- 1Third Hospital of Shanxi Medical University, Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, Tongji Shanxi Hospital, Taiyuan, China
- 2Department of Hepatobiliary Surgery, Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, Tongji Shanxi Hospital, Taiyuan, China
- 3Hepatic Surgery Center, Institute of Hepato-Pancreato-Biliary Surgery, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Circular RNAs (circRNAs) are endogenous non-coding RNAs (ncRNAs) with a closed-loop structure. In recent years, circRNAs have become the focus of much research into RNA. CircCCDC66 has been identified as a novel oncogenic circRNA and is up-regulated in a variety of malignant tumors including thyroid cancer, non-small cell carcinoma, gastric cancer, colorectal cancer, renal cancer, cervical cancer, glioma, and osteosarcoma. It mediates cancer progression by regulating epigenetic modifications, variable splicing, transcription, and protein translation. The oncogenicity of circCCDC66 suppresses or promotes the expression of related genes mainly through direct or indirect pathways. This finding suggests that circCCDC66 is a biomarker for cancer diagnosis, prognosis assessment and treatment. However, there is no review on the relationship between circCCDC66 and cancers. Thus, the expression, biological functions, and regulatory mechanisms of circCCDC66 in malignant tumor and non-tumor diseases are summarized. The clinical value and prognostic significance of circCCDC66 are also evaluated, which can provide insights helpful to those exploring new strategies for the early diagnosis and targeted treatment of malignancies.
Introduction
Malignant tumors remain diseases that threaten human life and health around the world. They are also one of the leading causes of human death (1, 2). Due to the novel coronavirus 2019 (COVID-19) pandemic, there has been a negative impact on the diagnosis and treatment of malignant tumors (3). The number of diagnosed cancer cases has reportedly decreased dramatically worldwide, with a significant increase in diagnosed cases expected in the near future (4). Therefore, there is an urgent need to identify potential highly specific biomarkers to improve the early diagnosis of cancer and enhance survival outcomes.
For many years, a great number of studies in tumor biology focused on the involvement of protein-coding genes, which accounted for less than 2% of the entire genome (5). With the development of whole genome sequencing, scientists have gained a better understanding of the transcriptome of organisms (6). More than 90% of the human genome contains non-coding RNAs (ncRNAs) that play an important role in biology, but ncRNAs were previously considered to be “transcriptional noise” or “transcriptional waste” (7). Circular RNAs (circRNAs) are a class of functionally diverse regulatory ncRNAs that are mainly produced by the circularization of exons or introns of precursor RNAs (pre-mRNAs) through alternative splicing followed by processing (8, 9). They are then reverse-spliced to form covalent closed-loop structures without a 5’ cap or 3’ Poly-A tail. In recent years, circRNAs have attracted much interest from scientists and there is a body of research evidence to the effect that circRNAs play an oncogenic or tumor suppressor role in the occurrence and development of cancers (10–12). For example, hsa_circ_0001666 inhibited epithelial-mesenchymal transition (EMT) and stemness of colorectal cancer (CRC) cells by competitively binding to miR-576-5p and high expression of hsa_circ_0001666 were associated with better clinical prognosis in CRC patients (13); CircZEB1 promoted the expression of PIK3CA by targeting miR-199a-3p to affect the proliferation and apoptosis of hepatocellular carcinoma (HCC) (14). This suggested that circZEB1 can be used as a biomarker for the diagnosis and treatment of HCC.
CircCCDC66 is a rising star in the circRNA family. CircCCDC66, also known as circ_0001313, is the full name of the circular RNA coiled-coil domain containing 66, which is the circular transcript from the CCDC66 pre-mRNA. CircCCDC66 was first reported to be up-regulated in CRC in 2017 (15). It was found to be closely related to the pathogenesis and prognosis of various human diseases (16–18). It not only participates in the regulation of the cell cycle (19), energy metabolism (20) and tumorigenesis through the network of competing endogenous RNAs (ceRNAs) (21, 22) but also regulates the expression of host proteins to exert a cis-transcriptional regulatory role (23). Due to its genome-wide expression patterns and tissue-specific expression signatures in various tissues, circCCDC66 has a strong potential as a novel biomarker and therapeutic target for cancers.
Here, we present a review of the latest progress in the biological functions and regulatory mechanisms of circCCDC66 in various malignant tumors. Furthermore, the role and significance of circCCDC66 in cancers are also evaluated and the prospects for future research are discussed.
The characterization and function of circRNAs
CircRNAs are widely distributed in the cytoplasm and nucleus of eukaryotic cells, where they are abundantly, robustly, and conserved expressed (24) Compared with linear RNAs, circRNAs are resistant to RNAases, causing circRNAs to be conserved across species and having higher stability and longer half-life (25).Moreover, circRNAs account for a considerable proportion of transcripts, and some are even expressed in significantly higher abundance than others (26). The expression of circRNAs can be tightly regulated by RNA binding proteins (RBPs), and the co-localization of circRNAs and RBPs suggests that circRNAs may also affect RBP activity (27, 28).
According to the sequence composition, circRNAs can be classified into three types (1): exonic circRNAs (ecircRNAs). EcircRNAs are exon-derived circRNAs consisting of single or multiple exons of a gene, and account for the majority of circRNAs (2); exon-intron circRNAs (eiciRNAs). EiciRNAs contain both exons and introns (3); intronic circRNAs (ciRNAs) (Figure 1). CiRNAs mainly derived from the lariat RNA and tRNA introns generated by pre-mRNA splicing. Another study reported that viral RNA genomes, rRNA, tRNA, and snRNA can also be circularized to generate circRNA (29). Recent studies have identified important regulatory functions of circRNAs. Cytoplasmic circRNAs (ecircRNAs) are mostly produced from exons and regulate gene expression at the post-transcriptional and translational levels, including interacting with different proteins in the cytoplasm, competing with microRNAs (miRNAs) to regulate mRNA metabolism, and encoding specific proteins or generating pseudogenes (30). The circRNAs (eiciRNA and ciRNA) distributed in the nucleus mainly regulate gene expression at the epigenetic and transcriptional levels, including interaction with proteins in the nucleus, regulation of parental gene expression, regulation of chromatin remodelling and processes of splicing and transcription (31, 32). Moreover, circRNAs can also be packaged in extracellular vesicles to form exosomes and transported in the circulation (33).
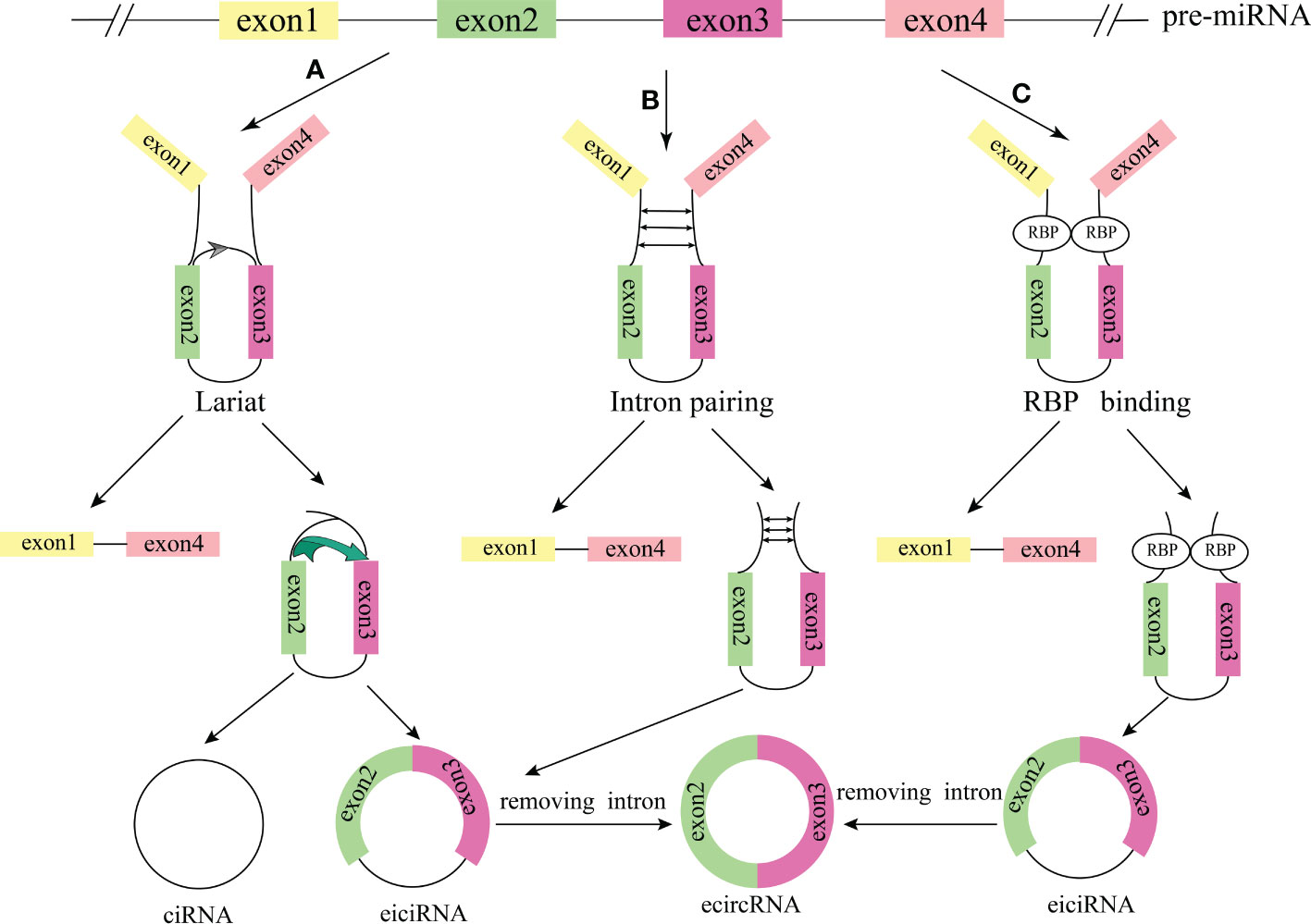
Figure 1 The formation process of the circRNAs. (A) Cyclization driven by lariat. (B) Intron pairing drives cyclization. (C) RBP was combined with cyclization to form a bridge. The pre-mRNA was spliced to produce an mRNA composed of exon 1 and exon 4 and an RBP binding RNA containing exons 2 and 3, and then generated ciRNA and eiciRNA. EiciRNA removed intron to form ecircRNA.
Accumulating evidence suggests that circRNAs are involved in the regulation of various tumor biological processes, especially playing a large regulatory role in important biological processes such as tumor proliferation, apoptosis, migration, invasion, chromatin remodeling, metabolism, and immune escape (32, 34). CircRPN2 accelerated ENO1 degradation and promoted glycolytic reprogramming through the AKT/mTOR pathway, thereby inhibiting HCC aerobic glycolysis and metastasis (35); plasma exosomal circLPAR1 specifically bound to eIF3h and inhibited the METTL3-eIF3h interaction, which reduced the translation of the oncogene BRD4 and inhibited CRC growth (36). These studies implied that circRNAs are critical for tumorigenesis and progression. Further exploration of the role of circRNAs in human cancer and their regulatory mechanisms could provide insights for exploring new strategies for early diagnosis and targeted therapy of cancers.
The source of the circCCDC66-CCDC66
CircCCDC66 was derived from exons 8-10 of the CCDC66 gene, located at chr3: 56626997-56628056. It has a full length of 468 nucleotides and is formed by non-linear splicing of CCDC66 pre-mRNA (15, 19) (Figure 2). According to the circRANA database (circBank and circBase), the circCCDC66 transcript was identified as hsa_circ_0001313 and showed high levels of conservation of among different species. Moreover, circCCDC66 is widely present in eukaryotic cells and is preferentially expressed in the cytoplasm.
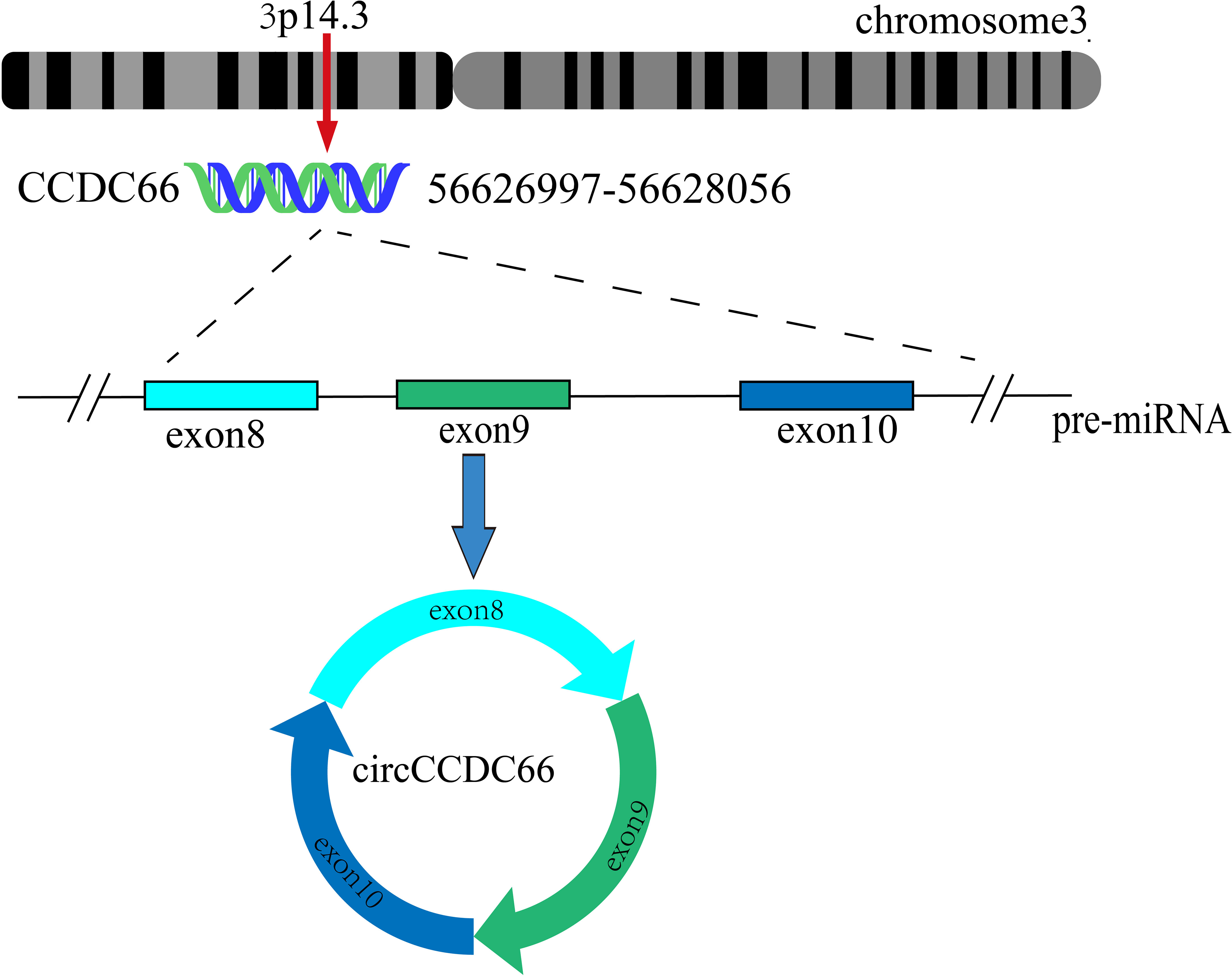
Figure 2 Schematic representation of the formation of circCCDC66. Exons 8-10 of CCDC66 located on chromosome 3 produce a 468-nucleotide circCCDC66.
The coiled-coil domain containing 66 (CCDC66) is a protein-coding gene located on chromosome 3p14.3. It was originally described as a gene mutated in retinal degeneration (37). Gerding et al. (38) used a CCDC66 mutant mouse model to reveal that CCDC66 deletion led to retinal degeneration and dysfunction. Subsequently, studies revealed that CCDC66 as a component of the centrosome and ciliary complex, co-localized with centriole satellites, microtubules, and molecular motors (39, 40). It also acts synergistically in ciliary formation, satellite organization, and ciliary recruitment in Bardet–Biedl syndrome4. Notably, retinal degeneration mutations disrupt the centrosome and ciliary protein localization and interaction of CCDC66. Moreover, Latour et al. (41) also found that CCDC66 was part of the Joubert syndrome (JBTS)-related protein module that maintains ciliary stability. In addition to ciliary function, CCDC66 could also control mitotic progression and cytokinesis by promoting microtubule nucleation and organization. Dysregulation of CCDC66 may contribute to carcinogenesis and ciliopathies (42).
CircCCDC66 expression in malignant tumors and its regulatory mechanism
Current research has confirmed that circCCDC66 is abnormally up-regulated in various types of cancers including thyroid cancer, non-small cell cancer, gastric cancer, colorectal cancer, renal cancer, cervical cancer, glioma, and osteosarcoma. In particular, circCCDC66 plays a key role in important biological processes such as tumor proliferation, migration, invasion, cell cycle and apoptosis, chemotherapy resistance, radiosensitivity, and immune response (Table 1). Next, the mechanism of circCCDC66 expression in cancers and regulation of tumor progression are reviewed and summarized and the clinical potential of circCCDC66 as a prognostic biomarker and cancer therapeutic agent are further expounded. The results will contribute to the development of more effective cancer treatment strategies.
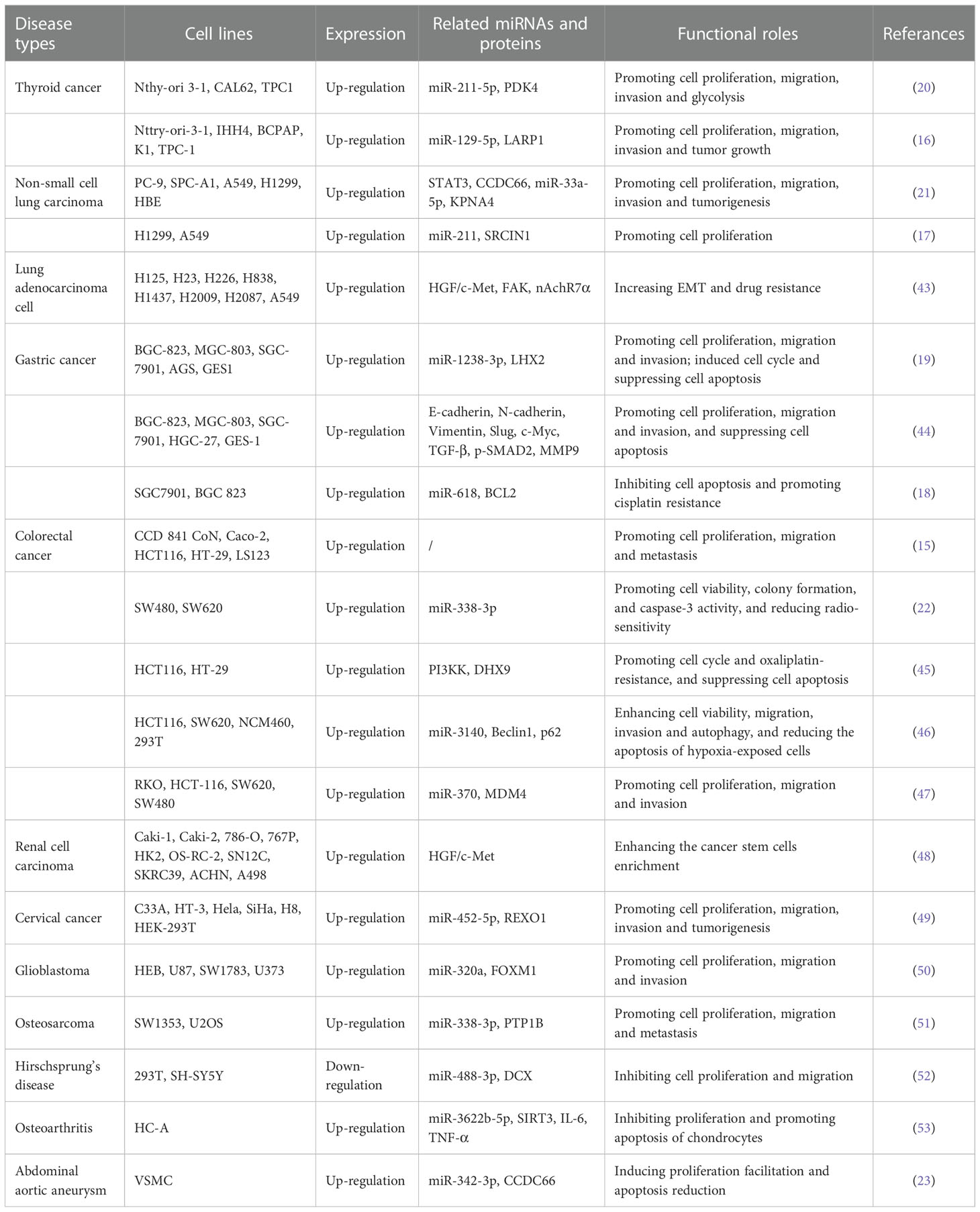
Table 1 Expression regulation and roles of circCCDC66 in various diseases including malignant tumors.
CircCCDC66 and thyroid cancer
Thyroid cancer (TC) is the most frequent endocrine malignancy worldwide and its incidence has steadily increased over several decades. Papillary thyroid cancer is the most common TC histotype, accounting for approximately 85% of all TCs (54). Differentiated TC behaviors vary from indolent to extremely aggressive, and TC prognosis is largely based on clinicopathological features that are a direct consequence of altered cellular and tissue microenvironment (55). Therefore, there is an urgent need for an in-depth understanding of the molecular mechanisms of TC invasion and metastasis for better assessment of invasion risk and clinical guidance.
CircRNAs play critical roles in gene regulation at the epigenetic, transcriptional, and post-transcriptional levels. Ren et al. (20) found that the level of expression of circCCDC66 was significantly up-regulated in TC tissues compared with normal thyroid tissues (n=9, P<0.05). CircCCDC66 might promote TC progression. In vitro, knockdown of circCCDC66 resulted in decreased expression of targeted miR-211-5p, which inhibited TC cell proliferation, migration, invasion, and glycolysis. miR-211-5p has been reported to be involved in the metabolic activity of various cancer cells (56). Overexpression of miR-211-5p significantly reduced the expression of pyruvate dehydrogenase kinase 4 (PDK4) and circCCDC66. Inhibition of PDK4 by miR-211 induced oxidative phosphorylation (20). These may reduce glucose and increase the expression of pyruvate dehydrogenase and key enzymes in the tricarboxylic acid cycle, which can affect the process of glucose metabolism and glucose homeostasis. Thus, circCCDC66 may upregulate the expression of PDK4 by binding to miR-211-5p by sponging, thereby the progression of TC.
Subsequently, Li et al. (16) also found that the level of expression of circCCDC66 was significantly increased in papillary TC (PTC) tissues (n=60, P<0.05) and cell lines. This study also investigated the relationship between the level of expression of circCCDC66 and clinicopathological features. The level of expression of circCCDC66 was positively correlated with tumor size (P=0.0099), TNM stage (P=0.0362), and lymph node metastasis (P=0.0089) in patients with PTC (16). Further mechanistic studies showed that circCCDC66 could bind miR-129-5p to promote the expression of La-related protein 1 (LARP1), which may accelerate the malignant progression of PTC. Therefore, targeting circCCDC66 may be considered a promising TC therapeutic strategy.
CircCCDC66 and lung cancer
Lung cancer (LC) is the most common cancer and the leading cause of cancer death worldwide. There are estimated 2.09 million new cases and 1.76 million deaths globally each year (57). According to histological type, lung cancer is divided into small-cell lung carcinoma (SCLC) and non-small cell lung carcinoma (NSCLC). NSCLC is the most common and deadliest type of primary lung cancer, accounting for 85% of all lung cancers. NSCLC includes lung adenocarcinoma (LADC), lung squamous cell carcinoma (LUSC), and large cell lung carcinoma (LCLC). These are usually diagnosed at a later stage. Despite significant advances in NSCLC surgery, chemotherapy, and targeted therapy over the past two decades, due to the difficulty of early diagnosis, easy metastasis, and poor prognosis, the current five-year survival rate of NSCLC remains below 20% (58). Therefore, it is necessary to reveal the occurrence and molecular mechanisms of lung cancer to find new and reliable biomarkers and therapeutic targets.
In recent times, a better understanding of disease biology and the identification of oncogenic driver alterations have altered the therapeutic landscape. Emerging evidence suggests that circRNAs play an important role in the initiation and progression of non-small cell lung cancer, with the potential to play a role in the development and progression of non-small cell lung cancer. Research has been undertaken to examine potential cancer biomarkers (59). Wang et al. (21) found that the expression level of circCCDC66 was up-regulated in NSCLC cells. Knockout of circCCDC66 could inhibit the proliferation, migration, and invasion of NSCLC cells while promoting the apoptosis of NSCLC cells. CircCCDC66 acts as a sponge of miR-33a-5p to mediate the ceRNA mechanism to regulate gene expression post-transcriptionally. Existing evidence proves the tumor suppressor role of miR-33a-5p in a variety of cancers (60). Karyopherin subunit alpha 4 (KPNA4) has been shown to be an mRNA with the potential to promote tumorigenesis and the development of various human malignancies (61). As revealed by the study, up-regulation of circCCDC66 accelerates the progression of NSCLC by binding miR-33a-5p to up-regulate KPNA4 expression. In addition, signal transducer and activator of transcription 3 (STAT3) was confirmed to activate CCDC66 transcription and increase the expression of circCCDC66 in non-small cell lung cancer cells. This provides evidence for the oncogenic role of circCCDC66 in NSCLC cells (21). Subsequently, Hong et al. (17) found that circCCDC66 can also bind miR-211 by acting as a competitive adsorbent for ceRNA, causing a significant increase in the expression of SRC kinase signaling inhibitor 1 (SRCIN1). This provides a new perspective on the post-transcriptional regulatory mechanism of circCCDC66. It is expected to be a prospective molecular biomarker for detection and treatment of NSCLC.
LADC is the most common histological subtype of non-small cell lung cancer. The morbidity and mortality of LADC are increasing year-on-year due to early tumor recurrence, enhanced resistance to chemoradiotherapy, and rapid progression of the disease (62). Chemotherapy targeting mutated tyrosine kinase inhibitors (TKIs) of the epidermal growth factor receptor (EGFR) has recently become the main strategic treatment for LADC patients, such as in gefitinib, erlotinib, and afatinib (63). Joseph et al. (43) discovered that SUMO-activating enzyme subunit 1 (SAE1) and circCCDC66 were highly expressed in LADC. Hepatocyte growth factor (HGF) and HGF receptor (or c-Met) positively regulates the expression of SAE2 and circCCDC66 to induce EGFR resistance and increase the EMT and metastatic potential of LADC cells. This finding is consistent with the mechanism of action reported in CRC (48). Designing multimodal drugs targeting multiple targets in EGFR and resistance-related feedback loops in the future may provide additional benefits to cancer patients.
CircCCDC66 and gastric cancer
Gastric cancer (GC) is one of the most common highly aggressive malignancies worldwide and the second leading cause of cancer-related death. GC remains a global health problem. The survival rate of GC patients has improved significantly over the past few decades (64). However, due to the lack of specific symptoms in the early stage of GC, most gastric cancer patients are often diagnosed at an advanced stage, and it is prone to peritoneal, local, and distant metastases. GC has a high recurrence rate and its prognosis remains suboptimal. The current 5-year survival rate for advanced gastric cancer is less than 20%. If timely and accurate diagnosis and comprehensive treatment are performed before the tumor invades the basement membrane, the 5-year survival rate of GC patients may be as high as 90% (65). Therefore, early detection with effective screening methods is critical to improve the long-term survival of patients.
Gastric cancer is a highly molecularly and phenotypically heterogeneous disease. Recent advances in the genomic analysis indicate that highly-expressed circCCDC66 enhances the malignant phenotype of tumor cells and is closely associated with poor prognosis (5, 18, 19). Yang et al. (60)19 found that circCCDC66 may promote cell proliferation and invasion by binding miRNA-1238-3p to up-regulate the expression of LIM-homeobox domain 2 (LHX2) to accelerate the progression of GC. LHX2 has been shown to be a member of the LIM homeodomain protein family involved in the formation and development of various tumor cells (66). Knockout of circCCDC66 can significantly inhibit the proliferation and invasion of GC cells, block cell cycle progression in G0/G1 phase and induce GC cell apoptosis. Overexpression of miRNA-1238-3p or silencing of LHX2 reversed the promotional effect of circCCDC66 on GC cell migration and invasion (19). This is the first report on the pattern of expression and biological function of circCCDC66 in gastric cancer. The circCCDC66/miRNA-1238-3p/LHX2 axis may be a promising target for GC therapy, providing new evidence that circRNAs function as miRNA sponges.
In addition, Xu et al. (44) also confirmed that circCCDC66 was up-regulated in GC tissues (n=70, P<0.001) and cell lines. Its level of expression is correlated with tumor stage (P=0.01) and lymphatic metastasis (P=0.014). Knockdown of circCCDC66 inhibited the activation of c-Myc and TGF-β signaling pathways. Both published reports and experimental evidence support c-Myc as a powerful oncogene (67). Overexpression of c-Myc can increase the ability of cancer cells to metastasise by directly or indirectly modulating the ability of EMT molecular markers to induce cell motility. TGF-β regulates tumorigenesis, progression, and metastasis through extensive and complex interdependent interactions (68). circCCDC66 induces EMT and promotes GC growth and metastasis by activating c-Myc and TGF-β signaling pathways. This suggests that it may serve as a potential biomarker for GC.
Cisplatin (CDDP) is a first-line chemotherapy drug for gastric cancer, which induces tumor cell apoptosis by interfering with DNA replication. Subsequently, Zhang et al. (18)proposed in a recent study that circCCDC66 is an important regulator of the development of CDDP resistance. Studies have shown that circCCDC66 significantly inhibits B-cell lymphoma-2 (BCL2) mRNA and protein levels by targeting miR-618 to induce cisplatin chemotherapy resistance in gastric cancer cells. For CDDP-sensitive cells, CDDP resistance resulted in marked up-regulation of BCL2. BCL2 was confirmed to be a key oncogene that regulates apoptosis signaling pathways in tumors (69). Overexpression of BCL2 can reverse the CDDP resistance of circCCDC66-suppressed GC cells by knocking down circCCDC66, which may provide a new theoretical basis for the treatment of GC.
CircCCDC66 and colorectal cancer
CRC is one of the most common and aggressive human malignancies and the third leading cause of cancer-related death worldwide. According to reports, the global burden of the disease will be increased by 60% by 2030, with more than 2.2 million new cases and 1.1 million deaths (70). Despite major advances in prevention and treatment, the 5-year survival rate for colon cancer patients remains at 30% due to recurrence and metastasis (71). Therefore, there is an increasing need to discover promising biomarkers for CRC diagnosis.
More studies have shown that abnormally expressed circRNAs are involved in the occurrence and development of CRC by acting as competing endogenous RNAs (72). CircCCDC66 is a recently discovered novel circRNA that is up-regulated in colon cancer tissues and promotes colon cancer progression. Hsiao et al. (15) performed RNA-Seq analysis on 12 pairs of CRCs and paired non-tumor samples to identify 74 novel circRNAs clinically relevant to CRC. They have further been validated by quantitative reverse transcription polymerase chain reaction (qRT-PCR). Among them, the expression of circCCDC66 was up-regulated in CRC cell lines and clinical specimens, which was negatively correlated with prognosis (n=12, P<0.05). In vitro and in vivo experimental studies showed that circCCDC66 regulates colorectal oncogenes with multiple tumor characteristics including cell proliferation, migration, invasion, and anchorage-independent growth. CircRNAs contain different binding sites for different miRNAs and have oncogenic capabilities by sponging to protect multiple oncogenes from miRNA attack (73). This is the first report on the regulatory role and clinical significance of circRNA CCDC66 in human cancer progression. It also highlights the importance of complex ceRNA-regulated gene networks in cancer progression and therapeutic efficacy.
Subsequently, Wang L. et al. (22) found that the expression of circCCDC66 in radioresistant colon cancer tissues was significantly higher than that in radiosensitive tissues and that circCCDC66 was significantly up-regulated in colon cancer cells exposed to radiation (n=40, P<0.001). Knockdown of circCCDC66 reduced cell viability, inhibited colony formation rate, and increased caspase-3 activity in irradiated colon cancer cells. While the expression of miR-338-3p was down-regulated, miR-338-3p could reverse the effects of circCCDC66 on cell viability, colony formation, and caspase-3 activity. miR-338-3p as considered a tumor suppressor is frequently found to be reduced in various cancer types. Previous studies have reported an important role for miR-338-3p in cancer chemotherapy resistance (74). This finding revealed the biological function and mechanism of circCCDC66 in the radiosensitivity of CRC. CircCCDC66 regulates the radioresistance of colon cancer by negatively regulating miR-338-3p expression, which may provide a potential therapeutic target for radioresistant colon cancer patients.
CircRNAs can be derived not only from cancer cells, but also from cells in the tumor microenvironment, immune cells, or cells of other organs (75). CircRNAs can be selectively secreted into the blood, and their expression in plasma differs from that in tissues. This is a phenomenon related to its active secretion mechanism and function. Recently, plasma circRNAs have been shown to act as biomarkers for CRC. Lin et al. (76) found that the expression levels of circCCDC66, circABCC1, and circSTIL were decreased in the plasma of CRC patients compared with healthy controls by the training cohort (n=15 vs.15) and the validation cohort (n=30 vs. 46) of CRC. The sensitivity and specificity of the three circRNA groups (circCCDC66 AUC=0.756 P<0.001; circABCC1 AUC=0.663 P=0.0041; circSTIL AUC=0.677 P=0.0018) were also evaluated together and had some diagnostic value. Notably, the combination of three circRNAs and the traditional serum tumor protein biomarkers CEA and CA19-9 will improve the rate of detection of early CRC and reduce the missed diagnosis rate (AUC=0.885, P=0.0021). High-throughput methods such as microarrays and next-generation sequencing should be used in the future to characterize the global landscape of plasma circRNAs in a cohort of human subjects. This may identify the most valuable diagnostic and prognostic factors for CRC.
The formation of CRC is a complex and dynamic process. Treatment failure in CRC is often associated with recurrent and drug-resistant cancer tissue. Lin et al. (45) found that the expression of circCCDC66 was up-regulated in oxaliplatin-resistant CRC cells. The induction of circCCDC66 is dependent on oxaliplatin-induced DExH-box helicase 9 (DHX9) phosphorylation, which is required for the establishment of chemoresistance to oxaliplatin. According to related reports, DHX9 has a phosphorylation site for the DNA-dependent protein kinase phosphatidylinositol 3-kinase-related kinases (PI3KKs). DHX9 is phosphorylated near the substrate-binding domain mediated by PI3KK upon DNA damage (77). Studies have shown that blocking PI3KK activity or DHX9 phosphorylation by point mutation reduces oxaliplatin-induced circCCDC66 expression and the ability to generate chemoresistant cells. This study proposes that DHX9 phosphorylation, which is impaired by cytotoxic stress, regulates chemotherapy-resistant CRC by promoting oncogenic circRNA expression, thereby providing mechanistic insights for the development of therapeutic strategies for recurrent and chemoresistant CRC.
Considering that intra-tumoral hypoxia is one of the driving forces of tumorigenesis and development, Feng et al. (46) demonstrated for the first time that hypoxia-induced up-regulation of circCCDC66. CircCCDC66 knockdown decreased the proliferation, migration, and invasion of CRC cells and increased apoptosis when exposed to hypoxic conditions. It has been reported that miR-3140 functions as an oncogene or tumor suppressor both in vitro and in vivo (78). Research shows that circCCDC66 activates the autophagy pathway by sponging and binds miR-3140 to promote the malignant phenotype of CRC, which provides a new therapeutic strategy for CRC. There is increasing evidence that the mouse double minute 4 (MDM4) and mouse double minute 2 (MDM2) oncoproteins are key negative regulators of the p53 tumor suppressor (79). In addition, Mo et al. (47) showed that circCCDC66 up-regulated the expression of MDM4 by targeting miR-370 to promote cell proliferation, migration, and invasion in CRC. In conclusion, circCCDC66 acts as an oncogenic circRNA in CRC tumor development. These studies revealed the potential functions of circRNAs in the occurrence and development of CRC, which can provide new insights into the treatment and prevention of CRC.
CircCCDC66 and renal cancer
Renal cancer is one of the most common and aggressive malignant tumors of the urinary system, accounting for more than 90% of all renal malignancies. Obesity and smoking are significantly associated with the pathogenesis of several systemic cancers, including renal cell carcinoma (RCC) (80). Changes in epigenetic phenomena, such as DNA methylation levels, and expression of lncRNAs and circRNAs, were observed early in the development of cancer (81). A deeper understanding of the pathophysiology of RCC may reveal relevant molecules able to advance the therapeutic management thereof.
A key focus of attention is the effect of circRNAs on renal cancer stem cells. Yang et al. (48) found that circCCDC66 was up-regulated not only in renal cancer cell lines, but also in cancer stem cell spheroids. More importantly, the results showed that circCCDC66 enhanced the enrichment of cancer stem cells. Further mechanistic studies indicated that the hepatocyte growth factor (HGF)/c-Met pathway was activated in cancer stem cell enrichment. The HGF/c-Met pathway could lead to up-regulation of circCCDC66. Inhibition of HGF/c-Met blocked circCCDC66-induced enrichment of cancer stem cells. According to related reports, HGF/c-Met positively regulates circCCDC66 to increase the EMT and metastatic potential of lung adenocarcinoma cells (43). If the combination of c-Met inhibitor and circCCDC66 inhibitor blocked cancer stem cell enrichment, it could provide new insights into cancer stem cells. Thus, circCCDC66 may be a promising biomarker and therapeutic target for renal cancer therapy.
CircCCDC66 and cervical cancer
Cervical cancer remains the fourth most common cancer in women worldwide. Almost all cases of cervical cancer are caused by human papillomavirus infection (82). Persistent infection with human papillomavirus can lead to cervical intraepithelial neoplasia or precancerous lesions of adenocarcinoma, evolving in situ into cervical cancer. Therefore, it is necessary and indeed pressing to detect biomarkers for early diagnosis and prognosis.
Recent studies have reported that circRNAs are abnormally expressed in cervical cancer and are important regulators of cervical cancer carcinogenesis and progression (83). Zhang et al. (49) found that circCCDC66 was highly expressed in cervical cancer tissues (n=36, P<0.01). Knockdown of circCCDC66 attenuated the proliferation, migration, and invasion of cervical cancer cells in vitro and inhibited cervical cancer tumor growth in vivo. This was correlated with advanced tumor stage and larger tumor size. The association between circCCDC66 and miR-452-5p or RNA exonuclease 1 homolog (REXO1) was assessed by way of bioinformatics analysis, biotinylated RNA pull-down, RNA immunoprecipitation, and dual-luciferase reporter assay analysis. miR-452-5p has been shown to be involved in the progression of a variety of cancers including renal carcinoma (84), hepatocellular carcinoma (85), and lung squamous cell carcinoma (86). It plays a specific role in tumorigenesis and progression. This study shows that circCCDC66 competitively binds miR-452-5p through spongeization, and up-regulates REXO1 expression to aggravate the progression of cervical cancer (49). This may provide new insights into new therapeutic targets for basic research and clinical management of cervical cancer.
CircCCDC66 and glioblastoma
Glioblastoma (GBM) is the most common and malignant primary brain tumor in adults. Its incidence increases with age, peaking at an age of 80 years. GBM accounts for 12% to 15% of all intracranial tumors and the five-year survival rate is only 5% (87). It is primarily characterized by extensive intra-tumoral and inter-tumoral genetic and epigenetic variability.
In recent years, a greater understanding of disease biology and the identification of oncogenic driver alterations have altered the therapeutic landscape. Emerging evidence suggests that aberrantly expressed circRNAs play a key role in the occurrence and development of GBM (50). Qi et al. (88) revealed that circCCDC66 was down-regulated in high-grade gliomas, and knockdown of circCCDC66 inhibited the proliferation, migration, and invasion of glioma cells. Co-transfection of miR-320a or forkhead box M1 (FOXM1) plasmids into glioma cells could rescue the consequences of si-circCCDC66-induced cell growth inhibition to some extent. Importantly, FOXM1 was identified as a key target of circCCDC66 involved in regulating DNA damage response pathways (89). This highlights the fact that circCCDC66 is overexpressed in gliomas and acts as an oncogene. It exerts oncogenic effects by regulating the ceRNA network, thus providing some new experimental evidence for its use in the clinical treatment of glioma.
CircCCDC66 and osteosarcoma
Osteosarcoma (OS) is deemed to be a tumor derived from osteogenic mesenchymal stem cells and is the most common primary bone malignancy. It primarily affects the metaphyseal region of long tubular bones (proximal tibia or humerus and distal femur) (90). The peak incidence of OS is mainly concentrated in children and adolescents, and its rate of growth is high. It also has the characteristics of strong invasiveness, strong resistance to chemotherapy, poor prognosis, and a high rate of recurrence (91).
The pathogenesis of OS is currently unclear, and it is important to identify new therapeutic targets to combat this devastating and potentially fatal disease. Recent reports indicate that circRNAs are abnormally expressed in OS and are important regulators of OS carcinogenesis and progression (51). Xiang et al. (92) found high expression of circCCDC66 in tissue samples (n=12, P<0.05) and cell lines derived from OS patients by qRT-PCR. Down-regulation of circCCDC66 enhanced the interaction between miR-338-3p and protein tyrosine phosphatase 1B (PTP1B) and inhibited OS cell proliferation and metastasis. PTP1B has been identified as an intracellular checkpoint that is up-regulated in tumor T cells (93). Further analysis showed that circCCDC66 up-regulated the expression of PTP1B by sponging miR-338-3p (92). It limits T cell expansion and cytotoxicity leading to the malignant phenotype of osteosarcoma. This suggests that the circCCDC66/miR-338-3p/PTP1B axis may be a potential therapeutic target. CircCCDC66 can be used as a novel biomarker for OS screening to predict poor prognosis and monitor cancer progression.
CircCCDC66 expression in human non-tumor disease and its regulatory mechanism
CircCCDC66 and Hirschsprung disease
Hirschsprung disease (HSCR) is a developmental disorder of the enteric nervous system. It is caused by defects in neural crest cell proliferation, migration, and differentiation during enteric nervous system formation (94). This neural crest disease affects 1 in 5000 births worldwide. It is 80% heritable and usually manifests quickly after birth (95). The pathogenesis of HSCR is a complex process that requires strict regulation.
There is increasing evidence that circRNAs have been shown to promote the growth and metastasis of malignant tumors by sponging and adsorbing miRNAs (28). This may also lead to the appearance of HSCR by affecting the basic functions of the cell. Wen et al. (52)found that circCCDC66 is widely present in enteric neurons, while circCCDC66 is absent in HSCR patient samples. Low expression of circCCDC66 in intestinal tissue can inhibit HSCR intestinal epithelial cell proliferation and migration. This result has important implications for the progression of HSCR. Mutations in doublecortin have been reported to cause defects in neuronal migration and proliferation (96). CircCCDC66 could regulate cell proliferation and migration in HSCR by attenuating doublecortin expression by sponging miR-488-3p. This is the first study to demonstrate a potential link between the pathogenesis of Hirschsprung’s disease and circCCDC66 and its associated RNAs and proteins. Therefore, this provides a new idea for the diagnosis of HSCR.
CircCCDC66 and osteoarthritis
Osteoarthritis (OA) is the most common chronic degenerative joint disease, which is characterized by articular cartilage degeneration and secondary bone hyperplasia (97). More than 500 million people worldwide are currently affected by OA. It peaks at an age of 75 years and is associated with high morbidity and disability (98), however, the exact molecular mechanisms that regulate the pathogenesis of OA remain unclear.
Importantly, studies have highlighted that circRNAs regulate their proliferation, apoptosis, autophagy, inflammation, or extracellular matrix reorganization by interacting with components in the OA microenvironment, such as chondrocytes, synoviocytes, and macrophages (99). Zhang et al. (53) first found that circCCDC66 was up-regulated in OA cartilage cells. Overexpression of circCCDC66 increased IL-6 and TNF-α levels in chondrocytes. Sirtuin 3 (SIRT3) as one of the most prominent sirtuins in mitochondria has been shown to be involved in various aspects of mitochondrial metabolism and homeostasis (100). Further mechanistic analysis implied that circCCDC66 promoted OA chondrocyte apoptosis by regulating the miR-3622b-5p/SIRT3 axis. This finding revealed novel aspects of the cellular functions and pathophysiological roles of circCCDC66 and miR-3622b-5p. CircCCDC66 can be considered as a potential molecular target for OA therapy.
CircCCDC66 and abdominal aortic aneurysm
Abdominal aortic aneurysm (AAA) is an asymptomatic and fatal cardiovascular disease characterized by localized dilation of the abdominal aortic vessel wall. Degradation of the aortic wall matrix and increased vascular smooth muscle cells (VSMCs) are important pathological features in the formation and exacerbation of AAA (101). Due to the lack of effective surgical methods and the unpredictability of the disease, the aortic wall is constantly enlarging. This leads to wall rupture and severe bleeding with a mortality rate of up to 80% (102).
Existing evidence suggests that circRNAs, as novel pathological regulators, may be involved in the pathogenesis of AAA (103). Yang et al. (23) found that circCCDC66 was significantly located in the VSMC cytoplasm in AAA. CircCCD66 was elevated in angiotensin II-stimulated VSMCs. Knockdown of circCCDC66 induced enhanced proliferation and decreased apoptosis in VSMCs. The study also showed that circCCDC66 positively regulated the expression of its host gene CCDC66. Moreover, miR-342-3p had the strongest binding affinity to circCCDC66 and CCDC66. Notably, miR-342-3p has been shown to be a potent tumor suppressor in non-small cell lung cancer (104), hepatocellular carcinoma (105), and glioma (106), among others. CCDC66 has only been reported in retinal degeneration and dysfunction (26). CircDC66 acts as a miR-34-3p sponge in AAA to regulate the CCDC66-dependent growth of VSMCs. The circCCDC66/miR-342-3p/CCDC66 axis was shown to play a role in regulating the proliferation and apoptosis of VSMCs. This provides us with a new molecular mechanism of action associated with AAA.
The roles of circCCDC66 in cancers
As a member of the circRNAs family, circCCDC66 is aberrantly expressed in thyroid cancer, non-small cell cancer, gastric cancer, colorectal cancer, renal cancer, cervical cancer, glioma, osteosarcoma and other cancers. It affects cancer development by regulating tumor malignant behavior. Mechanistically, circCCDC66 functions as an oncogene in cancer by acting as a molecular sponge competitively adsorbing miRNAs and thus regulating the expression of target genes (Figure 3). The networks of circCCDC66-regulated miRNAs are a key component of oncogene activation. CircCCDC66 promoted PDK4 expression by repressing miR-211-5 and up-regulated LARP1 expression by binding to miR-129-5p to accelerate thyroid cancer progression (16, 20). In NSCLC, circCCDC66 promoted cell proliferation through miR-211/SRCIN1 axis (17). Moreover, circCCDC66 was highly expressed in precancerous polyps and cancer tissues of colon cancer, and exacerbated the proliferation, migration and invasion of CRC cells by competitively binding miR-370 to upregulate MDM4 expression (15, 47). And circCCDC66 could also exert oncogenic roles by binding miR-1238-3p and miR-452-5p in gastric and cervical cancers (19, 49).
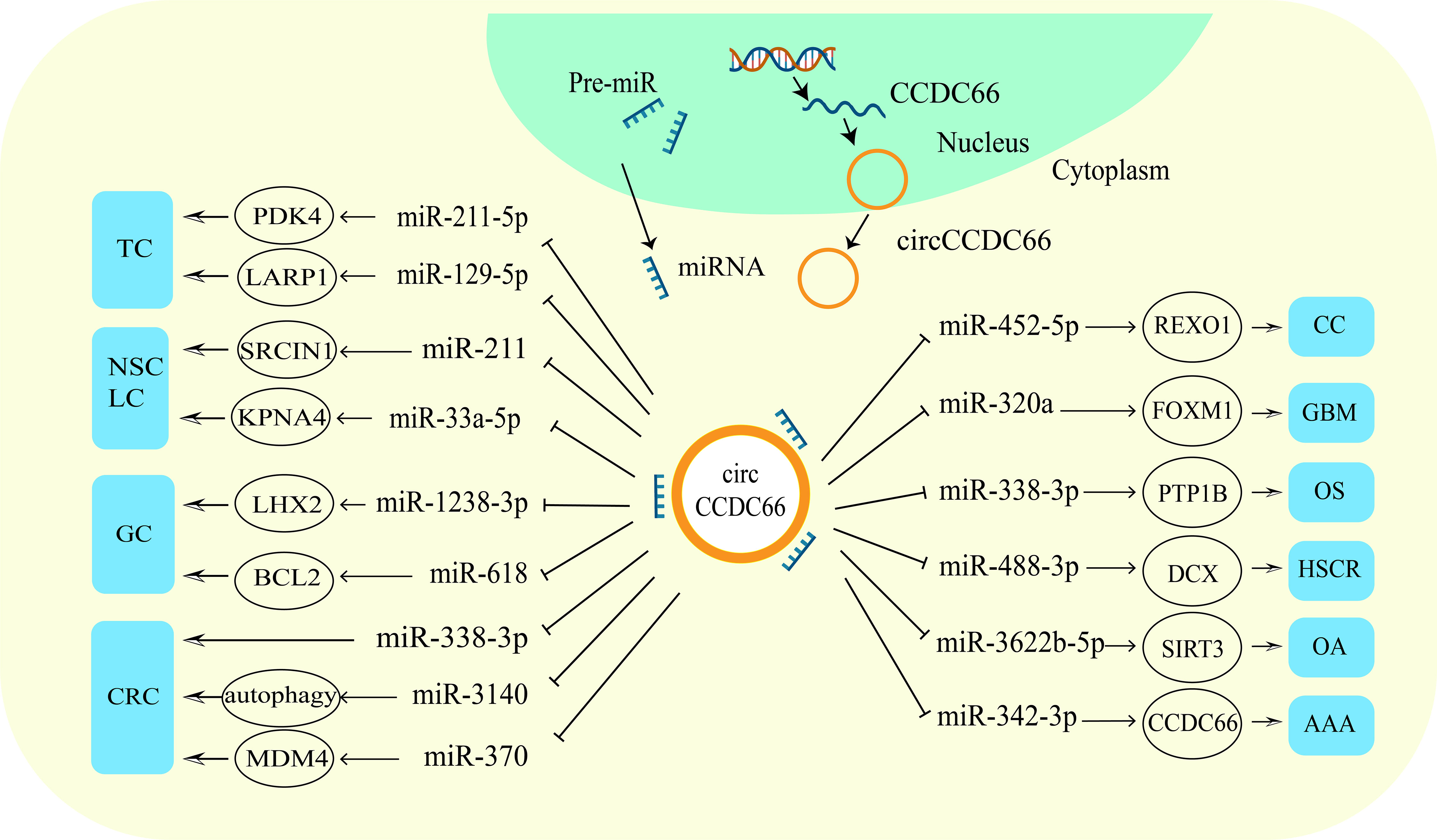
Figure 3 CircCCDC66 participates in various diseases through different ways. Up-regulation of circCCDC66 mediates the occurrence and development of malignant tumors.
On the other hand, circCCDC66 also acts as an miRNA sponge to regulate gene expression in non-neoplastic diseases. For example, circCCDC66 binding with miR-342-3p in abdominal aortic aneurysm up-regulated the host gene CCDC66 to induce cell proliferation and reduce apoptosis (23), while respectively binding with miR-488-3p and miR-3622b-5p suppressed the proliferation capacity of cells in Hirschsprung’s disease and osteoarthritis (52, 53). Table 1 shows the miRNAs and target proteins regulated by circCCDC66 in a variety of cancers.
Cancer stem cells (CSCs) can regulate the growth of cancers due to their self-renewal ability and high tumorigenic activity (107). Moreover, it plays an important role in chemotherapy resistance and metastatic recurrence. Recent studies have revealed that circRNAs regulate key gene expression and signaling pathways associated with CSCs, which has attracted significant attention. For example, circFAT1 promoted cancer stemness and immune evasion by activating STAT3 (108); circIPO11 recruited TOP1 to the GLI1 promoter to trigger its transcription and to drive self-renewal of the liver CSCs (109). The current study of circCCDC66 in CSCs has only been reported in renal cancer stem cells. HGF/c-MET-induced up-regulation of circCCDC66 expression promoted enrichment of renal cancer stem cells and accelerated the malignant process of cancer (48). Combined with the results of CSCs diversity and plasticity studies, blocking the source of CSCs and fully characterizing them may provide a prerequisite for therapeutic cancer strategies targeting CSCs.
Intratumoral hypoxia is one of the driving forces of cancer progression, triggering a variety of cellular metabolic stresses and also leading to increased autophagic activity (110). Exposure to hypoxic conditions increased the expression of circCCDC66 in CRC. Hypoxia-induced circCCDC66 combined with miR-3140 to activate the pathway of autophagy, which leaded to CRC cell growth and metastasis (46). Overexpression of circCCDC66 also acted as an miRNA sponge to interfering with miR-338-3p to promote cell viability and colony formation, and reduced radiosensitivity (22).
In addition, circCCDC66 plays a key role in cancer chemoresistance. CircCCD66 expression was dependent on oxaliplatin induction, and blocking PI3KK activity or DHX9 phosphorylation by point mutation decreased circCCDC66 expression and the ability to generate chemoresistant cells (45). Meanwhile, circCCDC66 is an important regulator in the progression of CDDP resistance. CircCCDC66 was overexpressed in CDDP-resistant gastric cancer cells and tissues. It promoted the up-regulation of BCL2 to inhibit apoptosis by targeting binding to miR-618 (18). However, there are no targeted inhibitory drugs for circCCDC66. Future research is needed not only to further explore the specific mechanisms by which circCCDC66 regulates cancer, but also to develop drugs that target circCCDC66 to reduce the organism’s resistance to chemotherapeutic agents and enhance the sensitivity of tumors to radiotherapy.
The clinical significance of circCCDC66 and its prognostic value
Human tumor specimens are used as the “gold standard”. Studies have found that circCCDC66 is up-regulated in a variety of solid tumors, including thyroid cancer, non-small cell cancer, gastric cancer, CRC, renal cancer, cervical cancer, glioma, osteosarcoma, etc. It is positively correlated with clinicopathological features such as tumor size, TNM stage, lymphatic metastasis, and vascular invasion. GC patients receiving cisplatin chemotherapy had decreased 5-year disease-free survival (DFS) in tissues with high expression of circCCC66 (n=53) compared to low expression of circCCC66 (n=52), which predicted a poorer prognosis (P=0.020) (18). Multivariate regression analysis showed that circCCD66 level was an independent risk factor for DFS (HR=1.775, P=0.011). Another study supported reduced plasma circCCDC66 levels in precursor lesions of CRC (colonic adenomas and adenomatous polyps), which may be a potential biomarker for CEA-negative or CA199-negative in CRC (76). Furthermore, circCCDC66 is sensitive to the state of tumor cells and may show the opposite state of expression when stimulated by apoptosis (Table 2).
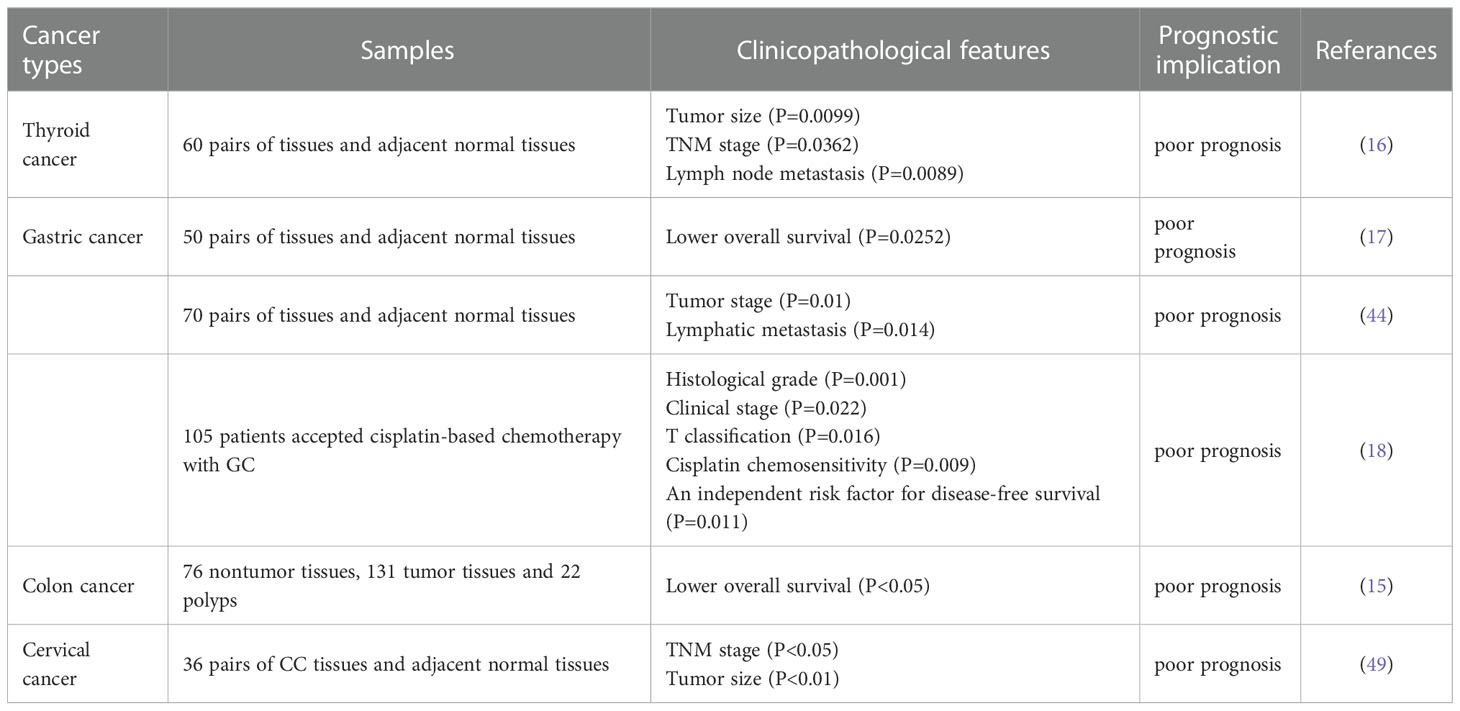
Table 2 Clinical pathological features and prognostic significance of circCCDC66 in malignant tumors.
Notably, the combined detection of circCCDC66 and traditional tumor markers can improve the specificity and sensitivity of diagnosis. Thus, circCCDC66 plays an important role in the occurrence, development, and prognosis of various cancers. In the future, we will expand the exploration of multiple areas of this important molecule, which will help to develop more effective cancer treatment strategies.
Conclusions and future expectations
As a new class of ncRNAs, thousands of circRNAs are important regulators of human gene expression. In recent years, circCCDC66 has been identified as a novel oncogenic circRNA with up-regulated expression in a variety of malignancies and significantly correlated with important clinical features such as tumor size, histological grade, TNM stage, and overall survival, among others. The current research on circCCDC66 mainly focuses on the molecular mechanism of action in cancers. To the best of our knowledge, circCCDC66 indirectly promotes oncogene expression by competitively binding to miRNAs (miR-211-5p/PDK4, miR-33a-5p/KPNA4, miR-320a/FOXM1, etc). At the same time, it activates the c-Myc/TGF-β signaling pathway and the autophagy pathway to regulate tumor cell proliferation, migration, invasion, drug resistance, autophagy, and EMT. In addition, circCCDC66 also plays an important regulatory role in non-tumor diseases such as Hirschsprung’s disease, osteoarthritis, and abdominal aortic aneurysm. These illustrate the heterogeneity of circRNA expression and the diversification of functions, however, research of the mechanism of action of circCCDC66 in cancers remains rare.
As an element in a dynamic network of ncRNA machinery, circCCDC66 has multiple functions in tumor progression. In the future, more detailed studies of the specific mechanism of circCCDC66 regulating tumorigenesis are required to provide new insights into the development of new biomarkers and personalized cancer treatments.
Author contributions
XW and CZ drafted and revised the manuscript. HS and JY collected relevant papers and helped to revise the manuscript. LZ and JH reviewed the article. XW designed tables and charts. All authors contributed to the article and approved the submitted version.
Funding
This article was supported by National Natural Science Fund, grant number 82073090; Shanxi Provincial Department of Human Resources and Social Security, grant number 20210001.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin (2021) 71:7–33. doi: 10.3322/caac.21654
2. Zhang Y, Liu Q, Liao Q. CircHIPK3: A promising cancer-related circular RNA. Am J Transl Res (2020) 12:6694–704. doi: 10.7150/jca.39722
3. Telenti A, Arvin A, Corey L, Corti D, Diamond MS, Garcia-Sastre A, et al. After the pandemic: Perspectives on the future trajectory of COVID-19. Nature (2021) 596:495–504. doi: 10.1038/s41586-021-03792-w
4. Ward ZJ, Walbaum M, Walbaum B, Guzman MJ, Jimenez DLJJ, Nervi B, et al. Estimating the impact of the COVID-19 pandemic on diagnosis and survival of five cancers in Chile from 2020 to 2030: A simulation-based analysis. Lancet Oncol (2021) 22:1427–37. doi: 10.1016/S1470-2045(21)00426-5
5. Martinez-Barriocanal A, Arango D, Dopeso H. PVT1 long non-coding RNA in gastrointestinal cancer. Front Oncol (2020) 10:38. doi: 10.3389/fonc.2020.00038
6. Zhou B, Yang H, Yang C, Bao YL, Yang SM, Liu J, et al. Translation of noncoding RNAs and cancer. Cancer Lett (2021) 497:89–99. doi: 10.1016/j.canlet.2020.10.002
7. Sun S, Wang W, Luo X, Li Y, Liu B, Li X, et al. Circular RNA circ-ADD3 inhibits hepatocellular carcinoma metastasis through facilitating EZH2 degradation via CDK1-mediated ubiquitination. Am J Cancer Res (2019) 9:1695–707. doi: 10.1101/027060
8. Kristensen LS, Andersen MS, Stagsted L, Ebbesen KK, Hansen TB, Kjems J. The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet (2019) 20:675–91. doi: 10.1038/s41576-019-0158-7
9. Liu CX, Chen LL. Circular RNAs: Characterization, cellular roles, and applications. Cell (2022) 185:2016–34. doi: 10.1016/j.cell.2022.04.021
10. Lei M, Zheng G, Ning Q, Zheng J, Dong D. Translation and functional roles of circular RNAs in human cancer. Mol Cancer (2020) 19:30. doi: 10.1186/s12943-020-1135-7
11. Meng S, Zhou H, Feng Z, Xu Z, Tang Y, Li P, et al. CircRNA: Functions and properties of a novel potential biomarker for cancer. Mol Cancer (2017) 16:94. doi: 10.1186/s12943-017-0663-2
12. Kristensen LS, Jakobsen T, Hager H, Kjems J. The emerging roles of circRNAs in cancer and oncology. Nat Rev Clin Oncol (2022) 19:188–206. doi: 10.1038/s41571-021-00585-y
13. Zhou J, Wang L, Sun Q, Chen R, Zhang C, Yang P, et al. Hsa_circ_0001666 suppresses the progression of colorectal cancer through the miR-576-5p/PCDH10 axis. Clin Transl Med (2021) 11:e565. doi: 10.1002/ctm2.565
14. Liu W, Zheng L, Zhang R, Hou P, Wang J, Wu L, et al. Circ-ZEB1 promotes PIK3CA expression by silencing miR-199a-3p and affects the proliferation and apoptosis of hepatocellular carcinoma. Mol Cancer (2022) 21:72. doi: 10.1186/s12943-022-01529-5
15. Hsiao KY, Lin YC, Gupta SK, Chang N, Yen L, Sun HS, et al. Noncoding effects of circular RNA CCDC66 promote colon cancer growth and metastasis. Cancer Res (2017) 77:2339–50. doi: 10.1158/0008-5472.CAN-16-1883
16. Li P, Chen J, Zou J, Zhu W, Zang Y, Li H. Circular RNA coiled-coil domain containing 66 regulates malignant development of papillary thyroid carcinoma by upregulating la ribonucleoprotein 1 via the sponge effect on miR-129-5p. Bioengineered (2022) 13:7181–96. doi: 10.1080/21655979.2022.2036304
17. Hong W, Yu S, Zhuang Y, Zhang Q, Wang J, Gao X. SRCIN1 regulated by circCCDC66/miR-211 is upregulated and promotes cell proliferation in non-Small-Cell lung cancer. BioMed Res Int (2020) 2020:5307641. doi: 10.1155/2020/5307641
18. Zhang Q, Miao Y, Fu Q, Hu H, Chen H, Zeng A, et al. CircRNACCDC66 regulates cisplatin resistance in gastric cancer via the miR-618/BCL2 axis. Biochem Biophys Res Commun (2020) 526:713–20. doi: 10.1016/j.bbrc.2020.03.156
19. Ren H, Song Z, Chao C, Mao W. CircCCDC66 promotes thyroid cancer cell proliferation, migratory and invasive abilities and glycolysis through the miR-211-5p/PDK4 axis. Oncol Lett (2021) 21:416. doi: 10.3892/ol.2021.12677
20. Wang L, Peng X, Lu X, Wei Q, Chen M, Liu L. Inhibition of hsa_circ_0001313 (circCCDC66) induction enhances the radio-sensitivity of colon cancer cells via tumor suppressor miR-338-3p: Effects of cicr_0001313 on colon cancer radio-sensitivity. Pathol Res Pract (2019) 215:689–96. doi: 10.1016/j.prp.2018.12.032
21. Wang Y, Zhao W, Zhang S. STAT3-induced upregulation of circCCDC66 facilitates the progression of non-small cell lung cancer by targeting miR-33a-5p/KPNA4 axis. BioMed Pharmacother (2020) 126:110019. doi: 10.1016/j.biopha.2020.110019
22. Yang R, Wang Z, Meng G, Hua L. Circular RNA CCDC66 facilitates abdominal aortic aneurysm through the overexpression of CCDC66. Cell Biochem Funct (2020) 38:830–8. doi: 10.1002/cbf.3494
23. Yang M, Wang GY, Qian H, Ji XY, Liu CY, Zeng XH, et al. Circ-CCDC66 accelerates proliferation and invasion of gastric cancer via binding to miRNA-1238-3p. Eur Rev Med Pharmacol Sci (2019) 23:4164–72. doi: 10.26355/eurrev_201905_17919
24. Chen LL, Yang L. Regulation of circRNA biogenesis. RNA Biol (2015) 12:381–8. doi: 10.1080/15476286.2015.1020271
25. Wen J, Liao J, Liang J, Chen XP, Zhang B, Chu L. Circular RNA HIPK3: A key circular RNA in a variety of human cancers. Front Oncol (2020) 10:773. doi: 10.3389/fonc.2020.00773
26. Chen J, Gu J, Tang M, Liao Z, Tang R, Zhou L, et al. Regulation of cancer progression by circRNA and functional proteins. J Cell Physiol (2022) 237:373–88. doi: 10.1002/jcp.30608
27. Huang A, Zheng H, Wu Z, Chen M, Huang Y. Circular RNA-protein interactions: Functions, mechanisms, and identification. Theranostics (2020) 10:3503–17. doi: 10.7150/thno.42174
28. Wang S, Sun Z, Lei Z, Zhang HT. RNA-Binding proteins and cancer metastasis. Semin Cancer Biol (2022) 86:748–68. doi: 10.1016/j.semcancer.2022.03.018
29. Yang T, Li Y, Zhao F, Zhou L, Jia R. Circular RNA foxo3: A promising cancer-associated biomarker. Front Genet (2021) 12:652995. doi: 10.3389/fgene.2021.652995
30. Qian Y, Li Y, Li R, Yang T, Jia R, Ge YZ. Circ-ZNF609: A potent circRNA in human cancers. J Cell Mol Med (2021) 25:10349–61. doi: 10.1111/jcmm.16996
31. Liu Y, Yang Y, Wang Z, Fu X, Chu XM, Li Y, et al. Insights into the regulatory role of circRNA in angiogenesis and clinical implications. Atherosclerosis (2020) 298:14–26. doi: 10.1016/j.atherosclerosis.2020.02.017
32. Chen L, Shan G. CircRNA in cancer: Fundamental mechanism and clinical potential. Cancer Lett (2021) 505:49–57. doi: 10.1016/j.canlet.2021.02.004
33. Wang Y, Liu J, Ma J, Sun T, Zhou Q, Wang W, et al. Exosomal circRNAs: Biogenesis, effect and application in human diseases. Mol Cancer (2019) 18:116. doi: 10.1186/s12943-019-1041-z
34. He AT, Liu J, Li F, Yang BB. Targeting circular RNAs as a therapeutic approach: Current strategies and challenges. Signal Transduct Target Ther (2021) 6:185. doi: 10.1038/s41392-021-00569-5
35. Li J, Hu ZQ, Yu SY, Mao L, Zhou ZJ, Wang PC, et al. CircRPN2 inhibits aerobic glycolysis and metastasis in hepatocellular carcinoma. Cancer Res (2022) 82:1055–69. doi: 10.1158/0008-5472.CAN-21-1259
36. Zheng R, Zhang K, Tan S, Gao F, Zhang Y, Xu W, et al. Exosomal circLPAR1 functions in colorectal cancer diagnosis and tumorigenesis through suppressing BRD4 via METTL3-eIF3h interaction. Mol Cancer (2022) 21:49. doi: 10.1186/s12943-021-01471-y
37. Dekomien G, Vollrath C, Petrasch-Parwez E, Boeve MH, Akkad DA, Gerding WM, et al. Progressive retinal atrophy in schapendoes dogs: Mutation of the newly identified CCDC66 gene. Neurogenetics (2010) 11:163–74. doi: 10.1007/s10048-009-0223-z
38. Gerding WM, Schreiber S, Schulte-Middelmann T, de Castro MA, Atorf J, Akkad DA, et al. Ccdc66 null mutation causes retinal degeneration and dysfunction. Hum Mol Genet (2011) 20:3620–31. doi: 10.1093/hmg/ddr282
39. Conkar D, Culfa E, Odabasi E, Rauniyar N, Yates JR, Firat-Karalar EN. The centriolar satellite protein CCDC66 interacts with CEP290 and functions in cilium formation and trafficking. J Cell Sci (2017) 130:1450–62. doi: 10.1242/jcs.196832
40. Conkar D, Bayraktar H, Firat-Karalar EN. Centrosomal and ciliary targeting of CCDC66 requires cooperative action of centriolar satellites, microtubules and molecular motors. Sci Rep (2019) 9:14250. doi: 10.1038/s41598-019-50530-4
41. Latour BL, Van De Weghe JC, Rusterholz TD, Letteboer SJ, Gomez A, Shaheen R, et al. Dysfunction of the ciliary ARMC9/TOGARAM1 protein module causes joubert syndrome. J Clin Invest (2020) 130:4423–39. doi: 10.1172/JCI131656
42. Batman U, Deretic J, Firat-Karalar EN. The ciliopathy protein CCDC66 controls mitotic progression and cytokinesis by promoting microtubule nucleation and organization. PloS Biol (2022) 20:e3001708. doi: 10.1371/journal.pbio.3001708
43. Joseph NA, Chiou SH, Lung Z, Yang CL, Lin TY, Chang HW, et al. The role of HGF-MET pathway and CCDC66 cirRNA expression in EGFR resistance and epithelial-to-mesenchymal transition of lung adenocarcinoma cells. J Hematol Oncol (2018) 11:74. doi: 10.1186/s13045-018-0557-9
44. Xu G, Chen Y, Fu M, Zang X, Cang M, Niu Y, et al. Circular RNA CCDC66 promotes gastric cancer progression by regulating c-myc and TGF-beta signaling pathways. J Cancer (2020) 11:2759–68. doi: 10.7150/jca.37718
45. Lin YC, Yu YS, Lin HH, Hsiao KY. Oxaliplatin-induced DHX9 phosphorylation promotes oncogenic circular RNA CCDC66 expression and development of chemoresistance. Cancers (Basel) (2020) 12:697. doi: 10.3390/cancers12030697
46. Feng J, Li Z, Li L, Xie H, Lu Q, He X. Hypoxiainduced circCCDC66 promotes the tumorigenesis of colorectal cancer via the miR3140/autophagy pathway. Int J Mol Med (2020) 46:1973–82. doi: 10.3892/ijmm.2020.4747
47. Mo Y, Lu Q, Zhang Q, Chen J, Deng Y, Zhang K, et al. Circular RNA CCDC66 improves murine double minute 4 (MDM4) expression through targeting miR-370 in colorectal cancer. Comput Math Methods Med (2022) 2022:7723995. doi: 10.1155/2022/7723995
48. Yang J, Yang L, Li S, Hu N. HGF/c-met promote renal carcinoma cancer stem cells enrichment through upregulation of cir-CCDC66. Technol Cancer Res Treat (2020) 19:1078168762. doi: 10.1177/1533033819901114
49. Zhang Y, Li X, Zhang J, Mao L. Circ-CCDC66 upregulates REXO1 expression to aggravate cervical cancer progression via restraining miR-452-5p. Cancer Cell Int (2021) 21:20. doi: 10.1186/s12935-020-01732-8
50. Salami R, Salami M, Mafi A, Vakili O, Asemi Z. Circular RNAs and glioblastoma multiforme: Focus on molecular mechanisms. Cell Commun Signal (2022) 20:13. doi: 10.1186/s12964-021-00809-9
51. Li Z, Li X, Xu D, Chen X, Li S, Zhang L, et al. An update on the roles of circular RNAs in osteosarcoma. Cell Prolif (2021) 54:e12936. doi: 10.1111/cpr.12936
52. Wen Z, Shen Q, Zhang H, Su Y, Zhu Z, Chen G, et al. Circular RNA CCDC66 targets DCX to regulate cell proliferation and migration by sponging miR-488-3p in hirschsprung’s disease. J Cell Physiol (2019) 234:10576–87. doi: 10.1002/jcp.27733
53. Zhang C, Lu Y, Yuan F, Jiang S. Circular RNA CCDC66 regulates osteoarthritis progression by targeting miR-3622b-5p. Gerontology (2022) 68:431–41. doi: 10.1159/000520325
54. Fallahi P, Ferrari SM, Galdiero MR, Varricchi G, Elia G, Ragusa F, et al. Molecular targets of tyrosine kinase inhibitors in thyroid cancer. Semin Cancer Biol (2022) 79:180–96. doi: 10.1016/j.semcancer.2020.11.013
55. Schlumberger M, Leboulleux S. Current practice in patients with differentiated thyroid cancer. Nat Rev Endocrinol (2021) 17:176–88. doi: 10.1038/s41574-020-00448-z
56. Chen LL, Zhang ZJ, Yi ZB, Li JJ. MicroRNA-211-5p suppresses tumour cell proliferation, invasion, migration and metastasis in triple-negative breast cancer by directly targeting SETBP1. Br J Cancer (2017) 117:78–88. doi: 10.1038/bjc.2017.150
57. Thai AA, Solomon BJ, Sequist LV, Gainor JF, Heist RS. Lung cancer. Lancet (2021) 398:535–54. doi: 10.1016/S0140-6736(21)00312-3
58. Singh T, Fatehi HM, Fatehi HA. Non-small cell lung cancer: Emerging molecular targeted and immunotherapeutic agents. Biochim Biophys Acta Rev Cancer (2021) 1876:188636. doi: 10.1016/j.bbcan.2021.188636
59. Liu Y, Ao X, Yu W, Zhang Y, Wang J. Biogenesis, functions, and clinical implications of circular RNAs in non-small cell lung cancer. Mol Ther Nucleic Acids (2022) 27:50–72. doi: 10.1016/j.omtn.2021.11.013
60. Pan J, Fang S, Tian H, Zhou C, Zhao X, Tian H, et al. LncRNA JPX/miR-33a-5p/Twist1 axis regulates tumorigenesis and metastasis of lung cancer by activating wnt/beta-catenin signaling. Mol Cancer (2020) 19:9. doi: 10.1186/s12943-020-1133-9
61. Feng L, Wang R, Yang Y, Shen X, Shi Q, Lian M, et al. KPNA4 regulated by miR-548b-3p promotes the malignant phenotypes of papillary thyroid cancer. Life Sci (2021) 265:118743. doi: 10.1016/j.lfs.2020.118743
62. Calvayrac O, Pradines A, Pons E, Mazieres J, Guibert N. Molecular biomarkers for lung adenocarcinoma. Eur Respir J (2017) 49:1601734. doi: 10.1183/13993003.01734-2016
63. Rosell R, Cardona AF, Arrieta O, Aguilar A, Ito M, Pedraz C, et al. Coregulation of pathways in lung cancer patients with EGFR mutation: Therapeutic opportunities. Br J Cancer (2021) 125:1602–11. doi: 10.1038/s41416-021-01519-2
64. Machlowska J, Baj J, Sitarz M, Maciejewski R, Sitarz R. Gastric cancer: Epidemiology, risk factors, classification, genomic characteristics and treatment strategies. Int J Mol Sci (2020) 21:4021. doi: 10.3390/ijms21114012
65. Matsuoka T, Yashiro M. Biomarkers of gastric cancer: Current topics and future perspective. World J Gastroenterol (2018) 24:2818–32. doi: 10.3748/wjg.v24.i26.2818
66. Shan G, Shao B, Liu Q, Zeng Y, Fu C, Chen A, et al. CircFMN2 sponges miR-1238 to promote the expression of LIM-homeobox gene 2 in prostate cancer cells. Mol Ther Nucleic Acids (2020) 21:133–46. doi: 10.1016/j.omtn.2020.05.008
67. Wang C, Zhang J, Yin J, Gan Y, Xu S, Gu Y, et al. Alternative approaches to target myc for cancer treatment. Signal Transduct Target Ther (2021) 6:117. doi: 10.1038/s41392-021-00500-y
68. Peng D, Fu M, Wang M, Wei Y, Wei X. Targeting TGF-beta signal transduction for fibrosis and cancer therapy. Mol Cancer (2022) 21:104. doi: 10.1186/s12943-022-01569-x
69. Radha G, Raghavan SC. BCL2: A promising cancer therapeutic target. Biochim Biophys Acta Rev Cancer (2017) 1868:309–14. doi: 10.1016/j.bbcan.2017.06.004
70. Gurba A, Taciak P, Sacharczuk M, Mlynarczuk-Bialy I, Bujalska-Zadrozny M, Fichna J. Gold (III) derivatives in colon cancer treatment. Int J Mol Sci (2022) 23:724. doi: 10.3390/ijms23020724
71. Wahab S, Alshahrani MY, Ahmad MF, Abbas H. Current trends and future perspectives of nanomedicine for the management of colon cancer. Eur J Pharmacol (2021) 910:174464. doi: 10.1016/j.ejphar.2021.174464
72. Huang J, Yu S, Ding L, Ma L, Chen H, Zhou H, et al. The dual role of circular RNAs as miRNA sponges in breast cancer and colon cancer. Biomedicines (2021) 9:1590. doi: 10.3390/biomedicines9111590
73. Lin YC, Lee YC, Chang KL, Hsiao KY. Analysis of common targets for circular RNAs. BMC Bioinf (2019) 20:372. doi: 10.1186/s12859-019-2966-3
74. Sun K, Su G, Deng H, Dong J, Lei S, Li G. Relationship between miRNA-338-3p expression and progression and prognosis of human colorectal carcinoma. Chin Med J (Engl) (2014) 127:1884–90. doi: 10.3748/wjg.v19.i14.2197
75. Wang S, Zhang K, Tan S, Xin J, Yuan Q, Xu H, et al. Circular RNAs in body fluids as cancer biomarkers: The new frontier of liquid biopsies. Mol Cancer (2021) 20:13. doi: 10.1186/s12943-020-01298-z
76. Lin J, Cai D, Li W, Yu T, Mao H, Jiang S, et al. Plasma circular RNA panel acts as a novel diagnostic biomarker for colorectal cancer. Clin Biochem (2019) 74:60–8. doi: 10.1016/j.clinbiochem.2019.10.012
77. Liu S, He L, Wu J, Wu X, Xie L, Dai W, et al. DHX9 contributes to the malignant phenotypes of colorectal cancer via activating NF-kappaB signaling pathway. Cell Mol Life Sci (2021) 78:8261–81. doi: 10.1007/s00018-021-04013-3
78. Tonouchi E, Gen Y, Muramatsu T, Hiramoto H, Tanimoto K, Inoue J, et al. MiR-3140 suppresses tumor cell growth by targeting BRD4 via its coding sequence and downregulates the BRD4-NUT fusion oncoprotein. Sci Rep (2018) 8:4482. doi: 10.1038/s41598-018-22767-y
79. Wade M, Li YC, Wahl GM. MDM2, MDMX and p53 in oncogenesis and cancer therapy. Nat Rev Cancer (2013) 13:83–96. doi: 10.1038/nrc3430
80. Barata PC, Rini BI. Treatment of renal cell carcinoma: Current status and future directions. CA Cancer J Clin (2017) 67:507–24. doi: 10.3322/caac.21411
81. Wang Y, Zhang Y, Wang P, Fu X, Lin W. Circular RNAs in renal cell carcinoma: Implications for tumorigenesis, diagnosis, and therapy. Mol Cancer (2020) 19:149. doi: 10.1186/s12943-020-01266-7
82. Ferrall L, Lin KY, Roden R, Hung CF, Wu TC. Cervical cancer immunotherapy: Facts and hopes. Clin Cancer Res (2021) 27:4953–73. doi: 10.1158/1078-0432.CCR-20-2833
83. Najafi S. Circular RNAs as emerging players in cervical cancer tumorigenesis; a review to roles and biomarker potentials. Int J Biol Macromol (2022) 206:939–53. doi: 10.1016/j.ijbiomac.2022.03.103
84. Zhai W, Li S, Zhang J, Chen Y, Ma J, Kong W, et al. Correction to: Sunitinib-suppressed miR-452-5p facilitates renal cancer cell invasion and metastasis through modulating SMAD4/SMAD7 signals. Mol Cancer (2022) 21:96. doi: 10.1186/s12943-022-01568-y
85. Zongqiang H, Jiapeng C, Yingpeng Z, Chuntao Y, Yiting W, Jiashun Z, et al. Exosomal miR-452-5p induce m2 macrophage polarization to accelerate hepatocellular carcinoma progression by targeting TIMP3. J Immunol Res (2022) 2022:1032106. doi: 10.1155/2022/1032106
86. Gan XN, Gan TQ, He RQ, Luo J, Tang RX, Wang HL, et al. Clinical significance of high expression of miR-452-5p in lung squamous cell carcinoma. Oncol Lett (2018) 15:6418–30. doi: 10.3892/ol.2018.8088
87. Hernandez MA, Madurga R, Garcia-Romero N, Ayuso-Sacido A. Unravelling glioblastoma heterogeneity by means of single-cell RNA sequencing. Cancer Lett (2022) 527:66–79. doi: 10.1016/j.canlet.2021.12.008
88. Qi L, Wang W, Zhao G, Jiang H, Zhang Y, Zhao D, et al. Circular RNA circCCDC66 promotes glioma proliferation by acting as a ceRNA for miR-320a to regulate FOXM1 expression. Aging (Albany NY) (2021) 13:17673–89. doi: 10.18632/aging.203258
89. Li C, Guan X, Jing H, Xiao X, Jin H, Xiong J, et al. Circular RNA circBFAR promotes glioblastoma progression by regulating a miR-548b/FoxM1 axis. FASEB J (2022) 36:e22183. doi: 10.1096/fj.202101307R
90. McKinnon C, Nandhabalan M, Murray SA, Plaha P. Glioblastoma: Clinical presentation, diagnosis, and management. BMJ (2021) 374:n1560. doi: 10.1136/bmj.n1560
91. Le Rhun E, Preusser M, Roth P, Reardon DA, van den Bent M, Wen P, et al. Molecular targeted therapy of glioblastoma. Cancer Treat Rev (2019) 80:101896. doi: 10.1016/j.ctrv.2019.101896
92. Xiang D, Li Y, Lin Y. Circular RNA circCCDC66 contributes to malignant phenotype of osteosarcoma by sponging miR-338-3p to upregulate the expression of PTP1B. BioMed Res Int (2020) 2020:4637109. doi: 10.1155/2020/4637109
93. Wiede F, Lu KH, Du X, Zeissig MN, Xu R, Goh PK, et al. PTP1B is an intracellular checkpoint that limits t-cell and CAR t-cell antitumor immunity. Cancer Discov (2022) 12:752–73. doi: 10.1158/2159-8290.CD-21-0694
94. Torroglosa A, Villalba-Benito L, Luzon-Toro B, Fernandez RM, Antinolo G, Borrego S. Epigenetic mechanisms in hirschsprung disease. Int J Mol Sci (2019) 20:3123. doi: 10.3390/ijms20133123
95. Klein M, Varga I. Hirschsprung’s disease-recent understanding of embryonic aspects, etiopathogenesis and future treatment avenues. Medicina (Kaunas) (2020) 56:611. doi: 10.3390/medicina56110611
96. Cheng L, Huang S, Chen L, Dong X, Zhang L, Wu C, et al. Research progress of DCLK1 inhibitors as cancer therapeutics. Curr Med Chem (2022) 29:2261–73. doi: 10.2174/0929867328666210709110721
97. Cho Y, Jeong S, Kim H, Kang D, Lee J, Kang SB, et al. Disease-modifying therapeutic strategies in osteoarthritis: Current status and future directions. Exp Mol Med (2021) 53:1689–96. doi: 10.1038/s12276-021-00710-y
98. Mao X, Cao Y, Guo Z, Wang L, Xiang C. Biological roles and therapeutic potential of circular RNAs in osteoarthritis. Mol Ther Nucleic Acids (2021) 24:856–67. doi: 10.1016/j.omtn.2021.04.006
99. Zhang W, Qi L, Chen R, He J, Liu Z, Wang W, et al. Circular RNAs in osteoarthritis: Indispensable regulators and novel strategies in clinical implications. Arthritis Res Ther (2021) 23:23. doi: 10.1186/s13075-021-02420-2
100. Zhang J, Xiang H, Liu J, Chen Y, He RR, Liu B. Mitochondrial sirtuin 3: New emerging biological function and therapeutic target. Theranostics (2020) 10:8315–42. doi: 10.7150/thno.45922
101. Golledge J. Abdominal aortic aneurysm: Update on pathogenesis and medical treatments. Nat Rev Cardiol (2019) 16:225–42. doi: 10.1038/s41569-018-0114-9
102. Sakalihasan N, Michel JB, Katsargyris A, Kuivaniemi H, Defraigne JO, Nchimi A, et al. Abdominal aortic aneurysms. Nat Rev Dis Primers (2018) 4:34. doi: 10.1038/s41572-018-0030-7
103. Han Y, Zhang H, Bian C, Chen C, Tu S, Guo J, et al. Circular RNA expression: Its potential regulation and function in abdominal aortic aneurysms. Oxid Med Cell Longev (2021) 2021:9934951. doi: 10.1155/2021/9934951
104. Komoll RM, Hu Q, Olarewaju O, von Dohlen L, Yuan Q, Xie Y, et al. MicroRNA-342-3p is a potent tumour suppressor in hepatocellular carcinoma. J Hepatol (2021) 74:122–34. doi: 10.1016/j.jhep.2020.07.039
105. Xue X, Fei X, Hou W, Zhang Y, Liu L, Hu R. MiR-342-3p suppresses cell proliferation and migration by targeting AGR2 in non-small cell lung cancer. Cancer Lett (2018) 412:170–8. doi: 10.1016/j.canlet.2017.10.024
106. Jiang Y, Zhou J, Zhao J, Zhang H, Li L, Li H, et al. The U2AF2/circRNA ARF1/miR-342-3p/ISL2 feedback loop regulates angiogenesis in glioma stem cells. J Exp Clin Cancer Res (2020) 39:182. doi: 10.1186/s13046-020-01691-y
107. Lagunas-Rangel FA. Circular RNAs and their participation in stemness of cancer. Med Oncol (2020) 37:42. doi: 10.1007/s12032-020-01373-x
108. Jia L, Wang Y, Wang CY. CircFAT1 promotes cancer stemness and immune evasion by promoting STAT3 activation. Adv Sci (Weinh) (2021) 8:2003376. doi: 10.1002/advs.202003376
109. Gu Y, Wang Y, He L, Zhang J, Zhu X, Liu N, et al. Circular RNA circIPO11 drives self-renewal of liver cancer initiating cells via hedgehog signaling. Mol Cancer (2021) 20:132. doi: 10.1186/s12943-021-01435-2
Keywords: circular RNA, circCCDC66, malignant tumors, endogenous competitive RNA, regulatory mechanism, cancer progression
Citation: Wang X, Zhang C, Song H, Yuan J, Zhang L and He J (2023) CircCCDC66: Emerging roles and potential clinical values in malignant tumors. Front. Oncol. 12:1061007. doi: 10.3389/fonc.2022.1061007
Received: 04 October 2022; Accepted: 16 December 2022;
Published: 09 January 2023.
Edited by:
Matiullah Khan, AIMST University, MalaysiaReviewed by:
Prasanna K. Santhekadur, JSS Academy of Higher Education and Research, IndiaRavindra Deshpande, Wake Forest University, United States
Anju Kumari, Center for Cancer Research, National Cancer Institute (NIH), United States
Copyright © 2023 Wang, Zhang, Song, Yuan, Zhang and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiefeng He, aGVqaWVmZW5nMjAwNUAxNjMuY29t
 Xiaoxiao Wang
Xiaoxiao Wang Chao Zhang1
Chao Zhang1