- 1Department of Oncological and Vascular Intervention, First Hospital of Shanxi Medical University, Taiyuan, China
- 2College of Medical Imaging, Shanxi Medical University, Taiyuan, China
Objective: Immunity and inflammation are key mediators of carcinoma development, invasion and metastasis. However, it remains unknown whether the systemic immune-inflammation index (SII) can be used as a prognostic indicator for cholangiocarcinoma. In this study, we investigated the association and predictive value of the SII with the prognosis of advanced perihilar cholangiocarcinoma (pCCA) after interventional therapy.
Methods: A retrospective cohort of patients with advanced pCCA treated with interventional therapy at the First Hospital of Shanxi Medical University enrolled in this study from January 2019 through January 2021 was examined. Cox regression models were used to analyze the relationship between the SII and overall survival (OS) of patients with advanced pCCA. Receiver operating characteristic (ROC) analysis was used to evaluate the predictive power of SII.
Results: Preoperative SII was positively associated with poor OS of pCCA after interventional therapy, with corresponding hazard ratios (HR) of 1.57 (95% CI: 1.17 - 2.10) for an inter-quartile range increase. The predictive power of SII was higher than that of other inflammation indexes based on ROC analysis (AUC = 0.835 [95% CI (0.731 - 0.940)]). The optimal cut-off values, sensitivity, and specificity with SII were 700, 0.774 and 0.846, respectively. An SII ≥ 700 was significantly associated with lymph node metastasis and high carbohydrate antigen199 (CA199) level. In multivariate analyses, total bilirubin, carbohydrate antigen 199, vascular invasion, and SII independently predicted overall survival (P < 0.05).
Conclusion: This is the first study demonstrating that an increase in the SII is associated with poor advanced pCCA prognosis, and could serve as a reliable prognostic indicator of pCCA after interventional therapy.
Introduction
Cholangiocarcinoma (CCA) is a rare digestive malignancy with limited therapeutic options and a poor prognosis (1). It is anatomically classified as intrahepatic, perihilar, and distal. Of these, perihilar cholangiocarcinoma (pCCA) accounts for 50-60% of all cholangiocarcinoma (2). Approximately 90% of pCCA patients present with biliary symptoms, including, most commonly, painless jaundice (3). Jaundice often reflects locally advanced or metastatic disease. Since early symptoms are not obvious, most patients have advanced-stage disease at presentation. Only 35% patients have early stage disease that is amenable to surgical resection with curative intent (4). For advanced pCCA, interventional therapy (percutaneous transhepatic cholangial drainage (PTCD) combined with hepatic artery infusion chemotherapy (HAIC)) can reduce jaundice and control the tumor size, which has become an important locoregional treatment and palliative care (5–7). It is important to identify patient subpopulations with poor prognosis for interventional therapy to optimize adjuvant treatments and provide new treatments to these subpopulations without delay.
The reasons for the poor prognosis in pCCA after interventional therapy are complex and multifactorial. It is largely recognized that poor prognosis in CCA are associated with immune and inflammation states (8, 9). Immune and inflammatory cells such as neutrophils, platelets, and lymphocytes also contribute to tumor cell invasion into the peripheral blood, where the tumor cells can survive and reseed distant organs. The systemic immune-inflammation index (SII), based on lymphocyte, neutrophil, and platelet counts, reflected comprehensively the balance of host inflammatory and immune status, which was developed to identify patients with poor prognosis (10, 11). Several studies have shown that SII is closely related to the prognosis of tumor and have some prognostic value in a variety of malignancies, including hepatocellular carcinoma (10), colon cancer (12), cervical cancer (13) and distal cholangiocarcinoma (14). However, for patients with advanced pCCA treated with interventional therapy, there is no data related to the relationship between prognosis and SII.
In this study, we investigated the relationship of between SII and the prognosis of advanced pCCA after interventional therapy. And the potential prognostic value of SII in patients with advanced pCCA who underwent interventional therapy was also evaluated in this study.
Patients and methods
Study population
This is a single-center retrospective study. The advanced pCCA refers to patients who cannot be resected with a negative margin (R0) and have no distant metastasis (locally advanced). In total, 76 patients with advanced pCCA who had undergone interventional therapy in the Department of Oncological and Vascular Intervention of the First Hospital of Shanxi Medical University from January 2019 to January 2021 were reviewed, of whom 10 were excluded due to uncompleted clinical information. Thus, the present retrospective study comprised 66 patients with advanced pCCA. Demographic and clinicopathological characteristics (age, gender, liver function, tumor markers, inflammation-based prognostic scores, tumor size, vascular invasion, and lymph node invasion) were collected from the medical records, and the study was approved by the Research Ethics Committee of the First Hospital of Shanxi Medical University. The inclusion criteria were that patients were ≥18 years of age, had received a histopathologic diagnosis of pCCA, were unable to a negative margin (R0) resection, and were treated with PTCD combined with HAIC. Exclusion criteria were: (1) patients with distant metastasis and recurrent pCCA after resection, (2) patients undergoing palliative resection, chemotherapy, radiotherapy and other anti-tumor treatments before this study, (3) patients with other inflammatory related diseases, blood diseases, immune diseases, and other malignant tumors, (4) patients with biliary stent and radioactive particle implantation, (5) using drugs that affect peripheral blood cell in recent 1 months, (6) patients with missing data, difficult follow-up and unknown cause of death.
Procedure and treatment
The PTCD and HAIC procedure was carried out according to the method described by Takada T et al. (15) and Wang X et al. (7). All patients underwent PTCD (routine placement of external drainage tube) and HAIC (local perfusion chemotherapy with 30 mg of lobaplatin and 1 g of gemcitabine pumped into the intrinsic hepatic artery; 1 cycle every 3-4 weeks, up to 6 cycles). After PTCD, sequential HAIC treatment was carried out according to the doctor’s judgment.
Definition of inflammation-based prognostic scores
The neutrophil-lymphocyte ratio (NLR) values were the ratio of neutrophil count and lymphocyte count. The platelet-lymphocyte ratio (PLR) values were the ratio of platelet count and lymphocyte count. The monocyte-lymphocyte ratio (MLR) values were the ratio of monocyte count and lymphocyte count. The SII was calculated as platelet * neutrophil/lymphocyte. The systemic inflammatory response index (SIRI) was calculated as (neutrophil * monocyte)/lymphocyte.
Statistical analysis
All analyses were conducted using SPSS (version 24.0) and SAS software (version 9.4). Before analysis, normality tests or P-P plots were used to check the normality of the variables. Continuous variables are expressed as mean ± standard deviation (SD) or median (inter-quartile range). Categorical variables are described as frequency (proportion). Cox proportional hazards models were used to analyze the relationship between the inflammation-based prognostic scores and overall survival (OS) of patients with advanced pCCA, and restricted cubic spline models were further used to examine the shapes of the dose-response association. Survival curves were plotted by Kaplan-Meier method and compared by Log-Rank test to compare between-group variability. Receiver operating characteristic (ROC) analysis was used to evaluate the predictive power of SII. In all cases, bilateral P values less than 0.05 were considered statistically significant.
Results
Characteristics of the participants in this study
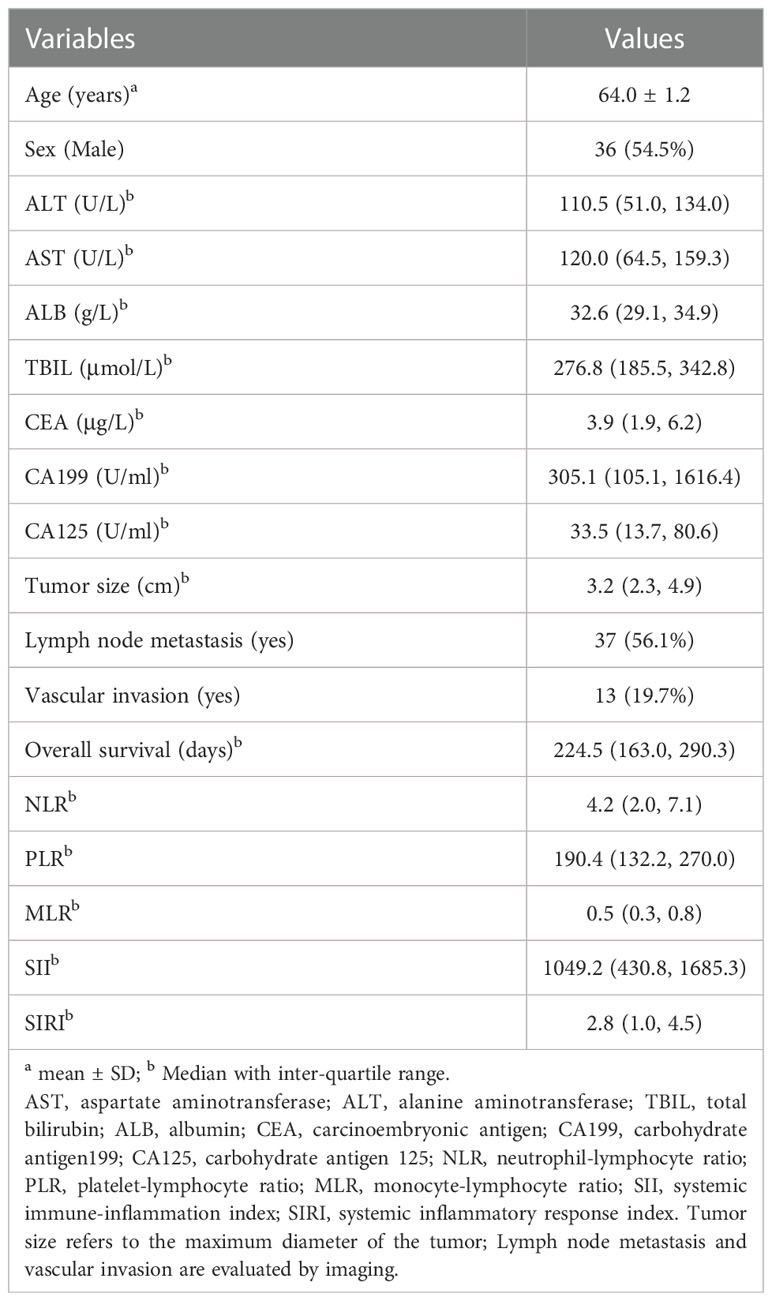
Of the 66 participants, 36 (54.5%) were male. The ages ranged from 35 to 84 years, with a mean of 64.0 years. The detailed baseline demographic and clinicopathological characteristics of the study population are listed in Table 1 and Supplementary Table S1.
The association of between inflammation-based prognostic scores and prognosis of pCCA after interventional therapy
According to the distribution of inflammation-based prognostic scores, we classified participants into 4 groups: Q1 (the 1st inter-quartile range (IQR)), Q2 (the 2nd IQR), Q3 (3rd IQR), and Q4 (the 4th IQR). The different models used to test the association between inflammation-based prognostic scores and HR of overall survival in advanced pCCA is presented in Table 2. We consistently observed that a monotonic and positive association of SII with HR of overall survival in different models, and p-value for trend test was less than 0.05. Further, the dose-response association of SII with increased HR of overall survival from advanced pCCA was confirmed in the restricted cubic spline models after adjusting for potential confounders (Figure 1). However, there were no significant trends among the HR of overall survival in participants stratified by NLR, PLR, MLR, and SIRI. These data suggested that preoperative SII was associated with the prognosis of advanced pCCA after interventional therapy and may be used as an early predictor to predict the prognosis and survival of advanced pCCA.
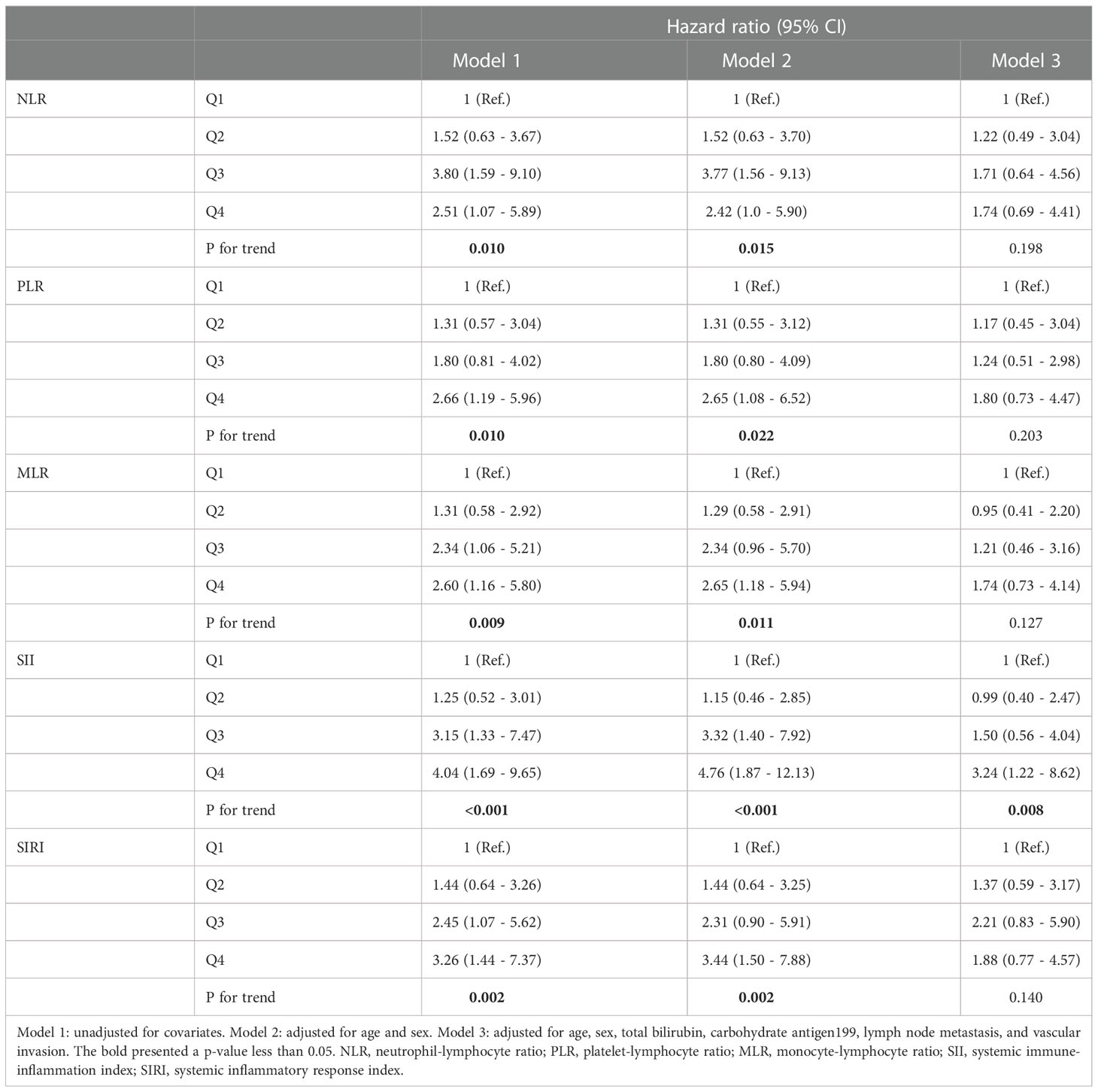
Table 2 Associations between inflammation-based prognostic scores and the prognosis of pCCA after interventional therapy by cox regression in different models.
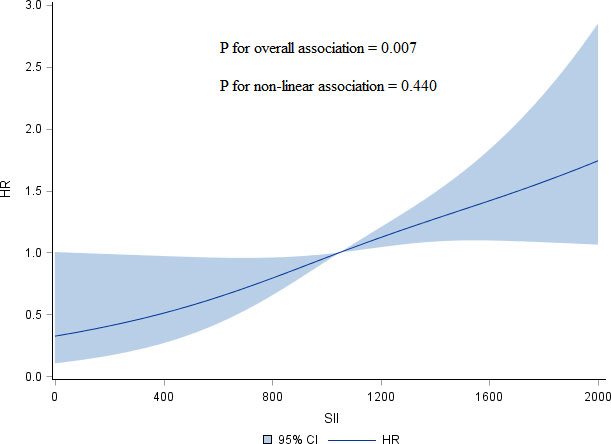
Figure 1 Restricted cubic spline models of association of SII with HR of overall survival for advanced pCCA after interventional therapy adjusted for age, sex, TBIL, CA199, lymph node metastasis, and vascular invasion. SII, systemic immune-inflammation index; HR, hazard ratio; pCCA, perihilar cholangiocarcinoma; TBIL, total bilirubin; CA199, carbohydrate antigen199.
Ability of SII to predict prognosis of advanced pCCA after interventional therapy
After confirming the relationship between SII and pCCA prognosis after interventional therapy, we further evaluated its ability to predict pCCA prognosis. We plotted the ROC curves and found the AUC value of SII (AUC = 0.835 [95% CI (0.731 - 0.940)]) was greater than the NLR (AUC = 0.816 [95% CI (0.712 - 0.921)]), PLR (AUC = 0.828 [95% CI (0.715 - 0.941)]), MLR (AUC = 0.673 [95% CI (0.499 - 0.848)]), and SIRI (AUC = 0.735 [95% CI (0.578 - 0.893)]) (Figure 2). The optimal cut-off values (SII = 700) for the SII in terms of prognosis of advanced pCCA was determined, with sensitivity values of 0.774 and specificity values of 0.846. The Kaplan-Meier analysis indicated that the high SII (SII ≥ 700) was associated with shorter overall survival (P for log-rank = 0.012) (Figure 3). The median of overall survival was significantly higher in patients in the SII < 700 group than those in the SII ≥700 group (324 days vs. 225 days).

Figure 2 ROC curves of the probability of inflammation-based prognostic scores in predicting prognosis of advanced pCCA after interventional therapy. ROC, receiver operating characteristic; pCCA, perihilar cholangiocarcinoma; NLR, neutrophil-lymphocyte ratio; SII, systemic immune-inflammation index; PLR, platelet-lymphocyte ratio, MLR, monocyte-lymphocyte ratio; SIRI, systemic inflammatory response index.
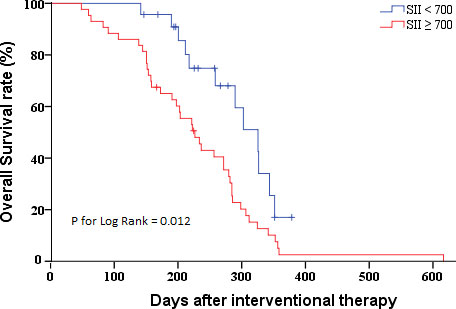
Figure 3 Kaplan-Meier survival curves of patients with pCCA after interventional therapy by SII distribution. pCCA, perihilar cholangiocarcinoma; SII, systemic immune-inflammation index.
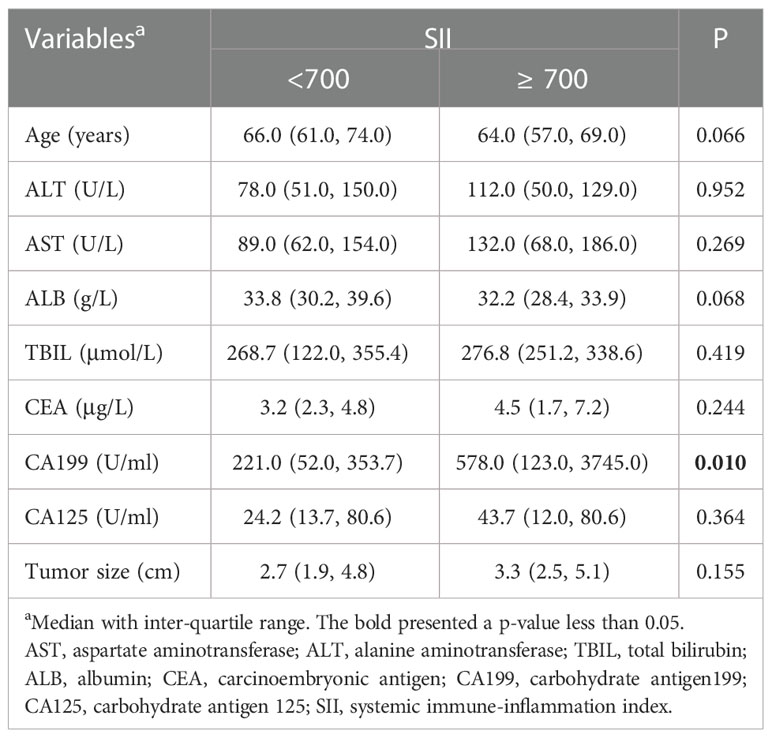
The correlation between the SII and clinicopathological characteristics
To further clarify the reasons for the association of SII with the prognosis of advanced pCCA, we investigate the association of SII with the clinicopathological characteristics of advanced pCCA. We found that SII was associated with lymph node metastasis and the carbohydrate antigen199 (CA199) level. Compared with SII < 700 group, participants in the SII ≥ 700 group had higher CA199 level (P = 0.010) (Table 3). Scatter plot analyses also revealed a significant positive correlation between the SII and CA199 level (r = 0.306; P = 0.001, Supplementary Figure S1). And the SII ≥ 700 group had more participants with lymph node metastasis than those in SII < 700 group (67.44% vs. 34.78%) (Figure 4).
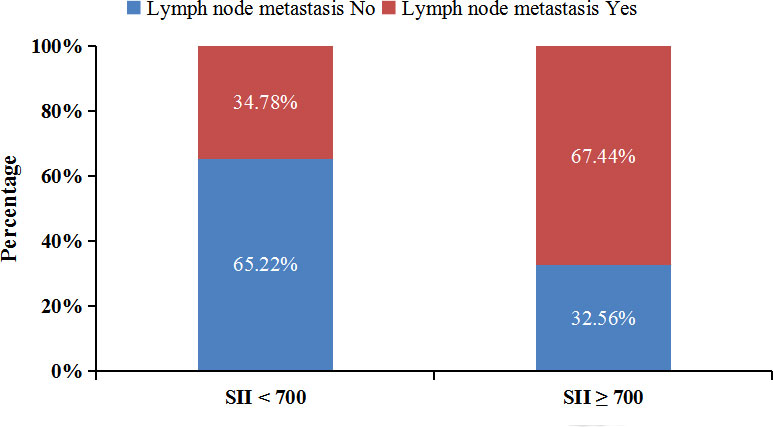
Figure 4 Lymph node metastasis rate of patients in different SII groups. SII, systemic immune-inflammation index.
Influencing factors of prognosis after interventional therapy for advanced pCCA
On the basis of the multiple cox regression analysis by forward method of factors, total bilirubin (TBIL), CA199, vascular invasion and SII were included in the final multivariate cox regression model. A higher TBIL, CA199, and SII and vascular invasion were found to be independent predictors of advanced pCCA after the interventional therapy. Under the premise that other variables remain unchanged, each an IQR increasing in SII, the hazard ratio of overall survival for advanced pCCA increased by 1.57 times (HR = 1.57, P < 0.05) (Table 4).

Table 4 Cox regression analyses the risk factors of prognosis for advanced pCCA after interventional therapy.
Discussion
To the best of our knowledge, this is the first report to demonstrate the prognostic value of the SII (a well-known immune inflammation index) for patients with advanced pCCA after interventional therapy. Advanced pCCA patients with a high SII before interventional therapy had a poor overall survival. Compared with inflammation-based prognostic scores (NLR, PLR, MLR, and SIRI), the SII was a powerful prognostic indicator of poor overall survival in patients with advanced pCCA after interventional therapy and was a promising tool for advanced pCCA treatment strategy decisions.
The SII was first proposed in 2014 by Hu et al. (10) as a prognostic indicator among patients with hepatocellular carcinoma. Subsequently, the role of SII in the prognosis of gastrointestinal malignancies has been repeatedly demonstrated, including intrahepatic and extrahepatic cholangiocarcinoma (16–18). But, pCCA patients are relatively scarce and mostly only included patients undergoing surgical resection. There were no studies on the association of SII and survival outcomes in patients with advanced pCCA after interventional therapy. Our conclusions were consistent with previous studies that high SII was independent predictors of poor prognosis for patients with CCA (17). But, compared with previous studies, the cut-off values of SII was different in predicting the prognosis of CCA. We believe the mainly reason was characteristics of the study population, in particular the type and severity of the disease. The cut-off values of SII reported by Hui Li et al. (19) and Jian Li et al. (20) were 450 and 412.6, respectively, which were lower than our report (SII= 700). We speculated that the cut-off value for SII was related to the stage of the disease, and the baseline level of SII was higher in our study.
Besides, the predictive power of SII was also superior to that of NLR, PLR, MLR, and SIRI. To clarify why SII can be used as a predictor, we further investigated the association of SII with demographic and clinicopathological characteristics. Notably, SII was associated with tumor-related features, such as CA199 level and lymph node metastasis. We speculate that this is related to an increase in platelets, a component of SII. Previous studies reported elevated platelet counts are significantly associated with a higher rate of lymph node metastasis, even if the platelet counts are within the reference range (21, 22). This is related to the fact that platelets can promote tumor cell migration and invasion into the surrounding microenvironment, as well as promote tumor endothelial cell arrest, extravasation and seeding. Platelets are known to contain important angiogenic factor, such as vascular endothelial growth factor (VEGF). The VEGF facilitate tumor metastasis via modulating the lymphatic vessels (23), and also can induce sentinel node lymphatic angiogenesis and promote lymphatic metastasis even before the tumor has metastasized (24, 25). This suggests that SII can be used as a prognostic predictor in advanced pCCA because of its association with inflammation and clinicopathological characteristics.
The mechanisms by which SII predicts survival outcomes in cancer patients are not clear. The predictive value of the SII for tumor prognosis might be elucidates by the function of the lymphocyte, neutrophil, and platelets cells, and their close relationship with tumor biomarkers (10). It is well known that inflammation plays a key role in tumorigenesis and progression, and the tumor microenvironment composed of inflammatory cells is increasingly recognized as an essential player in the tumor process. Studies shown neutrophils can promote ICC progression by activating STAT3 (26). Neutrophils can also release cytotoxic substances that cause DNA damage in epithelial cells and activate oncogenes (27), and it can suppress adaptive immune responses and reduce the level of activated lymphocytes (28). Platelets and their receptors can mediate cancer cell growth and angiogenesis. The interaction of tumor cells with platelets is a prerequisite for successful hematogenous metastatic dissemination (29). The lymphocyte is a key mediator of antitumor immunity (30). The B lymphocytes are necessary for establishing chronic inflammation, and it may regulate cancer progression by altering the levels of circulating cytokines and chemokines. The T lymphocytes not only destroy tumor cells directly, but also induce apoptosis of target cells by binding to Fas-FasL on the surface of tumor cells (31). High SII may represent an increased platelets-mediated tumor metastasis, an increased neutrophil-mediated inflammatory response, a decreased in lymphocyte-mediated antitumor immune response, and SII is a practical and comprehensive biomarker reflecting systemic inflammatory response, immune response, and tumor metastasis in tumor patients. The specific role and pathway of inflammatory response in tumor are still inconclusive, which needs further research and discussion. In addition to SII reflecting immune-inflammatory levels in patients, we also found that SII was positively associated several clinicopathological characteristics, including lymph node metastasis and high CA199 levels. CA199 is a tumor marker used in the management of gastrointestinal cancer, and elevated CA199 levels also occur in diseases of the bile ducts, especially, obstruction of the bile ducts (32). This also reflects why the predictive power of SII is better than that of NLR, because NLR only reflects the immune-inflammatory situation and does not take into account platelet-mediated tumor metastasis.
There are several limitations in this study. First, there was a rather small size sample study. But, this study gives some clues, suggesting that the prognosis predictors of interventional therapy for advanced pCCA. We should paid attention to the value of these convenient and accessible indictors in predictive pCCA prognosis. Second, this study was a single-center retrospective study. A prospective multi-center study may be required to validate our results. Third, we can not evaluate differences in therapeutic response such as RECIST between SII groups, due to missing imaging data. Finally, we did not perform a validation study on a separate patient cohort to verify the results of this study to confirm the value of the SII in pCCA prognosis. But, we conducted sensitivity analysis to validate the relationship between SII and pCCA prognosis. We obtained a consistent result in different models that pCCA patients with high SII level had poor prognosis after interventional therapy.
Conclusion
We first found high SII was an independent predictor of a short overall survival in patients with advanced pCCA after interventional therapy. The predictive power of SII was superior to that of NLR, PLR, MLR, and SIRI. SII can be used as a prognostic predictor in advanced pCCA due to its association with inflammation and clinicopathological characteristics.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Research Ethics Committee of the First Hospital of Shanxi Medical University. The ethics committee waived the requirement of written informed consent for participation.
Author contributions
JL designed the study, helped to draft the manuscript, and performed the statistical analysis. LG conducted data collection, management and revision guidance. TL performed the data collection and management. DF performed research design and analytical guidance. All authors contributed to the article and approved the submitted version.
Funding
This study was financially supported by the Shanxi provincial key research and development project (Grant No. 201903D321188; 202102130501014) and Natural Science Foundation of Shanxi Province (Grant No. 20210302123258).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1038759/full#supplementary-material
Supplementary Figure 1 | Scatter plots between the SII and CA199 level. SII, systemic immune-inflammation index; CA199, carbohydrate antigen199.
Abbreviations
SII, systemic immune-inflammation index; pCCA, perihilar cholangiocarcinoma; OS, overall survival; ROC, Receiver operating characteristic; HR, hazard ratios; CA199, carbohydrate antigen199; CCA, Cholangiocarcinoma; PTCD, percutaneous transhepatic cholangial drainage; HAIC, hepatic artery infusion chemotherapy; NLR, neutrophil-lymphocyte ratio; PLR, platelet-lymphocyte ratio; MLR, monocyte-lymphocyte ratio; SIRI, systemic inflammatory response index; SD, standard deviation; IQR, inter-quartile range; TBIL, total bilirubin.
References
1. Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet (London England) (2014) 21;383(9935):2168–79. doi: 10.1016/s0140-6736(13)61903-0
2. Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat Rev Clin Oncol (2018) 15(2):95–111. doi: 10.1038/nrclinonc.2017.157
3. Mansour JC, Aloia TA, Crane CH, Heimbach JK, Nagino M, Vauthey JN. Hilar cholangiocarcinoma: expert consensus statement. HPB Off J Int Hepato Pancreato Biliary Assoc (2015) 17(8):691–9. doi: 10.1111/hpb.12450
4. Jarnagin WR, Fong Y, DeMatteo RP, Gonen M, Burke EC, Bodniewicz BJ, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg (2001) 234(4):507–17; discussion 517-9. doi: 10.1097/00000658-200110000-00010
5. Qi S, Yan H. Effect of percutaneous transhepatic cholangial drainag + radiofrequency ablation combined with biliary stent implantation on the liver function of patients with cholangiocarcinoma complicated with malignant obstructive jaundice. Am J Trans Res (2021) 13(3):1817–24.
6. Cercek A, Boerner T, Tan BR, Chou JF, Gönen M, Boucher TM, et al. Assessment of hepatic arterial infusion of floxuridine in combination with systemic gemcitabine and oxaliplatin in patients with unresectable intrahepatic cholangiocarcinoma: A phase 2 clinical trial. JAMA Oncol (2020) 6(1):60–7. doi: 10.1001/jamaoncol.2019.3718
7. Wang X, Hu J, Cao G, Zhu X, Cui Y, Ji X, et al. Phase II study of hepatic arterial infusion chemotherapy with oxaliplatin and 5-fluorouracil for advanced perihilar cholangiocarcinoma. Radiology (2017) 283(2):580–9. doi: 10.1148/radiol.2016160572
8. Roy S, Glaser S, Chakraborty S. Inflammation and progression of cholangiocarcinoma: Role of angiogenic and lymphangiogenic mechanisms. Front Med (2019) 6:293. doi: 10.3389/fmed.2019.00293
9. Tamma R, Annese T, Ruggieri S, Brunetti O, Longo V, Cascardi E, et al. Inflammatory cells infiltrate and angiogenesis in locally advanced and metastatic cholangiocarcinoma. Eur J Clin Invest (2019) 49(5):e13087. doi: 10.1111/eci.13087
10. Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res (2014) 20(23):6212–22. doi: 10.1158/1078-0432.Ccr-14-0442
11. Dey S, Kashav R, Kohli JK, Magoon R, ItiShri, Walian A, et al. Systemic immune-inflammation index predicts poor outcome after elective off-pump CABG: A retrospective, single-center study. J cardiothoracic Vasc anesthesia. (2021) 35(8):2397–404. doi: 10.1053/j.jvca.2020.09.092
12. Chen JH, Zhai ET, Yuan YJ, Wu KM, Xu JB, Peng JJ, et al. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J gastroenterology. (2017) 23(34):6261–72. doi: 10.3748/wjg.v23.i34.6261
13. Huang H, Liu Q, Zhu L, Zhang Y, Lu X, Wu Y, et al. Prognostic value of preoperative systemic immune-inflammation index in patients with cervical cancer. Sci Rep (2019) 9(1):3284. doi: 10.1038/s41598-019-39150-0
14. Terasaki F, Sugiura T, Okamura Y, Ito T, Yamamoto Y, Ashida R, et al. Systemic immune-inflammation index as a prognostic marker for distal cholangiocarcinoma. Surg Today (2021) 51(10):1602–9. doi: 10.1007/s00595-021-02312-7
15. Takada T, Yasuda H, Hanyu F. Technique and management of percutaneous transhepatic cholangial drainage for treating an obstructive jaundice. Hepato-gastroenterology (1995) 42(4):317–22.
16. Hakeem AR, Marangoni G, Chapman SJ, Young RS, Nair A, Hidalgo EL, et al. Does the extent of lymphadenectomy, number of lymph nodes, positive lymph node ratio and neutrophil-lymphocyte ratio impact surgical outcome of perihilar cholangiocarcinoma? Eur J Gastroenterol Hepatol (2014) 26(9):1047–54. doi: 10.1097/meg.0000000000000162
17. Liu XC, Jiang YP, Sun XG, Zhao JJ, Zhang LY, Jing X. Prognostic significance of the systemic immune-inflammation index in patients with cholangiocarcinoma: A meta-analysis. Front Oncol (2022) 12:938549. doi: 10.3389/fonc.2022.938549
18. Tsilimigras DI, Moris D, Mehta R, Paredes AZ, Sahara K, Guglielmi A, et al. The systemic immune-inflammation index predicts prognosis in intrahepatic cholangiocarcinoma: an international multi-institutional analysis. HPB Off J Int Hepato Pancreato Biliary Assoc (2020) 22(12):1667–74. doi: 10.1016/j.hpb.2020.03.011
19. Li H, Wang JJ, Zhang M, Ren B, Li JX, Xu L, et al. Prognostic significance of systemic immune-inflammation index in patients with intrahepatic cholangiocarcinoma undergoing hepatic resection. World J gastrointestinal Oncol (2020) 12(4):467–82. doi: 10.4251/wjgo.v12.i4.467
20. Li J, Meng S, Bo K, Zhu R, Wang W, Liang R, et al. Relationship between systemic immune inflammation index and postoperative prognosis of patients with hilar cholangiocarcinoma. Chin J Hepatobiliary Surg (2021) 27(2):106–9. doi: 10.3760/cma.j.cn113884-20200423-00226
21. Qu CH, Li T, Tang ZP, Zhu XR, Han JY, Tian H. Platelet count is associated with the rate of lymph node metastasis in lung adenocarcinoma. Cancer Manage Res (2020) 12:9765–74. doi: 10.2147/cmar.S273328
22. Catal O, Ozer B, Sit M. Prediction of lymph node metastasis in colon cancer via platelet to lymphocyte ratio and platelet count. J Coll Physicians Surgeons–Pakistan JCPSP. (2020) 30(3):250–3. doi: 10.29271/jcpsp.2020.03.250
23. Karpanen T, Egeblad M, Karkkainen MJ, Kubo H, Ylä-Herttuala S, Jäättelä M, et al. Vascular endothelial growth factor c promotes tumor lymphangiogenesis and intralymphatic tumor growth. Cancer Res (2001) 61(5):1786–90.
24. Hirakawa S, Brown LF, Kodama S, Paavonen K, Alitalo K, Detmar M. VEGF-c-induced lymphangiogenesis in sentinel lymph nodes promotes tumor metastasis to distant sites. Blood (2007) 109(3):1010–7. doi: 10.1182/blood-2006-05-021758
25. Harrell MI, Iritani BM, Ruddell A. Tumor-induced sentinel lymph node lymphangiogenesis and increased lymph flow precede melanoma metastasis. Am J pathology. (2007) 170(2):774–86. doi: 10.2353/ajpath.2007.060761
26. Zhou Z, Wang P, Sun R, Li J, Hu Z, Xin H, et al. Tumor-associated neutrophils and macrophages interaction contributes to intrahepatic cholangiocarcinoma progression by activating STAT3. J immunotherapy Cancer (2021) 9(3):e001946. doi: 10.1136/jitc-2020-001946
27. Zhang X, Zhang W, Yuan X, Fu M, Qian H, Xu W. Neutrophils in cancer development and progression: Roles, mechanisms, and implications (Review). Int J Oncol (2016) 49(3):857–67. doi: 10.3892/ijo.2016.3616
28. Buettner S, Spolverato G, Kimbrough CW, Alexandrescu S, Marques HP, Lamelas J, et al. The impact of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio among patients with intrahepatic cholangiocarcinoma. Surgery (2018) 164(3):411–8. doi: 10.1016/j.surg.2018.05.002
29. Schlesinger M. Role of platelets and platelet receptors in cancer metastasis. J Hematol Oncol (2018) 11(1):125. doi: 10.1186/s13045-018-0669-2
30. Reading JL, Gálvez-Cancino F, Swanton C, Lladser A, Peggs KS, Quezada SA. The function and dysfunction of memory CD8(+) T cells in tumor immunity. Immunol Rev (2018) 283(1):194–212. doi: 10.1111/imr.12657
31. Tan DW, Fu Y, Su Q, Guan MJ, Kong P, Wang SQ, et al. Prognostic significance of neutrophil to lymphocyte ratio in oncologic outcomes of cholangiocarcinoma: A meta-analysis. Sci Rep (2016) 6:33789. doi: 10.1038/srep33789
Keywords: advanced perihilar cholangiocarcinoma, systemic immune-inflammation index, overall survival, prognosis, interventional therapy
Citation: Li J, Gao L, Liu T and Feng D (2022) Association of systemic inflammation index with survival in patients with advanced perihilar cholangiocarcinoma treated with interventional therapy. Front. Oncol. 12:1038759. doi: 10.3389/fonc.2022.1038759
Received: 07 September 2022; Accepted: 07 December 2022;
Published: 22 December 2022.
Edited by:
John Gibbs, Hackensack Meridian Health, United StatesReviewed by:
Eva Valentina Klocker, University Hospital Graz, AustriaTakahiro Ito, Mie University Hospital, Japan
Copyright © 2022 Li, Gao, Liu and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Duiping Feng, ZmVuZ2RwQHN4bXUuZWR1LmNu
†These authors have contributed equally to this work
 Jinyu Li
Jinyu Li Long Gao1†
Long Gao1†