
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 12 December 2022
Sec. Cancer Molecular Targets and Therapeutics
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.1042525
 Margherita Nannini1,2*
Margherita Nannini1,2* Andrea Repaci3
Andrea Repaci3 Gianluca Ricco2
Gianluca Ricco2 Manuela Ianni4
Manuela Ianni4 Arber Golemi5
Arber Golemi5 Vincenzo Maiolo6
Vincenzo Maiolo6 Marco Ferrari7
Marco Ferrari7 Filippo Natali7
Filippo Natali7 Elisa Lodi Rizzini8
Elisa Lodi Rizzini8 Fabio Monari8
Fabio Monari8 Erica Solaroli9
Erica Solaroli9 Antonio De Leo10,11
Antonio De Leo10,11 Thais Maloberti10
Thais Maloberti10 Maria A. Pantaleo1,2
Maria A. Pantaleo1,2 Dario De Biase11,12†
Dario De Biase11,12† Giovanni Tallini10,11†
Giovanni Tallini10,11†We are recently faced with a progressive evolution of the therapeutic paradigm for radioiodine refractory differentiated thyroid cancer (RAI-R DTC), since the advent of tissue agnostic inhibitors. Thus, tumor genotype assessment is always more relevant and is playing a crucial role into clinical practice. We report the case of an elderly patient with advanced papillary thyroid carcinoma (PTC) harboring RET-CCDC6 fusion with four co-occurring mutations involving PI3KCA, TP53, and hTERT mutations, treated with pralsetinib under a compassionate use program. Despite the high histological grade and the coexistence of aggressive RET co-mutations, an impressive metabolic and structural tumor response has been obtained, together with a patient’s prolonged clinical benefit. A timely comprehensive molecular testing of those cases wild-type for the common thyroid carcinoma BRAF V600E-like and RAS-like driver mutations may uncover actionable gene rearrangements that can be targeted by highly selective inhibitors with great potential benefit for the patients.
During the last decades, the biological background of differentiated thyroid cancer (DTC) has come into focus leading to significant therapeutic improvement of metastatic patients with a radioiodine refractory (RAI-R) disease. Indeed, the identification of genetic alterations in thyroid carcinogenesis, mainly involving the mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) signaling pathways, has been instrumental to explore the role of targeted therapies in DTC, making lenvatinib and sorafenib the current standard-of-care of treatment for metastatic/advanced RAI-R patients (1–4). More recently, therapeutic options of RAI-R DTC have further expanded with the advent of neurotrophic tyrosine receptor kinase (NTRK) and RET-inhibitors, which have shown marked and durable responses in patients with metastatic/advanced RAI-R DTC positive for NTRK or RET gene fusions, with a favorable safety profile (4–7). In this growing landscape, we are faced with a progressive evolution of the therapeutic paradigm for RAI-R DTC, for which tumor genotype assessment is thus playing crucial role. Herein, we report the case of an elderly patient with advanced papillary thyroid carcinoma (PTC) harboring RET::CCDC6 fusion with four co-occurring mutations involving PI3KCA, TP53, and hTERT mutations, with an impressive clinical and radiologic response to pralsetinib under a compassionate use program (AG43388 – Roche).
An 84-years old man, with a history of systemic hypertension, type II diabetes mellitus, ischemic cardiomyopathy and atrial fibrillation (AF) sought medical attention after two months exertional dyspnea and throat tightness. Thus, in August 2020 diagnostic investigations were performed. Neck ultrasonography showed a worrisome highly vascular nodule in the right thyroid lobe (45.5 x 41.1 x 58 mm) and right latero-cervical lymph nodes at III and IV levels (26.1 x 16.1 x 24.6 mm). Thyroid and nodal fine needle aspiration (FNA) cytology identified malignant cells (Bethesda VI) compatible with papillary thyroid carcinoma (Figure 1A). Basal levels of thyroid-stimulating hormone (TSH) and thyroglobulin (Tg) were 0.7 mcU/ml and 1026 ng/ml, respectively. A fibrobronchoscopy was performed due to patient’s symptoms, revealing ab estrinseco stenosis of the trachea with vegetating neoplastic tissue within the lumen. An endotracheal stent was placed, and the tumor was biopsied. The diagnosis was PTC with high grade features due to the presence of mitotic activity and necrosis (Figures 1B-D). Neoplastic cells were positive for Thyroid Transcription Factor- 1 (TTF-1) and Tg (Figures 1E, F).
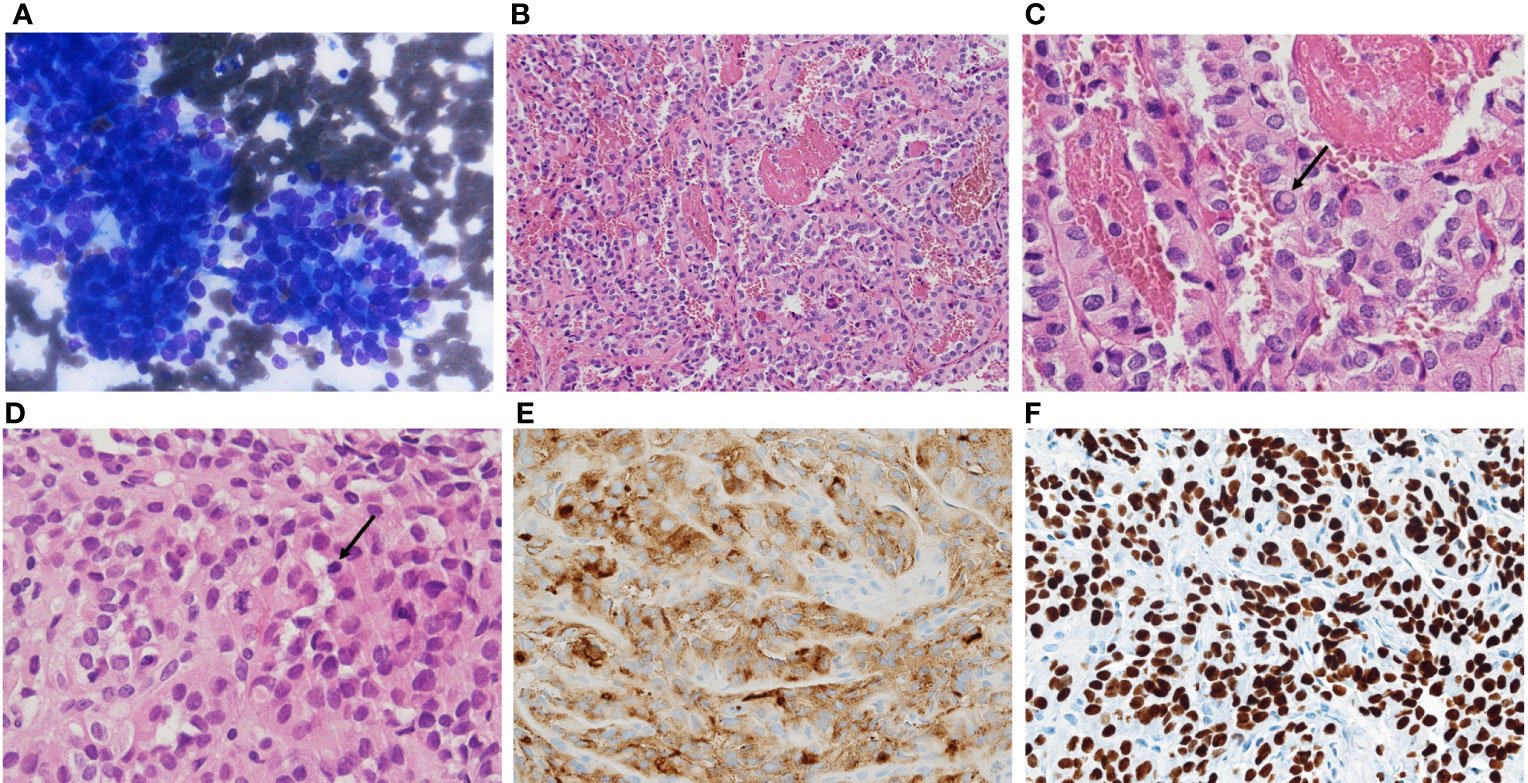
Figure 1 (A) fine needle aspiration showing atypical cells forming papillary clusters, diagnosed as TIR5-SIAPEC (Bethesda VI and to Thy5-BTA), compatible with papillary thyroid carcinoma. Histologically the tumor showed complex papillary architecture (B) intranuclear inclusions (C, arrow), mitotic activity (D, arrow), immunohistochemical thyroglobulin (E) and TTF1 expression (F).
Next-generation sequencing (NGS) analysis - performed on the thyroid cytology specimen using a laboratory-developed multi-gene panel (8) and the Oncomine Focus panel assay – demonstrated RET:CCDC6 rearrangement with three additional co-occurring mutations involving PI3KCA (p.N1044K c.3132T>A, Exon 21), TP53 (p.Y163C c.488A>G, Exon 5), and hTERT (C228T, Promoter).
Total-body computed tomography (TB-CT) scan demonstrated the tumor of the right thyroid lobe dislodging and infiltrating cervical esophagus and trachea, a partially necrotic confluent right latero-cervical lymph node metastases (maximum diameter of 5.8 cm), bilateral non-calcified pulmonary nodules, mediastinal lymph nodes, and a 12 mm liver lesion at V segment (Figure 2).
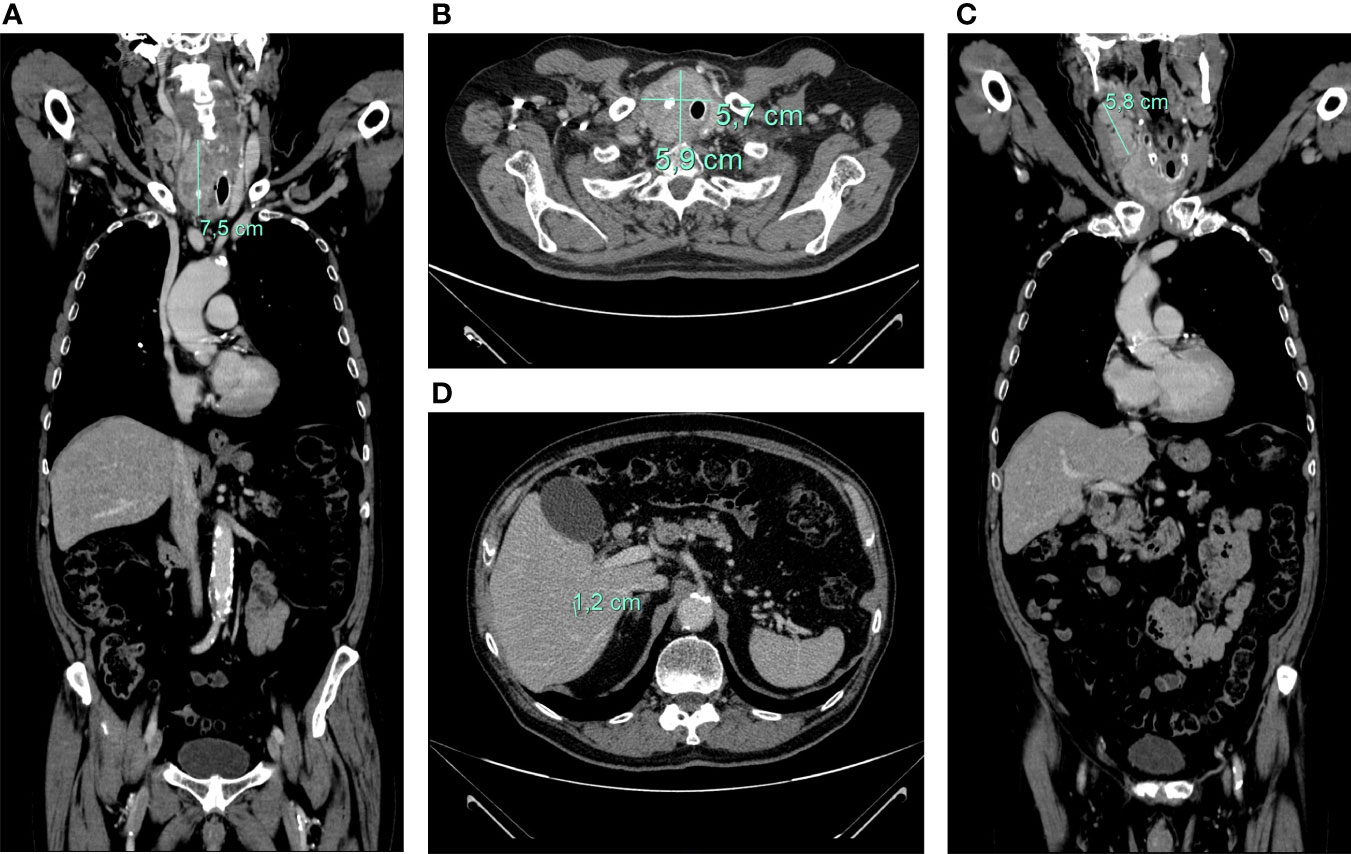
Figure 2 axial (A) and coronal (B) CT-scan showing large inhomogeneous tumor with calcifications of the right thyroid lobe with left tracheal displacement, infiltrating the trachea and esophagus; confluent neck lymph node metastases (maximum diameter of 58 mm) (C); hepatic lesion of 12 mm at V segment (D).
Total thyroidectomy and laryngectomy were not performed due to the extent of the disease, the patient’s age and his multiple comorbidities. Thus, in November 2020 systemic therapy with lenvatinib at personalized dose of 14 mg daily was started. The patient experienced several side-effects, such as asthenia grade 2 (G2), stomatitis G2, diarrhea G2, leading to transient treatment interruption and further dose reduction to 10 mg daily. At the first CT-scan evaluation, performed in March 2021, a dimensional reduction of latero-cervical lymphadenopathies (maximum diameter of 2x3 cm), and of left thyroid lobe was obtained, while a mild increase of the hepatic lesion was documented (17 mm vs 12 mm) (Figure 3). Thus, lenvatinib was continued until June 2021 when it was stopped due to the onset of severe anemia G4 (Hb 6 gr/dL, MCV 94 fL) from a gastrointestinal bleeding probably related to the concomitant anticoagulant therapy for AF.
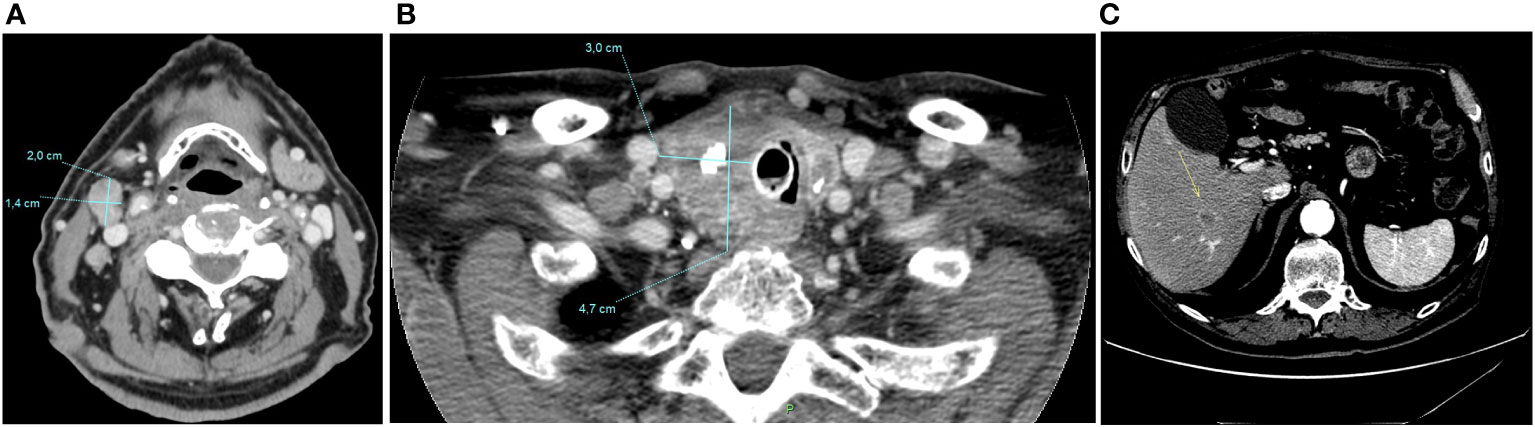
Figure 3 CT-scan performed in March 2021, after four months of Lenvatinib, showing a dimensional reduction of latero-cervical lymphadenopathies (maximum diameter of 2 cm) (A), and of left thyroid lobe (3x4,7 cm) (B), and a mild increase of the hepatic lesion (17 mm vs 12 mm) (C).
However, as a consequence of this treatment period-off, in August 2021, a CT-scan was performed due to markedly increased Tg levels (2495 vs 749 ng/ml), reveling disease progression with the onset of at least six bilateral brain and two cerebellar lesions, all with hemorrhagic features (Figure 4). Subsequent 18F-fluoro-deoxy-glucose positron emission tomography (18F-FDG-PET) showed high glucose uptake in the right thyroid lobe (SUVmax = 23.7), together with intense uptake at a very large right latero-cervical adenopathy (SUVmax = 44.5), and at several additional lymph nodal and visceral sites, including lung, bone and pancreas (Figure 5A).
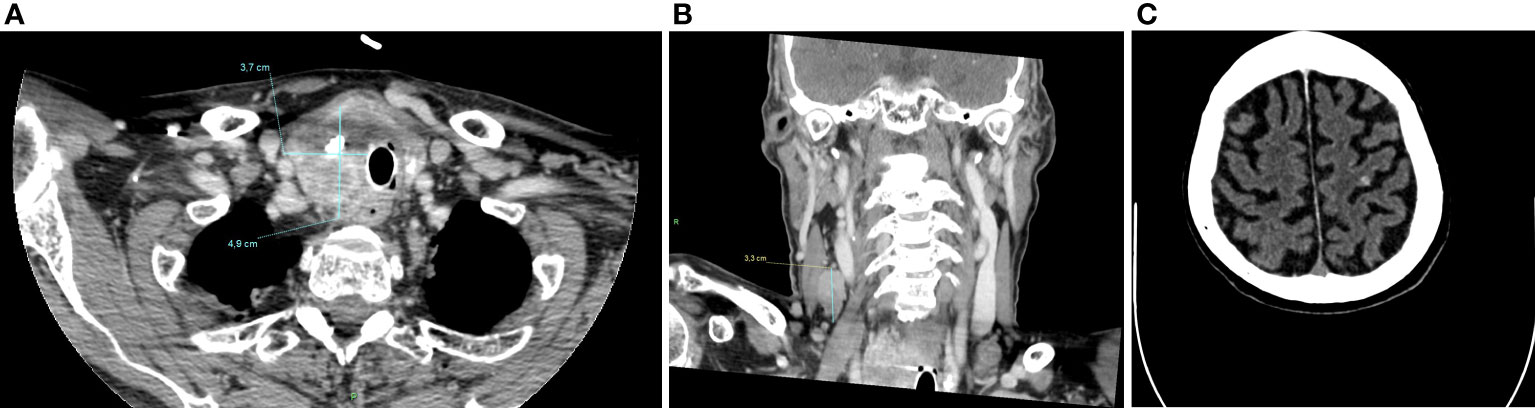
Figure 4 CT-scan performed in August 2021, after lenvatinib discontinuation, showing a dimensional increase of left thyroid lobe (4,9x3,7 cm) (A), latero-cervical lymphadenopathies (maximum diameter of 3 cm) (B) and multiple brain and cerebellar metastases (C).
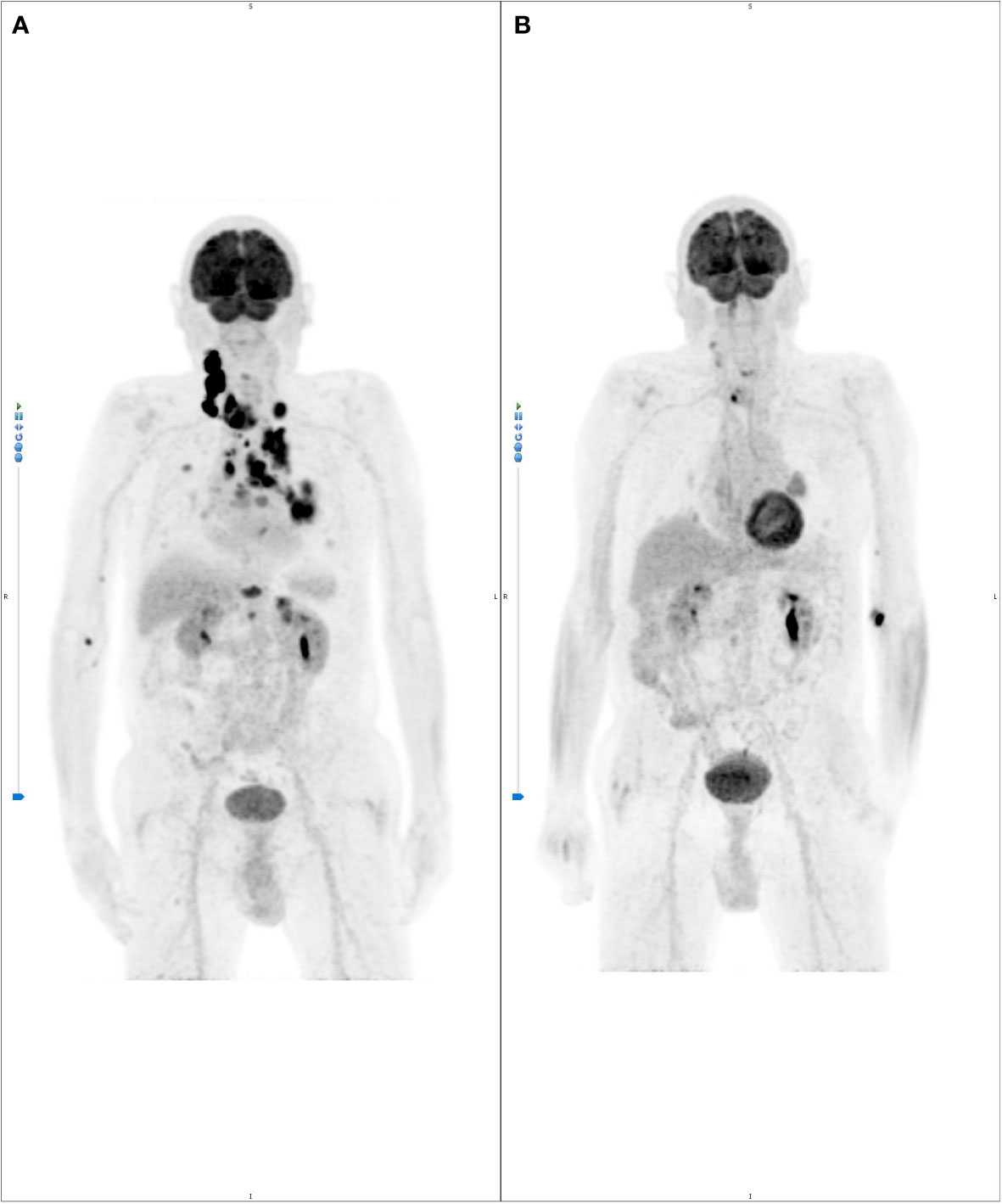
Figure 5 (A) basal 18FDG-PET scan showing intense signal in the right thyroid lobe (standardize uptake value, SUV max: 23.7), right neck metastasis (SUV max: 44.5), bilateral supraclavicular lymph nodes (SUV max: 31, right; SUV max: 15., left), lung nodules (Left lower lobe, SUV max: 15.1- Posterior and 17.0-Paravertebral; Right upper lobe, SUV max: 6.5 and 6.2), mediastinal lymph nodes (left pulmonary hilus, SUV max: 10.4; left peribronchial, bilateral tracheo-bronchial and subcarinal, SUV max: 7.3), upper thoracic outlet, paraesophageal, right para-tracheal lymph nodes (SUV max: 18.1); (B) remarkably rapid metabolic response within less than one month of pralsetinib therapy.
Given the tumor molecular profile, taking into account the patient’s good performance status and the gastrointestinal bleeding, a second line of treatment with pralsetinib within a compassionate use protocol (AG43388 – Roche) was considered. The study was approved by the local Ethic Committee of S. Orsola-Malpighi Hospital, Bologna (208/2021/Compass/AOUBo) and the patient provided informed consent. Pralsetinib was started in September 2021 at the standard dose of 400 mg daily, and subsequently personalized to 200 mg daily, due to the onset of G3 diarrhea and G2 asthenia. Noteworthy, the 18F-FDG-PET performed after one month of therapy for early treatment response evaluation, reported a complete metabolic response in almost all sites and a relevant partial response in the others (Figure 5B). CT-scan performed four months after the beginning of therapy showed dramatic tumor shrinkage of both primary tumor and of all metastatic lesions, including the brain metastases, for which an almost complete radiological response was obtained, with a consequent patient’s clinical benefit especially on dyspnea and throat tightness, and an important drop of Tg levels from 2495 to 485 ng/ml.
At present the patient is still in treatment with pralsetinib at the personalized dose of 200 mg daily, with an overall good tolerance. The last CT-scan performed in October 2022, 13 months after the beginning of treatment, showed a further tumor volume reduction and Tg levels have drop to 378 ng/ml (Figure 6).
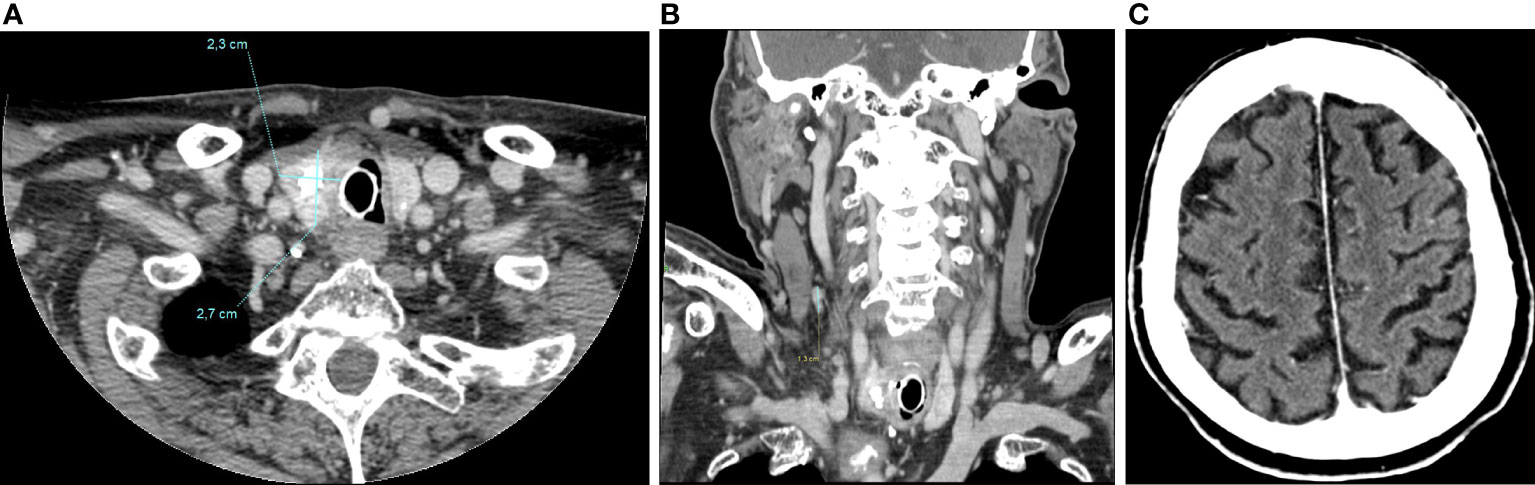
Figure 6 CT-scan performed in October 2022, 13 months after the beginning of treatment, showing a further tumor volume reduction of left thyroid lobe (2,3x2,7cm) (A), of latero-cervical lymphadenopathies (maximum diameter of 1,3 cm) (B), and a complete radiological response of brain metastases (C).
RET fusion represents the most common gene fusion event in DTC, present in about 10-20% of papillary thyroid carcinoma (9). The reported prevalence of RET fusion is very heterogeneous across studies, due to geographic variation, as well as to different sensitivities of detection methods used (10–12). So far, more than 20 fusions of the RET tyrosine kinase domain to the 5`-terminal region of heterologous genes have been reported resulting in RET/PTC chimeric oncogenes, of which CCDC6::RET (RET/PTC1) and NCOA4::RET (RET/PTC3) are the most common types (9, 13, 14).
RET rearrangements consist of the fusion of RET to the 5`-terminal region of partner genes, resulting in RET oncogenes and then into the corresponding fusion oncoproteins. These RET chimeric proteins dimerize, leading to the constitutive kinase activation of RET and to its autophosphorylation. This mechanism results in enhancing signal transduction along classical downstream pathways of both MAPK and the PI3K/AKT (9, 13).
RET-rearranged PTC develops frequently in pediatric and in radiation-exposed patients, whereas in older patients is rarer (15–17). Morphologically, RET-rearranged PTC are characterized by extensive lymph-vascular involvement, stromal sclerosis, prominent lymphocytosis, squamous metaplasia, and numerous psammoma bodies, features typical of diffuse sclerosing PTC that is indeed commonly associated with RET rearrangement (18–20). Similarly, to most other fusion-positive DTC, they thus share a unique histologic pattern, characterized by the triad of multinodularity, prominent fibrosis, and lymph-vascular invasion (18). Some RET fusion-positive PTC are aggressive with extrathyroidal extension, multifocality, lymph node involvement and distant metastases (18, 21–24). In some cases, aggressiveness is strongly associated with the high-grade features as well as RET co-alterations (18). Indeed, in the present case co-occurring PI3KCA, TP53, and hTERT mutations likely underlie the high-grade histologic features and the aggressive clinical course of the disease. The variant allele frequencies (VAF) of hTERT, TP53, and PI3KCA mutations (52%, 27%, and 8%, respectively) suggest that they occurred sequentially - in the order reported above - during tumor progression.
In the newborn era of tissue agnostic therapies, the treatment landscape of RET-rearranged PTC has been dramatically transformed by the advent of selective RET-inhibitors (pralsetinib and selpercatinib), whose impressive and durable response rates shown in clinical trials will inevitably change the treatment paradigm for this molecular subgroup of thyroid cancer (6, 7, 25). In particular, in phase I/II ARROW trial, among the RET-fusion positive TC cohort, including 10 PTC and 1 poorly differentiated thyroid cancer (PDTC), the overall response rate (ORR) was 89%, with a median duration of response (DOR) not reached with a median follow-up of 9.5 months and an estimated 1-year PFS of 81% [6]. Similarly, in phase I/II LIBRET0-001 trial among 19 RET fusion-positive patients, including 13 PTC, 3 PDTC, 2 ATC, 1 Hurthle cell, the ORR was 79%, with activity seen across all histologic types and a 1-year PFS of 64% (7). Notably the efficacy of both pralsetinib and selpercatinib treatment was observed regardless of the number of previous tyrosin kinase inhibitors received, radioiodine treatments, or type of RET mutation/fusion (6, 7).
Indeed, as demonstrated by the present case, in spite of the high histological grade and the coexistence of aggressive RET co-mutations, we have witnessed an impressive metabolic and structural tumor response. This response as well as the prolonged clinical benefit confirm the pathogenetic relevance of RET rearrangement as a oncogene-addicting driver mutation.
From a clinical perspective, two more issues deserve to be underlined. First, the dramatic response of brain metastases confirms the ability of this class of drugs to cross the blood-brain barrier (BBB). Second, as in other oncogene-driven solid tumors, early metabolic assessment of treatment response may help identify those patients with primarily resistance and select those that will benefit from RET-inhibitors.
In this scenario, early recognition of RET-rearranged PTC is crucial for several reasons. First of all, given their frequent extrathyroidal extension, multifocal presentation and lymph node involvement, RET-rearranged PTC deserve a tailored surgical strategy. Moreover, the identification of those cases with high-grade pathological and molecular features could help clinicians to set up intensive patient surveillance and thus promptly identify those patients who could benefit from early treatment with RET-inhibitors. Finally, given the high objective responses achievable, RET-inhibitors may be considered in the neoadjuvant setting or before the occurrence of RAI resistance.
In conclusion, the case presented here confirms that early and durable responses can be achieved with RET inhibitors in RET-rearranged thyroid carcinoma, even in elderly patients with multiple co-morbidities, and in spite of aggressive clinical, pathologic and molecular (PI3KCA, TP53, and hTERT co-mutations) features. It also demonstrates how timely comprehensive molecular testing of those cases wild-type for the common thyroid carcinoma BRAF V600E-like and RAS-like driver mutations uncovers actionable gene rearrangements that can be targeted by highly selective inhibitors with great potential benefit for the patients.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
The studies involving human participants were reviewed and approved by local Ethic Committee of S. Orsola-Malpighi Hospital, Bologna (208/2021/Compass/AOUBo). The patients/participants provided their written informed consent to participate in this study.
MN: conceptualization, writing-original draft; AR: writing – review and editing; GR: writing-original draft; MI: project administration; AG: resources; VM: resources; MF: resources; FN: resources; EL: resources; FM: supervision; ES: resources; AL: formal analysis; TM: formal analysis; MP: supervision, writing – review and editing; DB: conceptualization, writing-original draft, formal analysis, supervision; GT: conceptualization, writing-original draft, resources. All authors contributed to the article and approved the submitted version.
The work reported in this publication was funded by the Italian Ministry of Health, RC-2022-2773452.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: A randomised, double-blind, phase 3 trial. Lancet (2014) 384:319–28. doi: 10.1016/S0140-6736(14)60421-9
2. Cancer Genome Atlas Research N. Integrated genomic characterization of papillary thyroid carcinoma. Cell (2014) 159:676–90. doi: 10.1016/j.cell.2014.09.050
3. Filetti S, Durante C, Hartl DM, Leboulleux S, Locati LD, Newbold K, et al. ESMO clinical practice guideline update on the use of systemic therapy in advanced thyroid cancer. Ann Oncol (2022) 33:674–84. doi: 10.1016/j.annonc.2022.04.009
4. Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med (2015) 372:621–30. doi: 10.1056/NEJMoa1406470
5. Waguespack SG, Drilon A, Lin JJ, Brose MS, McDermott R, Almubarak M, et al. Efficacy and safety of larotrectinib in patients with TRK fusion-positive thyroid carcinoma. Eur J Endocrinol (2022) 186:631–43. doi: 10.1530/EJE-21-1259
6. Subbiah V, Hu MI, Wirth LJ, Schuler M, Mansfield AS, Curigliano G, et al. Pralsetinib for patients with advanced or metastatic RET-altered thyroid cancer (ARROW): A multi-cohort, open-label, registrational, phase 1/2 study. Lancet Diabetes Endocrinol (2021) 9:491–501. doi: 10.1016/S2213-8587(21)00120-0
7. Wirth LJ, Sherman E, Robinson B, Solomon B, Kang H, Lorch J, et al. Efficacy of selpercatinib in RET-altered thyroid cancers. N Engl J Med (2020) 383:825–35. doi: 10.1056/NEJMoa2005651
8. de Biase D, Malapelle U, De Leo A, Maloberti T, Visani M, Pisapia P, et al. Multi-gene custom panels for the characterization of metastatic colorectal carcinoma in clinical practice: Express the role of PIK3CA mutations. J Clin Pathol (2022) 75:488–92. doi: 10.1136/jclinpath-2021-207468
9. Santoro M, Moccia M, Federico G, Carlomagno F. RET gene fusions in malignancies of the thyroid and other tissues. Genes (Basel) (2020) 11(4):424. doi: 10.3390/genes11040424
10. Rhoden KJ, Johnson C, Brandao G, Howe JG, Smith BR, Tallini G. Real-time quantitative RT-PCR identifies distinct c-RET, RET/PTC1 and RET/PTC3 expression patterns in papillary thyroid carcinoma. Lab Invest (2004) 84:1557–70. doi: 10.1038/labinvest.3700198
11. Rhoden KJ, Unger K, Salvatore G, Yilmaz Y, Vovk V, Chiappetta G, et al. RET/papillary thyroid cancer rearrangement in nonneoplastic thyrocytes: Follicular cells of hashimoto's thyroiditis share low-level recombination events with a subset of papillary carcinoma. J Clin Endocrinol Metab (2006) 91:2414–23. doi: 10.1210/jc.2006-0240
12. Zhu Z, Ciampi R, Nikiforova MN, Gandhi M, Nikiforov YE. Prevalence of RET/PTC rearrangements in thyroid papillary carcinomas: Effects of the detection methods and genetic heterogeneity. J Clin Endocrinol Metab (2006) 91:3603–10. doi: 10.1210/jc.2006-1006
13. Tallini G, Asa SL. RET oncogene activation in papillary thyroid carcinoma. Adv Anat Pathol (2001) 8:345–54. doi: 10.1097/00125480-200111000-00005
14. Yoo SK, Lee S, Kim SJ, Jee HG, Kim BA, Cho H, et al. Comprehensive analysis of the transcriptional and mutational landscape of follicular and papillary thyroid cancers. PloS Genet (2016) 12:e1006239. doi: 10.1371/journal.pgen.1006239
15. Cordioli MI, Moraes L, Bastos AU, Besson P, Alves MT, Delcelo R, et al. Fusion oncogenes are the main genetic events found in sporadic papillary thyroid carcinomas from children. Thyroid (2017) 27:182–8. doi: 10.1089/thy.2016.0387
16. Elisei R, Romei C, Vorontsova T, Cosci B, Veremeychik V, Kuchinskaya E, et al. RET/PTC rearrangements in thyroid nodules: studies in irradiated and not irradiated, malignant and benign thyroid lesions in children and adults. J Clin Endocrinol Metab (2001) 86:3211–6. doi: 10.1210/jcem.86.7.7678
17. Hamatani K, Eguchi H, Ito R, Mukai M, Takahashi K, Taga M, et al. RET/PTC rearrangements preferentially occurred in papillary thyroid cancer among atomic bomb survivors exposed to high radiation dose. Cancer Res (2008) 68:7176–82. doi: 10.1158/0008-5472.CAN-08-0293
18. Chu YH, Wirth LJ, Farahani AA, Nosé V, Faquin WC, Dias-Santagata D, et al. Clinicopathologic features of kinase fusion-related thyroid carcinomas: an integrative analysis with molecular characterization. Mod Pathol (2020) 33:2458–72. doi: 10.1038/s41379-020-0638-5
19. Joung JY, Kim TH, Jeong DJ, Park SM, Cho YY, Jang HW, et al. Diffuse sclerosing variant of papillary thyroid carcinoma: Major genetic alterations and prognostic implications. Histopathology (2016) 69:45–53. doi: 10.1111/his.12902
20. Sheu SY, Schwertheim S, Worm K, Grabellus F, Schmid KW. Diffuse sclerosing variant of papillary thyroid carcinoma: lack of BRAF mutation but occurrence of RET/PTC rearrangements. Mod Pathol (2007) 20:779–87. doi: 10.1038/modpathol.3800797
21. Adeniran AJ, Zhu Z, Gandhi M, Steward DL, Fidler JP, Giordano TJ, et al. Correlation between genetic alterations and microscopic features, clinical manifestations, and prognostic characteristics of thyroid papillary carcinomas. Am J Surg Pathol (2006) 30:216–22. doi: 10.1097/01.pas.0000176432.73455.1b
22. Khan MS, Qadri Q, Makhdoomi MJ, Wani MA, Malik AA, Niyaz M, et al. RET/PTC gene rearrangements in thyroid carcinogenesis: Assessment and clinico-pathological correlations. Pathol Oncol Res (2020) 26:507–13. doi: 10.1007/s12253-018-0540-3
23. Ullmann TM, Thiesmeyer JW, Lee YJ, Beg S, Mosquera JM, Elemento O, et al. RET fusion-positive papillary thyroid cancers are associated with a more aggressive phenotype. Ann Surg Oncol (2022). doi: 10.1245/s10434-022-11418-2
24. Yip L, Nikiforova MN, Yoo JY, McCoy KL, Stang MT, Armstrong MJ, et al. Tumor genotype determines phenotype and disease-related outcomes in thyroid cancer: A study of 1510 patients. Ann Surg (2015) 262:519–25. doi: 10.1097/SLA.0000000000001420
Keywords: differentiated thyroid cancer, papillary thyroid carcinoma, RET, RET-rearrangement, RET-inhibitor, pralsetinib
Citation: Nannini M, Repaci A, Ricco G, Ianni M, Golemi A, Maiolo V, Ferrari M, Natali F, Rizzini EL, Monari F, Solaroli E, De Leo A, Maloberti T, Pantaleo MA, De Biase D and Tallini G (2022) Case report: Dramatic response to pralsetinib in an elderly patient with advanced RET-fusion positive papillary thyroid carcinoma. Front. Oncol. 12:1042525. doi: 10.3389/fonc.2022.1042525
Received: 12 September 2022; Accepted: 18 November 2022;
Published: 12 December 2022.
Edited by:
Vasyl Vasko, Uniformed Services University of the Health Sciences, United StatesReviewed by:
Dorina Ylli, MedStar Health Research Institute (MHRI), United StatesCopyright © 2022 Nannini, Repaci, Ricco, Ianni, Golemi, Maiolo, Ferrari, Natali, Rizzini, Monari, Solaroli, De Leo, Maloberti, Pantaleo, De Biase and Tallini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Margherita Nannini, bWFyZ2hlcml0YS5uYW5uaW5pQHVuaWJvLml0
†These authors have contributed equally to this work and share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.