
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 09 December 2022
Sec. Breast Cancer
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.1042451
This article is part of the Research Topic Novel Signaling Pathways and Therapy in Breast Cancer View all 6 articles
Objective: To evaluate the efficacy and safety of anlotinib-based treatment in metastatic breast cancer (MBC) patients with failure of standard treatment.
Methods: We collected the medical data of 56 female patients with the diagnosis of MBC and had failed the standard treatment before. These patients received at least two cycles of anlotinib-based treatment as the second-line or beyond treatment between October 2019 and April 2022 in Jiangsu Cancer Hospital. The primary endpoint of our study was progression-free survival (PFS), and it was estimated with Kaplan-Meier. The second end points were disease control rate (DCR), objective response rate (ORR), and side effects.
Results: The median PFS time of a total of 56 patients was 5.7 months (95% CI, 3.17-8.23 months). The ORR and DCR was 28.6% and 71.4%, respectively. In second-line, third-line, and beyond treatment, the median PFS was 11.7 months, 8.7 months, and 4.7 months, respectively. In different subtype of breast cancer, the median PFS was 5.6 months, 5.7months, and 6.4 months in human epidermal growth factor receptor 2 positive (HER2+), hormone receptor positive and HER2 negative (HR+/HER2-), and triple negative breast cancer (TNBC) patients, respectively. Most adverse effects were clinically manageable, and the most common events were platelet count decrease (35.7%), hand-foot syndrome (19.6%), diarrhea (19.6%), and fatigue (17.9%). The most common grade 3 and 4 adverse events were platelet count decrease (10.7%), diarrhea (7.1%), and oral mucositis (5.4%).
Conclusion: Anlotinib-based treatment showed good efficacy and manageable toxicity in multi-line treatment of MBC patients who failed the standard treatment.
Breast cancer is now the most common malignant tumor and the leading cause of cancer-related mortality in women. The 5-year relative survival rate for breast cancer is 90%, however, 30-40% of early-stage breast cancer patients still experience recurrence and metastasis, becoming incurable metastatic breast cancer (MBC) (1, 2). With the emerging targeted therapies such as CDK4/6 inhibitors, mTOR inhibitors, and anti-HER2 targeted therapy, the prognosis of breast cancer patients has been improved. Nevertheless, for patients with MBC and have received first- or second-line standard treatment, there is currently no standard treatment guideline for them. Treatment decisions in this setting have a great impact on the prognosis of the patients.
Breast cancer is an angiogenesis-dependent tumor (3), the rapid growth of the tumor requires sufficient blood supply to provide nutrients. Folkman proposed that angiogenesis is not only a prerequisite for tumor growth but also an essential factor in promoting tumor metastasis, we can inhibit tumor growth by inhibiting angiogenesis (4, 5). Vascular endothelial growth factor (VEGF) is essential for developing the vascular system at the early stage of the tumor and plays a crucial role in tumor proliferation and metastasis. The expression of VEGF in breast cancer tissue is 7 times higher than in normal tissue (6, 7). VEGF secreted by cancer cells acts on the vascular endothelial growth factor receptors (VEGFR) of vascular endothelial cells in the adjacent stroma, promotes the division and proliferation of vascular endothelial cells, induces tumor angiogenesis, and increases vascular permeability. It can also activate VEGFR-mediated downstream signal transduction. In addition, VEGF produced by cancer cells acts on the VEGF/VEGFR autocrine loop to promote cancer cell proliferation and facilitate evasion of apoptosis. The inhibition of tumor angiogenesis is an important mechanism and target of breast cancer anti-tumor therapy (7). The main categories of VEGF targeted therapy are currently monoclonal antibodies and small-molecule antiangiogenic tyrosine kinase inhibitors (TKIs). Monoclonal antibodies represented by bevacizumab showed conflicting results in clinical trials while the efficacy of anti-angiogenic TKIs also remains controversial. The role of anti-angiogenic therapy in breast cancer is still unclear.
Anlotinib is a new type of small-molecule antiangiogenic tyrosine kinase inhibitor that targets VEGFR, fibroblast growth factor receptor (FGFR), platelet-derived growth factor receptors (PDGFR), and c-kit (8). Anlotinib inhibits cell migration and capillary-like tube formation, and angiogenesis induced by VEGF.Anlotinib also decreased the expression of proangiogenic factors, enhanced the expression of immune cell adhesion molecules and chemokines and their receptors. It suppressed tumor angiogenesis and normalized the remaining blood vessels (8, 9). It has shown significant efficacy and been approved for indication in some malignant carcinomas such as advanced non-small cell lung cancer (NSCLC) and advanced soft tissue sarcoma in China (10–12). Professor Yuan had presented the good results of the treatment of anlotinib in advanced HER2 negative breast cancer in the SABCS meeting in 2019 (13). Based on these previous studies, we explored the use of anlotinib-based treatment in breast cancer patients who had failed standard therapy. In this study, we retrospectively analyzed the efficacy and safety of anlotinib-based treatment in these patients.
This study was a retrospective single-center analysis conducted in the Jiangsu Cancer Hospital. MBC patients with the failure of standard treatment and received anlotinib-based treatment at our hospital as the second-line or beyond treatment between October 2019 and April 2022 were enrolled. We collected the medical data of 56 female patients. These patients were aged 34-76 years old with Eastern Cooperative Oncology Group (ECOG) performance status of 0-2.
Patients used anlotinib in combination with chemotherapy or immunotherapy. Chemotherapy drugs mainly include capecitabine, nab-paclitaxel and vinorelbine. Capecitabine was given at 1000mg/m2 twice daily for 14 days on followed by 7 days off, nab-paclitaxel was given at 125 mg/m2 on days 1 and 8, and vinorelbine was given at 25 mg/m2 on days 1 and 8. We mainly use tislelizumab, an anti-human programmed cell death 1 (PD-1) monoclonal IgG4 antibody, at the dosage of 200 mg every 3 weeks for immunotherapy. HER2-targeting therapies were also used in HER2-positive breast cancer patients. All patients received anlotinib at a dosage of 12 mg, 10 mg, or 8 mg at the beginning. Anlotinib is an oral small molecule inhibitor of multiple receptor tyrosine kinases that is being co-developed by Jiangsu Chia-Tai Tianqing Pharmaceutical and Advenchen Laboratories for the treatment of advanced cancer (14). Anlotinib is available in three dose levels of 12 mg, 10 mg, and 8 mg. The recommended dosage of anlotinib is 12 mg once daily taken orally continuously for 2 weeks on and one week off, 21 days as one cycle. For patients with good general conditions and few adverse reactions to previous chemotherapy, physicians would use a standard dose (12 mg) of anlotinib. For patients with poor general conditions and significant adverse reactions to prior chemotherapy, considering that these patients may not be able to tolerate the 12mg dose, the physician would choose a 10mg or 8mg dose of the drug according to the specific situation the patient. Doses modifications were allowed when patients experienced drug-related toxicity (14, 15).Treatment was continued until disease progression or unacceptable toxicity.
Treatment response was assessed every two cycles according to the Response Evaluation Criteria in Solid Tumors (RECIST 1.1). The primary endpoint of our study was progression-free survival (PFS), and it was defined as the time from the onset of anlotinib to disease progression or death. Other evaluations of the therapeutic effects included the objective response rate (ORR) and disease control rate (DCR). Complete response (CR) was defined as complete resolution of all target foci; partial response (PR) refers to conditions with a ≥30% length reduction for the baseline foci; progression of disease (PD) refers to conditions with a total length increase of ≥20% for the baseline foci or the appearance of one or more new foci(s); stable disease (SD) refers to conditions between PD and PR. ORR was defined as the proportion of patients who had a PR or CR to therapy. DCR was defined as the proportion of patients who achieved CR, PR, and SD in response to therapy. Adverse events were evaluated according to the Common Terminology Criteria for Adverse Events version 5.0 (CTCAE 5.0).
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics board of Jiangsu Cancer Hospital (No. 2020-042), and individual consent for this retrospective analysis was waived.
PFS was estimated using the Kaplan-Meier method, and comparisons were calculated using the log-rank test. Data processing and analysis were performed using SPSS version 26.0 (IBM, New York, USA). The significance was accepted when P<0.05.
The median age of a total of 56 patients was 53 years. Among them, 14 patients (25.0%) had histologically confirmed HER2+, 15 patients (26.8%) were HR+/HER2-, and 27 patients (48.2%) were diagnosed with TNBC. Anlotinib-based treatment was used in second-line, third-line, and beyond treatment in 11 (19.6%), 15 (26.8%), and 30 (53.6%) patients, respectively. All patients received taxane in the (neo)adjuvant or metastatic setting. The median number of treatment lines was 4. Anlotinib was used in combination with chemotherapy or immunotherapy. A summary of patients’ characteristics was shown in Table 1.
The median PFS of patients treated with anlotinib-based treatment was 5.7 months (95% CI, 3.17-8.23 months). In the subgroup, the median PFS was 11.7 months, 8.7 months, and 4.7 months in second-line, third-line and later-line treatment, respectively. The median PFS was 5.6 months, 5.7months, and 6.4 months in HER2+, HR+/HER2-, and TNBC patients, respectively (Figure 1). Of the total of 56 patients, no patients achieved CR, 16 (28.6%) had PR, 24 (42.9%) had SD, and 12 (21.4%) had PD. The ORR was 28.6% and the DCR was 71.4% (Figure 2). In terms of treatment regimes, most patients (91.1%) used anlotinib in combination with chemotherapy. The combination with capecitabine presented longer PFS (9.6 months, 95% CI, 8.35-10.85 months). Five TNBC patient used anlotinib plus immunotherapy, the median PFS was 10.2 months (95% CI, 0.53-19.87 months). The results are presented in Table 2.
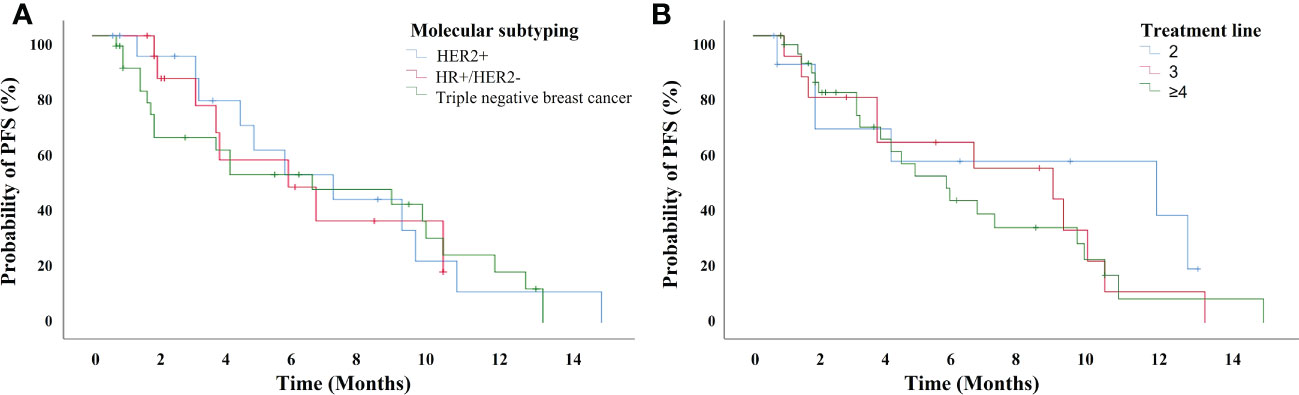
Figure 1 The progression-free survival for 56 breast cancer patients treated with anlotinib-based treatment (A) with different subtyping, and (B) in different treatment lines.
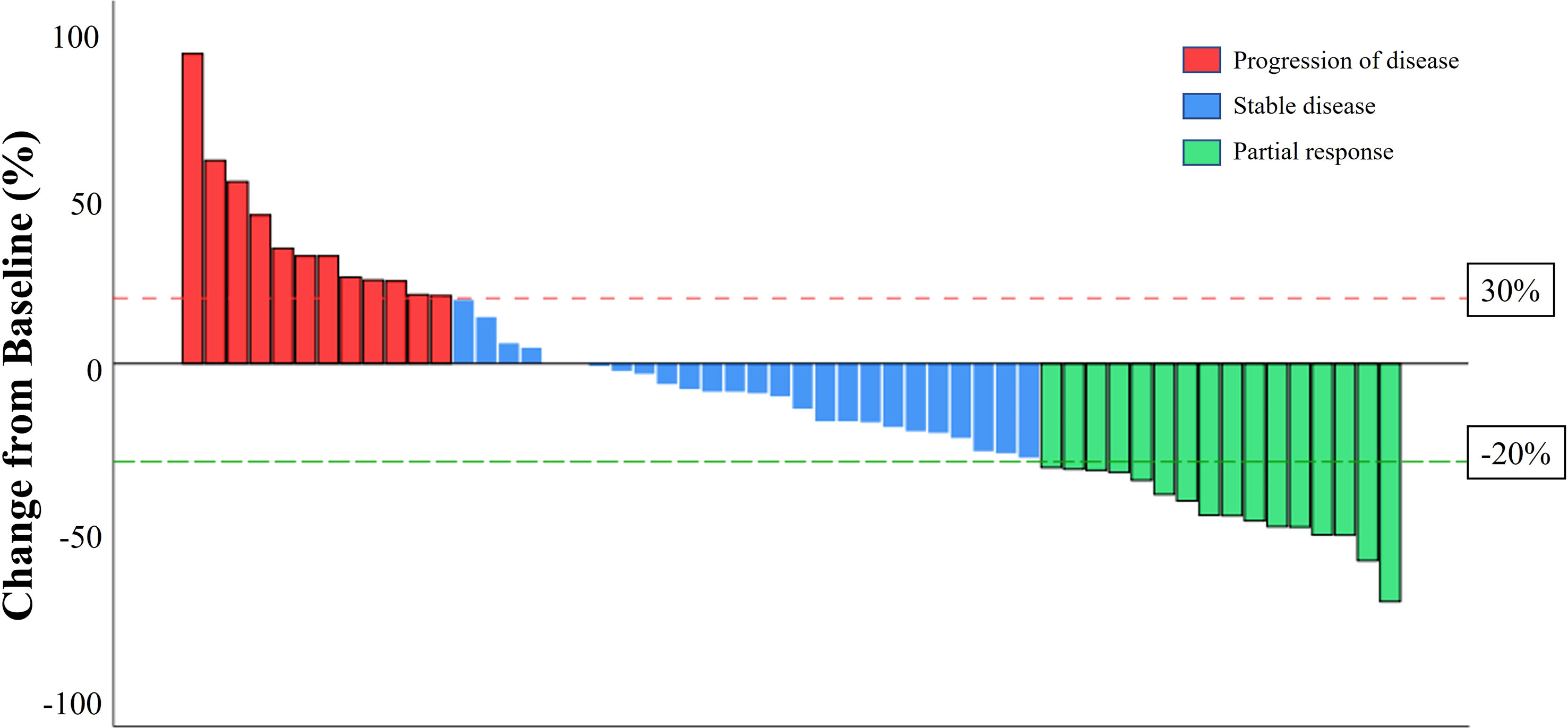
Figure 2 A waterfall plot of the best percentage change in the sum of the diameters of target lesions at any time in patient with measureable disease at baseline who received anlotinib-based treatment.
We also analyzed the efficacy of patients with brain metastases. In our study, 15 patients had breast cancer with brain metastases diagnosed at baseline and 2 patients developed brain metastases during the treatment with anlotinib. Of the 15 patients with baseline brain metastases, nine patients had prior brain radiation therapy and six patients had brain radiation therapy within the past 6 months. A total of 14 patients had measurable brain lesions, 6 patients had SD, 5 patients had PR, and 3 patients had PD. One patient with non-measurable disease at baseline had SD. The ORR in central nervous system (CNS) was 33.3% and the DCR in CNS was 80.0%. The CNS PFS was 9.4 months (95% CI, 4.04-14.76 months). As for the six patients had brain radiation therapy within the past 6 months, the CNS ORR was 33.3%, and the CNS DCR was 66.7%.
No patients experienced drug-related deaths. Five people (8.9%) discontinued the drug due to adverse reactions. Among them, grade 3-4 bone marrow suppression represented by neutropenia and thrombocytopenia are often the main reasons leading to dose reduction or drug withdrawal. Thrombocytopenia is the most common adverse event. Twenty patients (35.7%) had different degrees of platelet count decrease during the treatment of anlotinib. Among them, four patients (7.1%) had a grade 4 platelet count decrease. The platelet counts usually returned to normal after symptomatic treatment, including recombinant human thrombopoietin (rhTPO) and eltrombopag. Eleven people (19.6%) developed the hand-foot syndrome, and two (3.6%) of them developed grade 3 hand-foot syndrome. Gastrointestinal reactions such as anorexia (16.1%) and diarrhea (19.6%) are also common adverse reactions, but most patients deem these events tolerable. Other common adverse reactions include fatigue (17.9%), hypertension (16.1%), epistaxis (8.9%), and oral mucositis (7.1%) (Table 3).
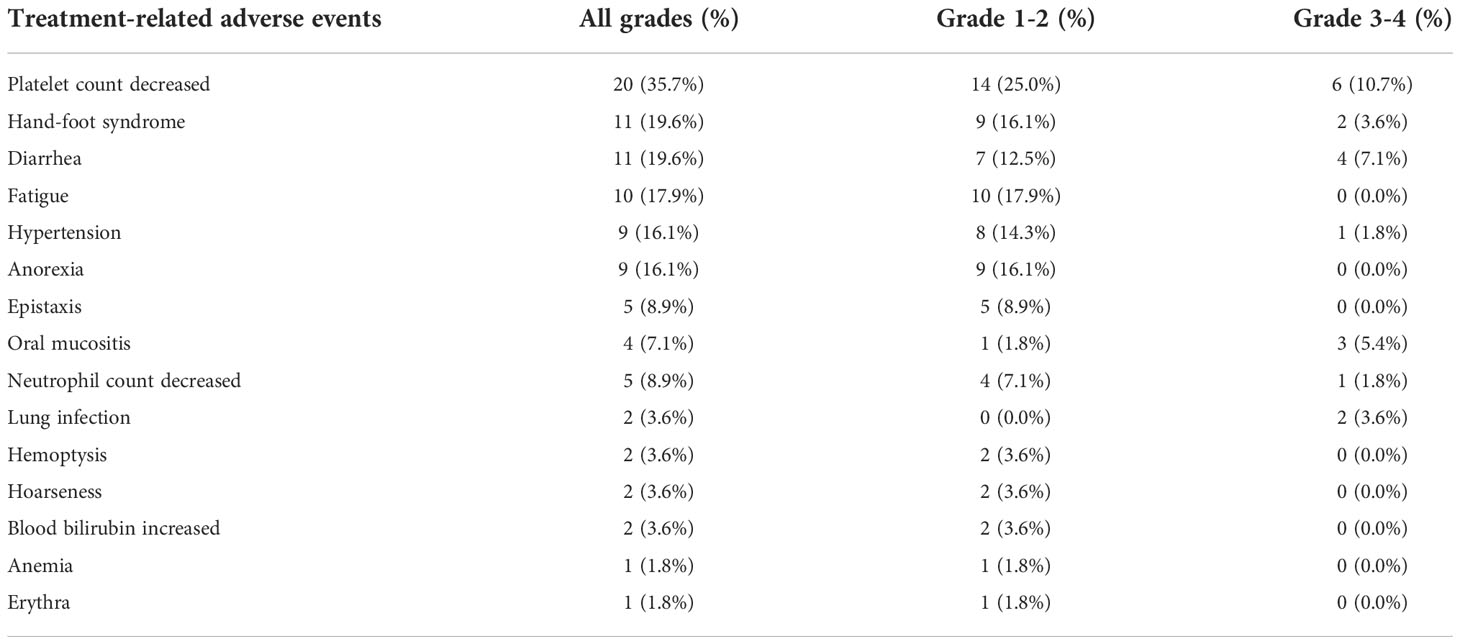
Table 3 Adverse events that occurred in 56 patients with metastatic breast cancer during treatment with anlotinib-based therapy.
There is currently no standard treatment for patients with MBC who have progressed after standard treatment. The inhibition of tumor angiogenesis can effectively inhibit breast cancer growth in preclinical studies (3). Monoclonal antibodies represented by bevacizumab have shown certain effects in clinical application. E2100, AVADO and RIBBON-1 trails confirmed that bevacizumab combined with chemotherapy can improve patients’ PFS and ORR, but not overall survival (OS) (16–18). However, in the RIBBON-2 study, the use of bevacizumab combined with chemotherapy in second-line and beyond patients showed no benefit in PFS, ORR or OS (19). Taking the inconsistent efficacy and relatively high rates of adverse events into account, U.S. Food and Drug Administration (FDA) revoked the approval of bevacizumab with paclitaxel for the treatment of women with HER2-negative MBC. In the studies of anti-angiogenic TKIs, the efficacy also remains controversial. The SOLTI-0701 study reported PFS benefit for sorafenib plus capecitabine as first- or second-line treatment, and the NU07B1 study showed a not statistically significant increase in PFS when combined with first-line paclitaxel (20, 21). With all that in mind, the efficacy is still ambiguous in clinic. Anlotinib is a new type of anti-angiogenic TKI. Yuan’s phase II study of anlotinib in pre-treated HER2 negative MBC showed that in patients had treatment failure after at least one prior chemotherapy regimen in the metastatic setting, the median PFS for anlotinib was 5.22 months (13), indicating a potential new treatment option for breast cancer. Thus, we began to try adding anlotinib to doctor’s choice of treatment, and we have indeed found that it has a good effect on some patients. However, few research on the efficacy of the clinical practice of anlotinib in MBC patients has been made. We hope that this retrospective study can provide clinicians with more clinical data about this drug and a new perspective for the treatment of MBC.
The median PFS time of a total of 56 patients was 5.7 months (95% CI, 3.17-8.23 months). In second-line, third-line, and beyond treatment, the median PFS was 11.7 months, 8.7 months, and 4.7 months, respectively. Since our study was a single-arm retrospective study with no control group, we investigated the previous single-arm retrospective study on MBC patients in our hospital. In the clinical study of platinum-based chemotherapy (not including anlotinib) for advanced MBC, the median time to progression (TTP) of second and third-line treatment was 6.0 and 3.7 months (22).
In our study, the median PFS of anlotinib-based treatment in HR+/HER2- and HER2+ patients are 5.7 months and 5.6 months while the median PFS of TNBC patients is 6.4 months. We believed that anlotinib may be more effective in TNBC. However, due to our small sample size, this hypothesis cannot be demonstrated through statistical data for the time being. The addition of angiogenic therapy extends PFS and OS in TNBC subgroup analysis of RIBBON-2 trial (23). We hope that prospective studies with large samples can help us further improve. TNBC lacks therapeutic targets. Although immunotherapy and PAPR inhibitors have changed the treatment pattern of metastatic TNBC in recent years, the efficacy of immunotherapy remains controversial and PAPR inhibitors are only suitable for patients with BRCA mutations. Currently, sacituzumab govitecan can be considered in metastatic TNBC with a median PFS of 5.6 months in patients have received at least one prior treatment for advanced disease (24). According to our study, anlotinib-based therapy showed comparable efficacy in TNBC with the median PFS of 6.4 months in second-line or beyond treatment. Although there is no randomized controlled trial comparing anlotinib versus sacituzumab govitecan, considering the high price and poor accessibility of sacituzumab govitecan in Chinese patients, anlotinib is more feasible for Chinese patients.
In terms of treatment regimes, 51 patients were treated in combination with chemotherapy. Among these patients, the capecitabine team showed better PFS benefit. The median PFS of capecitabine and anlotinib reached 9.6 months. Capecitabine reversed the inhibitory effects of tumor angiogenesis and tumor growth under anti-VEGF antibody treatment (25). The satisfying results of capecitabine and anlotinib may be attributed to the synergistic anti-angiogenesis effect of these two drugs. In addition, both anlotinib and capecitabine are oral drugs and the patients’ compliance will be improved. We also found that the median PFS of five TNBC patients who were treated with anlotinib combined with immunotherapy reached 10.2 months. Studies have shown that anlotinib can inhibit tumor growth by down-regulating the expression of programmed cell death ligand 1(PD-L1) on vascular endothelial cells, thereby improving the immune microenvironment (26). Synergistic antitumor therapeutic effect has been seen when anlotinib is used in combination with PD-1/PD-L1 blockade (27). The FUTURE-C-PLUS study also showed encouraging results of camrelizumab in combination with famitinib and nab-paclitaxel in TNBC (28). The regimen of anlotinib combined with immunotherapy deserves further explorations in TNBC patients based on our current findings.
Due to the existence of the blood-brain barrier, water-soluble and large anticancer agents have limited efficacy in the treatment of brain metastases. Angiogenesis inhibitors have showed some effect in the treatment of brain metastases (29). In NSCLC, anlotinib already showed improvement in the intracranial local control in patients with brain metastases (30). In the Phase III NALA Trial, the CNS ORR was 32.8% for neratinib plus capecitabine and 26.7% for lapatinib plus capecitabine (31). The intracranial PFS was about 4.2 months in HER2 positive MBC, and the addition of tucatinib improved intracranial PFS to 9.9 month in the HER2CLIMB study (32). In our study, for patients with brain metastases, the CNS ORR was 33.3%, the CNS DCR was 80.0%, and the median CNS PFS was 9.4 months. The addition of antiangiogenic drugs to the treatment of patients with brain metastases may be an effective means of controlling brain lesions. We still need to mention that 6 patients (40%) had received partial or whole brain radiation within 6 months before receiving anlotinib, which had an impact on our results.
Bone marrow suppression, represented by thrombocytopenia, is the most common adverse event and the leading cause of drug dose adjustment. It can often be controlled at an early stage through timely treatment due to periodic inspection of blood routine examination. Gastrointestinal reactions are also common AEs. Some patients experience a certain loss of appetite and/or mild to moderate diarrhea. Some patients complain of nosebleeds during the early stages of anlotinib treatment. The previous meta-analysis presented an increase in the incidence of bleeding events in patients with VEGFR TKIs compared to placebo treatment (33). In our study, bleeding symptoms are relatively light and can often be relieved after simple procedures such as compression. Hand-foot syndrome is usually found in patients with the treatment in combined with capecitabine. In summary, with regular blood routine examination, anlotinib is relatively safe, and most adverse events are manageable.
The main limitation of our study is that this is a single-center retrospective study with a small sample size. Due to the short follow-up time, we cannot prove whether anlotinib can affect the overall survival rate of MBC patients.
Anlotinib showed good efficiency and manageable toxicity in metastatic breast cancer patients with failure of standard treatment. It is emerging as a treatment option for metastatic breast cancer, but its efficacy needs to be further proven through prospective studies involving more patients.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the ethics board of Jiangsu Cancer Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Conception and design: YY; Administrative support: LZ; Provision of study materials or patients: LZ; Collection and assembly of data: YQ and HZ; Data analysis and interpretation: YQ and KL; Manuscript writing: All authors; Final approval of manuscript: All authors.
The work was supported by the National Youth Natural Science Foundation of China (Grant No. 81703548).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin (2022) 72(1):7–33. doi: 10.3322/caac.21708
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
3. Gasparini G. Prognostic value of vascular endothelial growth factor in breast cancer. Oncologist (2000) 5(Suppl 1):37–44. doi: 10.1634/theoncologist.5-suppl_1-37
4. Sitohy B, Nagy JA, Dvorak HF. Anti-VEGF/VEGFR therapy for cancer: reassessing the target. Cancer Res (2012) 72(8):1909–14. doi: 10.1158/0008-5472.CAN-11-3406
5. Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med (1971) 285(21):1182–6. doi: 10.1056/NEJM197111182852108
6. Yoshiji H, Gomez DE, Shibuya M, Thorgeirsson UP. Expression of vascular endothelial growth factor, its receptor, and other angiogenic factors in human breast cancer. Cancer Res (1996) 56(9):2013–6.
7. Young E, Miele L, Tucker KB, Huang M, Wells J, Gu JW. SU11248, a selective tyrosine kinases inhibitor suppresses breast tumor angiogenesis and growth via targeting both tumor vasculature and breast cancer cells. Cancer Biol Ther (2010) 10(7):703–11. doi: 10.4161/cbt.10.7.12904
8. Shen G, Zheng F, Ren D, Du F, Dong Q, Wang Z, et al. Anlotinib: A novel multi-targeting tyrosine kinase inhibitor in clinical development. J Hematol Oncol (2018) 11(1):120. doi: 10.1186/s13045-018-0664-7
9. Su Y, Luo B, Lu Y, Wang D, Yan J, Zheng J, et al. Anlotinib induces a T cell-inflamed tumor microenvironment by facilitating vessel normalization and enhances the efficacy of PD-1 checkpoint blockade in neuroblastoma. Clin Cancer Res (2022) 28(4):793–809. doi: 10.1158/1078-0432.CCR-21-2241
10. Zhou AP, Bai Y, Song Y, Luo H, Ren XB, Wang X, et al. Anlotinib versus sunitinib as first-line treatment for metastatic renal cell carcinoma: A randomized phase II clinical trial. Oncologist (2019) 24(8):e702–e8. doi: 10.1634/theoncologist.2018-0839
11. Wang HY, Chu JF, Zhang P, Wang JQ, Yan Z, Yao SN, et al. Safety and efficacy of chemotherapy combined with anlotinib plus anlotinib maintenance in Chinese patients with Advanced/Metastatic soft tissue sarcoma. Onco Targets Ther (2020) 13:1561–8. doi: 10.2147/OTT.S235349
12. Zhang K, Ma X, Gao H, Wang H, Qin H, Yang S, et al. Efficacy and safety of anlotinib in advanced non-small cell lung cancer: A real-world study. Cancer Manag Res (2020) 12:3409–17. doi: 10.2147/CMAR.S246000
13. Hu N, Si Y, Yue J, Sun T, Wang X, Jia Z, et al. Anlotinib has good efficacy and low toxicity: A phase II study of anlotinib in pre-treated HER-2 negative metastatic breast cancer. Cancer Biol Med (2021) 18(3):849–59. doi: 10.20892/j.issn.2095-3941.2020.0463
14. Syed YY. Anlotinib: First global approval. Drugs (2018) 78(10):1057–62. doi: 10.1007/s40265-018-0939-x
15. Sun Y, Niu W, Du F, Du C, Li S, Wang J, et al. Safety, pharmacokinetics, and antitumor properties of anlotinib, an oral multi-target tyrosine kinase inhibitor, in patients with advanced refractory solid tumors. J Hematol Oncol (2016) 9(1):105. doi: 10.1186/s13045-016-0332-8
16. Miles DW, Chan A, Dirix LY, Cortes J, Pivot X, Tomczak P, et al. Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol (2010) 28(20):3239–47. doi: 10.1200/JCO.2008.21.6457
17. Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med (2007) 357(26):2666–76. doi: 10.1056/NEJMoa072113
18. Robert NJ, Dieras V, Glaspy J, Brufsky AM, Bondarenko I, Lipatov ON, et al. RIBBON-1: Randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol (2011) 29(10):1252–60. doi: 10.1200/JCO.2010.28.0982
19. Brufsky AM, Hurvitz S, Perez E, Swamy R, Valero V, O'Neill V, et al. RIBBON-2: a randomized, double-blind, placebo-controlled, phase III trial evaluating the efficacy and safety of bevacizumab in combination with chemotherapy for second-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol (2011) 29(32):4286–93. doi: 10.1200/JCO.2010.34.1255
20. Baselga J, Rocheé H, Costa F, Getuélio Martins Segalla J, Pinczowski H, Ma Ciruelos E, et al. SOLTI-0701: A multinational double-blind, randomized phase 2b study evaluating the efficacy and safety of sorafenib compared to placebo when administered in combination with capecitabine in patients with locally advanced or metastatic breast cancer (BC). Cancer Res (2009) 69(24_Supplement):45. doi: 10.1158/0008-5472.SABCS-09-45
21. Bondarde S, Kaklamani V, Prasad Sahoo T, Lokanatha D, Raina V, Jain M, et al. Abstract P2-16-03: Sorafenib in combination with paclitaxel as a first-line therapy in patients with locally recurrent or metastatic breast cancer: Overall survival results from a double-blind, randomized, placebo-controlled, phase 2b trial. Cancer Res (2010) 70(24_Supplement):P2-16-03. doi: 10.1158/0008-5472.SABCS10-P2-16-03
22. Li L, Youqun W, Weili S, Yuan W, Delin L. Clinical study of platinum-based chemotherapy for advanced metastatic breast cancer. Chin J Cancer Prev Treat (2016) 23(10):657–62. doi: 10.16073/j.cnki.cjcpt.2016.10.009
23. Brufsky A, Valero V, Tiangco B, Dakhil S, Brize A, Rugo HS, et al. Second-line bevacizumab-containing therapy in patients with triple-negative breast cancer: Subgroup analysis of the RIBBON-2 trial. Breast Cancer Res Treat (2012) 133(3):1067–75. doi: 10.1007/s10549-012-2008-6
24. Bardia A, Hurvitz SA, Tolaney SM, Loirat D, Punie K, Oliveira M, et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med (2021) 384(16):1529–41. doi: 10.1056/NEJMoa2028485
25. Iwai T, Harada Y, Saeki H, Oki E, Maehara Y, Yonemitsu Y. Capecitabine reverses tumor escape from anti-VEGF through the eliminating CD11b(high)/Gr1(high) myeloid cells. Oncotarget (2018) 9(25):17620–30. doi: 10.18632/oncotarget.24811
26. Liu S, Qin T, Liu Z, Wang J, Jia Y, Feng Y, et al. Anlotinib alters tumor immune microenvironment by downregulating PD-L1 expression on vascular endothelial cells. Cell Death Dis (2020) 11(5):309. doi: 10.1038/s41419-020-2511-3
27. Yang Y, Li L, Jiang Z, Wang B, Pan Z. Anlotinib optimizes anti-tumor innate immunity to potentiate the therapeutic effect of PD-1 blockade in lung cancer. Cancer Immunol Immunother. (2020) 69(12):2523–32. doi: 10.1007/s00262-020-02641-5
28. Chen L, Jiang YZ, Wu SY, Wu J, Di GH, Liu GY, et al. Famitinib with camrelizumab and nab-paclitaxel for advanced immunomodulatory triple-negative breast cancer (FUTURE-C-PLUS): An open-label, single-arm, phase 2 trial. Clin Cancer Res: An official journal of the American Association for Cancer Research (2022) 28(13):2807–17. doi: 10.1158/1078-0432.CCR-21-4313
29. Kodack DP, Chung E, Yamashita H, Incio J, Duyverman AM, Song Y, et al. Combined targeting of HER2 and VEGFR2 for effective treatment of HER2-amplified breast cancer brain metastases. Proc Natl Acad Sci USA (2012) 109(45):E3119–27. doi: 10.1073/pnas.1216078109
30. Jiang S, Liang H, Liu Z, Zhao S, Liu J, Xie Z, et al. The impact of anlotinib on brain metastases of non-small cell lung cancer: Post hoc analysis of a phase III randomized control trial (ALTER0303). Oncologist (2020) 25(5):e870–e4. doi: 10.1634/theoncologist.2019-0838
31. Saura C, Oliveira M, Feng YH, Dai MS, Chen SW, Hurvitz SA, et al. Neratinib plus capecitabine versus lapatinib plus capecitabine in HER2-positive metastatic breast cancer previously treated with >/= 2 HER2-directed regimens: Phase III NALA trial. J Clin Oncol (2020) 38(27):3138–49. doi: 10.1200/JCO.20.00147
32. Lin NU, Borges V, Anders C, Murthy RK, Paplomata E, Hamilton E, et al. Intracranial efficacy and survival with tucatinib plus trastuzumab and capecitabine for previously treated HER2-positive breast cancer with brain metastases in the HER2CLIMB trial. J Clin Oncol (2020) 38(23):2610–9. doi: 10.1200/JCO.20.00775
Keywords: metastatic breast cancer, anlotinib, antiangiogenesis, efficacy, side effect
Citation: Qian Y, Lou K, Zhou H, Zhang L and Yuan Y (2022) Efficacy and safety of anlotinib-based treatment in metastatic breast cancer patients. Front. Oncol. 12:1042451. doi: 10.3389/fonc.2022.1042451
Received: 12 September 2022; Accepted: 24 November 2022;
Published: 09 December 2022.
Edited by:
Eriko Katsuta, University at Buffalo, United StatesReviewed by:
Vrinda Gote, Inventprise Inc., United StatesCopyright © 2022 Qian, Lou, Zhou, Zhang and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuan Yuan, eXVhbnl1YW44MTAxMjJAMTI2LmNvbQ==; Lili Zhang, bG9uZ3Jlbnl1QHNpbmEuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.