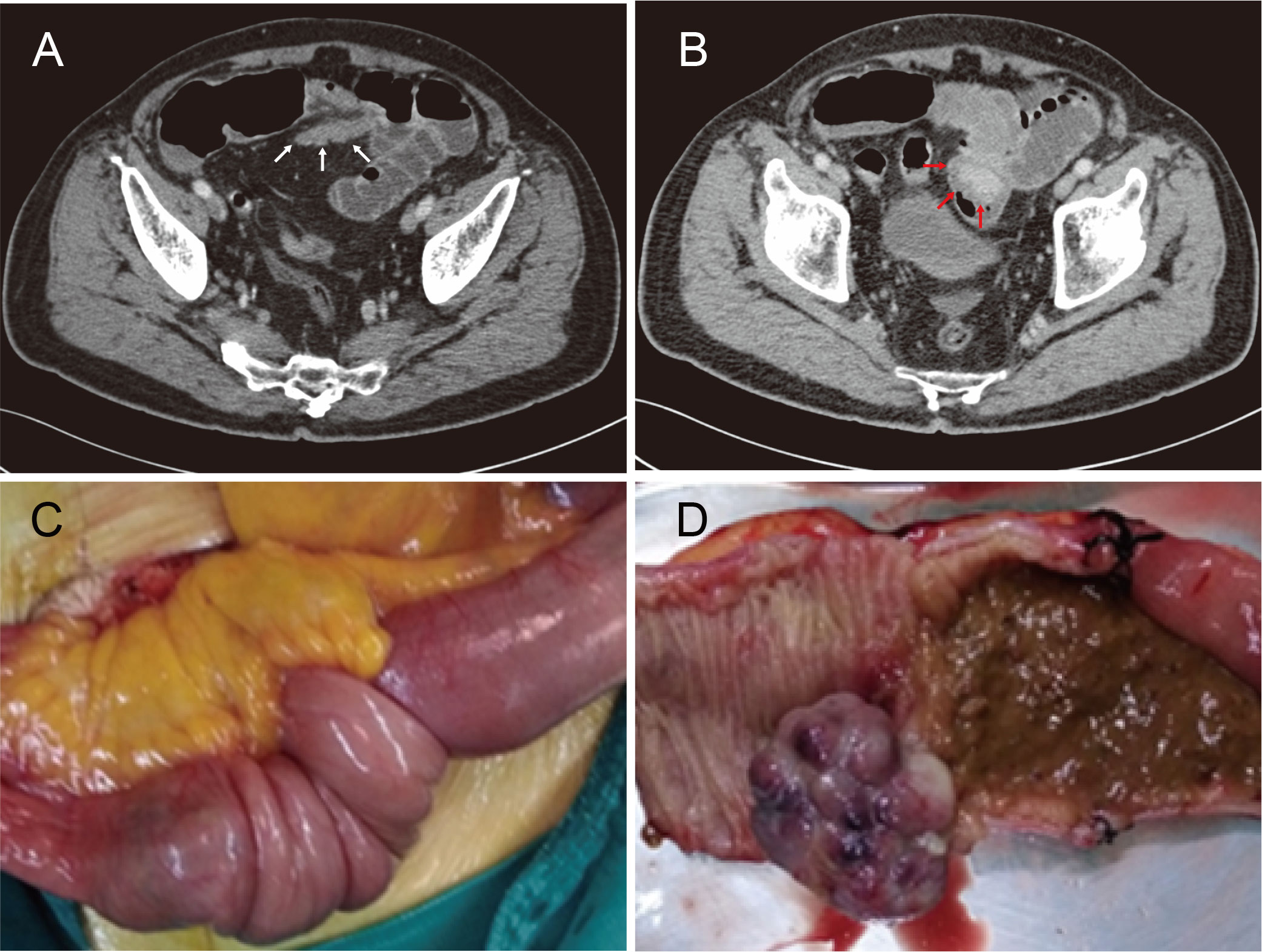
Figure 1 (A, B) Computed tomography images demonstrating small bowel obstruction due to intussusception of the distal ileum (white arrows) containing an approximately 4 × 4 cm intraluminal soft-tissue mass (red arrows). (C) An intraoperative photo confirming intussusception whose lead point was a solitary ileal neoplasm. (D) Gross image of the surgical specimen with a 4 × 3 × 3-cm taupe cauliflower-like, pedunculated tumor.
Complying with the multidisciplinary team’s decision, the patient underwent an urgent exploratory laparotomy. After the reduction of intestinal invagination, a solitary ileal neoplasm was found to be the lead point of the intussusception (Figure 1C). Hence, segmental resection of the ileum was performed together with primary functional end-to-end anastomosis. Gross examination of the surgical specimen indicated a 4 × 3 × 3-cm taupe cauliflower-like, pedunculated tumor protruding into the ileum (Figure 1D).
Postoperatively, the patient was transferred back to the gastrointestinal surgical ward after spending 1 day in the surgical intensive care unit. Under the guidance of the enhanced recovery after surgery protocol, the patient’s convalescence was unremarkable, with oral feeding beginning on postoperative day (POD) 3 and discharge on POD 6. The patient received no additional treatment due to his venerable age and underlying diseases. To date, the 1-year follow-up has been uneventful. The timeline with relevant data from the episode of care is shown in Figure 2.
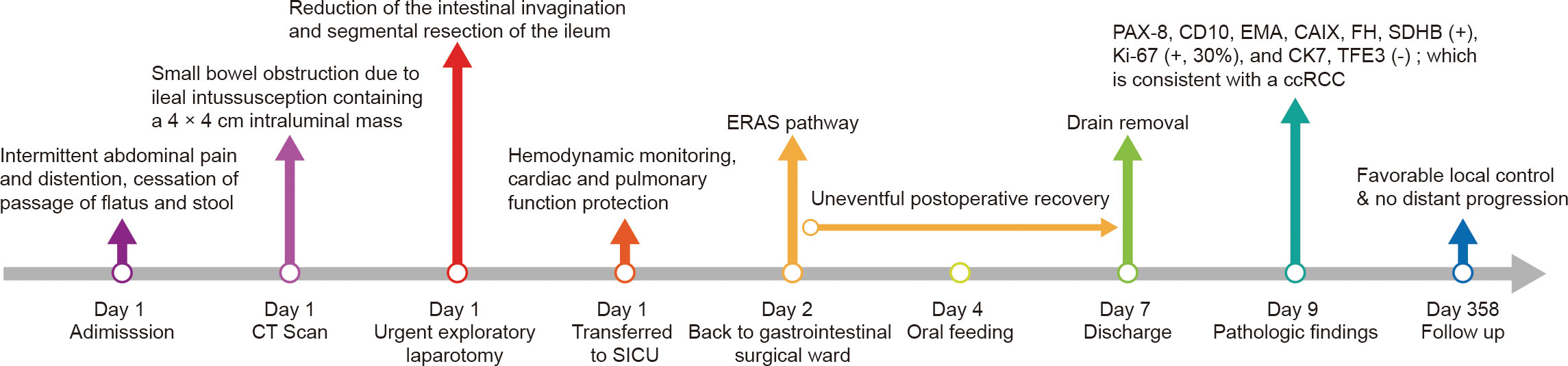
Figure 2 Timeline of the case presentation with relevant data. CT, computed tomography; SICU, surgical intensive care unit; ERAS, enhanced recovery after surgery; ccRCC, clear cell renal cell carcinoma.
Hematoxylin and eosin staining showed compact nests of tumor cells with clear cytoplasm separated by delicate vasculature (Figure 3A). The diagnosis of the ileal metastasis from ccRCC was established via the following immunohistochemical results: PAX-8 (+), CD10 (+), EMA (+), CK7 (-), CAIX (+), FH (+), SDHB (+), TFE3 (-), and Ki-67 (+, 30%) (Figures 3B-D). A total of 9 mesenteric lymph nodes were identified without tumor involvement. Additionally, genetic testing was declined by the patient’s family members.
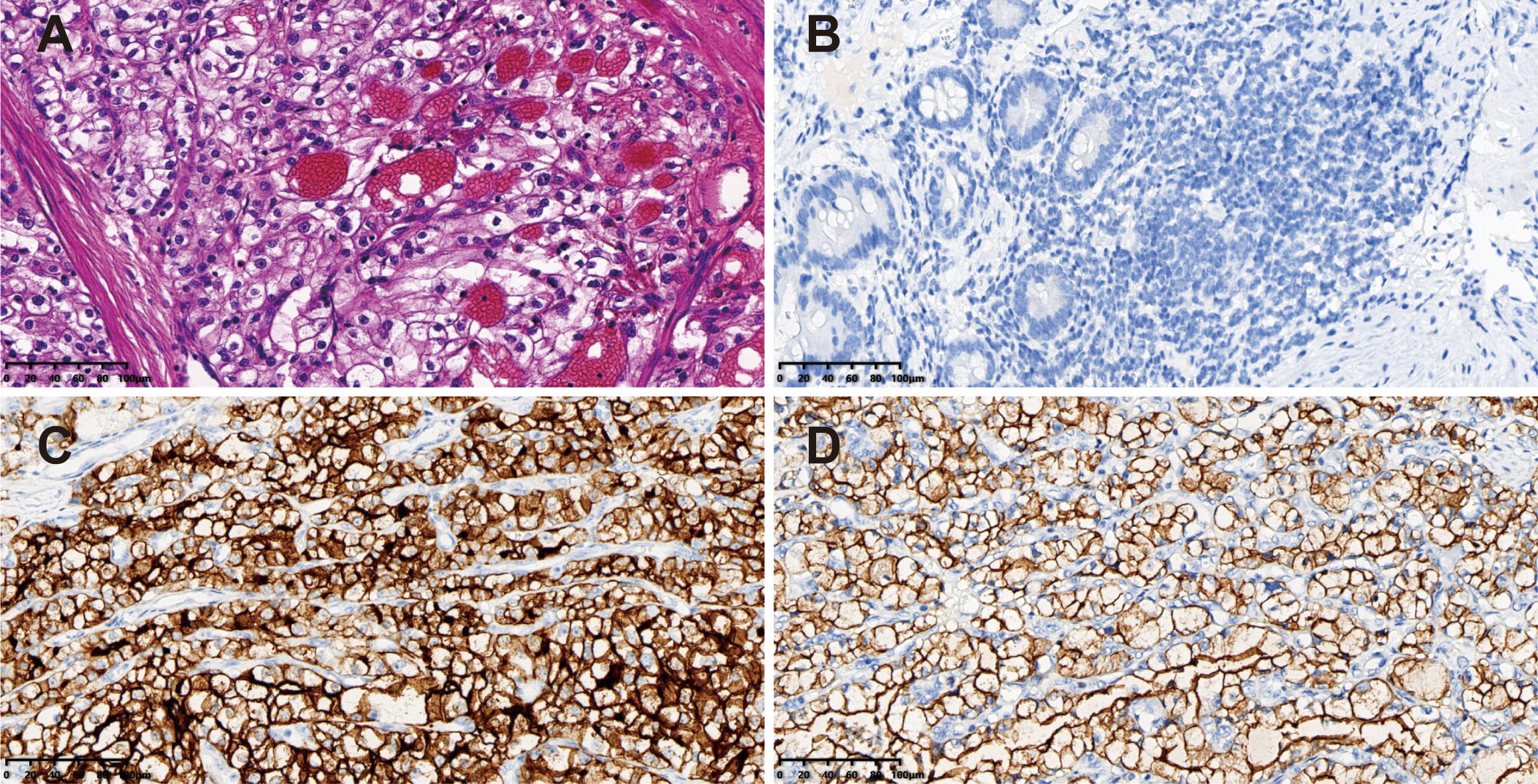
Figure 3 Pathological findings of the surgical specimen. (A) Hematoxylin & eosin staining showing compact nests of tumor cells with clear cytoplasm separated by delicate vasculature (magnification power: 20×). Immunohistochemical staining of the tumor revealing (B) PAX-8 (+), (C) CD10 (+), and (D) EMA (+) (magnification power: 20×).
Discussion
A systematic electronic literature search was conducted in the PubMed, Embase (OVID interface), and Cochrane Central Register of Controlled Trials databases using medical subject headings and text words related to “solitary metachronous small bowel metastasis from RCC” to obtain relevant case reports published through 10 May 2022. The term “solitary metachronous small bowel metastasis from RCC” was defined as the small bowel metastasis diagnosed more than 6 months after partial or radical nephrectomy for localized RCC. The search strategy and syntax for the PubMed database are shown in the Supplementary material. Papers with non-English language, animal subjects, or unavailable full text were excluded. The general characteristics of the included studies were extracted and entered into a preplanned electronic form (Table 1). The pooled data were interpreted in a descriptive and narrative manner.
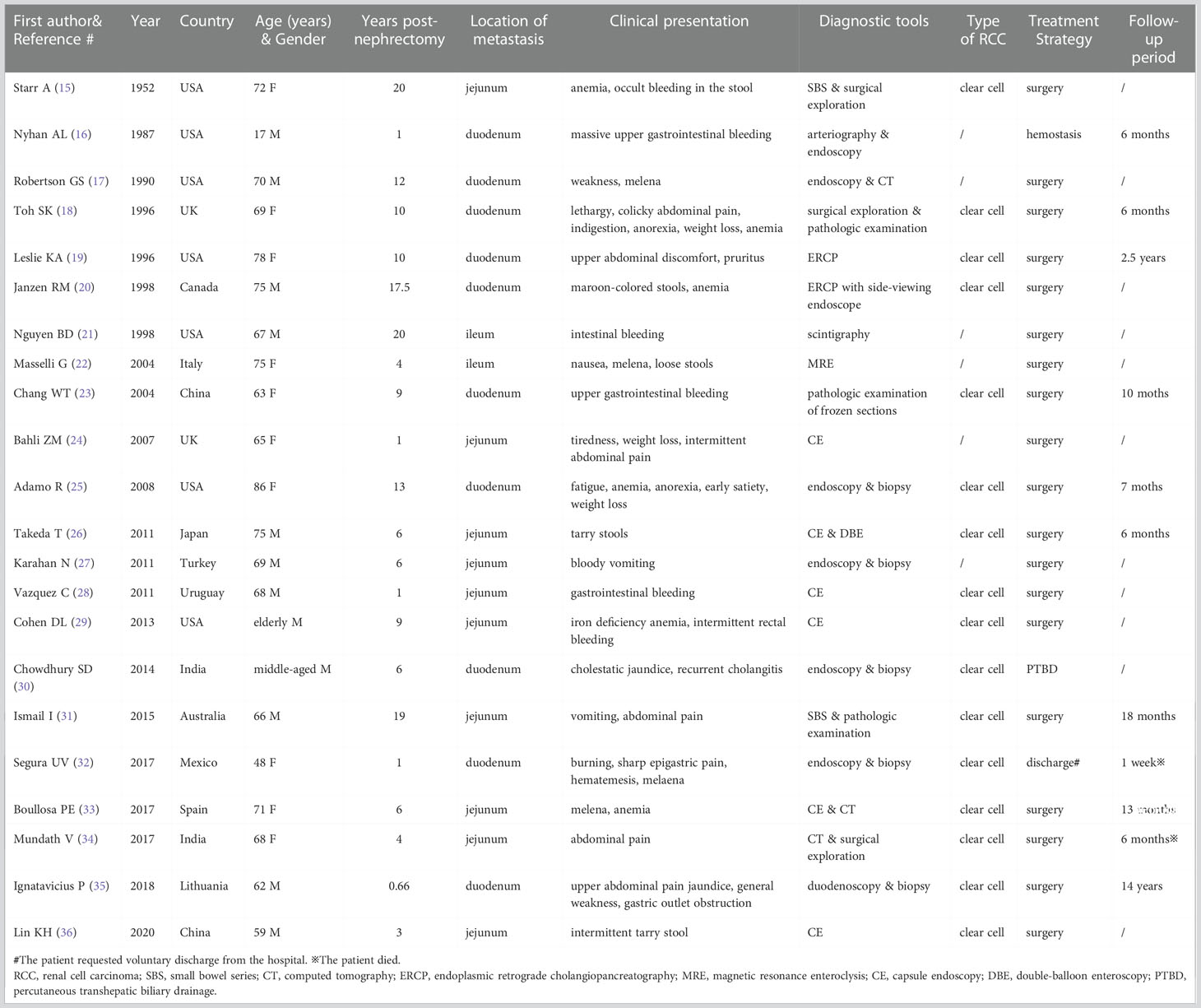
Table 1 General characteristics of the reported cases of solitary metachronous small bowel metastasis from RCC.
Finally, 48 potential studies were included for the evaluation, in which 21 cases with solitary metachronous small bowel metastases from RCC that had been reported between 1952 and 2020 met the inclusion criteria (15–34, 36, 37). The other cases in different publications were excluded due to synchronous, multiple metastases, or lack of additional radiological examination to check for metastases in other sites. The number of published case reports has increased in the last decade. Patients aged 60 years or older accounted for 81.8% of the enrolled instances (18/22). Like the primary RCCs, clear cell disease was the predominant histological subtype in these small bowel metastases (3). The disease course ranged from 8 months to 20 years post curative nephrectomy. Given the longer survival of RCC patients in the era of targeted therapy and immunotherapy, we are convinced that more late metastases will be identified (38, 39).
Ileal metastases from RCC were rather uncommon in our literature review when compared to duodenal and jejunal metastases. Although some subtypes might exhibit regional lymph node involvement, RCC is notable for hematogenous metastasis (40). Viadana et al. found that RCC first metastasizes to the lung via the renal vein and inferior vena cava before spreading systemically to multiple organs (41). However, our case revealed that small bowel metastasis may happen before lung metastasis. Furthermore, a clear cell sarcoma-like tumor of the gastrointestinal tract that primarily affects young-aged to middle-aged individuals is increasingly recognized as a primary neuroectodermal malignancy with clear cell morphology and a predilection for the small bowel (42). Besides, S-100 (+), SOX (+), and EWSR1 gene fusions are essential to confirming the diagnosis (43).
Small bowel metastases from RCC exhibit various biological characteristics (44). We found that the most typical clinical presentations were gastrointestinal bleeding, melena, and secondary iron deficiency anemia. Willis et al. discovered that metastatic small bowel lesions typically form in the submucosa and cause mucosal rupture and ulceration (Borrmann classification type II or III) and bleeding (11, 45). Some individuals might experience non-specific symptoms, such as intermittent blunt abdominal pain, weakness, and weight loss. In addition, intussusception and perforation could occur in rare cases. Intestinal intussusception is primarily caused by the pedunculated small bowel tumor that originates from the submucosa and represents dimple formation on the serosa. Unlike idiopathic intussusception in children, intussusception in adults and the elderly is uncommon but typically associated with an underlying pathology (37).
The significance of radiological screening should be emphasized in patients with atypical symptoms and an RCC history. Small bowel series is inferior to CT as a practical and effective tool in assessing suspicious small bowel lesions, especially in the emergency setting (34). Capsule endoscopy can facilitate the localization of occult bleeding and intraluminal masses and shorten the diagnosis time (36). Besides, single- or double-balloon enteroscopy can assist in hemostasis and biopsy (26). The Tc-99m tagged red blood cell scan can be used for gastrointestinal hemorrhage to show the hypervascular characteristics of the intestinal lesion and guide additional angiography (21). Exploratory laparoscopy and laparotomy can be performed to help verify the diagnosis after getting informed consent.
Systemic treatment should be considered for individuals with medically inoperable RCC (3). Several immune checkpoint inhibitors were initially tested for RCC in the adjuvant setting (46, 47). Nevertheless, more prospective phase III clinical trials are needed to provide high-quality evidence. It is necessary to conduct clinical and translational studies to determine the phenotypic predictors of response and resistance to each drug. Genetic testing can help select the optimal treatment regimen. Therefore, systemic and surgical treatments must be integrated to achieve a complete response and to obtain a thorough understanding of the biological features of RCC to explore new targets (48).
Meanwhile, complete metastasectomy of synchronous or metachronous solitary RCC metastasis could improve the patient survival (49). Thus, a conventional segmental resection of the ileum was performed with negative margins and lymph nodes in our case. Only one resected specimen reported by Mundath et al. in our literature review revealed the involvement of lymph nodes (34). Endoscopic hemostasis and transcatheter embolization can be utilized as a bridge to surgery in patients with massive gastrointestinal hemorrhage secondary to mRCC (16). Besides, diverting ostomy and gastrojejunostomy are recommended to ease obstruction-related symptoms in unresectable tumors. Definitive radiotherapy rather than no alternative treatment should be considered for RCC patients with medically inoperable duodenal metastases from RCC.
In conclusion, our case suggests that life-long follow-up of RCC patients is critical due to unpredictable tumor behavior and the possibility of a long period of dormancy. Complete resection of the solitary metachronous small bowel metastasis from RCC is indicated as a life-saving procedure and a curative treatment to cure metastasis-related complications. The present case report and review of the English-language literature emphasizes the rarity of this entity and recommends aggressive and tailored surgical treatment for these patients.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Ethical Committee on Biomedical Research, West China Hospital, Sichuan University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual for the publication of any potentially identifiable images or data included in this article.
Author contributions
LY, WY, ZC, and PN conceptualized and designed the study. WY and ZC drafted the manuscript. TY and HZ were responsible for the literature search. QD, SQ, and JZ collected the clinical data. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the 1·3·5 Project for Disciplines of Excellence–Clinical Research Incubation Project, West China Hospital, Sichuan University (Grant No. 2022HXFH028).
Acknowledgments
We would like to thank Dr. Da-Gang Zhou (Department of Pathology, The Third Affiliated Hospital of Chengdu University of TCM/Chengdu Pidu District Hospital of Traditional Chinese Medicine) for the professional and valuable interpretation of the pathologic findings.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Hsieh JJ, Purdue MP, Signoretti S, Swanton C, Albiges L, Schmidinger M, et al. Renal cell carcinoma. Nat Rev Dis Primers (2017) 3:17009. doi: 10.1038/nrdp.2017.9
3. Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet (2009) 373(9669):1119–32. doi: 10.1016/S0140-6736(09)60229-4
4. Ljungberg B, Bensalah K, Canfield S, Dabestani S, Hofmann F, Hora M, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol (2015) 67(5):913–24. doi: 10.1016/j.eururo.2015.01.005
5. Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: the SSIGN score. J Urol (2002) 168(6):2395–400. doi: 10.1097/01.ju.0000035885.91935.d5
6. Bianchi M, Sun M, Jeldres C, Shariat SF, Trinh QD, Briganti A, et al. Distribution of metastatic sites in renal cell carcinoma: a population-based analysis. Ann Oncol (2012) 23(4):973–80. doi: 10.1093/annonc/mdr362
7. Grüllich C, Vallet S, Hecht C, Duensing S, Hadaschik B, Jäger D, et al. Local salvage therapy for late (≥2 years) metastatic and local relapse of renal cell cancer is a potentially curative treatment irrespective of the site of recurrence. Urol Oncol (2016) 34(5):238.e9–17. doi: 10.1016/j.urolonc.2015.11.022
8. Azar T, Berger DL. Adult intussusception. Ann Surg (1997) 226(2):134–8. doi: 10.1097/00000658-199708000-00003
9. Cochran AA, Higgins GL 3rd, Strout TD. Intussusception in traditional pediatric, nontraditional pediatric, and adult patients. Am J Emerg Med (2011) 29(5):523–7. doi: 10.1016/j.ajem.2009.11.023
10. Chiarelli M, Zago M, Tagliabue F, Burati M, Riva C, Vanzati A, et al. Small bowel intussusception due to rare cardiac intimal sarcoma metastasis: A case report. Front Surg (2021) 8:743858. doi: 10.3389/fsurg.2021.743858
11. Willis RA. Secondary tumors of the intestine. In: Willis RA, editor. The spread of tumors in the human body, 3rd edition. London: Butterworth & Co (1973). p. 209–13.
12. Hsu CC, Chen JJ, Changchien CS. Endoscopic features of metastatic tumors in the upper gastrointestinal tract. Endoscopy (1996) 28(2):249–53. doi: 10.1055/s-2007-1005437
13. Hong KD, Kim J, Ji W, Wexner SD. Adult intussusception: a systematic review and meta-analysis. Tech Coloproctol (2019) 23(4):315–24. doi: 10.1007/s10151-019-01980-5
14. Riley DS, Barber MS, Kienle GS, Aronson JK, von Schoen-Angerer T, Tugwell P, et al. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol (2017) 89:218–35. doi: 10.1016/j.jclinepi.2017.04.026
15. Starr A, Miller GM. Solitary jejunal metastasis twenty years after removal of a renal-cell carcinoma; report of a case. N Engl J Med (1952) 246(7):250–1. doi: 10.1056/NEJM195202142460703
16. Nyhan AL, Fishman EK, Kadir S. Diagnosis and management of massive gastrointestinal bleeding owing to duodenal metastasis from renal cell carcinoma. J Urol (1987) 138(3):611–3. doi: 10.1016/s0022-5347(17)43275-7
17. Robertson GS, Gertler SL. Late presentation of metastatic renal cell carcinoma as a bleeding ampullary mass. Gastrointest Endosc (1990) 36(3):304–6. doi: 10.1016/s0016-5107(90)71032-2
18. Toh SK, Hale JE. Late presentation of a solitary metastasis of renal cell carcinoma as an obstructive duodenal mass. Postgrad Med J (1996) 72(845):178–9. doi: 10.1136/pgmj.72.845.178
19. Leslie KA, Tsao JI, Rossi RL, Braasch JW. Metastatic renal cell carcinoma to ampulla of vater: an unusual lesion amenable to surgical resection. Surgery (1996) 119(3):349–51. doi: 10.1016/s0039-6060(96)80122-x
20. Janzen RM, Ramj AS, Flint JD, Scudamore CH, Yoshida EM. Obscure gastrointestinal bleeding from an ampullary tumor in a patient with a remote history of renal cell carcinoma: a diagnostic conundrum. Can J Gastroenterol (1998) 12(1):75–8. doi: 10.1155/1998/429832
21. Nguyen BD, Port JD, Petronis JD. Scintigraphy of ileal hemorrhage from metastasis of renal cell carcinoma. Clin Nucl Med (1998) 23(7):464–5. doi: 10.1097/00003072-199807000-00014
22. Masselli G, Brizi MG, Restaino G, Vecchioli A. MR enteroclysis in solitary ileal metastasis from renal cell carcinoma. AJR Am J Roentgenol (2004) 182(3):828–9. doi: 10.2214/ajr.182.3.1820828
23. Chang WT, Chai CY, Lee KT. Unusual upper gastrointestinal bleeding due to late metastasis from renal cell carcinoma: a case report. Kaohsiung J Med Sci (2004) 20(3):137–41. doi: 10.1016/S1607-551X(09)70098-1
24. Bahli ZM, Panesar KJ. Solitary jejunal metastasis from renal cell carcinoma. J Ayub Med Coll Abbottabad (2007) 19(2):62–3.
25. Adamo R, Greaney PJ Jr, Witkiewicz A, Kennedy EP, Yeo CJ. Renal cell carcinoma metastatic to the duodenum: treatment by classic pancreaticoduodenectomy and review of the literature. J Gastrointest Surg (2008) 12(8):1465–8. doi: 10.1007/s11605-007-0426-2
26. Takeda T, Shibuya T, Osada T, Izumi H, Mitomi H, Nomura O, et al. Metastatic renal cell carcinoma diagnosed by capsule endoscopy and double balloon endoscopy. Med Sci Monit (2011) 17(2):CS15–7. doi: 10.12659/msm.881380
27. Karahan N, Bozkurt KK, Cırış IM, Songür Y, Akin M, Cetın M, et al. Duodenojejunal invagination caused by small bowel metastasis of renal cell carcinoma. Turk J Gastroenterol (2011) 22(3):355–7. doi: 10.4318/tjg.2011.0231
28. Vazquez C, Berrueta J, De Simone F, Tcheckmedyian A, Gonzalez N, Bernachin J, et al. Small-intestinal bleeding due to metastatic renal cell cancer. Endoscopy (2011) 43(Suppl 2 UCTN):E13. doi: 10.1055/s-0030-1255821
29. Cohen DL. Small bowel metastasis from renal cell carcinoma identified on capsule endoscopy. ACG Case Rep J (2013) 1(1):3. doi: 10.14309/crj.2013.3
30. Chowdhury SD, Masih D, Chawla G, Pal S, Kurien RT, Augustine J. Metastasis of renal cell carcinoma to the duodenal papilla. Indian J Gastroenterol (2014) 33(5):493–4. doi: 10.1007/s12664-013-0398-y
31. Ismail I, Neuen BL, Mantha M. Solitary jejunal metastasis from renal cell carcinoma presenting as small bowel obstruction 19 years after nephrectomy. BMJ Case Rep (2015) 2015:bcr2015210857. doi: 10.1136/bcr-2015-210857
32. Segura UV, García-Leiva J, Nuñez BPJ. Duodenal metastases from sarcomatoid renal cell carcinoma: Case report. Gastroenterol Hepatol (2017) 40(8):530–2. doi: 10.1016/j.gastrohep.2016.07.002
33. Boullosa PE, Turnes AM, Iglesias RC, Prada JIR. Jejunal metastasis of renal cell carcinoma. Rev Esp Enferm Dig (2017) 109(2):147–8.
34. Vani M, Nambiar A, Geetha K, Kundil B. Metastatic renal cell carcinoma causing small intestinal polyps with intussusception: A report of two cases. J Clin Diagn Res (2017) 11(4):ED13–5. doi: 10.7860/JCDR/2017/25513.9652
35. Ignatavicius P, Lizdenis P, Pranys D, Gulbinas A, Pundzius J, Barauskas G. Long-term survival of patient with ampulla of vater metastasis of renal cell carcinoma. Prague Med Rep (2018) 119(4):165–9. doi: 10.14712/23362936.2019.4
36. Lin KH, Hsu YC, Yen HH. Metastatic renal cell carcinoma of the jejunum: a rare cause of obscure gastrointestinal bleeding. ANZ J Surg (2020) 90(4):626–7. doi: 10.1111/ans.15270
37. Gayer G, Zissin R, Apter S, Papa M, Hertz M. Pictorial review: adult intussusception–a CT diagnosis. Br J Radiol (2002) 75(890):185–90. doi: 10.1259/bjr.75.890.750185
38. Albiges L, Tannir NM, Burotto M, McDermott D, Plimack ER, Barthélémy P, et al. Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: extended 4-year follow-up of the phase III CheckMate 214 trial. ESMO Open (2020) 5(6):e001079. doi: 10.1136/esmoopen-2020-001079
39. Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med (2019) 380(12):1116–27. doi: 10.1056/NEJMoa1816714
40. Dutcher JP. Update on the biology and management of renal cell carcinoma. J Investig Med (2019) 67(1):1–10. doi: 10.1136/jim-2018-000918
41. Viadana E, Bross ID, Pickren JW. The metastatic spread of kidney and prostate cancers in man. Neoplasma (1976) 23(3):323–32.
42. Wang J, Thway K. Clear cell sarcoma-like tumor of the gastrointestinal tract: an evolving entity. Arch Pathol Lab Med (2015) 139(3):407–12. doi: 10.5858/arpa.2013-0547-RS
43. Stockman DL, Miettinen M, Suster S, Spagnolo D, Dominguez-Malagon H, Hornick JL, et al. Malignant gastrointestinal neuroectodermal tumor: clinicopathologic, immunohistochemical, ultrastructural, and molecular analysis of 16 cases with a reappraisal of clear cell sarcoma-like tumors of the gastrointestinal tract. Am J Surg Pathol (2012) 36(6):857–68. doi: 10.1097/PAS.0b013e31824644ac
45. Borrmann R. Geschwulste des magens und des duodenums. In: Henke F, Lubarsch O, editors. Handbuch der speziellen pathologischen anatomie und histology, vol. Vol. IV/I . Berlin: Springer (1986). p. 812–1054.
46. Powles T, Tomczak P, Park SH, Venugopal B, Ferguson T, Symeonides SN, et al. Pembrolizumab versus placebo as post-nephrectomy adjuvant therapy for clear cell renal cell carcinoma (KEYNOTE-564): 30-month follow-up analysis of a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol (2022) 23(9):1133–44. doi: 10.1016/S1470-2045(22)00487-9
47. Pal SK, Uzzo R, Karam JA, Master VA, Donskov F, Suarez C, et al. Adjuvant atezolizumab versus placebo for patients with renal cell carcinoma at increased risk of recurrence following resection (IMmotion010): a multicentre, randomised, double-blind, phase 3 trial. Lancet (2022) 400(10358):1103–16. doi: 10.1016/S0140-6736(22)01658-0
48. Gao S, Yan L, Zhang H, Fan X, Jiao X, Shao F. Identification of a metastasis-associated gene signature of clear cell renal cell carcinoma. Front Genet (2021) 11:603455. doi: 10.3389/fgene.2020.603455
Keywords: surgical oncology, case report, renal cell carcinoma, solitary metastasis, adult intussusception
Citation: Yang W, Cai Z, Nie P, Yuan T, Zhou H, Du Q, Qiu S, Zhang J and Yang L (2022) Case report and literature review: Small bowel intussusception due to solitary metachronous metastasis from renal cell carcinoma. Front. Oncol. 12:1072485. doi: 10.3389/fonc.2022.1072485
Received: 17 October 2022; Accepted: 06 December 2022;
Published: 19 December 2022.
Edited by:
Ugo Grossi, University of Padua, ItalyReviewed by:
Firdaus Hayati, University of Malaysia Sabah, MalaysiaFaten Limaiem, Hôpital Mongi Slim, Tunisia
Copyright © 2022 Yang, Cai, Nie, Yuan, Zhou, Du, Qiu, Zhang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lie Yang, bGllXzIyMkAxNjMuY29t
 Wenming Yang
Wenming Yang Zhaolun Cai
Zhaolun Cai Pan Nie3
Pan Nie3 Qiang Du
Qiang Du Lie Yang
Lie Yang