
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol., 17 November 2022
Sec. Cancer Imaging and Image-directed Interventions
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.1043688
This article is part of the Research TopicMR-guided Focused Ultrasound Treatment Techniques in Cancers: from Physics to ClinicsView all 7 articles
Established therapies for prostate cancer (PCa), surgery and radiotherapy, treat the entire gland regardless of the location of the cancerous lesion within the prostate. Although effective, these methods include a significant risk of worsening genitourinary outcomes. Targeted image-guided cancer therapy has gained acceptance through improved PCa detection, localization, and characterization by magnetic resonance imaging (MRI). Minimally-invasive ablative techniques aim to achieve comparable oncological outcomes to radical treatment while preserving genitourinary function. Transurethral ultrasound ablation (TULSA) and next-generation transrectal high-intensity focused ultrasound (HIFU) utilize MRI guidance to thermally ablate prostate tissue under real-time MRI monitoring and active temperature feedback control. Previous trials performed by our group and others, including a large multicenter study in men with localized favorable-risk disease, have demonstrated that TULSA provides effective prostate ablation with a favorable safety profile and low impact on quality of life. Recently, MRI-guided HIFU focal therapy was also shown as a safe and effective treatment of intermediate-risk PCa. Here we review the current literature on ablative techniques in the treatment of localized PCa with a focus on TULSA and HIFU methods.
Prostate cancer (PCa) is the second most common cancer in men worldwide (1) and mainly a disease of older age groups. Due to the development of diagnostics and increased PCa awareness, the condition is diagnosed earlier and at a younger age (2). Moreover, the prostatic tumor affecting prognosis can be frequently visualized with newer imaging methods, becoming a target for imaging-guided cancer therapy.
Although PCa is the sixth leading cause of cancer-related death in men worldwide, the disease is frequently chronic in a large proportion, and many men die of reasons other than PCa (1, 3). In addition to the development of diagnostics and treatments, this is explained by the relatively slow natural course of PCa. The oncological benefits of local treatments with radical goals are visible after decades, while the genitourinary adverse effects typically appear shortly after these treatments (4). Given the relatively long PCa-specific survival, many radically treated men have to cope with treatment-related comorbidities for a long time (4).
At the time of diagnosis, many PCas are organ-confined with a favorable prognosis, lacking many of the characteristics typical of cancer; including the ability to grow beyond the prostate capsule or metastasize (5). These favorable risk cases can be safely monitored within surveillance protocols (6). More aggressive, Gleason grade ≥ 3 + 4 and ISUP (International Society of Urological Pathology) grade group (GG) ≥ 2 PCas are treated with curative goals if the patient’s life expectancy is long enough, 8-15 years, depending on the risk category. In addition to histopathology, the widely used risk classification of the European Association of Urology takes into account the level of prostate-specific antigen (PSA) and the local extent of PCa based on digital rectal examination of the prostate (6). Based on these variables, localized PCa is divided into a group of low (clinically insignificant), intermediate or high risk of recurrence after local radical intent therapy (6).
The established treatment methods for localized PCa, surgery and radiotherapy, are effective treatments but frequently cause significant long-term adverse effects on urinary, sexual, and bowel functions (7). In both treatment methods, the risk of recurrence increases according to the risk category of the disease. Local recurrence after surgery is treated with radiotherapy of the surgical bed, in some instances including pelvic lymph node regions, and with or without androgen deprivation therapy (ADT) (6). Local treatments for locally relapsing PCa after radiotherapy are more challenging. The available salvage treatments, surgery and additional radiotherapy, are associated with high risks of significant complications and quality of life harms concerning the benefits achieved. Therefore, in many cases, recurrence is first monitored and ADT is initiated as the disease progresses. In ADT, the serum testosterone is lowered to the castration level, or the effect of testosterone in the target tissue is prevented. The treatment is associated with significant deterioration of quality of life and an increased risk of cardiovascular problems (8). In some patients, the recurrence might be curable with local treatments and ADT could be postponed to the future. In addition to the toxicity of retreatments, the limited use of salvage treatments has partly been explained by the inability of traditional diagnostic methods to differentiate between local and metastatic recurrence reliably.
With new magnetic resonance imaging (MRI) methods and positron emission tomography-computed tomography (PET-CT), PCa detection and disease spread assessment has become more accurate. Recurrences can be located more reliably in PET-CT with PSMA (Prostate Specific Membrane Antigen) as a target, even with low PSA values. In this way, patients who might benefit from local re-treatments are found at an earlier stage. It has been hypothesized that new ablation treatment methods and focal therapy approaches can be used to treat primary localized PCa and locally recurrent PCa after radiotherapy radically, with less tissue damage, and improved quality of life. Preliminary scientific evidence has supported this assumption, and imaging-guided ablation treatments have gained approval and offer selected patients a potentially more optimal treatment option considering the benefit-risk ratio.
The purpose of this study is to review the current literature on ablation techniques for localized PCa with a particular focus on treatment methods that use therapeutic ultrasound and real-time MRI guidance for treatment delivery.
In the conventional diagnostic pathway, the suspicion of PCa is based on an elevated PSA value and abnormal palpation of the prostate gland. The diagnosis is confirmed by histopathological examination of tissue samples taken from the prostate under ultrasound guidance. It is often not possible to reliably distinguish PCa with ultrasound. Therefore, 10-12-core template systematic biopsies are typically taken to cover the peripheral zone of the prostate where up to 80% of PCas originate. The challenge of traditional diagnostic tools, however, is insufficient accuracy in finding clinically significant PCa affecting the patient’s prognosis.
Over the last ten years, prostate MRI has revolutionized PCa diagnostics. High-quality prospective studies have demonstrated the ability of MRI and MRI-targeted biopsy to detect and exclude clinically significant PCa and to ignore clinically insignificant PCa (9). MRI has also refined the assessment of the local spread of PCa (9).
Bone scintigraphy and CT of the body are used to investigate the distant spread of PCa. The methods have low sensitivity and specificity in identifying metastases (10). Alongside traditional methods, PSMA PET-CT has emerged and taken the diagnostics from the macro to the molecular level. PSMA is a type II transmembrane glycoprotein, the amount of which increases in PCa depending on the aggressiveness (11, 12). A recent randomized study showed that PSMA PET-CT is a 27% more accurate method than bone scintigraphy and CT in the primary staging of men with high-risk PCa (10). PSMA PET-CT has also proven to be a promising method for prostate tumor identification and local spread assessment (13–16).
In primary localized clinically significant PCa, the standard is the treatment of the entire gland, regardless of the location of the cancerous tumor in the prostate. Established treatment methods include surgery (open, laparoscopic, robot-assisted radical prostatectomy), external beam radiotherapy, and brachytherapy (6). Even though surgery and radiotherapy techniques have evolved, they still carry risks of adverse effects on the urinary and genital organs and the bowel as the structures that maintain these functions in the vicinity of the prostate will be damaged during the treatments (4, 7). In more detail, compared with active monitoring, there is a significantly higher risk of sexual dysfunction (95%) and urinary incontinence (55%) six months after surgery, and of sexual (88%) and bowel dysfunction (5%) after radiotherapy (4). In a more contemporary population-based observational study (n=2005), up to half of patients treated with surgery or radiotherapy experienced severe erectile dysfunction and 10% suffered from long-term urinary incontinence (7). This has aroused interest in prostate tissue-sparing treatment and focal therapy, which aims to selectively eradicate clinically significant cancer to reduce the risk of metastasis without causing adverse effects that affect the quality of life (17).
A minority (15-30%) of PCa are unifocal and/or unilateral, confined to a specific part of the prostate, and traditionally considered suitable for focal therapy (18–20). In multifocal PCa, the cancer foci differ in aggressiveness (21–23). Evidence indicates that the prognosis is determined mainly by the so-called index tumor, which is typically the largest in size and harbors the highest grade (24–28). MRI and PSMA PET-CT reliably identify this index tumor (9, 13). Treating the most aggressive tumor in multifocal diseases might be sufficient to control the disease with fewer adverse effects (28, 29).
Although imaging methods for PCa have developed tremendously, the challenge of focal therapy is the accuracy of the imaging methods to identify all clinically significant cancer foci that shall be treated to minimize the risk of recurrence and metastatic spread of the disease. For example, MRI reliably identifies the index tumor but frequently misses smaller significant lesions in multifocal diseases (30–33). The comparison of preoperative MRI data and histopathology of radical prostatectomy specimens has also shown that MRI significantly underestimates tumor size; a margin of up to 12 mm is needed around the tumor determined in MRI for cancer to be radically treated histopathologically (34–36).
In recent decades, imaging-guided ablative treatment methods have been developed (37). In addition to the aforementioned limitations of cancer imaging, another challenge has been the accuracy of the energy delivery system. These two requirements are necessary to estimate precise treatment margins and to optimize the oncological and functional outcomes. Promisingly, combining MRI with newer generation ablation methods has improved the treatment’s accuracy.
In ablation treatments, tissue-destroying energy is delivered to the prostate gland without a surgical wound. Most ablative methods utilize thermal energy to ablate prostate tissue, typically heating prostate tissue with radiofrequency, laser, or therapeutic ultrasound (37). Most research evidence has been accumulated on older generation ultrasound-guided high-intensity focused ultrasound (HIFU), cryoablation, irreversible electroporation, focal laser ablation and photodynamic therapy. High and low dose rate brachytherapy (HDR, high dose rate; LDR, low dose rate) and stereotactic ablative radiotherapy (SABR) have also been used in focal therapy. Other ablative treatment options, such as laser interstitial thermotherapy, radiofrequency ablation, and prostatic artery embolization have also been utilized for the treatment of localized PCa, but they are in the early phase of evaluation with a limited amount of data available (37). In general, ablative methods are less invasive than surgery and seem to have a more favorable side effect profile compared to traditional treatments (4, 7, 37). In focal ablative therapy, the treatment-emergent harm is expected to decrease even more. In the medium term, the oncological efficacy of focal ablative therapy seems non-inferior when compared to whole-gland treatment (38, 39). However, the patient selection criteria and the protocols for post-operative surveillance after ablation therapy and focal therapy approach remain to be established and it is evident that novel imaging methods will play a key role.
MRI-guided therapeutic ultrasound has been explored in the treatment of various benign and malignant solid tumors (40, 41). Recently, a novel MRI-guided transurethral ultrasound ablation (TULSA) (TULSA-PRO, Profound Medical Inc., Mississauga, Canada) has been evaluated in the treatment of various PCa conditions (42) (Figure 1). In contrast to a series of rapid small volume exposures in HIFU, directional ultrasound utilized in TULSA technology has distinct patterns of thermal dose and temperature deposition and subsequent tissue damage due to the use of continuous heating with an unfocused ultrasound beam (43). Both TULSA and HIFU performed inside the MRI scanner, known as magnetic resonance guided focused ultrasound (MRgFUS) (ExAblate; Insightec; Miami, FL, USA), exploit MRI guidance to thermally ablate prostate tissue under real-time MRI monitoring and active temperature feedback control. In addition to the MRI-thermometry derived thermal mapping, the treatment success can be assessed immediately post-treatment using gadolinium-enhanced images to visualize the acute perfusion defect caused by the treatment, denoted as non-perfused volume (NPV) (40, 43) (Figure 2 and Supplementary Figure S1). Immediate NPV, however, underestimates the extent of thermal injury substantially since it cannot observe delayed thermal injury (43).
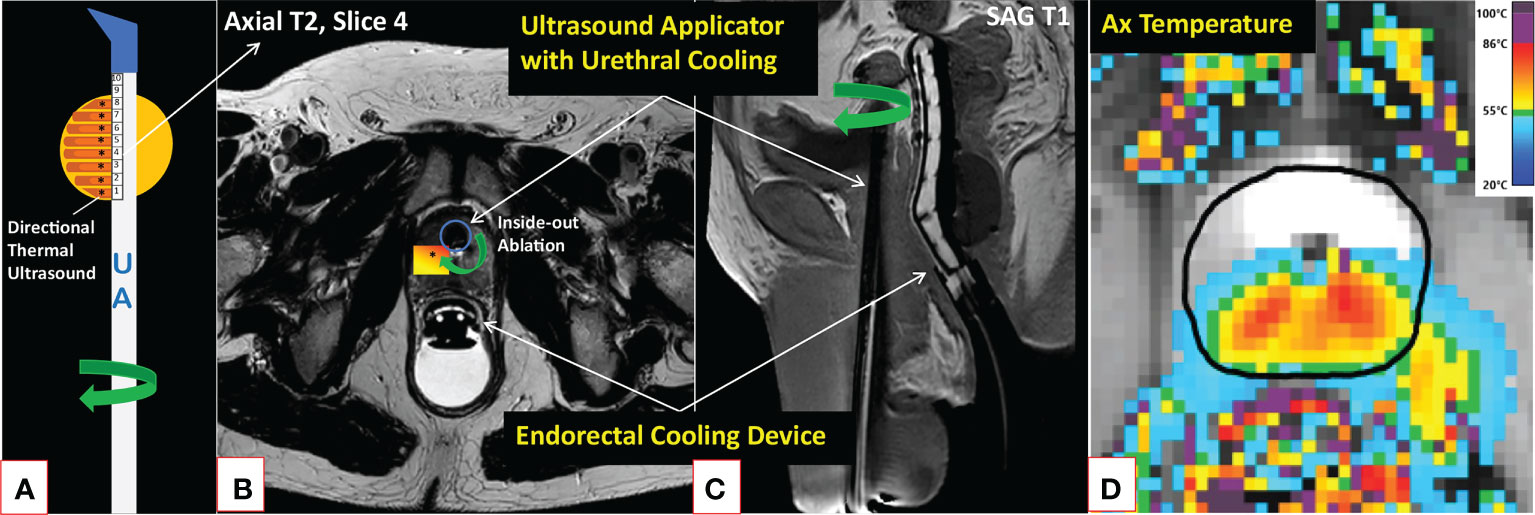
Figure 1 Description of the TULSA technology. MRI-guided TULSA is a minimally invasive ablation technique delivering directional high-intensity ultrasound energy (*) to the prostate (yellow circle) using a transurethral rotational UA comprised of 10 independently controlled ultrasound elements (A). By actively cooling both the urethra and rectum throughout the ablation, TULSA protects these structures from thermal injuries (B, C). Real-time MRI-thermometry is continuously acquired during the ablation to automatically control the delivered lethal thermal energy by adjusting each ultrasound element’s frequency and power and the UA’s rotation rate (D). On the axial maximum temperature image of a patient undergoing lesion-targeted TULSA of a posterior peripheral zone tumor (D), a minimum lethal temperature of 55°C reaches the drawn (black) boundary. Due to prostate swelling caused by the ablation, the catheter is kept in place for weeks after the procedure (see Figure 2 patient case with a suprapubic catheter). MRI, magnetic resonance imaging; TULSA, transurethral ultrasound ablation; UA, ultrasound applicator.
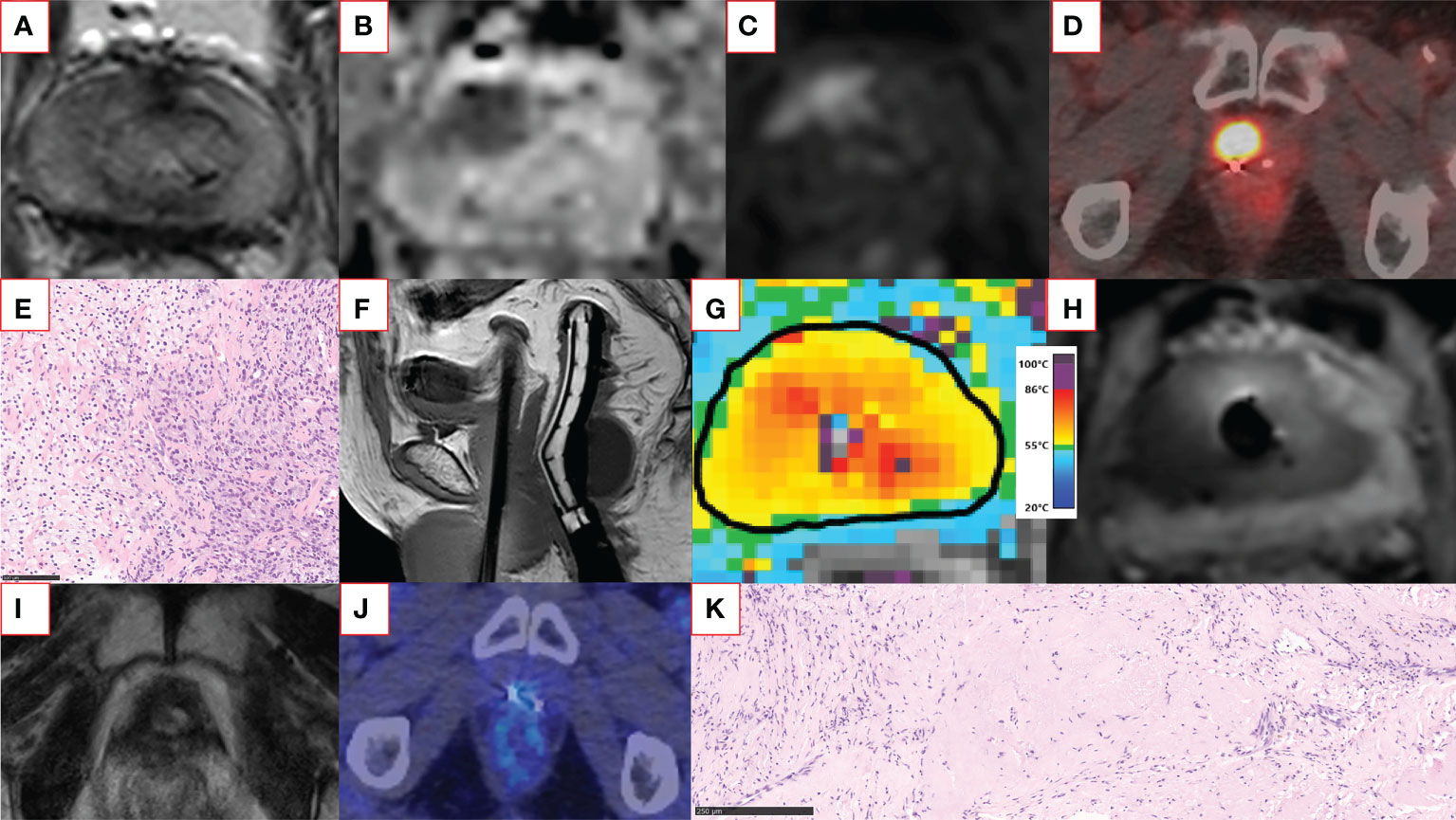
Figure 2 An example of a successful salvage TULSA patient case. The patient had a rising PSA of up to 13 ng/ml within six years after primary external beam radiotherapy. Screening T2-weighted (A) and diffusion-weighted (B) MRI showed a distinct anterior lesion with early enhancement on gadolinium-enhanced imaging (C) graded as PI-RR 5 lesion. The same lesion was also clearly visible in 18F-PSMA-1007 PET-CT (maximum standardized uptake value 23) (D). Two residual gold fiducial markers implanted before image-guided radiotherapy are also visible next to the PSMA-positive lesion (D). The MRI-targeted biopsy from the lesion revealed vital carcinoma resembling ISUP GG 5 disease (E). The patient underwent whole-gland TULSA. On the sagittal T1-weighted image (F), a transurethrally inserted ultrasound applicator, endorectal cooling device, and suprapubic catheter are in place. The targeted region reached a lethal minimum temperature of 55°C (G). The non-perfused volume can be visualized immediately after treatment, demonstrating the acute ablation effect covering the targeted lesion (H). At 12 months, the patient underwent follow-up imaging with multiparametric MRI and 18F-PSMA-1007 PET-CT (I, J), both negative for cancer. The prostate volume decreased from 20 cm3 to less than 1 cm3 at 12 months. The 12-month post-TULSA biopsy agreed with imaging findings and showed only a treatment effect with no signs of cancer (K). At the recent follow-up visit two years after TULSA, PSA is still low (PSA 0.067 ng/ml) and stable, and the patient has leak- and pad-free continence and erections sufficient for intercourse. The TULSA treatment report of the patient case, including treatment planning, thermal mapping, and post-treatment gadolinium-enhanced images, is provided in Supplementary Figure S1. CT, computed tomography; ISUP GG, International Society of Urological Pathology grade group; MRI, magnetic resonance imaging; PET, positron emission tomography; PI-RR, Prostate Imaging for Recurrence Reporting; PSA, prostate-specific antigen; PSMA, prostate-specific membrane antigen; TRUS, transrectal ultrasound; TULSA, Transurethral ultrasound ablation.
MRI-guided ultrasound ablation methods can be considered versatile, as they enable therapy for the entire prostate gland or more locally and in several different indications, such as palliation, salvage, and benign prostatic obstruction (42–46) (Figures 1, 2). MRI-guided ultrasound ablation can often also be renewed and the therapy does not prevent surgery or radiotherapy later (37, 42, 43). The main contraindications for MRI-guided ultrasound ablation are the same as for MRI. In addition, the size of the prostate, calcifications, cysts, or post-radiation fiducial seeds may limit the successful implementation of the treatment. Limitation of MRI-guided ultrasound ablation methods include the relative complex technical requirements of the devices including the prolonged in-bore magnet time and MR-compatible anesthesia equipment, which in turn carry additional costs.
The largest prospectively collected cohort from ablative therapy of localized PCa is for whole-gland ultrasound-guided HIFU. The medium-term results of the data set (n=1002) were published in 2014 (47). At eight years, 76% of low-risk, 63% of intermediate-risk, and 57% of high-risk patients were biochemically disease-free (PSA<nadir plus two ng/ml). At a ten-year follow-up, the cancer-specific survival rate was 97%, and metastases were found in 6%. The erectile function was preserved in half, and severe urinary incontinence occurred in 3-6% and urinary disorders in 6-35%, depending on whether older or newer ultrasound-guided HIFU technology was used. It is noteworthy that ADT was used to reduce the prostate size in 39% of patients, and 38% received two and 2% three HIFU treatments.
In Britain, high-quality, prospective cohort studies have been conducted on ablative methods in treating localized PCa. Failure-free survival (FFS) has been used as the primary endpoint, defined as survival without local or systemic treatments, metastases, or PCa death. Two ablation treatments were allowed to measure the effectiveness. A study published in 2018 reported the five-year PCa treatment results of focal HIFU (n=625) with five-year FFS of 88% (48). The corresponding result from whole-gland HIFU (n=754) was 70% (49). When taking the ISUP GG into account, FFS was 92% in GG 1 disease, 87% in GG 2-3 disease, and 59% in GG 4-5 disease (48). Metastases-free survival was 98%, cancer-specific survival 100%, and overall survival 99%. Complications included infections (~10%) and endoscopic procedures due to urinary disorders (~10%), while 30% of patients had to undergo endoscopic procedures in whole-gland HIFU. The rectourethral fistula was reported in two patients, similar to whole-gland HIFU. Urinary incontinence occurred in only 2% of patients, while in whole-gland HIFU, incontinence occurred in 12% of patients. Of note, in both studies ADT was used to downsize the prostate (48, 49).
Lately, Bründl et al. reported long-term oncological outcomes of 560 patients undergoing whole-gland ultrasound-guided HIFU with a median follow-up of 15 years and a range of up to 21 years. At 15 years, the cancer-specific and metastasis-free survival rates for low-, intermediate-, and high-risk patients were 95%, 89%, 65%, and 91%, 85%, and 58%, respectively (50). The corresponding percentages for salvage treatment-free survival were 67%, 52%, and 28%, respectively. Reddy et al. also recently reported longer-term oncological outcomes of 1379 patients undergoing focal ultrasound-guided HIFU with a median follow-up of 32 months (51). Seven-year FFS for low-, intermediate- and high-risk patients was 88%, 68%, and 65%, with 18% of patients undergoing repeat focal treatment and 7% undergoing salvage whole-gland treatment. At seven years, salvage whole-gland and systemic treatment-free, metastasis-free, cancer-specific, and overall survival rates were 75%, 100%, 100%, and 97%. Clavien Dindo adverse events greater than two occurred in 0.5% of patients.
In 2021, two retrospective studies compared the efficacy of focal therapy and traditional treatments in intermediate-risk PCa. Another study compared focal therapy (n=530, HIFU/cryotherapy/HDR-brachytherapy) with surgery (n=390) and radiotherapy (n=440) (52) and another compared focal therapy (n=246, HIFU/cryotherapy) with surgery alone (n= 246) (53). In both studies, the groups were compared using the propensity score matching method regarding significant prognostic factors. Neither study showed a demonstrable difference in efficacy (FFS) between the treatment groups at six and eight years. The only statistically significant difference was in the overall survival in favor of focal therapy. Of note, these two retrospective studies likely have included overlapping patient populations.
More recently, the results of a multicenter phase 2 study on the MRgFUS for patients with intermediate-risk PCa (n=101, 78% of patients with ISUP GG 2 disease) were published (54). Twenty-four-month biopsy outcomes demonstrated that the focal therapy with MRgFUS is a safe and effective treatment for ISUP GG 2 and 3 PCa with minimal deterioration of functional outcomes. Grade 1-2 urinary incontinence and erectile dysfunction occurred in 18% and 20% of patients. However, all patients reported pad-free continence and only a minor clinically insignificant decrease in functional erections by 24 months. At 24 months, 78 of 89 (88%) men had no evidence of ISUP GG 2 or higher PCa in the biopsies obtained from the treatment region and 59 of 98 (60%) men had no evidence of ISUP GG 2 or higher PCa anywhere in the prostate.
In a recent phase 2 multicenter study (TACT-trial), the efficacy of whole-gland TULSA treatment was demonstrated in low- and intermediate-risk PCa (n=115, 63% of patients with ISUP GG 2 disease) (55). At one-year biopsy , 72 of 111 (65%) men had no longer demonstrable cancer, and 14% had clinically insignificant disease (small volume ISUP 1 disease). Moreover, 52 of 68 (76%) men with ISUP GG 2 PCa at baseline were free of ISUP GG 2 disease anywhere in the prostate on 12-month biopsy. No severe complications occurred. Eight percent of patients had an infection or a urinary disorder as complications, 75% of patients maintained an erection sufficient for sexual intercourse without medication, and 96% of patients were continent at one year.
Safety and functional outcomes of the prospective and large retrospective studies evaluating ultrasound ablation of primary localized PCa are summarized in Table 1. The functional outcomes of these studies compare favorably to contemporary functional outcomes after surgery and radiotherapy (7). In a prospective, population-based cohort study from the United States including 1386 men with favorable-risk PCa, only 28% of men undergoing nerve-sparing radical prostatectomy and 51% of men undergoing external beam radiotherapy reported erections sufficient for penetration one year after treatment and 50% reported urinary incontinence requiring pad use one year after radical prostatectomy.
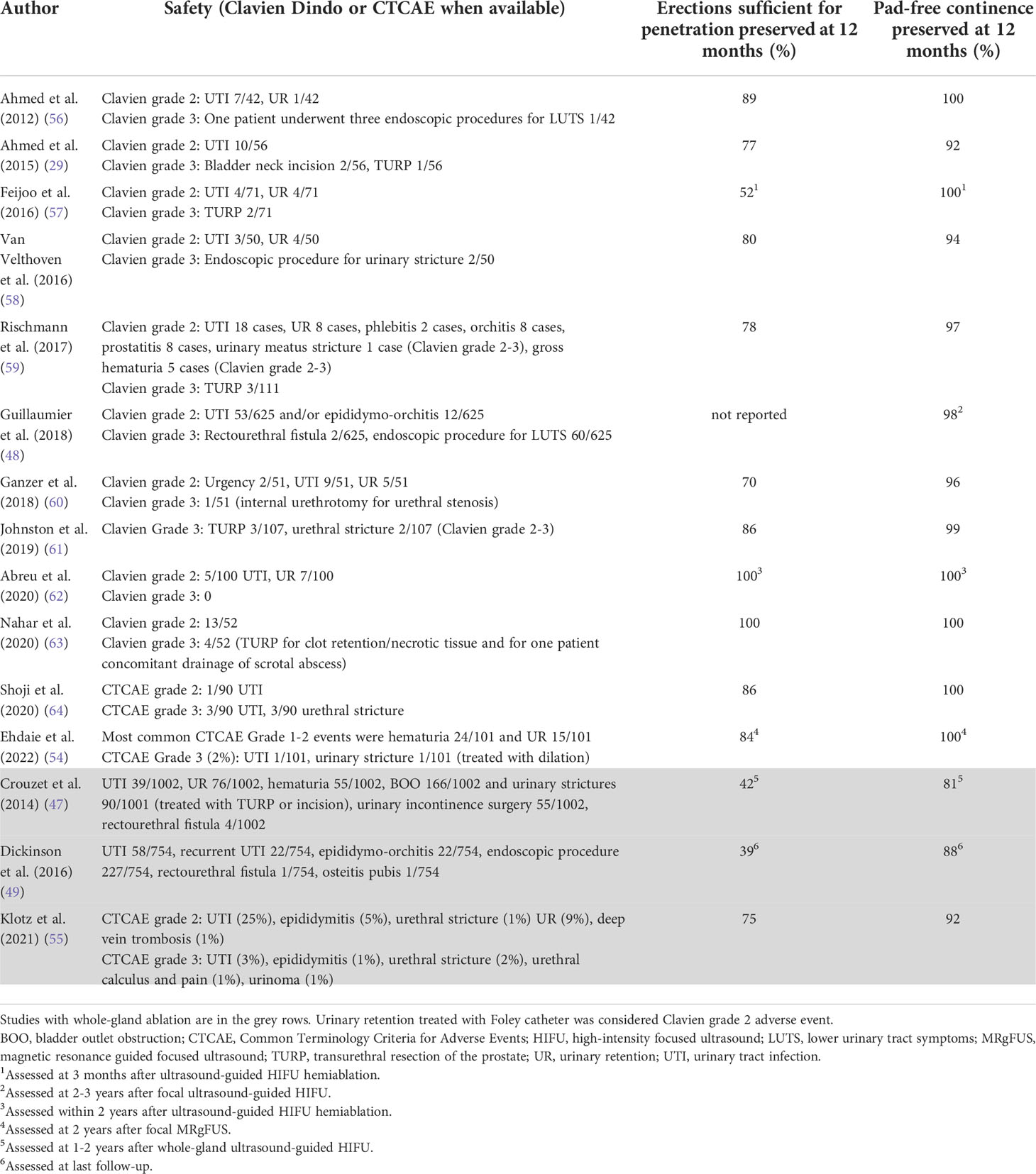
Table 1 Safety and functional outcomes of the studies evaluating ultrasound ablation of primary localized prostate cancer.
Focal and whole-gland prostate ablation treatment has been compared with traditional therapies in several systematic literature reviews and meta-analyses (37, 65, 66). However, without further prospective comparative, preferably randomized, studies, no firm conclusions can be drawn about the efficacy of ablation treatments relative to standard therapy. Most ablation studies have been done with small, single-center, and often retrospective datasets, where the follow-up periods have been short (Table 2). This might change shortly because, at least in the United States, a randomized multicenter study comparing TULSA treatment with surgery in intermediate-risk PCa is ongoing (NCT05027477).
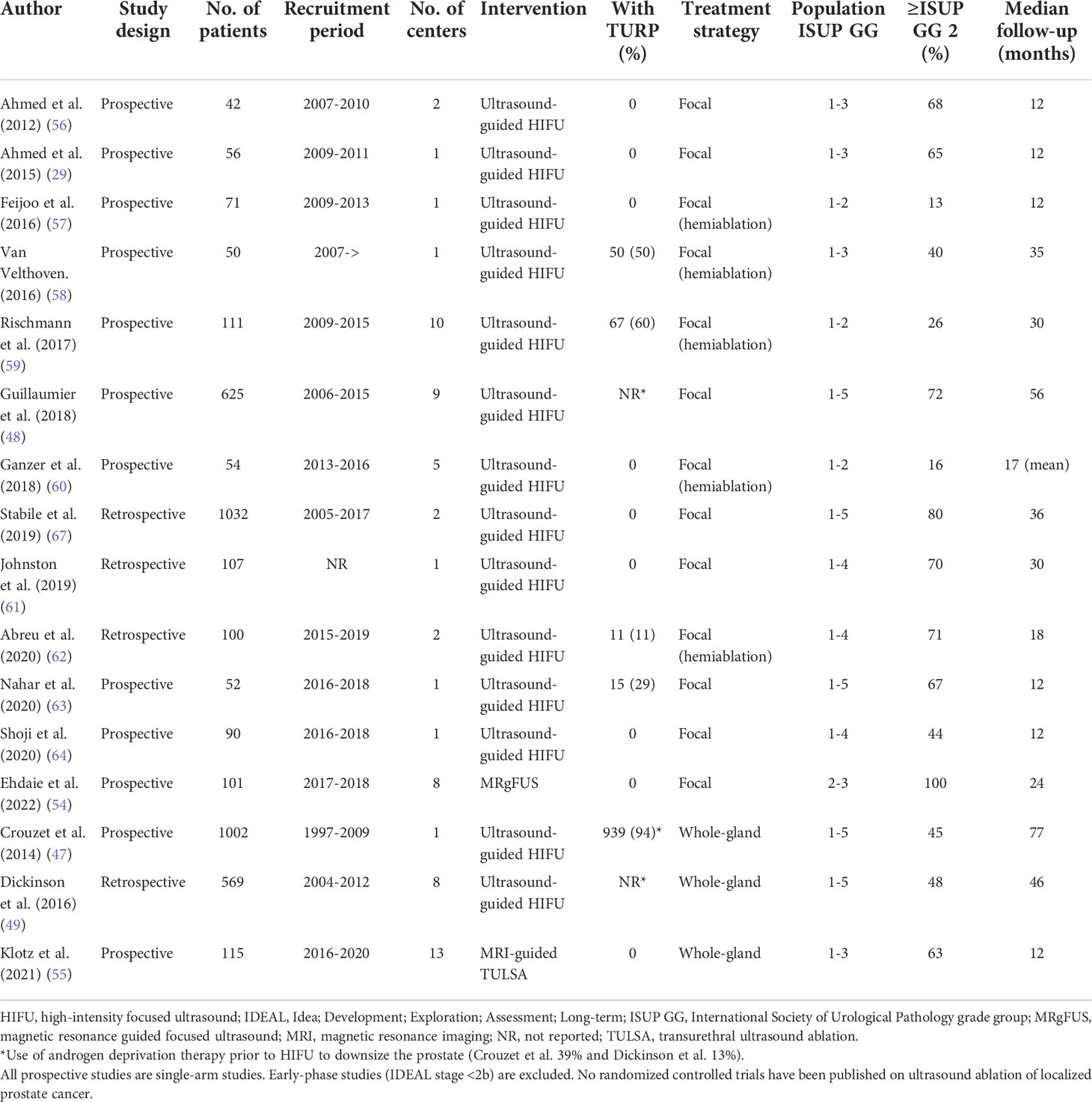
Table 2 Prospective and largest retrospective studies evaluating ultrasound ablation of primary localized prostate cancer.
Up to 50% of PCa patients treated with radiotherapy will eventually present recurrence biochemically, which typically precedes clinical relapse (68). However, only a few receive salvage treatments due to their significant toxicity and limited cancer therapeutic efficacy (68). Part of the reason has been the paucity of different salvage treatment methods available. Surgery and additional radiotherapy have been available for a long time but only a few patients are eligible for salvage radical prostatectomy due to their general condition and comorbidities. In addition to these methods, most research evidence has been accumulated on ultrasound-guided HIFU and cryotherapy. A recent meta-analysis has compared the salvage methods listed above (69). Depending on the method, 61-100% of salvage treatments were given to the entire gland. Severe urogenital adverse effects occurred in 4-23% and intestinal in 0-2% of the patients. Toxicity seems to be the least significantly associated with HDR brachytherapy and SBRT; however, these methods were mostly used for focal therapy which may explain the differences. There was no significant difference in the therapeutic efficacy of cancer control between the salvage methods, and the effectiveness can be considered limited. Depending on the salvage method, 40-50% of patients presented disease recurrence during the five-year follow-up. More accurate patient selection with modern imaging methods may improve treatment outcome in the future and with newer ablation methods and focal treatment strategy, a better benefit-harm ratio may be achieved even in the salvage setting. In an early phase study from our group TULSA was shown to be safe and feasible treatment approach for whole-gland and focal ablation of radiorecurrent PCa with promising one-year oncological control (45) (Figure 2 and Supplementary Figure S1). However, more studies with larger populations and longer follow-up are required to validate the efficacy of this treatment method in salvage indication.
Ablative methods and focal treatment strategy seem to have a more favorable side effect profile than standard whole-gland treatments in primary localized and locally recurrent PCa after radiotherapy. In the medium term, focal therapy seems to be as effective as a treatment of the whole gland, at least in the primary treatment of intermediate-risk PCa. Recent observational cohort studies have shown no clinically or statistically significant difference in treatment response between radical and focal therapy. However, the scientific evidence for ablative methods and the usefulness of focal therapy is still limited, and the long-term efficacy has not been demonstrated. Randomized controlled studies are still needed to compare standard whole-gland treatments to ablative methods. In locally recurrent PCa after radiotherapy, with limited treatment options, ablation therapy offers a new treatment option in well-selected patients.
MA: conceptualization, data curation, formal analysis, investigation, and writing—original draft. RBS: resources and investigation, funding acquisition, resources, supervision, writing—review and editing. PB: resources and investigation, funding acquisition, resources, supervision, writing—review and editing. PT: resources and investigation, funding acquisition, resources, supervision, writing—review and editing. All authors contributed to the article and approved the submitted version.
PB reports grants from the Cancer Foundation Finland and the Hospital District of Southwest Finland. PT reports grants from the Cancer Foundation Finland and the Hospital District of Southwest Finland.
MA reports grants from Profound Medical Inc, Finnish Urological Research Foundation, and Finnish Urological Association, and personal fees from Astellas, Bayer, Orion, and Janssen-Cilag, all outside the submitted work. PB reports personal fees from Profound Medical Inc and Janssen-Cilag Company outside the submitted work. PT reports personal fees from Roche, AstraZeneca, and MSD and non-financial support from MSD, all outside the submitted work.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1043688/full#supplementary-material
Supplementary Figure 1 | Intra-procedural MR images of the patient case (presented in Figure 2) undergoing salvage whole-gland TULSA for locally recurrent PCa after radiotherapy. On the top row, axial T2-weighted treatment planning images from all transducer elements (E1-E10) of the transurethral UA. The drawn yellow boundary on the prostate capsule (E3-E7) displays the target ablation area. On the second and third rows, maximum temperature and thermal dose images show a lethal minimum temperature of 55°C and a thermal dose of 240 CEM at 43°C covering the target area. On the fourth row, post-treatment contrast-enhanced images show the non-perfused volume with the rim of enhancement covering the target area. CEM, cumulative equivalent minute; MR, magnetic resonance; PCa, prostate cancer; TULSA, transurethral ultrasound ablation; UA, ultrasound applicator.
1. Culp MB, Soerjomataram I, Efstathiou JA, Bray F, Jemal A. Recent global patterns in prostate cancer incidence and mortality rates. Eur Urol (2020) 77(1):38–52. doi: 10.1016/j.eururo.2019.08.005
2. Bleyer A, Spreafico F, Barr R. Prostate cancer in young men: An emerging young adult and older adolescent challenge. Cancer (2020) 126(1):46–57. doi: 10.1002/cncr.32498
3. Ye Y, Zheng Y, Miao Q, Ruan H, Zhang X. Causes of death among prostate cancer patients aged 40 years and older in the united states. Front Oncol (2022) 12:914875. doi: 10.3389/fonc.2022.914875
4. Neal DE, Metcalfe C, Donovan JL, Lane JA, Davis M, Young GJ, et al. Ten-year mortality, disease progression, and treatment-related side effects in men with localised prostate cancer from the ProtecT randomised controlled trial according to treatment received. Eur Urol (2020) 77(3):320–30. doi: 10.1016/j.eururo.2019.10.030
5. Ahmed HU, Arya M, Freeman A, Emberton M. Do low-grade and low-volume prostate cancers bear the hallmarks of malignancy? Lancet Oncol (2012) 13(11):e509–17. doi: 10.1016/S1470-2045(12)70388-1
6. Guidelines EAU. Edn. presented at the EAU annual congress Amsterdam. (2022). Available at: https://uroweb.org/guidelines/prostate-cancer/chapter/citation-information.
7. Hoffman KE, Penson DF, Zhao Z, Huang LC, Conwill R, Laviana AA, et al. Patient-reported outcomes through 5 years for active surveillance, surgery, brachytherapy, or external beam radiation with or without androgen deprivation therapy for localized prostate cancer. Jama (2020) 323(2):149–63. doi: 10.1001/jama.2019.20675
8. Higano CS. Cardiovascular disease and androgen axis–targeted drugs for prostate cancer. New Engl J Med (2020) 382(23):2257–9. doi: 10.1056/NEJMe2016433
9. Drost FJH, Osses DF, Nieboer D, Steyerberg EW, Bangma CH, Roobol MJ, et al. Prostate MRI, with or without MRI-targeted biopsy, and systematic biopsy for detecting prostate cancer. CochraneDatabase Syst Rev (2019) 4(4):CD012663. doi: 10.1002/14651858.CD012663
10. Hofman MS, Lawrentschuk N, Francis RJ, Tang C, Vela I, Thomas P, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomised, multicentre study. Lancet (2020) 395(10231):1208–16. doi: 10.1016/S0140-6736(20)30314-7
11. Sweat SD, Pacelli A, Murphy GP, Bostwick DG. Prostate-specific membrane antigen expression is greatest in prostate adenocarcinoma and lymph node metastases. Urology (1998) 52(4):637–40. doi: 10.1016/S0090-4295(98)00278-7
12. Uprimny C, Kroiss AS, Decristoforo C, Fritz J, von Guggenberg E, Kendler D, et al. 68Ga-PSMA-11 PET/CT in primary staging of prostate cancer: PSA and Gleason score predict the intensity of tracer accumulation in the primary tumour. Eur J Nucl Med Mol Imaging (2017) 44(6):941–9. doi: 10.1007/s00259-017-3631-6
13. Kalapara AA, Nzenza T, Pan HY, Ballok Z, Ramdave S, O'Sullivan R, et al. Detection and localisation of primary prostate cancer using 68gallium prostate-specific membrane antigen positron emission tomography/computed tomography compared with multiparametric magnetic resonance imaging and radical prostatectomy specimen pathology. BJU Int (2020) 126(1):83–90. doi: 10.1111/bju.14858
14. Maurer T, Gschwend JE, Rauscher I, Souvatzoglou M, Haller B, Weirich G, et al. Diagnostic efficacy of 68gallium-PSMA positron emission tomography compared to conventional imaging for lymph node staging of 130 consecutive patients with intermediate to high risk prostate cancer. J Urol (2016) 195(5):1436–43. doi: 10.1016/j.juro.2015.12.025
15. Bahler CD, Green M, Hutchins GD, Cheng L, Magers MJ, Fletcher J, et al. Prostate specific membrane antigen targeted positron emission tomography of primary prostate cancer: assessing accuracy with whole mount pathology. J Urol (2020) 203(1):92–9. doi: 10.1097/JU.0000000000000501
16. Privé BM, Israël B, Schilham MG, Muselaers CH, Zámecnik P, Mulders PF, et al. Evaluating f-18-PSMA-1007-PET in primary prostate cancer and comparing it to multi-parametric MRI and histopathology. Prostate Cancer prostatic Dis (2021) 24(2):423–30. doi: 10.1038/s41391-020-00292-2
17. Ahmed HU. The index lesion and the origin of prostate cancer. New Engl J Med (2009) 361(17):1704–6. doi: 10.1056/nejmcibr0905562
18. Nevoux P, Ouzzane A, Ahmed HU, Emberton M, Montironi R, Presti JC Jr., et al. Quantitative tissue analyses of prostate cancer foci in an unselected cystoprostatectomy series. BJU Int (2012) 110(4):517–23. doi: 10.1111/j.1464-410X.2011.10776.x
19. Mouraviev V, Mayes JM, Sun L, Madden JF, Moul JW, Polascik TJ. Prostate cancer laterality as a rationale of focal ablative therapy for the treatment of clinically localized prostate cancer. Cancer: Interdiscip Int J Am Cancer Soc (2007) 110(4):906–10. doi: 10.1002/cncr.22858
20. Byar DP, Mostofi FK, Veterans Administration Cooperative Urological Research Group. Carcinoma of the prostate: prognostic evaluation of certain pathologic features in 208 radical prostatectomies. Cancer (1972) 30(1):5–13. doi: 10.1002/1097-0142(197207)30
21. Wei L, Wang J, Lampert E, Schlanger S, DePriest AD, Hu Q, et al. Intratumoral and intertumoral genomic heterogeneity of multifocal localized prostate cancer impacts molecular classifications and genomic prognosticators. Eur Urol (2017) 71(2):183–92. doi: 10.1016/j.eururo.2016.07.008
22. Løvf M, Zhao S, Axcrona U, Johannessen B, Bakken AC, Carm KT, et al. Multifocal primary prostate cancer exhibits high degree of genomic heterogeneity. Eur Urol (2019) 75(3):498–505. doi: 10.1016/j.eururo.2018.08.009
23. Arora R, Koch MO, Eble JN, Ulbright TM, Li L, Cheng L. Heterogeneity of Gleason grade in multifocal adenocarcinoma of the prostate. Cancer: Interdiscip Int J Am Cancer Soc (2004) 100(11):2362–6. doi: 10.1002/cncr.20243
24. McNeal JE. Cancer volume and site of origin of adenocarcinoma in the prostate: relationship to local and distant spread. Hum Pathol (1992) 23(3):258–66. doi: 10.1016/0046-8177(92)90106-D
25. Mouraviev V, Villers A, Bostwick DG, Wheeler TM, Montironi R, Polascik TJ. Understanding the pathological features of focality, grade and tumour volume of early-stage prostate cancer as a foundation for parenchyma-sparing prostate cancer therapies: active surveillance and focal targeted therapy. BJU Int (2011) 108(7):1074–85. doi: 10.1111/j.1464-410X.2010.10039.x
26. Algaba F, Montironi R. Impact of prostate cancer multifocality on its biology and treatment. J Endourol (2010) 24(5):799–804. doi: 10.1089/end.2009.0462
27. Wise AM, Stamey TA, McNeal JE, Clayton JL. Morphologic and clinical significance of multifocal prostate cancers in radical prostatectomy specimens. Urology (2002) 60(2):264–9. doi: 10.1016/S0090-4295(02)01728-4
28. Karavitakis M, Winkler M, Abel P, Livni N, Beckley I, Ahmed HU. Histological characteristics of the index lesion in whole-mount radical prostatectomy specimens: implications for focal therapy. Prostate Cancer Prostatic Dis (2011) 14(1):46–52. doi: 10.1038/pcan.2010.16
29. Ahmed HU, Dickinson L, Charman S, Weir S, McCartan N, Hindley RG, et al. Focal ablation targeted to the index lesion in multifocal localised prostate cancer: a prospective development study. Eur Urol (2015) 68(6):927–36. doi: 10.1016/j.eururo.2015.01.030
30. Le JD, Tan N, Shkolyar E, Lu DY, Kwan L, Marks LS, et al. Multifocality and prostate cancer detection by multiparametric magnetic resonance imaging: correlation with whole-mount histopathology. Eur Urol (2015) 67(3):569–76. doi: 10.1016/j.eururo.2014.08.079
31. Zhou Z, Zhou Y, Yan W, Sun H, Li Q, Li H, et al. Unilateral lesion detected on preoperative multiparametric magnetic resonance imaging and MRI/US fusion-guided prostate biopsy is not an appropriate indication for focal therapy in prostate cancer. In Urol Oncol: Semin Original Investi (2021) 39(10):730–e17. doi: 10.1016/j.urolonc.2021.04.021
32. Johnson DC, Raman SS, Mirak SA, Kwan L, Bajgiran AM, Hsu W, et al. Detection of individual prostate cancer foci via multiparametric magnetic resonance imaging. Eur Urol (2019) 75(5):712–20. doi: 10.1016/j.eururo.2018.11.031
33. Choi YH, Yu JW, Jeong BC, Seo SI, Jeon SS, Lee HM, et al. Histological characteristics of the largest and secondary tumors in radical prostatectomy specimens and implications for focal therapy. Diagn Pathol (2019) 14(1):1–6. doi: 10.1186/s13000-019-0782-8
34. Pooli A, Johnson DC, Shirk J, Markovic D, Sadun TY, Sisk AE Jr., et al. Predicting pathological tumor size in prostate cancer based on multiparametric prostate magnetic resonance imaging and preoperative findings. J Urol (2021) 205(2):444–51. doi: 10.1097/JU.0000000000001389
35. Priester A, Natarajan S, Khoshnoodi P, Margolis DJ, Raman SS, Reiter RE, et al. Magnetic resonance imaging underestimation of prostate cancer geometry: use of patient specific molds to correlate images with whole mount pathology. J Urol (2017) 197(2):320–6. doi: 10.1016/j.juro.2016.07.084
36. Merisaari H, Jambor I, Ettala O, Boström PJ, Montoya Perez I, Verho J, et al. IMPROD biparametric MRI in men with a clinical suspicion of prostate cancer (IMPROD trial): sensitivity for prostate cancer detection in correlation with whole-mount prostatectomy sections and implications for focal therapy. J Magnetic Reson Imaging (2019) 50(5):1641–50. doi: 10.1002/jmri.26727
37. Hopstaken JS, Bomers JG, Sedelaar MJ, Valerio M, Fütterer JJ, Rovers MM. An updated systematic review on focal therapy in localized prostate cancer: what has changed over the past 5 years? Eur Urol (2022) 81(1):5–33. doi: 10.1016/j.eururo.2021.08.005
38. Tay KJ, Polascik TJ, Elshafei A, Tsivian E, Jones JS. Propensity score-matched comparison of partial to whole-gland cryotherapy for intermediate-risk prostate cancer: an analysis of the cryo on-line data registry data. J Endourol (2017) 31(6):564–71. doi: 10.1089/end.2016.0830
39. Byun SS, Jin N, Lee H. High intensity focused ultrasound ablation for prostate cancer: Whole versus partial gland ablation. Clin Genitouri Cancer (2022) 20(1):e39–44. doi: 10.1016/j.clgc.2021.09.003
40. Keserci B, Duc NM, Nadarajan C, Huy HQ, Saizan A, Ahmed WAW, et al. Volumetric MRI-guided, high-intensity focused ultrasound ablation of uterine leiomyomas: ASEAN preliminary experience. Diagn Intervent Radiol (2020) 26(3):207. doi: 10.5152/dir.2019.19157
41. Duc NM, Huy HQ. A technical update of high-intensity focused ultrasound ablation for prostate cancer and benign prostatic hyperplasia. Imaging Med (2018) 10(5):139–42.
42. Dora C, Clarke GM, Frey G, Sella D. Magnetic resonance imaging-guided transurethral ultrasound ablation of prostate cancer: A systematic review. J Endourol (2022) 30(6):841–54. doi: 10.1089/end.2021.0866
43. Anttinen M, Mäkelä P, Suomi V, Kiviniemi A, Saunavaara J, Sainio T, et al. Feasibility of MRI-guided transurethral ultrasound for lesion-targeted ablation of prostate cancer. Scandinavian J Urol (2019) 53(5):295–302. doi: 10.1080/21681805.2019.1660707
44. Anttinen M, Mäkelä P, Nurminen P, Yli-Pietilä E, Suomi V, Sainio T, et al. Palliative MRI-guided transurethral ultrasound ablation for symptomatic locally advanced prostate cancer. Scandinavian J Urol (2020) 54(6):481–6. doi: 10.1080/21681805.2020.1814857
45. Anttinen M, Mäkelä P, Viitala A, Nurminen P, Suomi V, Sainio T, et al. Salvage magnetic resonance imaging–guided transurethral ultrasound ablation for localized radiorecurrent prostate cancer: 12-month functional and oncological results. Eur Urol Open Sci (2020) 22:79–87. doi: 10.1016/j.euros.2020.10.007
46. Viitala A, Anttinen M, Wright C, Virtanen I, Mäkelä P, Hovinen T, et al. Magnetic resonance imaging-guided transurethral ultrasound ablation for benign prostatic hyperplasia: 12-month clinical outcomes of a phase I study. BJU Int (2022) 129(2):208–16. doi: 10.1111/bju.15523
47. Crouzet S, Chapelon JY, Rouvière O, Mege-Lechevallier F, Colombel M, Tonoli-Catez H, et al. Whole-gland ablation of localized prostate cancer with high-intensity focused ultrasound: oncologic outcomes and morbidity in 1002 patients. Eur Urol (2014) 65(5):907–14. doi: 10.1016/j.eururo.2013.04.039
48. Guillaumier S, Peters M, Arya M, Afzal N, Charman S, Dudderidge T, et al. A multicentre study of 5-year outcomes following focal therapy in treating clinically significant nonmetastatic prostate cancer. Eur Urol (2018) 74(4):422–9. doi: 10.1016/j.eururo.2018.06.006
49. Dickinson L, Arya M, Afzal N, Cathcart P, Charman SC, Cornaby A, et al. Medium-term outcomes after whole-gland high-intensity focused ultrasound for the treatment of nonmetastatic prostate cancer from a multicentre registry cohort. Eur Urol (2016) 70(4):668–74. doi: 10.1016/j.eururo.2016.02.054
50. Bründl J, Osberghaus V, Zeman F, Breyer J, Ganzer R, Blana A, et al. Oncological long-term outcome after whole-gland high-intensity focused ultrasound for prostate cancer–21-yr follow-up. Eur Urol Focus (2022) 8(1):134–40. doi: 10.1016/j.euf.2020.12.016
51. Reddy D, Peters M, Shah TT, van Son M, Tanaka MB, Huber PM, et al. Cancer control outcomes following focal therapy using high-intensity focused ultrasound in 1379 men with nonmetastatic prostate cancer: a multi-institute 15-year experience. Eur Urol (2022) 81(4):407–13. doi: 10.1016/j.eururo.2022.01.005
52. van Son MJ, Peters M, Reddy D, Shah TT, Hosking-Jervis F, Robinson S, et al. Conventional radical versus focal treatment for localised prostate cancer: a propensity score weighted comparison of 6-year tumour control. Prostate Cancer Prostatic Dis (2021) 24(4):1120–8. doi: 10.1038/s41391-021-00369-6
53. Shah TT, Reddy D, Peters M, Ball D, Kim NH, Gomez EG, et al. Focal therapy compared to radical prostatectomy for non-metastatic prostate cancer: A propensity score-matched study. Prostate Cancer Prostatic Dis (2021) 24(2):567–74. doi: 10.1038/s41391-020-00315-y
54. Ehdaie B, Tempany CM, Holland F, Sjoberg DD, Kibel AS, Trinh QD, et al. MRI-Guided focused ultrasound focal therapy for patients with intermediate-risk prostate cancer: a phase 2b, multicentre study. Lancet Oncol (2022) 23(7):910–8. doi: 10.1016/S1470-2045(22)00251-0
55. Klotz L, Pavlovich CP, Chin J, Hatiboglu G, Koch M, Penson D, et al. Magnetic resonance imaging-guided transurethral ultrasound ablation of prostate cancer. J Urol (2021) 205(3):769–79. doi: 10.1097/JU.0000000000001362
56. Ahmed HU, Hindley RG, Dickinson L, Freeman A, Kirkham AP, Sahu, et al. Focal therapy for localised unifocal and multifocal prostate cancer: a prospective development study. Lancet Oncol (2012) 13(6):622–32. doi: 10.1016/S1470-2045(12)70121-3
57. Feijoo ERC, Sivaraman A, Barret E, Sanchez-Salas R, Galiano M, Rozet F, et al. Focal high-intensity focused ultrasound targeted hemiablation for unilateral prostate cancer: a prospective evaluation of oncologic and functional outcomes. Eur Urol (2016) 69(2):214–20. doi: 10.1016/j.eururo.2015.06.018
58. Van Velthoven R, Aoun F, Marcelis Q, Albisinni S, Zanaty M, Lemort M, et al. A prospective clinical trial of HIFU hemiablation for clinically localized prostate cancer. Prostate Cancer prostatic Dis (2016) 19(1):79–83. doi: 10.1038/pcan.2015.55
59. Rischmann P, Gelet A, Riche B, Villers A, Pasticier G, Bondil P, et al. Focal high intensity focused ultrasound of unilateral localized prostate cancer: A prospective multicentric hemiablation study of 111 patients. Eur Urol (2017) 71(2):267–73. doi: 10.1016/j.eururo.2016.09.039
60. Ganzer R, Hadaschik B, Pahernik S, Koch D, Baumunk D, Kuru T, et al. Prospective multicenter phase II study on focal therapy (hemiablation) of the prostate with high intensity focused ultrasound. J Urol (2018) 199(4):983–9. doi: 10.1016/j.juro.2017.10.033
61. Johnston MJ, Emara A, Noureldin M, Bott S, Hindley RG. Focal high-intensity focussed ultrasound partial gland ablation for the treatment of localised prostate cancer: a report of medium-term outcomes from a single-center in the united kingdom. Urology (2019) 133:175–81. doi: 10.1016/j.urology.2019.06.043
62. Abreu AL, Peretsman S, Iwata A, Shakir A, Iwata T, Brooks J, et al. High intensity focused ultrasound hemigland ablation for prostate cancer: initial outcomes of a united states series. J Urol (2020) 204(4):741–7. doi: 10.1097/JU.0000000000001126
63. Nahar B, Bhat A, Reis IM, Soodana-Prakash N, Becerra MF, Lopategui D, et al. Prospective evaluation of focal high intensity focused ultrasound for localized prostate cancer. J Urol (2020) 204(3):483–9. doi: 10.1097/JU.0000000000001015
64. Shoji S, Hiraiwa S, Uemura K, Nitta M, Hasegawa M, Kawamura Y, et al. Focal therapy with high-intensity focused ultrasound for the localized prostate cancer for Asian based on the localization with MRI-TRUS fusion image-guided transperineal biopsy and 12-cores transperineal systematic biopsy: prospective analysis of oncological and functional outcomes. Int J Clin Oncol (2020) 25(10):1844–53. doi: 10.1007/s10147-020-01723-9
65. Ramsay CR, Adewuyi TE, Gray J, Hislop J, Shirley MD, Jayakody S, et al. Ablative therapy for people with localised prostate cancer: a systematic review and economic evaluation. Health Technol Assess (2015) 19(49):1–490. doi: 10.3310/hta19490
66. Bates AS, Ayers J, Kostakopoulos N, Lumsden T, Schoots IG, Willemse PPM, et al. A systematic review of focal ablative therapy for clinically localised prostate cancer in comparison with standard management options: limitations of the available evidence and recommendations for clinical practice and further research. Eur Urol Oncol (2021) 4(3):405–23. doi: 10.1016/j.euo.2020.12.008
67. Stabile A, Orczyk C, Hosking-Jervis F, Giganti F, Arya M, Hindley RG, et al. Medium-term oncological outcomes in a large cohort of men treated with either focal or hemi-ablation using high-intensity focused ultrasonography for primary localized prostate cancer. BJU Int (2019) 124(3):431–40. doi: 10.1111/bju.14710
68. Tran H, Kwok J, Pickles T, Tyldesley S, Black PC. Underutilization of local salvage therapy after radiation therapy for prostate cancer. Urol Oncol: Semin Original Investi (2014) 32(5):701–6. doi: 10.1016/j.urolonc.2013.12.014
Keywords: ablation therapy, HIFU, high-intensity focused ultrasound, MRI, magnetic resonance imaging, Tulsa, transurethral ultrasound ablation, prostate cancer
Citation: Anttinen M, Blanco Sequeiros R, Boström PJ and Taimen P (2022) Evolving imaging methods of prostate cancer and the emergence of magnetic resonance imaging guided ablation techniques. Front. Oncol. 12:1043688. doi: 10.3389/fonc.2022.1043688
Received: 13 September 2022; Accepted: 21 October 2022;
Published: 17 November 2022.
Edited by:
Yonggang Lu, Medical College of Wisconsin, United StatesReviewed by:
Steven Yuvan, Medical College of Wisconsin, United StatesCopyright © 2022 Anttinen, Blanco Sequeiros, Boström and Taimen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mikael Anttinen, bWhqYW50QHV0dS5maQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.