
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 08 December 2022
Sec. Cancer Epidemiology and Prevention
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.1039930
 Yuzhuo Zhang1,2
Yuzhuo Zhang1,2 Nianci Xue3
Nianci Xue3 Wenyu Jia1,2
Wenyu Jia1,2 Xikang Chen1,2
Xikang Chen1,2 Xuezhang Chen1,2
Xuezhang Chen1,2 Hongliang Li1,2
Hongliang Li1,2 Bin Wang1,2
Bin Wang1,2 Yi Guo1,2
Yi Guo1,2 Ju Chen1,2*
Ju Chen1,2* Huaqin Tian1,2*
Huaqin Tian1,2*Background: As increasing experimental evidence suggests that iron metabolism play crucial roles in cancer and non-cancer conditions, there is a lack of data on serum soluble transferrin receptor (sTfR), a promising marker representing unmet cellular iron demands, between cancer risk from epidemiological studies. Here, we aimed to evaluate the predictive value of sTfR and cancer prevalence.
Materials and methods: We analyzed on 5,480 adult participants from 2015 to 2018 National Health and Nutrition Examination Survey (NHANES). Spearman correlation analysis was performed to investigate the correlations between sTfR and other characteristics. To identify the associations between sTfR and the prevalence of cancers, stratified multivariable logistic regression models, subgroup and sensitivity analyses were also performed.
Results: In tertile analyses, participants in the highest level of sTfR were significantly associated with increased prevalence of total cancers [odds ratio (OR) = 1.53, 95% confidence interval (CI): 1.15-2.02] as compared with those at the lowest tertile. Each unit increment in ln-transformed sTfR concentration was shown to be associated with 39% increased risks of total cancers. Similar associations were found in males rather than females. Further subgroup and sensitivity analyses indicated that, in continuous and tertile analyses, sTfR was more closely associated with male- and female-specific cancers of prostate and testis (2.35: 1.03-5.40; 2.03: 1.00-4.09; respectively), and breast, cervix, ovary and uterus (1.92: 1.11-3.35; 1.66: 1.02-2.69; respectively).
Conclusions: Our findings suggested that elevated level of sTfR was associated with the prevalence of cancers, especially in sex-specific cancers. In order to better determine them, further research in humans will be required.
Iron homeostasis involves in multiple cellular biological processes, including enzymatic activity, mitochondrial function and DNA synthesis (1). Unequivocally, disorders of iron metabolism, including iron deficiency and overload, and corresponding representing markers such as serum iron, ferritin, transferrin, and transferrin saturation (TSAT), closely relate with numerous cancer (2, 3). Mostly, high iron load, also called “ferrotoxic”, has been highlighted as a risk factor for cancer from basic science (4). However, evidence from several population-based prospective studies suggests mixed results on iron status and cancer risk. Some indicated that participants at higher levels of serum iron and TAST were subjected to increased risks of total cancers (5, 6), and especially breast cancer (5, 7, 8), whereas others could not deduce similar positive associations (8, 9). In addition, one recent study simultaneously analyzed the specific effects of above four markers on cancer incidence and mortality in European population, indicating that only elevated ferritin was related to lower risks of breast cancer and cancer mortality (2). Interestingly, they found that higher iron load might not contribute a cancer risk factor in the general European population.
In certain pathological conditions, such as inflammation, liver diseases and malnutrition, serum ferritin and TAST were easily affected and elevated, leading to diagnostic sophistication (10–12). Compared to them, serum soluble transferrin receptor (sTfR) seemed to be a superior and more sensitive marker representing cellular iron demands as it would not be affected by inflammation and return to normal physiological levels quickly after iron homeostasis (13, 14). TfR1 and TfR2 are two subtypes of sTfR that binds with iron-transferrin complex to facilitate serum iron into cells (15). Apart from expression on the surfaces of generic cells, TfR1 could highly express in tumor cells, resulting in higher level of sTfR (15). Thus, it attracted more attention than TfR2. Nowadays, accumulating preclinical evidence has identified that TfR1 played crucial roles in tumor onset, progression, treatment and prognosis (16–18). Notably, it is currently unknown whether these previous findings are generalizable to the results from epidemiological studies. Therefore, we report an epidemiological analysis with a large cross-sectional cohort to investigate the association between sTfR and cancer prevalence, aiming to fulfill the evidence of potential biomarkers for earlier identification of the disease.
We enrolled participants in this study from 2015 to 2018 National Health and Nutritional Examination Survey (NHANES), a population-based national survey that focused on the health and nutrition status among US citizens. The Centers for Disease Control and Prevention ratified the study protocols, and all participants provided informed consent. All survey data and details of the operation are publicly available at www.cdc.gov/nchs/nhanes/. Our mainly interested outcomes were serum sTfR and the prevalence of cancers. Thus, the total number of participants in the primary survey was 19,225. After excluding participants missing sTfR (n = 10,020), who were pregnant (n = 107), who had unavailable cancer information (n = 2,861), or without covariate data (n = 757), a total of 5,480 subjects were enrolled in the final analysis (Figure 1).
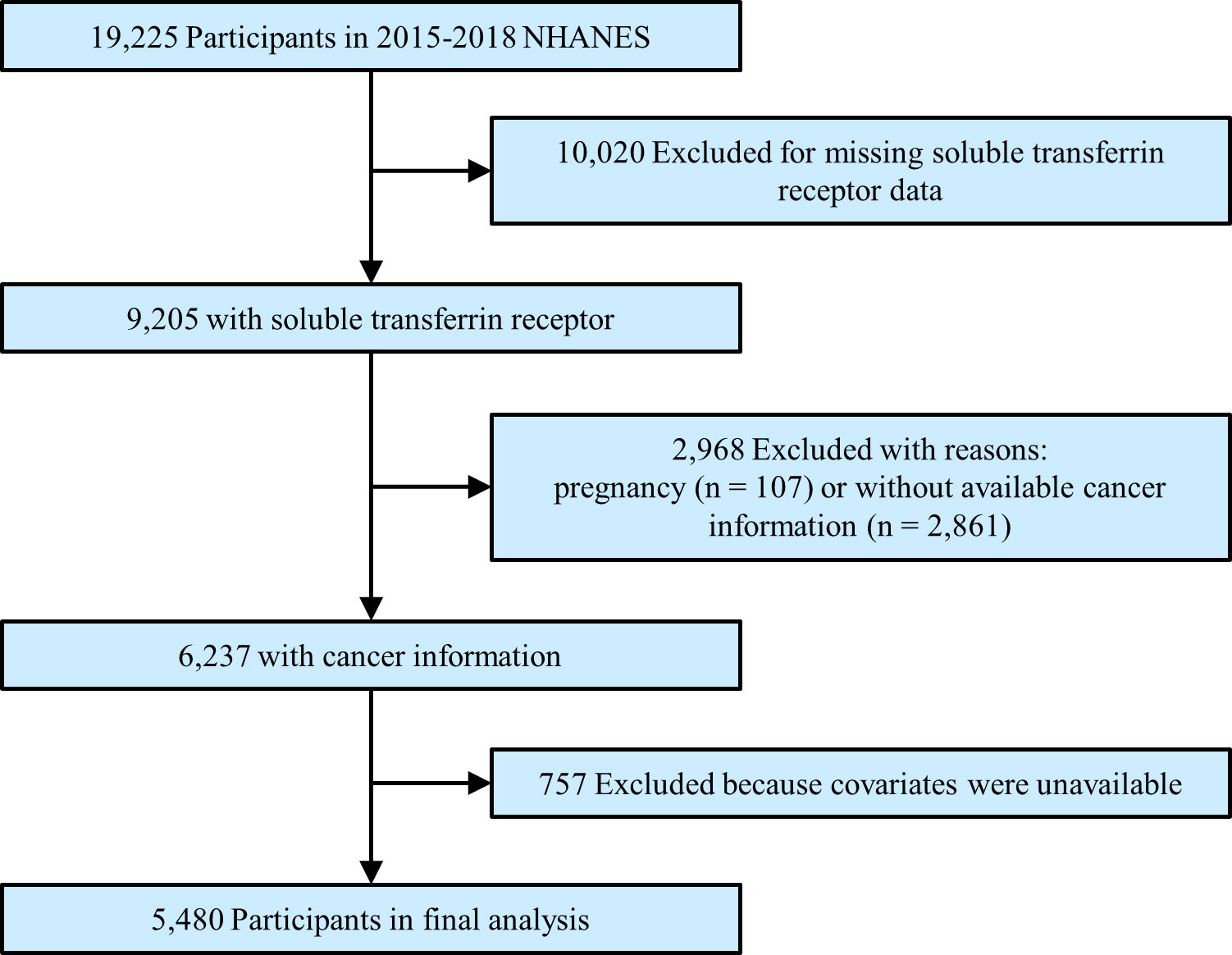
Figure 1 Study flowchart. Flow chart showing the process of participants selection. Of 19,225 participants from 2015 to 2018 of National Health and Nutrition Examination Survey (NHANES), 5,480 remained in the final analysis.
As reported by the protocol in NHANES, serum samples were collected, processed and stored under -30°C until they were shipped to National Center for Environmental Health, Atlanta, GA for testing. The method principle for measurement of sTfR was a particle enhanced immunoturbidimetric assay that used Roche kits on the Cobas® c501 clinical analyzer. The value of sTfR in mg/L was converted to nmol/L by multiplying 11.8. Other details of sTfR measurement are available at https://wwwn.cdc.gov/Nchs/Nhanes/2015-2016/TFR_I.htm.
Total cancers were defined as a total of self-reported physician diagnoses from at least 38 kinds of malignancies, such as bladder, blood, bone, brain, colon, kidney, liver, lung, stomach, skin and lymphoma. Sex-specific cancers were classified into male- and female-specific, including one composite of prostate and testis malignancies, and another combination of breast, cervix, ovary and uterus malignancies, respectively.
Following continuous and categorical covariates were included in our study, including age, body mass index, family income, systolic/diastolic blood pressure, serum iron, ferritin, hemoglobin, total protein, total cholesterol, high-density lipoprotein cholesterol, glycated hemoglobin (HbA1c), hypersensitive C-reactive protein (hs-CRP), systemic immune-inflammation index, energy intake, total fat intake, protein intake, iron intake, gender, ethnicity, education, smoking, drinking, diabetes, hypertension and cardiovascular diseases. Systemic immune-inflammation index was calculated using the peripheral blood cell counts with the calculation: (neutrophils × platelets)/lymphocytes, as it is a meaningful predictor for cancers (19). Cardiovascular diseases were defined as a composite of any self-report heart failure, coronary heart disease, myocardial infarction, angina pectoris and stroke, according to previous researches (20). The detailed acquisition process and measuring method of remaining variables are available at www.cdc.gov/nchs/nhanes.
Baseline characteristics of all included subjects were divided into cancer-free and cancer groups, with the continuous variables reported as the median (interquartile) and the categorical variables reported as numbers with percentages. Comparisons between the two groups were performed using the χ2 tests (categorical variables), one-way ANOVA tests (normal distribution), or Kruskal-Wallis tests (skewed distribution). On one hand, univariate and multivariate stepwise selection regression analyses were used to explore potential related clinical factors to total cancers. On the other hand, bivariate associations between sTfR and baseline characteristics were examined using Spearman correlation analyses for continuous variables and box plots for categorical variables. Moreover, in analyses examining associations with the prevalence of cancers, sTfR was treated as continuous independent variable, scaled per 1-unit increment in ln-transformed for the skewed distribution, or divided into tertiles, using multivariable logistic regression models with different adjustments to calculate odds ratios (ORs) and corresponding 95% confidence intervals (CIs). Model I was not adjusted for any confounders, and Model II was adjusted for age, gender and ethnicity. Model III was fully adjusted for age, gender, ethnicity, body mass index, family income, education, smoking, drinking, diabetes, hypertension, cardiovascular diseases, systolic/diastolic blood pressure, serum iron, ferritin, total protein, total cholesterol, high-density lipoprotein cholesterol, hemoglobin, HbA1c, hs-CRP, systemic immune-inflammation index and nutrition intake. Model IV was only adjusted for significant difference covariate at baseline.
Additionally, several subgroup and sensitivity analyses were performed by a multivariate regression analysis. First, we conducted different subgroups, such as gender, age, ethnicity, smoking, drinking and two levels of serum ferritin and hs-CRP, to identify potential effect modifiers. To test for statistical significance of interactions, interaction terms between sTfR and different subgroups were generated and examined by the Wald test for dichotomous variables and the likelihood ratio test for multilevel variables. If necessary, the possible interactions between all adjusted factors were also tested. Second, we performed a sensitivity analysis after exclusion of patients without diagnosis of male- and female-specific cancers, including prostate, testis, breast, cervix, ovary and uterus malignancies, to investigate possible associations between sTfR and sex-specific cancers. A value of p < 0.05 (two-sided) was considered statistically significant. All analyses were performed with EmpowerStats software with R (version 3.4.3).
Table 1 presents the baseline characteristics of a total of 5,480 participants enrolled in the study, including 498 and 4,982 with and without cancer, respectively. Among all the participants, 38.27% were males, 35.51% were Non-Hispanic Whites, and the median age at enrolment was 46 (33-62) years old. Overall, there were significant differences in baseline characteristics between the two groups, with the exception of body mass index, education, drinking, serum iron, hemoglobin, total cholesterol, high-density lipoprotein cholesterol, total fat intake and iron intake. Compared to the participants without cancer, subjects with cancer were older, smoker and had more comorbidities, such as diabetes, hypertension and cardiovascular diseases, with higher levels of systolic blood pressure, serum ferritin, sTfR and inflammation markers, such as hs-CRP and systemic immune-inflammation index.
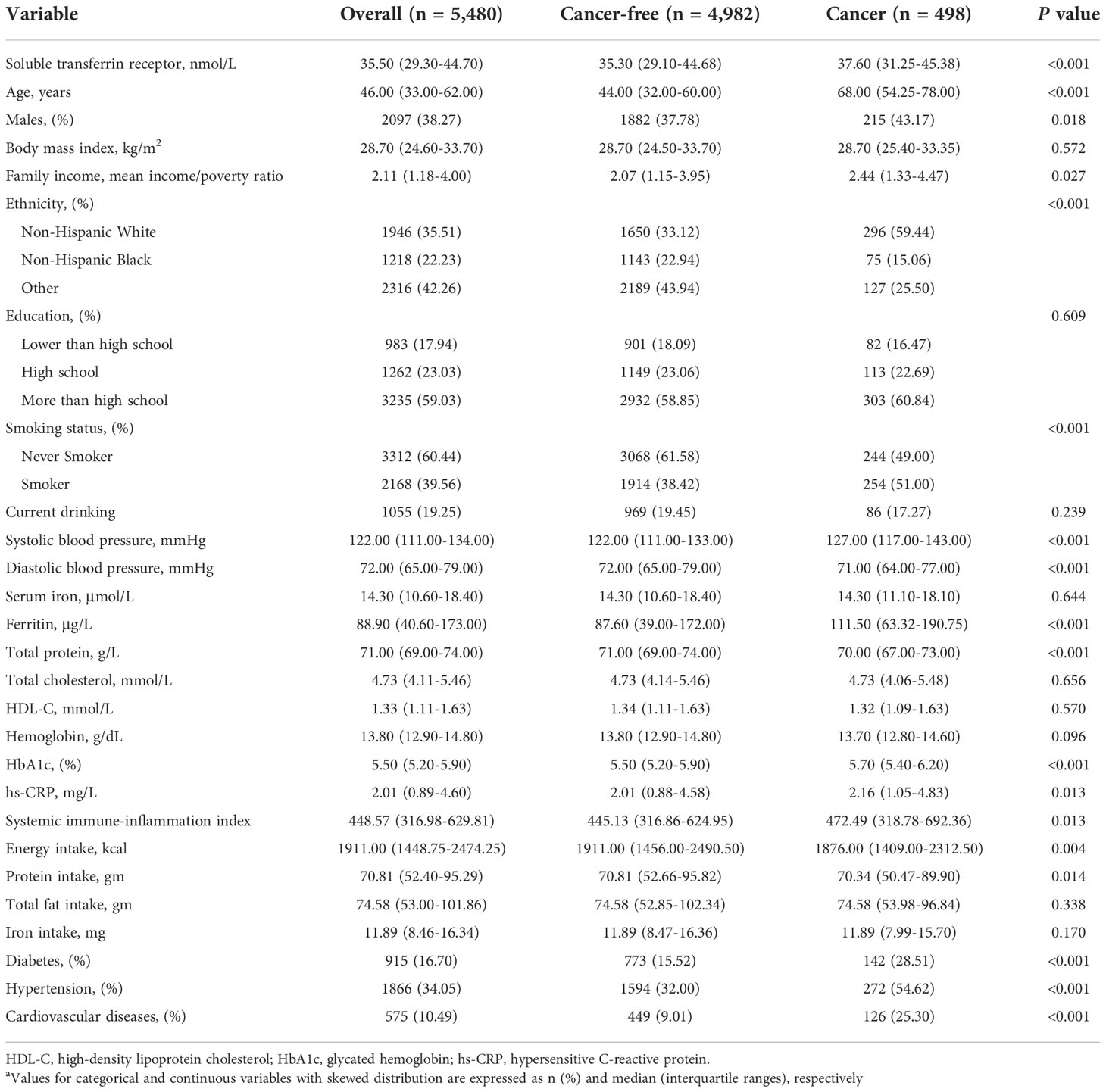
Table 1 Baseline characteristics of NHANES participants included in this studya.
As demonstrated in Table 2, univariate regression analyses revealed that numerous traditional risk factors, such as age, smoking, chronic diseases, systolic blood pressure, HbA1c, and iron markers like ferritin and sTfR were positively related to increased total cancers. Accordingly, after fully adjustment with stepwise selection in multivariate regression analyses, only age, smoking, ferritin and sTfR remained significantly associated with increased risks of total cancers, in which the OR of sTfR was highest (OR: 1.39, 95% CI: 1.01-1.91, p = 0.0443).
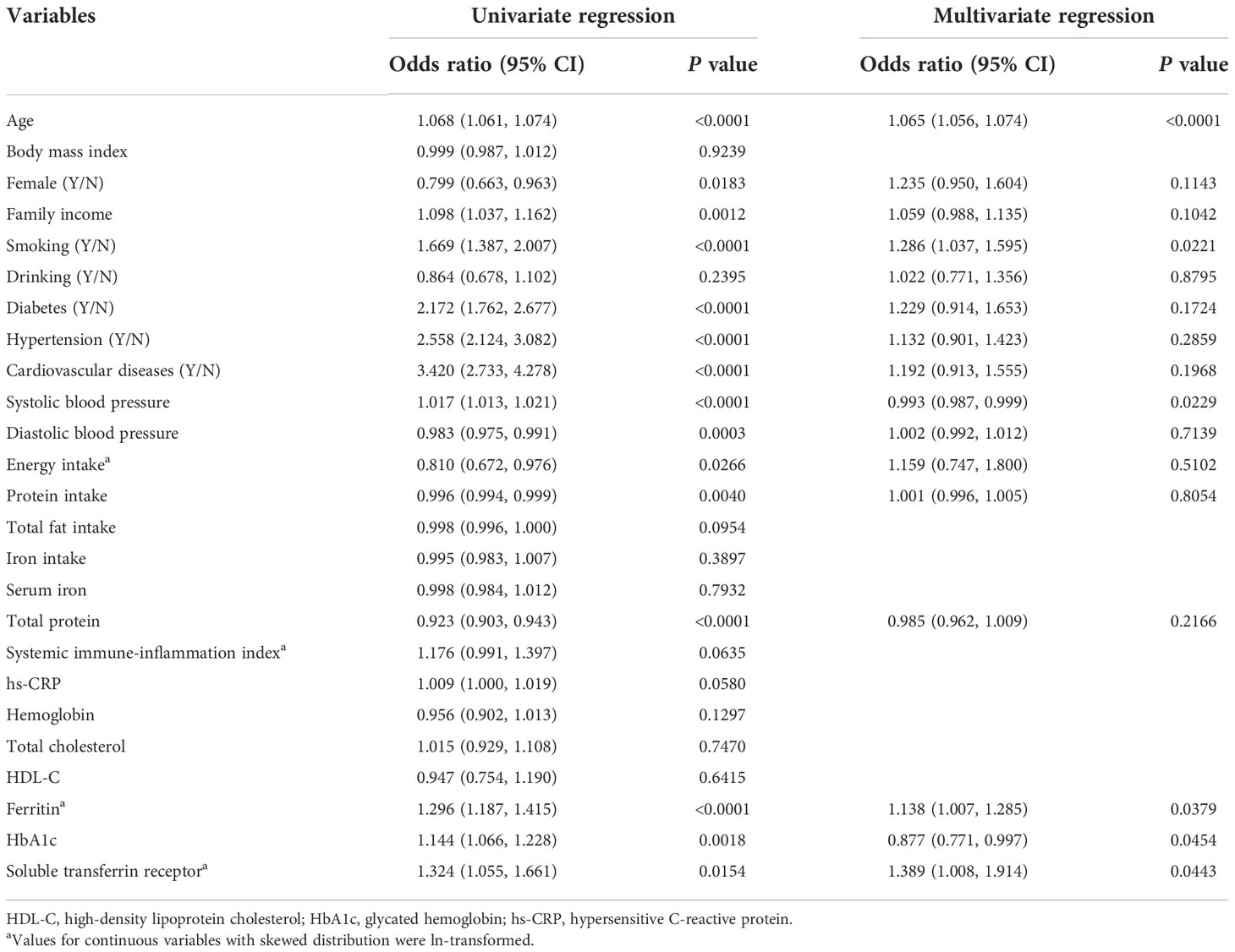
Table 2 Analysis of clinical factors related to total cancers in univariate and multivariate regression.
In Supplementary Figure S1 and Table S1, higher sTfR levels were observed in those who were female, non-Hispanic Blacks, non-smokers, non-drinkers, diabetes, hypertension, cardiovascular diseases and sex-specific cancers. Additionally, as shown in Figure 2 and Supplementary Tables S2, the results of Spearman correlation analysis indicated that age, body mass index, HbA1c and inflammation markers like hs-CRP and systemic immune-inflammation index were weakly positively correlated with sTfR (all p < 0.05), whereas high-density lipoprotein cholesterol, hemoglobin, serum iron, ferritin, and nutrition intake were weakly negatively correlated with sTfR (all p < 0.01). Moreover, there was no significant correlation between total cholesterol and sTfR (p = 0.342).
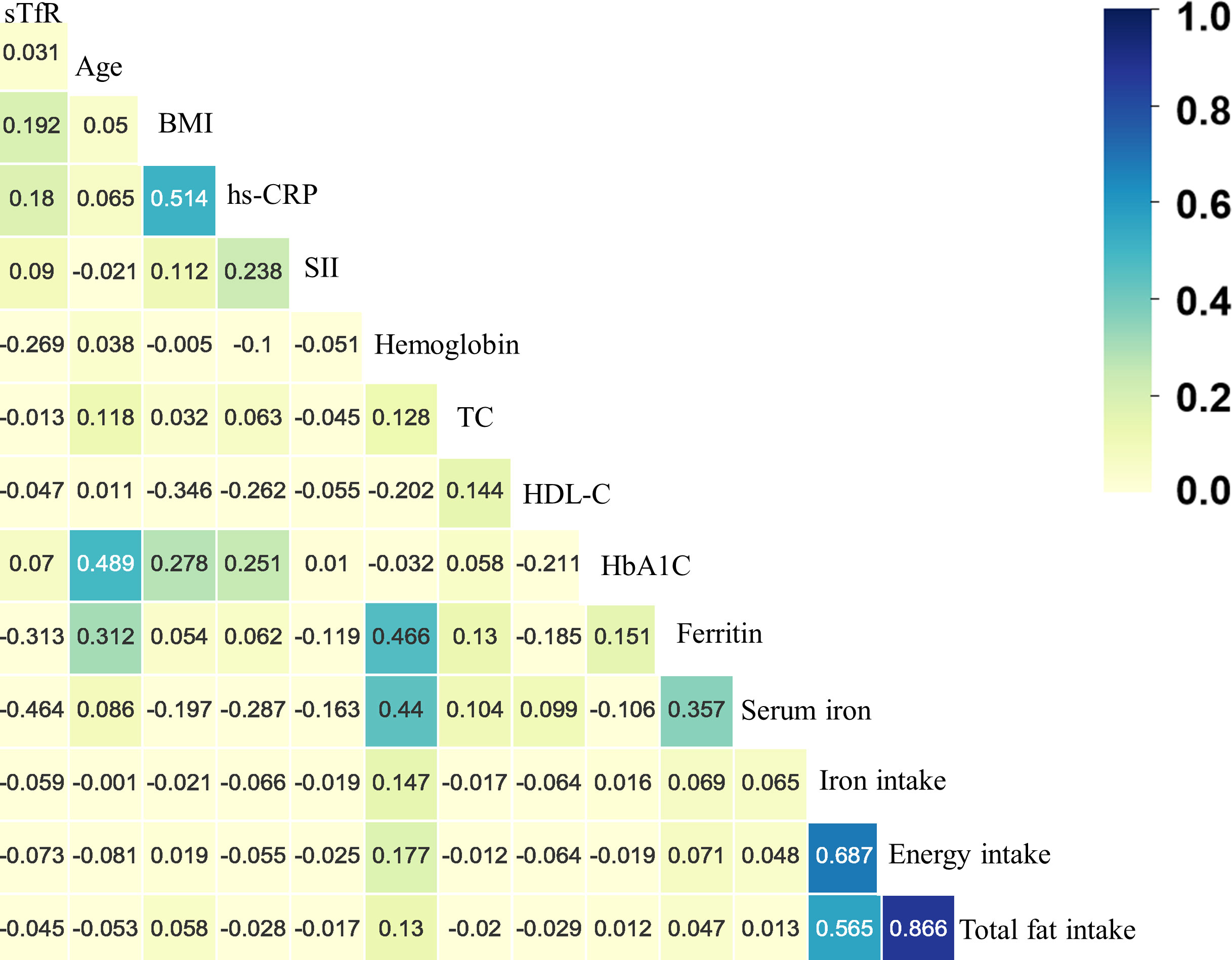
Figure 2 The heatmap of the correlation between covariates and serum soluble transferrin receptor (sTfR) using the Spearman correlation analysis among total participants. BMI, body mass index; hs-CRP, hypersensitive C-reaction protein; SII, systemic immune-inflammation index; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; HbA1c, glycated hemoglobin.
The multivariate logistic regression results are shown in Supplementary Table S3 and Figure 3. When treating serum sTfR as continuous variable, the increase in sTfR (per unit ln-transformed) was significant associated with the prevalence of total cancers in Models I to IV (OR: 1.32, 95% CI: 1.05-1.66, p = 0.0155; 1.53: 1.17-2.01, p = 0.0019; 1.39: 1.01-1.91, p = 0.0444; 1.53: 1.15-2.03, p = 0.0038; respectively). Compared to those at the lowest tertile, the prevalence of total cancers for participants in the highest level of sTfR was 1.66, 1.60, 1.53 and 1.59 in the four models, respectively. Nonetheless, after conducting a subgroup analysis by gender, for males, the positive associations of sTfR with total cancers remained obvious in continuous and tertile analysis regardless of any adjustments, which was not the case for females. Moreover, other subgroup analyses suggested that the associations between sTfR and increased prevalence of total cancers were still significant across subgroups with drinking, age over 45, higher levels of ferritin and hs-CRP (Figure 4 and Supplementary Table S4). In the sensitivity analysis that excluded subjects without diagnosis of sex-specific cancers (prostate and testis malignancies for males, n = 133; breast, cervix, ovary and uterus malignancies for females, n = 138), the ORs of sTfR for male- and female-specific cancers were 2.35 (1.03-5.40, p = 0.0431) and 1.92 (1.11-3.35, p = 0.0207), respectively, in fully adjusted Model III of continuous analysis (Figure 3 and Supplementary Table S5). Similarly, the positive associations were found for the top tertile of sTfR and increased prevalence of male- and female-specific cancers (2.03: 1.00-4.09, p = 0.0484; 1.66: 1.02-2.69, p = 0.0415; respectively). Furthermore, in Model IV that we only adjusted the significant difference covariates in Table 1, the associations between sTfR and the prevalence of sex-specific cancers were still robust in both continuous and tertile analyses.
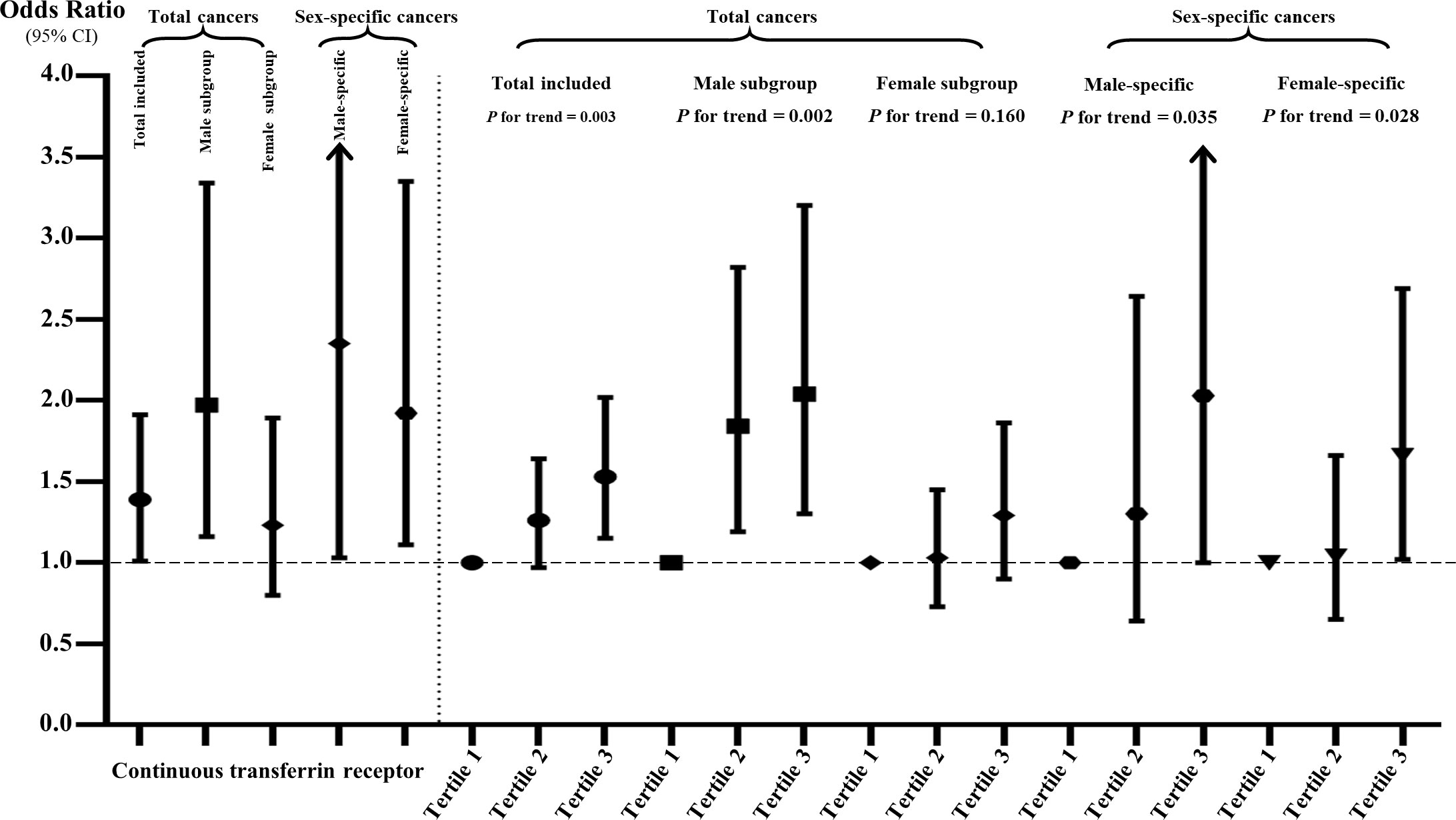
Figure 3 The associations of sTfR with total and sex-specific cancers in continuous and tertile analyses after full adjustment. The sTfR concentrations were ln-transformed in the continuous analysis.
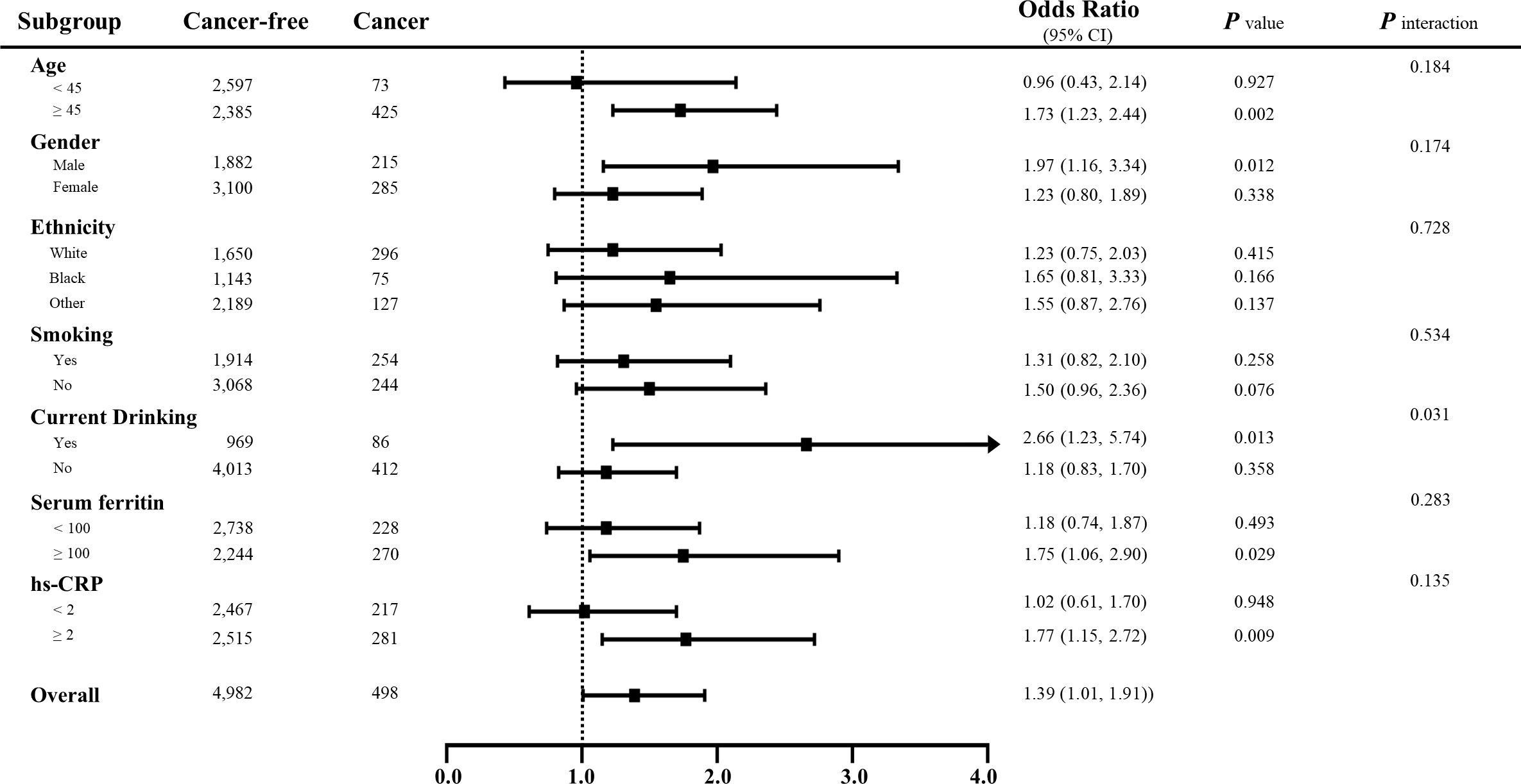
Figure 4 Subgroup analyses for the associations between sTfR and the prevalence of total cancers stratified by participant characteristics. Results are expressed as multivariable-adjusted odds ratio in continuous analyses after controlling covariates including age, gender, ethnicity, body mass index, family income, education, smoking, drinking, diabetes, hypertension, cardiovascular diseases, systolic/diastolic blood pressure, serum iron, ferritin, total protein, total cholesterol, high-density lipoprotein cholesterol, hemoglobin, HbA1c, hs-CRP, systemic immune-inflammation index and nutrition intake, where possible interactions between above factors are also adjusted if necessary. In the continuous analyses, the value of variables was ln-transformed.
In this study, out findings suggested: 1) elevated serum sTfR was associated with increased prevalence of total cancers, which was significant in males rather than females; 2) positive associations were found in both male- and female-specific cancers, including prostate, testis, breast, cervix, ovary and uterus malignancies; 3) serum sTfR was positively correlated to age, body mass index, HbA1c and inflammation while inversely correlated to HDL-C, serum iron, ferritin and iron intake. To our knowledge, this is the first study with large sample size to assess the associations between sTfR and the prevalence of cancers. More importantly, multivariate regression analyses with stepwise selection indicated the closest association between sTfR and total cancers compared to other common clinical tumorigenesis factors, and numerous crucial potential confounders such as comorbidities, lifestyles, nutrition intake, hs-CRP, systemic immune-inflammation index and previously reported iron status markers, have been adjusted in present study. Consistent with a previous study that revealed a potential sex dimorphism between cancer mortality and iron status (2), we did observe the significant association between sTfR and the prevalence of total cancers among men, but not the women. Probably, it was attributed to the inhibitory effect of estrogen and estrogen receptor 1 on TfR1 (21, 22). Traditionally, hormones could decrease with aging and the age of 45 to 50 was considered as a watershed threshold, which was more prevalent and fluctuated in female. Also, the larger proportion of female in the subgroup with age over 45 might account for the higher sTfR levels in total female participants. Likewise, it seems to be the evidence supporting the association between higher sTfR and increased total cancer prevalence in this subgroup. Collectively, age might affect sTfR expression and then lead to tumorigenesis. Besides, it is intriguing that when we only analyzed the sex-specific cancers, there was a positive value of sTfR to predict cancer prevalence among both men and women. On one hand, further separate analyses on type-specific cancer prevalence were restricted considering the limited number of individual cancer type. On the other hand, the interaction between gender, age and the detailed mechanism underlying the predictive value of sTfR in cancer merit further investigation.
There are many preclinical studies of iron overload and increased cancer risks. One of the most prominent was that iron stimulated hydroxyl radical formation, leading to oxidative tissue damage and subsequent carcinogenesis (23). Alternatively, iron could promote tumor growth through hypoxia-inducible factor (HIF) and WNT pathways (4). However, the results of Spearman correlation analysis in present study demonstrated inversed associations with sTfR of iron intake, serum iron and ferritin, which mean that higher levels of iron status might not always be a risk factor for cancer prevalence to some extent. A further line of evidence on inversely effect of ferritin between cancers comes from meta-analysis (24). Possible proposed mechanism underling such inverse associations was that iron deficiency could affect immune function and DNA biosynthesis (25, 26). In sight of this, we referred ferritin < 12 μg/l as anemia (prevalence of 5.88% in present study) and found it not associate increased total cancer risks. It is worth noting that elevated sTfR patients who were drinking or exposed in higher level of hs-CRP, had increased risks of total cancer prevalence. But interestingly, non-drinkers and even non-smokers in this study had higher sTfR levels instead. One previous study summarized that alcohol could alter the levels of some iron-related proteins, including serum iron, hepcidin and transferrin, which was not the case for sTfR (27). It indicated that there might be a more complex indirect interaction between alcohol consumption, sTfR and tumorigenesis. Currently, researches on the relationship between tobacco and sTfR always focused on newborns (28). Thus, further research on this topic in adults is meaningful in the future. Obviously, the burden of alcohol/tobacco-attributable cancers was explicit and highlighted endlessly. Hence, encouraging lifestyle improvements to the resolution of chronic inflammation remains an essential element for cancer prevention.
Inevitably, our study has some limitation. First, the nature of observational epidemiological study prevents us to draw any causal relationship between sTfR and cancer prevalence. Comparatively, serum sTfR as a potential earlier warning marker can be established. Second, the subtypes of sTfR were unavailable from NHANES raw data, which indicated that both TfR1 and TfR2 might be included in the study. Third, we tested all associations with single sTfR measurement but the fluctuation of individual sTfR levels was not available in our study, which restricted further investigation of the time-course associations between changes in sTfR and the incidence of new cancer events. Finally, on one hand, we only had sufficient statistical power for investigations on the prevalence of total and sex-specific cancers as the limited number of individual type-specific cancer. On other hand, some subgroups with a small number of events might exhibit a potentially statistical bias. Thus, the results should be interpreted with caution in clinical practice.
Overall, in this large cohort, we suggest that sTfR may be a potential earlier warning marker for the prevalence of total cancers, and particularly in sex-specific cancers. Obviously, further evidence on these potential links between sTfR and cancer risk from well-designed human studies should be warranted.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
This study was approved by the ethics review board of the National Center for Health Statistics and written informed consents were obtained from each participant. The patients/participants provided their written informed consent to participate in this study.
YZ, NX, WJ, XKC, XZC, HL, BW,YG, JC, and HT conceived and designed the study. YZ and NX collected and analyzed the data. YZ drafted the paper and HT revised the manuscript. All authors contributed to the article and approved the submitted version.
This research was funded by the Foshan “Summit Plan” of building high-level hospitals.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1039930/full#supplementary-material
1. Pasricha SR, Tye-Din J, Muckenthaler MU, Swinkels DW. Iron deficiency. Lancet (2021) 397(10270):233–48. doi: 10.1016/S0140-6736(20)32594-0
2. Quintana Pacheco DA, Sookthai D, Graf ME, Schübel R, Johnson T, Katzke VA , et al. Iron status in relation to cancer risk and mortality: Findings from a population-based prospective study. Int J Cancer (2018) 143(3):561–9. doi: 10.1002/ijc.31384
3. von Haehling S, Jankowska EA, van Veldhuisen DJ, Ponikowski P, Anker SD. Iron deficiency and cardiovascular disease. Nat Rev Cardiol (2015) 12(11):659–69. doi: 10.1038/nrcardio.2015.109
4. Torti SV, Torti FM. Iron and cancer: more ore to be mined. Nat Rev Cancer (2013) 13(5):342–55. doi: 10.1038/nrc3495
5. Wen CP, Lee JH, Tai YP, Wen C, Wu SB, Tsai MK, et al. High serum iron is associated with increased cancer risk. Cancer Res (2014) 74(22):6589–97. doi: 10.1158/0008-5472.CAN-14-0360
6. Knekt P, Reunanen A, Takkunen H, Aromaa A, Heliövaara M, Hakulinen T. Body iron stores and risk of cancer. Int J Cancer (1994) 56(3):379–82. doi: 10.1002/ijc.2910560315
7. Chua AC, Knuiman MW, Trinder D, Divitini ML, Olynyk JK. Higher concentrations of serum iron and transferrin saturation but not serum ferritin are associated with cancer outcomes. Am J Clin Nutr (2016) 104(3):736–42. doi: 10.3945/ajcn.115.129411
8. Gaur A, Collins H, Wulaningsih W, Holmberg L, Garmo H, Hammar N, et al. Iron metabolism and risk of cancer in the Swedish AMORIS study. Cancer Causes Control (2013) 24(7):1393–402. doi: 10.1007/s10552-013-0219-8
9. Wells BJ, Mainous AG 3rd, Everett CJ, Gill JM. Iron, cholesterol, and the risk of cancer in an 18-year cohort. Asian Pac J Cancer Prev (2005) 6(4):505–9.
10. Thomas DW, Hinchliffe RF, Briggs C, Macdougall IC, Littlewood T, Cavill I. British Committee for standards in haematology. guideline for the laboratory diagnosis of functional iron deficiency. Br J Haematol (2013) 161(5):639–48. doi: 10.1111/bjh.12311
11. Lopez A, Cacoub P, Macdougall IC, Peyrin-Biroulet L. Iron deficiency anaemia. Lancet (2016) 387(10021):907–16. doi: 10.1016/S0140-6736(15)60865-0
12. Wish JB. Assessing iron status: beyond serum ferritin and transferrin saturation. Clin J Am Soc Nephrol (2006) 1 Suppl 1:S4–8. doi: 10.2215/CJN.01490506
13. Beguin Y. Soluble transferrin receptor for the evaluation of erythropoiesis and iron status. Clin Chim Acta (2003) 329(1-2):9–22. doi: 10.1016/s0009-8981(03)00005-6
14. Dignass A, Farrag K, Stein J. Limitations of serum ferritin in diagnosing iron deficiency in inflammatory conditions. Int J Chronic Dis (2018) 2018:9394060. doi: 10.1155/2018/9394060
15. Shen Y, Li X, Dong D, Zhang B, Xue Y, Shang P. Transferrin receptor 1 in cancer: a new sight for cancer therapy. Am J Cancer Res (2018) 8(6):916–31.
16. Jeong SM, Hwang S, Seong RH. Transferrin receptor regulates pancreatic cancer growth by modulating mitochondrial respiration and ROS generation. Biochem Biophys Res Commun (2016) 471(3):373–9. doi: 10.1016/j.bbrc.2016.02.023
17. Buas MF, Rho JH, Chai X, Zhang Y, Lampe PD, Li CI. Candidate early detection protein biomarkers for ER+/PR+ invasive ductal breast carcinoma identified using pre-clinical plasma from the WHI observational study. Breast Cancer Res Treat (2015) 153(2):445–54. doi: 10.1007/s10549-015-3554-5
18. Li R, Ma Y, Dong Y, Zhao Z, You C, Huang S, et al. Novel paclitaxel-loaded nanoparticles based on human h chain ferritin for tumor-targeted delivery. ACS Biomater Sci Eng (2019) 5(12):6645–54. doi: 10.1021/acsbiomaterials.9b01533
19. Nøst TH, Alcala K, Urbarova I, Byrne KS, Guida F, Sandanger TM, et al. Systemic inflammation markers and cancer incidence in the UK biobank. Eur J Epidemiol (2021) 36(8):841–8. doi: 10.1007/s10654-021-00752-6
20. Abdalla SM, Yu S, Galea S. Trends in cardiovascular disease prevalence by income level in the united states. JAMA Netw Open (2020) 3(9):e2018150. doi: 10.1001/jamanetworkopen.2020.18150
21. Yu H, Yang C, Jian L, et al. Sulfasalazine−induced ferroptosis in breast cancer cells is reduced by the inhibitory effect of estrogen receptor on the transferrin receptor. Oncol Rep (2019) 42(2):826–38. doi: 10.3892/or.2019.7189
22. Liu L, Zhang C, Qu S, Guo S, Chen R, Li K, et al. ESR1 inhibits ionizing radiation-induced ferroptosis in breast cancer cells via the NEDD4L/CD71 pathway. Arch Biochem Biophys (2022) 725:109299. doi: 10.1016/j.abb.2022.109299
23. Toyokuni S. Role of iron in carcinogenesis: cancer as a ferrotoxic disease. Cancer Sci (2009) 100(1):9–16. doi: 10.1111/j.1349-7006.2008.01001.x
24. Fonseca-Nunes A, Jakszyn P, Agudo A. Iron and cancer risk–a systematic review and meta-analysis of the epidemiological evidence. Cancer Epidemiol Biomarkers Prev (2014) 23(1):12–31. doi: 10.1158/1055-9965.EPI-13-0733
25. Prá D, Rech Franke SI, Pegas Henriques JA, Fenech M. A possible link between iron deficiency and gastrointestinal carcinogenesis. Nutr Cancer (2009) 61(4):415–26. doi: 10.1080/01635580902803701
26. Fonseca-Nunes A, Agudo A, Aranda N, Arija V, Cross AJ, Molina E, et al. Body iron status and gastric cancer risk in the EURGAST study. Int J Cancer (2015) 137(12):2904–14. doi: 10.1002/ijc.29669
27. Ferrao K, Ali N, Mehta KJ. Iron and iron-related proteins in alcohol consumers: cellular and clinical aspects. J Mol Med (Berl) (2022) 100(12):1673–89. doi: 10.1007/s00109-022-02254-8
Keywords: biomarker, serum soluble transferrin receptor, cancer, NHANES, cross-section study
Citation: Zhang Y, Xue N, Jia W, Chen X, Chen X, Li H, Wang B, Guo Y, Chen J and Tian H (2022) Associations between serum soluble transferrin receptor and the prevalence of cancers. Front. Oncol. 12:1039930. doi: 10.3389/fonc.2022.1039930
Received: 08 September 2022; Accepted: 28 November 2022;
Published: 08 December 2022.
Edited by:
Jagdish Chandra, ESIC Model Hospital and PGIMSR, Basaidarapur, IndiaReviewed by:
Gustavo Helguera, Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), ArgentinaCopyright © 2022 Zhang, Xue, Jia, Chen, Chen, Li, Wang, Guo, Chen and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huaqin Tian, MTM5Mjk5NjkyNjJAMTYzLmNvbQ==; Ju Chen, ZHNqMDY0MDdAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.