
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 21 October 2022
Sec. Genitourinary Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.1046505
This article is part of the Research TopicMinimally-Invasive Treatment For Genitourinary Cancers: What Comes Next?View all 8 articles
 Lorenzo Bianchi1,2*
Lorenzo Bianchi1,2* Laura Cercenelli3
Laura Cercenelli3 Barbara Bortolani3
Barbara Bortolani3 Pietro Piazza1
Pietro Piazza1 Matteo Droghetti1
Matteo Droghetti1 Sara Boschi1
Sara Boschi1 Caterina Gaudiano4
Caterina Gaudiano4 Giulia Carpani4
Giulia Carpani4 Francesco Chessa1,2
Francesco Chessa1,2 Simone Lodi3
Simone Lodi3 Lorenzo Tartarini3
Lorenzo Tartarini3 Alessandro Bertaccini1,2
Alessandro Bertaccini1,2 Rita Golfieri4
Rita Golfieri4 Emanuela Marcelli3
Emanuela Marcelli3 Riccardo Schiavina1,2
Riccardo Schiavina1,2 Eugenio Brunocilla1,2
Eugenio Brunocilla1,2Objective: to evaluate the impact of 3D model for a comprehensive assessment of surgical planning and quality of partial nephrectomy (PN).
Materials and methods: 195 patients with cT1-T2 renal mass scheduled for PN were enrolled in two groups: Study Group (n= 100), including patients referred to PN with revision of both 2D computed tomography (CT) imaging and 3D model; Control group (n= 95), including patients referred to PN with revision of 2D CT imaging. Overall, 20 individuals were switched to radical nephrectomy (RN). The primary outcome was the impact of 3D models-based surgical planning on Trifecta achievement (defined as the contemporary absence of positive surgical margin, major complications and ≤30% postoperative eGFR reduction). The secondary outcome was the impact of 3D models on surgical planning of PN. Multivariate logistic regressions were used to identify predictors of selective clamping and Trifecta’s achievement in patients treated with PN (n=175).
Results: Overall, 73 (80.2%) patients in Study group and 53 (63.1%) patients in Control group achieved the Trifecta (p=0.01). The preoperative plan of arterial clamping was recorded as clampless, main artery and selective in 22 (24.2%), 22 (24.2%) and 47 (51.6%) cases in Study group vs. 31 (36.9%), 46 (54.8%) and 7 (8.3%) cases in Control group, respectively (p<0.001). At multivariate logistic regressions, the use of 3D model was found to be independent predictor of both selective or super-selective clamping and Trifecta’s achievement.
Conclusion: 3D-guided approach to PN increase the adoption of selective clamping and better predict the achievement of Trifecta.
Due to the effect of stage migration (1), increasing proportion of patients with renal tumour are diagnosed with T1 stage disease. Thus, partial nephrectomy (PN) is increasingly adopted as preferred treatment (2–4). Recently, the increasing experience with the robotic approach lead to expand the adoption of robotic PN even in complex T1 (5) and T2 renal mass (6–8). Moreover, the risk of unsuccessful PN with conversion to radical nephrectomy (RN) is higher in challenging cases (9, 10). Thus, a critical and detailed comprehension of tumour’s complexity is essential to achieve optimal success of PN. Nowadays, 3D models facilitate the understanding of renal anatomy (11, 12) and are more accurate to assess with higher accuracy surgical complexity of renal masses compared to 2D imaging (13, 14) and to predict surgical outcomes (15). Likewise in prostate cancer robotic surgery (16–19), 3D models may have strong implications for surgical planning.
Thus, the high-fidelity 3D reconstruction of renal vasculature allows to increase selective clamping (11–13, 20, 21) with potential improvement of functional outcomes. Thus, oncologic and functional outcomes of PN are dependent on quality of tumour resection, renal ischemia and quality and quantity of preserved renal parenchyma (22). Indeed, ideal outcomes of PN should comprehend maximal renal functional preservation, negative surgical margins and no complications: the simultaneous achievement of all three goals has been defined as Trifecta outcomes (23).
The aim of our study was to evaluate the impact of 3D virtual model for a comprehensive assessment of surgical planning and the improvement of the quality of PN.
We prospectively enrolled 195 consecutive patients with clinical diagnoses of single T1-T2 renal mass, scheduled for open, laparoscopic or robot assisted PN by experienced surgeons in each surgical technique at the end of the learning curve at our institution between December 2018 and August 2021. Before surgery, each patient was investigated with high quality chest and abdominal contrast-enhanced CT (slice thickness: 1.25 ÷ 2.5 mm, step interval: 0.8÷ 2.0 mm). Participants signed a written informed consent document. Patients with multiple synchronous renal tumours and with solitary kidney were excluded.The study was approved by our Institutional Ethics Committee (IRB approval 3386/2018).
To evaluate the impact of 3D virtual model on surgical planning and outcomes of PN, patients were stratified in two groups: Study Group (n= 100), including patients scheduled for PN in which the surgeon reviewed both the 2D CT imaging and the 3D virtual model before and during surgery; Control group (n= 95), including patients scheduled for PN in which the surgeon reviewed only the 2D CT imaging before surgery. Overall, 10 individuals referred to PN were switched to radical nephrectomy (RN) before surgery and 10 patients were switched to RN during surgery, thus the final population of patient underwent PN consisted of 175 patients (91 in Study group and 84 in Control group; Figure 1).
In Study group, all 3D virtual models, based on preoperative high-quality CT scan, were carried out by engineers at eDIMES Lab of the University of Bologna, located at IRCCS, Azienda Ospedaliero-Universitaria, S.Orsola-Malpighi Hospital, as previously described (11, 12).
Briefly, multiple imaging series with different contrast levels were used for the selective identification of each anatomical structure of interest (healthy parenchyma, tumour lesion, extra and intra-renal arterial and venous branches and urinary collecting system [UCS]) in the image segmentation process. Segmentation was achieved using D2P™ software (‘DICOM to PRINT’; 3D Systems Inc., Rock Hill, SC). The segmentation results and the anatomical correctness of the reconstructed 3D virtual models (14, 15) were reviewed and validated by surgeons and radiologists.
For each case in Study group, the surgeon viewed the 3D model before the intervention. Intraoperative viewing of the 3D models was performed at the surgeon’s discretion with the help of an assistant. In some cases, the creation of 3D model was not possible due to organisational reasons, since the reconstruction of 3D model required on average about 3 hours by the bioengineers who are involved also in 3D modelling for different surgical specialities. For these cases, patients underwent surgery with 2D imaging only and were included in the Control group.
PNs were performed with open, laparoscopic or robot-assisted approach by three dedicated surgeons with high experience in each surgical approach. The choice of surgical technique was left to the surgeon preference. Open PN was performed through a retroperitoneal flank incision between the XI and XII ribs, as previously described (24). Laparoscopic PN and robot-assisted PN were performed with transperitoneal approach as previously described (25, 26). Laparoscopic PN was performed using three 12 mm trocars and one 5 mm trocar. Robot-assisted PN was performed using the DaVinci® Xi™ Surgical System (Intuitive Surgical Inc., Sunnyvale, CA, USA) in a four-arm configuration with the integrated Firefly™ fluorescence-imaging mode (6). In case of clamping approach to the renal hilum we adopted warm ischemia: a selective (first branch) or super-selective (secondary and tertiary branches) clamping approach was preferred over non selective clamping whenever feasible according to preoperative imaging and intraoperative patients-specific surgical anatomy.
The preoperative surgical planning including the need of conversion to RN, the presumed type of arterial clamping technique and the need of UCS suture were evaluated by surgeons on 2D imaging in Control group and both on 2D imaging and 3D virtual models in Study group, consecutively. During surgery, the intraoperative conversion to RN, the effective intraoperative type of clamping and need of UCS suture were recorded and compared to the preoperative planning in both groups.
Demographic and clinical parameters were available for each patient, including age, body mass index, estimated glomerular filtration rate (eGFR; ml/min/1.73 m2) and comorbidities classified according to American Society of Anaesthesiologists (ASA) score. The surgical complexity of the renal masses was scored according to PADUA (27) and R.E.N.A.L (28). score based on 2D imaging in both groups. Prospective data were collected: global surgical time, intraoperative estimated blood loss (EBL), conversion to RN, type of arterial clamping, the need of UCS suture, histotype, pathologic stage and grade and positive surgical margin (PSM). The first postoperative eGFR was considered eGFR at discharge. The variation in eGFR from baseline at discharge was estimated for evaluating the impact of the surgical procedure on renal function. Complications within 30 d after surgery were recorded and graded according to the Clavien-Dindo classification (29). Major complications were categorized as Clavien grade III or higher according to European Association of Urology guidelines (30). During follow up, oncologic outcomes (disease recurrence) evaluated by conventional imaging and functional outcomes were recorded.
The primary outcome of the study was to determinate the impact of 3D virtual model to achieve the Trifecta for PN (defined as the contemporary absence of PSM, major complications and ≤30% postoperative eGFR reduction) (31).
The secondary outcomes were the impact of 3D models in the preoperative planning of PN with regards of rate of conversion to RN, type of arterial clamping and need of UCS suture and the success rate of the preoperative planning concerning the rate of conversion to RN, type of arterial clamping and need of UCS suture.
Chi-squared test, T-student test and Mann-Whitney U-test were used to compare proportion, means and medians, respectively. Statistical analyses consist of several steps. First, in the overall population of patients scheduled for PN, the effective rates of conversion to RN planned before surgery or unanticipated and performed during surgery was compared between Study and Control groups. Second, considering patients who effectively underwent PN, the preoperative planning concerning the type of arterial clamping and the need of UCS suture, the effective intraoperative type of arterial clamping and UCS and the success rate of preoperative planning were compared between Study and Control groups. Third, the Trifecta rate and the causes of Trifecta failure were analyzed between the two groups. Finally, multivariate logistic regressions were used to identify independent predictors of selective or super-selective clamping and of Trifecta’s achievement, basing on significant predictors at univariate analyses. Covariates consist of follows: age, ASA score, use of 3D models, surgical technique (open, laparoscopic or robotic), PADUA score (Model 1), RENAL score (Model 2). Multivariate logistic regression models to predict conversion to RN and UCS suture were not employed due to limited number of events.
A p value of <0.05 was considered statistically significant. All statistical tests were performed using SPSS 22.0 for Windows.
Table 1 depicts baseline characteristics in the overall population of patients scheduled for PN (n=195). Surgery was performed with open, laparoscopic or robotic approach in 37 (19%), 25 (12.8%) and 133 (68.2%) cases, respectively. The conversion to RN was planned before surgery in 77.8% and in 27.3% of cases in Study group after revision of the 3D model and in Control group after revision of 2D imaging, respectively (p=0.03). Considering the subgroup of patients referred to PN (n=175), the preoperative plan of arterial clamping was recorded as clampless, main artery, selective or super-selective in 22 (24.2%), 22 (24.2%), and 47 (51.6%) cases in Study group vs. 31 (36.9%), 46 (54.8%) and 7 (8.3%) cases in Control group, respectively (p<0.001). During surgery, the intraoperative management of the renal pedicle was done as preoperatively planned in 63.6% vs. 74.5% of cases for clampless approach (p=0.05), in 90.1% vs. 76% of cases for main artery clamping (p=0.005) and in 61.7% vs. 28.6% of cases for selective and super-selective clamping (p<0.001) in Study group vs. Control group, respectively. The effective intraoperative need of UCS suture was done as preoperatively planned in 66.6% and 26.1% in Study and Control group, respectively (p=0.004; Table 2). Table 3 shows intra, perioperative, pathologic and postoperative characteristics of individuals who effectively underwent PN (n=175). Overall, 11 and 6 patients in Study group vs. 13 and 4 individuals in Control group experienced Clavien grade I-II and Clavien grade III postoperative complications, respectively (p=0.5). Types of intra- and postoperative complications are reported in Table 4. At mean follow up of 12.5 months, patients underwent PN procedure experienced a mean decrease of eGFR value of -5.8% at discharge. Overall, 80.6% of patients in Study group and 73.4% in Control group had ≤30% postoperative eGFR reduction from baseline at discharge (p=0.01). Overall, 73 (80.2%) and 53 (63.1%) patients achieved the Trifecta for PN in Study and Control group, respectively (p=0.01). Figure 2 depicts the causes of Trifecta’s failure in the two groups. At multivariate logistic regressions, the use of 3D model was found to be independent predictor of both adoption of selective or super-selective clamping (Odd Radio [OR]:5.26 in model 1 and OR:5.04 in model 2; all p ≤ 0.001) and of Trifecta’s achievement (OR:2.42 in model 1 and OR:2.41 in model 2; all p ≤ 0.02; Table 5).
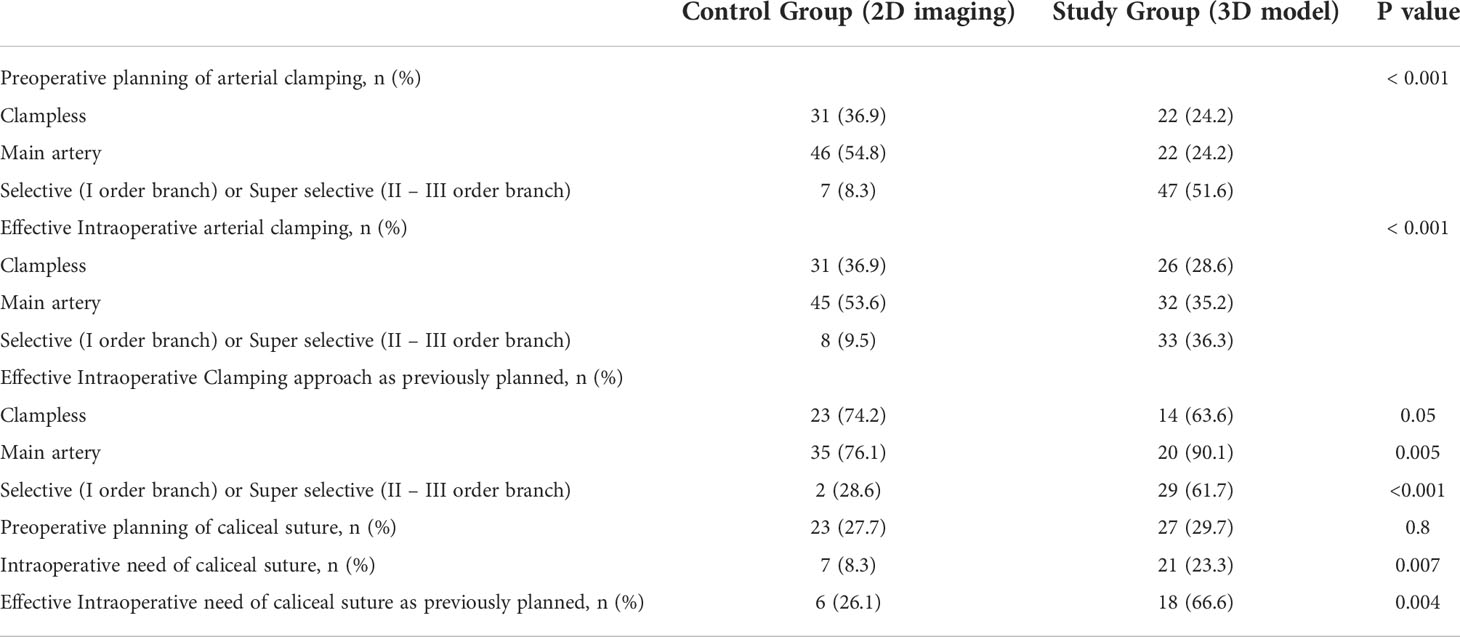
Table 2 Sub-analysis in patients underwent PN (n= 175) to compare the preoperative planning and the intraoperative approach to the renal hilum and caliceal system suturing.
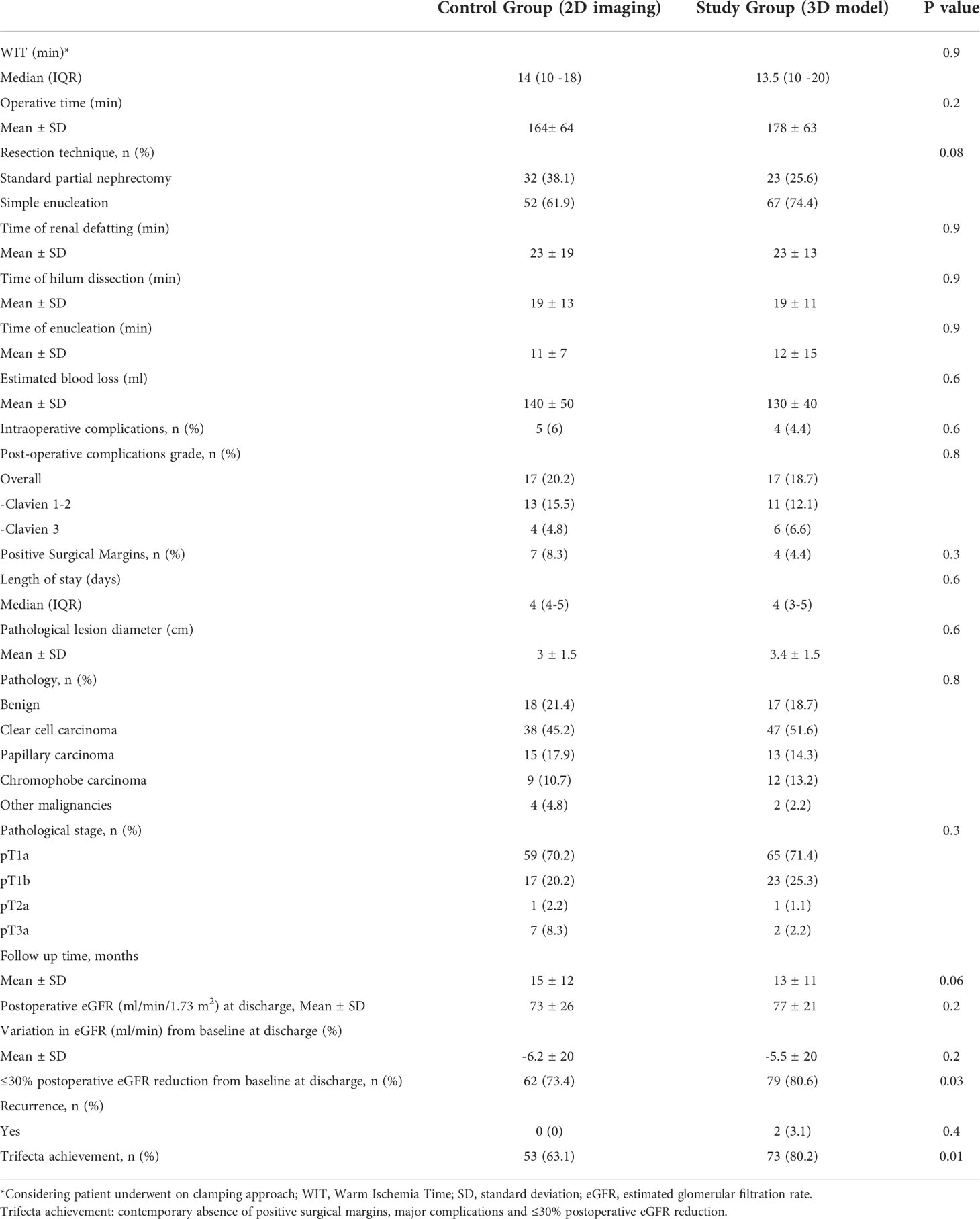
Table 3 Intraoperative, peri-operative, pathologic and postoperative characteristics in the overall population underwent PN (n=175).
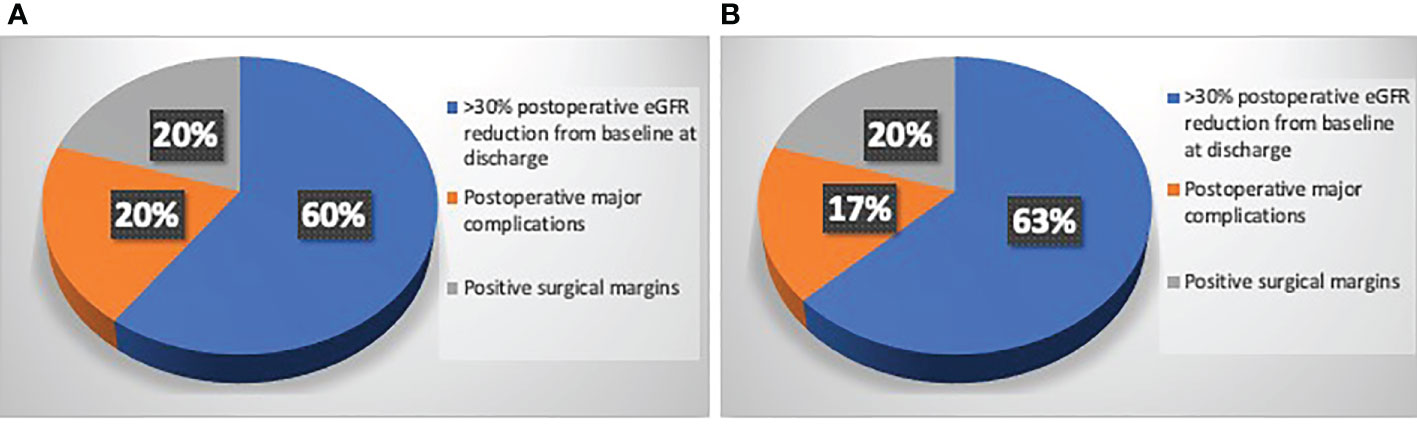
Figure 2 (A) Causes of Trifecta’s failure in patients underwent PN in Study group who did not reach the Trifecta (n=18/91); (B) Causes of Trifecta’s failure in patients underwent PN in Control group who did not reach the Trifecta (n=31/84).
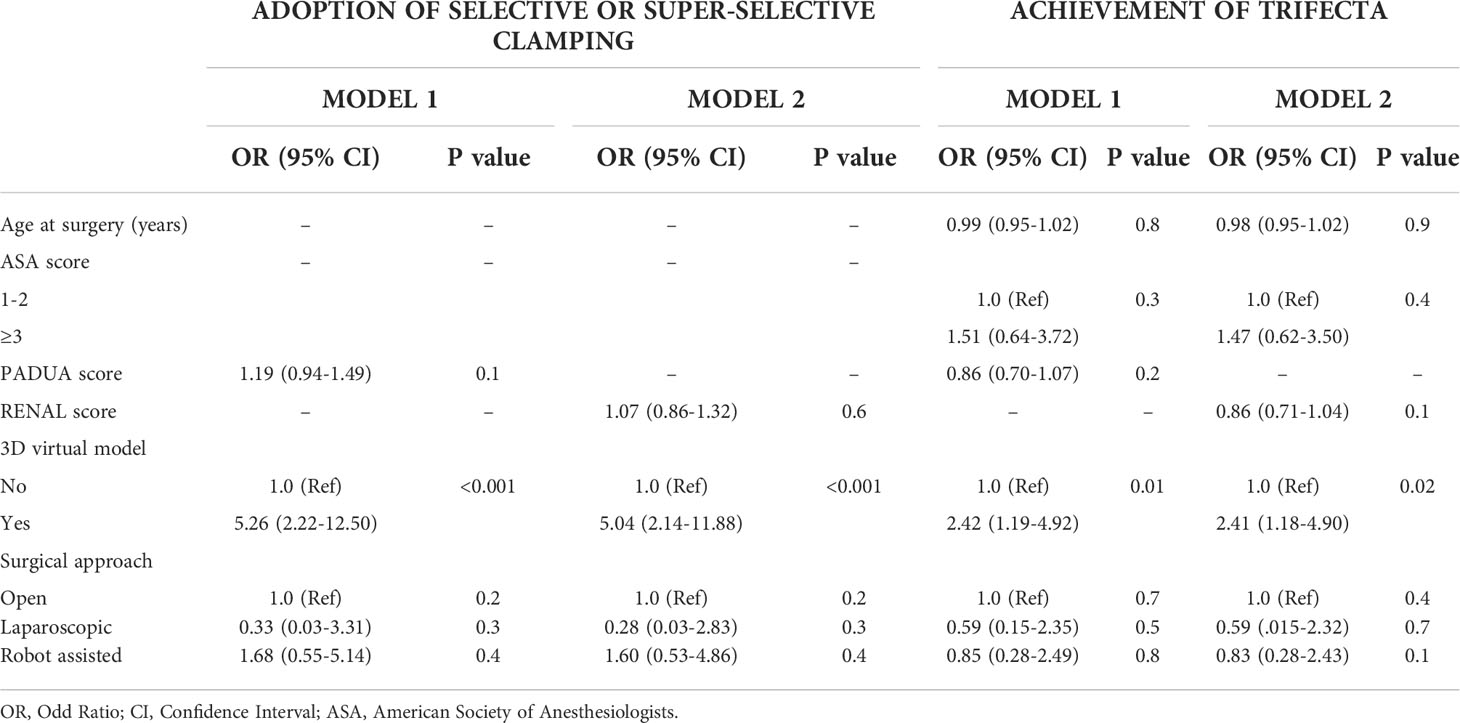
Table 5 Multivariate logistic regression analyses to predict the adoption of selective or super-selective clamping and the achievement of Trifecta in patients underwent PN (175), evaluating separately PADUA score (Model 1) and R.E.N.A.L. score (Model 2).
In recent years several technologic improvements have been introduced with the aim to increase the quality of renal surgery. The main goal of PN includes complete tumour excision with negative surgical margins, as well as reduced complications and damage to the healthy renal parenchyma as effect of resection of peritumoral renal tissue or ischemic damage (i.e. Trifecta). Indeed, to reduce the ischemic damage during PN, a non-global ischemia techniques have been proposed (32), despite the effect of selective clamping on renal function impairment is still debated. However, the adoption of selective clamping remained less popular in the pre-robotic era due to the need of precise dissection of segmental arterial branches. With the advent of robotics, a more precise surgery that allowed meticulous dissection of higher-order renal arteries was made possible (32). Nevertheless, PN is a complex surgical intervention and an accurate presurgical planning is the key for a good quality of PN outcomes. Before surgery, many aspects should be investigated: planning a conversion to RN, surgical technique, transperitoneal or retroperitoneal approach, selection of blood vessels for clamping, tumor resection margin and need of UCS suture. In the current era of precision surgery, the introduction of 3D models allow to simplify the anatomical knowledge of renal mass and to easily assess the surgical complexity (14), allowing a patient-tailored approach for PN (15).
Several points of our study are remarkable. First, the use of 3D model allows to significantly reduce the intraoperative conversions to RN. In our cohort the overall rate of conversion to RN (10.3%) is consistent with previously reported data from very high-volume centres in which it ranges from 3.1% (10) including only robotic cases to 12.4% (33) including both open, laparoscopic and robotic approach. Reason for unsuccessful PN may consist of patients-related factors, tumor-related features (9, 34) or surgeons and center experience (3, 33). As consequence, a precise evaluation of complexity of renal mass is the key to achieve successful PN. Our results suggest that the use of 3D model may predict the occurrence of unsuccessful PN, changing the indication from PN to RN before surgery allowing a better patients selection for PN, reducing the intraoperative conversion to RN that may be associated with longer operating times, increased blood loss, and worse postoperative renal function compared to non-converted RN (9, 10) (Figure 3). Second, our data confirmed that the effective intraoperative adoption of selective clamping was significantly higher in patients with 3D model available (36.3%) compared to patients with only 2D imaging (9.5%; p<0,001). Contrarily to previous reports by Michiels et al. (22) in which most patients with 3D model underwent PN without clamping (50.9%), while the vast majority of patients without 3D model underwent PN with main artery clamping (91.7%), in our cohort the rate of effective clampless approach was significantly higher in control group (36.9%) compared to patients with 3D model (28.6%). This could be due to surgeons’ and centres’ experience and preference: in our experience we noticed that when 3D model is not available the surgeon is more prone to clamp the main artery in case of complex mass or to perform clampless PN in case of easier cases. Besides, when the 3D model is available, a selective or super-selective clamping is planned and attempt even in easier cases that would have been treated with clampless approach whenever the 3D model was not available, to achieve better bleeding control. To note, the protective effect of 3D models on postoperative renal function may be due to higher adoption of selective clamping (36.3%) in our cohort and higher adoption of clampless approach (50.9%) in the series by Michiels et al (22). Moreover, the potential benefit of higher adoption of clampless approach (36.9%) in control group on postoperative renal function may be mitigated by significant adoption of main artery clamping (53.6%). Of note, the preoperative planning using the 3D virtual model is more accurate and surgeons revealed higher adherence to the preoperative planning during surgery, with lower risk of unexpected events or to change the predetermined plan of surgery (Figure 4).
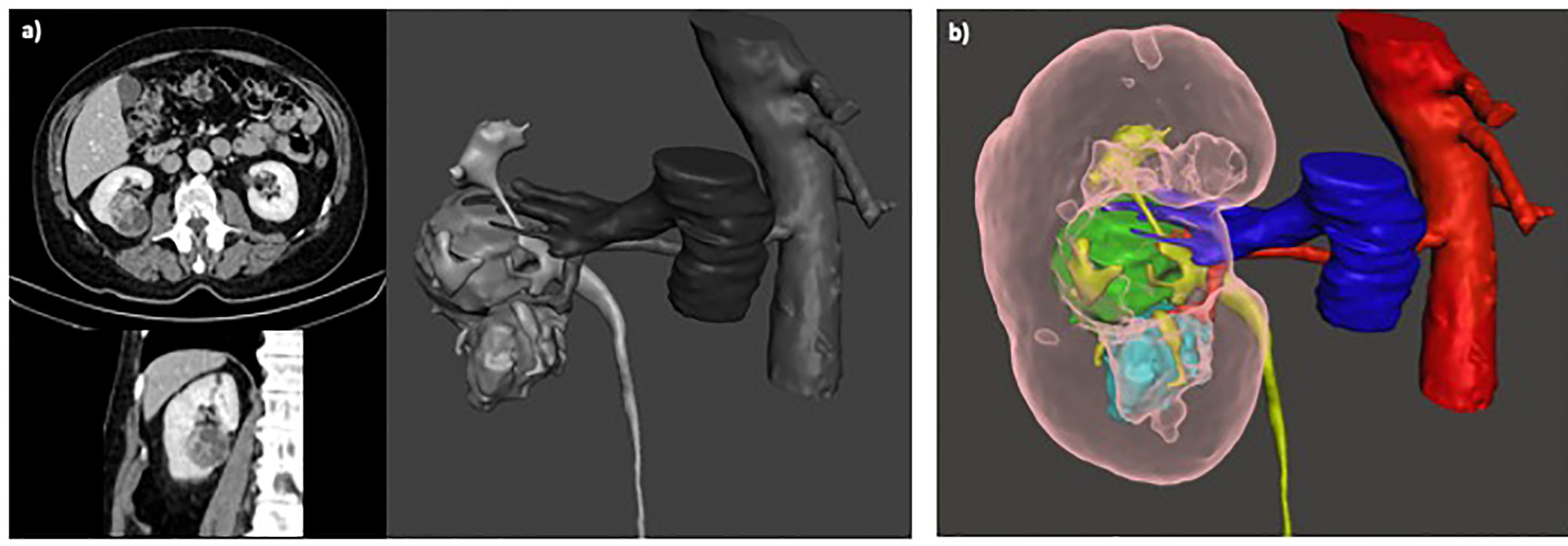
Figure 3 (A) Surgical planning of scheduled PN based on 2D imaging; (B) After revision of 3D model the planning of surgery was converted to RN before surgery, due to suspicious invasion of urinary collecting system and renal sinus.
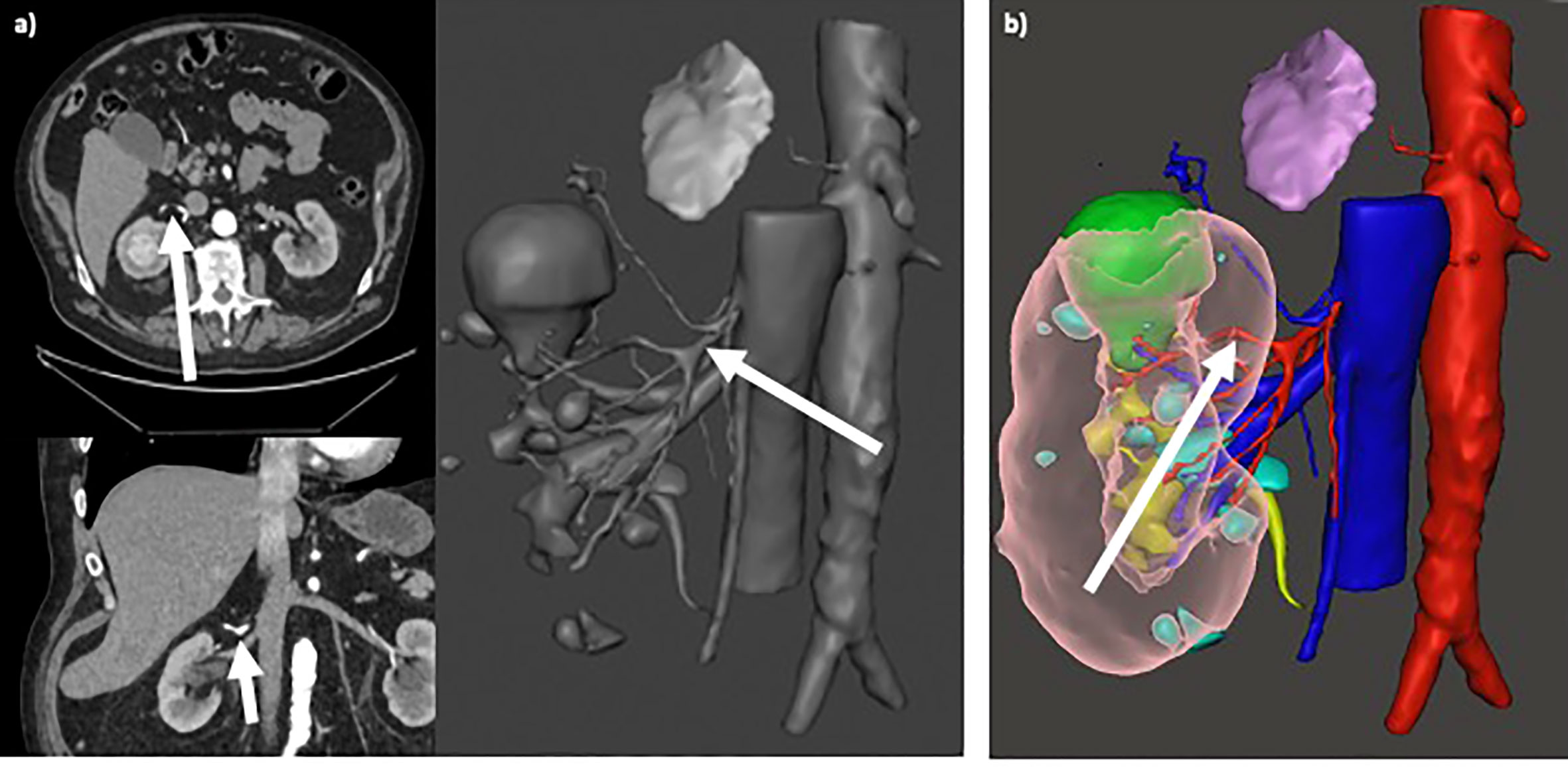
Figure 4 (A) Selective clamping of primary arterial branching (arrows in axial/coronal views and in 3D rendering), resulting from preoperative planning based on standard 2D CT imaging; (B) Super-selective clamping of tertiary arterial branching (arrow), resulting from preoperative planning based on 3D model.
Third, the use of 3D models led to improve quality of PN since the overall Trifecta achievement was significantly higher in study group (80.2%) compared to control group (63.1%). Similarly to other experience (22), in our cohort, the use of 3D model is independent predictor of Trifecta achievement even adjusting for surgical technique (6). To note, these findings may be limited by selection bias due to lacking of randomizations between the two groups, since patients in study group had higher use of robotic approach, better comorbidities profile and lower tumour’s volume compared to control group, despite no significant difference at baseline characteristics. Finally, the proportion of patients with ≤30% postoperative eGFR reduction from baseline at discharge was significantly higher in Study group. Thus, the higher adoption of selective clamping provided by 3D models may reduce the level of nephron loss due to hypoxia (32), however, long terms data are needed to assess the real effect of 3D-guided PN on renal functional outcomes.
Despite several strengths, our study is not avoided from limitations. First, the lack of randomization between the two groups. Second, the inclusion of patients who underwent surgery with open, laparoscopic and robotic approach may affect surgical outcomes. Third, 3D model reconstruction is intrinsically affected by inaccuracies due to errors that may occur for poor CT scan resolution and/or inaccurate image segmentation process. Finally, limited follow up did not allow to assess conclusion concerning the real impact of 3D-guided PN on long term residual renal function compared to standard 2D approach.
Despite such limitations, the technologic progress would improve the precision of 3D reconstruction to simplify surgical planning and the intraoperative 3D navigation during PN. In the next future, 3D virtual models may represent an essential tool for multiple needs of NSS: patients’ counseling, trainers’ education, standardize the surgical complexity, improve the efficiency of surgical planning and the quality of patient-tailored surgery.
In conclusion, the use of 3D models may improve the efficiency of surgical planning, reducing the risk of conversion to RN during surgery. Moreover, a 3D-guided approach to PN increase the adoption of selective clamping and better predict the achievement of Trifecta. Thus, the 3D virtual models applied to PN may improve the quality of surgery with potential implication on patient’s outcomes.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Approval of the research protocol by an Institutional Reviewer Board: IRB approval 3386/2018. The patients/participants provided their written informed consent to participate in this study.
LB, LC, FC, RS contributed to conception and design of the study. FC, GC, CG, AB, LT organized the database. BB, PP, MD, SB performed the statistical analysis. LB, BB, LC, EM, SL wrote the first draft of the manuscript. CG, GC, RG, EB, RS, LT, AB wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
The work reported in this publication was funded by the Italian Ministry of Health, RC-2022-2773318.
Authors acknowledge Fondazione CARISBO of Bologna (Italy).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Kane CJ, Mallin K, Ritchey J, Cooperberg MR, Carroll PR. Renal cell cancer stage migration: analysis of the national cancer data base. Cancer (2008) 113(1):78–83. doi: 10.1002/cncr.23518
2. Schiavina R, Mari A, Antonelli A, Bertolo R, Bianchi G, Borghesi M, et al. A snapshot of nephron-sparing surgery in Italy: a prospective, multicenter report on clinical and perioperative outcomes (the RECORd 1 project). Eur J Surg Oncol (2015) 41(3):346–52. doi: 10.1016/j.ejso.2014.12.001
3. Schiavina R, Mari A, Bianchi L, Amparore D, Antonelli A, Artibani W, et al. Predicting positive surgical margins in partial nephrectomy: A prospective multicentre observational study (the RECORd 2 project). Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol (2020) 46(7):1353–9. doi: 10.1016/j.ejso.2020.01.022
4. Ljungberg B, Albiges L, Abu-Ghanem Y, Bensalah K, Dabestani S, Fernandez-Pello S, et al. European Association of urology guidelines on renal cell carcinoma: The 2019 update. Eur Urol (2019) 75(5):799–810. doi: 10.1016/j.eururo.2019.02.011
5. Borghesi M, Schiavina R, Gan M, Novara G, Mottrie A, Ficarra V. Expanding utilization of robotic partial nephrectomy for clinical T1b and complex T1a renal masses. World J Urol (2013) 31(3):499–504. doi: 10.1007/s00345-013-1095-2
6. Bianchi L, Schiavina R, Borghesi M, Chessa F, Casablanca C, Angiolini A, et al. Which patients with clinical localized renal mass would achieve the trifecta after partial nephrectomy? the impact of surgical technique. Minerva Urol Nefrol (2019) 72(3):339–49. doi: 10.23736/S0393-2249.19.03485-4
7. Mir MC, Derweesh I, Porpiglia F, Zargar H, Mottrie A. Autorino r. partial nephrectomy versus radical nephrectomy for clinical T1b and T2 renal tumors: A systematic review and meta-analysis of comparative studies. Eur Urol (2017) 71(4):606–17. doi: 10.1016/j.eururo.2016.08.060
8. Bertolo R, Autorino R, Simone G, Derweesh I, Garisto JD, Minervini A, et al. Outcomes of robot-assisted partial nephrectomy for clinical T2 renal tumors: A multicenter analysis (ROSULA collaborative group). Eur Urol (2018) 74(2):226–32. doi: 10.1016/j.eururo.2018.05.004
9. Galvin DJ, Savage CJ, Adamy A, Kaag M, O’Brien MF, Kallingal G, et al. Intraoperative conversion from partial to radical nephrectomy at a single institution from 2003 to 2008. J Urol (2011) 185(4):1204–9. doi: 10.1016/j.juro.2010.11.077
10. Kara Ö, Maurice MJ, Mouracade P, Malkoç E, Dagenais J, Nelson RJ, et al. When partial nephrectomy is unsuccessful: Understanding the reasons for conversion from robotic partial to radical nephrectomy at a tertiary referral center. J Urol (2017) 198(1):30–5. doi: 10.1016/j.juro.2017.01.019
11. Bianchi L, Barbaresi U, Cercenelli L, Bortolani B, Gaudiano C, Chessa F, et al. The impact of 3D digital reconstruction on the surgical planning of partial nephrectomy: A case-control study. still time for a novel surgical trend? Clin Genitourin Cancer (2020) 18(6):e669–78. doi: 10.1016/j.clgc.2020.03.016
12. Schiavina R, Bianchi L, Borghesi M, Chessa F, Cercenelli L, Marcelli E, et al. Three-dimensional digital reconstruction of renal model to guide preoperative planning of robot-assisted partial nephrectomy. Int J Urol (2019) 26(9):931–2. doi: 10.1111/iju.14038
13. Porpiglia F, Amparore D, Checcucci E, Manfredi M, Stura I, Migliaretti G, et al. Three-dimensional virtual imaging of renal tumours: a new tool to improve the accuracy of nephrometry scores. BJU Int (2019) 124(6):945–54. doi: 10.1111/bju.14894
14. Bianchi L, Schiavina R, Bortolani B, Cercenelli L, Gaudiano C, Carpani G, et al. Interpreting nephrometry scores with three-dimensional virtual modelling for better planning of robotic partial nephrectomy and predicting complications. Urol Oncol (2021) 39(12):836.e1–9. doi: 10.1016/j.urolonc.2021.07.024
15. Bianchi L, Schiavina R, Bortolani B, Cercenelli L, Gaudiano C, Mottaran A, et al. Novel volumetric and morphological parameters derived from three-dimensional virtual modeling to improve comprehension of tumor’s anatomy in patients with renal cancer. Eur Urol Focus (2021) S2405-4569(21)00217. doi: 10.1016/j.euf.2021.08.002
16. Martini A, Falagario UG, Cumarasamy S, Jambor I, Wagaskar VG, Ratnani P, et al. The role of 3D models obtained from multiparametric prostate MRI in performing robotic prostatectomy. J Endourol (2022) 36(3):387–93. doi: 10.1089/end.2021.0541
17. Falagario U, Veccia A, Weprin S, Albuquerque EV, Nahas WC, Carrieri G, et al. Robotic-assisted surgery for the treatment of urologic cancers: recent advances. Expert Rev Med Devices (2020) 17(6):579–90. doi: 10.1080/17434440.2020.1762487
18. Schiavina R, Bianchi L, Lodi S, Cercenelli L, Chessa F, Bortolani B, et al. Real-time augmented reality three-dimensional guided robotic radical prostatectomy: Preliminary experience and evaluation of the impact on surgical planning. Eur Urol Focus (2020) 7(6):1260–7. doi: 10.1016/j.euf.2020.08.004
19. Bianchi L, Chessa F, Angiolini A, Cercenelli L, Lodi S, Bortolani B, et al. The use of augmented reality to guide the intraoperative frozen section during robot-assisted radical prostatectomy. Eur Urol (2021) 80(4):480–8. doi: 10.1016/j.eururo.2021.06.020
20. Porpiglia F, Fiori C, Checcucci E, Amparore D, Bertolo R. Hyperaccuracy three-dimensional reconstruction is able to maximize the efficacy of selective clamping during robot-assisted partial nephrectomy for complex renal masses. Eur Urol (2018) 74(5):651–60. doi: 10.1016/j.eururo.2017.12.027
21. Schiavina R, Bianchi L, Chessa F, Barbaresi U, Cercenelli L, Lodi S, et al. Augmented reality to guide selective clamping and tumor dissection during robot-assisted partial nephrectomy: A preliminary experience. Clin Genitourin Cancer (2020) 19(3):e149–55. doi: 10.1016/j.clgc.2020.09.005
22. Michiels C, Khene Z-E, Prudhomme T, Boulenger de Hauteclocque A, Cornelis FH, Percot M, et al. 3D-image guided robotic-assisted partial nephrectomy: a multi-institutional propensity score-matched analysis (UroCCR study 51). World J Urol (2021) 2. doi: 10.1007/s00345-021-03645-1
23. Hung AJ, Cai J, Simmons MN, Gill IS. “Trifecta” in partial nephrectomy. J Urol (2013) 189(1):36–42. doi: 10.1016/j.juro.2012.09.042
24. Borghesi M, Schiavina R, Chessa F, Bianchi L, La Manna G, Porreca A, et al. Retroperitoneal robot-assisted versus open partial nephrectomy for cT1 renal tumors: A matched-pair comparison of perioperative and early oncological outcomes. Clin Genitourin Cancer (2017) 16(2):e391–6. doi: 10.1016/j.clgc.2017.09.010
25. Schiavina R, Borghesi M, Chessa F, Rizzi S, Martorana G. [Predictors of positive surgical margins after nephron-sparing surgery for renal cell carcinoma: retrospective analysis on 298 consecutive patients]. Urologia (2014) 81(1):40–5. doi: 10.5301/uro.5000061
26. Novara G, La Falce S, Kungulli A, Gandaglia G, Ficarra V, Mottrie A. Robot-assisted partial nephrectomy. Int J Surg (2016) 36(Pt C):554–9. doi: 10.1016/j.ijsu.2016.05.073
27. Ficarra V, Novara G, Secco S, Macchi V, Porzionato A, De Caro R, et al. Preoperative aspects and dimensions used for an anatomical (PADUA) classification of renal tumours in patients who are candidates for nephron-sparing surgery. Eur Urol (2009) 56(5):786–93. doi: 10.1016/j.eururo.2009.07.040
28. Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol (2009) 182(3):844–53. doi: 10.1016/j.juro.2009.05.035
29. Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg (2004) 240(2):205–13. doi: 10.1097/01.sla.0000133083.54934.ae
30. Mitropoulos D, Artibani W, Graefen M, Remzi M, Rouprêt M, Truss M. Reporting and grading of complications after urologic surgical procedures: an ad hoc EAU guidelines panel assessment and recommendations. Eur Urol (2012) 61(2):341–9. doi: 10.1016/j.eururo.2011.10.033
31. Anceschi U, Ferriero MC, Tuderti G, Brassetti A, Bertolo R, Capitanio U, et al. Head to head impact of margin, ischemia, complications, score versus a novel trifecta score on oncologic and functional outcomes after robotic-assisted partial nephrectomy: Results of a multicenter series. Eur Urol Focus (2021) 7(6):1391–9. doi: 10.1016/j.euf.2020.06.021
32. Simone G, Gill IS, Mottrie A, Kutikov A, Patard J-J, Alcaraz A, et al. Indications, techniques, outcomes, and limitations for minimally ischemic and off-clamp partial nephrectomy: a systematic review of the literature. Eur Urol (2015) 68(4):632–40. doi: 10.1016/j.eururo.2015.04.020
33. Khandwala YS, Jeong IG, Kim JH, Han DH, Li S, Wang Y, et al. The incidence of unsuccessful partial nephrectomy within the united states: A nationwide population-based analysis from 2003 to 2015. Urol Oncol (2017) 35(12):672.e7–672.e13:. doi: 10.1016/j.urolonc.2017.08.014
Keywords: 3D model, surgical planning, surgical outcomes, renal cancer, partial nephrectomy
Citation: Bianchi L, Cercenelli L, Bortolani B, Piazza P, Droghetti M, Boschi S, Gaudiano C, Carpani G, Chessa F, Lodi S, Tartarini L, Bertaccini A, Golfieri R, Marcelli E, Schiavina R and Brunocilla E (2022) 3D renal model for surgical planning of partial nephrectomy: A way to improve surgical outcomes. Front. Oncol. 12:1046505. doi: 10.3389/fonc.2022.1046505
Received: 16 September 2022; Accepted: 07 October 2022;
Published: 21 October 2022.
Edited by:
Francesco Chierigo, San Martino Hospital (IRCCS), ItalyReviewed by:
Ugo Giovanni Falagario, University of Foggia, ItalyCopyright © 2022 Bianchi, Cercenelli, Bortolani, Piazza, Droghetti, Boschi, Gaudiano, Carpani, Chessa, Lodi, Tartarini, Bertaccini, Golfieri, Marcelli, Schiavina and Brunocilla. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lorenzo Bianchi, bG9yZW56by5iaWFuY2hpM0BnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.