
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 12 December 2022
Sec. Gastrointestinal Cancers: Hepato Pancreatic Biliary Cancers
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.1042493
Objective: To evaluate the consistencies and inconsistencies between distal cholangiocarcinoma (DCCA) and pancreatic ductal adenocarcinoma (PDCA) regarding their biological features and long-term prognosis.
Methods: PubMed, the Cochrane Library, and EMBASE were searched to find comparative studies between DCCA and PDCA. RevMan5.3 and Stata 13.0 software were used for the statistical analyses.
Results: Eleven studies with 4,698 patients with DCCA and 100,629 patients with PDCA were identified. Pooled results indicated that patients with DCCA had a significantly higher rate of preoperative jaundice (p = 0.0003). Lymphatic metastasis (p < 0.00001), vascular invasion (p < 0.0001), and peri-neural invasion (p = 0.005) were more frequently detected in patients with PDCA. After curative pancreaticoduodenectomy (PD), a significantly higher R0 rate (p < 0.0001) and significantly smaller tumor size (p < 0.00001) were detected in patients with DCCA. Patients with DCCA had a more favorable overall survival (OS) (p < 0.00001) and disease-free survival (DFS) (p = 0.005) than patients with PDCA. However, postoperative morbidities (p = 0.02), especially postoperative pancreatic fistula (POPF) (p < 0.00001), more frequently occurred in DCCA.
Conclusion: Patients with DCCA had more favorable tumor pathological features and long-term prognosis than patients with PDCA. An early diagnosis more frequently occurred in patients with DCCA. However, postoperative complications, especially POPF, were more frequently observed in patients with DCCA.
Distal cholangiocarcinoma (DCCA) and pancreatic ductal adenocarcinoma (PDCA) are both defined as peri-ampullary cancers, sharing incidences of 20% and 70%, respectively (1–3). Curative resection provides the only chance of curing these deadly malignancies, and pancreaticoduodenectomy (PD) has been widely applied in patients with DCCA and PDCA. However, although these two rare entities share similar surgical procedures and have a relatively indistinguishable tumor sites, whether they can be treated equally remains controversial.
Macroscopically, DCCA arises from the epithelium of the distal bile duct and often involves posterior pancreatic margins, while PDCA arises from the pancreatic ducts and can occur in any part of the pancreas (4). There are no specific tumor biomarkers to date to distinguish them clearly (4). Moreover, accumulating evidence has suggested that despite their similar origin and surgical techniques, their clinical-pathological features and long-term prognosis were reported to be inconsistent to some extent (4–9). For example, Andrianello et al. revealed that lymphatic invasion and neural invasion were more frequently detected in patients with PDCA, and patients with DCCA had a significantly better prognosis (5). However, the study by Guilbaud et al. revealed that both tumor types shared equal prognosis and oncological outcomes (8). The study introduced above either included a small sample size or just evaluated their differences from a few tumor-related features. The small sample size and the inadequate parameters continued undermining the validity of their results and conclusions. Obviously, powerful evidence is lacking, and our meta-analysis is performed to have a more comprehensive evaluation on their similarities and differences.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statements were the major guidelines observed (10). PubMed, EMBASE, and the Cochrane Library were searched until 1 May 2022. Eligible studies were restricted to comparative studies between DCCA and PDCA. The following keywords were used: distal cholangiocarcinoma, pancreatic ductal carcinoma, extra-hepatic cholangiocarcinoma, ampullary cancer, bile duct stenosis, and prognosis. Other relevant studies were also screened.
The following were the inclusion criteria: 1) comparative studies between DCCA and PDCA or various ampullary cancers, 2) studies that reported the tumor’s clinical-pathological features or oncological outcomes or long-term survival, and 3) published English articles. Abstracts, meetings, letters, reviews, or comments as well as studies that shared completely the same database were ruled out.
The specific modalities within our manuscript regarding the quality evaluation of identified studies and statistical analyses are similar to those of our previous series (11). In order to reduce the similarity index, no illustrations will be provided (Table 1).
A total of 6,932 studies were gained. Subsequently, under the inclusion and exclusion criteria, 11 studies were finally incorporated (Figure 1).
Initially, 12 studies (1, 4–9, 12–16) were identified through our search strategy. However, the study by Yeo et al. (16) (study period 1990–1996) and the study by He et al. (14) (study period 1980–2011) both came from Johns Hopkins Hospital. A complete overlap of the patient source was detected, and therefore, the study by Yeo et al. was excluded. Consequently, a total of 11 studies (1, 4–9, 12–15) with 4,698 patients with DCCA and 100,629 patients with PDCA were incorporated into our analysis. Except for the study by Garnier et al. (4), which was prospective, the remaining studies were retrospective cohort studies. All these studies reported the overall survival (OS) or disease-free survival (DFS) via the Kaplan–Meier curves. Nine studies also compared the clinical-pathological features between DCCA and PDCA (1, 4–8, 12–14). Additionally, the study by Hester et al. was based on the National Cancer Database (NCDB), and their patients were partly surgically treated and the others were undergoing palliative treatment (2004–2012) (7). Their study also analyzed the other two types of ampullary cancers, ampullary adenocarcinoma and duodenal adenocarcinoma, at the same time (7). The baseline characteristics of all studies are recorded in Table 1. There were a total of 13 measured parameters, including preoperative jaundice, preoperative stenting, R0 resection rate, lymph node metastasis, vascular invasion, neural invasion, tumor size, morbidities, postoperative biliary fistula (POBF), postoperative pancreatic fistula (POPF), mortalities, OS, and DFS. The pooled results of all available studies in measured outcomes are recorded in Table 2.
Four studies (1, 5, 8, 12) regarding patients with preoperative jaundice were incorporated, and the pooled result revealed no difference (87.2% versus 73.8%, OR = 2.39, 95% CI 0.74 to 7.74; p = 0.15) (Figure 2A). Significant heterogeneity (χ2 = 9.92, p = 0.02, I2 = 70%) was detected, and when the study by Guilbaud et al. (8) was removed, low heterogeneity with a statistical difference was then detected (94.2% versus 74.2%, OR = 4.42, 95% CI 1.96 to 9.95; p = 0.0003) (χ2 = 2.37, p = 0.31, I2 = 16%).
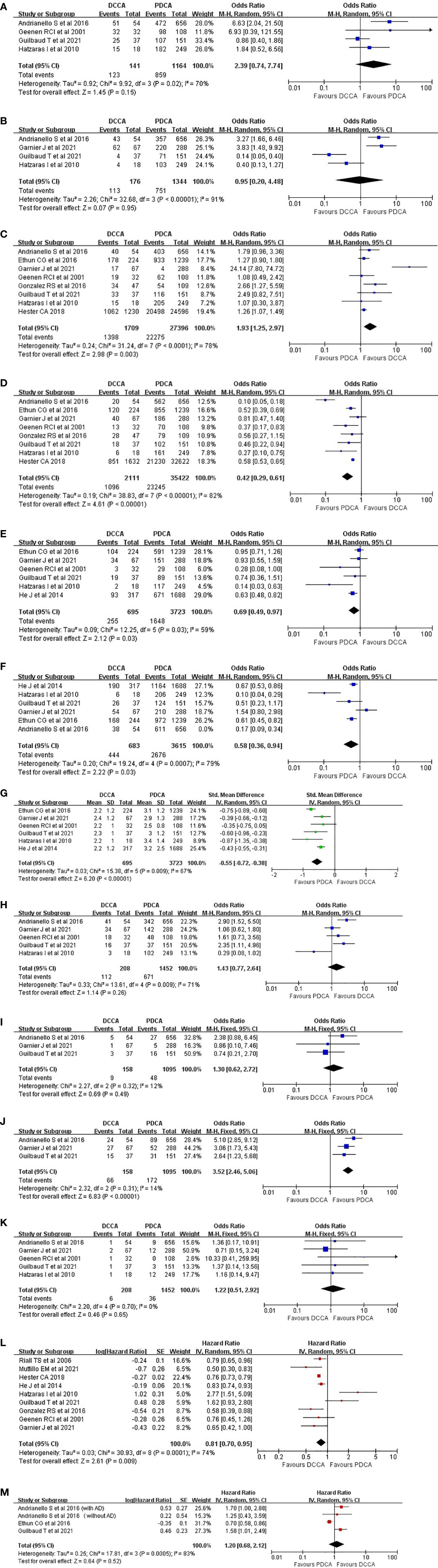
Figure 2 Pooled results regarding the tumor clinical-pathological features and long-term prognosis between DCCA and PDCA. (A) Preoperative jaundice. (B) Preoperative stenting. (C) R0 rate. (D) Lymph node metastasis. (E) Vascular invasion. (F) Neural invasion. (G) Tumor size. (H) Postoperative morbidities. (I) Postoperative biliary fistula. (J) Postoperative pancreatic fistula. (K) Mortalities. (L) Overall survival. (M) Disease-free survival. DCCA, distal cholangiocarcinoma; PDCA, pancreatic ductal adenocarcinoma.
Four studies (1, 4, 5, 8) reported the number of patients who received preoperative stenting, and the pooled result revealed no significant difference (64.2% versus 55.9%, OR = 0.95, 95% CI 0.20 to 4.48; p = 0.95) (χ2 = 32.68, p < 0.00001, I2 = 91%) (Figure 2B).
Eight studies (1, 4–8, 12, 13) were incorporated, and a significantly higher R0 rate in patients with DCCA was acquired (81.8% versus 81.3%, OR = 1.93, 95% CI 1.25 to 2.97; p = 0.003) (χ2 = 31.24, p < 0.0001, I2 = 78%) (Figure 2C). When the study by Garnier et al. (4) was removed, low heterogeneity with a more significant p-value was acquired (84.1% versus 82.2%, OR = 1.33, 95% CI 1.16 to 1.53; p < 0.0001) (χ2 = 6.27, p = 0.39, I2 = 4%).
Eight studies (1, 4–8, 12, 13) were incorporated, and a significantly higher incidence of node metastasis in patients with PDCA was observed (51.9% versus 65.6%, OR = 0.42, 95% CI 0.29 to 0.61; p < 0.00001) (χ2 = 38.83, p < 0.00001, I2 = 82%) (Figure 2D). Heterogeneity analysis indicated that the study by Andrianello et al. (5) was the major source of heterogeneity.
Six studies (1, 4, 6, 8, 12, 14) were incorporated, and a significantly higher incidence of vascular invasion was acquired in patients with PDCA (36.7% versus 44.3%, OR = 0.69, 95% CI 0.49 to 0.97; p = 0.03) (χ2 = 12.25, p = 0.03, I2 = 59%) (Figure 2E). Heterogeneity analysis indicated that the study by Ethun et al. (6) was the major source of heterogeneity (32.1% versus 42.6%, OR = 0.63, 95% CI 0.51 to 0.78; p < 0.0001) (χ2 = 7.75, p = 0.1, I2 = 48%).
Six studies (1, 4–6, 8, 14) were incorporated, and a significantly higher incidence of peri-neural infiltration in patients with PDCA was acquired (65.4% versus 77.0%, OR = 0.47, 95% CI 0.28 to 0.79; p = 0.005) (χ2 = 33.49, p < 0.00001, I2 = 85%) (Figure 2F). Heterogeneity analysis indicated the absence of a remarkable source of heterogeneity.
Six studies (1, 4, 6, 8, 12, 14) were incorporated, and the pooled result revealed that patients with PDCA had a significantly larger tumor size than patients with DCCA (weighted mean difference (WMD) = −0.55; 95% CI −0.72 to −0.38; p < 0.00001) (χ2 = 15.38, p = 0.009, I2 = 67%) (Figure 2G). Heterogeneity analysis indicated the absence of a remarkable source of heterogeneity.
Five studies (1, 4, 5, 8, 12) were incorporated, and no significant difference was acquired (53.8% versus 46.2%, OR = 1.43, 95% CI 0.77 to 2.64; p = 0.26) (Figure 2H). High heterogeneity (χ2 = 13.61, p = 0.009, I2 = 71%) was detected, and heterogeneity analysis indicated that when the study by Hatzaras et al. (1) was removed, a lower heterogeneity with a significantly higher incidence of postoperative morbidities in patients with DCCA was acquired (57.4% versus 47.3%, OR = 1.80, 95% CI 1.10 to 2.93; p = 0.02) (χ2 = 6.42, p = 0.09, I2 = 53%).
Three studies (4, 5, 8) were incorporated, and the pooled result revealed no significant difference between the two groups (5.7% versus 4.4%, OR = 1.30, 95% CI 0.62 to 2.72; p = 0.49) (χ2 = 2.27, p = 0.32, I2 = 12%) (Figure 2I).
Three studies (4, 5, 8) were incorporated, and a significantly higher incidence of pancreatic fistula in patients with DCCA was acquired (41.8% versus 15.7%, OR = 3.52, 95% CI 2.46 to 5.06; p < 0.00001) (χ2 = 2.32, p = 0.31, I2 = 14%) (Figure 2J).
Five studies (1, 4, 5, 8, 12) were incorporated, and no significant difference was detected (2.9% versus 2.5%, OR = 1.22, 95% CI 0.51 to 2.92; p = 0.65) (χ2 = 2.20, p = 0.70, I2 = 0%) (Figure 2K).
Nine studies (1, 4, 7–9, 12–15) were incorporated, and a significantly better OS was acquired in patients with DCCA (hazard ratio (HR) = 0.81, 95% CI 0.70 to 0.95, p = 0.009) (χ2 = 30.93, p = 0.0001, I2 = 74%) (Figure 2L). Significant heterogeneity was detected, and heterogeneity analysis indicated that when the study by Hatzaras I et al. (1) was removed, patients with DCCA had a much better prognosis than patients with PDCA (HR = 0.77, 95% CI 0.74 to 0.80, p < 0.00001) (χ2 = 13.84, p = 0.05, I2 = 49%).
Three studies (5, 6, 8) with four outcomes (two outcomes from the study by Andrianello et al.: with and without adjuvant therapies) were incorporated, and no significant difference was acquired (HR = 1.20, 95% CI 0.68 to 2.12, p = 0.52) (χ2 = 17.81, p = 0.0005, I2 = 83%) (Figure 2M). However, when the study by Ethun et al. was removed, low heterogeneity with a significantly better DFS was achieved in patients with DCCA (HR = 1.69, 95% CI 1.15 to 2.20, p = 0.005) (χ2 = 0.26, p = 0.88, I2 = 0%).
As shown in Table 2, among all the comparisons except for mortalities, the p-values in Egger’s test were all higher than 0.05, indicating the absence of remarkable bias. As for the comparison of postoperative mortalities, the p-value of Egger’s test was <0.05 (p = 0.011). Begg’s funnel plot and filled funnel plot (meta-trim command) were used for further evaluation (Figure S1). The result after trimming was similar to the result before trimming, indicating the absence of remarkable bias. The results of sensitivity analyses and heterogeneity analyses are recorded in the Results section of our manuscript.
DCCA and PDCA have similar malignancies, sharing the common pancreatico-biliary epithelium. Owing to the rarity of DCCA, especially in western countries, little has been known about the similarities and differences between DCCA and PDCA. Previous studies have indicated that DCCA and PDCA shared similar tumor biological features (17, 18). However, the World Health Organization (WHO) has indicated that DCCA and PDCA are two independent entities (19). Therefore, we performed the current meta-analysis to systematically evaluate the consistencies and inconsistencies of these two rare entities. Our major findings are as follows:
1. Obstructive jaundice and subsequent preoperative stenting are more frequently detected in patients with DCCA.
2. Patients with PDCA tend to have a larger tumor size, and PDCA exhibited more aggressively, with node metastasis and neural invasion more frequently detected in patients with PDCA. Patients with DCCA had a significantly higher R0 resection rate.
3. After curative-intent resection, patients with DCCA have more morbidities, and the incidence of POPF is significantly higher in patients with DCCA.
4. Patients with DCCA had a more favorable prognosis than patients with PDCA.
To accurately distinguish DCCA from PDCA preoperatively is rather technically challenging and confusing. Regarding preoperative laboratory examinations, such as tumor biomarker CA199, previous observations often showed no meaningful results (1). As for radiological approaches, such as computed tomography (CT) and magnetic resonance imaging, these modalities can contribute to confirming the site of the tumor origin of intra-pancreatic lesions to some extent. However, regarding lesions within the pancreatic head, the correct localization could be more difficult (20). Both tumors often present as a solitary mass, adjacent to the ampullary or within the pancreas in the imaging of CT scan or ultrasound. Moreover, an endoscopic exploration often failed to provide valuable information, for they are both adenocarcinomas (21). Hence, accurate diagnosis mainly relies on intra- or postoperative pathological specimen evaluation. Macroscopically, the location of the tumor epicenter may contribute to a better distinction because DCCA mainly arises from the bile duct wall, until the posterior-cranial aspect of the pancreatic head, above or at the level of the ampulla (4). The cancer tissue of DCCA often spreads in a circumferential manner and therefore forms a constrictive lesion along the bile duct. Obstructive jaundice due to tumor infiltration often occurs earlier and is rather severe, which has been validated in our study that the proportion of patients with preoperative jaundice is extremely higher in patients with DCCA (p = 0.0003). As was acquired in our analysis, the tumor size of patients with DCCA was often smaller than that of patients with PDCA (p < 0.00001). A smaller tumor size in DCCA would cause earlier-period jaundice and would lead to an early diagnosis. In contrast to DCCA, pancreatic tumors can be found in any part of the pancreas, and the obstructive is often much later due to a delayed infiltration of the common bile duct. It is also unusual for PDCA to involve the distal bile duct circumferentially in a rather disorganized manner (13). Microscopically, there is no valid evidence suggesting the accurate differentiation of DCCA and PDCA, except for cases with precursor lesions (bile ductal or pancreatic) identified. It is worth mentioning that there are no specific immune-histochemical markers for distinguishing DCCA from PDCA (4). Additionally, although the ultrasound-guided invasive tissue biopsy may provide pathological confirmation, the risk of tumor dissemination, hemorrhage, organ injuries, or inflammation has made this medical procedure more technically challenging and less widely applicable (22–24). These factors all make the precise diagnosis of DCCA and PDCA more confusing. However, recently, promising results were reported by Gkolfakis et al.; in their study, the results of a network meta-analysis indicated that 22-gauge size end-cutting fine-needle biopsy needles showed the most favorable diagnostic performance for pancreatic masses with an extremely low false-negative rate (25). This unexpected finding may help clinicians better distinguish DCCA from PDCA.
In our meta-analysis, a total of 2,261 patients with DCCA and 29,996 patients with PDCA received curative-intent PD. Pooled results revealed that patients with DCCA had a significantly higher R0 resection rate than patients with PDCA (p < 0.0001). A similar result was also reported by other authors (4, 6). Further exploring its potential reasons, we accounted for it for the following reasons. First, due to the tumor location of DCCA, that is, its location is often within the bile duct lumen, DCCA tends to cause symptomatic obstructive jaundice earlier with a less advanced stage and a smaller tumor size at the time of diagnosis as well as patients receiving curative surgery. Numerous studies have also reported that patients with DCCA might present with more symptomatic pancreatic symptoms, including pain, abdominal fullness, early satiety, and weight loss (26, 27). Second, previous studies have proved that patients with PDCA had a significantly higher incidence of portal vein reconstruction (4, 6). Moreover, lymph node metastasis (p < 0.00001) and neural invasion (p = 0.005) were more frequently detected in patients with PDCA, reflecting the fact that the majority of patients with PDCA were diagnosed in a more advanced stage, and therefore, achieving a negative margin could be more difficult. Our meta-analysis revealed a significantly lower R0 rate in patients with PDCA (p < 0.0001). However, conversely, the study by Garnier et al. (4) reported a similar R0 resection rate among patients with DCCA and PDCA. Garnier et al. analyzed its potentially reasonable reasons and accounted for the modern cohort (2010–2018) of their study. Compared with the study by Ethun et al. (6) (2000–2015), the great evolvement in PD specimen analyses in the late 2000s, that is, the highlight of the venous groove invasion in patients with DCCA since then, would increase the R1 rate of DCCA (28). Additionally, in the study by Garnier et al., the application of neo-adjuvant therapies in patients with PDCA might also cause a lower R1 rate (4). After neo-adjuvant therapies, residual cancer often consists of scattered tumor foci separated by stretches of non-neoplastic tissue, which would lead to a higher possibility of negative margins (29). In short, based on our findings as well as the observations reported by others, PDCA tends to be more advanced with a lower R0 resection rate and higher incidences of node metastasis and neural invasion. Evaluating the surgical margins can be problematic after the application of neo-adjuvant therapies, and future well-designed studies are required for further exploration.
After the application of curative PD, patients with DCCA had a significantly higher incidence of postoperative complications, especially the incidence of POPF (Clavien grade B to C) (p < 0.00001) and other pancreas-associated complications. Our findings were consistent with the observations reported in previously published literature (16, 30, 31). Based on the International Study Group on Pancreatic Fistula, pancreatic duct size <3 mm and soft pancreatic parenchyma are all risk factors for POPF (32). As was acquired in our analysis, patients with DCCA had a smaller tumor size than patients with PDCA (p < 0.00001). The DCCA would mainly infiltrate the main bile duct rather than cause obstructive pancreatitis. An un-dilated pancreatic duct and the soft pancreatic parenchyma without tumor infiltration would greatly increase the risk of POPF. One previous study has demonstrated that a hard pancreas is advantageous for surgeons because the infiltration of the pancreatic duct seemed to have increased the mechanical strength, leading to a more solid pancreaticojejunostomy (33). Consequently, the earlier diagnosis of DCCA tends to cause more severe post-anastomosis pancreatic fistula, linked with postoperative mortalities, various complications (delayed gastric emptying), and a longer postoperative hospital stay (34).
The prognosis of patients with DCCA is significantly better than that of patients with PDCA in our analysis (p < 0.00001), which is in line with the observations reported by others (4–6, 9, 35). The most likely explanation for this phenomenon is that patients with PDCA were more frequently diagnosed in an advanced stage with delayed-observed obstructive jaundice. The incidences of node metastasis and neural invasion were significantly higher in patients with PDCA. Node metastasis and neural invasion have both been demonstrated as independent prognostic factors for peri-ampullary cancers (1). With regard to patients with DCCA, earlier-detected obstructive jaundice would introduce an earlier diagnosis as well as more timely curative surgery. Consequently, a significantly higher R0 rate could be detected more frequently in patients with DCCA. One previous meta-analysis with 2,063 patients with DCCA included has indicated that peri-neural invasion, R0 resection rate, and node metastasis were all independent prognostic factors (36). Lymph node status, margin status, and neural invasion have also been regarded as the prognostic factors for peri-ampullary cancers in general (37, 38). Interestingly, in the study by Guilbaud et al., after controlling the factors margin status, node metastasis, and tumor size via propensity score matching analysis, patients with DCCA had a similar prognosis versus patients with PDCA (8), which further validated the fact that tumor biology seemed to have the strongest weight of evidence of predicting survival (39–41). Moreover, the earlier diagnosis of DCCA with less advanced disease often leads to a higher R0 rate, which further promotes a better prognosis of DCCA.
There are several limitations to our study. First, the retrospective nature of the majority of included studies would introduce bias. Second, owing to the rarity of DCCA, especially in western countries, the comparison between DCCA and PDCA would be less convincing. Third, the estimation of HRs via Tierney’s method might introduce bias. Fourth, the deficiency of the original date also hindered deeper exploration.
DCCA had more favorable tumor pathological features and prognosis than PDCA, and preoperative jaundice was more common in patients with DCCA. Moreover, even after the same surgical procedure, PD, a significantly higher incidence of postoperative complications, especially POPF, was more common in patients with DCCA.
T-RL and J-MW contributed equally to the study. T-RL contributed to data acquisition and drafted the manuscript. W-JM, Y-FH, Y-SD and Y-WJ were involved in editing the manuscript. F-YL contributed to the study design and revision of the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYJC21046); 1.3.5 project for disciplines of excellence-Clinical Research Incubation Project, West China Hospital, Sichuan University (2021HXFH001); Natural Science Foundation of Sichuan Province (2022NSFSC0806); National Natural Science Foundation of China for Young Scientists Fund (82203650, 82203782), Sichuan Science and Technology Program (2021YJ0132, 2021YFS0100); the fellowship of China Postdoctoral Science Foundation (2021M692277); Sichuan University-Zigong School-local Cooperation project (2021CDZG-23); Science and Technology project of the Health planning committee of Sichuan (21PJ046); and Post-Doctor Research Project, West China Hospital, Sichuan University (2021HXBH127). The funding source had no role in the design and preparation of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1042493/full#supplementary-material
Supplementary Figure 1 | Further evaluation on the potential publication bias within the comparison of Mortalities. (A) Begg’s funnel plot. (B) Filled funnel plot (meta-trim command).
1. Hatzaras I, George N, Muscarella P, Melvin WS, Ellison EC, Bloomston M. Predictors of survival in periampullary cancers following pancreaticoduodenectomy. Ann Surg Oncol (2010) 17(4):991–7. doi: 10.1245/s10434-009-0883-9
2. Cameron JL, He J. Two thousand consecutive pancreaticoduodenectomies. J Am Coll Surg (2015) 220(4):530–6. doi: 10.1016/j.jamcollsurg.2014.12.031
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin (2016) 66(1):7–30. doi: 10.3322/caac.21332
4. Garnier J, Ewald J, Poizat F, Traversari E, Marchese U, Palen A, et al. Prospective evaluation of resection margins using standardized specimen protocol analysis among patients with distal cholangiocarcinoma and pancreatic ductal adenocarcinoma. J Clin Med (2021) 10(15):3247. doi: 10.3390/jcm10153247
5. Andrianello S, Marchegiani G, Malleo G, Rusev BC, Scarpa A, Bonamini D, et al. Over 700 whipples for pancreaticobiliary malignancies: Postoperative morbidity is an additional negative prognostic factor for distal bile duct cancer. J Gastrointest Surg (2017) 21(3):527–33. doi: 10.1007/s11605-016-3328-3
6. Ethun CG, Lopez-Aguiar AG, Pawlik TM, Poultsides G, Idrees K, Fields RC, et al. Distal cholangiocarcinoma and pancreas adenocarcinoma: Are they really the same disease? a 13-institution study from the US extrahepatic biliary malignancy consortium and the central pancreas consortium. J Am Coll Surg (2017) 224(4):406–13. doi: 10.1016/j.jamcollsurg.2016.12.006
7. Hester CA, Dogeas E, Augustine MM, Mansour JC, Polanco PM, Porembka MR, et al. Incidence and comparative outcomes of periampullary cancer: A population-based analysis demonstrating improved outcomes and increased use of adjuvant therapy from 2004 to 2012. J Surg Oncol (2019) 119(3):303–17. doi: 10.1002/jso.25336
8. Guilbaud T, Girard E, Lemoine C, Schlienger G, Alao O, Risse O, et al. Intra-pancreatic distal cholangiocarcinoma and pancreatic ductal adenocarcinoma: A common short and long-term prognosis? Updates Surg (2021) 73(2):439–50. doi: 10.1007/s13304-021-00981-0
9. Muttillo EM, Ciardi A, Saullo P, Troiano R, Masselli G, Guida M, et al. A prognostic score for predicting survival in patients with pancreatic head adenocarcinoma and distal cholangiocarcinoma. In Vivo (Athens Greece) (2021) 35(1):507–15. doi: 10.21873/invivo.12285
10. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PloS Med (2009) 6(7):e1000100. doi: 10.1371/journal.pmed.1000100
11. Lv TR, Liu F, Hu HJ, Regmi P, Ma WJ, Yang Q, et al. The role of extra-hepatic bile duct resection in the surgical management of gallbladder carcinoma. a first meta-analysis. Eur J Surg Oncol (2022) 48(3):482–91. doi: 10.1016/j.ejso.2021.11.131
12. van Geenen RC, van Gulik TM, Offerhaus GJ, de Wit LT, Busch OR, Obertop H, et al. Survival after pancreaticoduodenectomy for periampullary adenocarcinoma: An update. Eur J Surg Oncol (2001) 27(6):549–57. doi: 10.1053/ejso.2001.1162
13. Gonzalez RS, Bagci P, Basturk O, Reid MD, Balci S, Knight JH, et al. Intrapancreatic distal common bile duct carcinoma: Analysis, staging considerations, and comparison with pancreatic ductal and ampullary adenocarcinomas. Modern Pathol (2016) 29(11):1358–69. doi: 10.1038/modpathol.2016.125
14. He J, Ahuja N, Makary MA, Cameron JL, Eckhauser FE, Choti MA, et al. 2564 Resected periampullary adenocarcinomas at a single institution: Trends over three decades. HPB (Oxford) (2014) 16(1):83–90. doi: 10.1111/hpb.12078
15. Riall TS, Cameron JL, Lillemoe KD, Winter JM, Campbell KA, Hruban RH, et al. Resected periampullary adenocarcinoma: 5-year survivors and their 6- to 10-year follow-up. Surgery (2006) 140(5):764–72. doi: 10.1016/j.surg.2006.04.006
16. Yeo CJ, Cameron JL, Sohn TA, Lillemoe KD, Pitt HA, Talamini MA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: Pathology, complications, and outcomes. Ann Surg (1997) 226(3):248–57; discussion 57-60. doi: 10.1097/00000658-199709000-00004
17. Bledsoe JR, Shinagare SA, Deshpande V. Difficult diagnostic problems in pancreatobiliary neoplasia. Arch Pathol Lab Med (2015) 139(7):848–57. doi: 10.5858/arpa.2014-0205-RA
18. Nakanuma Y, Sato Y. Hilar cholangiocarcinoma is pathologically similar to pancreatic duct adenocarcinoma: Suggestions of similar background and development. J Hepatobiliary Pancreat Sci (2014) 21(7):441–7. doi: 10.1002/jhbp.70
19. Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology (2020) 76(2):182–8. doi: 10.1111/his.13975
20. Mangiavillano B, Mariani AA, Petrone MC. An intrapancreatic cholangiocarcinoma detected with optical coherence tomography during endoscopic retrograde cholangiopancreatography. Clin Gastroenterol Hepatol (2008) 6(6):A30. doi: 10.1016/j.cgh.2008.02.004
21. Dumonceau JM, Polkowski M, Larghi A, Vilmann P, Giovannini M, Frossard JL, et al. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European society of gastrointestinal endoscopy (ESGE) clinical guideline. Endoscopy (2011) 43(10):897–912. doi: 10.1055/s-0030-1256754
22. Lewis AR, Valle JW, McNamara MG. Pancreatic cancer: Are "liquid biopsies" ready for prime-time? World J Gastroenterol (2016) 22(32):7175–85. doi: 10.3748/wjg.v22.i32.7175
23. Fujii LL, Levy MJ. Basic techniques in endoscopic ultrasound-guided fine needle aspiration for solid lesions: Adverse events and avoiding them. Endoscopic Ultrasound (2014) 3(1):35–45. doi: 10.4103/2303-9027.123006
24. Rimbas M, Deaconu M, Croitoru A, Haidar A. Sudden appearance of free fluid during endoscopic ultrasound-guided fine-needle aspiration. Endoscopic Ultrasound (2016) 5(1):55–7. doi: 10.4103/2303-9027.175900
25. Gkolfakis P, Crinò SF, Tziatzios G, Ramai D, Papaefthymiou A, Papanikolaou IS, et al. Comparative diagnostic performance of end-cutting fine-needle biopsy needles for EUS tissue sampling of solid pancreatic masses: A network meta-analysis. Gastrointest Endosc (2022) 95(6):1067–77.e15. doi: 10.1016/j.gie.2022.01.019
26. Veillette G, Castillo CF. Distal biliary malignancy. Surg Clinics North America (2008) 88(6):1429–47. doi: 10.1016/j.suc.2008.07.003
27. DiMagno EP. Pancreatic cancer: Clinical presentation, pitfalls and early clues. Ann Oncol (1999) 10 Suppl 4:140–2. doi: 10.1093/annonc/10.suppl_4.S140
28. Kamposioras K, Anthoney A, Fernández Moro C, Cairns A, Smith AM, Liaskos C, et al. Impact of intrapancreatic or extrapancreatic bile duct involvement on survival following pancreatoduodenectomy for common bile duct cancer. Br J Surg (2014) 101(2):89–99. doi: 10.1002/bjs.9367
29. Kleive D, Labori KJ, Line PD, Gladhaug IP, Verbeke CS. Pancreatoduodenectomy with venous resection for ductal adenocarcinoma rarely achieves complete (R0) resection. HPB (Oxford) (2020) 22(1):50–7. doi: 10.1016/j.hpb.2019.05.005
30. Vollmer CM Jr., Sanchez N, Gondek S, McAuliffe J, Kent TS, Christein JD, et al. A root-cause analysis of mortality following major pancreatectomy. J Gastrointest Surg (2012) 16(1):89–102; discussion -3. doi: 10.1007/s11605-011-1753-x
31. Čečka F, Jon B, Šubrt Z, Ferko A. Clinical and economic consequences of pancreatic fistula after elective pancreatic resection. Hepatobiliary Pancreatic Dis Int (2013) 12(5):533–9. doi: 10.1016/s1499-3872(13)60084-3
32. Pratt WB, Callery MP, Vollmer CM Jr. Risk prediction for development of pancreatic fistula using the ISGPF classification scheme. World J Surg (2008) 32(3):419–28. doi: 10.1007/s00268-007-9388-5
33. Belyaev O, Rosenkranz S, Munding J, Herzog T, Chromik AM, Tannapfel A, et al. Quantitative assessment and determinants of suture-holding capacity of human pancreas. J Surg Res (2013) 184(2):807–12. doi: 10.1016/j.jss.2013.04.017
34. Callery MP, Pratt WB, Kent TS, Chaikof EL, Vollmer CM Jr. A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J Am Coll Surg (2013) 216(1):1–14. doi: 10.1016/j.jamcollsurg.2012.09.002
35. Oh TG, Chung MJ, Bang S, Park SW, Chung JB, Song SY, et al. Comparison of the sixth and seventh editions of the AJCC TNM classification for gallbladder cancer. J Gastrointest Surg (2013) 17(5):925–30. doi: 10.1007/s11605-012-2134-9
36. Wellner UF, Shen Y, Keck T, Jin W, Xu Z. The survival outcome and prognostic factors for distal cholangiocarcinoma following surgical resection: A meta-analysis for the 5-year survival. Surg Today (2017) 47(3):271–9. doi: 10.1007/s00595-016-1362-0
37. Bergeat D, Turrini O, Courtin-Tanguy L, Truant S, Darnis B, Delpero JR, et al. Impact of adjuvant chemotherapy after pancreaticoduodenectomy for distal cholangiocarcinoma: A propensity score analysis from a French multicentric cohort. Langenbecks Arch Surg (2018) 403(6):701–9. doi: 10.1007/s00423-018-1702-1
38. Jun SY, Sung YN, Lee JH, Park KM, Lee YJ, Hong SM. Validation of the eighth American joint committee on cancer staging system for distal bile duct carcinoma. Cancer Res Treat (2019) 51(1):98–111. doi: 10.4143/crt.2017.595
39. Cameron JL, Crist DW, Sitzmann JV, Hruban RH, Boitnott JK, Seidler AJ, et al. Factors influencing survival after pancreaticoduodenectomy for pancreatic cancer. Am J Surg (1991) 161(1):120–4; discussion 4-5. doi: 10.1016/0002-9610(91)90371-J
40. Yeo CJ, Sohn TA, Cameron JL, Hruban RH, Lillemoe KD, Pitt HA. Periampullary adenocarcinoma: Analysis of 5-year survivors. Ann Surg (1998) 227(6):821–31. doi: 10.1097/00000658-199806000-00005
Keywords: pancreatic ductal adenocarcinoma, pancreaticoduodenectomy, prognosis, distal cholangiocarcinoma, ampullary cancer
Citation: Lv T-R, Wang J-M, Ma W-J, Hu Y-F, Dai Y-S, Jin Y-W and Li F-Y (2022) The consistencies and inconsistencies between distal cholangiocarcinoma and pancreatic ductal adenocarcinoma: A systematic review and meta-analysis. Front. Oncol. 12:1042493. doi: 10.3389/fonc.2022.1042493
Received: 12 September 2022; Accepted: 09 November 2022;
Published: 12 December 2022.
Edited by:
Zhe Cao, Peking Union Medical College Hospital (CAMS), ChinaReviewed by:
Lingdi Yin, Nanjing Medical University, ChinaCopyright © 2022 Lv, Wang, Ma, Hu, Dai, Jin and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan-Wen Jin, eWFud2ppbkAxMjYuY29t; Fu-Yu Li, bGZ5Xzc0QGhvdG1haWwuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.