- 1Department of Obstetrics and Gynecology, Taizhou Women and Children’s Hospital of Wenzhou Medical University, Taizhou, Zhejiang, China
- 2Department of Textile Engineering, Akesu Regional Vocational and Technical College, Akesu, Xinjiang, China
- 3Department of Pathology, Taizhou Women and Children’s Hospital of Wenzhou Medical University, Taizhou, Zhejiang, China
Background: The efficacy of adjuvant radiotherapy for postoperative patients with early-stage cervical adenocarcinoma who are lymph node-negative is still inconclusive. Establishing a nomogram to predict the prognosis of such patients could facilitate clinical decision-making.
Methods: We recruited 4636 eligible patients with pT1-T2aN0M0 cervical adenocarcinoma between 2004 and 2016 from the Surveillance, Epidemiology and End Results (SEER) database. Random survival forest (RSF) and conditional survival forest (CSF) model was used to assess the prognostic importance of each clinical characteristic variable. We identified independent prognostic factors associated with overall survival (OS) by univariate and multivariate Cox regression risk methods and then constructed a nomogram. We stratified patients based on nomogram risk scores and evaluated the survival benefit of different adjuvant therapies. To reduce confounding bias, we also used propensity score matching (PSM) to match the cohorts before performing survival analyses.
Results: The RSF and CSF model identified several important variables that are associated with prognosis, including grade, age, radiotherapy and tumor size. Patients were randomly divided into training and validation groups at a ratio of 7:3. Multivariate cox analysis revealed that age, grade, tumor size, race, radiotherapy and histology were independent prognostic factors for overall survival. Using these variables, we then constructed a predictive nomogram. The C-index value for evaluating the prognostic nomogram fluctuated between 0.75 and 0.91. Patients were divided into three subgroups based on risk scores, and Kaplan-Meier (K-M) survival analysis revealed that in the low-risk group, postoperative chemotherapy alone was associated with a significantly worse OS than surgery alone. Following PSM, survival analysis showed that compared with surgery alone, radiotherapy was associated with a worse OS in the training group although there was no significant difference in the validation group.
Conclusions: For patients with pT1-T2aN0M0 cervical adenocarcinoma, adjuvant treatments such as postoperative radiotherapy or chemotherapy, compared with surgery alone, are of no benefit with regards to patient survival. Our prognostic nomogram exhibits high accuracy for predicting the survival of patients with early-stage postoperative cervical adenocarcinoma.
Introduction
Cervical cancer is one of the most common malignancies of the female reproductive tract and the fourth leading cause of cancer-related death in women. An estimated 341,831 people worldwide were predicted to die from this form of cancer in 2020 (1). Cervical cancer mainly originates from the squamous epithelium and glandular epithelium of the cervix, of which adenocarcinoma accounts for approximately 25% (2, 3). In Western countries, such as the United Kingdom and the United States, the incidence of cervical adenocarcinoma is gradually increasing (3, 4). The prognosis of patients with cervical adenocarcinoma is significantly worse than that of patients with squamous cell carcinoma, furthermore, these patients are prone to distant metastasis. Furthermore, the pathological type of adenocarcinoma is an independent risk factor for the poor prognosis of patients with cervical cancer (5–8).
For locally advanced cervical carcinoma, there is a consensus that whole-pelvic radiation therapy has the best outcome when combined with cisplatin-based chemotherapy (9). The best treatment for earlier stage cervical carcinoma is optimized surgery (9–12). Postoperative high-risk factors for early cervical cancer include lymph node metastasis, parametrial invasion and positive surgical margins. The American Society for Radiation Oncology (ASTRO) guidelines propose that patients with high-risk factors after cervical cancer surgery should receive postoperative supplemental pelvic radiotherapy combined with cisplatin chemotherapy (9). However, in patients with intermediate risk factors after surgery, such as tumor size, pathological type and tumor differentiation, there is no conclusive evidence of the need for radiotherapy and chemotherapy. In a previous study, Mabuchi considered adenocarcinoma to be an independent prognostic indicator for poor survival in patients with early-stage cervical cancer with both intermediate and high-risk factors, regardless of the type of adjuvant radiotherapy after radical hysterectomy (13). In another study, Ryud et al. considered that adenocarcinoma represents a moderate risk factor and that whole pelvic radiotherapy should be performed following surgery (14). Zhou et al. suggested that for patients with lymph node-positive early-stage cervical cancer, cervical adenocarcinoma had a significantly worse survival rate than cervical squamous cell carcinoma that could benefit from concurrent chemoradiotherapy (CCRT); however, patients with adenocarcinoma did not benefit from CCRT (15).
However, some researchers believe that the addition of CCRT after radical hysterectomy does not significantly improve the survival of patients with pelvic lymph node-positive FIGO stage IIIC1 cervical adenocarcinoma (16). For example, Mahmoud et al. concluded that adjuvant chemoradiotherapy did not have a significant survival advantage over adjuvant radiotherapy in postoperative women with early-stage cervical cancer with intermediate risk factors (17). Compared with cervical squamous cell carcinoma, cervical adenocarcinoma exhibits obvious heterogeneity in terms of tissue anatomy, prognosis and recurrence, although current treatment is unable to distinguish cervical adenocarcinoma. At present, there is no evidence to indicate which clinicopathological risk factors affect the prognosis of lymph node-negative and non-metastatic early-stage cervical adenocarcinoma after surgery or whether these patients can benefit from adjuvant therapies such as postoperative radiotherapy and chemotherapy.
Large-scale clinical trials and studies are restricted due to the low incidence of cervical adenocarcinoma. The Surveillance, Epidemiology and End Results (SEER) database is a nationwide cancer dataset compiled in the United States and features a wealth of relevant information about patients with different types of cancer, thus providing appropriate real-world population data for our research. In this study, we used RSF and CSF model to evaluate the importance of clinicopathological variables in the prognosis of cervical adenocarcinoma patients identified in the SEER database. Then, we constructed a predictor nomogram of overall survival (OS) for patients with pT1-T2aN0M0 after surgery to facilitate clinical decision making. In addition, we also investigated whether postoperative adjuvant chemoradiotherapy can benefit early cervical adenocarcinoma after surgery.
Methods
Data collection
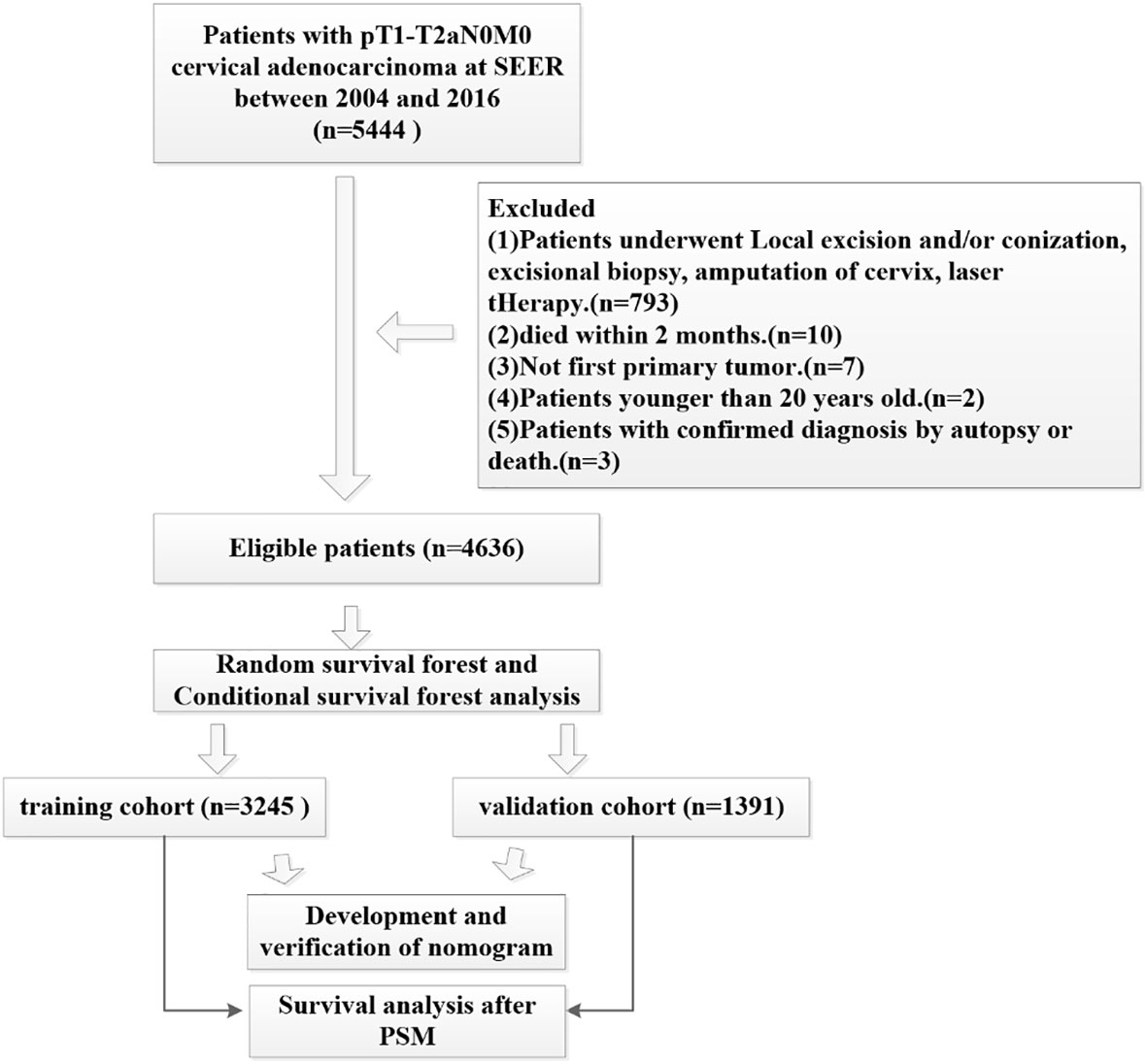
This study analyzed data from the publicly available SEER database from 2004 to 2016. We extracted data using SEER*Stat Software Version 8.3.9.2 (https://seer.cancer.gov/data-software/) with a permitted SEER ID (19968-Nov2020). Because this data is open access, no ethical approval was required. Our study cohort applied that following inclusion criteria (1): All patients underwent hysterectomy and radical hysterectomy (surgery encode: 30-70) (2); none of the patients had lymph node metastasis or distant metastasis (3); cervical adenocarcinoma was the first primary tumor (4); the primary sites for all patients were C53.0-endocervix, C53.1-exocervix, C53.9-cervix uteri and C53.8-overlapping lesions of cervix uteri (5); all patients showed histology codes 8140 to 8389 with adenomas and adenocarcinomas based on ICD-O-3 (6); according to the ACJJ 8, T staging was T1-T2a, and (7) complete follow-up data was available. The exclusion criteria were as follows (1): patients younger than 20 years-of-age (2); all patients who died within 2 months were excluded (3); patients undergoing local excision and/or conization, excisional biopsy, amputation of the cervix or those undergoing laser therapy (4), not first primary tumor, and (5) patients with a confirmed diagnosis by autopsy or death. We used the random forest method to replace missing data with multiple imputation; a specific flowchart describing patient recruitment is given in Figure 1.
We extracted the following demographic and clinicopathological characteristics for analysis: age at diagnosis, marital status, histological grade, race, histopathological type, surgery, AJCC Seventh T stage, tumor size, chemotherapy and radiotherapy. Overall survival (OS) was used to evaluate patient prognosis. The death outcome for OS was death by any cause.
Random survival forest analysis
Random survival forest (RSF) analysis is an ensemble machine learning method that adds survival analysis to random forest analysis (18). This strategy is bound by the hazard proportionality assumption and the log linearity assumption. In addition, RSF can also rank the importance of variables according to the minimal depth of the largest subtree in which the variable is located. In this cohort, we adopted the random forest algorithm to fill in some missing values. We also explored the prognostic importance of clinical feature variables in early-stage cervical adenocarcinoma using RSF models.
Conditional survival forest analysis
Disadvantages of RSF include a bias towards inclusion of variables with many split points (19–21). This effect leads to deviations in the estimates of the summary of results, such as variable importance. And conditional inference forest (CIF) can reduce this selection bias by separating the algorithm for selecting the best covariate to be segmented from the algorithm for searching the best split point. Therefore, CSF can select variables according to the importance of variables, and then select the split area. We then used the CSF model to explore the importance of clinical feature variables.
Nomogram construction
We screened eligible patients for inclusion in our study. Then, these patients were randomly assigned to training and validation groups in a 7:3 ratio. A nomogram was subsequently constructed and validated with the Cox method. Cox proportional hazards regression was then used to identify independent prognostic factors. Based on the variables screened by Cox multivariate analysis, a nomogram was established to predict the prognosis of patients with early-stage cervical cancer. The concordance index (C-index), receiver operating characteristic (ROC) curve and calibration curves were used to evaluate the robustness of the nomogram. Based on the nomogram, we then calculated a risk score for each patient. The optimal cut-off value for patient risk scores was determined using X-tile software (https://medicine.yale.edu/lab/rimm/research/software/) and patients were divided into three risk subgroups.
Statistical analysis
All analyses were performed using R statistical software (http://www.r-project.org, version 3.6.1). Clinicopathological baseline characteristics of patients were described using proportions and frequencies, and comparisons between different subgroups were performed using Fisher’s exact test. Kaplan-Meier (K-M) survival curves were evaluated using the log-rank test. The proportional risk hypothesis for each covariate included in the Cox model fitting was tested. Hazard ratio (HR) values were calculated using Cox proportional hazard regression models. Variables with a P value <0.1 in the Cox univariate analysis were then included in Cox multivariate analysis. To reduce selection bias due to unbalanced perioperative factors, propensity score matching (PSM) was used in the analyses of non-radiotherapy and radiotherapy groups. We use the “nearest neighbor method” method for matching according to a ratio of 1:1. A two-sided P<0.05 was considered to be statistically significant. The CSF model was analyzed using “PySurvival” in Python version 3.6 (Python Software Foundation for Statistical Computing, Wilmington, Delaware, USA). All other analyses were performed using packages in the R software (http://www.r-project.org, version 3.6.1), including “tableone”, “randomForestSRC”, “survival”, “survminer”, “rms”, “Cairo”, “pec” and “MatchIt”.
Results
Patient characteristics
A total of 4636 patients diagnosed with pT1-2aN0M0 cervical adenocarcinoma were identified in the SEER database and met our inclusion criteria. Of these patients, 3953 were white and 3278 were younger than 50 years-of-age, with a mean follow-up of 62 months (range: 2 - 155 months). In our cohort, there were many missing values for key variables such as tumor size and grade (see Figure 2A). To avoid reducing the robustness of our model, we used a random forest method to fill in these missing values. This is a common method in machine learning and represents an ensemble learning model based on decision tree classifiers. We implemented random forest filling using the misforest function in the misforest package in R. Subsequently, patients were randomly divided into training (n=3245) and validation (n=1391) sets at a ratio of 7:3. Detailed baseline demographic and clinical characteristics are summarized in Table 1. There were no significant differences in the demographic and clinicopathological characteristics when compared between the training and validation sets. The OS rates at 1, 3 and 5 years were 99.0%, 95.6% and 92.7% in the training cohort and 98.9%, 96.3% and 93.7% in the validation cohort, respectively.
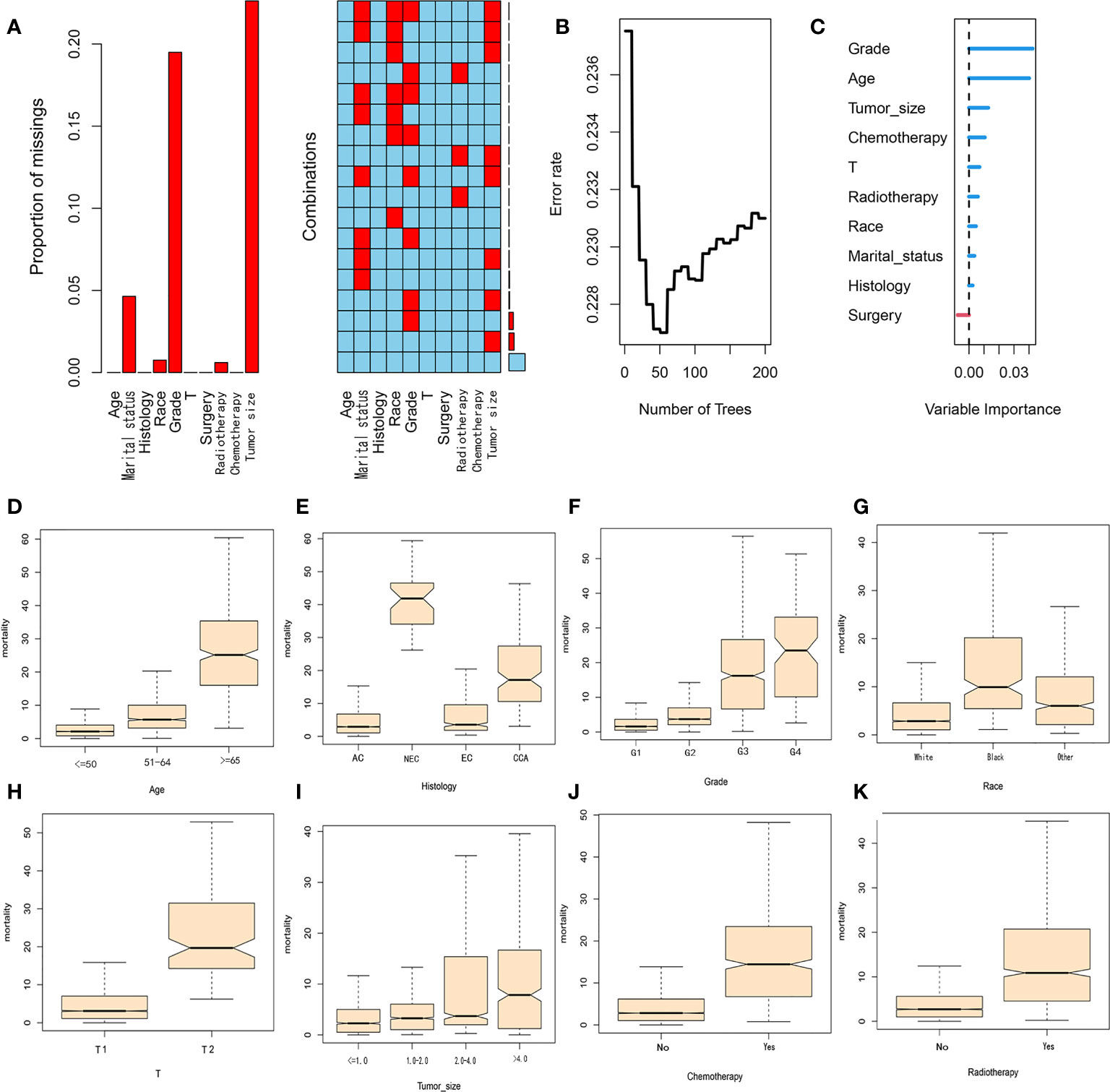
Figure 2 (A) Proportion and combination of missing values in the cohort. (B) Random forest plot. (C) Variable importance score. (D–K) The contribution of variables in each subgroup to mortality.
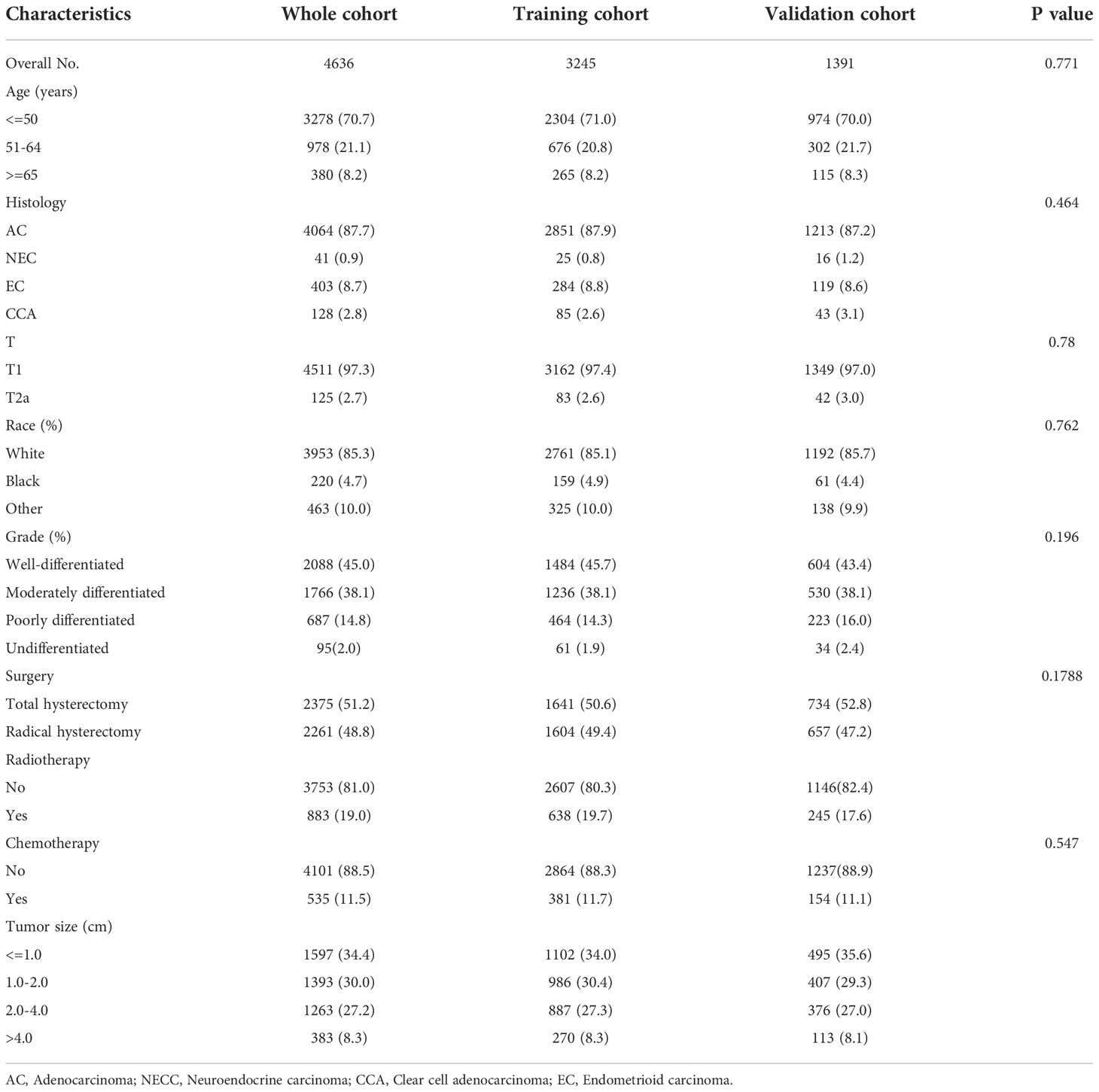
Table 1 Demographics and clinicopathological characteristics of patients with pT1-T2aN0M0 cervical adenocarcinoma.
RSF and CSF analysis
The key advantage of the random survival forest model is that it is not constrained by the proportional hazard assumption and the logarithmic linear assumption. Furthermore, RSF analysis can avoid the overfitting problem associated with some other algorithms by applying two random sampling processes (22). We trained a RSF with 200 trees in the entire dataset with a terminal node size of 15 and randomly selected mtry=3 variables per iteration. RSF analysis uses Harrell’s concordance index to calculate accuracy. From Figure 2B, it can be concluded that the C-index of the model is approximately 0.77 and when the survival tree increased to a certain number, the error rate curve tended to be stable (Figure 2B). The variable importance of predicting patient prognosis is demonstrated in Figure 2C. The variable importance score, obtained by the minimum depth method, was determined to be grade, age, tumor-size, chemotherapy and T-stage (from high to low). The contribution of each subgroup variable to mortality is shown in Figures 2D–K. Subgroup variables such as age (>=65 years), NEC, G3, G4 and T2 contributed the most to mortality. In the CSF model, we used 20 minimum node sizes, 200 trees, and a 0.05 alpha. We calculated the correlation between clinical variables, and the results are shown in Figure 3A. Subsequently, we compared the prediction errors of COX regression model and RSF model based on brief score. As shown in Figure 3B, both have similar prediction performance. We calculated the patient’s risk factors using the CSF model (Figure 3C), The order of importance of variables is grade, Radiotherapy, Chemotherapy, Tumor Size, age, etc. We also tested the predictive errors of the model. The results showed that when the time was 60 months, the Brief score was 0.03 (Figure 3D). The comparison between the overall prediction and the actual number of deaths is shown in the Figure 3E. The above show that the CSF model has good prediction ability.

Figure 3 Conditional survival forest analysis (A) Correlation of clinical feature variables, (B) Prediction errors of Cox regression model and RSF model, (C) Variable importance score, (D) Prediction error curve with Integrated Brier Score (IBS), (E) Number of dead patients.
Prognostic analysis of patients with pT1-2aN0M0 cervical adenocarcinoma
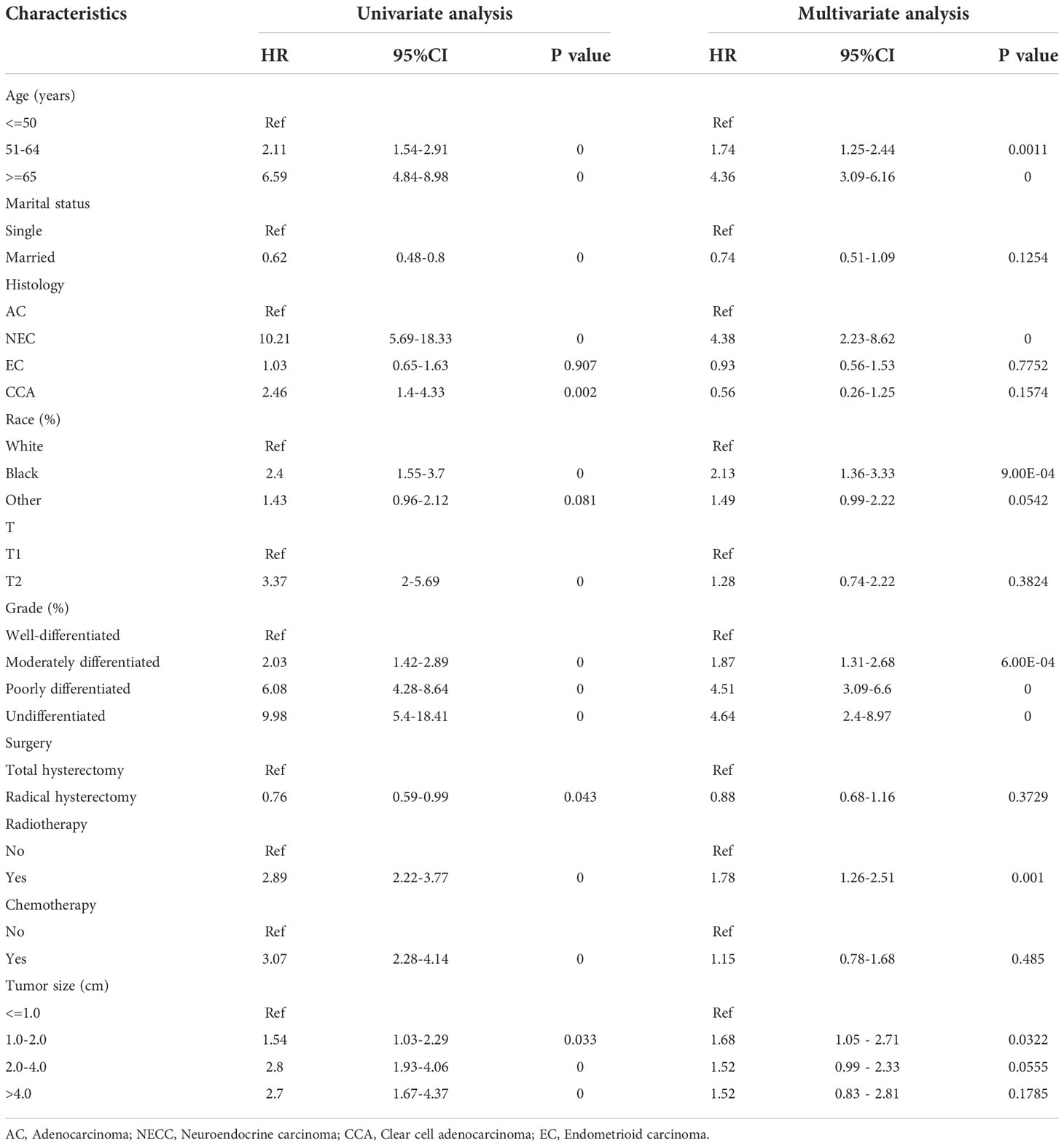
Kaplan-Meier curves were used to compare the OS of patients with pT1-T2aN0M0 cervical adenocarcinoma, as stratified by clinical variables; the results are shown in Figures 4A–H. Patients in the radiotherapy or chemotherapy groups had significantly worse survival outcomes when compared to the non-radiotherapy or non-chemotherapy groups (P < 0.001; Figures 4G, H). We used univariate and multivariate analyses to further explore the prognostic factors affecting the training cohort of patients with cervical adenocarcinoma identified from the SEER database. Table 2 shows results for the training cohort. Each covariate included in the Cox model fitting conforms to the proportional risk assumption. Cox multivariate hazard ratio analysis showed that several variables were independent prognostic risk factors for overall survival, including age at diagnosis, race, grade, histology, tumor size and radiotherapy
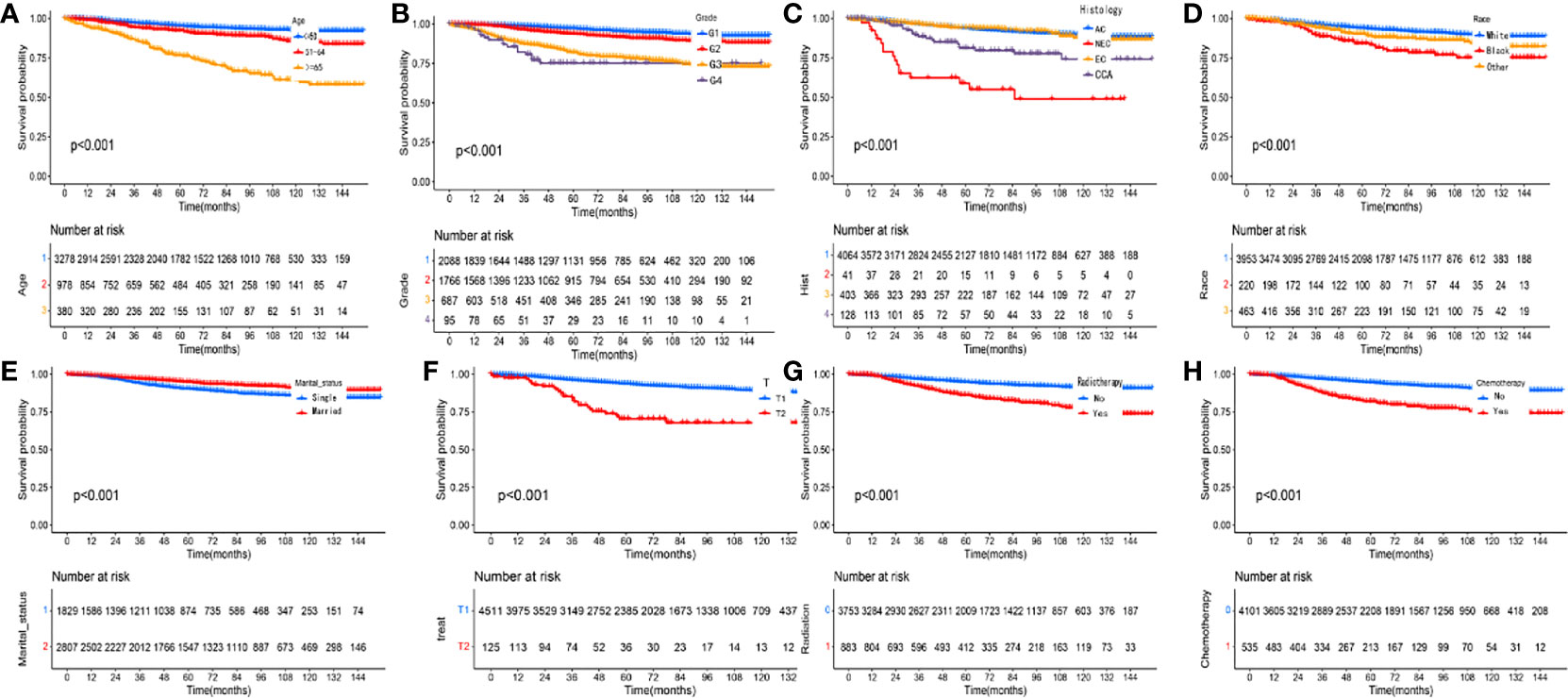
Figure 4 Kaplan-Meier curves comparing overall survival (OS) for pT1-T2aN0M0 cervical adenocarcinoma patients stratified by (A) age, (B) grade, (C) histology, (D) race, (E) marital status, (F) T stage, (G) radiotherapy and (H) chemotherapy, respectively.
Construction and validation of a predictive nomogram
Among the variables identified by multivariate analysis, there were no T stage and chemotherapy. Combined with the screening of previous survival forest models, and considering the importance of T staging in clinical practice, we added T stage to the variables identified by cox multivariate analysis to be included in the predictive nomogram. Adjuvant therapy such as radiotherapy and chemotherapy will be further studied in the follow-up analysis. Based on these variables, we constructed a nomogram to assess 1-, 3- and 5-year survival in the training cohort with early-stage pT1-2aN0M0 cervical adenocarcinoma (Figure 5). The model assigned beta-coefficients to each of the included variables. In this manner, we created a linear equation and calculated each patient’s risk score to predict her prognosis. Then we verified the prediction nomogram in the verification cohort. Calibration curves for the training and validation groups showed that the survival rate, as predicted by the model, was consistent with actual observations (Figures 6A–F). ROC curves for the training set and validation group are shown in Figures 7A, C, respectively. The C-index of the model fluctuated between 0.75 and 0.91 in the training set and between 0.72 and 0.81 in the validation group (Figures 7B, D).
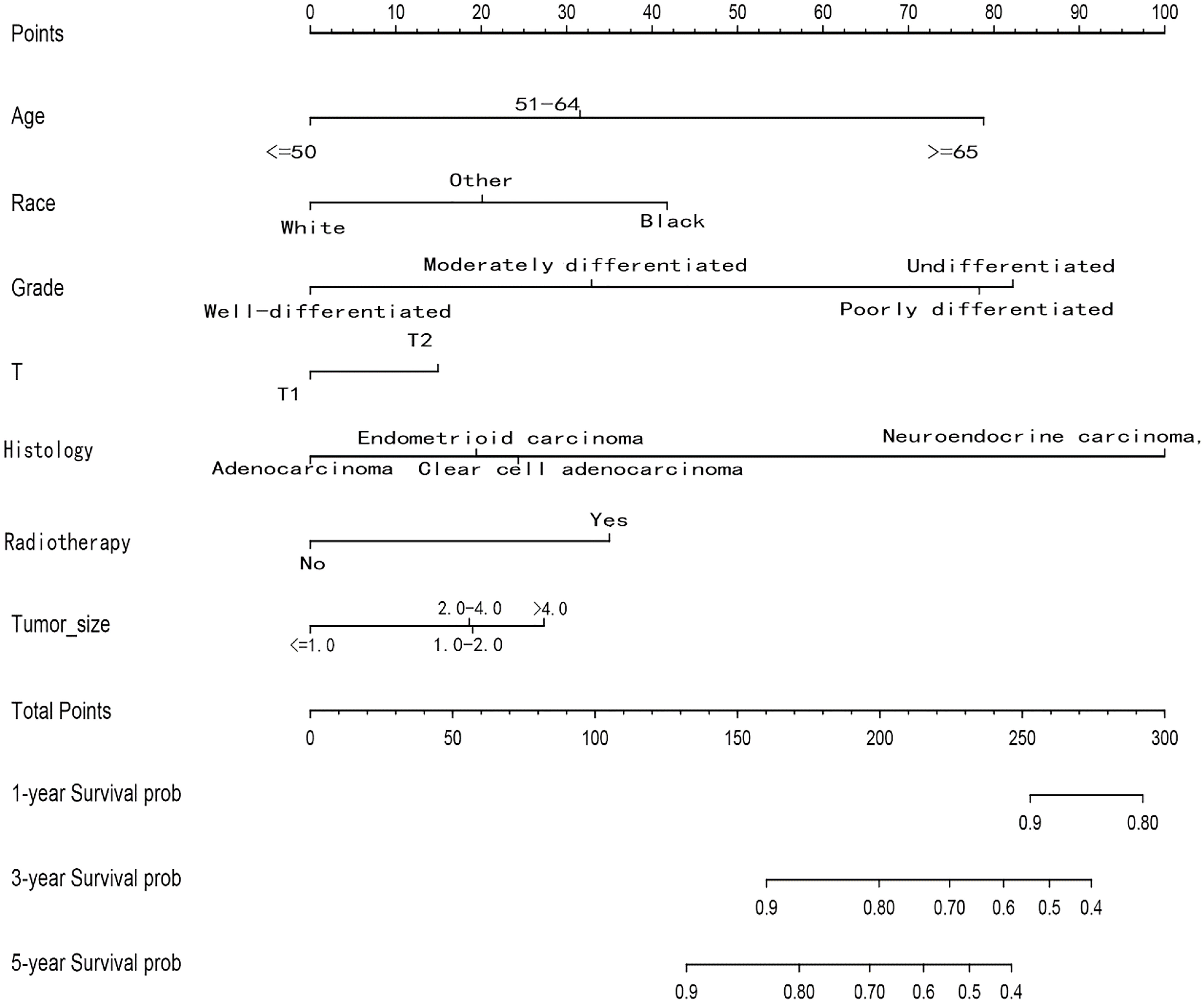
Figure 5 Nomograms for predicting the 1-, 3- and 5-year OS rates of patients with pT1-T2aN0M0 cervical adenocarcinoma undergoing surgery.
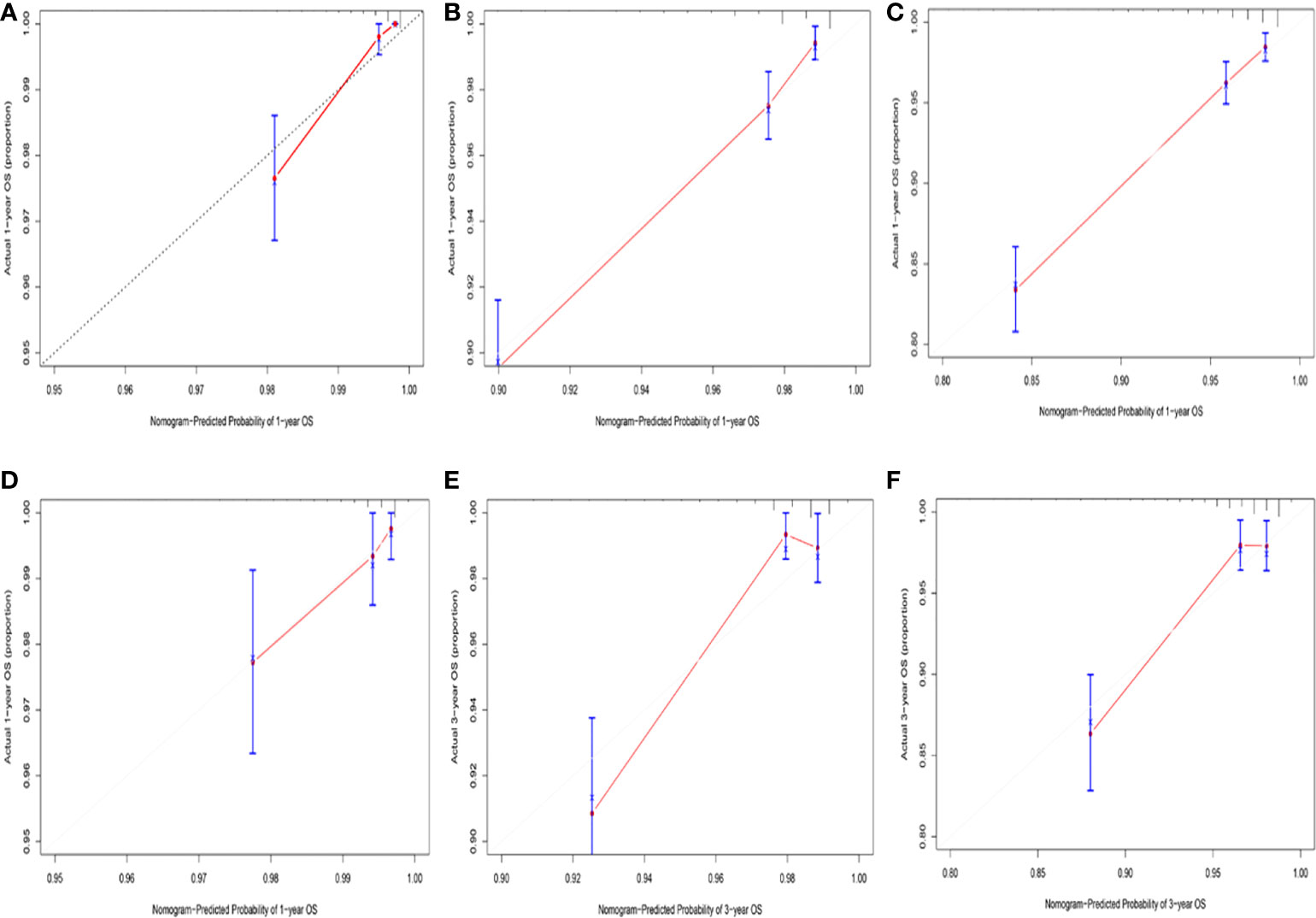
Figure 6 (A) Calibration curve for predicting 1-year OS rates in the training cohort. (B) Calibration curve for predicting 3-year OS rates in the training cohort. (C) Calibration curve for predicting 5-year OS rates in the training cohort. (D) Calibration curve for predicting 1-year OS rates in the validation cohort. (E) Calibration curve for predicting 3-year OS rates in the validation cohort. (F) Calibration curve for predicting 5-year OS rates in the validation cohort.
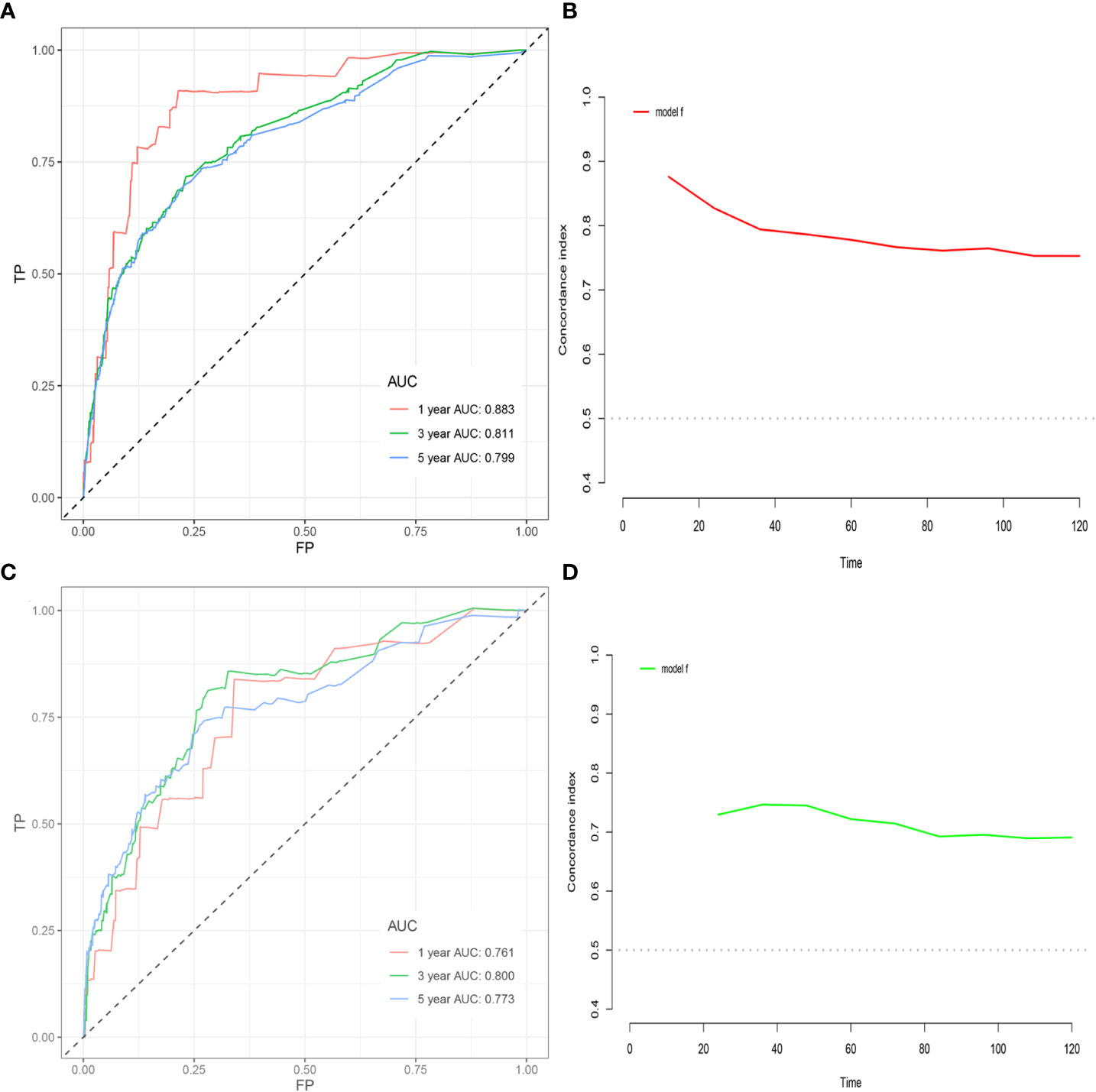
Figure 7 (A) ROC curve based on the nomogram for the training cohort. (B) C-index value based on nomogram in the training cohort. (C) ROC curve based on nomogram in the validation cohort. (D) C-index value based on the nomogram in the validation cohort.
Risk stratification according to the nomogram
We calculated a risk score for each patient based on the nomogram and then performed risk stratification analysis based on risk scores using X-tile software (23). Cut-offs were set at 0.25 and 1.30, respectively, with a risk score < 0.25 as a low-risk group, between 0.25 and 1.30 as a medium-risk group and > 1.30 as a high-risk group. Patients with pT1-2aN0M0 cervical adenocarcinoma could be divided into three sub-groups according to risk scores. In the training cohort, the 5-year OS decreased significantly with increasing risk and was 92.7%, 88.5% and 74.9% in the low, moderate and high-risk groups, respectively (p < 0.01). In the validation cohort, the 5-year OS rates were 97.6%, 89.4% and 71.6% in the low, moderate and high-risk groups, respectively (p < 0.001). As shown in Figure 8G, after risk stratification, the different groups of patients showed significant differences following K-M curve survival analysis.
Subgroup analysis after risk stratification
To assess whether postoperative adjuvant therapies, such as radiotherapy and chemotherapy, could benefit survival in each subgroup, we plotted KM survival curves for different adjuvant treatments in each subgroup and then calculated hazard ratios for each adjuvant therapy. For patients in the low-risk training and validation cohorts, postoperative chemotherapy alone exhibited a significantly worse OS than surgery alone (Figures 8A, D, p<0.001). In the moderate risk validation group, postoperative chemotherapy alone had a worse OS when compared with surgery alone (Figure 8E, p=0.044). In the other groups, there was no significant difference in terms of OS when compared between surgery alone and other adjuvant therapies (Figures 8B, C, F). In each subgroup, the hazard ratios for each adjuvant therapy are detailed in Table 3.
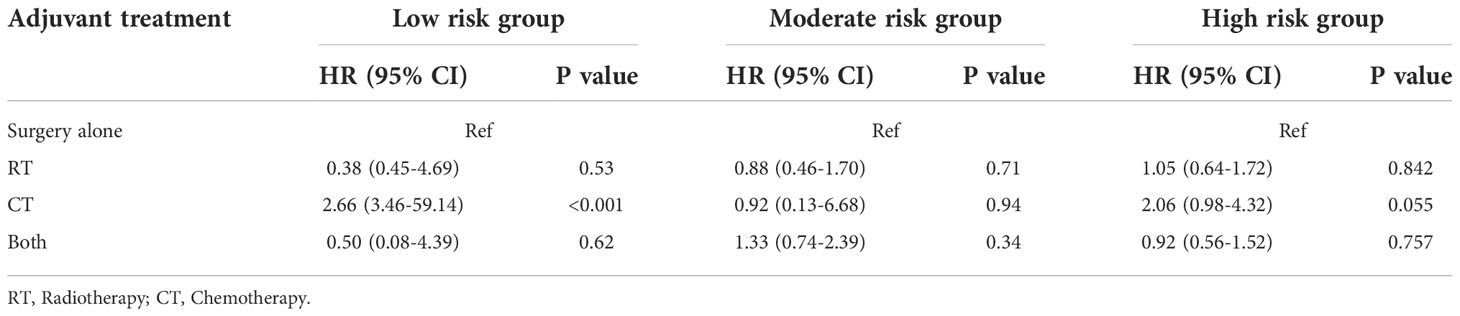
Table 3 Univariate analysis evaluating the effect of adjuvant treatment strategies stratified by subgroups.
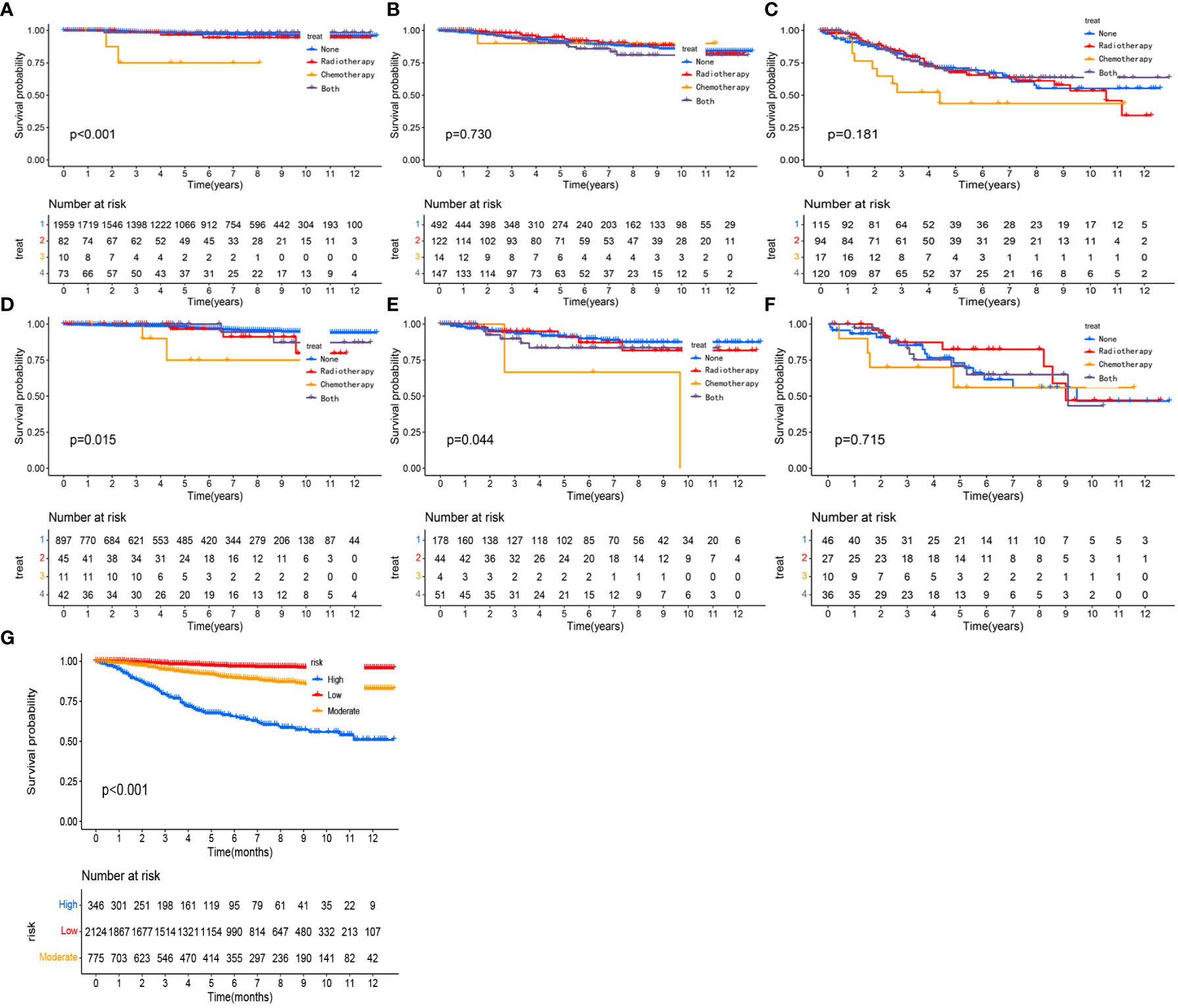
Figure 8 Kaplan-Meier curves comparing the survival effects of different adjuvant treatment strategies for patients with pT1-T2aN0M0 cervical adenocarcinoma in the low-risk training cohort (A), moderate-risk training cohort (B), the high-risk training cohort (C), the low-risk validation cohort (D), the moderate-risk validation cohort (E), and the high-risk validation cohort (F). Kaplan-Meier curves after the risk stratification of patients in the training cohort (G).
Survival analysis after PSM
Both our previous survival analysis and Cox multivariate analysis suggested that radiotherapy was a poor prognostic factor. Radiotherapy failed to improve prognosis; rather, it appeared to increase the poor survival outcomes of patients. When we analyzed different groups of radiotherapy patients, we found that high-risk groups were more likely to receive radiotherapy (Tables 4, 5). To reduce bias, we used propensity score matching (PSM) for subsequent survival analyses. Patients in the training set and validation set were matched in a 1:1 ratio according to the PSM method, respectively (Tables 4, 5). After matching patients 1:1 with PSM to control for confounding bias. In the training cohort, KM survival analysis revealed significantly worse survival in the radiotherapy group than in the non-radiotherapy group (Figure 9A, p=0.019). However, in the validation cohort, KM survival curves revealed no significant differences in terms of survival between the radiotherapy groups compared with the non-radiotherapy groups (Figure 9B, P >0.05).
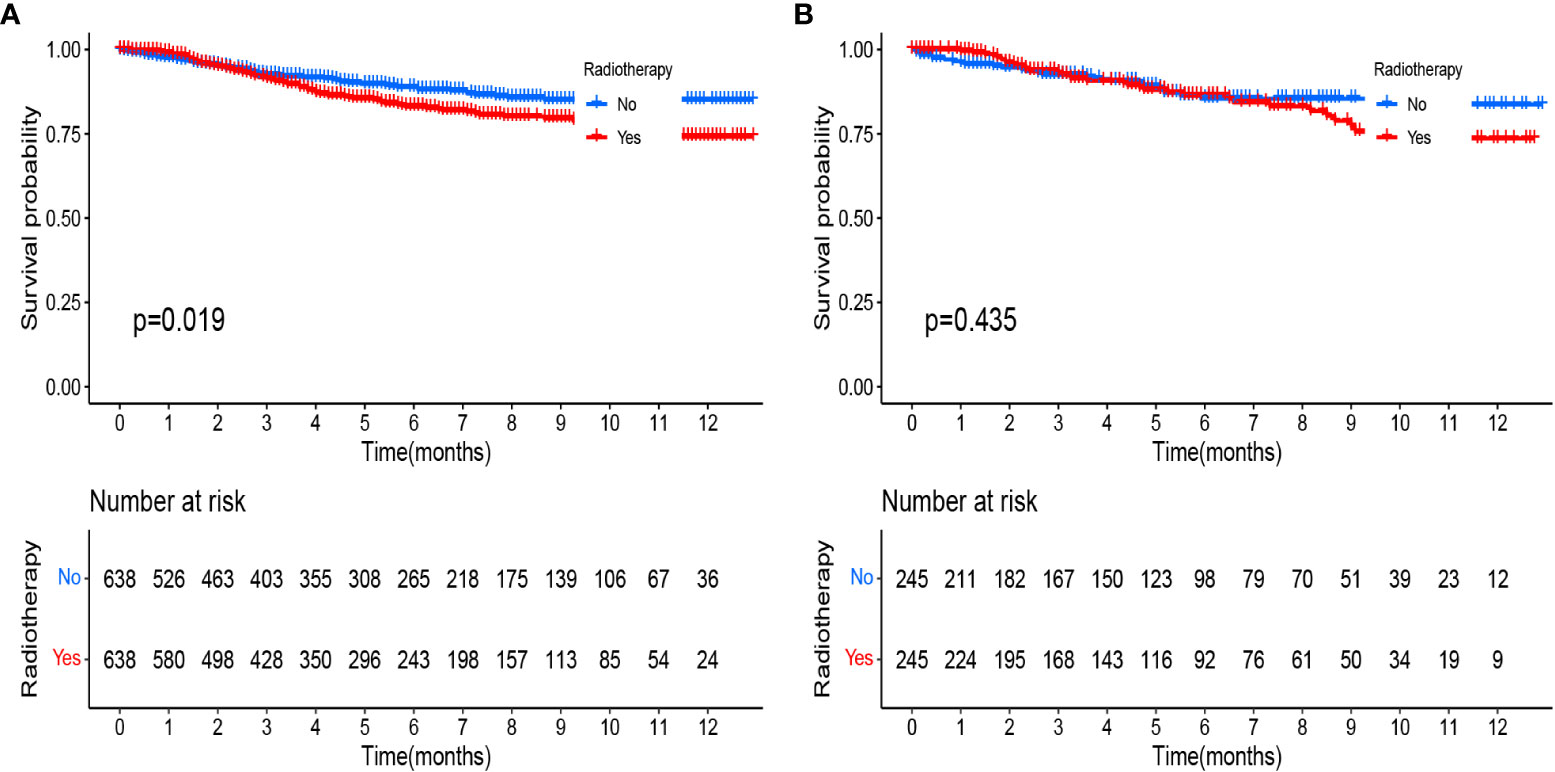
Figure 9 Kaplan-Meier curves comparing the survival effects of radiotherapy for patients with pT1-T2aN0M0 cervical adenocarcinoma after PSM (A) in the training cohort and (B) in the validation cohort.
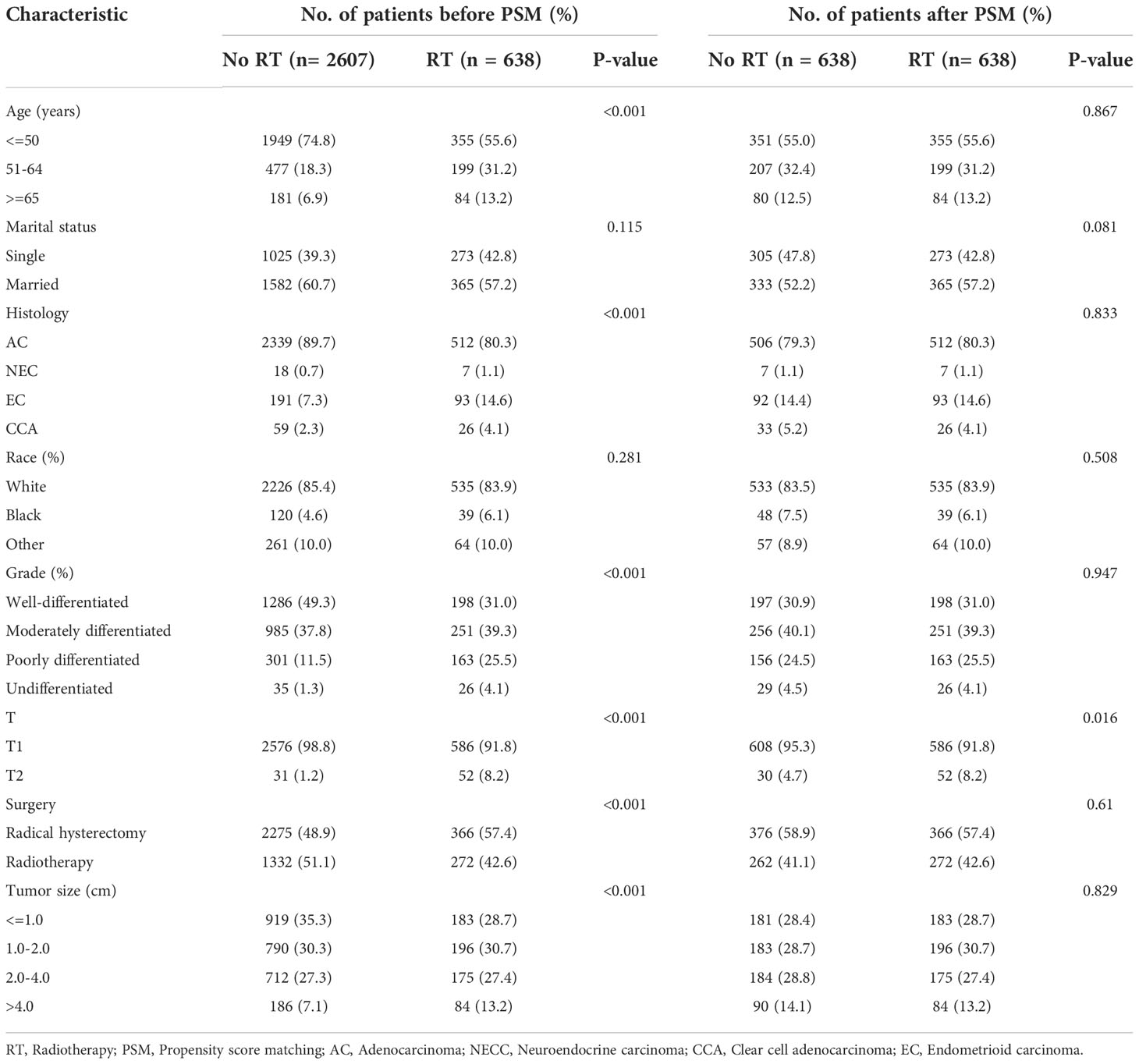
Table 4 Baseline characteristics of patients with pT1-T2aN0M0 cervical adenocarcinoma in the training cohort.
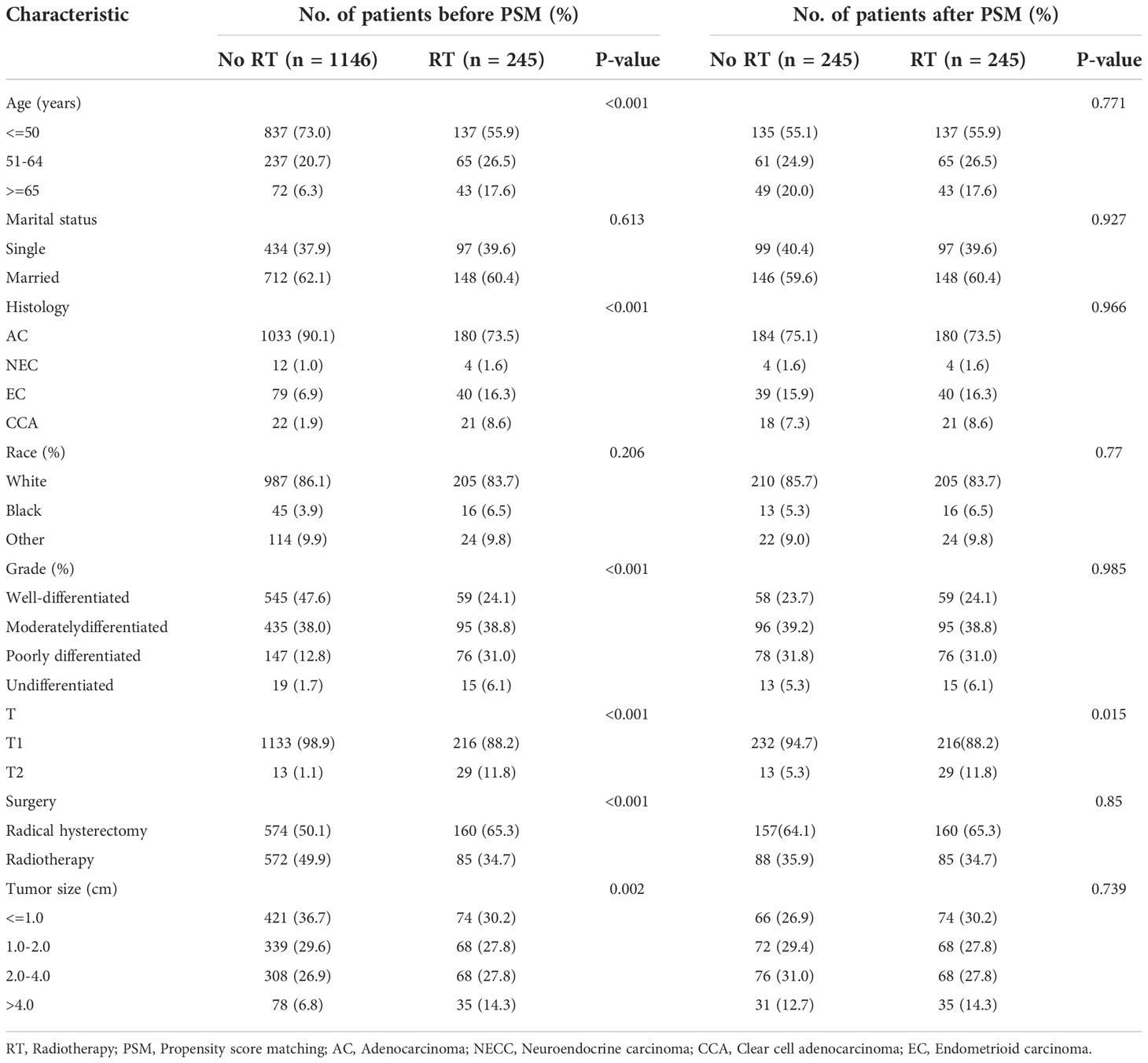
Table 5 Baseline characteristics of patients with pT1-T2aN0M0 cervical adenocarcinoma in the validation cohort.
Discussion
In the present study, we investigated a cohort of 4636 patients with early postoperative cervical adenocarcinoma and constructed and internally validated 1-, 3- and 5-year nomograms to predict patient OS. This nomogram can be personalized to accurately predict the prognosis of the disease and facilitate clinical decision-making. Using the SEER database, some previous studies constructed nomograms of early forms of postoperative cancers, including non-small cell lung cancer (24), triple-negative breast cancer (25) and esophageal cancer (26) to predict patient outcomes. To date, no specific nomogram has been reported for surgically resected early-stage cervical adenocarcinoma.
In this study, RSF analysis revealed that several subgroup variables, including age (>=65 years), NECC, G3, G4 and T2 contributed the most to mortality. NECC is a rare but highly malignant cervical tumor with a poor prognosis. In a previous study, Intaraphet et al. suggested that adjuvant chemotherapy may provide a survival benefit for patients with early-stage small cell neuroendocrine carcinoma of the uterine cervix (SNECC) when treated with surgery (27). In another study, Lee et al. also recommended that systemic chemotherapy should be used as part of the initial treatment of SNECC (28). However, Prodromidou et al. reviewed the literature and concluded that for large cell cervical neuroendocrine carcinoma, surgery and lymphadenectomy had a significant effect on survival while chemotherapy and radiotherapy did not appear to have a significant effect on prognosis (29). By applying multivariate Cox analysis, we identified age, race, histology, grade, tumor size and radiotherapy as independent prognostic factors in patients with early-stage surgical resection. RSF analysis also demonstrated the importance of different factors to prognosis, including grade, age and tumor size. In the CSF model, the prognostic importance of variables is in order of grade, radiotherapy, chemotherapy, tumor Size, age, etc. Previous studies of the prognostic factors related to cervical adenocarcinoma have focused on lymph node status, tumor size, tumor grade, depth of cervical invasion, lymphovascular invasion (LVSI) and parametrial invasion (30–35). However, there is a notable lack of prospective studies featuring large sample sizes. It is worth noting that with the release of the new FIGO staging system in 2018, cervical cancer patients with nodal disease detected by imaging are now included in stage IIIC, thus implying that the best treatment for these patients is concurrent chemoradiotherapy, rather than surgery.
In this study, we focused on patients with early-stage cervical adenocarcinoma without nodal metastasis and parametrial invasion after surgery. Notably, tumor size (>2.0cm) was not found to be an independent prognostic factor for early-stage cervical adenocarcinoma in our study. However, previous studies suggested that tumor size was associated with early cervical adenocarcinoma metastasis and recurrence (36–38). In our cohort, there were missing values for tumor size in some patients; although we used the random forest algorithm to fill in these missing values, this practice may have interfered with the results we obtained. Therefore, whether tumor size (>2.0cm) is an independent prognostic factor for cervical adenocarcinoma still needs further verification.
In addition, we also constructed a nomogram that combined the risk predictors identified by our analysis. Calibration curves showed excellent consistency between predicted survival and actual observations. Moreover, both the nomogram C-index and ROC curve analysis revealed that the nomogram had a good predictive effect. However, the robustness of our model can be further improved if risk factors such as potential serum biomarkers and vascular invasion are included.
Currently, adjuvant therapy for cervical cancer, especially adenocarcinoma with intermediate-risk factors is still debatable. According to NCCN guidelines, patients with cervical cancer and negative nodes, margins and parametrium after surgery, should receive pelvic EBRT with or without concurrent platinum-containing chemotherapy if the patient meets the risk factors of the Sedlis criteria (i.e., primary tumor size, stromal invasion, and/or LVSI) (11). However, to the best of our knowledge, the current clinical evidence to support this recommendation remains insufficient. Cao et al. believe that patients with intermediate-risk cervical cancer (according to Sedlis criteria) can still achieve good survival with radical hysterectomy alone without adjuvant therapy (39). Some researchers have also analyzed patients with FIGO 2009 IB1 cervical cancer post-surgery and found that intermediate risk patients who did not receive adjuvant therapy after surgery did not show an increased recurrence rate and LVSI was the only risk factor affecting PFS and DSS (40). Okazawa et al. suggested that postoperative concurrent chemoradiotherapy can improve the prognosis of patients with FIGO stage IB1-IIB cervical cancer in high-risk groups and patients with two or more intermediate-risk factors (41). For early-stage cervical clear cell adenocarcinoma with moderate risk factors, Liu et al. concluded that radiotherapy did not improve prognosis; therefore, radical surgery alone was recommended for early-stage patients without high-risk factors (42). In another study, Glaze et al. analyzed 166 patients with cervical adenocarcinoma. Univariate analysis showed that premenopausal status, tumor size, first-line chemotherapy, LVSI, rare histological subtypes, FIGO stage and the receipt of second-line treatment were significantly associated with a lower OS. However, multivariate analysis further showed that only FIGO stage was an independent factor (43).
In this study, postoperative cervical adenocarcinoma patients with pT1-2aN0M0 were divided into three subgroups based on risk scores calculated from nomograms. The effects of different adjuvant treatments on survival were compared in each subgroup. For patients in the low-risk group, adjuvant chemotherapy had a worse prognosis than surgery alone. In the moderate- and high-risk groups, adjuvant chemoradiotherapy did not benefit patients, thus implying that a comprehensive risk assessment should be made when considering the therapy/benefit ratio of adjuvants. Because of the difficulties associated with randomized controlled trial (RCT), to compare therapeutic outcomes between individuals who have received a particular treatment regimen to those who have not. However, PSM analysis of patients can be used as a substitute for a RCT, at least in part (44). PSM has been proven to reduce the bias caused by confounding variables; thus, in our study, we were able to use PSM to produce reliable results and conclusions. In the present study, survival analysis showed that adjuvant chemoradiotherapy not only failed to improve OS; it was also associated with a worse prognosis. Then, we used the PSM method to further conduct survival analysis. Prior to PSM, high-risk groups, such as grade, histology and T stage were more inclined to receive adjuvant chemoradiotherapy. After matching, survival analysis found no significant difference between adjuvant radiotherapy and surgery alone in the validation cohort. In the training cohort, adjuvant radiotherapy was associated with a worse prognosis than surgery alone. Previous studies also found that radiation therapy only plays a limited role in early-stage clear cell adenocarcinoma of the uterine cervix with risk factors (42, 45). The results of our present analysis suggest that postoperative adjuvant chemoradiotherapy does not provide a survival benefit for patients with early-stage pT1-T2aN0M0 cervical adenocarcinoma.
To our knowledge, this is the largest study (4636 cases) to compare the prognosis of patients with early postoperative cervical adenocarcinoma. However, there are some limitations that need to be considered. First, we lacked information related to the prognosis after surgical resection, including potential biological biomarkers, the depth of cervical invasion, LVSI and HPV infection. Second, our training and validation sets were created by the same database and were limited to the United States; thus, datasets from other countries are also required for external validation to improve the robustness of our model. Third, the SEER database has not yet collected tumor recurrence data, so it is impossible to evaluate the progression free survival, and the effectiveness of rescue therapy. Finally, our study was retrospective; inherent selection biases are known to exist in any retrospective study. Therefore, further prospective clinical studies are still needed for further verification.
Conclusion
We identified several clinicopathological variables that were independent risk factors for pT1-2aN0M0 stage cervical adenocarcinoma, including grade, age, T stage and histology. Postoperative patients did not benefit from adjuvant chemoradiotherapy. Whether patients with positive lymph nodes and incisional margins can benefit from adjuvant therapy, we are prepared to further present it in the follow-up study. Our findings may provide useful references and guidelines for future treatment decisions.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
Conception and design: DO. Development of methodology: DO. Extracted and analyzed the data: DO, MS, and LL. Writing, review, and/or revision of the manuscript: All authors. All authors read and approved the final manuscript.
Funding
This work was supported by grants from Taizhou "500 Elite Plan" high-level talent project.
Acknowledgments
We are very grateful to the staff in Surveillance, Epidemiology, and End Results Program (SEER) for their kind work in data collection and delivery. And we thank Professor Xueqiong Zhu at the Department of Obstetrics and Gynecology, The Second Affiliated Hospital of Wenzhou Medical Universit, China, for guiding us.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Small W Jr., Bacon MA, Bajaj A, Chuang LT, Fisher BJ, Harkenrider MM, et al. Cervical cancer: A global health crisis. Cancer (2017) 123(13):2404–12. doi: 10.1002/cncr.30667
3. Williams NL, Werner TL, Jarboe EA, Gaffney DK. Adenocarcinoma of the cervix: Should we treat it differently? Curr Oncol Rep (2015) 17(4):17. doi: 10.1007/s11912-015-0440-6
4. Castanon A, Landy R, Sasieni PD. Is cervical screening preventing adenocarcinoma and adenosquamous carcinoma of the cervix? Int J Cancer (2016) 139(5):1040–5. doi: 10.1002/ijc.30152
5. Hu K, Wang W, Liu X, Meng Q, Zhang F. Comparison of treatment outcomes between squamous cell carcinoma and adenocarcinoma of cervix after definitive radiotherapy or concurrent chemoradiotherapy. Radiat Oncol (2018) 13(1):249. doi: 10.1186/s13014-018-1197-5
6. Noh JM, Park W, Kim YS, Kim JY, Kim HJ, Kim J, et al. Comparison of clinical outcomes of adenocarcinoma and adenosquamous carcinoma in uterine cervical cancer patients receiving surgical resection followed by radiotherapy: A multicenter retrospective study (Krog 13-10). Gynecol Oncol (2014) 132(3):618–23. doi: 10.1016/j.ygyno.2014.01.043
7. Rose PG, Java JJ, Whitney CW, Stehman FB, Lanciano R, Thomas GM. Locally advanced adenocarcinoma and adenosquamous carcinomas of the cervix compared to squamous cell carcinomas of the cervix in gynecologic oncology group trials of cisplatin-based chemoradiation. Gynecol Oncol (2014) 135(2):208–12. doi: 10.1016/j.ygyno.2014.08.018
8. Sakuragi N, Kato T, Shimada C, Kaneuchi M, Todo Y, Mitamura T, et al. Oncological outcomes after okabayashi-Kobayashi radical hysterectomy for early and locally advanced cervical cancer. JAMA Netw Open (2020) 3(5):e204307. doi: 10.1001/jamanetworkopen.2020.4307
9. Chino J, Annunziata CM, Beriwal S, Bradfield L, Erickson BA, Fields EC, et al. The astro clinical practice guidelines in cervical cancer: Optimizing radiation therapy for improved outcomes. Gynecol Oncol (2020) 159(3):607–10. doi: 10.1016/j.ygyno.2020.09.015
10. Wipperman J, Neil T, Williams T. Cervical cancer: Evaluation and management. Am Fam Phys (2018) 97(7):449–54.
11. Koh WJ, Abu-Rustum NR, Bean S, Bradley K, Campos SM, Cho KR, et al. Cervical cancer, version 3.2019, nccn clinical practice guidelines in oncology. J Natl Compr Canc Netw (2019) 17(1):64–84. doi: 10.6004/jnccn.2019.0001
12. Zhou J, Wu SG, Sun JY, Li FY, Lin HX, Chen DH, et al. The effect of local treatment modalities in patients with early-stage adenocarcinoma of the uterine cervix: A population-based analysis. Int J Surg (2017) 41:16–22. doi: 10.1016/j.ijsu.2017.03.024
13. Mabuchi S, Okazawa M, Matsuo K, Kawano M, Suzuki O, Miyatake T, et al. Impact of histological subtype on survival of patients with surgically-treated stage Ia2-iib cervical cancer: Adenocarcinoma versus squamous cell carcinoma. Gynecol Oncol (2012) 127(1):114–20. doi: 10.1016/j.ygyno.2012.06.021
14. Ryu SY, Kim MH, Nam BH, Lee TS, Song ES, Park CY, et al. Intermediate-risk grouping of cervical cancer patients treated with radical hysterectomy: A Korean gynecologic oncology group study. Br J Cancer (2014) 110(2):278–85. doi: 10.1038/bjc.2013.716
15. Zhou J, Zhang WW, Wu SG, He ZY, Sun JY, Yang GF, et al. The prognostic value of histologic subtype in node-positive early-stage cervical cancer after hysterectomy and adjuvant radiotherapy. Int J Surg (2017) 44:1–6. doi: 10.1016/j.ijsu.2017.05.074
16. Suzuki K, Nagao S, Narita M, Nakazawa H, Shibutani T, Yamamoto K, et al. Survival impact of adjuvant concurrent chemoradiotherapy after radical hysterectomy in figo stage Iiic1 cervical adenocarcinoma. Int J Clin Oncol (2021) 26(7):1322–9. doi: 10.1007/s10147-021-01904-0
17. Mahmoud O, Hathout L, Shaaban SG, Elshaikh MA, Beriwal S, Small W Jr. Can chemotherapy boost the survival benefit of adjuvant radiotherapy in early stage cervical cancer with intermediate risk factors? a population based study. Gynecol Oncol (2016) 143(3):539–44. doi: 10.1016/j.ygyno.2016.10.022
18. Taylor JM. Random survival forests. J Thorac Oncol (2011) 12:1974–5. doi: 10.1097/JTO.0b013e318233d835
19. Strobl C, Boulesteix AL, Zeileis A, Hothorn T. Bias in random forest variable importance measures: Illustrations, sources and a solution. BMC Bioinforma (2007) 8(1):1. doi: 10.1186/1471-2105-8-25
20. Wright MN, Dankowski T, Ziegler A. Unbiased split variable selection for random survival forests using maximally selected rank statistics. Stat Med (2017) 36(8):1272–84. doi: 10.1002/sim.7212
21. Nasejje JB, Mwambi H, Dheda K, Lesosky M. A comparison of the conditional inference survival forest model to random survival forests based on a simulation study as well as on two applications with time-to-event data. BMC Med Res Methodol (2017) 17(1):115. doi: 10.1186/s12874-017-0383-8
22. Strobl C, Malley J, Tutz G. An introduction to recursive partitioning: Rationale, application, and characteristics of classification and regression trees, bagging, and random forests. Psychol Methods (2009) 14(4):323–48. doi: 10.1037/a0016973
23. Camp RL, Dolled-Filhart M, Rimm DL. X-Tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res (2004) 10(21):7252–9. doi: 10.1158/1078-0432.CCR-04-0713
24. Xu Y, Wan B, Zhu S, Zhang T, Xie J, Liu H, et al. Effect of adjuvant chemotherapy on survival of patients with 8th edition stage ib non-small cell lung cancer. Front Oncol (2021) 11:784289. doi: 10.3389/fonc.2021.784289
25. Mao Q, Liu S, Lv M, Sun Y, Zhang C, Li L. Nomogram for predicting overall survival and assessing the survival benefit of adjuvant treatment in Pt1-2n0m0 triple-negative breast cancer: A surveillance, epidemiology, and end results-based study. Front Oncol (2021) 11:663621. doi: 10.3389/fonc.2021.663621
26. Yu X, Gao S, Xue Q, Tan F, Gao Y, Mao Y, et al. Development of a predictive nomogram for cause-specific mortality in surgically resected early-stage oesophageal cancer: A surveillance, epidemiology, and end results (Seer) analysis. J Thorac Dis (2020) 12(5):2583–94. doi: 10.21037/jtd.2020.03.25
27. Intaraphet S, Kasatpibal N, Siriaunkgul S, Chandacham A, Sukpan K, Patumanond J. Prognostic factors for small cell neuroendocrine carcinoma of the uterine cervix: An institutional experience. Int J Gynecol Cancer (2014) 24(2):272–9. doi: 10.1097/IGC.0000000000000059
28. Lee SW, Lim KT, Bae DS, Park SY, Kim YT, Kim KR, et al. A multicenter study of the importance of systemic chemotherapy for patients with small-cell neuroendocrine carcinoma of the uterine cervix. Gynecol Obstet Invest (2015) 79(3):172–8. doi: 10.1159/000367920
29. Prodromidou A, Phelps DL, Pergialiotis V, Cunnea P, Thomakos N, Rodolakis A, et al. Clinicopathological characteristics and survival outcomes of patients with Large cell neuroendocrine carcinoma of the uterine cervix: A systematic review and meta-analysis. Eur J Obstetrics Gynecol Reprod Biol (2022) 270:212–20. doi: 10.1016/j.ejogrb.2022.01.014
30. Park JY, Kim DY, Kim JH, Kim YM, Kim YT, Nam JH. Outcomes after radical hysterectomy in patients with early-stage adenocarcinoma of uterine cervix. Br J Cancer (2010) 102(12):1692–8. doi: 10.1038/sj.bjc.6605705
31. Kim JH, Shim SH, Nam SH, Lee SW, Park JY, Kim DY, et al. Prognostic factors and impact of minimally invasive surgery in early-stage neuroendocrine carcinoma of the cervix. J Minim Invasive Gynecol (2020) 27(7):1558–65. doi: 10.1016/j.jmig.2020.02.004
32. Nosaka K, Horie Y, Shiomi T, Itamochi H, Oishi T, Shimada M, et al. Cytoplasmic maspin expression correlates with poor prognosis of patients with adenocarcinoma of the uterine cervix. Yonago Acta Med (2015) 58(4):151–6.
33. Gadducci A, Guerrieri ME, Cosio S. Adenocarcinoma of the uterine cervix: Pathologic features, treatment options, clinical outcome and prognostic variables. Crit Rev Oncol Hematol (2019) 135:103–14. doi: 10.1016/j.critrevonc.2019.01.006
34. Wang M, Yuan B, Zhou ZH, Han WW. Clinicopathological characteristics and prognostic factors of cervical adenocarcinoma. Sci Rep (2021) 11(1):7506. doi: 10.1038/s41598-021-86786-y
35. Baalbergen A, Ewing-Graham PC, Hop WC, Struijk P, Helmerhorst TJ. Prognostic factors in adenocarcinoma of the uterine cervix. Gynecol Oncol (2004) 92(1):262–7. doi: 10.1016/j.ygyno.2003.09.001
36. Cheng H, Huo L, Zong L, Kong Y, Yang J, Xiang Y. Oncological outcomes and safety of ovarian preservation for early stage adenocarcinoma of cervix: A systematic review and meta-analysis. Front Oncol (2019) 9:777. doi: 10.3389/fonc.2019.00777
37. Levinson K, Beavis AL, Purdy C, Rositch AF, Viswanathan A, Wolfson AH, et al. Beyond sedlis-a novel histology-specific nomogram for predicting cervical cancer recurrence risk: An Nrg/Gog ancillary analysis. Gynecol Oncol (2021) 162(3):532–8. doi: 10.1016/j.ygyno.2021.06.017
38. Casarin J, Buda A, Bogani G, Fanfani F, Papadia A, Ceccaroni M, et al. Predictors of recurrence following laparoscopic radical hysterectomy for early-stage cervical cancer: A multi-institutional study. Gynecol Oncol (2020) 159(1):164–70. doi: 10.1016/j.ygyno.2020.06.508
39. Cao L, Wen H, Feng Z, Han X, Zhu J, Wu X. Role of adjuvant therapy after radical hysterectomy in intermediate-risk, early-stage cervical cancer. Int J Gynecol Cancer (2021) 31(1):52–8. doi: 10.1136/ijgc-2020-001974
40. Haesen J, Salihi R, Van Gorp T, Van Nieuwenhuysen E, Han SN, Christiaens M, et al. Radical hysterectomy without adjuvant radiotherapy in patients with cervix carcinoma figo 2009 Ib1, with or without positive sedlis criteria. Gynecol Oncol (2021) 162(3):539–45. doi: 10.1016/j.ygyno.2021.06.026
41. Okazawa M, Mabuchi S, Isohashi F, Suzuki O, Yoshioka Y, Sasano T, et al. Impact of the addition of concurrent chemotherapy to pelvic radiotherapy in surgically treated stage Ib1-iib cervical cancer patients with intermediate-risk or high-risk factors: A 13-year experience. Int J Gynecol Cancer (2013) 23(3):567–75. doi: 10.1097/IGC.0b013e31828703fd
42. Liu Z, Li J, Gu H, Tu H, Liu G, Liu J. Clear cell adenocarcinoma of uterine cervix: A single institution retrospective experience. Front Oncol (2020) 10:532748. doi: 10.3389/fonc.2020.532748
43. Glaze S, Duan Q, Sar A, Lee S, Kobel M, Park E, et al. Figo stage is the strongest prognostic factor in adenocarcinoma of the uterine cervix. J Obstet Gynaecol Can (2019) 41(9):1318–24. doi: 10.1016/j.jogc.2019.01.026
44. Austin PC. A critical appraisal of propensity-score matching in the medical literature between 1996 and 2003. Stat Med (2008) 27(12):2037–49. doi: 10.1002/sim.3150
Keywords: adjuvant treatment, prognosis, cervical adenocarcinoma, random survival forest, nomogram, SEER, conditional survival forest
Citation: Ouyang D, Shi M, Wang Y, Luo L and Huang L (2022) Prognostic analysis of pT1-T2aN0M0 cervical adenocarcinoma based on random survival forest analysis and the generation of a predictive nomogram. Front. Oncol. 12:1049097. doi: 10.3389/fonc.2022.1049097
Received: 20 September 2022; Accepted: 31 October 2022;
Published: 24 November 2022.
Edited by:
Jamshid Abdul-Ghafar, French Medical institute for Mothers and Children (FMIC), AfghanistanReviewed by:
Yanal Alnimer, Virginia Commonwealth University, United StatesZubair Ahmad, Aga Khan University, Pakistan
Copyright © 2022 Ouyang, Shi, Wang, Luo and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dong Ouyang, eHNzbXRAc2luYS5jb20uY24=
 Dong Ouyang
Dong Ouyang Mengting Shi2
Mengting Shi2