- 1Department of Gynecology, Anhui Medical University Affiliated Maternity and Child Healthcare Hospital, Anhui Province Maternity and Child Healthcare Hospital, Hefei, Anhui, China
- 2Division of Molecular Virology, SR Life Sciences Institute, Clarksburg, MD, United States
- 3Key Laboratory of Protein Engineering and Drug Development of Hainan, Haikou, China
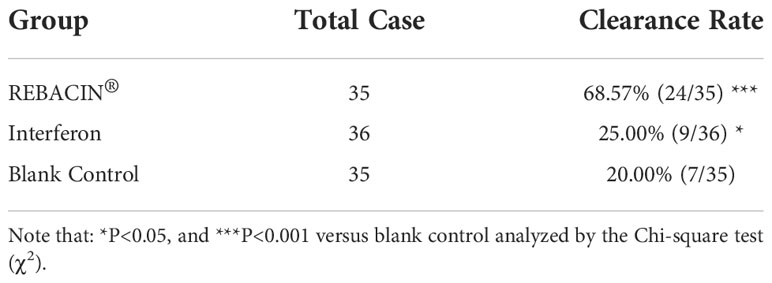
Previous studies have demonstrated that REBACIN® intervention eliminates persistent high-risk human papillomavirus (hrHPV) infection. The initial establishment and subsequent progression of cervical cancer mainly depends on two major oncogenes, E6/E7, and previous studies have proposed E6/E7 oncogenes as a target for therapeutic drug development. The aim of this study was to investigate in vitro and in vivo whether REBACIN® inhibits E6/E7 oncogenes for elucidating the mechanism of REBACIN® in the clearance of persistent hrHPV infection. In vitro, after REBACIN® treatment, the growth of both Ca Ski and HeLa cervical cancer cells containing the E6/E7 oncogenes was prevented. In line with this finding is that E6/E7 expression was inhibited, which can be counteracted by the co-application of anti-REBACIN® antibody. These studies demonstrated that REBACIN® can effectively inhibit the growth of cervical cancer cells via targeting HPV E6/E7 expression. To further verify this finding in clinic, 108 volunteer patients with persistent hrHPV infections were randomly divided into REBACIN®, recombinant human interferon alpha-2b (Immunological drug control), or no-treatment blank control groups, received intravaginal administration of REBACIN®, interferon or no-treatment every other day for three months, and then followed up for E6/E7 mRNA assay. In REBACIN® group, 68.57% of patients showed complete clearance of HPV E6/E7 mRNA, which was significantly higher compared to 25.00% in the interferon immunological drug control group and 20.00% in blank control group, confirming that REBACIN® is potently efficacious on clearing persistent hrHPV infections via inhibition of HPV E6/E7 oncogenes.
Clinical trial registration: http://www.chictr.org.cn/historyversionpuben.aspx?regno=ChiCTR2100045911, identifier ChiCTR2100045911.
Introduction
Human papilloma virus (HPV) is a highly conserved double-stranded DNA virus (1, 2), which is one of the most common sexually transmitted viruses (3). Although most infections are naturally cleared by the host’s immune system within 1-2 years, about 10% of the infected people become persistent infected (4). Studies have shown that high-risk HPV (hrHPV) persistent infection is the main cause of cervical cancer (5, 6). It is widely known that cervical cancer is the fourth most common cancer among women in the world (7). According to statistics, there were about 570,000 new cases and 311,000 deaths worldwide in 2018 (8). Persistent infection of hrHPV and integration of its DNA into the human genome are the key carcinogenic basis and mechanism of cervical cancer (9). HPV16 and HPV18 are the most common types resulting in about 70-75% of cervical cancer and 40-60% of cervical precancerous lesions in the world (10, 11), while HPV negative people have almost no risk of cervical cancer (12). Studies have shown that HPV E6 and E7 are the two most important oncogenes in the process of HPV induced lesions or carcinogenesis, which are easy to integrate with host genes in the nucleus and then synthesize oncoproteins E6 and E7 (13). It has been proved that their protein products interact with two key factors p53 and pRb of the tumor-associated signaling pathway respectively, leading to the continuous proliferation and carcinogenesis of cervical malignant cells (14–16).
Currently, the conventional treatments for persistent hrHPV infections include cryotherapy, carbon dioxide therapy, laser therapy, loop electrosurgical excision procedure, cold knife conization, et al. (17). These therapies without tissue specificity are invasive in different degrees. Since no anti-HPV drugs have been approved by FDA so far, interferon, a typical immune antiviral medicine, is occasionally used as an alternative drug applied to anti-hrHPV in China (18–21), which plays a critical role in the innate immune response against viral infections by inhibiting virus replication (22). However, the clinical efficacy of interferon on persistent hrHPV infections was controversial (23).
As a recent coming out antiviral biologic, REBACIN® was reported to be high clinical efficacy on the clearance of persistent hrHPV infections (24, 25). The aim of this study is to further investigate the mechanism of REBACIN® in clearing the persistent hrHPV infection.
Materials and methods
Cell culture
Ca Ski (ATCC® CRL1550™) is a human epidermoid cervical cancer cell line, which derived from an epidermoid carcinoma of the cervix metastatic to the small bowel mesentery of a 40 year old Caucasian. HeLa (ATCC® CCL2™) is the first immortal human cell line to be grown in culture, which was isolated in 1951 from a cervical carcinoma derived from a 31-year-old patient. 293T (ATCC® CRL-3216™) is a highly transfectable derivative cell line of human embryonic kidney 293 cells. All these cells were purchased from Shanghai Enzyme Research Biotechnology Co., Ltd, cultured in HyClone Dulbecco’s Modified Eagle medium (DMEM)/High glucose containing 10% Fetal Bovine Serum (FBS), 100 units/mL of penicillin and 100 µg/mL of streptomycin, and incubated at 37°C in an atmosphere of 5% CO2 (100% relative humidity).
SDS-PAGE and western blotting
Ca Ski, HeLa or 293T cells were split and approximately 2.5 x 105 cells per well were plated onto 6-well culture plate containing fresh media with or without 50 µg/ml REBACIN®, and then the cells were cultured continuously up to 6 days. After every one day’s culture, the total proteins in each wells were extracted with RIPA Lysis and Extraction Buffer containing 1x Halt™ Protease Inhibitor Cocktail an 5 mM EDTA Solution, separated by 10% regular SDS-PAGE, and transferred to nitrocellulose membrane. The membranes were blocked for 1 h with 5% non-fat milk in KPL wash solution (Beijing XMJ Scientific Co., Ltd) and processed with primary monoclonal antibodies (anti-HPV16 E7 and anti-β-Actin, Santa Cruz Biotechnology; anti-HPV18 E6, Merck Millipore) and subsequently with anti-mouse horseradish peroxidase-conjugated secondary antibody (1:500, Santa Cruz Biotechnology, Inc). The membranes were developed using West Femto ECL Substrate (Beijing Solarbio Science&Technology Co., Ltd), and pictures were taken using Amersham Imager 600 (GE Healthcare Life Sciences). The signal of western blotting was quantified using the ImageJ (National Institutes of Health, USA), and the β-actin, a traditional housekeeping protein, was used as an internal control to normalize total protein loading in each lane. Note that the β-Actin and HPV18 E6 was detected respectively to reduce background in Figure 4C.
Cell viability test
Ca Ski, HeLa or 293T cells were split and approximately 5 x 104 cells per well were plated onto 24-well culture plate containing fresh media with or without 50 µg/ml REBACIN® in the presence or absence of anti-REBACIN® antibody, and then the cells were cultured continuously up to 6 days. After every one day’s culture, the cells in each well were split and stained with Trypan Blue Solution, and the living cells were counted according to the manufacturer’s protocol (Gibco).
Participants
This clinical observation was approved by the Institutional Ethics Committee of Anhui Province Maternity and Child Healthcare Hospital. Between June 2019 and December 2020, participants with persistent infections of high-risk HPV (hrHPV) for at least twelve months, but without a high level of cervical lesions [no more than grade one cervical intraepithelial neoplasia (CIN 1) according to the examination of colposcopy and biopsy] were enrolled in the Department of Gynecology at Anhui Province Maternity and Child Healthcare Hospital.
Inclusion criteria of participants: (1) women between 18 and 70 years old with sexual life, but non-pregnant or lactating; (2) persistent hrHPV infection for more than twelve months; (3) without a high level of cervical lesions [no more than grade one cervical intraepithelial neoplasia (CIN 1) according to the examination of colposcopy and biopsy]; (4) no previous anti-HPV intervention. Exclusion criteria of participants: (1) with a history of allergy, smoking, oral contraceptive and HPV vaccination; (2) with severe heart, lung, liver and kidney dysfunction; (3) women who can’t complete the designed drug administration due to menstrual disorder. There was no significant difference (p>0.05) in the age, E6/E7 mRNA expression level, cytological abnormality of the participants among REBACIN®, recombinant human interferon alpha-2b and no-treatment blank control group (Data not shown).
Study design
After understanding details of the investigation and agreeing to comply with the research process, patients signed a written informed consent form and then participated in this clinical observation. Eligible volunteers were screened out according to the inclusion and exclusion criteria. A random digital table was generated via a computer randomized numbering system. According to the digital table, eligible patients were randomly assigned into experimental groups.
All eligible participants received intravaginal administration of either REBACIN® (0.5 g compounding agent per dose, the Key Laboratory of Protein Engineering and Drug Development of Hainan, Haikou, China) or recombinant human interferon alpha-2b (100,000 IU per suppository, Anhui Anke Bioengineering (Group) Co., Ltd., Anhui, China) every other day for three months, except during the menstrual period. The patients were then followed-up during four months after their drug withdrawal. The expression level of hrHPV E6/E7 mRNA was detected using HPV E6/E7 mRNA assay as shown in the following.
HPV E6/E7 mRNA assay by QuantiVirus
HPV E6/E7 mRNA analysis were carried out according to the manufacturer’s instructions of QuantiVirus HPV E6/E7 mRNA assay (Kodia Biotechnology, China), which detects HPV oncogenes E6/E7 mRNA of 14 high-risk HPV subtypes (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68). This test utilizes branched DNA (bDNA) to capture the target mRNA without either mRNA purification or reverse transcription−polymerase chain reaction. The E6/E7 mRNAs were quantified as relative luminescence units (RLU) by the KODIA QuantiViurs Luminometer. If RLU was more then 1.0, the patient was considered as positive of high-risk HPV E6/E7 mRNAs in the assay (26). Otherwise, the patient was negative.
Cervical cytology
According to routine method, thinPrep cytologic test (TCT) was used to identify abnormal cells at the squamocolumnar junction, where cervical dysplasia and cancers mostly develop. The observations were reported according to the Bethesda 2014 guidelines (27).
Statistical analysis
According to significance level (α) and power (1-β) requirements of the clinical observation, the number of cases for the REBACIN® group, interferon alpha-2b group and no-treatment blank control group was determined in a ratio of 1:1:1. The evaluation indicators are qualitative indicators. According to the efficacy of pre-test and statistical requirements (α=0.05, and β=10%), the minimum sample size required for this clinical observation was obtained.
The data from cell viability test and western blotting were analyzed by Repeated Measures ANOVA followed by Bonferroni test (GraphPad Prism 6). The counting data was analyzed via the chi-square test. All the p-values reported were two-sided, and p-values < 0.05 indicated statistical significance.
Results
REBACIN® inhibits cell growth of cervical cancer cell line Ca Ski
HPV E6/E7 oncoproteins are thought to immortalize cells and maintenance of HPV-associated cancer, primarily through interference with the p53 and pRB tumor suppressor proteins (28–30). We therefore investigated whether REBACIN® treatment affects the growth of a human epidermoid cervical cancer cell line of Ca Ski cells, which stably express HPV16 E6/E7 oncoproteins. As shown in Figure 1A, the treatment of REBACIN® did significantly inhibit the growth of Ca Ski cells. Five days after the treatment, there were only few cells alive compared to the confluence cells in control without the treatment of REBACIN®, and this growth inhibition can be almost completely counteracted under co-application of anti-REBACIN® antibody. Otherwise, in a cancer cell line of 293T derived from human embryonic kidney 293 epidermoid cells without containing HPV E6/E7 genes, REBACIN® almost has no influence in 293T cell growth (Figure 1B). In quantitative analysis, six days after the culture, the total cells of Ca Ski proliferated almost 14 folds under normal growth condition whereas alive cells decreased around 75% in the presence of REBACIN®, which the growth inhibition in the presence of REBACIN® could be blocked with co-application of anti-REBACIN® antibody (Figure 2A). In other words, the growth of Ca Ski cells was inhibited up to 98% compared to the control (Figure 2C). For the cancer cells of 293T without expression of the E6/E7 oncoproteins, REBACIN® only slightly influenced cell growth, and anti-REBACIN® antibody didn’t significantly counteract this slight inhibition (Figures 2B, D).
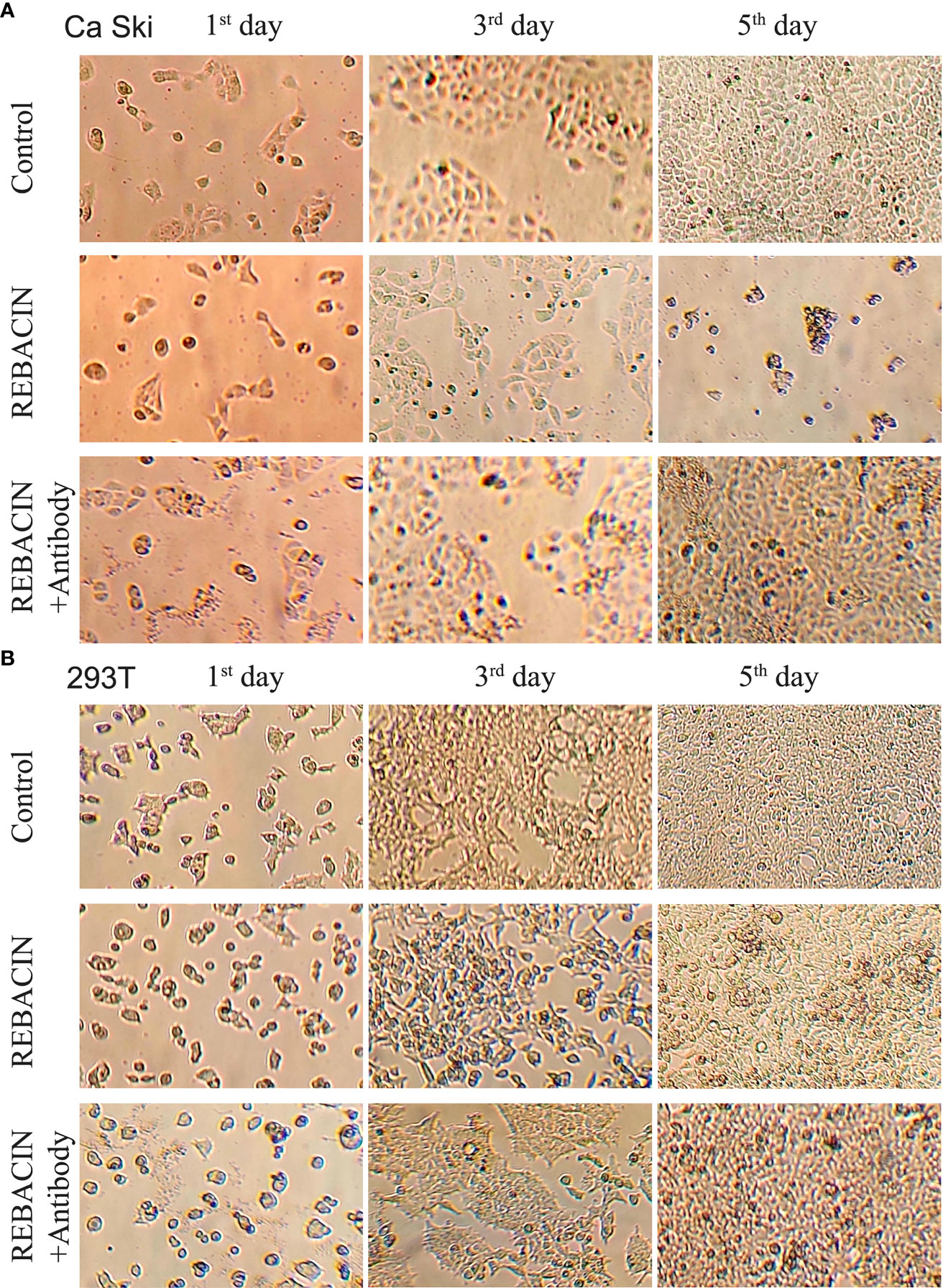
Figure 1 Effect of REBACIN® on the growth of different cell lines. Ca Ski (A) or 293T (B) cells in 24-well plate were treated or not treated by 50 μg/ml REBACIN® or REBACIN®/anti-REBACIN® antibody for up to 6 days, and the representative images in the 1st, 3rd, and 5th day were shown.
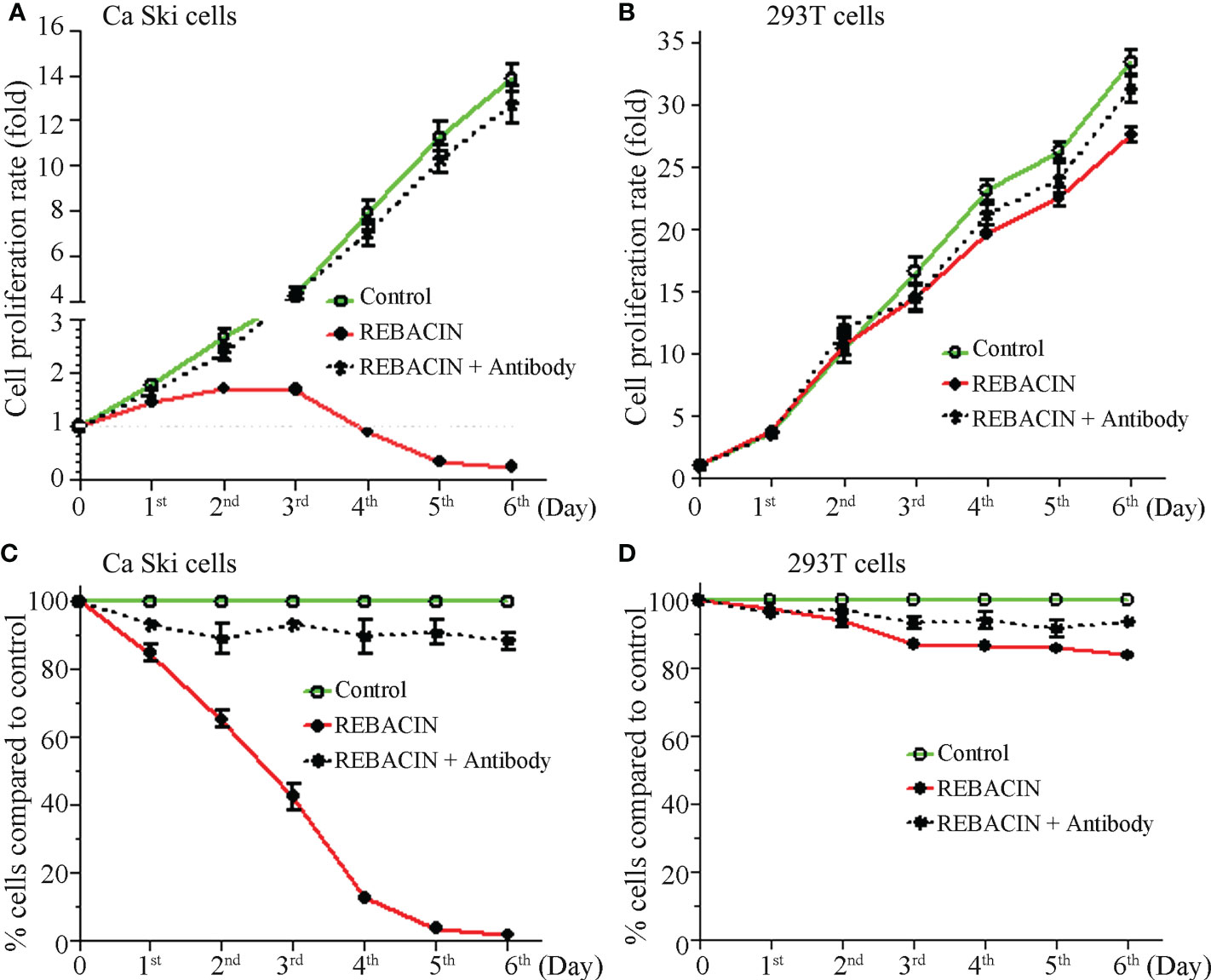
Figure 2 Effect of REBACIN® on the growth of different cell lines. Ca Ski (A, C) or 293T (B, D) cells in 24-well plate were treated or not treated by 50 μg/ml REBACIN® or REBACIN®/anti-REBACIN® antibody for up to 6 days, and the total amount of alive cells in each well was counted every 24 hours. During each count, the cell proliferation rate at the beginning stage was set as 1 (A, B); the total alive cells in each well without any treatment (control) were set as 100%, and the total alive cells in treated well were normalized to its control (C, D). Data show mean ± SEM of seven independent experiments. REBACIN® versus control: p < 0.05 in (A) and p < 0.01 in (C).
REBACIN® inhibits the expression of HPV16 E7 oncoprotein
Since REBACIN® influenced the growth of Ca Ski cells with expression of HPV16 E6/E7 oncoproteins, and only had slight influence in the growth of 293T cells without expression of HPV16 E6/E7 oncoproteins. We therefore investigated whether REBACIN®-mediated inhibition of Ca Ski’s growth is via the inhibition of E6/E7 oncoproteins. As expected, HPV16 oncoprotein E7 was detected in Ca Ski cells, and was under detectable in 293T cells (Figure 3A). The expression of HPV16 E7 oncoprotein was relative stable during six-day culture (Figure 3B). However, in the presence of REBACIN®, the expression of HPV16 E7 oncoprotein was gradually decreased as the culture time increased. Five days after the treatment, oncoprotein E7 was under detectable in Ca Ski cells (Figures 3C, E). The REBACIN®-mediated inhibition of HPV16 E7 expression could be counteracted under co-application of anti-REBACIN® antibody (Figures 3D, E).
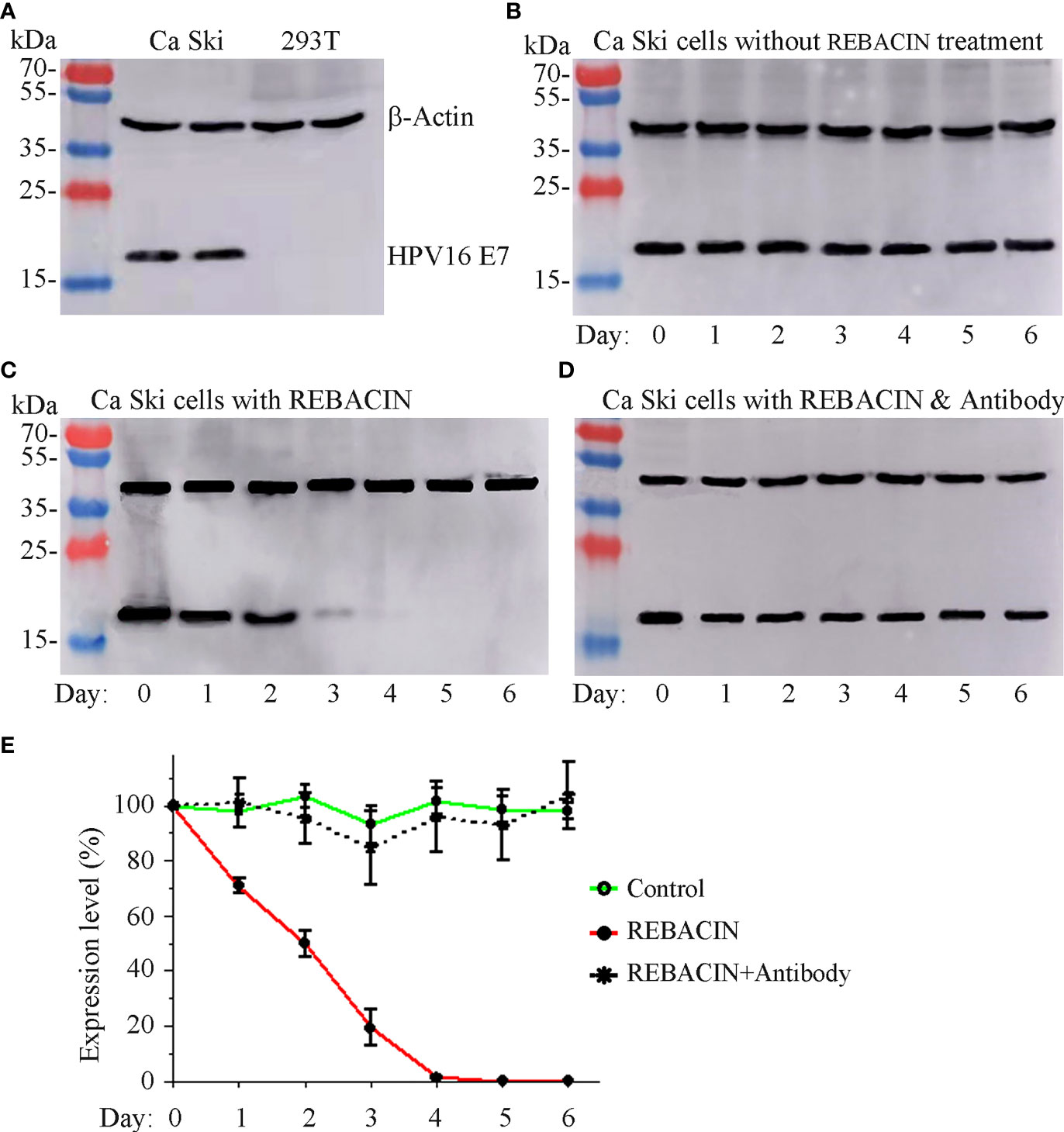
Figure 3 Effect of REBACIN® on the expression of HPV16 E7. Ca Ski or 293T cells in 6-well plate were treated or not treated by 50 μg/ml REBACIN® for up to 6 days. The expression of β-actin and HPV16 E7 in different cell lines was detected by western-blot in (A), and their expression levels in each well every 24 hours under different conditions were shown in (B–D), respectively. Representative images were shown in (A–D), and the relative quantitative data from (B–D) were analyzed and shown in (E), which the expression level at 0 day was set as 100%. Data show mean ± SEM of three independent experiments. REBACIN® versus control: p < 0.001 in (E).
REBACIN® influences the growth of HeLa cells
HeLa is a cervical cancer cell line, and have been reported to contain human papilloma virus 18 (HPV18) sequences. As expected, the treatment of REBACIN® did influence this cervical cancer cell’s growth. As shown in Figure 4A, REBACIN® treatment markedly inhibited the cell growth, which exhibited a time-dependent manner, and this inhibition can be blocked in the presence of anti-REBACIN® antibody (Figures 4A, B). In line with this find is that the expression level of HPV18 E6 oncoprotein was impressively decreased after the REBACIN® treatment, and this effect can be blocked by the addition of anti-REBACIN® antibody (Figure 4C), indicating that REBACIN® inhibits HeLa cell’s growth via the suppression of HPV18 E6 oncoprotein.
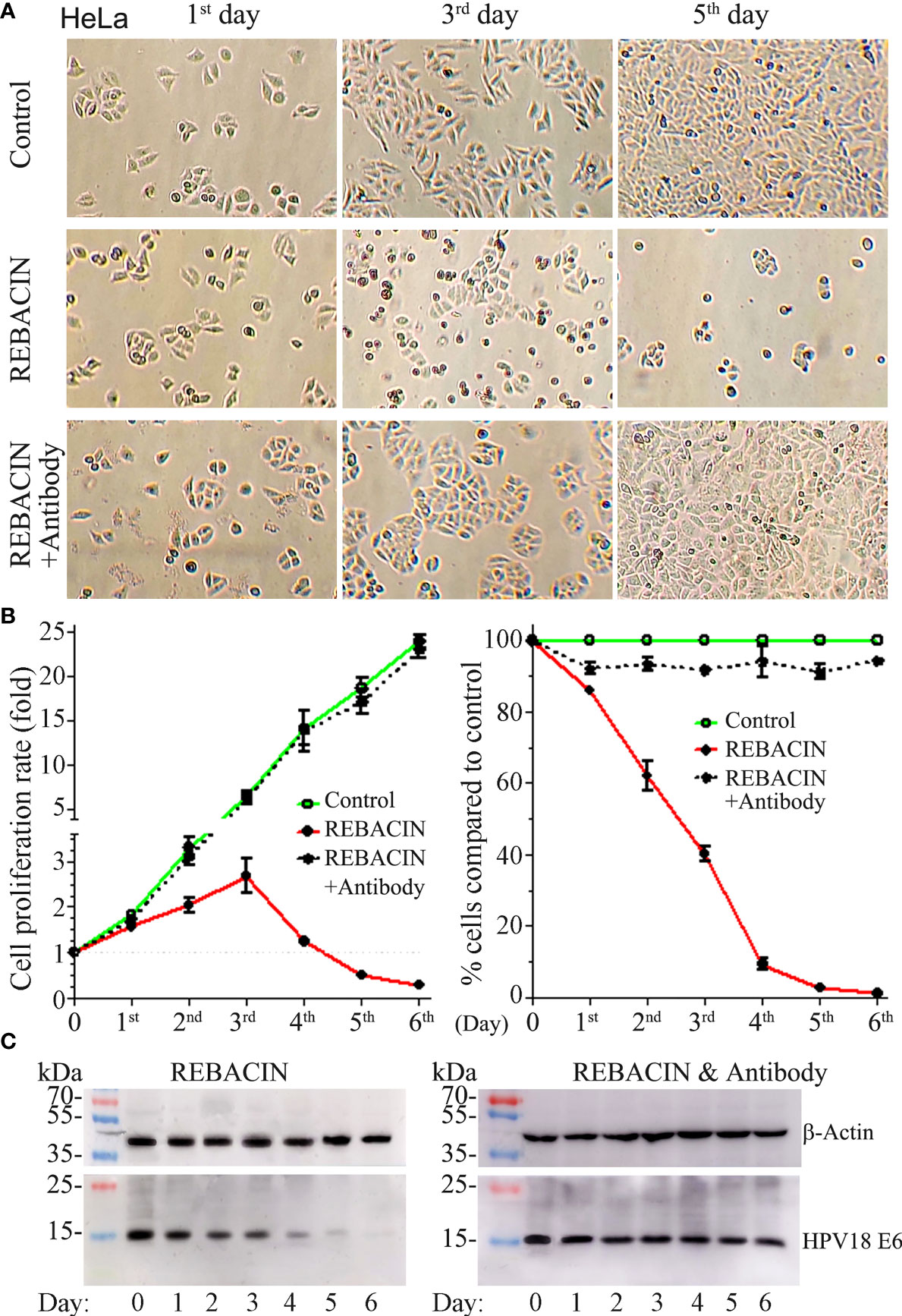
Figure 4 REBACIN® inhibits HeLa cell’s growth via the suppression of HPV18 E6. HeLa cells in 24-well plate were treated or not treated by 50 μg/ml REBACIN® or REBACIN®/anti-REBACIN® antibody for up to 6 days, and representative images in the 1st, 3rd, and 5th day were shown in (A). The total amount of alive cells in each well was counted every 24 hours, and their quantitative data show mean ± SEM of four independent experiments. REBACIN® versus control: p < 0.05 in (B). The expression level of β-actin and HPV18 E6 was detected by western-blot, and representative images were showed in (C).
Strong effect of REBACIN® on hrHPV E6/E7 mRNA suppression
In vitro study in cervical cancer cells demonstrated that REBACIN® inhibited cell growth via inhibition of HPV E6/E7 oncoproteins, we thus further investigated in clinical observation whether REBACIN® affects the expression level of hrHPV E6/E7 mRNA. In this study, the participants were randomly divided into REBACIN®, interferon or no-treatment blank control groups, and were treated intravaginally by REBACIN®, recombinant human interferon alpha-2b or no-treatment every other day for three months, except their menstrual period. All patients were then followed up for a test of hrHPV E6/E7 mRNA during four months after the intervention, and the effect of REBACIN® on the expression of hrHPV E6/E7 mRNA were evaluated. Of the 108 participants, 106 (98.15%) patients had all information available for statistical analysis. As shown in Table 1, 68.57% (24/35) of patients were tested as negative for hrHPV E6/E7 mRNA after three-month administration in REBACIN® treatment group, which was significantly higher in comparison with 20.00% (7/35) in the blank control group and also 25.00% (9/36) in the interferon alpha-2b control group. This finding demonstrates that REBACIN® markedly inhibits the expression of hrHPV E6/E7 oncogenes.
No significant side effect was observed in this study
Clinical observation has shown that, for all participants, there was no significant side effect during and after the intervention in this clinical observation. Moreover, routine physical examinations in all participants before and after intervention indicated that the participant’s blood routine, liver function (ALT and AST), kidney function (BUN and Cr) and other tests were in the normal range. However, REBACIN® or interferon alpha-2b induced mild vaginal itch (five patients) was occasionally observed. No other side effects were reported so far.
Discussion
Although the incidence of cervical cancer has dropped in industrialized countries over the past decade, the rate of decline has been much slower and even increasing in some of developing countries. In developing countries, the cervical cancer accounts for about 12% of all female cancers, and up to 90% mortality rate occurs disproportionately (31). The number of patients diagnosed with persistent hrHPV infections in hospitals has been kept high in China during recent several years, becoming a high-risk population of cervical cancer. The standard clinical therapy of serious hrHPV infection and hrHPV-induced cervical intraepithelial lesion is surgery, which could lead to potential adverse effects including future fertility (32–34). However, there are currently no available FDA-approved antiviral drugs targeting HPV infections (35).
REBACIN® was recently reported to be efficacious on the clearance of persistent hrHPV infection (24, 25), and this innovative non-invasive clinical intervention creates a new clinical approach and option in addition to standard invasive treatment of surgery for the patients with persistent hrHPV infection and its related diseases. In particular, patients, especially in the developing country, can use it by themselves under the guidance of doctors without hospitalization.
On the basis of previous research (24, 25), this study attempts to further confirm and verify that REBACIN® eliminates persistent high-risk HPV infection via inhibition of hrHPV E6 and E7 oncogenes. We found in vitro that REBACIN® inhibits and even blocks the growth of human cervical cancer cell lines of both Ca Ski and HeLa by inhibition of HPV E6/E7 expression. Moreover, in a clinical observation, three-month treatment of REBACIN® resulted in 68.57% of the patients with a completed suppression of hrHPV E6/E7 mRNA in REBACIN® treatment group, while 25.00% in the interferon alpha-2b control group (a typical immunological drug), and 20.00% in the no-treatment blank control group. This finding strongly supports that the effect of REBACIN® on the clearance of hrHPV infection is via inhibition of hrHPV E6/E7 expression.
High-risk HPV E6/E7 oncogenes play a key role not only in cervical intraepithelial lesion and the development of cervical cancer (36), but also in viral replication and maintenance (37, 38). HPV E6/E7 RNA interference was suggested as a promising therapeutic candidate for a wide variety of HPV-associated cancers (28–30). Several strategies targeted at HPV E6/E7 have been investigated for the development of therapeutic vaccines (39). However, all these strategies are still under investigation. A certain level of E6/E7 expression is required for HPV16 replication and maintenance in the host cells (38). If the expression of the E6/E7 oncogenes is completely suppressed, the viral replication and maintenance will not continue and the hrHPV persistent infection will be eliminated. Thus, this study demonstrated REBACIN® as a non-invasive intervention therapy with high clinical efficacy in the clearance of persistent high-risk HPV infection via inhibition of hrHPV E6/E7 expression. As for how REBACIN® inhibits the expression of E6/E7 oncogenes, further investigation is needed.
Data availability statement
The original data presented in the study are included in the article material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by The Institutional Ethics Committee of Anhui Province Maternity and Child Healthcare Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
C-FZ and GC conceived and designed the study. S-GZ, D-FW, HY, W-YZ and F-JT performed the experiments and carried out the data collection. S-GZ and D-FW analyzed the data and drafted the original manuscript. C-FZ and GC edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Applied Medicine Research Project of Hefei Health Commission (Grant No. HWKJ2019-172-14), the Research Fund Project of Anhui Medical University (Grant No. 2020xkj236), the Natural Science Foundation of Higher Education Institutions of Anhui Province (Grant No. KJ2021A0352) and the Funding Project of Haikou City Patent Implementation Guidance (Grant No. 2020ZLSSYD0101).
Acknowledgments
The authors are grateful to the volunteer patients and their families who have participated in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ramqvist T, Grün N, Dalianis T. Human papillomavirus and tonsillar and base of tongue cancer. Viruses (2015) 7:1332–43. doi: 10.3390/v7031332
2. Van Doorslaer K. Evolution of the papillomaviridae. Virology (2013) 445:11–20. doi: 10.1016/j.virol.2013.05.012
3. Boda D, Docea AO, Calina D, Ilie MA, Caruntu C, Zurac S, et al. Human papilloma virus: Apprehending the link with carcinogenesis and unveiling new research avenues (Review). Int J Oncol (2018) 52:637–55. doi: 10.3892/ijo.2018.4256
4. Veldhuijzen NJ, Snijders PJ, Reiss P, Meijer CJ, van de Wijgert JH. Factors affecting transmission of mucosal human papillomavirus. Lancet Infect Dis (2010) 10:862–74. doi: 10.1016/S1473-3099(10)70190-0
5. Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, et al. A review of human carcinogens–part b: biological agents. Lancet Oncol (2009) 10:321–22. doi: 10.1016/s1470-2045(09)70096-8
6. Zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer (2002) 2:342–50. doi: 10.1038/nrc798
7. Arbyn M, Weiderpass E, Bruni L, de Sanjosé S, Saraiya M, Ferlay J, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health (2020) 8:e191–203. doi: 10.1016/S2214-109X(19)30482-6
8. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68:394–24. doi: 10.3322/caac.21492
9. Hu Z, Zhu D, Wang W, Li W, Jia W, Zeng X, et al. Genome-wide profiling of HPV integration in cervical cancer identifies clustered genomic hot spots and a potential microhomology-mediated integration mechanism. Nat Genet (2015) 47:158–63. doi: 10.1038/ng.3178
10. de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol (2010) 11:1048–56. doi: 10.1016/S1470-2045(10)70230-8
11. Bzhalava D, Guan P, Franceschi S, Dillner J, Clifford G. A systematic review of the prevalence of mucosal and cutaneous human papillomavirus types. Virology (2013) 445:224–31. doi: 10.1016/j.virol.2013.07.015
12. Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol (1999) 189:12–9. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F
13. D'Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch M, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med (2007) 356:1944–56. doi: 10.1056/NEJMoa065497
14. Doorbar J, Egawa N, Griffin H, Kranjec C, Murakami I. Human papillomavirus molecular biology and disease association. Rev Med Virol (2015) 25:2–23. doi: 10.1002/rmv.1822
15. Gupta S, Kumar P, Das BC. HPV: Molecular pathways and targets. Curr Probl Cancer (2018) 42:161–74. doi: 10.1016/j.currproblcancer.2018.03.003
16. Yeo-The NSL, Ito Y, Jha S. High-risk human papillomaviral oncogenes E6 and E7 target key cellular pathways to achieve oncogenesis. Int J Mol Sci (2018) 19:1706. doi: 10.3390/ijms19061706
17. Rositch AF, Soeters HM, Offutt-Powell TN, Wheeler BS, Taylor SM, Smith JS. The incidence of human papillomavirus infection following treatment for cervical neoplasia: a systematic review. Gynecol Oncol (2014) 132:767–79. doi: 10.1016/j.ygyno.2013.12.040
18. Liu Y, Li H, Pi R, Yang Y, Zhao X, Qi X. Current strategies against persistent human papillomavirus infection (Review). Int J Oncol (2019) 55:570–84. doi: 10.3892/ijo.2019.4847
19. Shi HJ, Song H, Zhao QY, Tao CX, Liu M, Zhu QQ. Efficacy and safety of combined high-dose interferon and red light therapy for the treatment of human papillomavirus and associated vaginitis and cervicitis: A prospective and randomized clinical study. Med (Baltimore) (2018) 97:e12398. doi: 10.1097/MD.0000000000012398
20. Xiong Y, Cu L, Bian C, Zhao X, Wang X. Clearance of human papillomavirus infection in patients with cervical intraepithelial neoplasia: A systemic review and meta-analysis. Med (Baltimore) (2020) 99:e23155. doi: 10.1097/MD.0000000000023155
21. Zhao HD, Feng XL, Zhao Y, Li N. Randomized controlled study: Sophora flavescens gel in treatment of cervical HPV infection. China J Chin materia Med (2016) 41:4072–75. doi: 10.4268/cjcmm20162129
22. Terenzi F, Saikia P, Sen GC. Interferon-inducible protein, P56, inhibits HPV DNA replication by binding to the viral protein E1. EMBO (2008) 27:3311–21. doi: 10.1038/emboj.2008.241
23. Koromilas AE, Li S, Matlashewski G. Control of interferon signaling in human papillomavirus infection. Cytokine Growth Factor Rev (2001) 12:157–70. doi: 10.1016/s1359-6101(00)00023-x
24. Wang F, Liu R, Ma Y, Wu DF, Deng LH, Wang S, et al. Case report: Noninvasive clinical intervention of REBACIN on histologic regression of high grade cervical intraepithelial neoplasia. Front Med (Lausanne) (2021) 8:627355. doi: 10.3389/fmed.2021.627355
25. Yang Y, Meng YL, Duan SM, Zhan SB, Guan RL, Yue TF, et al. REBACIN® as a noninvasive clinical intervention for high-risk human papillomavirus persistent infection. Int J Cancer (2019) 145:2712–19. doi: 10.1002/ijc.32344
26. Ren C, Zhu Y, Yang L, Zhang X, Liu L, Wang Z, et al. Prognostic and diagnostic validity of p16/Ki-67, HPV E6/E7 mRNA, and HPV DNA in women with ASCUS: a follow-up study. Virol J (2019) 16:143. doi: 10.1186/s12985-019-1251-4
27. Nayar R, Wilbur DC. The pap test and Bethesda 2014. Cancer Cytopathol (2015) 123:271–81. doi: 10.1002/cncy.21521
28. Jung HS, Rajasekaran N, Ju W, Shin YK. Human papillomavirus: Current and future RNAi therapeutic strategies for cervical cancer. J Clin Med (2015) 4:1126–55. doi: 10.3390/jcm4051126
29. Nishida H, Matsumoto Y, Kawana K, Christie RJ, Naito M, Kim BS, et al. Systemic delivery of siRNA by actively targeted polyion complex micelles for silencing the E6 and E7 human papillomavirus oncogenes. J Control Release (2016) 231:29–37. doi: 10.1016/j.jconrel.2016.03.016
30. Yamato K, Yamada T, Kizaki M, Ui-Tei K, Natori Y, Fujino M, et al. New highly potent and specific E6 and E7 siRNAs for treatment of HPV16 positive cervical cancer. Cancer Gene Ther (2008) 15:140–53. doi: 10.1038/sj.cgt.7701118
31. Gurram L, Kalra B, Mahantshetty U. Meeting the global need for radiation therapy in cervical cancer-an overview. Semin Radiat Oncol (2020) 30:348–54. doi: 10.1016/j.semradonc.2020.05.004
32. Bjørge T, Skare GB, Bjørge L, Tropé A, Lönnberg S. Adverse pregnancy outcomes after treatment for cervical intraepithelial neoplasia. Obstet Gynecol (2016) 128:1265–73. doi: 10.1097/AOG.0000000000001777
33. Ciavattini A, Clemente N, Delli Carpini G, Gentili C, Di Giuseppe J, Barbadoro P, et al. Loop electrosurgical excision procedure and risk of miscarriage. Fertil Steril (2015) 103:1043–48. doi: 10.1016/j.fertnstert.2014.12.112
34. Heinonen A, Gissler M, Riska A, Paavonen J, Tapper AM, Jakobsson M. Loop electrosurgical excision procedure and the risk for preterm delivery. Obstet Gynecol (2013) 121:1063–68. doi: 10.1097/AOG.0b013e31828caa31
35. Hutter JN, Decker CF. Human papillomavirus infection. Dis Mon (2016) 62:294–300. doi: 10.1016/j.disamonth.2016.03.014
36. Hoppe-Seyler K, Bossler F, Braun JA, Herrmann AL, Hoppe-Seyler F. The HPV E6/E7 oncogenes: Key factors for viral carcinogenesis and therapeutic targets. Trends Microbiol (2018) 26:158–68. doi: 10.1016/j.tim.2017.07.007
37. Roman A, Munger K. The papillomavirus E7 proteins. Virology (2013) 445:138–68. doi: 10.1016/j.virol.2013.04.013
38. Murakami I, Egawa N, Griffin H, Yin W, Kranjec C, Nakahara T, et al. Roles for E1-independent replication and E6-mediated p53 degradation during low-risk and high-risk human papillomavirus genome maintenance. PloS Pathog (2019) 15:e1007755. doi: 10.1371/journal.ppat.1007755
Keywords: REBACIN, human papilloma virus (HPV), E6/E7, clearance, cervical cancer
Citation: Zhou S-G, Wu D-F, Yao H, Zhang W-Y, Tian F-J, Chen G and Zhang C-F (2022) REBACIN® inhibits E6/E7 oncogenes in clearance of human papillomavirus infection. Front. Oncol. 12:1047222. doi: 10.3389/fonc.2022.1047222
Received: 26 September 2022; Accepted: 16 November 2022;
Published: 06 December 2022.
Edited by:
Jinhui Liu, Nanjing Medical University, ChinaReviewed by:
Bo Li, Hainan Women and Children’s Medical Center, ChinaHuaiwu Lu, Sun Yat-sen Memorial Hospital, China
Copyright © 2022 Zhou, Wu, Yao, Zhang, Tian, Chen and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chun-Fa Zhang, emhhbmdfY3NAaG90bWFpbC5jb20=; Guo Chen, ZmN6eDIwMjBAMTI2LmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Shu-Guang Zhou
Shu-Guang Zhou Dai-Fei Wu
Dai-Fei Wu Hui Yao
Hui Yao Wei-Yu Zhang
Wei-Yu Zhang Feng-Jiao Tian3
Feng-Jiao Tian3 Guo Chen
Guo Chen