- 1Department of Oncology, Hillman Cancer Center, University of Pittsburgh Medical Center, Pittsburgh, PA, United States
- 2Dana-Faber Cancer Institute and Harvard Medical School, Boston, MA, United States
- 3Division of Cardiology, University of Washington, Seattle, WA, United States
- 4MedStar Heart and Vascular Institute, Georgetown University, Washington, DC, United States
- 5Department of Biobehavioral Nursing and Health Informatics, School of Nursing, University of Washington, Seattle, WA, United States
- 6Division of Cardiovascular Medicine, Department of Internal Medicine, University of Michigan, Ann Arbor, MI, United States
- 7Department of Surgery, Wake Forest University School of Medicine, Winston-Salem, NC, United States
- 8Department of Hematology/Oncology, Ascension Providence Hospital/Michigan State University College of Human Medicine, Southfield, MI, United States
- 9Department of Oncology, Weill Cornell Medical College, New York, NY, United States
- 10Department of Oncology, Karmanos Cancer Institute at Wayne State University, Detroit, MI, United States
- 11Population Studies and Disparities Research Program, Karmanos Cancer Institute, Detroit, MI, United States
Both obesity and metabolic syndrome are linked to increased incidence of type 2 diabetes, cardiovascular disease (CVD), and cancers of the breast (post-menopausal), and other obesity-related cancers. Over the past 50 years, the worldwide prevalence of obesity and metabolic syndrome has increased, with a concomitant higher incidence of associated co-morbidities and mortality. The precise mechanism linking metabolic syndrome to increased cancer incidence is incompletely understood, however, individual components of metabolic syndrome have been linked to increased breast cancer incidence and worse survival. There is a bidirectional relationship between the risk of CVD and cancer due to a high burden of shared risk factors and higher rates of CVD among cancer survivors, which may be impacted by the pro-inflammatory microenvironment associated with metabolic syndrome and cancer-directed therapies. The Women’s Health Initiative (WHI) is an excellent resource to study a dual relationship between cancer and CVD (cardio-oncology) with extensive information on risk factors and long-term outcomes. The purpose of this review is to provide an overview of research on cardio-oncology conducted utilizing WHI data with focus on studies evaluating both breast cancer and CVD including shared risk factors and outcomes after cancer. The review also includes results on other obesity related cancers which were included in the analyses of breast cancer, articles looking at cancer after heart disease (reverse cardio-oncology) and the role of Clonal Hematopoiesis of Indeterminate Potential (CHIP) as a shared risk factor between CVD and cancer. A summary of pertinent WHI literature helps to delineate the direction of future research evaluating the relationship between CVD and other cancer sites, and provides information on the opportunity for other novel analyses within the WHI.
Introduction
Over the past 50 years, obesity has increased in prevalence, with consequent increases in morbidity and mortality (1, 2). From 2017-2018, the prevalence of obesity in the United States was estimated at approximately 42% (2) with a projected increase to above 50% after 2030 (3). In addition, over the last decade, metabolic syndrome (MS), defined by the presence of at least three out of five cardiometabolic abnormalities [high waist circumference (WC), triglycerides, blood pressure, fasting blood glucose, and low high-density lipoprotein cholesterol (HDL-C)], has also increased in prevalence (4).
Both obesity and MS have been linked to increased incidence of type 2 diabetes, cardiovascular disease (CVD), and cancers of the breast (post-menopausal), endometrium, adenocarcinoma of the esophagus, kidney, liver, gallbladder, pancreas, ovaries, small intestine, thyroid, stomach, multiple myeloma and non-Hodgkin’s lymphoma (5–9). The precise pathophysiology driving the increased incidence of cancer is incompletely understood but proposed mechanisms include shared predisposing factors such as sedentary lifestyle, and lower quality diet, or common cellular pathways related to systemic inflammation (10). Individual components of MS have been linked to higher breast cancer (BC) incidence, and worse survival among cancer survivors (11, 12). There is a proposed bidirectional relationship between risk of CVD and cancer with shared risk factors and higher rates of CVD among cancer survivors, which may be worsened by a pro-inflammatory microenvironment (10) as well as cardiotoxic cancer therapies (13).
In this review, we provide a summary of published studies within the Women’s Health Initiative (WHI) which focus on the area of “cardio-oncology” defined as intersection between cancer and CVD. The review focuses on the relationship between BC and CVD and includes studies evaluating shared risk factors and outcomes after cancer as well as “reverse cardio-oncology” investigating the risk of cancer among women with CVD. The review also covers the role of Clonal Hematopoiesis of Indeterminate Potential (CHIP) and risk of subsequent cancer (8, 12, 14–67). A PubMed search of WHI articles related to CVD and cancer, as well as other non-indexed articles were selected for the review using keywords including BC, cardio-oncology, CHIP, and WHI. When applicable, results for other obesity related cancers reported in the studies evaluating BC are also included.
The WHI includes an observational study (OS) and 3 clinical trials (CT) including the dietary modification trial (DM), the hormone therapy trial (HT) and the Calcium/Vitamin D trial (CaD). Participants could be included in one or more CT. Women were included in the OS if they were not eligible or not interested in participating in a CT. The WHI study included 161,808 postmenopausal women, aged 50-79 at enrollment, and as part of the protocol, detailed information on CVD, cancer risk factors and long-term outcomes were collected (68, 69). Participants were recruited from one of 40 U.S. clinical centers between October 1, 1993, and December 31, 1998 and had a predicted survival of at least 3 years at enrollment. Follow-up was initially through March 2005, followed by two 5-year extension periods and currently ongoing through 2027 (68, 69). The review includes publications inclusive of the entire cohort, the OS, CT or from smaller groups of participants included in ancillary studies which collected biologic or clinical information which was not part of the original protocol.
A. Shared risk-factors
Several predisposing risk-factors and/or protective factors have been linked to CVD and cancer including physical activity, obesity, body composition, hypertension, diet, lipids, circulating cytokines and insulin resistance (70, 71). Table 1 includes WHI studies which address shared risk factors.
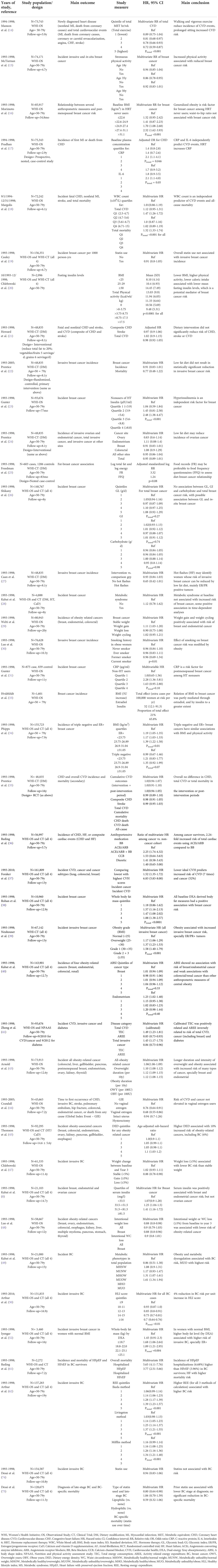
Table 1 Summary of WHI publications on shared risk factors between cardiovascular disease and cancer, with a focus on breast cancer.
Physical activity
In an analysis of 73,743 women in the OS, high levels of physical activity, reported as both walking and vigorous exercise, were associated with lower incidence of CVD, irrespective of race or ethnicity, age and body mass index (BMI), with increasing quintiles of energy expenditure associated with lower risk (Ptrend <0.001) (14). In an analysis of self-reported physical activity at age 35, and cancer risk among 74,171 women in the OS, there was a lower risk of BC for active vs. inactive women [Relative risk (RR) 0.86, 95% confidence interval (CI) 0.78-0.95] and similar trends for physical activity reported at age 18 and 50 (15). These findings were also demonstrated in a WHI analysis which showed that higher physical activity was inversely associated with all types of BC (34). As suggested by these studies, higher levels of physical activities have the potential to lower risk of both CVD and BC.
Obesity and body composition
Obesity and body size are well-established risk-factors for CVD (74, 75) and cancers including BC (6–9). In the OS among non-hormone therapy (HT) users, women with BMI > 31.1 had a higher risk of BC (RR 2.52, 95%CI, 1.62-3.93) (16) and in another analysis, weight cycling over 4 to 6 times was associated with a higher BC risk [Hazard ratio (HR) 1.11, 95%CI 1.03-1.20] (29). Among non-HT users, the proposed mechanism for increased risk is thought to be increased peripheral conversion of androgens to estrogen by the aromatase enzyme in adipose tissue (76). Another study using data from the CT also demonstrated a significant relationship between baseline overweight/obesity and BC risk with higher risk associated with overweight/obese status compared to normal weight [HR 1.58; 95%CI 1.4-1.79] (39). Also using OS data, longer duration of being overweight was associated with a greater risk of all obesity-related cancers [Per 10-year increment HR 1.07, 95%CI 1.06-1.09], and 5% higher risk of BC (43).
Other studies have shown that both smoking and obesity are independent risk-factors for CVD (77) and cancer (7, 8, 78). In evaluating a possible synergistic effect between smoking and obesity among 76,628 women in the OS, there was a greater BC risk noted only among non-obese women (HR 1.24, 95% CI 1.05-1.47) (30) suggesting the possibility that the anti-estrogenic effects of smoking in obese women counterbalances the carcinogenic effects of tobacco (79).
It has been proposed that the obesity - cancer association may be due to the fact that adipose tissue is metabolically active, secreting cytokines and adipokines, which play a role in breast tumorigenesis (80, 81). Supportive of this hypothesis are results in the OS which demonstrated an association between higher levels of C-reactive protein (CRP) and increased BC risk among non-HT users (HR 1.67, 95%CI 1.04-2.68) (31). Similar findings, demonstrating a relationship between higher CRP and CVD risk have also been reported in the Women's Health Study (82).
Adiposity is also associated with higher levels of endogenous estrogen and insulin, both of which are known to play a role in breast tumorigenesis (23, 83). In a study of 1,601 OS women, a 5-unit increase in BMI was associated with 50 additional BC cases per 100,000 women per year, of which 65.8% was mediated by insulin and 23.8% by estrogen (33). In contrast, the use of vaginal estrogen among OS women with or without an intact uterus was not associated with greater risk of CVD, or breast cancer (44) suggesting the lack of a systemic effect of vaginally administered estrogen.
In an analysis of both anthropometric measures and physical activity in the OS and CT, women with the highest BMI quartile compared to the two lowest quartiles had a 1.35 and 1.39-fold higher risk of triple-negative-BC (TNBC) and estrogen receptor (ER)+ tumors, respectively (34).
In an attempt to develop a more valid measure of body fat distribution, a WHI study assessed the relationship between body fat distribution and central obesity (38) using baseline dual energy X-ray absorptiometry (DXA) scans. Results from this study demonstrated a positive association between central obesity and BC risk (1.5-2 fold higher), while analyses only using anthropometric measures showed no differences in risk (38). Another analysis using a body shape index (ABSI), an index hypothesized to be an improved marker of abdominal obesity, showed no association with BC risk (40).
Other studies evaluated the impact of weight change on BC risk. In one OS analysis weight loss (≥ 5%) at 3-years was associated with a significantly lower risk compared to stable weight (< 5% loss) (HR 0.88, p=0.02), and weight gain was associated with a higher risk for TNBC (HR 1.54, 95% CI 1.16-2.05) (47). Similarly, in another OS analysis, intentional weight loss (> 5%) was associated with a lower risk of 11 obesity-related cancers (including BC) compared to stable weight [HR 0.88, 95%CI 0.8-0.98] (48).
Lastly, in another analysis, both obesity and metabolically unhealthy categories were independently associated with increased BC risk, but the metabolically unhealthy obese (MUO) phenotype demonstrated the highest risk (HR 1.62, 95%CI 1.33-1.96) (49). Also an ancillary study of 3,460 women demonstrated that higher whole body fat measured by DXA, was associated with higher BC risk among women with normal BMI (HR 1.89, 95%CI 1.21-2.95) (52).
In conclusion, while obesity and body composition are known risk factors for CVD, WHI research also demonstrates the relationship between obesity, body composition and cancer risk and provides evidence that measures of body composition utilizing DXA provides a more refined method in which to investigate this relationship. In addition, the WHI biospecimen repository has enabled research further investigating the relationship between insulin, inflammatory cytokines, hormones and cancer risk.
Hyperlipidemia
Hyperlipidemia is a known risk-factor for CVD (84), and its association with BC has also been investigated (71, 85–87). Studies in the WHI have evaluated the relationship between statin use and BC risk. In an evaluation of 156,351 women in the WHI, there was no association between statin use and BC risk overall [HR 0.91, 95% CI 0.8-1.05] however hydrophobic statins were associated with an 18% lower risk of BC [0.82, 95% CI 0.7-0.97] (19). The essentially null results were corroborated in a later follow-up analysis (72). In another study (73) lipophilic statins were associated with a reduction in diagnosis of late-stage BC (HR 0.80, 95% CI 0.64-0.98, p = 0.035) and by a marginally lower risk of breast cancer mortality (HR 0.59, 95% CI 0.32-1.06, p = 0.075). While a protective effect of statins and breast cancer risk has not been clearly demonstrated in the WHI, other ongoing research is investigating the relationship between lipid biomarkers measured at baseline and outcomes after cancer (unpublished).
Hyperinsulinemia, insulin resistance and impaired glucose tolerance
Fasting hyperinsulinemia is a potential mediator for breast carcinogenesis (88), and insulin and insulin-like growth factor-1 (IGF-1) may synergistically increase BC risk (70, 89). In an analysis of 2,996 women in a WHI ancillary study, lower BMI (p<0.0001), higher physical activity (p<0.001) and lower caloric intake (p<0.02) were independently associated with lower mean fasting insulin levels (20). Another OS analysis among women without diabetes showed that higher fasting insulin, but not total IGF-1 was associated with a higher BC risk (HR 1.46, Ptrend=0.02) (23). Similarly, hyperglycemia resulting from impaired glucose tolerance has been shown in other non-WHI analyses to be a risk-factor for both CVD (90) and BC (91). In another WHI ancillary study of 21,103 women, higher levels of serum insulin was associated with higher BC risk (HR 1.41, Ptrend<0.0003) (8). In another overall WHI analysis there was no significant association between dietary glycemic load (GL), glycemic index (GI), or carbohydrate intake with total BC risk (26). The WHI has added to the literature on insulin resistance and impaired glucose tolerance and BC risk suggesting a relationship between diabetes and risk of BC.
Cardiometabolic abnormalities and heart failure
Metabolic Syndrome (MS) has been shown by others to be associated with higher risk of type 2 diabetes and CVD (92). In an analysis of MS as measured at baseline among 4,888 women in the overall WHI cohort, there was no overall relationship between MS and risk of BC, however diastolic blood pressure (DBP) showed a borderline positive association among women without diabetes (28).
Hypertension is a known risk factor for CVD (86). In a study of 56,997 cancer survivors in the overall WHI, use of angiotensin-converting-enzyme inhibitors and angiotensin-receptor-blockers was associated with 2.24-fold risk of total cardiac events, and a 1.87-fold increase in heart failure (HF) risk compared to use of beta-blockers; however, these findings were only seen among women with cancer (36).
In another analysis of 2,272 women with BC hospitalized for HF, (61) the incidence of HF with preserved ejection fraction (HFpEF) was higher (6.68%) than the incidence of HF with reduced ejection fraction (HFrEF) (3.96%). Factors associated with HFpEF included prior myocardial infarction (HR 2.83), greater WC (HR 1.99) and smoking history (HR 1.65), however these variables were not associated with HFrEF. Overall mortality among BC survivors was 5.65-fold and 3.77-fold higher among women with HFpEF and HFrEF respectively, compared to those without HF. In summary, the WHI has contributed research on the relationship between CVD and various components of CVD and BC risk. In addition, WHI investigators have emphasized the importance of differentiating the specific HF phenotype (93).
Diet
In the WHI, several measures of dietary intake have been used to investigate the relationship between diet, CVD and cancer. An investigation of 131,833 women reported a 4% reduction in BC risk per unit increase in healthy lifestyle index (HLI) scores (94) based on factors including diet and exercise (50). Another analysis (37) demonstrated that a lower cardiovascular health (CVH) score (95) was associated with a 7-fold greater risk of incident CVD, and a 52% greater risk of incident cancer, with lung cancer having the strongest association (37).
The WHI Dietary Modification (DM) CT randomly assigned 48,835 postmenopausal women to usual diet (60%) vs intervention (40%) that focused on reduction of total fat intake to 20% of energy intake, increased vegetable and fruit intake to 5-servings and grains to 6-servings/day. As measured by food frequency questionnaire (FFQ), at baseline, women consumed 32% or more of their total energy from fat (FFQ) (96, 97).
Several DM analyses evaluated the relationship between dietary intervention, CVD and incident cancer risk (21, 22, 24, 27, 35). After 8.1 years of follow-up, the dietary intervention was not associated with a reduction in CVD (21), invasive BC (22), or ovarian or endometrial cancer (24); however, risk of ovarian cancer decreased with increased duration of dietary intervention (24). In another analysis, there were no differences in CHD, total CVD, or total all-cause mortality in either the intervention or post-intervention periods after 16-years of follow-up (35). Finally, among women on a low-fat diet, baseline vasomotor symptoms, particularly hot flashes, were associated with a lower BC risk, particularly for women with ER/progesterone receptor (PR)+ tumors, thought to be due to modulation of estrogen metabolism by diet (27).
An analysis comparing two dietary instruments (4-day food records [FR] and FFQs) among women in the non-intervention DM arm, showed that the FR over the FFQ, was a preferred method of dietary assessment for all types of dietary fats (25). Another study using data from the entire WHI, demonstrated that higher dietary energy density was associated with a 10% increased risk of any obesity-related cancer among women with a normal BMI (45).
In another OS analysis, various reductions in energy consumption were associated with lower risk of major incident CVD events and cancer. Specifically, a 20% reduction in total energy consumption (TEC) was associated with one-third lower risk, 20% increase in activity-related energy expenditure (AREE) one-fourth lower risk, and simultaneous TEC and AREE, a 50% lower risk (41). Another analysis of 137,283 women demonstrated that predicted resting energy expenditure (REE) was positively associated with invasive BC risk (62).
In summary, results from the OS strongly support a relationship between fat and energy consumption and risk of CVD and cancer, including alternative measures of healthy eating and lifestyle including the HLI and CVH. These results however have not been replicated in the DM thought to be at least in part due to poor dietary compliance among participants randomized to the intervention (21, 22). The interaction between diet and other shared risk factors for CVD and cancer, including weight loss, physical activity and body composition is complex and requires further evaluation regarding synergistic relationships or whether outcomes may differ depending on timing, pre-, during or post-cancer.
B. Shared outcomes between cancer and CVD
Table 2 lists WHI studies on shared outcomes between CVD and cancer with a focus on BC. In the DM, low fat dietary intervention did not result in significant changes in CHD, total CVD, or all-cause mortality in the intervention, post-intervention and cumulative (intervention + post-intervention) periods (35). In an analysis of incident CVD and total and cause-specific death rates among women with and without incident BC, over 10-years post-diagnosis, there was an increase in total mortality (HR 1.20, 95%CI 1.04-1.39) for women with localized BC, aged 70-79, compared to those with no BC. While the risk for coronary heart disease was the same for women with and without BC, CVD was the leading cause of death for women with BC diagnosed between age 70-79 (42).
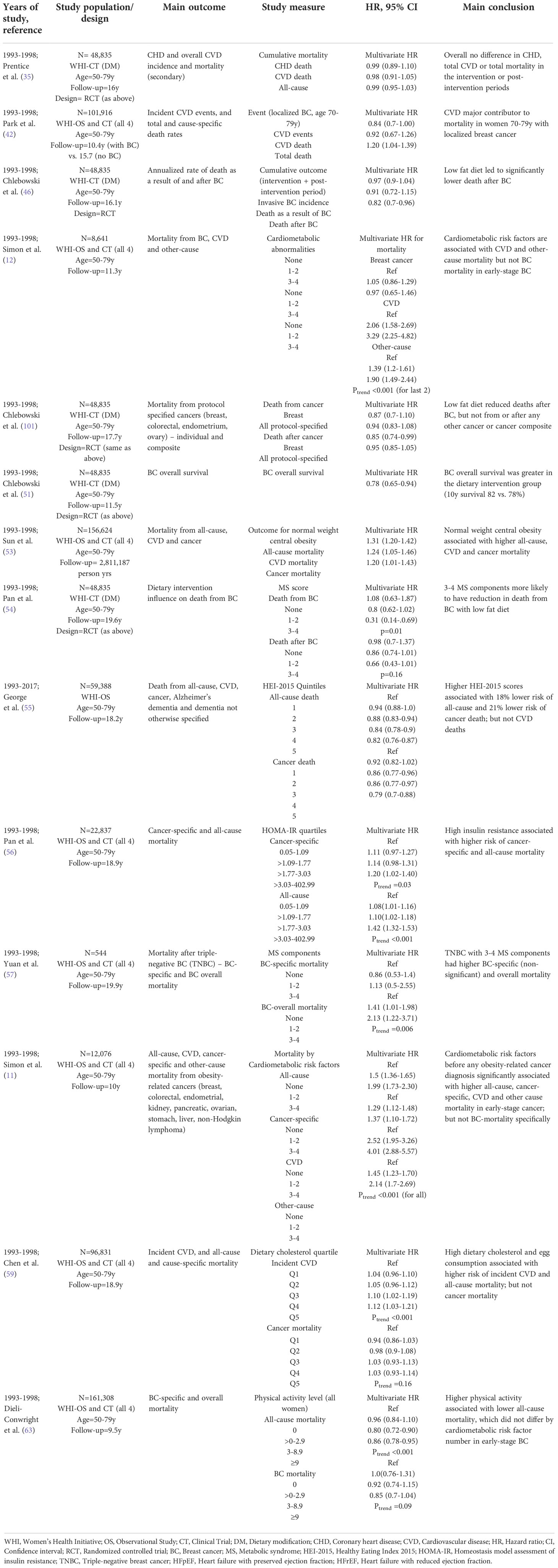
Table 2 Summary of WHI publications on shared outcomes between cardiovascular disease and cancer, focusing on breast cancer.
In contrast to the findings above, showing no effect of low-fat dietary intervention on CVD and all-cause mortality in the overall DM cohort (35), post-hoc analyses among women with subsequent diagnosis of BC demonstrated, fewer deaths after BC among women randomized to the intervention (low fat dietary intake) (46). Consistent with this, in an analysis of overall survival among women randomized to the dietary intervention, survival among those diagnosed with BC was significantly higher in the intervention group (10-year survival of 82 vs. 78%). There were fewer deaths from BC (68 vs. 120), other cancers (36 vs. 65) and CVD (27 vs. 64) in the intervention arm which could partly explain the improved survival (51). Lastly, in an evaluation of the influence of the dietary intervention on BC mortality by MS components, only women with 3-4 MS components had a significant reduction in BC mortality in the intervention arm (HR 0.31, p=0.01), compared to those with 0 or 1-2 MS components (54). This latter result suggests that the DM intervention may be more effective among women in the highest risk group.
In a targeted analysis of 8,641 women with early-stage BC, a higher number of CM risk-factors including high waist circumference, blood pressure, cholesterol and history of type-2 diabetes, was associated with a higher risk of CVD and other-cause mortality (Ptrend<0.001) but not BC mortality (Ptrend=0.86) (12). A similar analysis on 12,076 women with early-stage obesity-related cancers (11) showed that women with 3-4 CM abnormalities (vs. none) had 1.5, 1.37, 4.0, and 2.14-fold greater risk of death from any-cause, cancer, CVD and other-causes respectively, with no specific increase in BC-specific mortality as shown in the earlier report.
In another analysis of 156,262 women in the entire cohort, those that were normal-weight, with central obesity, compared with women that were normal-weight and no central obesity, had a higher risk of mortality due to CVD (HR 1.25; 95%CI, 1.05-1.46) as well as mortality due to cancer (HR 1.20; 95%CI, 1.01-1.43) (53). These findings support non-WHI studies which have demonstrated that excessive visceral fat is a risk-factor for greater risk of CVD and cancer (98).
Other WHI analyses have looked at diet and cancer outcomes (55, 56, 59). In a study of 59,388 women in the OS, women who had higher measured Healthy Eating Index-2015 (HEI-2015) scores, reflecting more optimal diet quality, had a 21% lower risk of all-cause mortality, and an 18% lower risk of cancer mortality, but there was no association with mortality due to CVD (55). In another analysis of 22,837 women, high baseline insulin resistance, measured as higher homeostasis model assessment of insulin resistance (HOMA-IR) scores was associated with higher cancer-specific mortality (HR 1.26, Ptrend=0.003) and all-cause mortality (HR 1.63, Ptrend<0.001) (56). Lastly in a study of 96,831 women, both higher dietary cholesterol and egg intake was associated with modestly elevated risk of incident CVD, CVD mortality, and all-cause mortality, but not cancer mortality (p=0.16 and p=0.26 respectively) (59).
An analysis of 544 women with non-metastatic TNBC showed that those with a greater number of MS components had a 27% lower 10-year BC-overall survival, non-significantly higher BC-specific mortality (HR 2.05, Ptrend=0.114) and significantly higher BC-overall mortality (HR 2.13, Ptrend=0.006), likely because of reduction in other causes of death (57); while another report showed that higher physical activity was associated with lower all-cause (HR 0.86, Ptrend<0.001), but not BC-specific mortality (HR 0.85, p=0.09) (63).
In summary, WHI analyses support the notion that shared risk-factors representing lifestyle and body composition impact both cancer and CVD outcomes, largely due to risk-factor burden. It is important for investigators interested in both CVD and cancer outcomes to investigate the impact of lifestyle interventions known to modify these risk factors, which may improve outcomes from both cancer and CVD.
C. Reverse cardio-oncology and the role of clonal hematopoiesis of indeterminate potential
While the increased risk of CVD in cancer survivors is well described for certain cancers (13), the term “reverse cardio-oncology” describes the increased risk of cancer, among individuals with CVD, compared to the general population (100). Factors linking CVD and cancer risk as addressed in the WHI (Supplementary Table 1) include treatment as well as pathophysiologic pathways related to inflammation, clonal hematopoiesis of indeterminate potential (CHIP), hypoxia, microRNAs, extracellular vesicles, and circulating “cardiokines” (100).
In an analysis of 93,676 women assessing the association between baseline self-reported atrial fibrillation (AF) and incident invasive breast over 15-years follow-up, there was a 19% excess risk of subsequent BC among women with AF (HR 1.19, 95%CI 1.03-1.38). While the excess BC risk was mitigated by baseline cardiac glycoside use, the use of glycosides was also independently associated with increased BC risk (HR 1.68, 95% CI1.33-2.12), but not CRC (32). In an analysis of the relationship between HF and incident cancer over 22-years follow-up, HFpEF was associated with increased total cancer incidence (HR 1.34, 95%CI 1.06-1.67), but not HFrEF (HR 0.99, 95%CI 0.74-1.34) (58). HF overall was also associated with an increased risk of obesity-related cancers but not BC specifically.
Aging is associated with acquisition of somatic mutations in the absence of neoplasia, known as clonal hematopoiesis of indeterminate potential (CHIP), which has been linked to a higher risk of cancers as well as CVD (64, 65). In the WHI, CHIP has been shown to be associated with a greater risk of leukemias, as well as solid tumor-specific mortality, but not CVD mortality post cancer diagnosis (66, 67). In an analysis of 8,709 women with data on CHIP, the prevalence of CHIP among women free of CVD and cancer was 8.7%. Further analysis of the relationship between a healthy lifestyle score (BMI, physical activity, diet and smoking) and CHIP showed that both normal BMI and never-smoking were associated with lower odds for CHIP (OR 0.71, 95%CI 0.57-0.88) (60). Since obesity is associated with both breast cancer risk and CHIP, the relationship of CHIP with breast cancer risk is of scientific interest. In fact, recent analyses of UK biobank data suggest an increased risk of breast cancer in CHIP carriers (101) and similar analyses are ongoing in the WHI with longer follow up data with more incident breast cancer cases.
In summary WHI studies demonstrate a possible relationship between pre-existing CVD and increased cancer risk. In addition, CHIP is a shared risk-factor between CVD and cancer. More importantly, several clinical associations that are seen with breast cancer are also shared with CHIP. For example, CHIP has been associated with diabetes in several cohorts and heart failure in the TOPMed consortium (that included WHI data) (102). The complex associations of CVD risk factors, CHIP and breast cancer deserve further evaluation both in terms of mediation as well as interaction together, to lead to potential worsening of outcomes. These risk factors particularly are relevant in survivorship cohorts where shared risk factors interact further with a post chemotherapy state that can impact both cardiovascular risk and CHIP penetrance.
Future direction
This review provides an overview of published literature on shared risk-factors and outcomes between CVD and BC, highlighting a likely bidirectional risk and adding information to a recent over-arching summary of cardiovascular research in the WHI (103). The WHI findings presented here provide a unique insight into complex associations between lifestyle risk factors, CVD and BC, and long-term outcomes including CV and cancer-specific mortality. The potential clinical and public health implications of the WHI results are significant and suggest that promotion of healthy lifestyle, and behaviors in at-risk post-menopausal women, may reduce cardiovascular and cancer mortality. Importantly, this literature provides a foundation for ongoing and future research of the association between shared risk factors between CVD and cancers of other primary sites (Supplementary Table 1).
Author contributions
SR and MS developed the hypothesis, rationale, helped with data gathering, analysis, writing and editing. CD-C, RC, AB, KR, AV, KC, PD and VN helped with writing and editing the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1039246/full#supplementary-material
References
1. Obesity and Overweight. World Health Organization. Available from: www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
2. Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. In: NCHS Data Brief, no 360. Hyattsville, MD: National Center for Health Statistics (2020). Available from: https://www.cdc.gov/nchs/data/databriefs/db360-h.pdf.
3. Ward ZJ, Bleich SN, Cradock AL, Barrett JL, Giles CM, Flax C, et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med (2019) 381(25):2440–50. doi: 10.1056/NEJMsa1909301
4. Shin D, Kongpakpaisarn K, Bohra C. Trends in the prevalence of metabolic syndrome and its components in the united states 2007–2014. Int J Cardiol (2018) 259:216–9. doi: 10.1016/j.ijcard.2018.01.139
5. Simon S. Obesity Rates Continue to Rise Among Adults in the US. American Cancer Society (2018). Available from: https://www.cancer.org/latest-news/obesity-rates-continue-to-rise-among-adults-in-the-us.html
6. Esposito K, Chiodini P, Colao A, Lenzi A, Giugliano D. Metabolic syndrome and risk of cancer: A systematic review and meta-analysis. Diabetes Care (2012) 35:2402–11. doi: 10.2337/dc12-0336
7. Kabat GC, Xue X, Kamensky V, Lane D, Bea JW, Chen C, et al. Risk of breast, endometrial, colorectal, and renal cancers in postmenopausal women in association with a body shape index and other anthropometric measures. Cancer Causes Control (2015) 26:219–29. doi: 10.1007/s10552-014-0501-4
8. Kabat GC, Kim MY, Lane DS, Zaslavsky O, Ho GYF, Luo J, et al. Serum glucose and insulin and risk of cancers of the breast, endometrium, and ovary in postmenopausal women. Eur J Cancer Prev (2018) 27:261–8. doi: 10.1097/CEJ.0000000000000435
9. Mitri J, Castillo J, Pittas AG. Diabetes and risk of non-hodgkin’s lymphoma: a meta-analysis of observational studies. Diabetes Care (2008) 31:2391–7. doi: 10.2337/dc08-1034
10. de Boer RA, Aboumsallem JP, Bracun V, Leedy D, Cheng R, Patel S, et al. A new classification of cardio-oncology syndromes. Cardiooncology (2021) 7(1):24. doi: 10.1186/s40959-021-00110-1
11. Simon MS, Hastert TA, Barac A, Banack HR, Caan BJ, Chlebowski RT, et al. Cardiometabolic risk factors and survival after cancer in the women’s health initiative. Cancer (2020), cncr.33295. doi: 10.1002/cncr.33295
12. Simon MS, Beebe-Dimmer JL, Hastert TA, Manson JE, Cespedes-Feliciano EM, Neuhouser ML, et al. Cardiometabolic risk factors and survival after breast cancer in the women’s health initiative. Cancer (2018) 124:1798–807. doi: 10.1002/cncr.31230
13. Okwuosa TM, Anzevino S, Rao R. Cardiovascular disease in cancer survivors. Postgrad Med J (2017) 93(1096):82–90. doi: 10.1136/postgradmedj-2016-134417
14. Manson JE, Greenland P, LaCroix AZ, Stefanick ML, Mouton CP, Oberman A, et al. Walking compared with vigorous exercise for the prevention of cardiovascular events in women. N Engl J Med (2002) 347(10):716–25. doi: 10.1056/NEJMoa021067
15. McTiernan A, Kooperberg C, White E, Wilcox S, Coates R, Adams-Campbell LL, et al. Women's health initiative cohort study. recreational physical activity and the risk of breast cancer in postmenopausal women: The women's health initiative cohort study. JAMA (2003) 290(10):1331–6. doi: 10.1001/jama.290.10.1331
16. Morimoto LM, White E, Chen Z, Chlebowski RT, Hays J, Kuller L, et al. Obesity, body size, and risk of postmenopausal breast cancer: The women's health initiative (United states). Cancer Causes Control (2002) 13(8):741–51. doi: 10.1023/a:1020239211145
17. Pradhan AD, Manson JE, Rossouw JE, Siscovick DS, Mouton CP, Rifai N, et al. Inflammatory biomarkers, hormone replacement therapy, and incident coronary heart disease: Prospective analysis from the women's health initiative observational study. JAMA (2002) 288(8):980–7. doi: 10.1001/jama.288.8.980
18. Margolis KL, Manson JE, Greenland P, Rodabough RJ, Bray PF, Safford M, et al. Leukocyte count as a predictor of cardiovascular events and mortality in postmenopausal women: The women's health initiative observational study. Arch Intern Med (2005) 165(5):500–8. doi: 10.1001/archinte.165.5.500
19. Cauley JA, McTiernan A, Rodabough RJ, LaCroix A, Bauer DC, Margolis KL, et al. Statin use and breast cancer: Prospective results from the women's health initiative. J Natl Cancer Inst (2006) 98(10):700–7. doi: 10.1093/jnci/djj188
20. Chlebowski RT, Pettinger M, Stefanick ML, Howard BV, Mossavar-Rahmani Y, McTiernan A. Insulin, physical activity, and caloric intake in postmenopausal women: Breast cancer implications. J Clin Oncol (2004) 22(22):4507–13. doi: 10.1200/JCO.2004.04.119
21. Howard BV, Van Horn L, Hsia J, Manson JE, Stefanick ML, Wassertheil-Smoller S, et al. Low-fat dietary pattern and risk of cardiovascular disease: The women's health initiative randomized controlled dietary modification trial. JAMA (2006) 295(6):655–66. doi: 10.1001/jama.295.6.655
22. Prentice RL, Caan B, Chlebowski RT, Patterson R, Kuller LH, Ockene JK, et al. Low-fat dietary pattern and risk of invasive breast cancer: The women's health initiative randomized controlled dietary modification trial. JAMA (2006) 295(6):629–42. doi: 10.1001/jama.295.6.629
23. Gunter MJ, Hoover DR, Yu H, Wassertheil-Smoller S, Rohan TE, Manson JE, et al. Insulin, insulin-like growth factor-I, and risk of breast cancer in postmenopausal women. J Natl Cancer Inst (2009) 101(1):48–60. doi: 10.1093/jnci/djn415
24. Prentice RL, Thomson CA, Caan B, Hubbell FA, Anderson GL, Beresford SA, et al. Low-fat dietary pattern and cancer incidence in the women's health initiative dietary modification randomized controlled trial. J Natl Cancer Inst (2007) 99(20):1534–43. doi: 10.1093/jnci/djm159
25. Freedman LS, Potischman N, Kipnis V, Midthune D, Schatzkin A, Thompson FE, et al. A comparison of two dietary instruments for evaluating the fat-breast cancer relationship. Int J Epidemiol (2006) 35(4):1011–21. doi: 10.1093/ije/dyl085
26. Shikany JM, Redden DT, Neuhouser ML, Chlebowski RT, Rohan TE, Simon MS, et al. Dietary glycemic load, glycemic index, and carbohydrate and risk of breast cancer in the women's health initiative. Nutr Cancer (2011) 63(6):899–907. doi: 10.1080/01635581.2011.587227
27. Caan BJ, Aragaki A, Thomson CA, Stefanick ML, Chlebowski R, Hubbell FA, et al. Vasomotor symptoms, adoption of a low-fat dietary pattern, and risk of invasive breast cancer: A secondary analysis of the women's health initiative randomized controlled dietary modification trial. J Clin Oncol (2009) 27(27):4500–7. doi: 10.1200/JCO.2008.20.0493
28. Kabat GC, Kim M, Chlebowski RT, Khandekar J, Ko MG, McTiernan A, et al. A longitudinal study of the metabolic syndrome and risk of postmenopausal breast cancer. Cancer Epidemiol Biomarkers Prev (2009) 18(7):2046–53. doi: 10.1158/1055-9965.EPI-09-0235
29. Welti LM, Beavers DP, Caan BJ, Sangi-Haghpeykar H, Vitolins MZ, Beavers KM. Weight fluctuation and cancer risk in postmenopausal women: The women's health initiative. Cancer Epidemiology Biomarkers Prev Publ Am Assoc Cancer Research Cosponsored by Am Soc Prev Oncol (2017) 26(5):779–86. doi: 10.1158/1055-9965.epi-16-0611
30. Luo J, Horn K, Ockene JK, Simon MS, Stefanick ML, Tong E, et al. Interaction between smoking and obesity and the risk of developing breast cancer among postmenopausal women: The women's health initiative observational study. Am J Epidemiol (2011) 174(8):919–28. doi: 10.1093/aje/kwr192
31. Gunter MJ, Wang T, Cushman M, Xue X, Wassertheil-Smoller S, Strickler HD, et al. Circulating adipokines and inflammatory markers and postmenopausal breast cancer risk. J Natl Cancer Inst 107(9):djv169. doi: 10.1093/jnci/djv169
32. Wassertheil-Smoller S, McGinn AP, Martin L, Rodriguez BL, Stefanick ML, Perez M. The associations of atrial fibrillation with the risks of incident invasive breast and colorectal cancer. Am J Epidemiol (2017) 185(5):372–84. doi: 10.1093/aje/kww185
33. Hvidtfeldt UA, Gunter MJ, Lange T, Chlebowski RT, Lane D, Farhat GN, et al. Quantifying mediating effects of endogenous estrogen and insulin in the relation between obesity, alcohol consumption, and breast cancer. Cancer Epidemiol Biomarkers Prev (2012) 21(7):1203–12. doi: 10.1158/1055-9965.EPI-12-0310
34. Phipps AI, Chlebowski RT, Prentice R, McTiernan A, Stefanick ML, Wactawski-Wende J, et al. Body size, physical activity, and risk of triple-negative and estrogen receptor-positive breast cancer. Cancer Epidemiol Biomarkers Prev (2011) 20(3):454–63. doi: 10.1158/1055-9965.EPI-10-0974
35. Prentice RL, Aragaki AK, Van Horn L, Thomson CA, Beresford SA, Robinson J, et al. Low-fat dietary pattern and cardiovascular disease: results from the women's health initiative randomized controlled trial. Am J Clin Nutr (2017) 106(1):35–43. doi: 10.3945/ajcn.117.153270
36. Reding KW, Aragaki AK, Cheng RK, Barac A, Wassertheil-Smoller S, Chubak J, et al. Cardiovascular outcomes in relation to antihypertensive medication use in women with and without cancer: Results from the women's health initiative. Oncologist (2020) 25(8):712–21. doi: 10.1634/theoncologist.2019-0977
37. Foraker RE, Abdel-Rasoul M, Kuller LH, Jackson RD, Van Horn L, Seguin RA, et al. Cardiovascular health and incident cardiovascular disease and cancer: The women's health initiative. Am J Prev Med (2016) 50(2):236–40. doi: 10.1016/j.amepre.2015.07.039
38. Rohan TE, Heo M, Choi L, Datta M, Freudenheim JL, Kamensky V, et al. Body fat and breast cancer risk in postmenopausal women: A longitudinal study. J Cancer Epidemiol (2013) 2013:754815. doi: 10.1155/2013/754815
39. Neuhouser ML, Aragaki AK, Prentice RL, Manson JE, Chlebowski R, Carty CL, et al. Overweight, obesity, and postmenopausal invasive breast cancer risk: A secondary analysis of the women's health initiative randomized clinical trials. JAMA Oncol (2015) 1(5):611–21. doi: 10.1001/jamaoncol.2015.1546
40. Kabat GC, Xue X, Kamensky V, Lane D, Bea JW, Chen C, et al. Risk of breast, endometrial, colorectal, and renal cancers in postmenopausal women in association with a body shape index and other anthropometric measures. Cancer Causes Control (2015) 26(2):219–29. doi: 10.1007/s10552-014-0501-4. Erratum in: Cancer Causes Control. 2017.
41. Zheng C, Beresford SA, Van Horn L, Tinker LF, Thomson CA, Neuhouser ML, et al. Simultaneous association of total energy consumption and activity-related energy expenditure with risks of cardiovascular disease, cancer, and diabetes among postmenopausal women. Am J Epidemiol (2014) 180(5):526–35. doi: 10.1093/aje/kwu152
42. Park NJ, Chang Y, Bender C, Conley Y, Chlebowski RT, van Londen GJ, et al. Cardiovascular disease and mortality after breast cancer in postmenopausal women: Results from the women's health initiative. PloS One (2017) 12(9):e0184174. doi: 10.1371/journal.pone.0184174
43. Arnold M, Jiang L, Stefanick ML, Johnson KC, Lane DS, LeBlanc ES, et al. Duration of adulthood overweight, obesity, and cancer risk in the women's health initiative: A longitudinal study from the united states. PloS Med (2016) 13(8):e1002081. doi: 10.1371/journal.pmed.1002081
44. Crandall CJ, Hovey KM, Andrews CA, Chlebowski RT, Stefanick ML, Lane DS, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the women's health initiative observational study. Menopause (2018) 25(1):11–20. doi: 10.1097/GME.0000000000000956
45. Thomson CA, Crane TE, Garcia DO, Wertheim BC, Hingle M, Snetselaar L, et al. Association between dietary energy density and obesity-associated cancer: Results from the women's health initiative. J Acad Nutr Diet (2018) 118(4):617–26. doi: 10.1016/j.jand.2017.06.010
46. Chlebowski RT, Anderson GL, Manson JE, Prentice RL, Aragaki AK, Snetselaar L, et al. Low-fat dietary pattern and cancer mortality in the women's health initiative (WHI) randomized controlled trial. JNCI Cancer Spectr (2019) 2(4):pky065. doi: 10.1093/jncics/pky065
47. Chlebowski RT, Luo J, Anderson GL, Barrington W, Reding K, Simon MS, et al. Weight loss and breast cancer incidence in postmenopausal women. Cancer. (2019) 125(2):205–12. doi: 10.1002/cncr.31687
48. Luo J, Hendryx M, Manson JE, Figueiredo JC, LeBlanc ES, Barrington W, et al. Intentional weight loss and obesity-related cancer risk. JNCI Cancer Spectr (2019) 3(4):pkz054. doi: 10.1093/jncics/pkz054
49. Kabat GC, Kim MY, Lee JS, Ho GY, Going SB, Beebe-Dimmer J, et al. Metabolic obesity phenotypes and risk of breast cancer in postmenopausal women. Cancer Epidemiol Biomarkers Prev (2017) 26(12):1730–5. doi: 10.1158/1055-9965.EPI-17-0495
50. Arthur R, Wassertheil-Smoller S, Manson JE, Luo J, Snetselaar L, Hastert T, et al. The combined association of modifiable risk factors with breast cancer risk in the women's health initiative. Cancer Prev Res (Phila) (2018) 11(6):317–26. doi: 10.1158/1940-6207.CAPR-17-0347
51. Chlebowski RT, Aragaki AK, Anderson GL, Simon MS, Manson JE, Neuhouser ML, et al. Association of low-fat dietary pattern with breast cancer overall survival: A secondary analysis of the women's health initiative randomized clinical trial. JAMA Oncol (2018) 4(10):e181212. doi: 10.1001/jamaoncol.2018.1212. Erratum in: JAMA Oncol. 2019 Apr 1;5(4):580.
52. Iyengar NM, Arthur R, Manson JE, Chlebowski RT, Kroenke CH, Peterson L, et al. Association of body fat and risk of breast cancer in postmenopausal women with normal body mass index: A secondary analysis of a randomized clinical trial and observational study. JAMA Oncol (2019) 5(2):155–63. doi: 10.1001/jamaoncol.2018.5327
53. Sun Y, Liu B, Snetselaar LG, Wallace RB, Caan BJ, Rohan TE, et al. Association of normal-weight central obesity with all-cause and cause-specific mortality among postmenopausal women. JAMA Netw Open (2019) 2(7):e197337. doi: 10.1001/jamanetworkopen.2019.7337
54. Pan K, Aragaki AK, Neuhouser ML, Simon MS, Luo J, Caan B, et al. Low-fat dietary pattern and breast cancer mortality by metabolic syndrome components: a secondary analysis of the women's health initiative (WHI) randomised trial. Br J Cancer (2021) 125(3):372–9. doi: 10.1038/s41416-021-01379-w
55. George SM, Reedy J, Cespedes Feliciano EM, Aragaki A, Caan BJ, Kahle L, et al. Alignment of dietary patterns with the dietary guidelines for americans 2015-2020 and risk of all-cause and cause-specific mortality in the women's health initiative observational study. Am J Epidemiol (2021) 190(5):886–92. doi: 10.1093/aje/kwaa268
56. Pan K, Nelson RA, Wactawski-Wende J, Lee DJ, Manson JE, Aragaki AK, et al. Insulin resistance and cancer-specific and all-cause mortality in postmenopausal women: The women's health initiative. J Natl Cancer Inst (2020) 112(2):170–8. doi: 10.1093/jnci/djz069
57. Yuan Y, Pan K, Mortimer J, Chlebowski RT, Luo J, Yan JE, et al. Metabolic syndrome risk components and mortality after triple-negative breast cancer diagnosis in postmenopausal women in the women's health initiative. Cancer (2021) 127(10):1658–67. doi: 10.1002/cncr.33407
58. Leedy DJ, Reding KW, Vasbinder AL, Anderson GL, Barac A, Wactawski-Wende J, et al. The association between heart failure and incident cancer in women: an analysis of the women's health initiative. Eur J Heart Fail (2021) 23(10):1712–21. doi: 10.1002/ejhf.2207
59. Chen GC, Chen LH, Mossavar-Rahmani Y, Kamensky V, Shadyab AH, Haring B, et al. Dietary cholesterol and egg intake in relation to incident cardiovascular disease and all-cause and cause-specific mortality in postmenopausal women. Am J Clin Nutr (2021) 113(4):948–59. doi: 10.1093/ajcn/nqaa353
60. Haring B, Reiner AP, Liu J, Tobias DK, Whitsel E, Berger JS, et al. Healthy lifestyle and clonal hematopoiesis of indeterminate potential: Results from the women's health initiative. J Am Heart Assoc (2021) 10(5):e018789. doi: 10.1161/JAHA.120.018789
61. Reding KW, Cheng RK, Vasbinder A, Ray RM, Barac A, Eaton CB, et al. Lifestyle and cardiovascular risk factors associated with heart failure subtypes in postmenopausal breast cancer survivors. JACC CardioOncol (2022) 4(1):53–65. doi: 10.1016/j.jaccao.2022.01.099
62. Arthur RS, Mossavar-Rahmani Y, Prentice RL, Shadyab AH, Luo J, Sattari M, et al. The association of predicted resting energy expenditure with risk of breast cancer among postmenopausal women in the women's health initiative cohort. Cancer Prev Res (Phila) (2022) 15(4):255–64. doi: 10.1158/1940-6207.CAPR-21-0467
63. Dieli-Conwright CM, Nelson RA, Simon MS, Irwin ML, Neuhouser ML, Reding KW, et al. Cardiometabolic risk factors, physical activity, and postmenopausal breast cancer mortality: Results from the women's health initiative. BMC Womens Health (2022) 22(1):32. doi: 10.1186/s12905-022-01614-3
64. Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med (2014) 371:2488–98. doi: 10.1056/NEJMoa1408617
65. Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med (2017) 377:111–21. doi: 10.1056/NEJMoa1701719
66. Desai P, Mencia-Trinchant N, Savenkov O, Simon MS, Cheang G, Lee S, et al. Somatic mutations precede acute myeloid leukemia years before diagnosis. Nat Med (2018) 24(7):1015–23. doi: 10.1038/s41591-018-0081-z
67. Desai P, Handelman S, Wu A, Christos PJ, Lee S, Samuel MB, et al. Antecedent clonal hematopoiesis and risk of and mortality after solid and hematological malignancies: Analyses from the women’s health initiative study. Blood. (2019) 134(1):1199. doi: 10.1182/blood-2019-131862
68. Lund B, Hall D, Davis S, Shumaker S, Wang CY, Stein E, et al. Implementation of the women’s health initiative study design. Ann Epidemiol (2003) 13:S5–S17.
69. Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The women’s health initiative observational study: Baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol (2003) 13:S107–21. doi: 10.1016/S1047-2797(03)00047-4
70. Lawlor DA, Smith GD, Ebrahim S, Hyperinsulinaemia and increased risk of breast cancer: Findings from the British Women’s Heart and Health Study Cancer Causes Control 15:267-275, 2004,
71. Karr S. Epidemiology and management of hyperlipidemia. Am J Manag Care (2017) 23(9 Suppl):S139–48.
72. Desai P, Chlebowski R, Cauley JA, Manson JE, Wu C, Martin LW, et al. Prospective analysis of association between statin use and breast cancer risk in the women's health initiative. Cancer Epidemiol Biomarkers Prev (2013) 22(10):1868–76. doi: 10.1158/1055-9965.EPI-13-0562
73. Desai P, Lehman A, Chlebowski RT, Kwan ML, Arun M, Manson JE, et al. Statins and breast cancer stage and mortality in the women's health initiative. Cancer Causes Control (2015) 26(4):529–39. doi: 10.1007/s10552-015-0530-7
74. Kachur S, Lavie CJ, de Schutter A, Milani RV, Ventura HO. Obesity and cardiovascular diseases. Minerva Med (2017) 108(3):212–28. doi: 10.23736/S0026-4806.17.05022-4
75. Marcus JB. Weight management: Finding the healthy balance. In: Culinary nutrition (2013). p. 431–73.
76. Silteri PK. Adipose tissue as a source of hormones. Am J Clin Nutr (1987) 45:277–82. doi: 10.1093/ajcn/45.1.277
77. Kondo T, Nakano Y, Adachi S, Murohara T. Effects of tobacco smoking on cardiovascular disease. Circ J (2019) 83(10):1980–5. doi: 10.1253/circj.CJ-19-0323
78. Luo J, Margolis KL, Wactawski-Wende J, Horn K, Messina C, Stefanick ML, Tindle HA, et al. Association of active and passive smoking with risk of breast cancer among postmenopausal women: a prospective cohort study [electronic article]. BMJ (2011) 342:d1016. doi: 10.1136/bmj.d1016
79. Michnovicz JJ, Hershcopf RJ, Naganuma H, Bradlow L, Fishman J. Increased 2-hydroxylation of estradiol as a possible mechanism for the anti-estrogenic effect of cigarette smoking. N Engl J Med (1986) 315(21):1305–9. doi: 10.1056/NEJM198611203152101
80. Perks CM, Holly JM. Hormonal mechanisms underlying the relationship between obesity and breast cancer. Endocrinol Metab Clin North Am (2011) 40:485–507:vii. doi: 10.1016/j.ecl.2011.05.010
81. Roberts DL, Dive C, Renehan AG. Biological mechanisms linking obesity and cancer risk: new perspectives. Annu Rev Med (2010) 61:301–16. doi: 10.1146/annurev.med.080708.082713
82. Ridker PM, Hennekens CH, Buring JE, et al. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med (2000) 342:836–43. doi: 10.1056/NEJM200003233421202
83. Rouleau JL, Rutherford JD, Cole TG, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med (1996) 335:1001 – 9. doi: 10.1056/NEJM199610033351401
84. Okorodudu DO, Jumean MF, Montori VM, Romero-Corral A, Somers VK, Erwin PJ, et al. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: a systematic review and metaanalysis. Int J Obes (2010) 34:791–9. doi: 10.1038/ijo.2010.5
85. Cauley JA, Zmuda JM, Lui LY, Hillier TA, Ness RB, Stone KL, et al. Lipid lowering drug use and breast cancer in older women: A prospective study. J Womens Health (Larchmt) (2003) 12:749 – 56. doi: 10.1089/154099903322447710
86. Hu FB. Measurements of adiposity and body composition. In: Hu FB, editor. Obesity epidemiology. New York, NY, USA: Oxford University Press (2008). p. 53–83.
87. Koene RJ, Prizment AE, Blaes A, Konety SH. Shared risk factors in cardiovascular disease and cancer. Circulation. (2016) 133(11):1104–14. doi: 10.1161/CIRCULATIONAHA.115.020406
88. Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. cholesterol and recurrent events trial investigators. N Engl J Med (1996) 335(14):1001–9. doi: 10.1056/NEJM199610033351401
89. Malin A, Dai Q, Yu H, Shu XO, Jin F, Gao YT, et al. Evaluation of the synergistic effect of insulin resistance and insulin-like growth factors on the risk of breast carcinoma. Cancer (2004) 100:694–700. doi: 10.1002/cncr.20023
90. Chia CW, Egan JM, Ferrucci L. Age-related changes in glucose metabolism, hyperglycemia, and cardiovascular risk. Circ Res (2018) 123(7):886–904. doi: 10.1161/CIRCRESAHA.118.312806
91. Augustin LS, Dal Maso L, Franceschi S, Parpinel M, Negri E, Vaccarella S, et al. Dietary glycemic index and glycemic load and breast cancer risk: a case–control study. Ann Oncol (2001) 12:1533–8. doi: 10.1023/A:1013176129380
92. Alexander CM, Landsman PB, Teutsch SM, Haffner SM. NCEPdefined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes (2003) 52:1210 – 4. doi: 10.2337/diabetes.52.5.1210
93. Reding KW, Cheng RK, Barac A, Vasbinder A, Hovsepyan G, Stefanick M, et al. Toward a better understanding of the differential impact of heart failure phenotypes after breast cancer. J Clin Oncol (2022) 10:JCO2200111. doi: 10.1200/JCO.22.00111
94. McKenzie F, Ellison-Loschmann L, Jeffreys M, Firestone R, Pearce N, Romieu I. Healthy lifestyle and risk of breast cancer for indigenous and non-indigenous women in new Zealand: a case control study. BMC Cancer (2014) 14:12. doi: 10.1186/1471-2407-14-12
95. Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction. Circulation. (2010) 121(4):586–613. doi: 10.1161/CIRCULATIONAHA.109.192703
96. Women’s Health Initiative Study Group. Design of the women’s health initiative clinical trial and observational study. Control Clin Trials (1998) 19:61–109. doi: 10.1016/S0197-2456(97)00078-0
97. Ritenbaugh C, Patterson R, Chlebowski RT, Caan B, Fels-Tinker L, Howard B, et al. The women’s health initiative dietary modification trial: Overview and baseline characteristics of participants. Ann Epidemiol (2003) 13:S87–97. doi: 10.1016/S1047-2797(03)00044-9
98. Despres JP. Intra-abdominal obesity: An untreated risk factor for type 2 diabetes and cardiovascular disease. J Endocrinol Invest (2006) 29(3):77–82.
99. Harvie M, Hooper L, Howell AH. Central obesity and breast cancer risk: a systematic review. Obes Rev (2003) 4(3):157–73. doi: 10.1046/j.1467-789X.2003.00108.x
100. Aboumsallem JP, Moslehi J, de Boer RA. Reverse cardio-oncology: Cancer development in patients with cardiovascular disease. J Am Heart Assoc (2020) 9(2):e013754. doi: 10.1161/JAHA.119.013754
101. Kessler MD, Damask A, O’Keeffe S, et al. Exome sequencing of 628,388 individuals identifies common and rare variant associations with clonal hematopoiesis phenotypes. MedRxiv (2021) 12:29.21268342. doi: 10.1101/2021.12.29.21268342. [Preprint].
102. Yu B, Roberts MB, Raffield LM, Zekavat SM, Nguyen NQH, Biggs ML, et al. Supplemental association of clonal hematopoiesis with incident heart failure. J Am Coll Cardiol (2021) 78(1):42–52. doi: 10.1016/j.jacc.2021.04.085. Erratum in: J Am Coll Cardiol. 2021 Aug 17;78(7):762.
Keywords: breast cancer, cardiovascular disease, cancer treatment, risk factors, cancer survivors
Citation: Raychaudhuri S, Dieli-Conwright CM, Cheng RK, Barac A, Reding KW, Vasbinder A, Cook KL, Nair V, Desai P and Simon MS (2023) A review of research on the intersection between breast cancer and cardiovascular research in the Women’s Health Initiative (WHI). Front. Oncol. 12:1039246. doi: 10.3389/fonc.2022.1039246
Received: 07 September 2022; Accepted: 29 November 2022;
Published: 21 March 2023.
Edited by:
Dana Kristjansson, Norwegian Institute of Public Health (NIPH), NorwayReviewed by:
Zohre Momenimovahed, Qom University of Medical Sciences, IranVincenzo Quagliariello, G. Pascale National Cancer Institute Foundation (IRCCS), Italy
Copyright © 2023 Raychaudhuri, Dieli-Conwright, Cheng, Barac, Reding, Vasbinder, Cook, Nair, Desai and Simon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sreejata Raychaudhuri, c3JlZWphdGExQGdtYWlsLmNvbQ==
 Sreejata Raychaudhuri
Sreejata Raychaudhuri Christina M. Dieli-Conwright
Christina M. Dieli-Conwright Richard K. Cheng
Richard K. Cheng Ana Barac
Ana Barac Kerryn W. Reding5
Kerryn W. Reding5 Alexi Vasbinder
Alexi Vasbinder Katherine L. Cook
Katherine L. Cook Pinkal Desai
Pinkal Desai Michael S. Simon
Michael S. Simon