
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 04 January 2023
Sec. Cancer Imaging and Image-directed Interventions
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.1061242
This article is part of the Research TopicIncorporation of Reporting and Data Systems into Cancer RadiologyView all 8 articles
 Quanyang Wu1
Quanyang Wu1 Shijun Zhao1
Shijun Zhao1 Yao Huang1
Yao Huang1 Jianwei Wang1
Jianwei Wang1 Wei Tang1
Wei Tang1 Lina Zhou1
Lina Zhou1 Linlin Qi1
Linlin Qi1 Zewei Zhang2
Zewei Zhang2 Yuting Xie3
Yuting Xie3 Jiaxing Zhang1
Jiaxing Zhang1 Hongjia Li2
Hongjia Li2 Ning Wu1,4*
Ning Wu1,4*Background: Screening for lung cancer with LDCT detects a large number of nodules. However, it is unclear whether nodule number influences lung cancer probability. This study aimed to acquire deeply insight into the distribution characteristics of nodule number in the Chinese population and to reveal the association between the nodule number and the probability of lung cancer (LC).
Methods: 10,167 asymptomatic participants who underwent LDCT LC screening were collected. Noncalcified nodules larger than 4 mm were included. The nodule number per participant was determined. We defined five categories according to the number of nodules (based on nodule type and size): one, two, three, four, and more than four nodules. We stratified the nodules as groups A, B, and C and participants as Amax, Bmax, and Cmax groups, and explored the association between nodule number and the probability of LC on nodule and participant levels.
Results: 97 participants were confirmed to have LC. The probabilities of LC were 49/1719, 22/689, 11/327, 6/166, and 9/175 in participants with one, two, three, four, and more than four nodules (p>0.05), respectively. In the Bmax group, the probability of LC was significantly higher in participants with one nodule than those with >4 nodules (p<0.05), and the probability of LC showed a negative linear trend with increasing nodule numbers (p<0.05). Based on the nodule-level analyses, in Group B, LC probability was significantly higher when participants had a solitary nodule than when they had >4 nodules (p<0.05).
Conclusion: LC probability does not significantly change with the number of nodules. However, when stratified by the nodule size, the effect of nodule number on LC probability was nodule-size dependent, and greater attention and active follow-up are required for solitary nodules especially SNs/solid component of PSNs measuring 6-15 mm or NSNs measuring 8-15 mm. Assessing the nodule number in conjunction with nodule size in baseline LDCT LC screening is considered beneficial.
According to GLOBOCAN 2020, lung cancer (LC) ranks second in the world’s newly occurring malignant tumors and remains the leading cause of cancer-related mortality worldwide (1). Most patients with LC are already in the moderate to late stages at diagnosis (2, 3), and their 5-year survival rate are only 21% (4). Therefore, performing LC screening to improve the rate of early diagnosis and treatment is of great significance to improve patient survival and reduce LC mortality. Data from a nonrandomized controlled study of the International Early Lung Cancer Action Program (I-ELCAP) indicate that LC can be detected at an early stage during low-dose computed tomography (LDCT) screening, with stage I LC numbers more than 80% of cases. With timely treatment, the expected 10-year survival rate is as high as 88% (5). The results of the National Lung Screening Trial (NLST) in the United States and the Dutch-Belgian Randomized Lung Cancer Screening Trial (NELSON) in Europe showed that LDCT screening can reduce LC mortality (6, 7). The characteristics of LC screening in Asian populations differ from those in Europe and America, especially the proportion of non-smokers in female lung cancer participants is significantly higher than that in Europe and America (8). The results of the NLST showed that 26.8% of the participants had pulmonary nodules at the 4mm threshold criterion and pulmonary nodules are clinically significant because they may be the first presentation of LC (9). The main concern of LC screening is the early identification of their differentiation between benign and malignant in indeterminate pulmonary nodules. Some nodule characteristics, such as nodule diameter, speculation, location of the superior lobe, and the solid components, may be associated with an increased probability of LC (10, 11). Volume and mass also has been reported to potentially reflect the natural growth history of nodules (12, 13). However, nodule size is still the first consideration in LC risk assessment.
Malignancy estimation and management recommendations are primarily based on expert consensus or data from research trials, and nodule-grade classification is often based on nodule size. Risk assessment of participants with multiple nodules is often based on the highest risk nodule, usually the largest nodule, but smaller nodules should not be ignored. However, one of the neglected aspects in real-world clinical implementation is the nodule number detected in patients during LDCT LC screening. Although multiple nodules are often identified during LDCT screening, the number of nodules is frequently ignored in the risk assessment of LC. A limited analysis of multinodularity and LC risk was conducted for nodules detected in NELSON trial. Nodule number was found to be ambiguous in relation to LC probability in participants, with LC probability varying with the number of nodules (14). The effect of the number of nodules on the probability of LC deserves further study. This study aimed to explore the correlation between the probability of LC and number of nodules detected during baseline LDCT LC screening within the Chinese population.
LDCT screening data were collected and analyzed for 10167 asymptomatic participants undergoing early LC screening at the National Cancer Center from 2010 to 2018. The opportunistic LDCT LC screening inclusion criteria were as follows: (a) aged 40–80 years; (b) participants with no clinical symptoms; (c) participants with no cancer history within 5 years (via registry); and (d) participants with good general physical condition and willingness to undergo invasive diagnostic assessment and treatment, such as biopsy, puncture, and surgery, required for positive screening results. Participants who met the basic eligibility criteria were included in the study if they met both of the following requirements: (a) aged 50–75 years; (b) CT images are available. The institutional review board of Cancer Hospital, Chinese Academy of Medical Sciences approved this retrospective study and waived the requirement for informed consent.
In this study, we included noncalcified nodules ≥ 4 mm in diameter (nodule diameter refers to the maximum diameter) and defined these nodules as positive nodules. The correlation between nodule number and LC at the nodule and participant levels was explored. According to the needs of the research, the grouping criteria were developed through the integration of NCCN guidelines, Lung-RADS guidelines, and Chinese lung cancer screening guidelines (15–17). On the nodule level, the detected nodules were divided into three groups (diameter cut-off refer to maximum diameter): solid nodules (SNs)/solid components of partial SNs < 6 mm or non-SNs (NSNs) < 8 mm (Group A), SNs/solid components of partial SNs measuring 6-15 mm or NSNs measuring 8-15 mm (Group B), and SNs/solid components of partial SNs or NSNs ≥ 15 mm (Group C). On the participant level, the largest nodules of each participant were considered as the study object. The participants were divided into three groups based on the largest nodule: SNs/solid components of partial SNs < 6 mm or NSNs < 8 mm (Group Amax), SNs/solid components of partial SNs measuring 6-15 mm, or NSNs measuring 8-15 mm (Group Bmax), and SN/solid components of partial SNs or NSNs ≥ 15 mm (Group Cmax).
All CT scans were obtained using 64-detector row scanners (LightSpeed VCT, Discovery CT750 HD or Optima CT660, General Electric Medical Systems; Healthineer or Edge, Siemens Medical Systems) at full inspiration. The CT scanning parameters were as follows: tube voltage, 120 kVp; automatic current time, 20–250 mA with rotation time of 0.5 s; and thickness, 5 mm. Reconstruction thicknesses were 1.0 and 1.25 mm, and the interval was 0.8 mm using a standard reconstruction algorithm. The “Dose Report” function of the spiral CT was turned on to record the dose parameters during the scan. The image data were transferred to the Picture Archiving and Communication System (PACS).
Every baseline LDCT images were read by two chest radiologists (3-5 years of experience in thoracic radiology). All noncalcified nodules ≥ 4mm in diameter on LDCT images were identified, including intraparenchymal and endobronchial nodules. The number, size, and type of each nodule were recorded. LC nodules are confirmed by pathological examination, and a nodule is considered benign if it has not been confirmed as LC after the second round of screening. According to whether the nodule covered the lung parenchyma, the nodule was divided into the following: SN, part-solid nodule (PSN), or NSN. The number of nodules was recorded in strict accordance to the imaging record standard of the National Cancer Center. Each nodule was assigned a serial number requiring a detailed recording of its size (including the solid components size), shape, density, location, and distance from the pleura. The nodule number was defined as the number of noncalcified pulmonary nodules detected at the baseline screening. We defined five categories according to the number of nodules: one, two, three, four, and more than four nodules. If the two radiologists did not reach an agreement, a senior radiologist (at least 20 years of experience in thoracic radiology) makes the final decision.
Normally distributed continuous variables are shown as mean ± standard deviation or 95% confidence interval (95%CI), whereas non-normally distributed continuous variables are presented as median (range). Categorical variables are shown as numbers (percentages). LC probability by nodule number groups for participants at baseline screening was evaluated by Chi-square test. In the stratified groups by participant and nodule risk levels, one-factor binary logistic regression analysis was performed to analyze the correlation between nodule number and LC probability. The Cochran–Armitage test for trend was conducted to assess LC trends over nodule number. All statistical data were analyzed using the Statistical Package for the Social Sciences version 26. The level of significance was defined as p < 0.05.
In this study, 10167 participants underwent LDCT examination including 4629 males and 5538 females, age range from 50-75 years (Figure 1). A total of 3076 participants with at least one non-calcified nodule were included in the study, 1486 males (48.3%) and 1590 females (51.7%), median age was 60 years old (IQR 57-63). The number of participants with noncalcified nodules decreased curvilinearly as the nodule numbers increased. The detailed distribution is shown in Figure 2. 1719 (55.9%), 689 (22.4%), 327 (10.6%), 166 (5.4%), and 175 (5.7%) participants had one, two, three, four, and more than four nodules, respectively. There were 1926 (62.6%), 1052 (34.2%), and 98 (3.2%) participants in the Amax, Bmax, and Cmax groups, respectively. In the Bmax group, with the increase in the nodule number, the proportion of participants increased from 25.2% to 63.4%. In the Amax group, with the increase in nodule numbers, the proportion of participants decreased from 72.1% to 29.1%. the proportion of participants in the Cmax group was also slightly increased (Table 1). In total, 5875 positive nodules were detected in 3076 participants, including 4611 SNs (78.5%), 437 PSNs (7.4%), and 827 NSNs (14.1%). As the number of nodules increased, the proportion of solid nodules increased from 76.5% to 80.6%. PSNs had the highest proportion in single nodules (8.9%), and NSNs had the highest proportion with > 4 nodules (15.3%) (Table 2). The largest nodules size increased linearly with more nodules detected (p < 0.05).
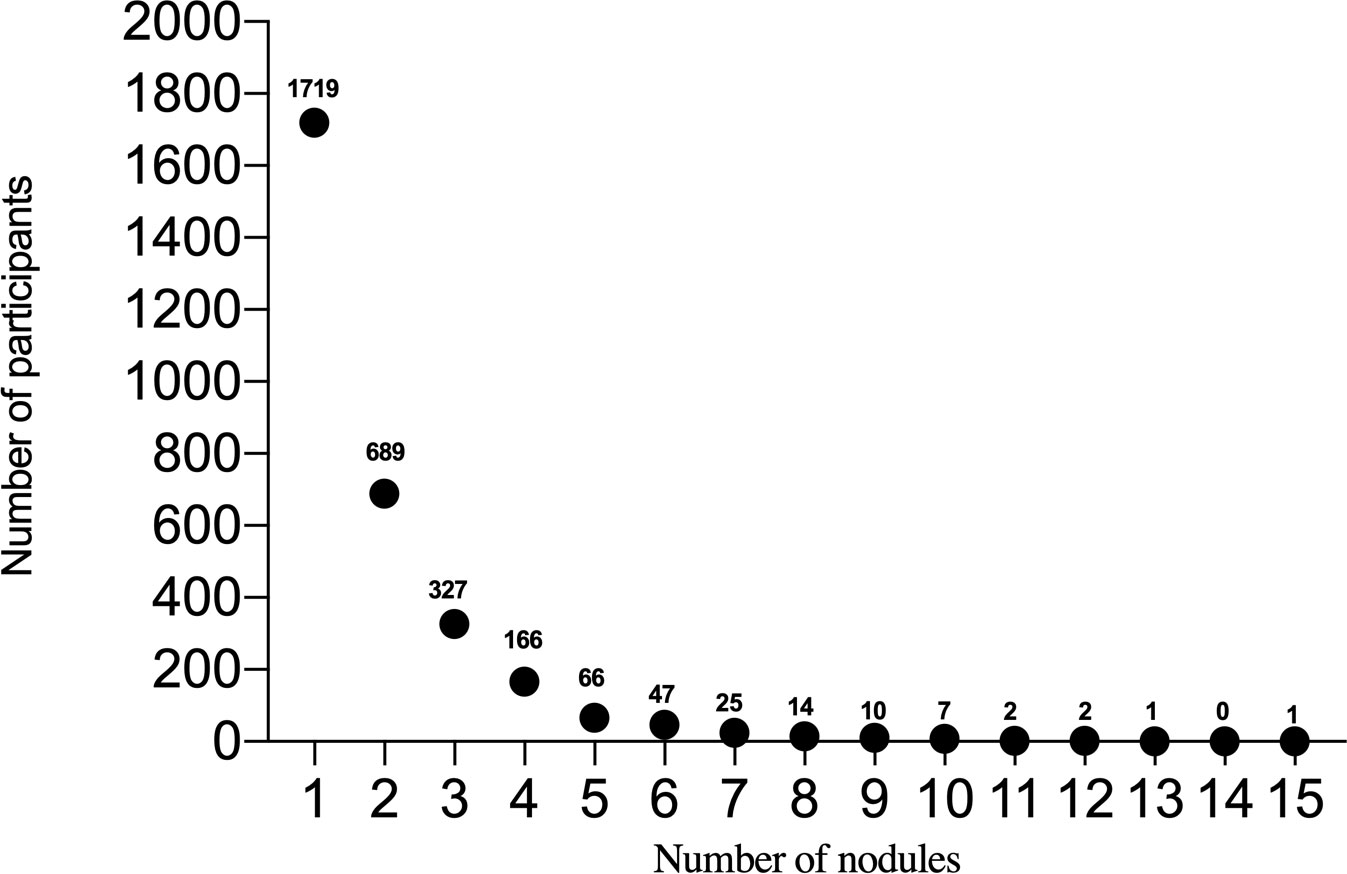
Figure 2 The horizontal axis represents the number of nodules, and the vertical axis represents the number of participants.
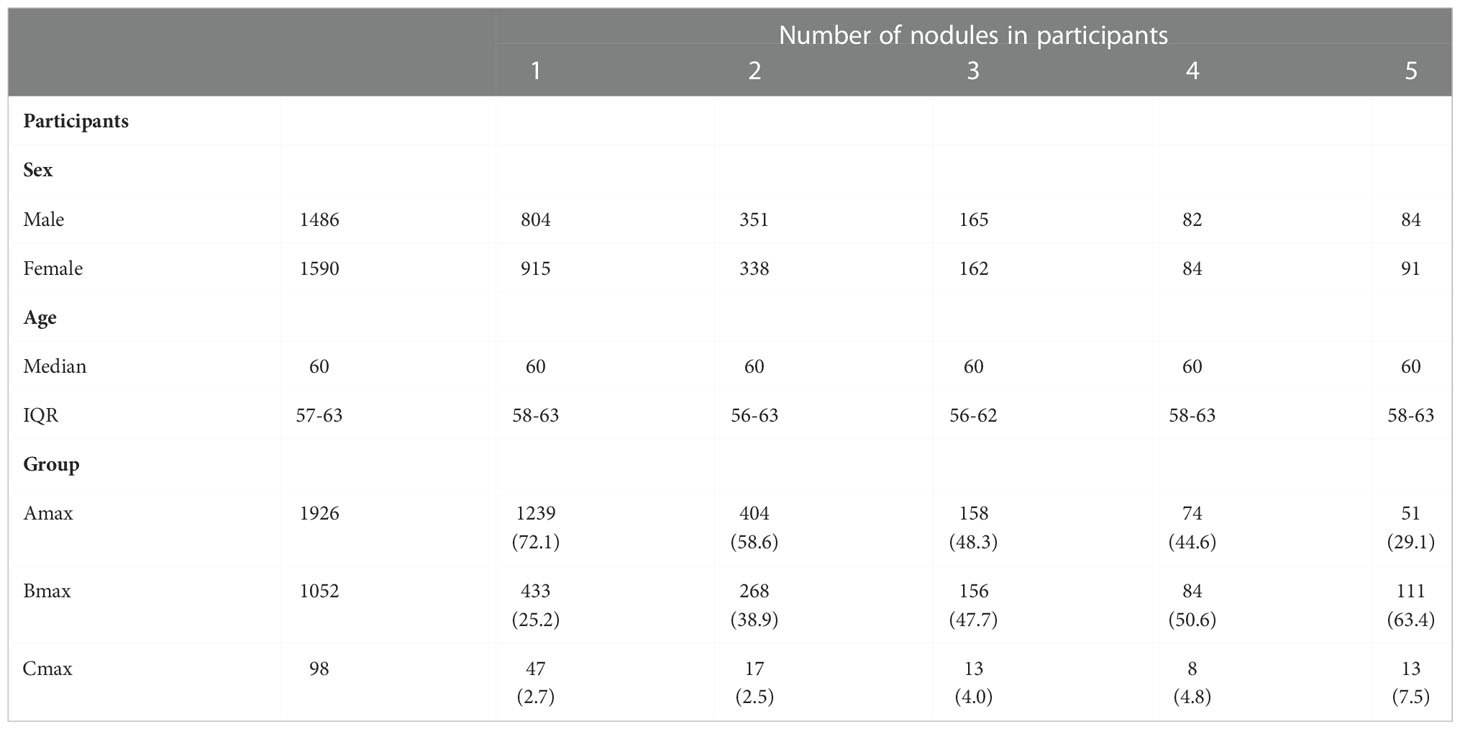
Table 1 Characteristics of participants with at least one pulmonary nodule at baseline screening round.
97 participants were pathologically confirmed to have LC, 37 (38.1%) males, median age was 61 years (IQR 58.5-64); 60 (61.9%) females, median age was 60 years (IQR 55-63). There were 87 people with stage I lung cancer, accounting for about 90.0%(87/97). 93, 90, 3, 2, 1, and 1 participant had non-small cell lung cancer, adenocarcinoma, squamous-cell carcinoma, small cell lung cancer, neuroendocrine carcinoma, and sarcoma, respectively. Simultaneous multiple tumors were found in 18 participants. In total, 15, 2, and 1 participant had two, three, and four tumors, respectively, all those multiple malignant tumors were pathologically confirmed to be adenocarcinoma. LC was confirmed with histopathology in the largest, second largest, and third largest nodules in 94/97 (96.9%), 2/97 (2.1%), and 1/97(1.0%) of the participants, respectively. The average nodule number was equal for both participants with and without LC (median nodule number, 1). The nodule-number ranges were 1–11 and 1–15 in participants with and without LC, respectively.
119 malignant nodules were confirmed in 97 participants with LC. Among the malignant nodules, 33 (27.7%), 37 (31.1%), and 49 (41.2%) were SNs, PSNs, and NSNs, respectively. The size of malignant nodules on thin-slice CT were 7–50 mm, and the median diameter was 12 mm.
One nodule was found in 1719 participants, and 49 participants were diagnosed with LC (2.9%; 95% confidence interval [CI], 2.1–3.6%). Of the 689 participants with two nodules, 22 were diagnosed with LC (3.2%; 95% CI, 1.9–4.5%). Eleven of 327 participants with three nodules (3.4%; 95% CI, 1.4-5.3%), 6 of 166 with four nodules (3.6%; 95% CI, 0.7-6.5%) and 9 of 175 with more than four nodules (5.1%; 95% CI, 1.8-8.4%) were confirmed with LC. The nodule number was not associated with LC probability, although the LC probability slightly increased with the number of nodules. (Table 3) (p > 0.05).
When the participants were stratified according to their largest nodule (Table 4), in the Bmax groups, the probability of LC was significantly higher in participants with only one nodule than for those with >4 nodules (p < 0.05, Figure 3), and the probability of LC showed a negative linear trend with increased nodule numbers (p < 0.05). For the Cmax group, there was no difference in the probability of LC among participants with different numbers of nodules, although the probability of LC was increased to a certain extent (p > 0.05). In addition, we performed a subgroup analysis based on the female population. Only in the Bmax group, participants with isolated nodules had a higher probability of LC than participants with more than 4 nodules, with statistically significant differences (Table S1).
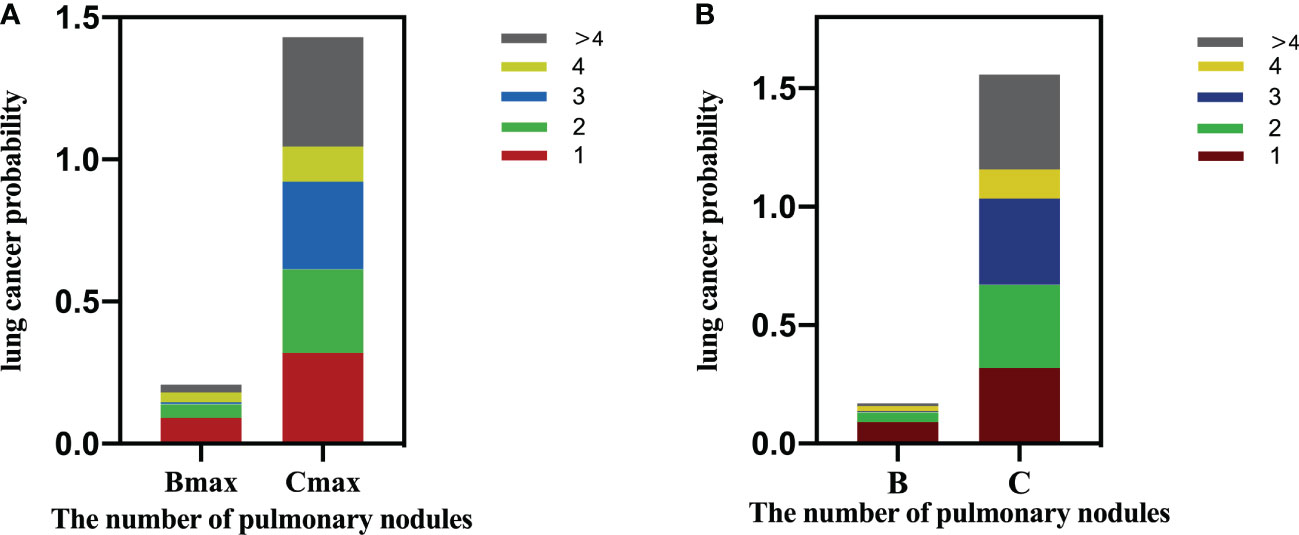
Figure 3 (A) Lung cancer probability by nodule count for Amax/Bmax/Cmax based on participant level. (B) Lung cancer probability by nodule count for A/B/C nodules based on nodule level.
Table 5 illustrates that as the nodule grade increased, the probability of LC also increased, displaying a positive linear trend (overall: Group A, 0.4%; Group B, 4.4%; and Group C, 32.7%; p < 0.01). For Group B nodules, the probability of malignancy was significantly higher when the number of nodules was 1 than those with more than four nodules (p < 0.01, Figure 3). However, for the nodules in groups A and C, the number of nodules had limited utility for distinguishing LC.
Many pulmonary nodules can be detected during LC screening, with most being benign. In this study, the detection rate of participants with positive nodules was 30.2% (3076/10167). The detection rates of LC in positive participants were 3.2% (97/3076) and approximately 0.95% (97/10167) in the entire population. The number of pulmonary nodules is frequently ignored during LC screening. A few studies suggest that the number of nodules does not differentiate benign from malignant nodules, but more in-depth research is still required (18, 19). At present, only a few risk-prediction models have considered the number of nodules as a risk factor (20, 21). This study innovatively stratified nodules according to the nodule size and determined the correlation between the nodule number and the probability of LC. Our principal findings include the following. First, in 55.9% (1719/3076) of the participants, a solitary nodule was detected at baseline. Second, the size of the largest nodule increased with the number of detected nodules increasing, and this trend was linear. At present, the risk of LC is often evaluated according to the largest nodule (22). When the largest nodule diameter increases with the number of detected nodules increasing, this may indicate an indirect association between nodule number and LC incidence. Third, we also found that not all LC nodules detected in this study were the largest nodules (94/97), but the largest nodules had the highest possibility of malignancy (Group C, 32.7%). Fourth, generally, although the LC probability slightly increased with the number of nodules, there is no statistically significant difference. Fifth, when stratified by nodule size, the effect of nodule number on LC probability was nodule-size dependent. On the participant level, in the Bmax group, the probability of LC was significantly higher in participants with only one nodule than in participants with >4 nodules, and the number of nodules showed a negative linear trend with the probability of LC. On the nodule level, in the Group B, the probability of LC in participants with only one nodule was significantly higher than those with >4 nodules. For nodules in Group A or C, the number of nodules had a limited association on LC probability at both the participant and nodule levels.
Multiple noncalcified nodules in Group A were usually < 6 mm in diameter. Small nodules in this size range are frequently observed in routine clinical practice and are usually benign (23). In our study, only 8 of 1926 people were confirmed with LC. They typically reflect previous infection or healing granulomas of lymph nodes in the lungs. For multiple noncalcified nodules with at least one Group B nodule (SNs/solid component of PSNs measuring 6-15 mm or NSNs measuring 8-15 mm), follow-up at approximately 3-6 months is recommended. Our findings demonstrate that the risk of primary cancer decreased as the total number of nodules increased from 1 to 4. When participants had only one nodule, LC probability was significantly higher than when participants had >4 nodules. In participants with multiple nodules with at least one nodule that was 15 mm or larger (Group C nodules), infectious causes or multiple primary adenocarcinomas are considered (24). In this case, our results indicated that the number of nodules has limited utility for the prediction of LC. There are two possible explanations. First, it may be due to insufficient sample size, as the number of nodules detected in Group C was much less than the other two groups. Second, the nodule diameter of the Cmax group was > 15 mm (categorized regularly as high-risk nodule grade classification). This may contribute to the limited effect of the number of nodules on the probability of LC.
The average nodule number of each screening participant was significantly lower in the present study than in McWilliams et al.’s study (25). The participants without LC in the British Columbia Cancer Agency (BCCA) study had an average of 10 nodules, and the participants without LC in the Pan-Canadian Early Detection of Lung Cancer Study (PanCan) study had an average of 6.2 nodules. The average nodule number of participants without LC in our study was 1.9. The average numbers of participants with LC in the BCCA study, PanCan study, and our study were 4.7, 4.8, and 2.1, respectively. The above differences can be explained by the different inclusion criteria. A risk prediction model determined the inclusion criteria in the PanCan study, and the risk of LC in the participants included in the study for 3 years was at least 2%. The participants included in the BCCA study had at least 30 years of current or previous smoking history. The present study was an opportunistic screening study with no specific requirements for smoking history, and the overall LC risk of the study participants in this study was lower than those of the BCCA and PanCan studies. Moreover, all noncalcified nodules larger than 1 mm in diameter were included in PanCan and BCCA study, and we included noncalcified nodules ≥ 4 mm in diameter. A smaller threshold will undoubtedly increase the number of positive nodules detected.
Although a considerable amount of time is spent on numbering all nodules (≥ 4 mm in diameter), this is necessary for screening. First, smaller nodules may still grow during follow-up, and the changes between the two scans will affect follow-up and clinical decisions. Moreover, new nodules are regularly detected in annual screening and carry a higher LC probability than baseline nodules, even for smaller sizes (9, 26). However, some studies have also reported that new subsolid nodules are associated with a lower incidence of LC and a higher spontaneous regression rate, indicating that they have greater inflammatory potential (27, 28). Although it remains debatable, considering the potential risk of LC, it is necessary to record baseline nodules in detail as a reference.
This study had some limitations. First, this study included all noncalcified nodules and did not differentiate SNs, PSNs, and NSNs. The influence of nodule numbers from different types of nodules (solid, subsolid) on the probability of LC should be further studied in detail. Second, participants’ smoking status were not included in this study because this part of the data was not available for some reasons. Since opportunistic screening does not restrict for smoking levels, it does not affect the experimental results and we will refine it in future trials. Finally, this was a single-center study, and multicenter studies are required to further verify our results.
In LDCT lung cancer screening, lung cancer probability does not significantly change with the number of nodules. However, assessing the nodule number in conjunction with nodule size in baseline LDCT lung cancer screening is considered beneficial. More attention should be paid to and active follow-up should be provided for solitary nodules, especially SNs/solid component of PSNs measuring 6–15 mm or NSNs measuring 8–15 mm.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The institutional review board of Cancer Hospital, Chinese Academy of Medical Sciences approved this retrospective study and waived the requirement for informed consent.
Conceptualization: QW, NW. Methodology: QW, SZ, NW. Methodology validation: SZ, NW. Investigation: QW, SZ, LQ, LZ. Data Curation: ZZ, JZ, HL. Formal analysis:QW, YX, LQ. Writing - Original Draft: QW. Writing - Review and Editing: YH, JW, WT, NW. Visualization: YX, QW. Supervision: SZ, NW. Funding acquisition: NW. All authors contributed to the article and approved the submitted version.
This work was supported by National Key R&D Program of China (No. 2020AAA0109504, No. 2017YFC1308700) and CAMS Innovation Fund for Medical Sciences (No.2021-I2M-C&T-B-063).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1061242/full#supplementary-material
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 numberries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Ettinger DS. Ten years of progress in non-small cell lung cancer. J Natl Compr Canc Netw (2012) 10(3):292–5. doi: 10.1200/JCO.2011.39.8594
3. Gettinger S, Horn L, Jackman D, Spigel D, Antonia S, Hellmann M, et al. Five-year follow-up of nivolumab in previously treated advanced non–small-cell lung cancer: results from the CA209-003 study. J Clin Oncol (2018) 36(17):1675–84. doi: 10.1200/JCO.2017.77.0412
4. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin (2021) 71(1):7–33. doi: 10.3322/caac.21654
5. International Early Lung Cancer Action Program Investigators. Survival of patients with stage I lung cancer detected on CT screening. New Engl J Med (2006) 355(17):1763–71. doi: 10.1056/NEJMoa060476
6. National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med (2011) 365(5):395–409. doi: 10.1056/NEJMoa1102873
7. Wu FZ, Huang YL, Wu CC, Tang EK, Chen CS, Mar GY, et al. Assessment of selection criteria for low-dose lung screening CT among Asian ethnic groups in Taiwan: From mass screening to specific risk-based screening for non-smoker lung cancer. Clin Lung Cancer (2016) 17(5):e45–56. doi: 10.1016/j.cllc.2016.03.004
8. Yousaf-Khan U, van der Aalst C, de Jong PA, Heuvelmans M, Scholten E, Lammers JW, et al. Final screening round of the NELSON lung cancer screening trial: the effect of a 2.5-year screening interval. Thorax (2017) 72(1):48–56. doi: 10.1136/thoraxjnl-2016-208655
9. Walter JE, Heuvelmans MA, Oudkerk M. Small pulmonary nodules in baseline and incidence screening rounds of low-dose CT lung cancer screening. Transl Lung Cancer Res (2017) 6(1):42–51. doi: 10.21037/tlcr.2016.11.05
10. Swensen SJ, Silverstein MD, Ilstrup DM, Schleck CD, Edell ES. The probability of malignancy in solitary pulmonary nodules. application to small radiologically indeterminate nodules. Arch Intern Med (1997) 157(8):849–55. doi: 10.1001/archinte.1997.00440290031002
11. Reid M, Choi HK, Han X, Wang X, Mukhopadhyay S, Kou L, et al. Development of a risk prediction model to estimate the probability of malignancy in pulmonary nodules being considered for biopsy. Chest (2019) 156:367–75. doi: 10.1016/j.chest.2019.01.038
12. Qi LL, BT Wu, Tang W, Zhou LN, Huang Y, Zhao SJ, et al. Long-term follow-up of persistent pulmonary pure ground-glass nodules with deep learning-assisted nodule segmentation. Eur Radiol (2020) 30(2):744–55. doi: 10.1007/s00330-019-06344-z
13. Qi LL, JW W, Yang L, Huang Y, Zhao SJ, Tang W, et al. Natural history of pathologically confirmed pulmonary subsolid nodules with deep learning-assisted nodule segmentation. Eur Radiol (2021) 31(6):3884–97. doi: 10.1007/s00330-020-07450-z
14. Horeweg N, van Rosmalen J, Heuvelmans MA, van der Aalst CM, Vliegenthart R, Scholten ET, et al. Lung cancer probability in patients with CT-detected pulmonary nodules: a prespecified analysis of data from the NELSON trial of low-dose CT screening. Lancet Oncol (2014) 15:1332–41. doi: 10.1016/S1470-2045(14)70389-4
15. Wood DE, Kazerooni EA, Aberle D, Berman A, Brown LM, Eapen GA, et al. NCCN guidelines® insights: Lung cancer screening, version 1.2022. J Natl Compr Canc Netw (2022) 20(7):754–64. doi: 10.6004/jnccn.2022.0036
16. American College of radiology committee on lung-RADS website. lung-RADS assessment categories version1.1 . Available at: www.acr.org/-/media/ACR/Files/RADS/Lung-RADS/LungRADSAssessmentCategoriesv1-1.pdf (Accessed December 27, 2020).
17. Chinese Preventive medicine association. [Lung cancer screening guideline of China(T/CPMA 013-2020)]. Zhonghua Zhong Liu Za Zhi (2021) 43(1):1–7. doi: 10.3760/cma.j.cn112152-20210106-00019
18. Heuvelmans MA, Walter JE, Peters RB, Bock GH, Yousaf-Khan U, Aalst CMV, et al. Relationship between nodule count and lung cancer probability in baseline CT lung cancer screening: The NELSON study. Lung Cancer (2017) 113:45–50. doi: 10.1016/j.lungcan.2017.08.023
19. Walter JE, Heuvelmans MA, de Bock GH, Yousaf-Khan U, Groen HJM, van der Aalst CM, et al. Relationship between the number of new nodules and lung cancer probability in incidence screening rounds of CT lung cancer screening: The NELSON study. Lung Cancer (2018) 125:103–8. doi: 10.1016/j.lungcan.2018.05.007
20. Chang HT, Wang PH, Chen WF, Lin CJ. Risk assessment of early lung cancer with LDCT and health examinations. Int J Environ Res Public Health (2022) 19(8):4633. doi: 10.3390/ijerph19084633
21. Ye M, Tong L, Zheng X, Wang H, Zhou H, Zhu X, et al. A classifier for improving early lung cancer diagnosis incorporating artificial intelligence and liquid biopsy. Front Oncol (2022) 12:853801. doi: 10.3389/fonc.2022.853801
22. Baldwin DR, Callister ME, Guideline Development Group. British Thoracic society guidelines for the investigation and management of pulmonary nodules. Thorax (2015) 70 Suppl 2:ii1–ii54. doi: 10.1136/thoraxjnl-2015-207221
23. National Lung Screening Trial Research Team, Church TR, Black WC, Aberle DR, Berg CD, Clingan KL. National lung screening trial research team. results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med (2013) 368(21):1980–91. doi: 10.1056/NEJMoa1209120
24. MacMahon H, Naidich DP, Goo JM, Lee KS, Leung ANC, Mayo JR, et al. Guidelines for management of incidental pulmonary nodules detected on CT images: From the fleischner society 2017. Radiology (2017) 284(1):228–43. doi: 10.1148/radiol.2017161659
25. McWilliams A, Tammemagi MC, Mayo JR, Roberts H, Liu G, Soghrati K, et al. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med (2013) 369(10):910–9. doi: 10.1056/NEJMoa1214726
26. Pinsky PF, Gierada DS, Nath PH, Munden R. Lung cancer risk associated with new solid nodules in the national lung screening trial. Am J Roentgenol (2017) 209(5):1009–14. doi: 10.2214/AJR.17.18252
27. Kim YW, Kwon BS, Lim SY, Lee YJ, Park JS, Cho YJ, et al. Lung cancer probability and clinical outcomes of baseline and new subsolid nodules detected on low-dose CT screening. Thorax (2021) 76(10):980–8. doi: 10.1136/thoraxjnl-2020-215107
Keywords: lung cancer probability, low-dose computed tomography, pulmonary nodules, nodule numbers, screening
Citation: Wu Q, Zhao S, Huang Y, Wang J, Tang W, Zhou L, Qi L, Zhang Z, Xie Y, Zhang J, Li H and Wu N (2023) Correlation between lung cancer probability and number of pulmonary nodules in baseline computed tomography lung cancer screening: A retrospective study based on the Chinese population. Front. Oncol. 12:1061242. doi: 10.3389/fonc.2022.1061242
Received: 04 October 2022; Accepted: 06 December 2022;
Published: 04 January 2023.
Edited by:
Pilar López-Larrubia, Spanish National Research Council (CSIC), SpainReviewed by:
Yihui Du, University Medical Center Groningen, NetherlandsCopyright © 2023 Wu, Zhao, Huang, Wang, Tang, Zhou, Qi, Zhang, Xie, Zhang, Li and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ning Wu, Y2pyLnd1bmluZ0B2aXAuMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.