
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 09 January 2023
Sec. Thoracic Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.1036906
This article is part of the Research Topic Real-World Data and Real-World Evidence in Lung Cancer View all 23 articles
 Zhiting Zhao1†
Zhiting Zhao1† Luqing Zhao1†
Luqing Zhao1† Guohao Xia1
Guohao Xia1 Jianwei Lu1,2
Jianwei Lu1,2 Bo Shen1
Bo Shen1 Guoren Zhou1
Guoren Zhou1 Fenglei Wu3
Fenglei Wu3 Xiao Hu2
Xiao Hu2 Jifeng Feng1*
Jifeng Feng1* Shaorong Yu1,2*
Shaorong Yu1,2*Background: Bevacizumab has played an important role in the systemic treatment of patients with advanced non-small-cell lung cancer (NSCLC) without gene mutation. In recent years, bevacizumab biosimilar has received marketing approval based on the results of phase III clinical studies. However, more clinical data are needed to verify the efficacy and safety of bevacizumab biosimilar in clinical application.
Materials and methods: We identified 946 patients with locally advanced or metastatic NSCLC who were treated with bevacizumab biosimilar or bevacizumab from January 1, 2019 to November 30, 2021. Comparisons and statistical analyses of bevacizumab biosimilar and bevacizumab were made in terms of efficacy and safety. Efficacy evaluation was performed directly in accordance with RECIST v1.1. Adverse events were graded following the National Cancer Institute Common Terminology Criteria for Adverse Events v5.0.
Results: The objective response rates (ORRs) were 28.9% in the biosimilar group (n=551) and 30.9% in the reference group (n=395; unstratified ORR risk ratio: 0.934, 95% confidence interval [CI]: 0.677–1.138; unstratified ORR risk difference: −0.020, 95% CI: −0.118–0.035). The estimated median progression-free survival (mPFS) were 6.27 (95% CI: 5.53–7.01) and 4.93 (95% CI: 4.24–5.62) months in the biosimilar and reference groups, respectively (P=0.296). The number of treatment lines, combined treatment regimens and with or without radiotherapy were significant factors affecting the PFS of both groups (P<0.001, P=0.001, P=0.039). Different genetic mutations and dose intensity were not the main factors affecting PFS (P=0.627, P=0.946). The incidences of treatment-emergent adverse events (TEAEs) were 76.41% in the biosimilar group and 71.65% in the reference group (P=0.098). The incidences of grade 3 or higher TEAEs were 22.14% and 19.49% in the biosimilar and reference groups, respectively (P=0.324).
Conclusions: Bevacizumab biosimilar is equivalent in efficacy to bevacizumab in patients with locally advanced and advanced NSCLC. It showed acceptable toxicity profile and no new adverse events. Patients who were excluded by clinical trials can also benefit from bevacizumab biosimilar.
Lung cancer is the leading cause of cancer-related deaths worldwide with an estimated 1.8 million deaths each year (1). Approximately 57% of patients have distant metastasis at the time of initial diagnosis and lose surgical indications (2). Therefore, systemic therapy plays an important role in lung cancer treatment. Vascular endothelial growth factor (VEGF) acts as a key regulator of tumor angiogenesis and is associated with increased risks of recurrence, metastasis, and death (3, 4). Bevacizumab is a recombinant humanized monoclonal antibody that specifically interrupts the interaction between human VEGF and endothelial cell surface receptors, inhibiting the biological activity of VEGF and limiting angiogenesis (5). Randomized controlled trials have demonstrated that bevacizumab in combination with chemotherapy improves overall survival (OS) and progression-free survival (PFS) relative to chemotherapy alone (6, 7). In addition, recent evidence points to novel combinations of bevacizumab with another targeted therapy or immunotherapy, as well as maintenance therapy after disease progression (8).
A biosimilar is a biological product that is highly similar to the reference product with no clinically remarkable differences in safety, purity, or potency (9). A biosimilar is an important avenue to reduce patient expenditure and the financial burden of national healthcare systems while maintaining therapeutic effect (10). However, a biosimilar is not an exact copy of its reference product and thus requires vigilance and concern in clinical application (11, 12).
Currently, prospective clinical trials have confirmed that bevacizumab biosimilar in combination with platinum-containing two-drug chemotherapy has similar efficacy and safety compared with bevacizumab in patients with untreated advanced non-squamous non-small-cell lung cancer (NSCLC) (13, 14). In 2019, China National Medical Products Administration approved the marketing of the bevacizumab biosimilar developed by Qilu Pharmaceutical Co., Ltd. (trade name: Encoda) with all indications approved for bevacizumab in China. However, no clinical data has verified the efficacy and safety of this biosimilar in clinical application. Existing trials (15–17) have excluded patients receiving previous treatment, patients receiving combination with targeted therapy or immunotherapy, patients with brain metastases, patients with rare genetic mutations (such as EML4-ALK rearrangement), and patients with Eastern Cooperative Oncology Group (ECOG) scores greater than 2. The efficacy and safety of bevacizumab biosimilar have no concensus in these populations. The purpose of the current investigation was to retrospectively analyze and compare the efficacy and safety of bevacizumab biosimilar and reference bevacizumab in patients with locally advanced and advanced NSCLC in clinical application and to provide reference for clinical decision-making.
We studied the medical records of all patients with locally advanced and advanced non-squamous NSCLC treated at Jiangsu Cancer Hospital from January 1, 2019 to November 30, 2021. We screened patients who received bevacizumab biosimilar or bevacizumab treatment. All patients included in the study had at least one measurable disease. This study was approved by the Academic Ethics Committee of Jiangsu Cancer Hospital (reference No.036 (2022)). All patients gave informed consent and signed the consent form.
Medical records were examined and separated by clinical pathologic features and treatment histories. Radiographic examinations were performed to assess the efficacy at two cycles after initiation, and then the state of the tumor was assessed every two cycles or when symptoms of suspected disease progression occurred. Data and follow-up records were updated as of December 1, 2021. Efficacy evaluation was performed directly in accordance with the Response Evaluation Criteria in Solid Tumor 1.1. The best response to bevacizumab biosimilar or bevacizumab treatment was defined as a complete response (CR) or partial response (PR) and stable disease achieved at least once during therapy. The primary efficacy endpoint was the objective response rate (ORR) defined as the CR or PR rate. The secondary efficacy endpoint was the progression-free survival (PFS) defined as the time from treatment initiation to clinical or radiographic progression or death. Adverse events (AEs) were graded by the National Cancer Institute Common Terminology Criteria for Adverse Events v5.0.
Differences in baseline clinical features, efficacy, and safety between groups were measured by χ2 test or independent T test. Survival data were estimated using the Kaplan–Meier method, including 95% confidence intervals (CIs). Significant differences between these curves were determined using log-rank test. Multivariate analysis of PFS was conducted by Cox proportional risk analysis. All statistical analyses were conducted using the SPSS (version 25.0) and R software (version 3.6.3). Statistical significance was set at P<0.05.
A total of 946 patients were included in this study, including 551 patients who received bevacizumab biosimilar (biosimilar group) and 395 patients who received bevacizumab (reference group). The baseline characteristics of the patients are summarized in Table 1. The subjects’ demographics and baseline disease characteristics were well balanced between the treatment groups with no statistical differences. Overall, the median age of the 946 patients was 60.5 years, including 533 males (56.34%) and 413 females (43.66%). Adenocarcinoma was the most common pathological type (98.52%). The remaining 14 nonadenocarcinomas included 7 adenosquamous carcinoma, 3 sarcomatoid carcinoma, 2 large cell carcinoma, and 2 undifferentiated carcinoma. The proportion of patients without gene mutation was the highest (44.83%), followed by patients with EGFR L858R mutation (22.69%) and patients with EGFR exon 19 deletion (17.12%). Before initial treatment with bevacizumab or bevacizumab biosimilar, 209 patients (21.88%) had brain metastases, 136 patients (14.38%) had liver metastases, and 136 patients (14.38%) had clinically diagnosed hypertension.
Exposure to treatment agents was comparable between the groups. The mean durations of exposure were 6.98 (standard deviation [SD], 6.03) and 8.31 (9.74) months (P=0.121) and the mean number of doses were 7.5 (5.45) and 7.8 (6.02, P=0.364) in the biosimilar and reference groups, respectively. The mean dose intensities in the biosimilar and reference groups were 8.37 and 7.64 mg/kg per cycle, respectively (P=0.823).
No patient achieved CR. In the biosimilar group (n=551), 159 patients experienced PR and 334 patients experienced SD with an ORR of 28.9% (95% CI: 25.1%–32.7%) and a disease control rate (DCR) of 89.5% as shown in Figure 1A. In the reference group (n=395), 122 patients developed PR and 223 patients developed SD with an ORR of 30.9% (95% CI: 26.3%–35.5%) and a DCR of 87.3%. As is shown in Table 2, the unstratified ORR risk ratio was 0.934 with a 95% CI of 0.767–1.138 and a 90% CI of 0.792–1.103. The unstratified ORR risk difference was −0.020 with a 95% CI of −0.118–0.035 and a 90% CI of −0.105–0.023. The results fell within the range prescribed by the Food and Drug Administration (FDA), Japan’s Pharmaceuticals and Medical Devices Agency (PMDA), and the European Medicines Agency (EMA). This result indicates that the bevacizumab biosimilar showed similar efficacy to bevacizumab. As shown in Table 3, the subgroup analyses were performed based on the following subgroups: sex, age, pathology, stage, number of treatment lines, radiotherapy, combined treatment regimens, combined chemotherapy regimens (if combined with chemotherapy), dose intensity, genetic mutations, brain metastasis, liver metastasis, and history of hypertension. In a subgroup analysis of combined treatment regimens, the ORR of the biosimilar group was lower than that of the reference group when combined with chemotherapy and immunotherapy (22.58% vs. 33.64%, P=0.048, Figure 1B). Further logistic regression analysis showed that the influencing factors of ORR in patients under the biosimilar combined with chemotherapy and immunotherapy were the number of treatment lines and combination with radiotherapy (P=0.021, 0.008). Product type (i.e., bevacizumab or bevacizumab biosimilar) was not a main factor (P=0.604). Overall, patients in the first-line treatment group revealed relatively higher ORR and DCR than those in the second- or later-line therapy (biosimilar group: ORR: 37.29% vs. 19.72% vs. 16.98%, P<0.001, Figure 1C; DCR: 93.07% vs. 85.92% vs. 83.96%, P=0.009; reference group: ORR: 35.96% vs. 26.00% vs. 25.00%, P=0.080; DCR: 93.10% vs. 82.00% vs. 80.43%, P=0.002). Patients with combined radiotherapy showed higher ORR than those without combined radiotherapy (biosimilar group: 34.55% vs. 28.23%, P=0.326; reference group: 47.92% vs. 28.53%, P=0.006; Figure 1D). Remarkable differences in ORR and DCR were observed in the different combination groups as shown in Figure 1E. Patients in the combination chemotherapy and another targeted therapy had the highest ORR (56.34%) and patients in the combination of another targeted therapy had the highest DCR (92.50%). In addition, patients without brain metastases showed higher DCR than patients with brain metastases (84.69% vs. 89.69%, P=0.045, Figure 1F).
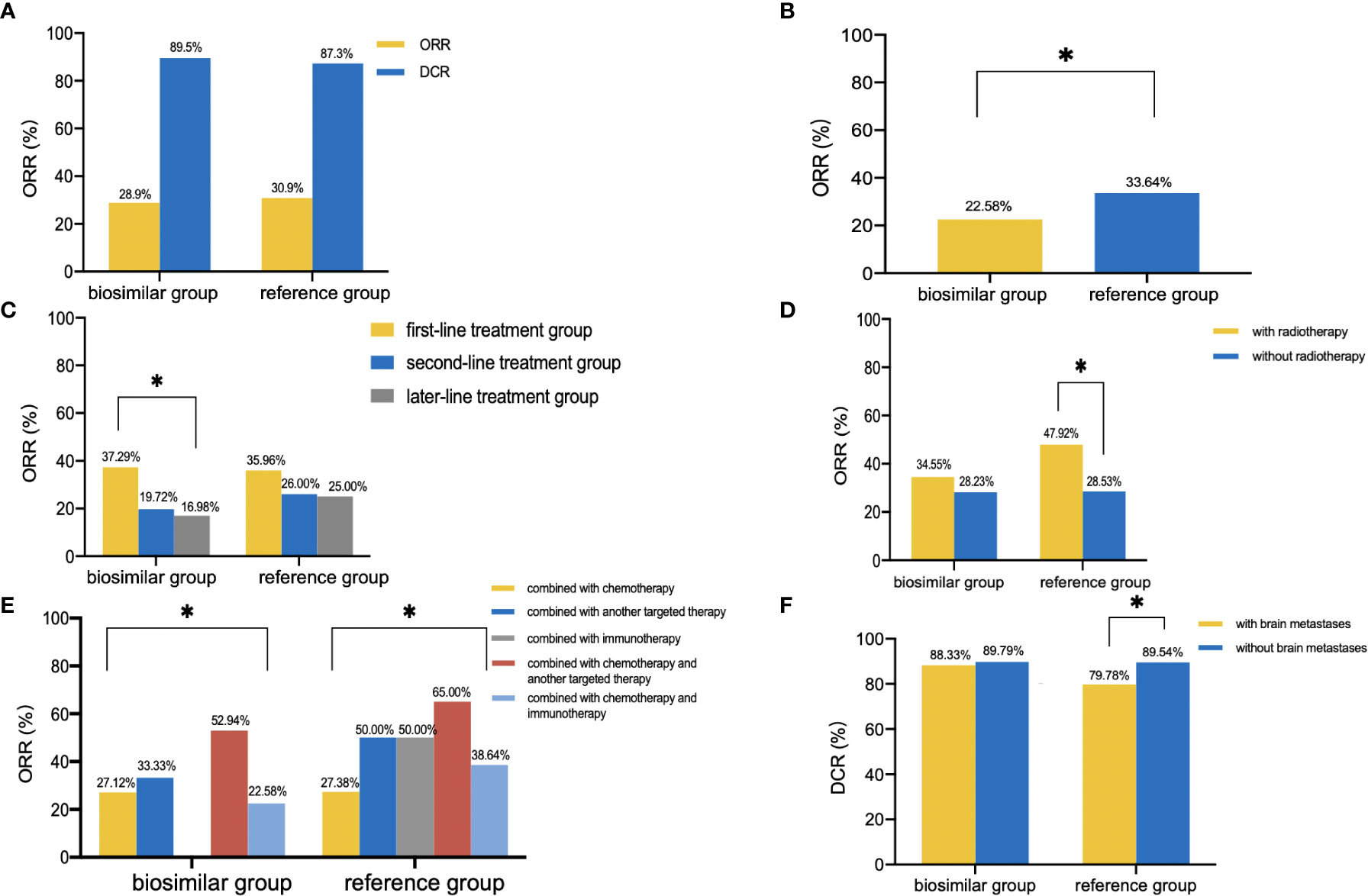
Figure 1 Clinical outcomes. (A), ORR and DCR of the biosimilar and reference groups. (B), ORR of patients treated with bevacizumab (or biosimilar) in combination with chemotherapy and immunotherapy. (C), ORR of different number of treatment lines in the two groups. (D), ORR of patients with or without radiotherapy in the two groups. (E), ORR of different combined treatment regimens in the two groups. (F), DCR of patients with or without brain metastases in the two groups. *P<0.05.
A total of 385 (69.9%) patients progressed or died in the biosimilar group compared with 363 (91.9%) patients in the reference group. Based on the Kaplan–Meier analysis, the estimated median PFS (mPFS) values were 6.27 (95% CI: 5.53–7.01) months in the biosimilar group and 4.93 (95% CI: 4.24–5.62) months in the reference group as shown in Figure 2. Based on the Cox regression model, the estimated hazard ratio (HR) for bevacizumab biosimilar and bevacizumab comparison was 1.084 (95% CI: 0.932–1.260, P=0.296). The analyses showed that the long-term efficacies of the two treatment groups were similar. Overall, the mPFS was 5.53 months (95% CI: 4.98–6.09) for all patients. The number of treatment lines, radiotherapy, and combined treatment regimens were statistically significant for PFS as shown in Table 4. In addition, based on the Cox regression analysis of the subgroups, no statistical difference was observed in the PFS of the bevacizumab biosimilar and bevacizumab groups except for the subgroup with a history of hypertension as shown in Figure 3. In a subgroup of patients with a history of hypertension, bevacizumab biosimilar obtained longer PFS than reference bevacizumab (8.85 vs. 6.63 months, P=0.047). The mean dose intensity of the biosimilar group was significantly higher than that of the reference group (8.28 vs. 7.37 mg/kg, P=0.007) in this subgroup. Based on multivariate analysis, the product type was not the main factor affecting PFS (P=0.595).
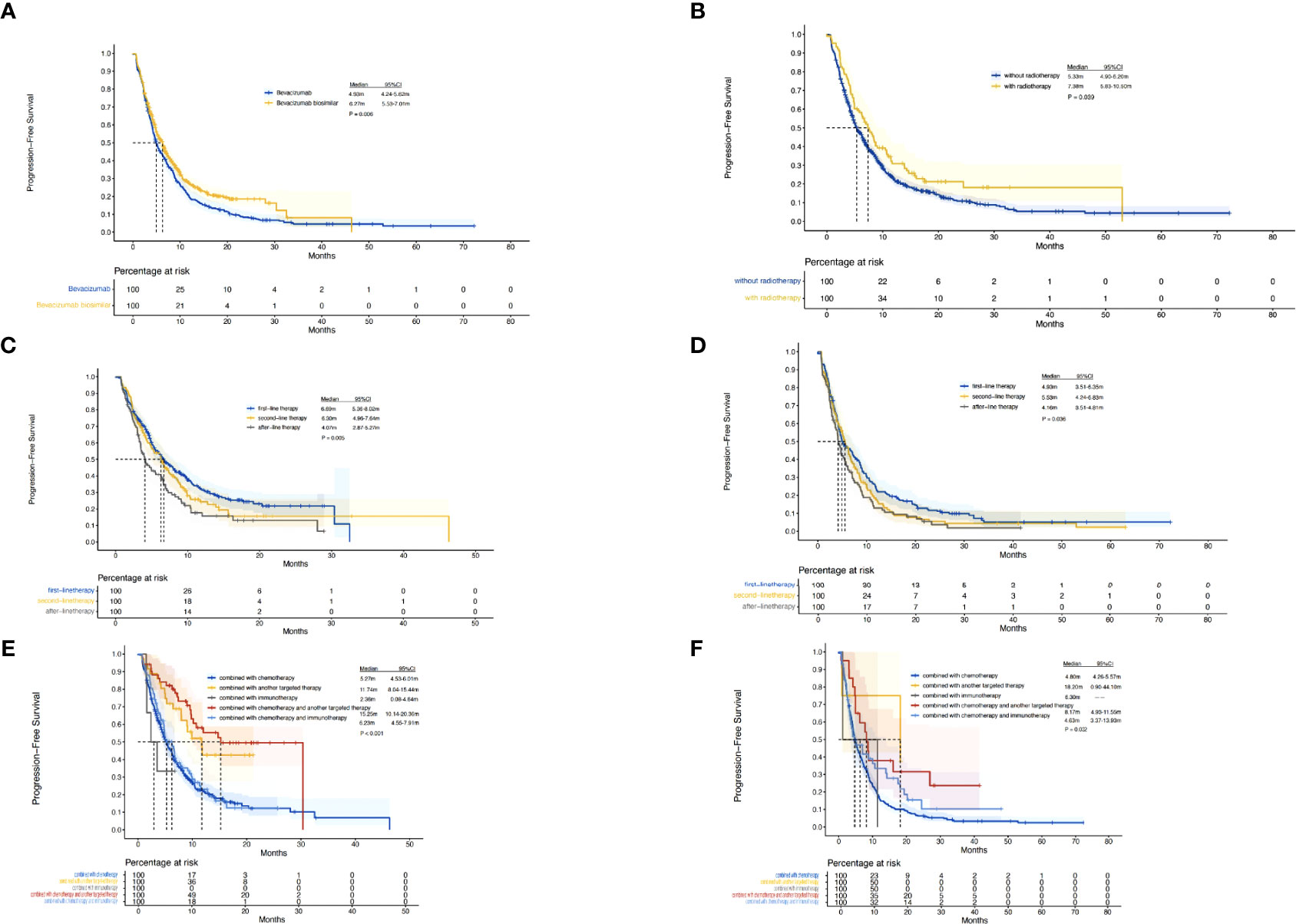
Figure 2 Kaplan–Meier curves. (A), PFS of the biosimilar and reference groups. (B), PFS of all the patients with or without radiotherapy. (C), PFS of patients in the biosimilar group with different number of treatment lines. (D), PFS of patients in the reference group with different number of treatment lines. (E), PFS of patients in the biosimilar group with different combined treatment regimens. (F), PFS of patients in the reference group with different combined treatment regimens.
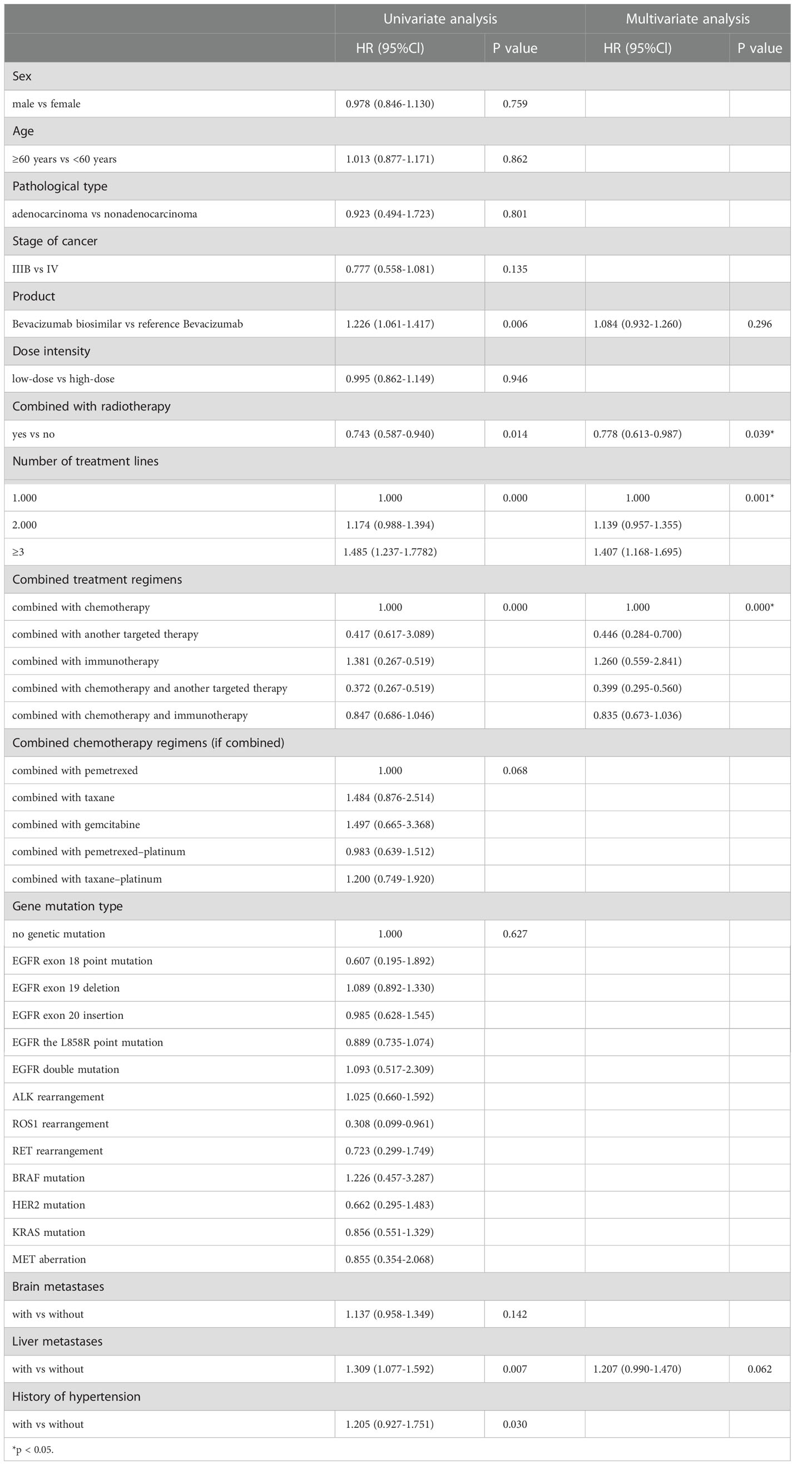
Table 4 Univariate analysis and multivariate analysis of factors affecting the progression-free survival (PFS) of all patients.
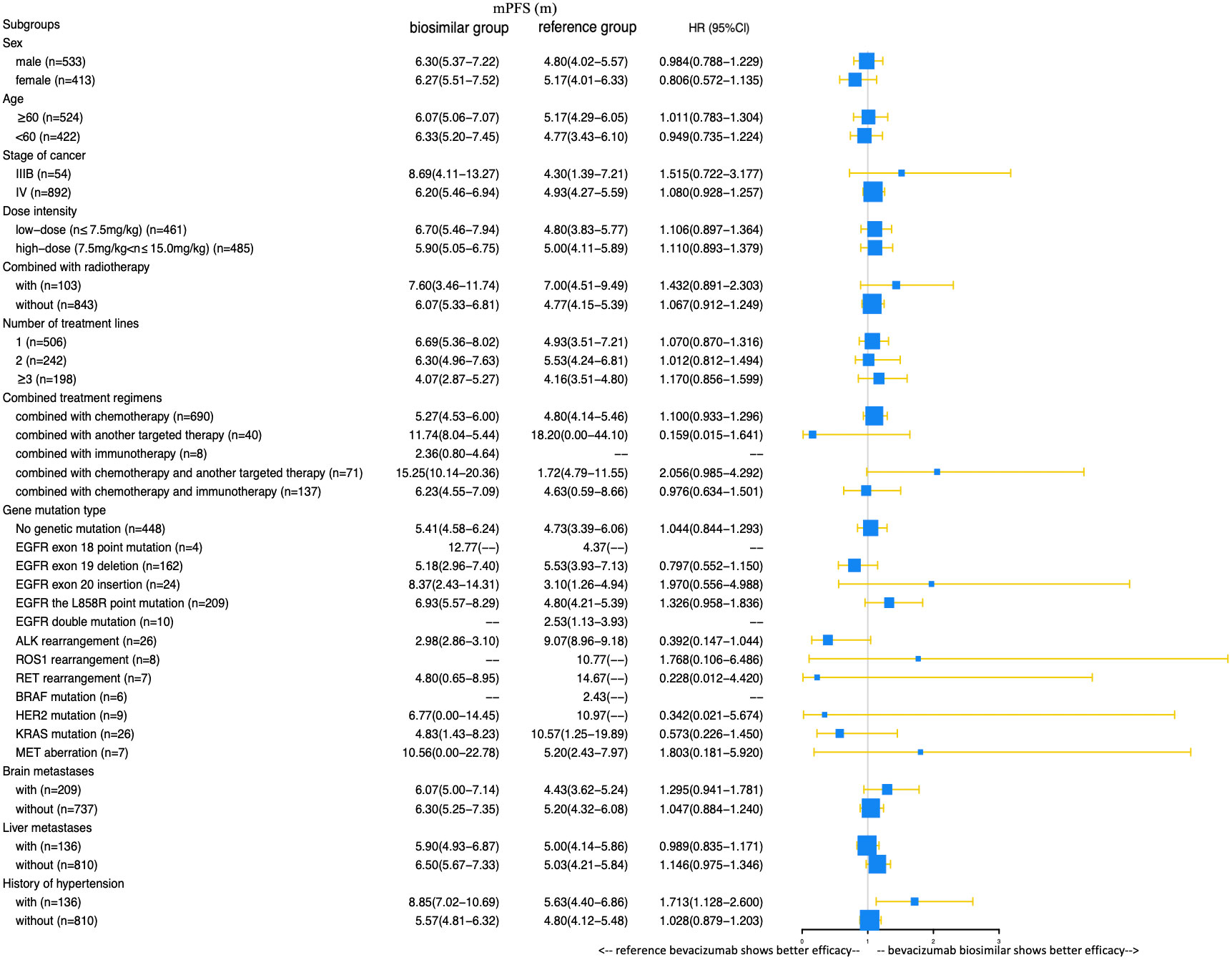
Figure 3 Comparison of the effects of bevacizumab biosimilar and reference bevacizumab on the PFS of each subgroup.
As shown in Table 5, the incidence of treatment-related grade AEs in all patients was 74.42% (704/946). Among which, 199 cases (21.04%) were more than grade 3 AEs. No fatal effects happened. Similar incidences of TEAEs at any grade were observed in the biosimilar and reference groups (421 subjects [76.41%] vs. 283 subjects [71.65%], P=0.098), and most of them were classified as grade 1 or 2 AEs. No statistically significant differences in the incidence of grade 3 and 4 TEAEs were observed between the two groups (22.14% vs. 19.49%, P=0.324). Among the treatment-related AEs in the biosimilar group, neutropenia had the highest incidence (22.87%), followed by anemia (16.15%), alopecia (14.70%), nausea (12.89%), fatigue (10.16%), thrombocytopenia (10.16%), hypertension (9.62%), and fever (8.71%). In the reference group, neutropenia had the highest incidence (24.05%), followed by anemia (16.46%), alopecia (13.67%), fatigue (11.90%), thrombocytopenia (10.89%), nausea (10.63%), loss of appetite (9.37%), and bleeding (7.34%). The incidence of hypertension at any grade in the biosimilar group was higher than that in the reference group (9.62% vs. 5.82%, P=0.033), but no difference in the incidence of hypertension was found at grade 3 or 4 (1.63% vs. 0.51%, P=0.093). We further analyzed the clinical data to understand the origin of the differences. Among patients over 70 years of age who received high-dose treatment, the incidence of hypertension caused by the biosimilar (n=48) was significantly higher than that of reference bevacizumab (n=32; 37.5% vs. 15.6%, P=0.034). Among patients over 70 years of age with a history of hypertension, the incidence of hypertension caused by the biosimilar (n=25) was higher than that of reference bevacizumab (n=17; 40% vs. 23.5%, P=0.266). Among these patients, we first hypothesized that differences in the incidence of hypertension might be attributed to some patients in the subgroup over 70 years of age. We then compared the incidence of hypertension in patients over 70 years of age after excluding patients with high-dose therapy and a history of hypertension. The results showed that the incidence of hypertension was similar between the two products (17.3% vs. 10.0%, P=0.511). After imbalanced factors were eliminated, no statistically significant difference in the incidence of hypertension of any grade was found between the biosimilar group (n=541) and reference group (n=392; 7.9% vs. 5.1%, P=0.087).
The results of this study demonstrated that bevacizumab biosimilar is equivalent in efficacy to bevacizumab in locally advanced and advanced NSCLC. Patients receiving previous treatment, patients receiving regimens other than in combination with chemotherapy, patients with rare genetic mutations, and patients with brain metastases also benefit clinically from both products. The AE spectra and incidence rates of the two products were similar.
Bevacizumab is a humanized monoclonal antibody targeting VEGF and is prepared by recombinant DNA technology. By combining with VEGF, it can inhibit the binding of VEGF to its receptor and block the signaling pathway of angiogenesis in tumor tissues. Bevacizumab has become an important component of systemic therapy for advanced NSCLC without genetic mutations. A biosimilar is an approved biological product that is highly similar to the original drug with no clinically remarkable difference in safety, purity, or potency. Biosimilar therapy is an important avenue to reduce patient expenditure and the financial burden of national healthcare systems while maintaining therapeutic effect (18). However, a biosimilar is not an exact copy of its reference product and requires vigilance and concern in clinical application.
Current prospective clinical trials have confirmed that bevacizumab biosimilar in combination with platinum-containing two-drug chemotherapy has similar efficacy and safety compared with bevacizumab in patients with untreated advanced non-squamous NSCLC. In 2019, China National Medical Products Administration approved the marketing of bevacizumab biosimilar developed by Qilu Pharmaceutical Co., Ltd. (trade name: Encoda) with all indications approved for bevacizumab in China. However, no clinical data has verified the efficacy and safety of the biosimilar in clinical application. Existing trials have excluded patients receiving previous treatment, patients receiving combination with targeted therapy or immunotherapy, patients with brain metastases, patients with rare genetic mutations (such as EML4–ALK rearrangement), and patients with ECOG scores greater than 2. No consensus has been reached on the efficacy and safety of bevacizumab biosimilar in these populations. Among the published retrospective studies comparing bevacizumab biosimilar with bevacizumab, most have focused on patient clinical characteristics, cost-effectiveness, and economic impact rather than efficacy and safety. Only one study mentioned the statistical analysis of treatment modalities for 18 patients with NSCLC who used bevacizumab (19). Our study is not comparable to this one because of the lack of other information and its limited patient population. Our study greatly expanded the sample size and provided detailed information. It is the first retrospective study to evaluate the efficacy and safety of bevacizumab biosimilar.
Subject demographics, baseline disease characteristics. And exposure to treatment were well balanced and comparable between the two treatment groups. In the biosimilar group, the ORR was 28.9% (95% CI: 25.1%–32.7%), and the DCR was 89.5%. The ORR and DCR of the control group were 30.9% (95% CI: 26.3%–35.5%) and 87.3%, respectively. The unstratified ORR risk ratio was 0.934 with a 95% CI of 0.767–1.138 and a 90% CI of 0.792–1.103. The unstratified ORR risk difference was −0.020 with a 95% CI of −0.118–0.035 and a 90% CI of −0.105–0.023. The definition of equivalence by FDA, PMDA, and EMA corresponds to the 90% CI of ORR hazard ratio in the range of 0.73–1.37; the 95% CI of the ORR HR is 0.729–1.371, and the 95% CI of the ORR risk difference is −13%–13% (20, 21). In the present study, the CIs of ORR risk ratio and ORR risk difference were within these predefined equivalence margins. The estimated mPFS values were 6.27 (95% CI: 5.53–7.01) months in the biosimilar group and 4.93 (95% CI: 4.24–5.62) months in the reference group. The estimated HR for bevacizumab biosimilar and bevacizumab comparison was 1.084 (95% CI: 0.932–1.260, P=0.296). Therefore, bevacizumab biosimilar and reference bevacizumab are equivalent in efficacy.
In different historical clinical trial results for bevacizumab biosimilar, the ORR ranges from 41.5% to 53.1%, and the mPFS is about 7.5 months (22–24). Numerically, our study showed a lower ORR and a shorter PFS than other studies. In view of the differences between clinical application and clinical trials, such as the number of treatment lines, drug combinations, and dose intensity, the above results are not highly comparable. The subgroup analyses were performed based on the following subgroups: sex, age, pathology, stage, number of treatment lines, radiotherapy, combined treatment regimens, combined chemotherapy regimens (if combined with chemotherapy), dose intensity, genetic mutations, brain metastasis, liver metastasis, and history of hypertension. To avoid the increased risk of potential bias associated with a small sample size, subgroups with less than 3 cases were not included in the subgroup analysis.
In the subgroup analysis of combined treatment regimens, the ORR of the biosimilar group was lower than that of the reference group when combined with chemotherapy and immunotherapy (22.58% vs. 33.64%, P=0.048). Further logistic regression analysis showed that the influencing factors of ORR in patients combined with chemotherapy and immunotherapy were the number of treatment lines and combination with radiotherapy (P=0.021, 0.008). Product type (i.e., bevacizumab or bevacizumab biosimilar) was not a main factor (P=0.604). In a subgroup of patients with a history of hypertension, bevacizumab biosimilar obtained a longer PFS than reference bevacizumab (8.85 vs. 6.63 months, P=0.047). The mean dose intensity of the biosimilar group was significantly higher than that of the reference group (8.28 vs. 7.37 mg/kg, P=0.007) in this subgroup. Based on multivariate analysis, the product type was not the main factor affecting PFS (P=0.595). Among the remaining subgroups, the ORR and PFS analyses support the equivalence between the two groups.
The number of treatment lines, combined treatment regimens, and radiotherapy were the significant factors affecting the PFS of both groups (P<0.001, P=0.001, P=0.039). Combined chemotherapy regimens (if combined with chemotherapy), different genetic mutations, dose intensity, brain metastases, liver metastases, and a history of hypertension were not the main factors affecting PFS (P=0.104, 0.627, 0.946, 0.142, 0.062, 0.030).
The treatments combined with chemotherapy and another targeted therapy (biosimilar group: ORR=52.94%, mPFS=15.25 months; reference group: ORR=65.00%, mPFS=8.17 months) and combined with another targeted therapy group (biosimilar group: ORR=33.33%, mPFS=11.74 months; reference group: ORR=50.00%, mPFS=18.20 months) showed better efficacy than other treatment regimens. Among these treatments, approximately 80% of other targeted therapies are EGFR–TKI. Preclinical studies have shown that VEGF and EGFR share a common downstream signaling pathway (25–28), but the clinical trial data of EGFR–TKI combined with bevacizumab are still immature and have many uncertainties (29). Our study confirmed the benefits of bevacizumab (or bevacizumab biosimilar) combined with EGFR–TKI in ORR and PFS but failed to obtain OS results for all patients because of the short follow-up period. Previous studies have shown that patients with exon 19 deletions have a better prognosis after EGFR–TKI treatment than those with 21 p.L858 mutations (30). However, our study revealed similar ORR and PFS benefits between the two mutation types in both groups. The synergistic antiproliferative effects of EGFR–TKI and antiangiogenic treatment might eliminate the prognostic differences caused by genetic mutations. More prospective studies or in-depth retrospective clinical data are expected to better explore the above conclusions.
In addition to EGFR mutations, studies on patients with other rare mutations receiving targeted therapy combined with bevacizumab are few, and all of which are exploratory studies with small samples. The American Society of Clinical Oncology reported a study from China in which 16 patients with EML4–ALK rearrangement receiving crizotinib and bevacizumab have a mPFS of 13.0 months. Our study included 89 patients that harbor other rare driving gene mutations, including ALK rearrangement, ROS1 rearrangement, RET rearrangement, BRAF mutation, HER2 mutation, KRAS mutation, and MET aberration. These populations have also been proven to benefit from bevacizumab or bevacizumab biosimilar.
In the subgroup analysis of combined with chemotherapy, multivariate analysis showed the combined chemotherapy regimen was not the main factor affecting PFS (P=0.068). But it is worth noting that, numerically, the treatment combined with gemcitabine showed the shortest PFS and the treatment combined with pemetrexed–platinum showed the longest PFS in both the biosimilar and reference groups (biosimilar group: pemetrexed vs taxane vs gemcitabine vs pemetrexed–platinum vs taxane–platinum: 3.17m vs 3.20m vs 1.15m vs 4.43m vs 3.41m, P=0.119; reference group:1.97m vs 2.27m vs 1.59m vs 4.23m vs 3.57m, P=0.055). Previous studies (14) have confirmed that gemcitabine is inferior to pemetrexed or paclitaxel in advanced NS-NSCLC patients. Our results suggest that this trend may remain in the context of bevacizumab in combination. Whether the efficacy of bevacizumab in combination with different platinum-based doublets is different is still controversial. PointBreak trial (31) confirmed that pemetrexed–platinum combined with bevacizumab regimen obtained significantly longer PFS than taxane–platinum combined with bevacizumab regimen, but no statistical difference was observed in PRONOUNCE trial (32)and ERACLE trial (33). In our study, no statistical difference was obtained on this question, probably due to the inherent influence of selection bias and missing data. Larger prospective studies are expected to investigate this issue.
In the previous phase III clinical trial studies, only the AVAiL and AVAPERL studies used bevacizumab at 7.5 mg/kg, and the other studies used 15 mg/kg. These studies lacked the ability to directly compare the two doses of bevacizumab. With 7.5 mg/kg as the boundary line, our study showed that no statistical differences in the ORR and PFS between the low- and high-dose groups (biosimilar group: 33.19% vs. 25.85%, 6.70 vs. 5.90 months; reference group: 29.79% vs. 32.50%, 4.80 vs. 5.00 months). In addition, the patients with the brain metastases (BMS) also benefit from bevacizumab or biosimilar (biosimilar group: ORR=26.67%, mPFS=6.07 months; reference group: ORR=23.60%, mPFS=4.43 months).
Similar incidences of TEAEs at any grade were observed in the biosimilar group and the reference group (421 [76.41%] vs. 283 subjects [71.65%], P=0.098), and most of them were classified as grade 1 or 2 events. No statistically significant difference in the incidence of grade 3 or 4 TEAEs was observed between the two groups (22.14% vs. 19.49%, P=0.324). No fatal effects happened. Among the treatment-related AEs in the biosimilar group, neutropenia had the highest incidence (22.87%), followed by anemia (16.15%), alopecia (14.70%), nausea (12.89%), fatigue (10.16%), thrombocytopenia (10.16%), hypertension (9.62%), and fever (8.71%). In the reference group, neutropenia had the highest incidence (24.05%), followed by anemia (16.46%), alopecia (13.67%), fatigue (11.90%), thrombocytopenia (10.89%), nausea (10.63%), loss of appetite (9.37%), and bleeding (7.34%). In general, the AE spectra and AE rates of bevacizumab biosimilar and reference bevacizumab were similar. The overall incidences of grade 3 and 4 TEAEs were low and similar, indicating that bevacizumab biosimilar and bevacizumab have favorable safety profiles.
The incidence of hypertension at any grade in the biosimilar group was higher than that in the reference group (9.62% vs. 5.82%, P=0.033), but no differences in the incidence of hypertension were observed at grades 3 and 4 (1.63% vs. 0.51%, P=0.093). We further analyzed the clinical data to understand the origin of the differences. Among patients over 70 years of age who received high-dose treatment, the incidence of hypertension caused by the biosimilar (n=48) was significantly higher than that of reference bevacizumab (n=32; (37.5% vs. 15.6%, P=0.034). Among patients over 70 years of age with a history of hypertension, the incidence of hypertension caused by biosimilar (n=25) was higher than that of reference bevacizumab (n=17; 40% vs. 23.5%, P=0.266). Among these patients, we first hypothesized that differences in the incidence of hypertension might be attributed to some patients in the subgroup over 70 years of age. We then compared the incidence of hypertension in patients over 70 years of age after excluding patients with high-dose therapy and a history of hypertension. The results showed that the incidence of hypertension was similar between the two products (17.3% vs. 10.0%, P=0.511). After imbalanced factors were eliminated, no statistically significant difference in the incidence of hypertension of any grade was found between the biosimilar group (n=541) and reference group (n=392; 7.9% vs. 5.1%, P=0.087).
In the subgroup analysis of combined with chemotherapy, similar incidences of TEAEs at any grade were observed (pemetrexed vs taxane vs gemcitabine vs pemetrexed–platinum vs taxane–platinum: 69.2% vs 78.3% vs 55.6% vs 71.9% vs 72.6%, P=0.695). Among these treatment-related AEs, neutropenia had the highest incidence (28.92%), followed by anemia (18.92%), thrombocytopenia (11.54%) and fatigue (10.77%). The incidence of a few adverse reactions varied with chemotherapy regiments, and these differences existed in both the biosimilar and reference groups. The incidence of anemia at any grade was significantly higher in the treatment combined with pemetrexed–platinum than in the other chemotherapy regimens (biosimilar group:10.0% vs 13.3% 0.00% vs 26.8% vs 12.1%, P=0.042; reference group:12.5% vs 13.3% vs 0.00% vs 23.1% vs.10.8%, P=0.039). All 4 cases of sensory neuropathy were in the taxane–platinum combined with bevacizumab regimen (biosimilar group vs reference group: 5.2% vs 2.7%, P=0.654), but all were classified as grade 1 or grade 2 events. The above adverse reactions were consistent with previous studies on corresponding chemotherapy regimens. These were not attributed to the type of bevacizumab product. The toxicity of these adverse reactions is considered acceptable, but close monitoring of these patients is still required in clinical practice.
We acknowledge the limitations of the retrospective study design, which is inherently affected by selection bias and missing data. Moreover, reliance on electronic health records may mean that some events may be underestimated. Therefore, larger prospective studies are needed to confirm our findings.
The results of this study demonstrated that bevacizumab biosimilar is equivalent in efficacy to bevacizumab in patients with locally advanced and advanced NSCLC. Bevacizumab biosimilar showed acceptable toxicity profile and no new AEs. Patients receiving previous treatment, patients receiving regimens other than in combination with chemotherapy, patients with rare genetic mutations, and patients with brain metastases can also benefit clinically from both products. The number of treatment lines, radiotherapy, and combined treatment regimens were the substantial factors affecting the ORR and PFS of bevacizumab or biosimilar. Different genetic mutations and dose intensity were not the main factors affecting PFS.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Academic Ethics Committee of Jiangsu Cancer Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
This work was supported by the National Natural Science Foundation of China, 82172872, 81871873; Social Development Project of Jiangsu Province, BE2021746; "Six Talent Peaks" of Jiangsu Province, WSN-039; Key topic of social development project of Suqian City, S202208; Guangzhou Life Oasis Public Service Center, Health Research Exchange Project 2-38.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin (2022) 72(1):7–33. doi: 10.3322/caac.21708
3. Toi M, Matsumoto T, Bando H. Vascular endothelial growth factor: its prognostic, predictive, and therapeutic implications. Lancet Oncol (2001) 2(11):667–73. doi: 10.1016/s1470-2045(01)00556-3
4. Lee SH, Jeong D, Han YS, Baek MJ. Pivotal role of vascular endothelial growth factor pathway in tumor angiogenesis. Ann Surg Treat Res (2015) 89(1):1–8. doi: 10.4174/astr.2015.89.1.1
5. Garcia J, Hurwitz HI, Sandler AB, Miles D, Coleman RL, Deurloo R, et al. Bevacizumab (Avastin(R)) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat Rev (2020) 86:102017. doi: 10.1016/j.ctrv.2020.102017
6. Reck M, von Pawel J, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V, et al. Overall survival with cisplatin-gemcitabine and bevacizumab or placebo as first-line therapy for nonsquamous non-small-cell lung cancer: results from a randomised phase III trial (AVAiL). Ann Oncol (2010) 21(9):1804–9. doi: 10.1093/annonc/mdq020
7. Zhou C, Wu YL, Chen G, Liu X, Zhu Y, Lu S, et al. BEYOND: A randomized, double-blind, placebo-controlled, multicenter, phase III study of first-line Carboplatin/Paclitaxel plus bevacizumab or placebo in Chinese patients with advanced or recurrent nonsquamous non-Small-Cell lung cancer. J Clin Oncol (2015) 33(19):2197–204. doi: 10.1200/JCO.2014.59.4424
8. Shukla NA, Yan MN, Hanna N. The story of angiogenesis inhibitors in non-small-cell lung cancer: The past, present, and future. Clin Lung Cancer (2020) 21(4):308–13. doi: 10.1016/j.cllc.2020.02.024
9. He K, Chen H, Gwise T, Casak S, Lemery S, Keegan P, et al. Statistical considerations in evaluating a biosimilar product in an oncology clinical study. Clin Cancer Res (2016) 22(21):5167–70. doi: 10.1158/1078-0432.CCR-16-1010
10. Endrenyi L, Markus R. Interchangeability of biological drug products-FDA draft guidance. J Biopharm Stat (2019) 29(6):1003–10. doi: 10.1080/10543406.2019.1607369
11. Bloomfield D, D'Andrea E, Nagar S, Kesselheim A. Characteristics of clinical trials evaluating biosimilars in the treatment of cancer: A systematic review and meta-analysis. JAMA Oncol (2022) 8(4):537–45. doi: 10.1001/jamaoncol.2021.7230
12. Saleem T, Qurashi H, Jamali M, Chan Gomez J, Kanderi T. Biosimilars as a future, promising solution for financial toxicity: A review with emphasis on bevacizumab. Cureus (2020) 12(7):e9300. doi: 10.7759/cureus.9300
13. Cohen MH, Gootenberg J, Keegan P, Pazdur R. FDA Drug approval summary: bevacizumab (Avastin) plus carboplatin and paclitaxel as first-line treatment of advanced/metastatic recurrent nonsquamous non-small cell lung cancer. Oncologist (2007) 12(6):713–8. doi: 10.1634/theoncologist.12-6-713
14. Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, et al. Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol (2018) 29:iv192–237. doi: 10.1093/annonc/mdy275
15. Luo X, Liu Q, Zhou Z, Yi L, Peng L, Wan X, et al. Cost-effectiveness of bevacizumab biosimilar LY01008 combined with chemotherapy as first-line treatment for Chinese patients with advanced or recurrent nonsquamous non-small cell lung cancer. Front Pharmacol (2022) 13:832215. doi: 10.3389/fphar.2022.832215
16. Yang J, Liu R, Ektare V, Stephens J, Shelbaya A. Does biosimilar bevacizumab offer affordable treatment options for cancer patients in the USA? a budget impact analysis from US commercial and Medicare payer perspectives. Appl Health Econ Health Policy. (2021) 19(4):605–18. doi: 10.1007/s40258-021-00637-5
17. Jin R, Mahtani RL, Accortt N, Lawrence T, Sandschafer D, Loaiza-Bonilla A. Clinical and treatment characteristics of patients treated with the first therapeutic oncology biosimilars bevacizumab-awwb and trastuzumab-anns in the US. Ther Adv Med Oncol (2021) 13:17588359211041961. doi: 10.1177/17588359211041961
18. Science IIfHD. The global use of medicine in 2019 and outlook to 2023. Available at: https://www.iqvia.com/insights/the-iqvia-institute/reports/the-global-use-of-medicine-in-2019-and-outlook-to-2023 (Accessed January 2021).
19. Yang J, Kelton JM, Thompson J, Alvir JMJ, Maculaitis MC, Shelbaya A. Real-world usage of bevacizumab-bvzr biosimilar in US oncology practice. Am J Manag Care (2022) 28(4):160–6. doi: 10.37765/ajmc.2022.88831
20. Syrigos K, Abert I, Andric Z, Bondarenko IN, Dvorkin M, Galic K, et al. Efficacy and safety of bevacizumab biosimilar FKB238 versus originator bevacizumab: Results from AVANA, a phase III trial in patients with non-squamous non-Small-Cell lung cancer (non-sq-NSCLC). BioDrugs (2021) 35(4):417–28. doi: 10.1007/s40259-021-00489-4
21. Trukhin D, Poddubskaya E, Andric Z, Makharadze T, Bellala RS, Charoentum C, et al. Efficacy, safety and immunogenicity of MB02 (Bevacizumab biosimilar) versus reference bevacizumab in advanced non-small cell lung cancer: A randomized, double-blind, phase III study (STELLA). BioDrugs (2021) 35(4):429–44. doi: 10.1007/s40259-021-00483-w
22. Reinmuth N, Bryl M, Bondarenko I, Syrigos K, Vladimirov V, Zereu M, et al. PF-06439535 (a bevacizumab biosimilar) compared with reference bevacizumab (Avastin((R))), both plus paclitaxel and carboplatin, as first-line treatment for advanced non-squamous non-Small-Cell lung cancer: A randomized, double-blind study. BioDrugs (2019) 33(5):555–70. doi: 10.1007/s40259-019-00363-4
23. Reck M, Luft A, Bondarenko I, Shevnia S, Trukhin D, Kovalenko NV, et al. A phase III, randomized, double-blind, multicenter study to compare the efficacy, safety, pharmacokinetics, and immunogenicity between SB8 (proposed bevacizumab biosimilar) and reference bevacizumab in patients with metastatic or recurrent nonsquamous non-small cell lung cancer. Lung Cancer (2020) 146:12–8. doi: 10.1016/j.lungcan.2020.05.027
24. Chu T, Lu J, Bi M, Zhang H, Zhuang W, Yu Y, et al. Equivalent efficacy study of QL1101 and bevacizumab on untreated advanced non-squamous non-small cell lung cancer patients: a phase 3 randomized, double-blind clinical trial. Cancer Biol Med (2021) 18(3):816–24. doi: 10.20892/j.issn.2095-3941.2020.0212
25. Jiang T, Zhang Y, Li X, Zhao C, Chen X, Su C, et al. EGFR-TKIs plus bevacizumab demonstrated survival benefit than EGFR-TKIs alone in patients with EGFR-mutant NSCLC and multiple brain metastases. Eur J Cancer (2019) 121:98–108. doi: 10.1016/j.ejca.2019.08.021
26. Yang JC-H. Bevacizumab in EGFR-positive NSCLC: time to change first-line treatment? Lancet Oncol (2019) 20(5):602–3. doi: 10.1016/s1470-2045(19)30085-3
27. Larsen AK, Ouaret D, El Ouadrani K, Petitprez A. Targeting EGFR and VEGF(R) pathway cross-talk in tumor survival and angiogenesis. Pharmacol Ther (2011) 131(1):80–90. doi: 10.1016/j.pharmthera.2011.03.012
28. Le X, Nilsson M, Goldman J, Reck M, Nakagawa K, Kato T, et al. Dual EGFR-VEGF pathway inhibition: A promising strategy for patients with EGFR-mutant NSCLC. J Thorac Oncol (2021) 16(2):205–15. doi: 10.1016/j.jtho.2020.10.006
29. Ma JT, Guo YJ, Song J, Sun L, Zhang SL, Huang LT, et al. Rational application of first-line EGFR-TKIs combined with antiangiogenic inhibitors in advanced EGFR-mutant non-Small-Cell lung cancer: A systematic review and meta-analysis. BioMed Res Int (2021) 2021:8850256. doi: 10.1155/2021/8850256
30. Hsu WH, Yang JC, Mok TS, Loong HH. Overview of current systemic management of EGFR-mutant NSCLC. Ann Oncol (2018) 29(suppl_1):i3–9. doi: 10.1093/annonc/mdx702
31. Patel JD, Socinski MA, Garon EB, Reynolds CH, Spigel DR, Olsen MR, et al. PointBreak: A randomized phase III study of pemetrexed plus carboplatin and bevacizumab followed by maintenance pemetrexed and bevacizumab versus paclitaxel plus carboplatin and bevacizumab followed by maintenance bevacizumab in patients with stage IIIB or IV nonsquamous non–Small-Cell lung cancer. J Clin Oncol (2013) 31(34):4349–57. doi: 10.1200/jco.2012.47.9626
32. Zinner RG, Obasaju CK, Spigel DR, Weaver RW, Beck JT, Waterhouse DM, et al. PRONOUNCE: randomized, open-label, phase III study of first-line pemetrexed + carboplatin followed by maintenance pemetrexed versus paclitaxel + carboplatin + bevacizumab followed by maintenance bevacizumab in patients ith advanced nonsquamous non-small-cell lung cancer. J Thorac Oncol (2015) 10(1):134–42. doi: 10.1097/JTO.0000000000000366
33. Galetta D, Cinieri S, Pisconti S, Gebbia V, Morabito A, Borsellino N, et al. Cisplatin/Pemetrexed followed by maintenance pemetrexed versus Carboplatin/Paclitaxel/Bevacizumab followed by maintenance bevacizumab in advanced nonsquamous lung cancer: The GOIM (Gruppo oncologico italia meridionale) ERACLE phase III randomized trial. Clin Lung Cancer (2015) 16(4):262–73. doi: 10.1016/j.cllc.2014.12.002
Keywords: antiangiogenic treatment, bevacizumab, biosimilar, non-small cell lung cancer, lung cancer
Citation: Zhao Z, Zhao L, Xia G, Lu J, Shen B, Zhou G, Wu F, Hu X, Feng J and Yu S (2023) Efficacy and safety of bevacizumab biosimilar compared with reference bevacizumab in locally advanced and advanced non-small cell lung cancer patients: A retrospective study. Front. Oncol. 12:1036906. doi: 10.3389/fonc.2022.1036906
Received: 05 September 2022; Accepted: 05 December 2022;
Published: 09 January 2023.
Edited by:
Valerio Gristina, University of Palermo, ItalyReviewed by:
Luis Mas, Instituto Nacional de Enfermedades Neoplásicas (INEN), PeruCopyright © 2023 Zhao, Zhao, Xia, Lu, Shen, Zhou, Wu, Hu, Feng and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaorong Yu, eXVzaGFvcm9uZzIwMDlAMTYzLmNvbQ==; Jifeng Feng, amlmZW5nX2ZlbmdAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.