- Department of Radiation Oncology, Ruijin Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China
Background: Many studies have shown that the peripheral blood inflammatory index and nutritional index, such as the platelet lymphocyte ratio (PLR), neutrophil lymphocyte ratio (NLR), lymphocyte monocyte ratio (LMR), systemic inflammation response index (SIRI), pan-immune-inflammation value (PIV), systemic immune-inflammation index (SII), and prognostic nutrition index (PNI), are independent prognostic factors for tumors. The present study aimed to investigate the prognostic role of these peripheral blood indexes before treatment in locally advanced gastric cancer (LAGC) treated with adjuvant chemoradiotherapy after D2 dissection.
Methods: A total of 89 patients with LAGC who underwent D2 gastrectomy and adjuvant chemoradiotherapy at our hospital from 2010–2018 were eligible. Systemic inflammatory indicators before treatment were evaluated. Receiver operating characteristic curve (ROC), Kaplan–Meier analysis, and Cox regression were utilized for prognosis evaluation.
Results: The median follow-up time was 29.1 (4.1–115.8) months. The overall survival at 3 years (OS) and the disease-free survival (DFS) were 78.9% and 59.1%, respectively. According to the ROC curve for 3-year DFS, the best cut-off values of pre-treatment NLR, PLR, LMR, SII, SIRI, PIV and PNI were 1.7, 109.3, 2.9, 369.2, 0.58, 218.7, and 48, respectively. Multivariate Cox regression analysis showed that NLR was an independent prognostic factor for DFS (HR 2.991, 95%CI 1.085–8.248, P = 0.034). Kaplan-Meier analysis showed that a higher NLR (>1.70) was significantly associated with a poorer OS (3-year OS: 68.8% vs 92.9%, P = 0.045) and DFS (3-year DFS: 47.5% vs 80.9%, P = 0.005). In terms of the free locoregional recurrence rate (LRR), the prognosis of patients with high NLR was also significantly worse than those with low NLR (70.2% vs 96.0%, P = 0.017). Paraaortic lymph nodes were the most common site of LRR (7/14 patients). The seven cases of paraaortic lymph node metastasis occurred in patients with high NLR.
Conclusions: In our retrospective analysis, we found that pretreatment NLR could serve as a prognostic factor for survival in LAGC treated with adjuvant chemoradiotherapy after D2 dissection, especially for the prediction of LRR and paraaortic lymph node metastasis. Prospective studies are needed to confirm our findings.
Introduction
Gastric cancer is one of the leading causes of cancer mortality in China and worldwide (1, 2). Even with curative resection, 5-year survival in patients with locally advanced gastric cancer (LAGC) remains at 30–50% (3). Therefore, researchers have turned their attention to adjuvant and neoadjuvant treatment to improve both overall survival (OS) and quality of life in patients with LAGC (4).
According to early studies, more than 80% of patients who died from gastric cancer presented locoregional recurrence (LRR) (5), which led to the introduction of radiotherapy. The positive impact of adjuvant and neoadjuvant therapies on survival in patients with resectable LAGC has become increasingly clear. The INT 0116 trial (6, 7), which demonstrated a survival benefit from postoperative chemoradiotherapy, was compromised by the fact that >50 percent of the enrolled patients had an insufficient (less than D1) lymph node dissection, suggesting that adjuvant chemoradiotherapy could be primarily compensating for suboptimal lymph node surgery. As one of the largest trials for D2 lymph node dissection patients, the Adjuvant Chemoradiation Therapy in Stomach Cancer (ARTIST) trial (8) failed to show that the addition of radiotherapy to chemotherapy has a significant overall survival benefit. Only subgroup analyses showed that chemoradiotherapy significantly improved disease-free survival (DFS) in patients with gastric cancer-node positive disease. However, in the successor trial ARTIST 2 (9), the addition of radiation therapy to chemotherapy did not appear to provide additional benefit in patients with node-positive disease. Through the above studies, we found that the clinical benefits brought by postoperative radiotherapy are not universal for all patients after D2 lymphadenectomy.
Therefore, it is essential to find effective predictors and to accurately stratify patients with gastric cancer who may benefit from chemoradiotherapy. Inflammation plays a key role in the occurrence and development of tumors. Recently, several new indicators have been identified as independent prognostic markers for tumor outcome, such as the platelet lymphocyte ratio (PLR) (10), neutrophil lymphocyte ratio (NLR) (10–13), systemic immune inflammation index (SII) (14, 15), lymphocyte monocyte ratio (LMR) (16), systemic inflammation response index (SIRI) (17, 18), pan-immune-inflammation value (PIV) (19, 20), and prognostic nutrition index (PNI) (21, 22). Although some studies (12, 21) have also suggested that nutritional and inflammatory indexes are closely related to the prognosis of gastric cancer, the role of these indexes in gastric cancer treated with D2 dissection and chemoradiotherapy remains undetermined. Therefore, we conducted this study to evaluate the prognostic value of peripheral blood inflammatory and nutritional indicators in LAGC treated with adjuvant chemoradiotherapy following D2 dissection.
Materials and methods
Patient characteristics
The medical records of 89 patients with gastric or gastroesophageal junction adenocarcinoma who underwent radical D2 gastrectomy and adjuvant chemoradiotherapy at our hospital between January 2010 and December 2018, were retrospectively reviewed. The inclusion criteria were as follows: patients diagnosed with adenocarcinoma; stage IB-IIIC according to the eighth edition of the American Joint Committee on Cancer (AJCC) 8th edition (23); R0 and D2 was confirmed by postoperative pathology; presence of positive pathological lymph nodes; and received adjuvant chemoradiotherapy after the operation. This study was approved by the Institutional Medicine Review Board and a waiver for patient consent was obtained.
Adjuvant chemotherapy and chemoradiotherapy
All patients received 6 (range 4–8) cycles of treatment regimens involving fluoropyrimidine after surgery. Concurrent chemotherapy regimens included tegafur or capecitabine (24).
All patients received 6MV linear accelerator radiotherapy with a total tumor dose of 41.4-54 Gy (1.8-2 Gy/time, 5 times/week). The tumor bed area, the anastomotic site, and the regional drainage lymph nodes (including paraaortic nodes) were defined as the clinical target volume (CTV). Radiotherapy plans were confirmed by senior radiotherapy clinicians. Before treatment, treatment fields, radiation dosimetry, surgical and pathological information, and preoperative imaging were checked.
Data collection
All medical and surgical records were retrospectively reviewed, including baseline neutrophil, lymphocytes, monocytes, platelets, and albumin (g/L) levels, depth of tumor infiltration, number of positive lymph nodes, chemoradiotherapy, recurrence, and survival information. Recurrence of the tumor bed, anastomotic stoma, duodenal stump, gastric remnant, and regional lymph nodes were defined as LRR. Peritoneal dissemination referred to the recurrence that occurred in the peritoneum. All recurrences at distant sites were recorded as distant metastases (excluding the peritoneum) (25).
Data definition
Laboratory examinations were performed before treatment. The calculation formula of each indicator of inflammatory markers was as follows: NLR = neutrophil count/lymphocyte count (21); PLR = platelet count/lymphocyte count; LMR = lymphocyte count/monocyte count (26); SII = platelet count × neutrophil count/lymphocyte count (27); SIRI = neutrophil count × monocyte count/lymphocyte count (28); PIV = neutrophil count × platelet count × monocyte count/lymphocyte count (19). PNI = serum albumin value + 5 × lymphocyte count (21).
Statistical analysis
All statistical analyses were conducted with SPSS 26 software (IBM Corp., Armonk, NY, USA). Continuous variables were described using median and range, and categorical variables were presented as frequency and percentage. The receiver operating characteristic (ROC) curve was applied to determine the optimal cut-off value of these inflammatory markers based on the maximum Youden index. ROC curves were created by plotting the sensitivity against (1-specificity) for each parameter. The optimal cutoff value represented the maximum Youden index (sensitivity + specificity − 1). Prognostic factors for DFS were investigated using Cox regression analyses. Factors with a p-value <0.05 in the univariate analysis were then entered in the multivariate analysis, to identify independent prognostic factors. Survival curves were plotted using the Kaplan–Meier method and were compared using the logarithmic rank test. A P-value less than 0.05 was considered statistically significant.
Results
Patient characteristics
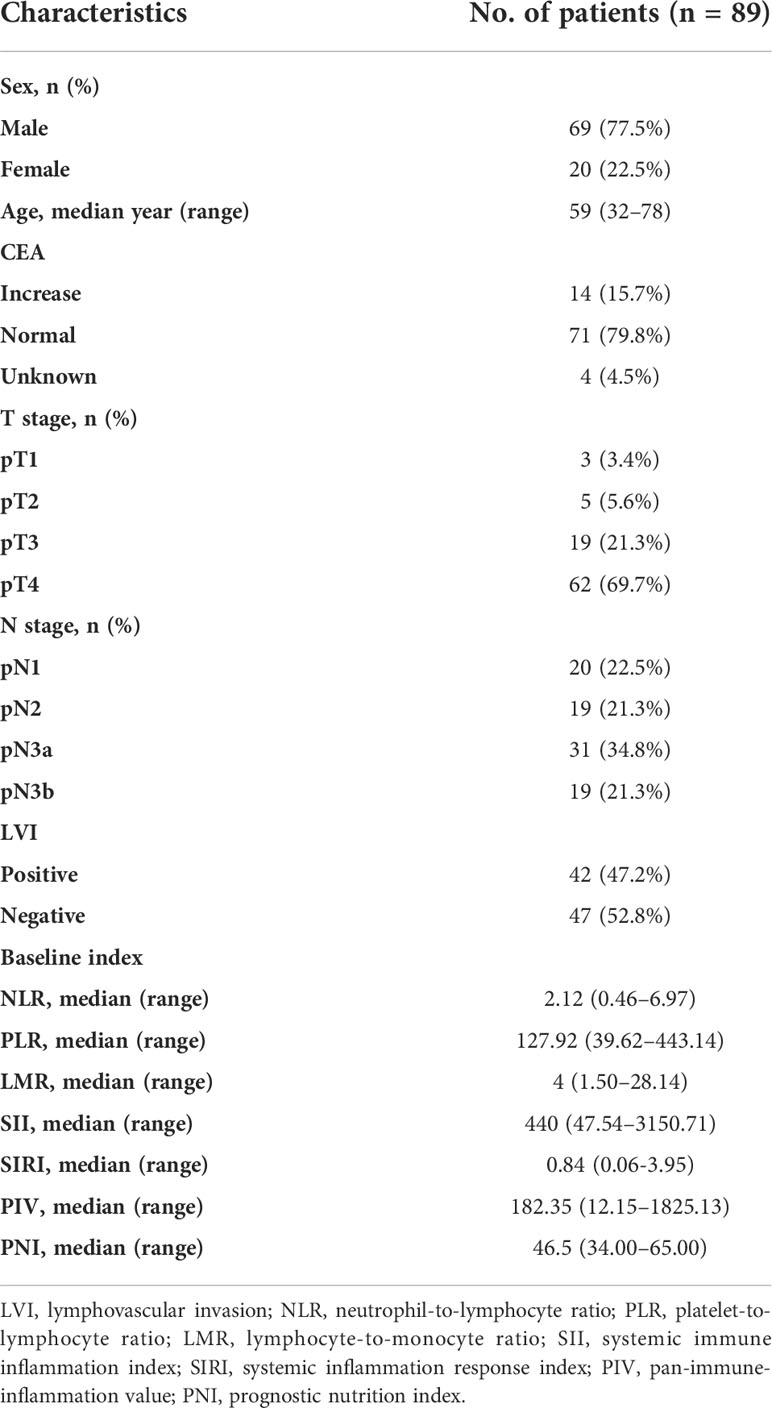
Table 1 summarizes the baseline characteristics of the 89 patients included in the study. All patients received surgery plus chemotherapy and radiation therapy. The median age was 59 years (range, 32–78), 69 men (77.5%) and 20 women (22.5%). More than two thirds of the patients were in the stage T4 (69.7%) and more than half of the patients were in the stage N3.
The cut-off value of inflammatory markers
The optimal cutoff value of these inflammatory markers for the 3-year DFS was obtained using ROC curve analysis. The cut-off values for NLR, PLR, LMR, SII, SIRI, PIV, and PNI were 1.7, 109.3, 2.9, 369.2, 0.58, 218.7, 48, respectively (Figure 1).
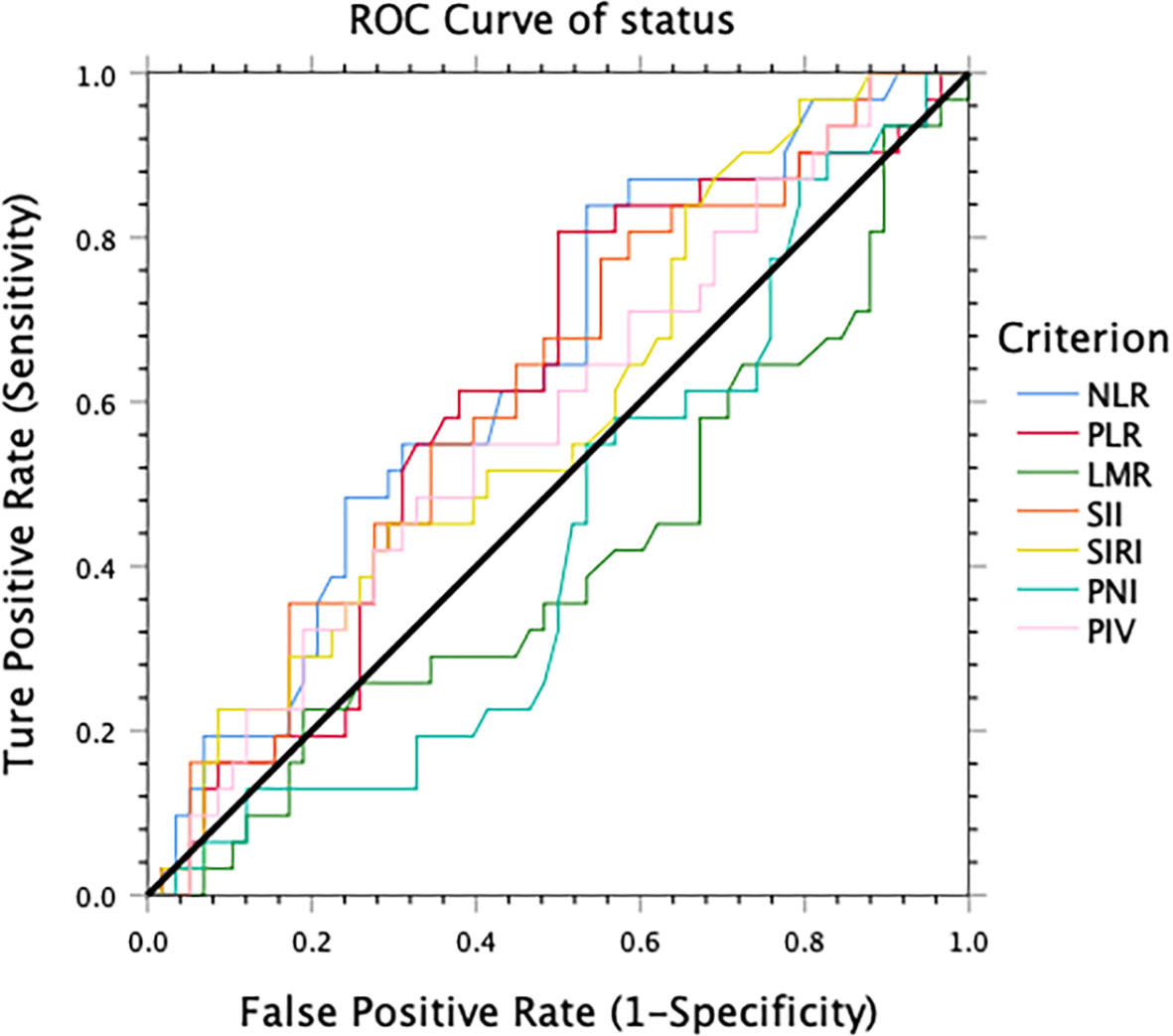
Figure 1 The optimal cut-off value for 3-year disease-free survival obtained using receiver operating characteristic analysis.
Relationship between clinicopathological factors and 3-year DFS
Univariate Cox regression analysis was performed using age, sex, stage T, stage N, LVI, CEA level, NLR, PLR, LMR, SII, SIRI, PIV, and PNI (Table 2). Univariate analysis showed that stage N (HR 2.656, P = 0.012), LVI (HR 2.621, P = 0.011), NLR (HR 3.642, P = 0.008), PLR (HR 3.137, P = 0.012), LMR (HR 0.406, P = 0.017), and PNI (HR 0.399, P = 0.033) were associated with the 3-year DFS of patients with LAGC treated with adjuvant chemoradiotherapy. There was no significant difference between individuals ≥65 years and those <65 years of age. Factors with p-value <0.05 in the univariate analysis were also included in the multivariate analysis. Univariate analysis showed that there was no statistically significant difference in the 3-year DFS among patients with different neutrophil counts or platelet counts. Multivariable Cox analysis (Table 2) indicated that the NLR (HR 2.991, P = 0.034) and pN3b (HR 2.463, P = 0.026) were independent predictors for DFS among gastric cancer patients treated with adjuvant chemoradiotherapy.
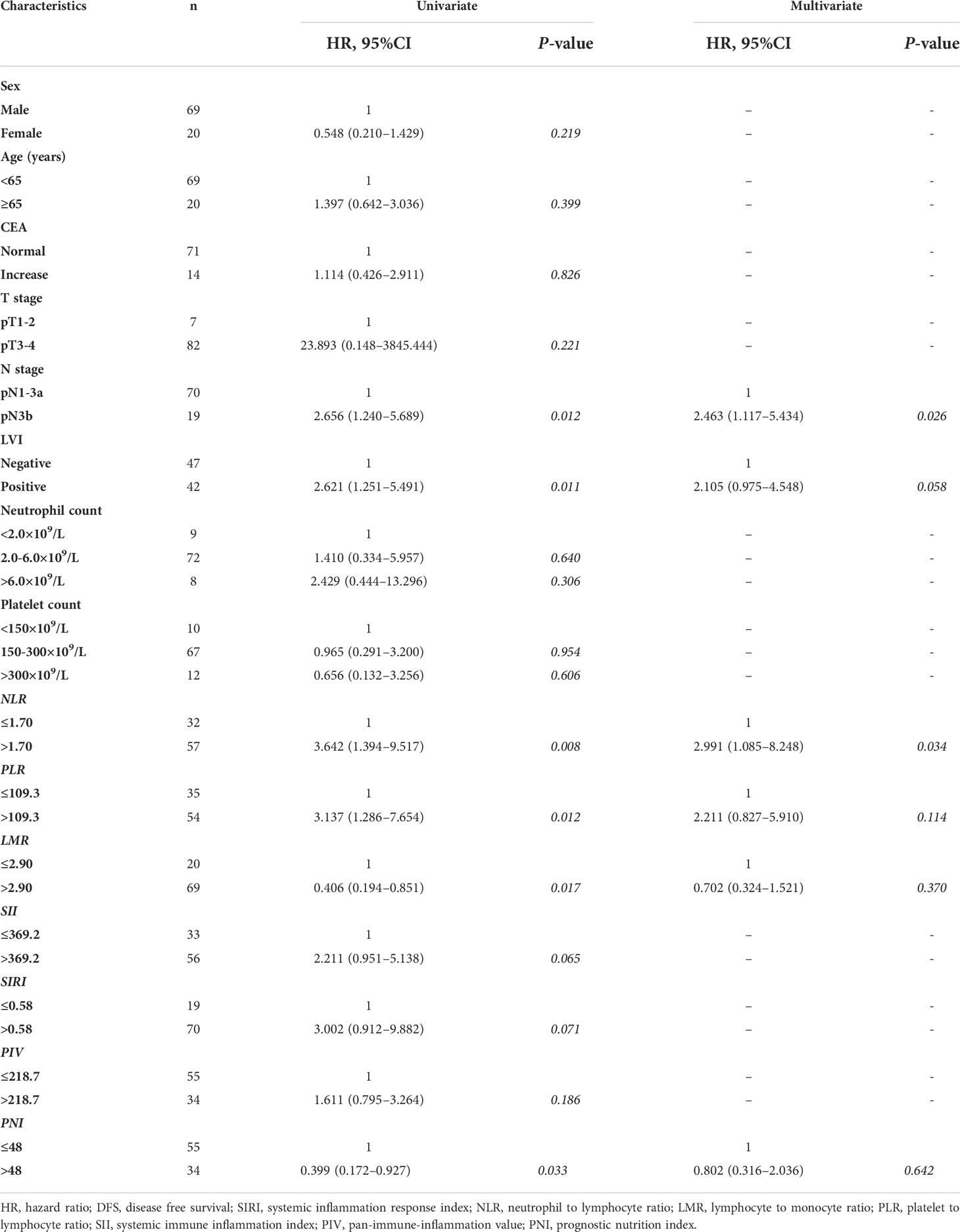
Table 2 Univariate and multivariate analysis for 3-year DFS in patients with gastric cancer treated with D2 gastrectomy and chemoradiotherapy.
NLR and survival outcomes
With a median follow-up of 29.1 months (range 4.1 to 115.8 months), the 3-year OS was 78.9% and the 3-year DFS was 59.1%. Patients were divided into two groups according to the optimal cut-off value: the low NLR group (NLR 1.7) and the high NLR group (NLR>1.7). In total, 32 (36.0%) and 57 (64.0%) patients were divided into the low and high NLR groups, respectively. The survival rate of these two groups was significantly different (Figures 2A, B). The 3-year OS rate of the patients in the low NLR group and the high NLR group was 92.9% and 68.8%, respectively (P = 0.045); the 3-year DFS rate in the low NLR group and the high NLR group was 80.9% and 47.5%, respectively (P = 0.005). To identify a better model for predicting the outcome of patients, we also calculated the NLR-PLR score ranging from 0 to 2 as follows: score of 2, high NLR (>1.7) and high PLR (>109.3); score of 1, high NLR or high PLR; score of 0, neither high NLR nor high PLR. NLR-PLR scores of 0, 1, and 2 were observed in 18 (20.2%), 31 (34.8%), and 40 (44.9%) patients, respectively. However, overall survival differences according to the NLR-PLR score were not significant (P > 0.05).
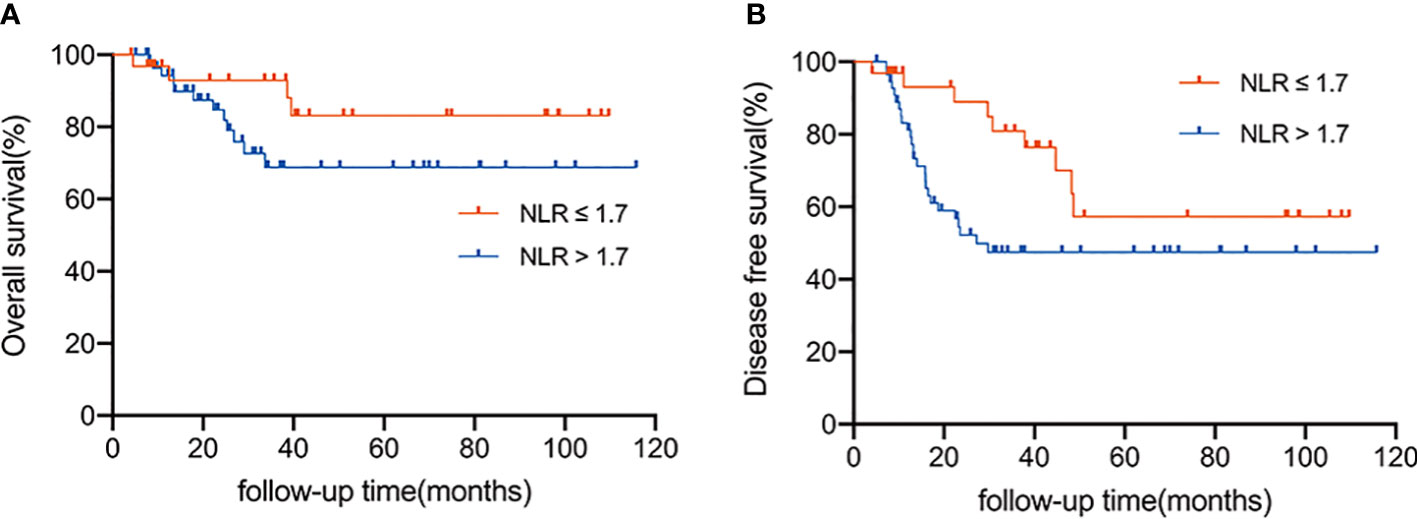
Figure 2 (A) Overall survival according to the NLR status (≤ 1.7 vs. > 1.7). (B) Disease-free survival according to the status of NLR status (≤ 1.7 vs. > 1.7).
Role of NLR in initial failure patterns
We also analyzed the first site of recurrence, LRR was more common in the high NLR group. The LRR-free survival rate of patients in the low NLR group was much higher than those in the high NLR group (96.0% vs. 70.2%, P = 0.017, Figure 3). The aortic lymph node was the most common site of LRR (7/14). But no patient in the low NLR group experienced para-aortic lymph node recurrence. In contrast, the distant or peritoneal metastasis rate was not significantly different between the high NLR group and the low NLR group (P = 0.066, 0.117, respectively).
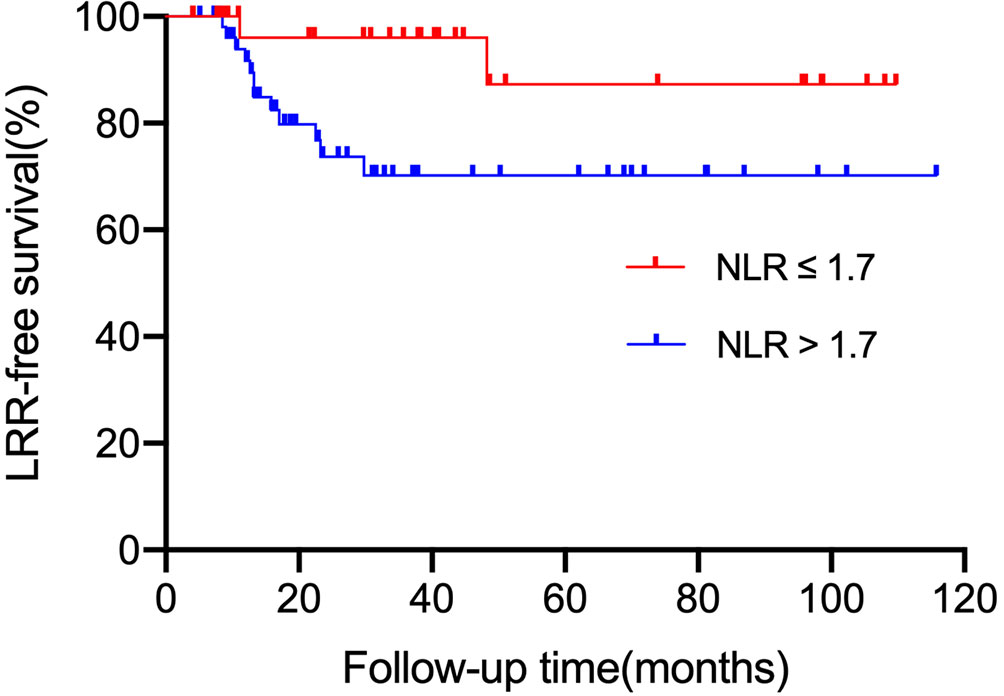
Figure 3 LRR-free survival according to NLR status (≤ 1.7 vs. > 1.7). LRR, local regional recurrence.
Discussion
Despite the large number of clinical trials, it remains uncertain whether patients can benefit from chemoradiotherapy after D2 dissection for gastric cancer. Many studies have shown that systemic inflammatory and nutritional biomarkers are independent predictors of various malignancies, including gastric cancer (15, 29–35). Furthermore, several inflammatory indicators, such as the NLR (13, 36), PLR (36), nutritional index (37), and LMR (38) have also been reported as potential prognostic indices for chemoradiotherapy. However, it remains to be determined whether inflammatory indicators can predict the prognosis of gastric cancer after D2 dissection with chemoradiotherapy. To our knowledge, this is the first study to define the prognostic value of peripheral blood inflammation indices in gastric cancer after D2 dissection treated with chemoradiotherapy. Our study found that N stage (pN3b) and NLR were independent prognostic factors for DFS in patients with LAGC after D2 dissection treated with chemoradiotherapy. In our previous study, we showed that pN3b is an independent adverse prognostic factor in gastric cancer after D2 dissection (24). Thus, in this study, we focused mainly on the correlation between NLR and the prognosis of patients with LAGC after D2 gastrectomy.
Several researchers have demonstrated a close relationship between NLR and tumor prognosis in various malignancies, including gastric cancer (10–13). Similar to previous studies (11, 39), our current research also showed that patients with high NLR have relatively shorter OS and DFS than patients with low NLR. Furthermore, our study found that the baseline NLR value was strongly associated with gastric cancer LRR after D2 dissection and chemoradiotherapy. Before this study, several retrospective studies explored the prognostic role of NLR in patients with gastric cancer undergoing radiation therapy and chemotherapy. The multicenter study by Meral et al. (39) showed that NLR was significantly correlated with lymph node status and prognosis in gastric carcinoma. Subsequently, Tetsushi et al. (36) found that baseline NLR was closely related to the clinical outcomes of gastric cancer after chemotherapy or chemoradiotherapy. Recently, Liu et al. (40) also found that NLR could be considered a routine potential prognostic factor for gastric cancer after surgery. According to our study, baseline NLR could be a new prognostic factor for disease progression and survival in patients with LAGC after D2 dissection and chemoradiotherapy.
There are several possible explanations for the relationship between increased NLR and poor tumor prognosis. Increased expression of tumor-related inflammatory mediators and cytokines, such as tumor necrosis factor-α, interleukin-1 (IL-1), and IL-6, have been reported to be increased in gastric cancer cases and other cancers (41). These inflammatory mediators may cause neutrophilia and lymphocytopenia, leading to a higher NLR (42). The host immune response to cancer is lymphocyte-dependent. Conversely, neutrophils are reported to be the main source of circulating chemokines and cytokines, and are major contributors to tumor-related angiogenesis (43). Meanwhile, consistent with our findings, patients with an elevated NLR can experience a relatively poor oncologic outcome and need more intensive treatment.
There is no generally recognized cut-off value for these indexes; some studies have selected the median value of each inflammatory and nutritional index as the cut-off level, and others have set the cut-off value based on existing literature. In previous published studies, the NLR cutoff values ranged from 2–6 (13, 36, 39). In this study, we draw ROC curves to determine the best cut-off value. A cutoff value of 1.7 for NLR could be optimal for LAGC patients treated with adjuvant chemoradiotherapy after D2 dissection. According to this cut-off value, patients were divided into two groups: low NLR group (NLR ≤1.7) and high NLR group (NLR >1.7).
However, there are several inherent limitations to our study. First, our study was a small retrospective study based on a single center, which may have potential selection bias. Another limitation was the short follow-up time.
In conclusion, the present study confirmed that baseline NLR could serve as a valuable independent prognostic factor for LAGC patients after D2 dissection who receive adjuvant chemoradiotherapy. However, large prospective studies with long-term follow-up should be performed to confirm our findings.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
This study was approved by the Institutional Medicine Review Board and a waiver for patient consent was obtained.
Author contributions
S-BW conducted data extraction, quality appraisal, data synthesis and analysis, and drafted the manuscript. W-GC designed the protocol, performed the search, data extraction, quality appraisal, data synthesis and interpretation, and drafted the manuscript. J-YC and CX contributed to writing and editing the manuscript. LC and RC determined the scope of the review and contributed to protocol design and writing and editing the manuscript. GC had full access to the data, takes responsibility for data integrity, and is the guarantor of the review. All authors contributed to the article and approved the submitted version.
Funding
This study was supported in part by the National Natural Science Foundation of China (grant numbers 81803164).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer (2015) 136(5):E359–86. doi: 10.1002/ijc.29210
2. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin (2016) 66(2):115–32. doi: 10.3322/caac.21338
3. Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol (2006) 24(18):2903–9. doi: 10.1200/jco.2005.05.0245
4. Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. New Engl J Med (2006) 355(1):11–20. doi: 10.1056/NEJMoa055531
5. Gunderson LL, Sosin H. Adenocarcinoma of the stomach: areas of failure in a re-operation series (second or symptomatic look) clinicopathologic correlation and implications for adjuvant therapy. Int J Radiat Oncol Biol Phys (1982) 8(1):1–11. doi: 10.1016/0360-3016(82)90377-7
6. Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. New Engl J Med (2001) 345(10):725–30. doi: 10.1056/NEJMoa010187
7. Smalley SR, Benedetti JK, Haller DG, Hundahl SA, Estes NC, Ajani JA, et al. Updated analysis of SWOG-directed intergroup study 0116: a phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J Clin Oncol (2012) 30(19):2327–33. doi: 10.1200/jco.2011.36.7136
8. Park SH, Sohn TS, Lee J, Lim DH, Hong ME, Kim KM, et al. Phase III trial to compare adjuvant chemotherapy with capecitabine and cisplatin versus concurrent chemoradiotherapy in gastric cancer: Final report of the adjuvant chemoradiotherapy in stomach tumors trial, including survival and subset analyses. J Clin Oncol (2015) 33(28):3130–6. doi: 10.1200/jco.2014.58.3930
9. Park SH, Lim DH, Sohn TS, Lee J, Zang DY, Kim ST, et al. A randomized phase III trial comparing adjuvant single-agent S1, s-1 with oxaliplatin, and postoperative chemoradiation with s-1 and oxaliplatin in patients with node-positive gastric cancer after D2 resection: the ARTIST 2 trial(☆). Ann Oncol (2021) 32(3):368–74. doi: 10.1016/j.annonc.2020.11.017
10. Zhang N, Jiang J, Tang S, Sun G. Predictive value of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in non-small cell lung cancer patients treated with immune checkpoint inhibitors: A meta-analysis. Int Immunopharmacol (2020) 85:106677. doi: 10.1016/j.intimp.2020.106677
11. Alexiou GA, Vartholomatos E, Zagorianakou P, Voulgaris S. Prognostic significance of neutrophil-to-lymphocyte ratio in glioblastoma. Neuroimmunol Neuroinflamm (2014) 1(3):131–4. doi: 10.4103/2347-8659.143666
12. Miyamoto R, Inagawa S, Sano N, Tadano S, Adachi S, Yamamoto M. The neutrophil-to-lymphocyte ratio (NLR) predicts short-term and long-term outcomes in gastric cancer patients. Eur J Surg Oncol (2018) 44(5):607–12. doi: 10.1016/j.ejso.2018.02.003
13. Ng SP, Bahig H, Jethanandani A, Sturgis EM, Johnson FM, Elgohari B, et al. Prognostic significance of pre-treatment neutrophil-to-lymphocyte ratio (NLR) in patients with oropharyngeal cancer treated with radiotherapy. Br J Cancer (2021) 124(3):628–33. doi: 10.1038/s41416-020-01106-x
14. Shi H, Jiang Y, Cao H, Zhu H, Chen B, Ji W. Nomogram based on systemic immune-inflammation index to predict overall survival in gastric cancer patients. Dis Markers (2018) 2018:1787424. doi: 10.1155/2018/1787424
15. Wang K, Diao F, Ye Z, Zhang X, Zhai E, Ren H, et al. Prognostic value of systemic immune-inflammation index in patients with gastric cancer. Chin J Cancer (2017) 36(1):75. doi: 10.1186/s40880-017-0243-2
16. Zhou X, Du Y, Xu J, Huang Z, Qiu T, Wang X, et al. The preoperative lymphocyte to monocyte ratio predicts clinical outcomes in patients with stage II/III gastric cancer. Tumour Biol (2014) 35(11):11659–66. doi: 10.1007/s13277-014-2504-x
17. Li S, Lan X, Gao H, Li Z, Chen L, Wang W, et al. Systemic inflammation response index (SIRI), cancer stem cells and survival of localised gastric adenocarcinoma after curative resection. J Cancer Res Clin Oncol (2017) 143(12):2455–68. doi: 10.1007/s00432-017-2506-3
18. Chen Y, Jin M, Shao Y, Xu G. Prognostic value of the systemic inflammation response index in patients with adenocarcinoma of the oesophagogastric junction: A propensity score-matched analysis. Dis Markers (2019) 2019:4659048. doi: 10.1155/2019/4659048
19. Ligorio F, Fucà G, Zattarin E, Lobefaro R, Zambelli L, Leporati R, et al. The pan-Immune-Inflammation-Value predicts the survival of patients with human epidermal growth factor receptor 2 (HER2)-positive advanced breast cancer treated with first-line taxane-Trastuzumab-Pertuzumab. Cancers (Basel) (2021) 13(8):1964. doi: 10.3390/cancers13081964
20. Lin F, Zhang LP, Xie SY, Huang HY, Chen XY, Jiang TC, et al. Pan-Immune-Inflammation value: A new prognostic index in operative breast cancer. Front Oncol (2022) 12:830138. doi: 10.3389/fonc.2022.830138
21. Gao J, Wang Y, Li F, Zhu Z, Han B, Wang R, et al. Prognostic nutritional index and neutrophil-to-Lymphocyte ratio are respectively associated with prognosis of gastric cancer with liver metatasis undergoing and without hepatectomy. BioMed Res Int (2019) 2019:4213623. doi: 10.1155/2019/4213623
22. Wen J, Bedford M, Begum R, Mitchell H, Hodson J, Whiting J, et al. The value of inflammation based prognostic scores in patients undergoing surgical resection for oesophageal and gastric carcinoma. J Surg Oncol (2018) 117(8):1697–707. doi: 10.1002/jso.25057
23. Amin MB, Edge SB, Greene FL, Brierley JD. AJCC cancer staging manual [M], 8th Ed. New York: Springer.
24. Wang SB, Qi WX, Chen JY, Xu C, Cao WG, Cai R, et al. Identification of patients with locally advanced gastric cancer who may benefit from adjuvant chemoradiotherapy after D2 dissection: A propensity score matching analysis. Front Oncol (2021) 11:648978. doi: 10.3389/fonc.2021.648978
25. Fan M, Li G, Shen L, Zhang H, Liang L, Zhang Z. Identification of patients with lymph node metastasis from gastric cancer who may benefit from adjuvant chemoradiotherapy after D2 dissection–do N3 patients benefit from additional radiation? Br J Radiol (2016) 89(1059):20150758. doi: 10.1259/bjr.20150758
26. Mandaliya H, Jones M, Oldmeadow C, Nordman II. Prognostic biomarkers in stage IV non-small cell lung cancer (NSCLC): neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), platelet to lymphocyte ratio (PLR) and advanced lung cancer inflammation index (ALI). Transl Lung Cancer Res (2019) 8(6):886–94. doi: 10.21037/tlcr.2019.11.16
27. Yang R, Chang Q, Meng X, Gao N, Wang W. Prognostic value of systemic immune-inflammation index in cancer: A meta-analysis. J Cancer (2018) 9(18):3295–302. doi: 10.7150/jca.25691
28. Valero C, Pardo L, Sansa A, Garcia Lorenzo J, López M, Quer M, et al. Prognostic capacity of systemic inflammation response index (SIRI) in patients with head and neck squamous cell carcinoma. Head Neck (2020) 42(2):336–43. doi: 10.1002/hed.26010
29. Hu H, Yao X, Xie X, Wu X, Zheng C, Xia W, et al. Prognostic value of preoperative NLR, dNLR, PLR and CRP in surgical renal cell carcinoma patients. World J Urol (2017) 35(2):261–70. doi: 10.1007/s00345-016-1864-9
30. Dolan RD, Laird BJA, Horgan PG, McMillan DC. The prognostic value of the systemic inflammatory response in randomised clinical trials in cancer: A systematic review. Crit Rev Oncol Hematol (2018) 132:130–7. doi: 10.1016/j.critrevonc.2018.09.016
31. Duan J, Pan L, Yang M. Preoperative elevated neutrophil-to-lymphocyte ratio (NLR) and derived NLR are associated with poor prognosis in patients with breast cancer: A meta-analysis. Med (Baltimore) (2018) 97(49):e13340. doi: 10.1097/md.0000000000013340
32. Lee GW, Park SW, Go SI, Kim HG, Kim MK, Min CK, et al. The derived neutrophil-to-Lymphocyte ratio is an independent prognostic factor in transplantation ineligible patients with multiple myeloma. Acta Haematol (2018) 140(3):146–56. doi: 10.1159/000490488
33. Liu JX, Li A, Zhou LY, Liu XF, Wei ZH, Wang XZ, et al. Significance of combined preoperative serum alb and dNLR for diagnosis of pancreatic cancer. Future Oncol (2018) 14(3):229–39. doi: 10.2217/fon-2017-0339
34. Liu XF, Zhou LY, Wei ZH, Liu JX, Li A, Wang XZ, et al. The diagnostic role of circulating inflammation-based biomarker in gallbladder carcinoma. biomark Med (2018) 12(10):1095–103. doi: 10.2217/bmm-2018-0049
35. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature (2008) 454(7203):436–44. doi: 10.1038/nature07205
36. Hirahara T, Arigami T, Yanagita S, Matsushita D, Uchikado Y, Kita Y, et al. Combined neutrophil-lymphocyte ratio and platelet-lymphocyte ratio predicts chemotherapy response and prognosis in patients with advanced gastric cancer. BMC Cancer (2019) 19(1):672. doi: 10.1186/s12885-019-5903-y
37. Hua X, Long ZQ, Huang X, Deng JP, He ZY, Guo L, et al. The value of prognostic nutritional index (PNI) in predicting survival and guiding radiotherapy of patients with T1-2N1 breast cancer. Front Oncol (2019) 9:1562. doi: 10.3389/fonc.2019.01562
38. Abe S, Kawai K, Nozawa H, Hata K, Kiyomatsu T, Morikawa T, et al. LMR predicts outcome in patients after preoperative chemoradiotherapy for stage II-III rectal cancer. J Surg Res (2018) 222:122–31. doi: 10.1016/j.jss.2017.09.053
39. Gunaldi M, Goksu S, Erdem D, Gunduz S, Okuturlar Y, Tiken E, et al. Prognostic impact of platelet/lymphocyte and neutrophil/lymphocyte ratios in patients with gastric cancer: a multicenter study. Int J Clin Exp Med (2015) 8(4):5937–42.
40. Liu Z, Ge H, Miao Z, Shao S, Shi H, Dong C. Dynamic changes in the systemic inflammation response index predict the outcome of resectable gastric cancer patients. Front Oncol (2021) 11:577043. doi: 10.3389/fonc.2021.577043
41. Hussain SP, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer (2007) 121(11):2373–80. doi: 10.1002/ijc.23173
42. Arigami T, Uenosono Y, Matsushita D, Yanagita S, Uchikado Y, Kita Y, et al. Combined fibrinogen concentration and neutrophil-lymphocyte ratio as a prognostic marker of gastric cancer. Oncol Lett (2016) 11(2):1537–44. doi: 10.3892/ol.2015.4049
Keywords: chemoradiotherapy, locally advanced gastric cancer, neutrophil-lymphocyte ratio (NLR), prognosis, para-aortic lymph nodes
Citation: Wang S-B, Chen J-Y, Xu C, Cao W-G, Cai R, Cao L and Cai G (2022) Evaluation of systemic inflammatory and nutritional indexes in locally advanced gastric cancer treated with adjuvant chemoradiotherapy after D2 dissection. Front. Oncol. 12:1040495. doi: 10.3389/fonc.2022.1040495
Received: 09 September 2022; Accepted: 17 October 2022;
Published: 27 October 2022.
Edited by:
Yanqing Liu, Columbia University, United StatesReviewed by:
Hehai Pan, University of Pennsylvania, United StatesAnqi Li, The Ohio State University, United States
Zhongzheng Cao, Amgen, United States
Yating Xiao, University of Chinese Academy of Sciences, China
Copyright © 2022 Wang, Chen, Xu, Cao, Cai, Cao and Cai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gang Cai, Y2FpZ2FuZzExODU1QDE2My5jb20=
 Shu-Bei Wang
Shu-Bei Wang Jia-Yi Chen
Jia-Yi Chen Cheng Xu
Cheng Xu Lu Cao
Lu Cao