
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 19 January 2023
Sec. Thoracic Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.1070761
This article is part of the Research Topic KRAS in Stage IV Non-Small Cell Lung Cancer View all 10 articles
 Lixiu Peng1†
Lixiu Peng1† Jun Guo1†
Jun Guo1† Li Kong2
Li Kong2 Yong Huang3
Yong Huang3 Ning Tang1
Ning Tang1 Juguang Zhang1
Juguang Zhang1 Minglei Wang1
Minglei Wang1 Xiaohan He4
Xiaohan He4 Zhenzhen Li5
Zhenzhen Li5 Yonggang Peng4
Yonggang Peng4 Zhehai Wang1*
Zhehai Wang1* Xiao Han1*
Xiao Han1*Background: Immunotherapy has improved the clinical outcomes of patients with advanced non-small cell lung cancer (NSCLC). However, in patients with Kirsten rat sarcoma viral oncogene homolog (KRAS) mutations, the superior efficacy of immunotherapy has not been elucidated and especially in real-world practice. Our study aimed to use real-world data to assess the efficacy of immunotherapy in KRAS-mutant NSCLC in a Chinese cohort.
Methods: In this retrospective cohort study, we extracted the clinical, molecular, and pathologic data from the electronic health records of patients with advanced KRAS-mutant NSCLC at Shandong Cancer Hospital between January 2018 and May 2022. Furthermore, we evaluated the progression-free survival (PFS) and overall survival (OS) of the included patients.
Results: Between January 2018 and November 2020, 793 patients were identified with stage IIIB-IV NSCLC and a total of 122 patients with KRAS mutations were included in the analysis. The majority of patients were diagnosed with stage IV (82.0%) adenocarcinoma (93.4%), along with a history of smoking (57.4%). Of these, 42% of patients received anti-PD-(L)1 with or without chemotherapy (Immunotherapy-based regimens), while 58.2% of patients received chemotherapy (Chemotherapy-based regimens). The median overall survival (mOS) in this cohort was 22.9 months (95% CI: 14.1–31.7), while the median-progression-free survival (mPFS) was 9.4 months (95% CI: 6.6–12.1). Patients receiving immunotherapy-based regimens displayed better mOS than those receiving chemotherapy-based regimens (45.2 vs. 11.3 months; P=1.81E-05), with no statistical difference observed in the mPFS (10.5 vs. 8.2 months; P=0.706). Patients receiving immunotherapy-based regimens either in the first line (P=0.00038, P=0.010, respectively) or second-line setting (P=0.010, P=0.026, respectively) showed benefits in both PFS and OS. Subgroup analysis indicated that in patients having KRAS G12C or non-KRAS G12C mutant types, immunotherapy showed benefits of better OS (P=0.0037, P=0.020, respectively) than chemotherapy. Moreover, in advanced NSCLCs patients with or without KRAS/TP53 co-mutation the immunotherapy-based regimen achieved longer OS and PFS than chemotherapy-based regimens.
Conclusions: In the Chinese population of patients with KRAS-mutant advanced NSCLC, immunotherapy-based regimens achieved longer OS than chemotherapy-based regimens, which was independent of first or second-line setting, as well as KRAS mutational subtypes.
Non-small cell lung cancer (NSCLC) remains one of the major causes of cancer-related deaths in China and worldwide (1). The most common oncogenic driver in NSCLC is the mutation of Kirsten rat sarcoma viral oncogene homolog (KRAS), exhibiting approximately 20–30% prevalence among Western countries and 10–15% among Asian countries (2). KRAS mutant NSCLC is considered a heterogeneous disease regarding KRAS mutant subtypes, co-mutations (3), and immunogenic profiles (4). Biological heterogeneity is suggested to play a role in the vulnerability to therapy, tumor microenvironment, and immune modulatory effects. For instance, patients with KRAS/TP53 co-mutations were reported to be sensitive to immunotherapy (Objective Response Rate[ORR]: 35.7%), while patients with KRAS/STK11 displayed poorer outcomes upon treatment with immunotherapy (ORR: 7.4%) (5). However, a retrospective study showed that KRAS-mutant NSCLC might benefit from chemo-immunotherapy (6). KRAS has long been considered ‘undruggable’ (7), and the management of KRAS-addicted lung cancer is considered the same as that of non-oncogene-addicted cancer (8). Furthermore, limited treatment options and high heterogeneity may increase the difficulties of managing advanced KRAS-mutant patients.
Research on optimal management of KRAS-mutant NSCLC is still in progress. However, a breakthrough was achieved in the treatment landscape when the US Food and Drug Administration (FDA) approved direct KRAS G12C inhibitor Sotorasib for advanced or metastatic NSCLC adult patients having KRAS G12C local mutation, with patients receiving one prior systemic therapy. Immunotherapy is considered promising cancer therapy. Although most oncogene-addicted tumors, including EGFR-or ALK-driven lung cancer, do not respond to immunotherapy (9), even at >50% of PD-L1 expression. However, this is not the case in KRAS mutant NSCLC. The response rate to immunotherapy in such patients is shown to be at least the same or even better than that of KRAS-wild type patients (10–13). Few studies have also confirmed the superior efficacy of immunotherapy over chemotherapy in the KRAS-mutant NSCLC population. For instance, in one meta-analysis including three clinical trials, Kim et al. showed the superior efficacy of immunotherapy over chemotherapy in KRAS-mutant patients in the second-line setting (14). Similarly, a recent meta−analysis including six randomized controlled trials with 386 KRAS-mutant NSCLC patients suggested that anti-PD-(L)1 with or without chemotherapy displayed a significant association with prolonged OS (HR=0.59, 95%CI: 0.49–0.72; P<0.00001) and PFS (HR=0.58, 95%CI:0.43–0.78; P=0.0003) compared to chemotherapy alone (15).
However, since these findings were from the subgroup analysis of clinical studies, validating them in a real-world setting was necessary. Therefore, we conducted a real-world study in a Chinese population to verify the efficacy of immunotherapy with or without chemotherapy in KRAS-mutated advanced NSCLC patients.
The data for this retrospective observational cohort analysis was extracted from the electronic health records of patients at Shandong First Medical University Cancer Hospital and Shandong Cancer Hospital. The study was approved by the Ethics Committee of Shandong First Medical University Cancer Hospital and Shandong Cancer Hospital. Between January 2018 and November 2020, the patient records with stage IIIB-IV NSCLC were included in the study. The cohort used in this study was based on 793 patients. The above-mentioned clinical information mainly included baseline characteristics (sex, age, smoking status, histological subtype, ECOG PS, and tumor stage), KRAS mutation status, and treatment history of the patients. Furthermore, the patients were followed up from the date of diagnosis till the date of death due to all causes or up to the latest available follow-up.
Initially, patients included in the cohort met the following inclusion criteria: Age 18 years or older; diagnosed with stage IIIB to stage IV NSCLC with evidence of mutation in KRAS; receiving treatments from diagnosis to the end of follow-up. The exclusion criteria included records with no adequate information of pathological diagnosis, evidence of mutation in EGFR or ALK gene arrangement and ROS1 translocation, and records of EGFR TKIs treatment. The chemotherapy-based regimen was defined as the non-addition of anti-PD(L) 1 in the management of patients during the period of treatment.
Of the 51 immunotherapy-based patients, 6 received ICI monotherapy and 45 received ICI combination therapy with the following regimens: monotherapy: sintilimab, pembrolizumab, tislelizumab, and camrelizumab; combination therapy: sintilimab plus pemetrexed/platinum-based, sintilimab plus nab-paclitaxel/platinum-based, sintilimab plus docetaxel, pembrolizumab plus pemetrexed/platinum-based, tislelizumab plus pemetrexed/platinum-based, atelelizumab plus nab-paclitaxel/platinum-based, atelelizumab combined with bevacizumab and paclitaxel and platinum-based, toripalizumab combined with pemetrexed/platinum-based. Of the 71 patients treated with chemotherapy received the following conventional chemotherapy regimens: pemetrexed plus carboplatin or cisplatin, paclitaxel plus carboplatin or cisplatin, docetaxel plus carboplatin or cisplatin, gemcitabine plus carboplatin or cisplatin, bevacizumab combined with pemetrexed/platinum-based or paclitaxel/platinum-based.
Among the 122 patients, 24 patients were treated with first-line immunotherapy, 98 patients were treated with first-line chemotherapy, 21 patients were treated with second-line immunotherapy, and 26 patients were treated with second-line chemotherapy.
The primary endpoint was OS, which was defined as the period starting from the diagnosis till death or the date of the last follow-up. The secondary endpoint was real-world progression-free survival (rwPFS), defined as the time from diagnosis until objective tumor progression or death, whichever occurs first. Our study used a clinician-anchored approach supported by radiology data. Based on the radiology scan and pathologic confirmation via tissue biopsy or through clinical assessment, the clinician-recorded assessment was used to determine disease progression. Patients with missing information regarding the date of the last clinical note and progression were excluded from the rwPFS analysis.
Amplification refractory mutation system-polymerase chain reaction (ARMS-PCR) was used to identify KRAS mutation status. Genomic alterations were detected in patient samples using targeted sequencing panels (BerryOncology, Beijing), including a 456-gene (BerryOncology, Beijing) and a 36-gene test panel (BerryOncology, Beijing).
Standard descriptive statistics were used to compare the cohort characteristics between the chemotherapy- and immunotherapy-based regimen groups. The Fisher’s exact test and Mann-Whitney Wilcoxon test were used to compare the differences among variables of both groups, which included age, gender, smoking history, clinical stage, KRAS mutation subtype, KRAS gene co-mutation, distant metastasis, and the presence or absence of radiotherapy. Kaplan–Meier analysis was performed to estimate the survival rate, while the log-rank test was performed to test the differences in survival distribution among the subgroups. Moreover, the Cox proportional hazard regression model was used for univariate analyses. All statistical analyses were performed using the SPSS version 23.0, IBM software. The difference was considered statistically significant if the P-value was less than 0.05.
Of 632 patients with available gene test results, KRAS mutation was identified in a total of 142 advanced NSCLC patients. Among them, 20 patients did not receive any treatment at our hospital. Hence, we finally included 122 patients in our retrospective analysis, as shown in Figure 1, whose detailed clinical characteristics are summarized in Table 1. The cohort comprised 100 (82.0%) males and 22 (18.0%) females having an average age of 62 years. The major histological subtype included adenocarcinoma (n=114, 93.4%). Of these, 100 (82.0%) patients had recurrent or stage IV disease at the time of diagnosis. Additionally, 57.4% of the patients had a history of smoking. Finally, all patients were treated based on their clinical staging status. Our results showed no significant differences in clinical characteristics, except for anti-angiogenesis therapy (P=0.03)
Our study showed the median overall survival (mOS) of KRAS-mutant advanced NSCLC patients as 22.9 months (95% CI: 14.07–31.67) and the median progression-free survival (mPFS) as 9.4 months (95% CI: 6.60–12.14) (Figures 2A, B). While 51 (41.8%) patients received immunotherapy-based regimens, 71 (58.2%) received chemotherapy-based regimens (Table 1). Patients receiving immunotherapy-based regimens displayed significantly longer mOS compared to patients receiving chemotherapy-based regimens (45.2 vs. 11.3 months; P=1.81E-5), with no significant difference observed in the mPFS (10.5 vs. 8.2 months; P=0.706) (Figures 2C, D). Additionally, immunotherapy from both first-and second-line treatments showed survival benefits. Patients receiving immunotherapy-based regimens as the first line of treatment displayed better mOS and mPFS than those receiving chemotherapy-based regimens (mOS: 33.5 vs. 16.1 months; P=0.010, mPFS: 32.2 vs. 6.9 months; P=0.00038) (Figures 3A, B). Similarly, the patients receiving immunotherapy as the second line of treatment also displayed significant improvement in the mOS and mPFS compared to those receiving chemotherapy (mOS: NR vs. 9.23 months; P=0.026, mPFS: 10.8 vs. 5.5 months; P=0.010) (Figures 3C, D).
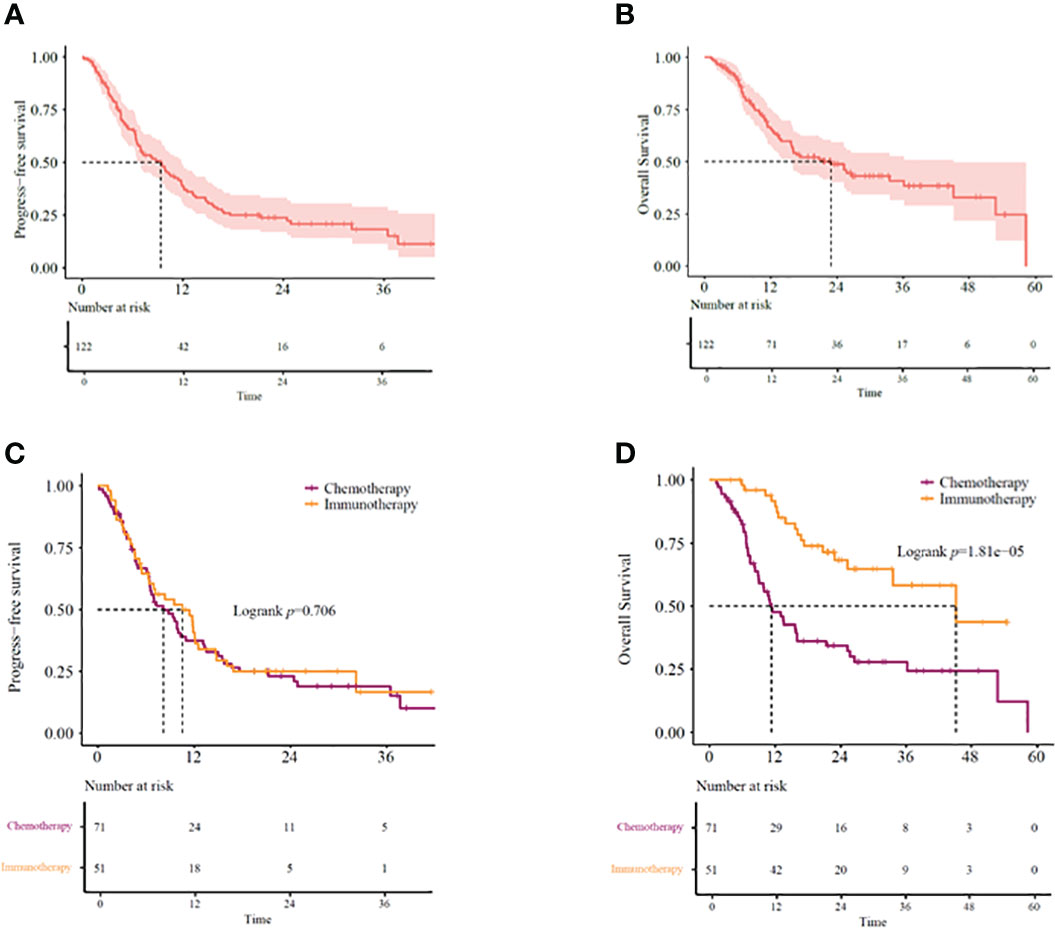
Figure 2 (A) PFS and (B) OS in KRAS-mutant advanced NSCLC patients. (C) PFS and (D) OS in KRAS-mutant advanced NSCLC patients receiving immunotherapy- or chemotherapy-based regimens. OS, overall survival; PFS, progression-free survival.
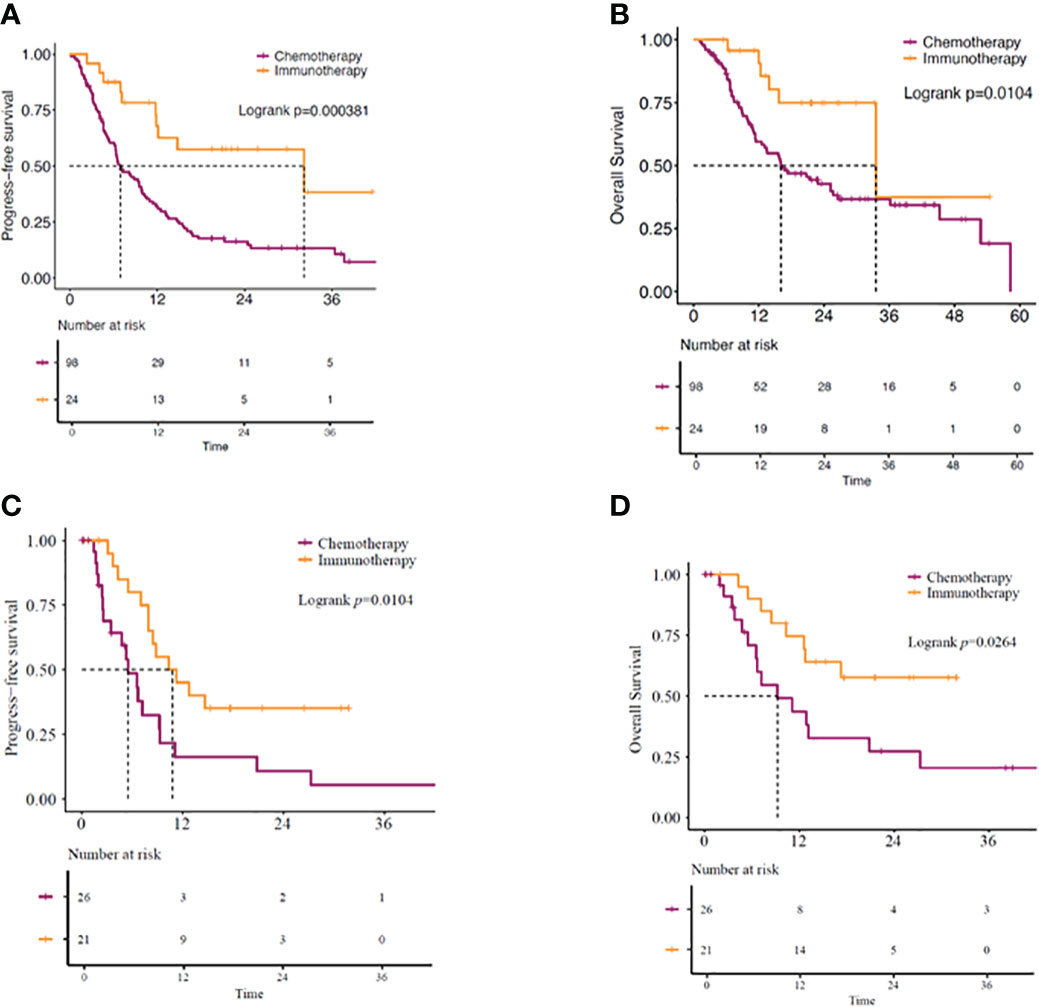
Figure 3 (A) PFS and (B) OS in KRAS-mutant advanced NSCLC patients. Patients receiving immunotherapy- or chemotherapy-based regimens as first-line of treatment. (C) PFS and (D) OS in KRAS-mutant advanced NSCLC patients receiving immunotherapy- or chemotherapy-based regimens as second-line of treatment. OS, overall survival; PFS, progression-free survival.
Since specific KRAS mutational subtypes may exert different effects on treatment response and survival, we aimed to characterize the effects of KRAS mutation subtypes on the OS and treatment response of these patients. With the available information on mutation revealed by molecular characterization, we stratified the patients into KRAS G12C and KRAS non-G12C subgroups. Genomic profiles of 64 KRAS mutant patients were analyzed using next-generation sequencing (Berryoncology, Beijing), which detected two major mutation subtypes, including G12C (20.5%) and non-G12C (32.0%). The G12C status was unknown for 47.5% of the patients. Among the four different categories of KRAS-mutant NSCLCs, significant differences were observed in both mOS and mPFS (mOS: log-rank test, P=0.00020; mPFS: log-rank test, P=0.026) (Figures 4A, B). Further analysis revealed that KRAS G12C and non-G12C subtype patients treated with immunotherapy-based regimens showed significantly better mOS compared to the same patients receiving chemotherapy-based regimens (G12C group HR=0.23,95%CI:0.08-0.67, P=0.0074; mOS: 25.2 vs. 9.1 months, P=0.0037; non-G12C group HR=0.13,95%CI:0.02-0.99, P=0.049; mOS: NR vs. 25.7 months, P=0.020). However, significant difference for PFS was observed in G12C group but not in non-G12C group (G12C group HR=0.38,95%CI:0.14-0.99, P=0.047; mPFS: 12.1 vs. 5.0 months, P=0.039; non-G12C group HR=0.73,95%CI:0.3-1.75, P=0.48; mPFS: 14.8 vs. 10.3 months, P=0.48).
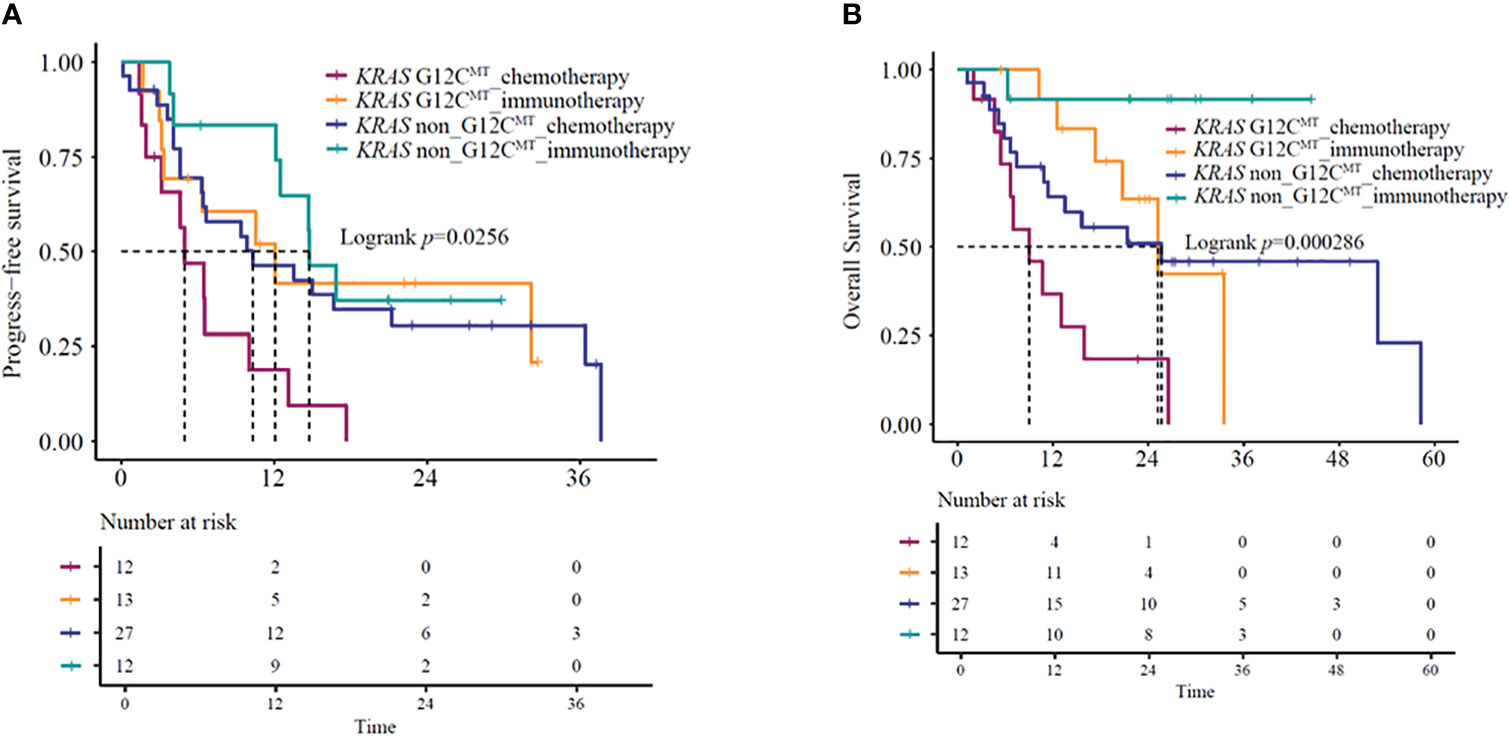
Figure 4 (A) PFS and (B) OS in KRAS G12C-mutant and KRAS non-G12C mutant NSCLC patients receiving immunotherapy- or chemotherapy-based regimens. OS, overall survival; PFS, progression-free survival.
Several studies (16–18) have indicated that under immunotherapy, the co-mutation status of advanced KRAS-mutant type exerts an impact on the patient’s clinical outcomes. Based on the co-mutation status, we used the NGS results of 64 patients for survival analysis. The identified co-mutations included TP53 (20.3%), PIK3CA (1.6%), and STK11 (0.8%). Kaplan-Meier curves based on TP53 mutation status and treatment group showed a significant difference in mOS (P=0.035) (Figure 5A) but not in mPFS (P = 0.41) (Figure 5B). Further analysis suggested that KRAS/TP53 co-mutation group and non- KRAS/TP53 mutation group patients treated with immunotherapy-based regimens showed significantly better mOS compared to the same patients receiving chemotherapy-based regimens (KRAS/TP53 co-mutation group HR=0.32, 95%CI:0.1-0.98, P=0.047; mOS:33.5 vs. 11.8 months, P=0.036; non-KRAS/TP53 co-mutation group HR=0.23,95%CI:0.05-0.99, P=0.049; mOS: NA vs. 16 months, P=0.031). However, no significant difference was observed in the mPFS (KRAS/TP53 co-mutation group HR=0.78,95%CI:0.31-1.96, P=0.59; mPFS:12.5 vs.10.0 months, P=0.59; non- KRAS/TP53 co-mutation group HR=0.49,95%CI:0.2-1.23, P=0.13; mPFS: 16.9 vs. 6.7 months, P=0.12).
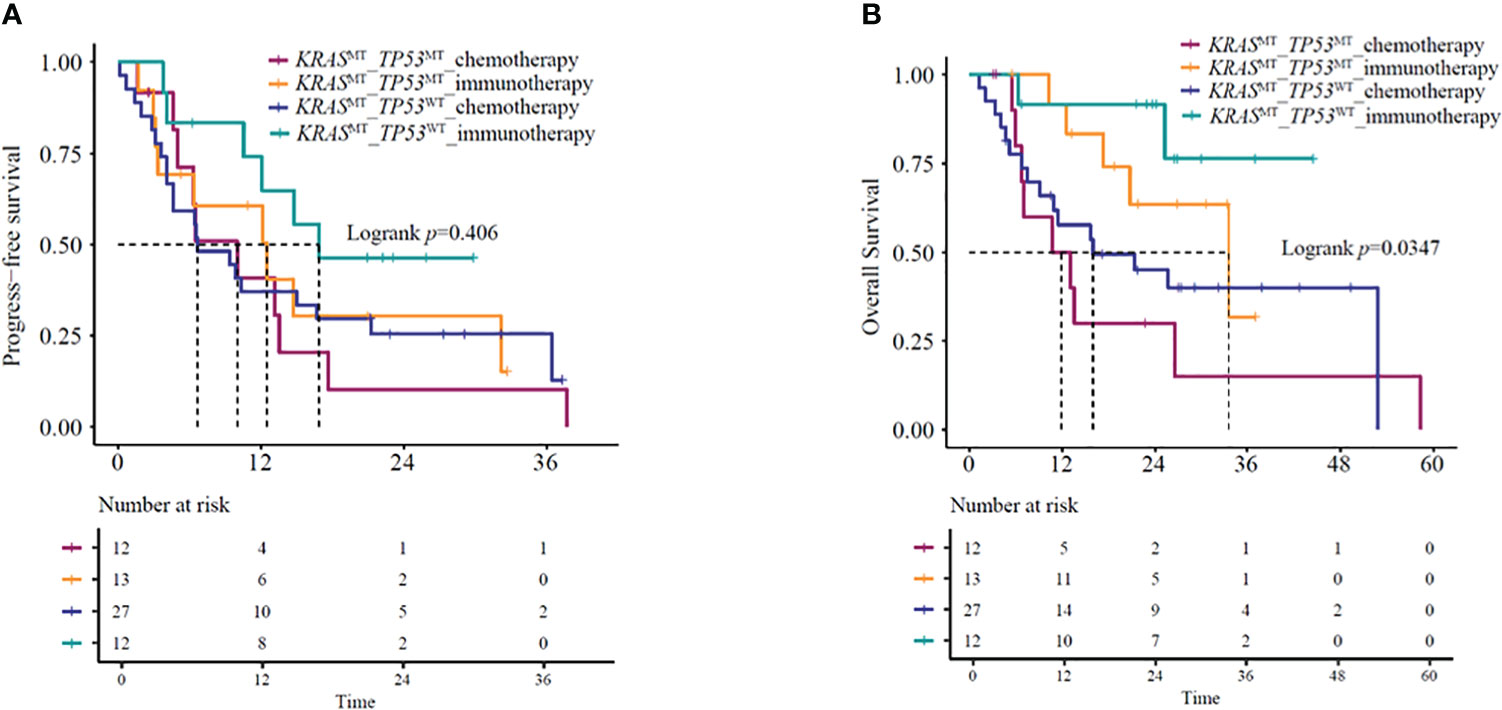
Figure 5 (A) PFS and (B) OS in KRAS/TP53 co-mutation or KRAS mutant/TP53 wild-type patients receiving immunotherapy- or chemotherapy-based regimens. OS, overall survival; PFS, progression-free survival.
KRAS-mutant NSCLC is a genetically heterogeneous disease with distinct biology and therapeutic vulnerabilities. An effective choice of treatment for this disease is immunotherapy. However, further investigation, especially in real-world settings, may be required to verify the efficacy of immunotherapy in KRAS-mutant NSCLC patients. Therefore, we retrospectively studied 122 advanced NSCLC patients with KRAS mutations for their prognosis and obtained the mOS at 22.9 months (Figure 2B). This result was similar to a previous study, where mOS was 28 months (19). Furthermore, the mOS was 25.8 months in the study of El Osta., et al, which was similar to our study (20). In our study, patients receiving immunotherapy-based regimes displayed a significantly longer OS than those receiving chemotherapy-based regimens (45.2 vs. 11.3 months, P=0.001) (Figure 2D). Moreover, the survival benefits were independent of whether it was the first-line setting or second-line setting, which was also consistent with the subgroup analysis results of previous clinical trials (15). In addition, outcomes of the KEYNOTE189 shows that patients receiving immunotherapy plus chemotherapy have longer mPFS than those receiving chemotherapy (9 vs. 5 months, HR=0.47,95%CI [0.29-0.77]) in KRAS -mutated lung cancer (21). In the 2022 ASCO meeting, data scientists from the FDA conducted a large retrospective analysis, including 555 metastatic NSCLC patients with KRAS mutations. Their analysis concluded that the chemo-immune checkpoint inhibitor combination produced the greatest survival benefit compared to the treatment with immune checkpoint inhibitors (ICIs) or chemotherapy alone and hence, should be given to such patients upfront (22). Specifically, chemo-ICIs as the first line of treatment were linked to a response rate of 46%, while ICI alone generated a response rate of 37%, indicating that chemo-immunotherapy may be the optimal management option for the advanced KRAS-mutant NSCLC patients both in white and Asian populations.
The enhanced survival benefits in this study can be explained using several biological rationales. KRAS mutations in NSCLC were associated with tobacco smoking, a high tumor mutational burden (TMB), and an inflammatory tumor microenvironment, along with high T-cell infiltration (23). Importantly, compared to the wild-type counterparts, KRAS-mutant tumors showed higher expression of PD-L1, with the median PD-L1 tumor proportion scores ranging between 30–60% and 5–35% in patients with and without KRAS mutations, respectively (21, 24). One study suggested that the activation of the KRAS-signaling pathway resulted in the inhibition of tristetraprolin activity, which is important for the stabilization of PD-L1-mRNA and, thus, its synthesis (25). Another study showed that KRAS mutations were correlated to an inflammatory tumor microenvironment and tumor immunogenicity, which benefitted the response to ICIs (23). Since KRAS-mutated NSCLC is typically smoking-related lung cancer, with more than 90% of patients having a history of smoking, it is more likely that such patients will respond to ICI treatment.
Notably, the patients treated with a combination of anti-PD(L)1 and chemotherapy (immunotherapy-based regimens) showed an mOS of 45 months, which was longer than most previous studies (21, 26). This may be because the Eastern Cooperative Oncology Group performance score (ECOG PS) of the patients was between 1 and 2. The value of ECOG PS was 0~1 in 84.4% of patients and 2 in 15.6% of patients. Multiple retrospective cohort studies across different tumor types have suggested that patients with ECOG PS ≥2 showed worse response rates, faster progression, and shorter OS (27–29). Additionally, a recent study showed that mOS of advanced NSCLC patients with good performance status was 30 months (95% CI 16.6–42.3), but in patients with poor performance status, it was only 4 months (95% CI 3.2–8.1) (30), which was similar to our results.
No significant difference was observed in PFS between immunotherapy and chemotherapy. Studies suggested no correlation between the mOS and mPFS (31, 32) in immunotherapy-related clinical trials. Moreover, in randomized clinical trials of PD-1 inhibitors, the effect of treatment was higher on OS than on PFS (31), which was consistent with our results. This suggested that PFS may not be able to capture the benefits of immune checkpoint inhibitors. PD-1 inhibitors have residual efficacy for a longer duration, and even after the discontinuation of treatment, these drugs could affect OS more than PFS. Therefore, the RECIST criteria may not be completely suitable to measure the immunotherapy response.
Previous studies demonstrated that KRAS G12C mutations and TP53 co-mutations were correlated to benefits obtained from anti-PD-1/PD-L1 immunotherapy (18). Similar results were also found in this study, where patients with KRAS-G12C mutation receiving immunotherapy with or without chemotherapy achieved more survival benefits than chemotherapy alone. This indicated the significant role of immunotherapy in the clinical management of these patients. The combination strategy may abolish the adverse OS impact of the KRAS G12C mutant. A preclinical study suggested that KRAS G12C inhibition can swiftly change the tumor’s immune-suppressive microenvironment to the one that allows effective anti-tumor immunity (33). In addition, a phase 2 trial results of sotorasib for lung cancers with KRAS G12C mutation showed that the mPFS was 6.8 months (95% CI, 5.1 to 8.2) and the mOS was 12.5 months (95% CI, 10.0 to could not be evaluated) (34).Due to a higher level of PD-L1 expression, T cell infiltration, and tumor immunogenicity, the KRAS/TP53 co-mutation in NSCLC exhibited sensitivity to anti-PD-1/PD-L1 immunotherapy (17). In our study, the advanced NSCLCs patients with or without KRAS/TP53 co-mutation benefitted more from the immunotherapy-based regimens than chemotherapy-based regimens in mOS (KRASMTTP53WT mOS: P=0.36; KRASMTTP53WT mOS: P=0.049). Furthermore, no significant differences were observed in mPFS between immunotherapy receiving KRASMTTP53MT and KRASMTTP53WT patients (KRASMTTP53MT mPFS: P=0.59; KRASMTTP53WT mPFS: P=0.12), which may be due to the small size of our study sample. Hence, this aspect may require further investigation.
The first limitation of our study was the insufficient characterization of the genomic profiles of the patients, with ARMS-PCR being applied to only nearly half of the patients. Also, performing survival analyses in subgroups based on KRAS-mutation and co-mutation status was challenging. Second, since heterogeneous patients with various levels of PD-L1 expression and TMB status, KRAS mutation status may have affected the survival outcomes of ICIs differently as per the expression level of PD-L1. For example, patients with high PD-L1 levels receiving immunotherapy as the first line of treatment may have fared as well as those who received chemo-immunotherapy (35). Also, these levels were only available in a small proportion of patients. Hence, whether the superior efficacy of ICIs observed in this study was independent of TMB status and/or PD-L1 expression remains unknown. Thirdly, our study was a single-center study and not fully representative of the broader population of cancer patients in China, which in some cases, may limit the generalizability of the obtained data. Therefore, to make informed clinical decisions, further studies may be needed to provide sufficient evidence.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by Ethics Committee of Shandong First Medical University Cancer Hospital and Shandong Cancer Hospital. The patients/participants provided their written informed consent to participate in this study.
ZHW and XH designed the research and revised the article. LXP and JG analyzed the data and wrote the manuscript. ZZL analyzed the data. LK, YH, NT, JGZ and MLW collected the data. XHH and YGP revised the article. All authors contributed to the article and approved the submitted version.
The authors thank Berry Oncology Corporation for the sequencing and analysis of tumor samples.
Author YGP, XHH, and ZZL were employed by Berry Oncology Corporation.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
2. Dearden S, Stevens J, Wu YL, Blowers D. Mutation incidence and coincidence in non small-cell lung cancer: Meta-analyses by ethnicity and histology (mutMap). Ann Oncol (2013) 24:2371–6. doi: 10.1093/annonc/mdt205
3. Scheffler M, Ihle MA, Hein R, Merkelbach-Bruse S, Scheel AH, Siemanowski J, et al. K-Ras mutation subtypes in NSCLC and associated Co-occuring mutations in other oncogenic pathways. J Thorac Oncol (2019) 14:606–16. doi: 10.1016/j.jtho.2018.12.013
4. Falk AT, Yazbeck N, Guibert N, Chamorey E, Paquet A, Ribeyre L, et al. Effect of mutant variants of the KRAS gene on PD-L1 expression and on the immune microenvironment and association with clinical outcome in lung adenocarcinoma patients. Lung Cancer (2018) 121:70–5. doi: 10.1016/j.lungcan.2018.05.009
5. Skoulidis F, Goldberg ME, Greenawalt DM, Hellmann MD, Awad MM, Gainor JF, et al. STK11/LKB1 mutations and PD-1 inhibitor resistance in KRAS-mutant lung adenocarcinoma. Cancer Discov (2018) 8:822–35. doi: 10.1158/2159-8290.CD-18-0099
6. Chen H, Huang D, Lin G, Yang X, Zhuo M, Chi Y, et al. The prevalence and real-world therapeutic analysis of Chinese patients with KRAS-mutant non-small cell lung cancer. Cancer Med (2022) 11:3581–92. doi: 10.1002/cam4.4739
7. Reck M, Carbone DP, Garassino M, Barlesi F. Targeting KRAS in non-small-cell lung cancer: Recent progress and new approaches. Ann Oncol (2021) 32:1101–10. doi: 10.1016/j.annonc.2021.06.001
8. Ghimessy A, Radeczky P, Laszlo V, Hegedus B, Renyi-Vamos F, Fillinger J, et al. Current therapy of KRAS-mutant lung cancer. Cancer Metastasis Rev (2020) 39:1159–77. doi: 10.1007/s10555-020-09903-9
9. Negrao MV, Skoulidis F, Montesion M, Schulze K, Bara I, Shen V, et al. Oncogene-specific differences in tumor mutational burden, PD-L1 expression, and outcomes from immunotherapy in non-small cell lung cancer. J Immunother Cancer (2021) 9(8):e002891. doi: 10.1136/jitc-2021-002891
10. Cinausero M, Laprovitera N, De Maglio G, Gerratana L, Riefolo M, Macerelli M, et al. KRAS and ERBB-family genetic alterations affect response to PD-1 inhibitors in metastatic nonsquamous NSCLC. Ther Adv Med Oncol (2019) 11:1758835919885540. doi: 10.1177/1758835919885540
11. Jeanson A, Tomasini P, Souquet-Bressand M, Brandone N, Boucekine M, Grangeon M, et al. Efficacy of immune checkpoint inhibitors in KRAS-mutant non-small cell lung cancer (NSCLC). J Thorac Oncol (2019) 14:1095–101. doi: 10.1016/j.jtho.2019.01.011
12. Noordhof AL, Damhuis RAM, Hendriks LEL, de Langen AJ, Timens W, Venmans BJW, et al. Prognostic impact of KRAS mutation status for patients with stage IV adenocarcinoma of the lung treated with first-line pembrolizumab monotherapy. Lung Cancer (2021) 155:163–9. doi: 10.1016/j.lungcan.2021.04.001
13. Passiglia F, Cappuzzo F, Alabiso O, Bettini AC, Bidoli P, Chiari R, et al. Efficacy of nivolumab in pre-treated non-small-cell lung cancer patients harbouring KRAS mutations. Br J Cancer (2019) 120:57–62. doi: 10.1038/s41416-018-0234-3
14. Kim JH, Kim HS, Kim BJ. Prognostic value of KRAS mutation in advanced non-small-cell lung cancer treated with immune checkpoint inhibitors: A meta-analysis and review. Oncotarget (2017) 8:48248–52. doi: 10.18632/oncotarget.17594
15. Landre T, Justeau G, Assie JB, Chouahnia K, Davoine C, Taleb C, et al. Anti-PD-(L)1 for KRAS-mutant advanced non-small-cell lung cancers: a meta-analysis of randomized-controlled trials. Cancer Immunol Immunother (2022) 71:719–26. doi: 10.1007/s00262-021-03031-1
16. Shepherd FA, Lacas B, Le Teuff G, Hainaut P, Jänne PA, Pignon JP, et al. Pooled analysis of the prognostic and predictive effects of TP53 comutation status combined with KRAS or EGFR mutation in early-stage resected non–small-cell lung cancer in four trials of adjuvant chemotherapy. J Clin Oncol (2017) 35:2018–27. doi: 10.1200/JCO.2016.71.2893
17. Dong ZY, Zhong WZ, Zhang XC, Su J, Xie Z, Liu SY, et al. Potential predictive value of TP53 and KRAS mutation status for response to PD-1 blockade immunotherapy in lung adenocarcinoma. Clin Cancer Res (2017) 23:3012–24. doi: 10.1158/1078-0432.CCR-16-2554
18. Aredo JV, Padda SK, Kunder CA, Han SS, Neal JW, Shrager JB, et al. Impact of KRAS mutation subtype and concurrent pathogenic mutations on non-small cell lung cancer outcomes. Lung Cancer (2019) 133:144–50. doi: 10.1016/j.lungcan.2019.05.015
19. Amanam I, Mambetsariev I, Gupta R, Achuthan S, Wang Y, Pharaon R, et al. Role of immunotherapy and co-mutations on KRAS-mutant non-small cell lung cancer survival. J Thorac Dis (2020) 12:5086–95. doi: 10.21037/jtd.2020.04.18
20. El Osta B, Behera M, Kim S, Berry LD, Sica G, Pillai RN, et al. Characteristics and outcomes of patients with metastatic KRAS-mutant lung adenocarcinomas: The lung cancer mutation consortium experience. J Thorac Oncol (2019) 14:876–89. doi: 10.1016/j.jtho.2019.01.020
21. Gadgeel S, Rodriguez-Abreu D, Felip E, Esteban E, Speranza G, Reck M, et al. LBA5-KRAS mutational status and efficacy in KEYNOTE-189: Pembrolizumab (pembro) plus chemotherapy (chemo) vs placebo plus chemo as first-line therapy for metastatic non-squamous NSCLC. Annals of Oncology (2019) 30:xi64–5. doi: 10.1093/annonc/mdz453.002
22. Nakajima EC, Ren Y, Vallejo JJ, Akinboro O, Mishra-Kalyani PS, Larkins EA, et al. Outcomes of first-line immune checkpoint inhibitors with or without chemotherapy according to KRAS mutational status and PD-L1 expression in patients with advanced NSCLC: FDA pooled analysis. J Clin Oncol (2022) 40:9001–1. doi: 10.1200/JCO.2022.40.16_suppl.9001
23. Liu C, Zheng S, Jin R, Wang X, Wang F, Zang R, et al. The superior efficacy of anti-PD-1/PD-L1 immunotherapy in KRAS-mutant non-small cell lung cancer that correlates with an inflammatory phenotype and increased immunogenicity. Cancer Lett (2020) 470:95–105. doi: 10.1016/j.canlet.2019.10.027
24. Herbst R, Lopes G, Kowalski D, Kasahara K, Wu Y-L, De Castro G Jr., et al. LBA4 association of KRAS mutational status with response to pembrolizumab monotherapy given as first-line therapy for PD-L1-positive advanced non-squamous NSCLC in keynote-042. Ann Oncol (2019) 30:xi63–4. doi: 10.1093/annonc/mdz453.001
25. Coelho MA, de Carne Trecesson S, Rana S, Zecchin D, Moore C, Molina-Arcas M, et al. Oncogenic RAS signaling promotes tumor immunoresistance by stabilizing PD-L1 mRNA. Immunity (2017) 47:1083–1099 e1086. doi: 10.1016/j.immuni.2017.11.016
26. Gianoncelli L, Spitaleri G, Passaro A, Radice D, Fumagalli C, Del Signore E, et al. Efficacy of anti-PD1/PD-L1 therapy (IO) in KRAS mutant non-small cell lung cancer patients: A retrospective analysis. Anticancer Res (2020) 40:427–33. doi: 10.21873/anticanres.13970
27. Khaki AR, Li A, Diamantopoulos LN, Bilen MA, Santos V, Esther J, et al. Impact of performance status on treatment outcomes: A real-world study of advanced urothelial cancer treated with immune checkpoint inhibitors. Cancer (2020) 126:1208–16. doi: 10.1002/cncr.32645
28. Petrillo LA, El-Jawahri A, Nipp RD, Lichtenstein MRL, Durbin SM, Reynolds KL, et al. Performance status and end-of-life care among adults with non-small cell lung cancer receiving immune checkpoint inhibitors. Cancer (2020) 126:2288–95. doi: 10.1002/cncr.32782
29. Sehgal K, Gill RR, Widick P, Bindal P, McDonald DC, Shea M, et al. Association of performance status with survival in patients with advanced non-small cell lung cancer treated with pembrolizumab monotherapy. JAMA Network Open (2021) 4:e2037120. doi: 10.1001/jamanetworkopen.2020.37120
30. Kawsar H, Gaudel P, Suleiman N, Al-Jumayli M, Huang C, Neupane P. 221 poor performance status negatively affects survival benefit of immunotherapy in non-small cell lung cancer. J Immunother Cancer (2020) 8:A131–2. doi: 10.1136/jitc-2020-SITC2020.0221
31. Gyawali B, Hey SP, Kesselheim AS. A comparison of response patterns for progression-free survival and overall survival following treatment for cancer with PD-1 inhibitors: A meta-analysis of correlation and differences in effect sizes. JAMA Network Open (2018) 1:e180416. doi: 10.1001/jamanetworkopen.2018.0416
32. Mushti SL, Mulkey F, Sridhara R. Evaluation of overall response rate and progression-free survival as potential surrogate endpoints for overall survival in immunotherapy trials. Clin Cancer Res (2018) 24:2268–75. doi: 10.1158/1078-0432.CCR-17-1902
33. Canon J, Rex K, Saiki AY, Mohr C, Cooke K, Bagal D, et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature (2019) 575:217–23. doi: 10.1038/s41586-019-1694-1
34. Skoulidis F, Li BT, Dy GK, Price TJ, Falchook GS, Wolf J, et al. Sotorasib for lung cancers with KRAS p. G12C mutation. N Engl J Med (2021) 384:2371–81. doi: 10.1056/NEJMoa2103695
35. Perol M, Felip E, Dafni U, Polito L, Pal N, Tsourti Z, et al. Effectiveness of PD-(L)1 inhibitors alone or in combination with platinum-doublet chemotherapy in first-line (1L) non-squamous non-small-cell lung cancer (Nsq-NSCLC) with PD-L1-high expression using real-world data. Ann Oncol (2022) 33:511–21. doi: 10.1016/j.annonc.2022.02.008
Keywords: NSCLC, KRAS, immunotherapy, co-mutation, KRAS-mutant subtypes
Citation: Peng L, Guo J, Kong L, Huang Y, Tang N, Zhang J, Wang M, He X, Li Z, Peng Y, Wang Z and Han X (2023) Efficacy of immunotherapy in KRAS-mutant advanced NSCLC: A real-world study in a Chinese population. Front. Oncol. 12:1070761. doi: 10.3389/fonc.2022.1070761
Received: 15 October 2022; Accepted: 28 December 2022;
Published: 19 January 2023.
Edited by:
Wouter Van Geffen, Medisch Centrum Leeuwarden, NetherlandsReviewed by:
Armin Frille, University Hospital Leipzig, GermanyCopyright © 2023 Peng, Guo, Kong, Huang, Tang, Zhang, Wang, He, Li, Peng, Wang and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhehai Wang, d3poYWk4Nzc4QHNpbmEuY29t; Xiao Han, aHh6YmIxOTgzQDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.