
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 23 November 2022
Sec. Genitourinary Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.1058700
This article is part of the Research Topic Case Reports in Genitourinary Oncology : 2022 View all 38 articles
Background: Endometrial stromal sarcoma is a relatively rare malignant tumor that derives from the malignant transformation of primitive uterine mesenchymal cells. It can lead to distant metastases. High-grade endometrial stromal sarcoma is extremely rare. The adrenal glands are an unreported site of metastasis.
Case summary: A 71-year-old woman with a diagnosis of endometrial stromal sarcoma 30 months ago. After receiving treatment with radiotherapy and chemotherapy, the patient was kept asymptomatic during the follow-up until 2 years later, when she complained of dyspnea. Pulmonary and right adrenal gland metastases were detected by 18F-FDG PET/CT. The right upper lobe mass was diagnosed as a high-grade endometrial stromal sarcoma metastasis after postoperative pathology. Due to the patient’s high risk of surgery, as she had many underlying diseases, we performed adequate preoperative preparation. The physical examination revealed that a hard mass was palpable in the right renal area. The right adrenal mass was resected in our hospital. Immunohistochemistry showed ER (-), PR (-), CD10 (+), P16 (+), Ki-67 (50%). The final diagnosis on pathological examination was a high-grade ESS metastatic to the right adrenal gland. The patient continued treatment in other hospitals after surgical resection. After four months of postoperative follow-up, metastasis was detected again during a PET/CT examination at an outside hospital.
Conclusion: Endometrial stromal sarcoma is rare, and the adrenal glands are an unreported site of metastasis. It has no specific clinical symptoms and mainly found for other reasons. The diagnosis still depends on pathology and immunohistochemistry. If there is no relevant past history, it is difficult to exclude a primary adrenal tumor.
Endometrial stromal sarcoma (ESS) is a rare malignant tumor of mesenchymal origin (1). Currently, there are four types of ESS (2, 3). It is a rare aggressive tumor, known to metastasize to lymph nodes, bones and lungs (2). There are no reports in the literature that ESS metastasizes to the adrenal glands. In this article, we retrospectively analyzed the clinical data of a patient with high-grade ESS metastasis to the right adrenal gland (2), which we cannot exclude as primary adrenal tumors before surgical resection. Therefore, we analyzed the clinical features of this disease in order to further improve our understanding of the disease and thus improve the diagnosis and treatment of this disease.
A 71-year-old woman with diagnosis of ESS 30 months ago. After receiving treatment with radiotherapy and chemotherapy, the patient remained asymptomatic during the follow-up until 2 years later, when she complained of dyspnea.
18F-FDG PET/CT was performed to identify if there was a potential malignancy tumor. The maximum intensity projection image (Figure 1A) revealed hypermetabolic lesions in the right thorax ((long arrow) and the right upper abdomen (short arrow). On the axial CT (Figure 1B), PET (Figure 1C), and fused PET/CT (Figure 1D) showed a large irregular lesion in the right lung upper lobe, measuring 8.4 cm×10.2 cm×8.1 cm showing intense 18F-FDG uptake with SUVmax of 13.4. The axial CT (Figure 1E), PET (Figure 1F), and fused PET/CT (Figure 1G) revealed the increased activity corresponded to a slight hypodense right adrenal gland soft tissue mass, measuring 5.1 cm×6.3 cm×6.5 cm showing intense 18F-FDG uptake with SUVmax of 13.4. The patient underwent thoracoscopic resection of the right upper lobe at an outside hospital, and postoperative pathology diagnosed high-grade ESS metastasis to the right side of the lung. The patient had follow-up outside the hospital. After 2 months, the ultrasound revealed a right adrenal mass that was 9.1 × 6.5 cm. After 5 months, the 18F-FDG PET/CT revealed a right adrenal mass that was 11.4 cm × 11.0 cm × 10.8 cm. After a period of recuperation at home, she was came to our hospital for further treatment.
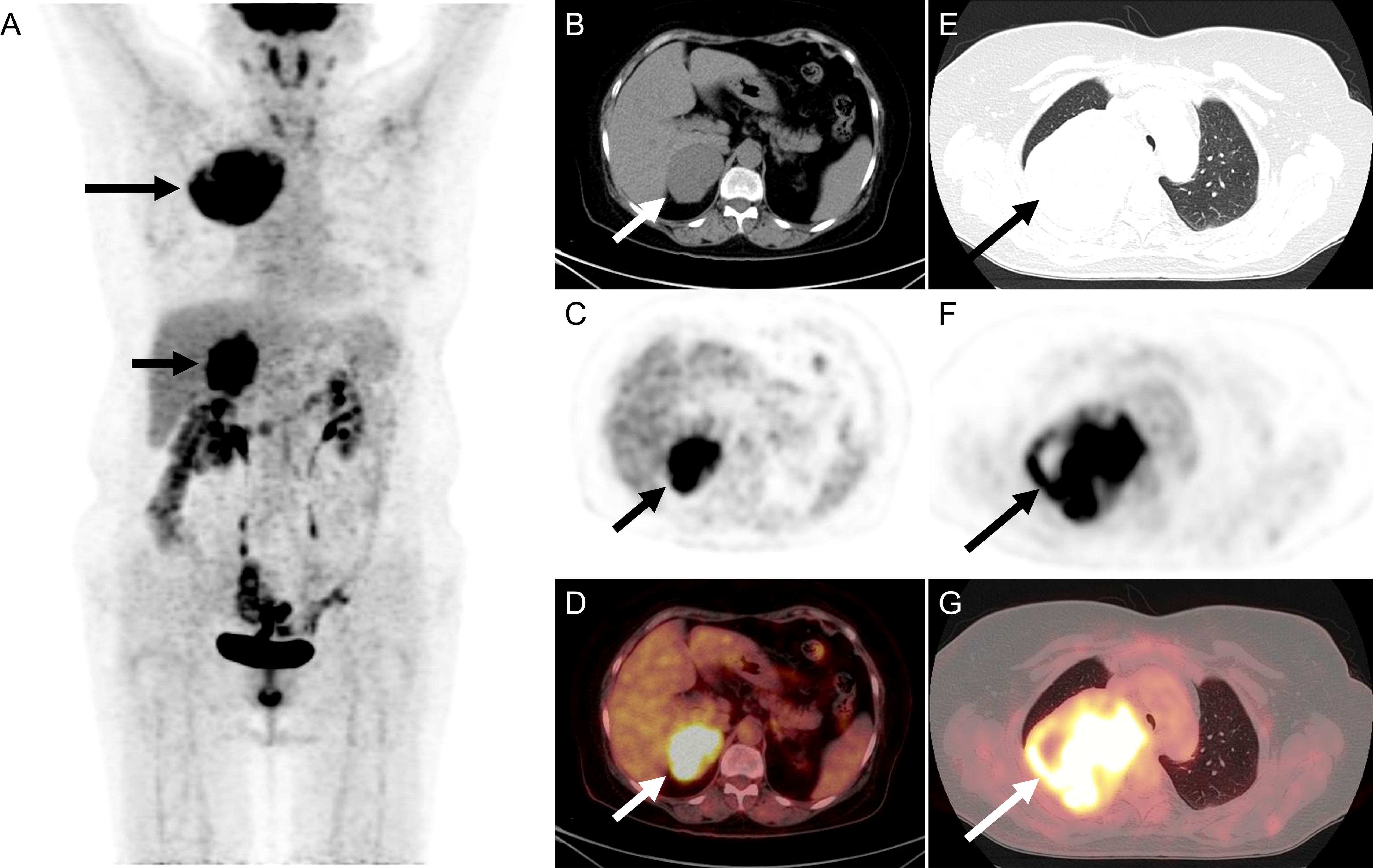
Figure 1 The 18F-FDG PET/CT of patient before operation. (A) The image revealed hypermetabolic lesions in the right thorax (long arrow) and the right upper abdomen (short arrow). (B–D) On the axial CT, PET, and fused PET/CT showed a large irregular lesion in the right lung upper lobe, measuring 8.4 cm×10.2 cm×8.1 cm showing intense 18F-FDG uptake with SUVmax of 13.4. (E–G) The axial CT, PET, and fused PET/CT revealed the increased activity corresponded to a slight hypodense right adrenal gland soft tissue mass, measuring 5.1 cm×6.3 cm×6.5 cm showing intense 18F-FDG uptake with SUVmax of 13.4.
The past medical history was significant for hypertension and coronary artery disease. Coronary stenting was performed, and she was usually treated with oral aspirin anticoagulation and avastatin lipid regulation. Physical examination revealed a palpable and hard mass, in the right area of her kidney. The levels of CA-125 were normal (17.49 U/mL, normal reference values: 0-35 U/mL). The levels of CA-199 were abnormal (36.74 U/mL, normal reference values: 0-35 U/mL). Plasma ACTH, angiotensin and renin concentrations were within the normal range. Other laboratory tests showed no significant abnormalities.
With adequate preoperative preparation, an open right adrenal tumor resection was performed. The intraoperative exploration revealed a cystic solid mass with a size of about 11.0 cm×10.5 cm. In a pathological view, the lesion was a round, dark red in color, and surrounded by a thick membrane and adhered to the surrounding tissues. On histopathological examination, the tumor cells were seen arranged in a swirling flow structure (Figures 2A, B). Immunohistochemistry showed: ER (-), PR (-), CD10 (+) (Figure 2C), P16 (+), Ki-67 (50%). The postoperative pathological diagnosis confirmed the high-grade ESS with right adrenal gland metastases. After 4 months of postoperative follow-up, metastasis was detected again during a 18F-FDG PET/CT examination at an outside hospital. The PET/CT of the outside hospital showed thickening of the left adrenal gland union with increased metabolism.
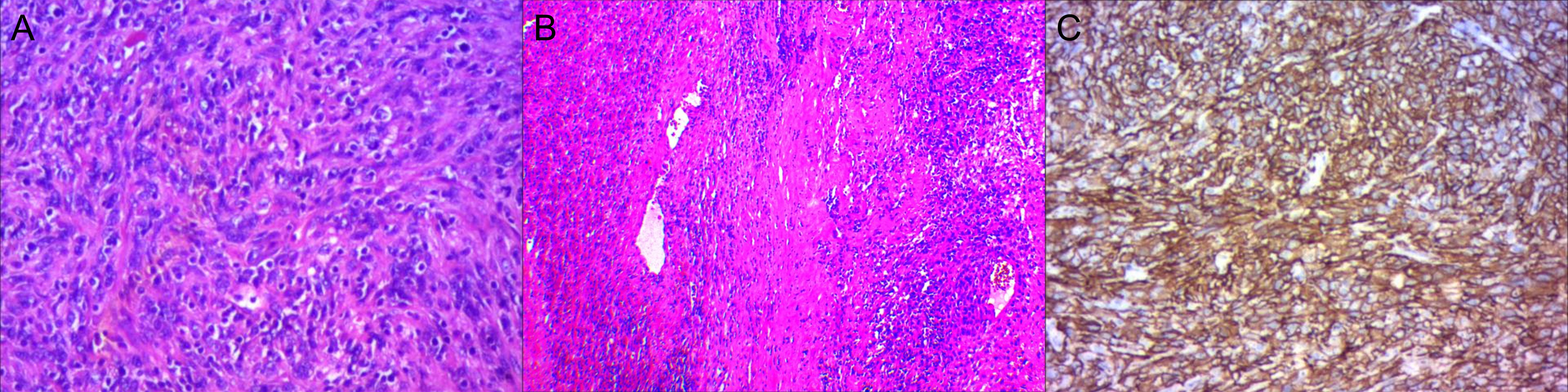
Figure 2 High-grade Endometrial Stromal Sarcoma in the right adrenal gland. (A, B) The tumor cells were seen in a swirling flow structure. (C) CD10 (+).
Timeline: Diagnosed ESS for 30 months—Pulmonary and Adrenal Glands Metastases were detected by 18F-FDG PET/CT before 6 months—She had undergone thoracoscopic right upper lobe resection 5 months ago, and postoperative pathology diagnosed high-grade ESS metastasis in the right lung—Right adrenal tumor resection was performed—After 4 months of postoperative follow-up, metastasis was detected again during a 18F-FDG PET/CT examination at an outside hospital (Figure 3).
Endometrial stromal sarcoma (ESS) is a relatively rare malignant tumor, which accounts for 0.2% of all uterine malignancies. The annual incidence of ESS is 1-2 per million women (1). There are four types of ESS: endometrial stromal nodule, undifferentiated uterine sarcoma, high-grade ESS and low-grade ESS (2, 3).
Chromosome translocations and rearrangements are common events in ESS and reflect distinct mechanisms of occurrence (4). Low-grade ESS is most commonly characterized by JAZF1-SUZ12 fusions followed by rearrangements involving PHF1 and multiple fusion partners (including JAZF1, EPC1, EPC2 and MEAF6). Furthermore, YWHAE gene rearrangement may be associated with high-grade ESS (5). However, genetic testing was not performed on our patients. The mean age of patients was 50 years and ranged from 28 to 67 years old. In addition to occurring around the uterus, it can also occur in the ovaries, rectal wall, pelvic peritoneum, and vagina. ESS occurring outside the uterus may be associated with endometriosis. However, it may also be unrelated to endometriosis (6). Clinical symptoms are variable, non-specific, and largely depend upon the location of ESS. When ESS is located in the uterus, abnormal vaginal bleeding and abdominal pain are the most common symptoms (3, 6).
Owing to its aggressive behavior, ESS can metastasize to lymph nodes, lungs, and bones, but it is relatively rare (2). Meanwhile, the incidence of high-grade ESS is extremely low. There was no report of adrenal gland metastases. In this case, we reported a very rare case of ESS with simultaneous metastasis to the right adrenal gland and right lung. The patient had been previously diagnosed with ESS and had received several cycles of chemotherapy (due to the absence of surgical guidelines). PET/CT imaging revealed occupations of the right lung and right adrenal gland (both considered metastases). The lung metastasis was confirmed. Surgical resection for pulmonary metastasis was performed. The exact details of the surgery, which were performed at an outside hospital, were not known.Adrenal occupancy was seen to be progressively larger on multiple postoperative reviews. So she was seen at our hospital for further treatment. The patient who has a history of ESS would be highly considered as a possible case of adrenal gland metastasis.In the literature, there was no reported ESS case that had metastasized to the adrenal gland (2). Primary adrenal malignancies could not be excluded in this patient.
ESS lacks specific clinical manifestations and imaging features. Microscopic examination showed that the tumor cells are arranged in a swirling flow structure and are located around the small arterial network of the hyaline wall (7). Necrosis may be seen, but is less common in low-grade ESS (7). High-grade ESS cells have irregular nuclear outlines, lack nucleoli, and have a high nuclear division index (but it is meaningless to distinguish between low-level and high-level ESS). High-grade ESS is the most malignant of the four classifications. Low-grade ESS characteristically shows diffuse positivity for CD10, ER, and PR, while high-grade ESS typically shows absent or only focal and weak staining for CD10, ER and PR (8, 9). In this case, macroscopically, the lesion was a gray-brown mass with an envelope, measuring about 12cm×12cm×6.5cm. Tumor cells mainly arranged in a swirling pattern. Immunohistochemistry: ER (-), PR (-), CD10 (+), P16 (+), Ki-67 (50%). The final diagnosis was that high-grade ESS metastasized to the right adrenal gland.
If there is no history of endometriosis, diagnosing ESS remains challenging. Some patients have no history of endometriosis (6, 10). Patients with high-grade ESS need to be distinguished from those with the following other diseases. 1) Undifferentiated uterine sarcoma: This is a high-grade sarcoma that is extremely rare and lacks a specific line of differentiation. The morphology and immunohistochemistry lack evidence of differentiation. Therefore, the prognosis of the disease is poor (11–13). Chemotherapy, hormonal therapy, and radiotherapy are the existing treatment options currently available. 2) Leiomyosarcoma: Leiomyosarcoma is a malignant mesenchymal tumor that originates from the smooth muscle. The incidence increases with age. Leiomyosarcoma can occur in the gastrointestinal tract and uterus. The adrenal glands can be seen (14). The clinical presentation is not specific. It is commonly discovered incidentally. On microscopic analysis, tumor cells show cytological atypia with cigar-shaped nuclei and abundant eosinophilic and fibrillar cytoplasm. When the cells were well differentiated, the cells were seen to be smooth muscle-like (15). The diagnosis is based on the demonstration of desmin, smooth muscle actin, actin HHF-35, and h-caldesmon. The main treatment method is surgery-assisted chemotherapy (14).
Because of the low incidence, there has been no consensus on the standard treatment of high-grade ESS (15). For ESS, complete surgery is the main treatment. Hormone therapy is recommended for patients with low-grade ESS. Chemotherapy with gemcitabine and docetaxel is used in high-grade ESS (8). Immunotherapy of high-grade ESS is still under investigation (16). High-grade ESS, with a median survival time of 11-23 months, is more aggressive and has a lower prognosis than low-grade ESS (7, 16, 17). The patient underwent surgery at our hospital, while the continuation phase was given in another hospital. After 4 months of postoperative follow-up, metastasis was detected again during a PET/CT examination at an outside hospital.
In conclusion, ESS is a rare malignancy, and the symptoms differ greatly depending on the location. High-grade ESS can metastasize throughout the lungs, lymph nodes, and bone. High-grade ESS is particularly rare and this is the first report of a metastasis to the adrenal glands. The diagnosis of metastatic ESS should be considered when the patient has a history of ESS and presents with a mass at a distant site. However, the diagnosis depends on the pathology and needs to be differentiated from other diseases. Surgical resection is used as the first treatment of choice, and high-grade ESS needs to be followed by concurrent chemotherapy and requires follow-up after surgery.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
TZ, R-LF and S-FY collected data and drafted the manuscript. R-LF and HW provided figures and pathology results and drafted the manuscript. W-BF and Z-YY review the manuscript. HW and C-XK edited the manuscript and critically revised the draft. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Park GE, Rha SE, Oh SN, Lee A, Lee KH, Kim MR. Ultrasonographic findings of low-grade endometrial stromal sarcoma of the uterus with a focus on cystic degeneration. Ultrasonography (2016) 35(2):124–30. doi: 10.14366/usg.15045
2. Sreerekha J, Gochhait D, Dk K, Siddaraju N, T P, Kayal S. Endometrial stromal sarcoma metastasis in a supraclavicular lymph node: A diagnostic conundrum on cytology. Cytopathology (2019) 30(5):567–9. doi: 10.1111/cyt.12733
3. Ginter PS, Pirog EC, Hoda RS. High grade endometrial stromal sarcoma on thinprep. Diagn Cytopathol (2015) 43(9):756–62. doi: 10.1002/dc.23316
4. Micci F, Heim S, Panagopoulos I. Molecular pathogenesis and prognostication of "Low-grade'' and "High-grade" endometrial stromal sarcoma. Genes Chromosomes Cancer (2021) 60(3):160–7. doi: 10.1002/gcc.22907
5. Lin DI, Huang RSP, Mata DA, Decker B, Danziger N, Lechpammer M, et al. Clinicopathological and genomic characterization of Bcorl1-driven high-grade endometrial stromal sarcomas. Mod Pathol (2021) 34(12):2200–10. doi: 10.1038/s41379-021-00873-z
6. Sharma A, Bal M, Menon S. Extragenital endometrial stromal sarcoma of transverse mesocolon: A diagnostic conundrum. Int J Surg Pathol (2022) 30(5):551–6. doi: 10.1177/10668969211070485
7. Alan S, Yilmaz E, Tecellioglu F, Akatli AN, Inci Coskun E, Gokce H. Multifocal low-grade endometrial stromal sarcoma arising from pre-existing endometriosis in a hysterectomised patient: A case report. J Obstet Gynaecol (2019) 39(8):1177–80. doi: 10.1080/01443615.2019.1587599
8. Aisagbonhi O, Harrison B, Zhao L, Osgood R, Chebib I, Oliva E. Ywhae rearrangement in a purely conventional low-grade endometrial stromal sarcoma that transformed over time to high-grade sarcoma: Importance of molecular testing. Int J Gynecol Pathol (2018) 37(5):441–7. doi: 10.1097/PGP.0000000000000451
9. Xie W, Bi X, Cao D, Yang J, Shen K, You Y. Primary endometrioid stromal sarcomas of the ovary: A clinicopathological study of 14 cases with a review of the literature. Oncotarget (2017) 8:64435–3352. doi: 10.18632/oncotarget.18805
10. Deb PQ, Heller DS. Extrauterine endometrial stromal sarcoma: A systematic review and outcome analysis. Ann Diagn Pathol (2022) 59:151966. doi: 10.1016/j.anndiagpath.2022.151966
11. Allanson ER, Cummings M, Kuchel A, Wastney T, Nicklin J. Undifferentiated uterine sarcoma: A multidisciplinary challenge. Int J Gynecol Cancer (2022) 32(2):198–202. doi: 10.1136/ijgc-2021-003215
12. Tanner EJ, Garg K, Leitao MM Jr., Soslow RA, Hensley ML. High grade undifferentiated uterine sarcoma: Surgery, treatment, and survival outcomes. Gynecol Oncol (2012) 127(1):27–31. doi: 10.1016/j.ygyno.2012.06.030
13. Cabrera S, Bebia V, Acosta U, Franco-Camps S, Manalich L, Garcia-Jimenez A, et al. Survival outcomes and prognostic factors of endometrial stromal sarcoma and undifferentiated uterine sarcoma. Clin Transl Oncol (2021) 23(6):1210–9. doi: 10.1007/s12094-020-02512-6
14. Devaud N, Vornicova O, Abdul Razak AR, Khalili K, Demicco EG, Mitric C, et al. Leiomyosarcoma: Current clinical management and future horizons. Surg Oncol Clin N Am (2022) 31(3):527–46. doi: 10.1016/j.soc.2022.03.011
15. Recine F, Bongiovanni A, Casadei R, Pieri F, Riva N, De Vita A, et al. Primary leiomyosarcoma of the bone: A case report and a review of the literature. Med (Baltimore) (2017) 96(45):e8545. doi: 10.1097/MD.0000000000008545
16. Kim Y, Kim D, Sung WJ, Hong J. High-grade endometrial stromal sarcoma: Molecular alterations and potential immunotherapeutic strategies. Front Immunol (2022) 13:837004. doi: 10.3389/fimmu.2022.837004
17. Gremel G, Liew M, Hamzei F, Hardell E, Selling J, Ghaderi M, et al. A prognosis based classification of undifferentiated uterine sarcomas: Identification of mitotic index, hormone receptors and ywhae-Fam22 translocation status as predictors of survival. Int J Cancer (2015) 136(7):1608–18. doi: 10.1002/ijc.29141
Keywords: endometrial stromal sarcoma, adrenal glands, diagnosis, treatment, clinical features
Citation: Zhang T, Feng R-l, Yin S-f, Feng W-b, Yin Z-y, Wang H and Ke C-X (2022) Case report: High-grade endometrial stromal sarcoma with adrenal glands metastases: An unreported site of metastasis. Front. Oncol. 12:1058700. doi: 10.3389/fonc.2022.1058700
Received: 05 October 2022; Accepted: 04 November 2022;
Published: 23 November 2022.
Edited by:
Maribel Acién, Miguel Hernández University of Elche, SpainReviewed by:
Kehua Jiang, Guizhou Provincial People’s Hospital, ChinaCopyright © 2022 Zhang, Feng, Yin, Feng, Yin, Wang and Ke. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chang-Xing Ke, a2VubmUxMjZAMTYzLmNvbQ==; Hao Wang, MTg4MTA1NjYxNTVAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.