- 1Department of Interventional Radiology, Central South University, Xiangya School of Medicine Affiliated Haikou Hospital, Haikou, Hainan, China
- 2Department of Hepatobiliary Surgery, Hainan General Hospital, Haikou, Hainan, China
Background: The pan-immune-inflammation value (PIV) has been reported as a novel prognostic biomarker in multiple malignancies. The aim of this study is to investigate the prognostic value of the PIV in patients with colorectal cancer.
Methods: We comprehensively searched electronic databases including PubMed, Embase and Web of Science up to August 2022. The endpoints were survival outcomes. Hazard ratios (HRs) with 95% confidence intervals (CIs) for survival data were collected for analysis.
Results: Six studies including 1879 participants were included. A significant heterogeneity in the PIV cut-off value among studies was observed. The combined results indicated that patients in the high baseline PIV group had a worse overall survival (HR=2.09; 95%CI: 1.67-2.61; P<0.0001; I2 = 7%) and progression-free survival (HR=1.82; 95%CI: 1.49-2.22; P<0.0001; I2 = 15%). In addition, early PIV increase after treatment initiation was significantly associated with decreased overall survival (HR=1.79; 95%CI: 1.13-2.93; P=0.01; I2 = 26%), and a trend toward poor progression-free survival (HR=2.00; 95%CI: 0.90-4.41; P=0.09; I2 = 70%).
Conclusion: Based on existing evidence, the PIV could act as a valuable prognostic index in patients with colorectal cancer. However, the heterogeneity in the PIV cut-off value among studies should be considered when interpreting these findings.
1 Background
Colorectal cancer is one of the most common malignancies in the world, accounting for about 10% of newly diagnosed cancers and cancer-related deaths (1). Despite significant advances in surgery-based multimodal therapy for colorectal malignancy, the prognosis of most patients, especially those with advanced stages, is still unsatisfactory (2–4). Consequently, it is essential to develop a useful prognostic index to predict postoperative recurrence and survival in colorectal cancers, aiming to formulate treatment plans for patients in the clinic.
Cancer-related inflammation is prevalent in most patients with malignancy, which can promote tumor progression and suppress treatment response (5, 6). Increasing evidence has reported that cancer-related inflammation plays an important role in postoperative recovery and prognosis of cancer patients (7, 8). Therefore, inflammation-based biomarkers are expected to be valuable predictors of surgical and long-term outcomes. For example, as the most common indicators of systemic inflammation, neutrophil (9), platelet (10) and monocyte (11) have been reported as strong indicators for increased postoperative complications, prolongation of hospital stays and poor survival outcomes in several types of malignancies. On the contrary, tumor-infiltrating lymphocyte subsets, such as CD8+ T cells and memory T cells, are associated with better prognosis in various tumors (12, 13).
In recent years, a novel biomarker, the pan-immune-inflammation value (PIV), which integrates peripheral neutrophil, platelet, monocyte and lymphocyte (neutrophil x platelet x monocyte/lymphocyte), has been reported as a promising predictor of long-term outcomes in cancers, because it can precisely reflect the inflammatory and immune status of patients with malignancy (14–17). A recent meta-analysis demonstrated that high PIV before treatment indicates poor prognosis in cancer patients (18). Nevertheless, the role of the PIV in survival outcomes of colorectal cancer remains inconclusive and no meta-analysis is available so far. In addition, emerging studies on the PIV and survival outcomes in colorectal cancer have been reported in recent years. Thus, we performed a systematic review and meta-analysis based on existing evidence to investigate the value of the PIV in long-term survival outcomes in patients with colorectal cancer.
2 Methods
2.1 Search strategy
The current study was performed in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to identify studies that assess the association of PIV with survival outcomes in colorectal cancer patients. Relevant studies from PubMed, Embase and Web of Science were comprehensively examined up to August 20, 2022. Published language was not restricted during the search process. The MeSH term “pan-immune-inflammation value” was used to comprehensively identify potential studies. In addition, the references of the included studies were scanned for additional reports. The search was independently performed by two investigators (XC-Y and H-L).
2.2 Inclusion and exclusion criteria
The inclusion criteria were as follows:
(1) Studies examined the relationship between the PIV and long-term survival of patients with colorectal cancer;
(2) Hazard ratios (HRs) with 95% confidence intervals (CIs) were available;
(3) The cutoff value of the PIV was clearly reported.
The exclusion criteria were as follows:
(1) Studies were reported as case reports, reviews and letters;
(2) Duplicated data.
2.3 Data extraction and quality assessment
Two reviewers (XC-Y and H-L) conducted the data extraction independently and cross-checked all the results. The extracted data included first author, publication year, study interval, country, study design and sample size, selection method, cut-off value, clinicopathological features like age, sex and tumor stage, and survival data.
The quality of included studies were also evaluated following this method described by Lin et al. (19), which contains predefined nine items. A study could get a final score from 0 to 9 after assessment.
2.4 Outcomes
In the present study, the primary outcomes were to investigate the relationship between the PIV and long-term survival in patients with colorectal cancer. Long-term outcomes included OS and PFS. Of note, since disease-free survival (DFS), recurrence-free survival (RFS) and PFS share the similar endpoints, they were analyzed together as one outcome, PFS, as previously suggested (20).
2.5 Statistical analysis
The HRs with their 95% CIs were used as the effect size for OS and PFS. Statistical heterogeneity among enrolled studies was assessed using I2 statistic. All pooled analyses were conducted assuming the random-effects model, which accounts for variance across included studies. Subgroup analysis and sensitivity analysis were utilized to evaluate the credibility of pooled results. Begg’s funnel plot was applied to assess the possibility of publication bias. A two-tailed P value <0.05 was considered statistically significant. All of these statistical analyses were performed by Review Manager Software, version 5.3 (Cochrane, London, UK) and Stata, version 12.0 (Statacorp, College Station, TX).
3 Results
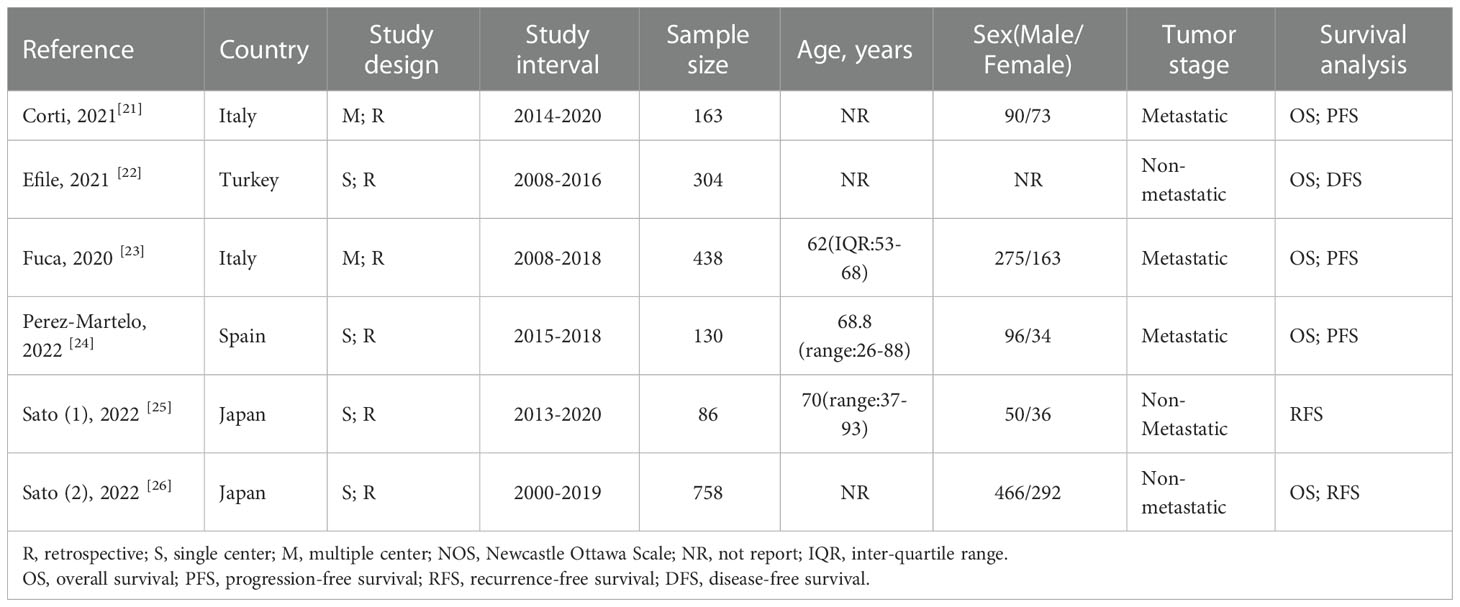
3.1 Study characteristics
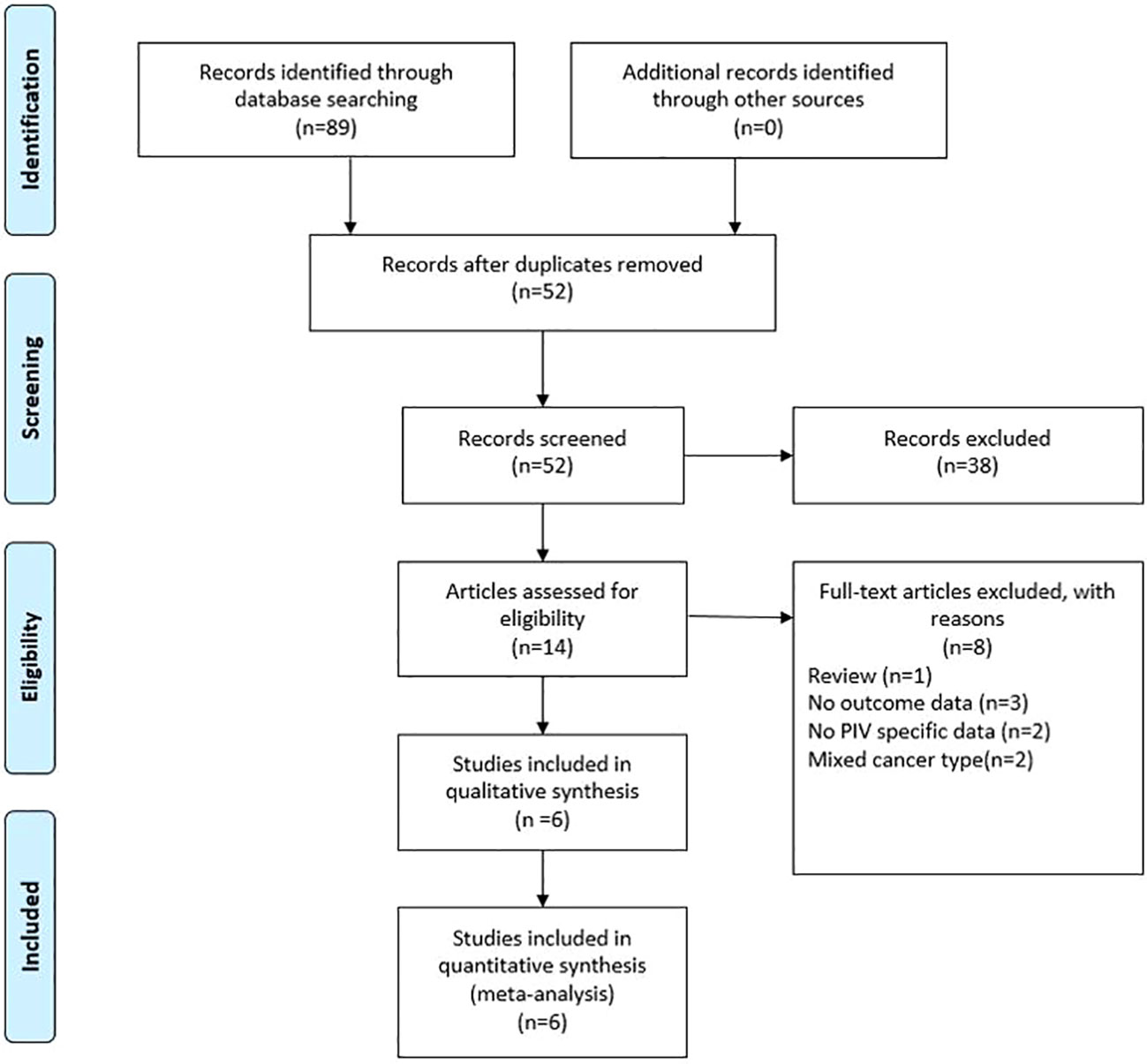
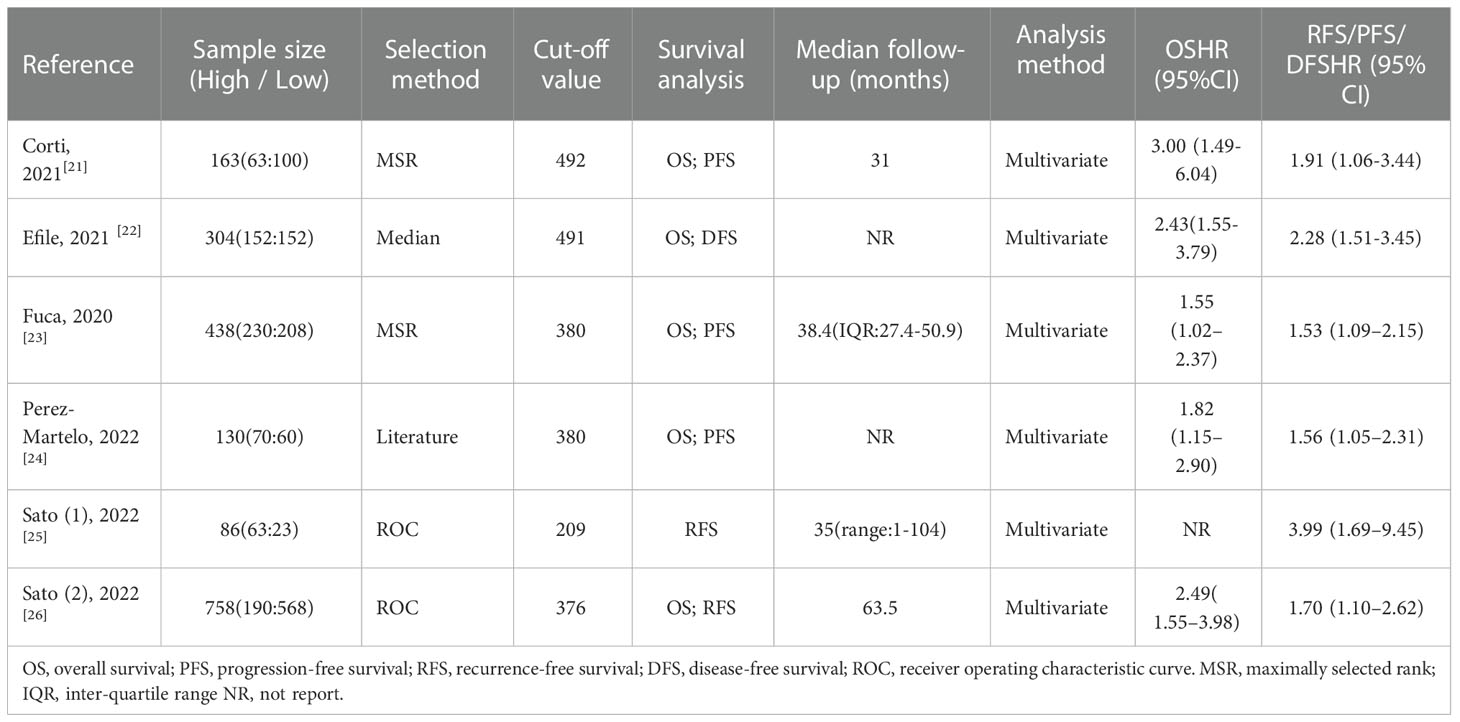
As shown in Figure 1, the search strategy yielded a total of 89 records. After careful title, abstract assessment and full text assessment, 6 studies (21–26) were finally included in the present study. The basic information of the included studies was shown in Tables 1, 2. A total of 1879 patients from Italy (21, 23), Turkey (22), Spain (24) and Japan (25, 26) were included in this study. These studies were published from 2020 to 2022 with a sample size ranging from 86 to 758. Among these studies, two studies (21, 23) were designed as multicenter studies, and another four studies (22, 24–26) were single-center studies. In addition, five (21–24, 26) and six studies (21–26) reported the relationship between baseline PIV and OS and PFS, respectively; and two studies (21, 24) reported the relationship between early PIV increase after treatment initiation and OS and PFS, respectively. Moreover, the cut-off value of the PIV varies a lot among these studies, ranging from 209 to 492. The quality of the included studies was good with a median score of 8 (range: 6-9, Figure 2 and Table S1).
3.2 Relationship between baseline PIV and OS
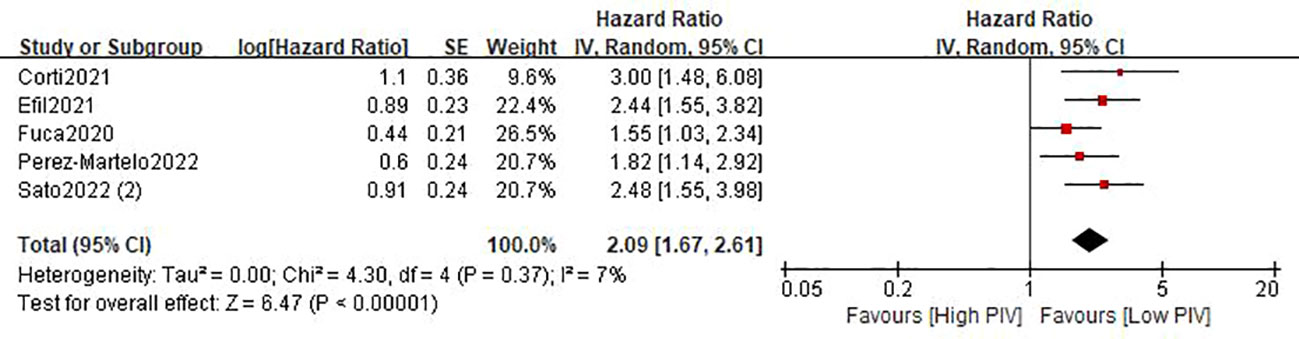
Five studies (21–24, 26) involving 1793 patients described the association between the baseline PIV and OS. The pooled HR was 2.09 (95%CI: 1.67-2.61; P<0.0001; I2 = 7%), which indicated that a high PIV was significantly associated with decreased OS in patients with colorectal cancer (Figure 3 and Table 3). Furthermore, subgroup analyses based on country, study design, sample size, and tumor stage were performed. As shown in Table 3 and Figure S1, the pooled results of all subgroup analyses revealed that patients in the high PIV group had a substantially reduced OS when compared with these in the low PIV group. Additionally, sensitivity analysis by deleting one study at a time showed that the pooled outcome did not substantially change (Figure S3A).
3.3 Relationship between baseline PIV and PFS
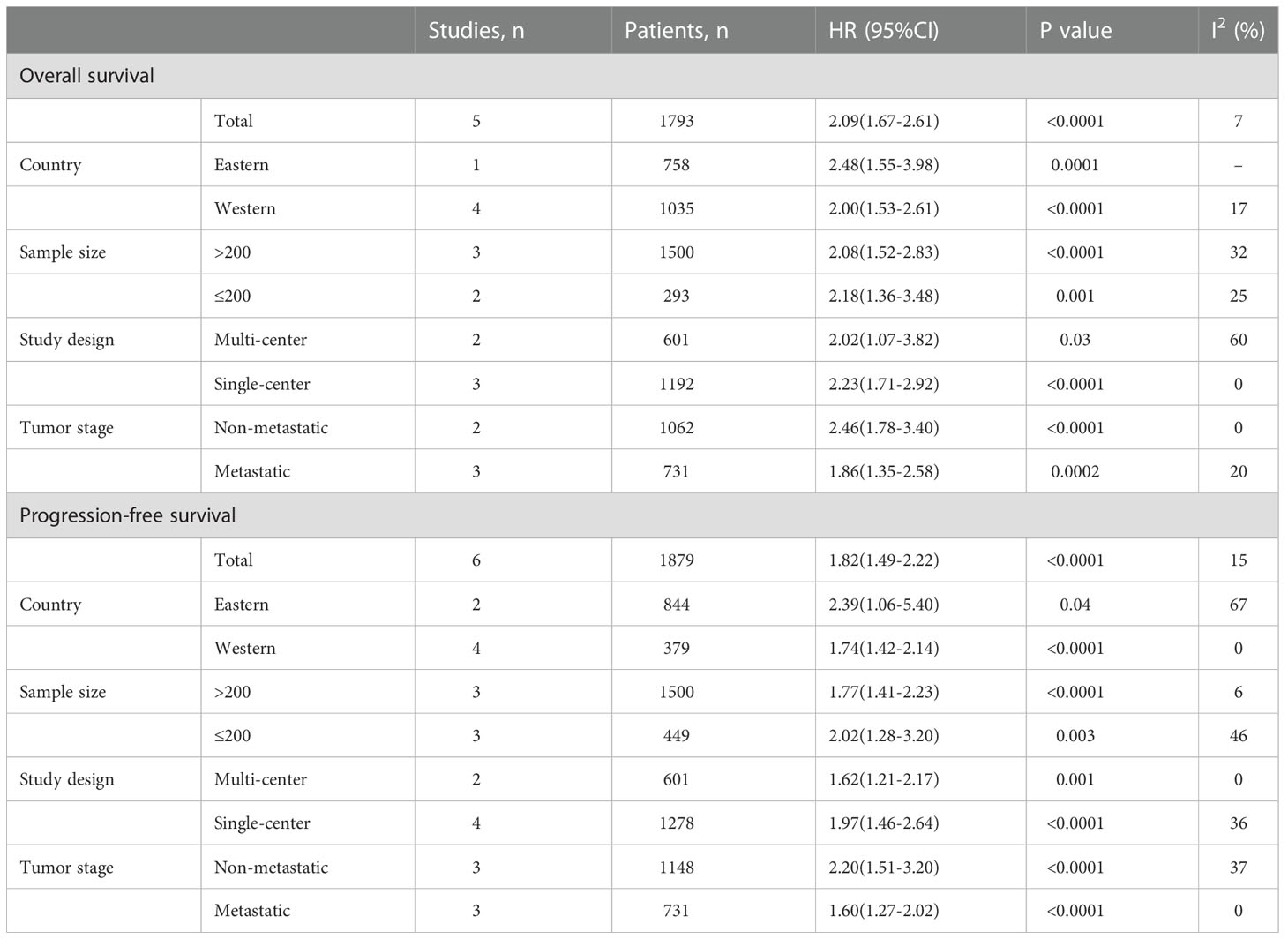
A total of six studies (21–26) involving 1879 patients reported on PFS. The pooled HR was 1.82 (95%CI: 1.49-2.22; P<0.0001; I2 = 15%), which suggested that patients in the high PIV group had a worse PFS when compared with patients in the low PIV group (Figure 4 and Table 3). Similarly, subgroup analyses based on country, study design, sample size, and tumor stage demonstrated that the pooled results remained consistent in each subgroup (Table 3 and Figure S2). Sensitivity analysis showed that the combined effect was not significantly changed (Figure S3B).
3.4 Relationship between early PIV increase and OS/PFS
Only two studies (21, 24) involving 277 cases reported the relationship between early PIV increase after the treatment initiation and survival outcomes. As shown in Figure 5, the combined results suggested that early PIV increase was substantially correlated with decreased OS (HR=1.79; 95%CI: 1.13-2.93; P=0.01; I2 = 26%), and a trend toward poor PFS (HR=2.00; 95%CI: 0.90-4.41; P=0.09; I2 = 70%).
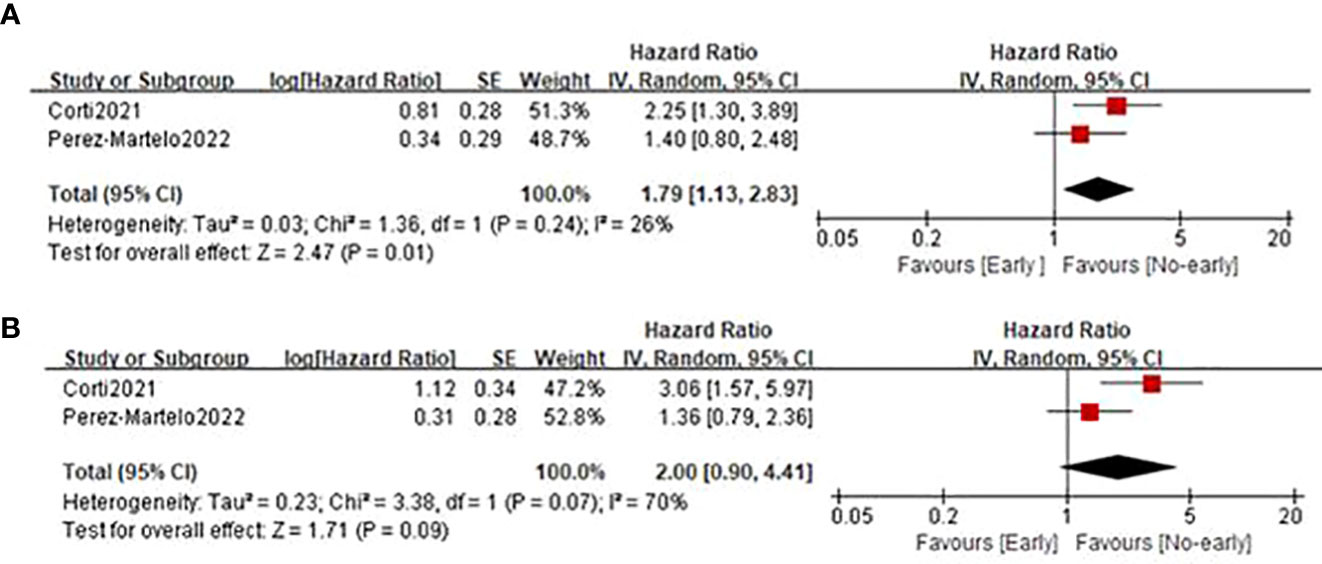
Figure 5 Forest plot assessing the relationship between PIV dynamics and survival outcomes including OS (A) and PFS (B).
3.5 Publication bias
The Begg’s funnel plot was performed to assess the possibility of publication bias. As shown in Figure S4, the funnel plots of OS and PFS were symmetric, and the P values of Begg’s test were 0.130 and 0.060, respectively, indicating that these pooled outcomes were absence of publication bias.
4 Discussion
In 2020, Fuca et al. (23) first developed the PIV based on commonly used peripheral blood count parameters as a systemic inflammation-related prognostic biomarker for metastatic colorectal cancer. Since then, the PIV has been widely used as a cheap, readily available and reliable index to evaluate the prognosis of various cancers (27–29). In the present study, we included six studies with 1879 patients with colorectal cancer and found that high PIV was significantly associated with decreased OS and PFS. Meanwhile, we have further identified that the early PIV increase after the treatment initiation was also associated with significantly poor OS and a trend toward worse PFS in colorectal cancer patients. Therefore, the PIV may have a good discriminatory value and remains an effective inflammatory index for predicting long-term survival outcomes in colorectal cancer.
Systemic inflammatory reflection has been well confirmed to be closely associated with the occurrence and progression of malignancies (5). Increased neutrophils and monocytes in the tumor microenvironment have been reported to induce myeloid-derived suppressor cells, thereby suppressing the host immunity and prompting the tumor growth (30, 31). In addition, monocytes can differentiate into tumor-associated macrophages, which is associated with creating a favorable microenvironment for cancer development (32). Platelets are reported to secrete TGF-β, FGF and VEGF, which contribute to the epithelial–mesenchymal transition and angiogenic process (33, 34). Moreover, the interaction between platelets and tumor cells recruits and activates neutrophils and monocytes, which is required for the formation of distal metastasis sites (34). While lymphocytes, especially cytotoxic T lymphocytes, as the most important cell-mediated anti-tumor immune cells, inhibit tumor cell proliferation and metastasis by inducing the lysis and apoptosis of tumor cells (35, 36). Low lymphocyte counts have been demonstrated to lead to poor prognosis in colorectal cancer patients (37). Reasonably, the PIV, combined with neutrophils, monocytes, lymphocytes, and platelets, may enable better understanding of the functional state of patients and predict the prognosis of patients with colorectal cancer.
In our combined analysis involving 1793 samples, we identified that the baseline PIV is an independent prognostic factor of OS in patients with colorectal cancer. Furthermore, subgroup analyses based on country, study design, sample size and tumor stage showed our results were consistent and robust. Meanwhile, the sensitivity analysis showed that there was no significant change in the correlation between high PIV and decreased OS. Additionally, we have further investigated the relationship between the PIV and PFS. The pooled result including 1879 patients showed that patients in the high PIV group has a substantially decreased PFS. Similarly, the subgroup analyses and sensitivity analysis supported the reliability of this incorporated result. Furthermore, we have also preliminarily explored the relationship between the early PIV increase after the treatment initiation and survival outcomes. The integrated results showed that the early PIV increase was correlated with decreased OS and tended to have a poor PFS. However, given that there were only two studies with small samples included, these results should be interpreted with caution and more studies with big sample size were required to further classify this issue. Based on these results, the PIV may be regarded as an effective prognostic indicator of long-term results of colorectal cancer.
There are some limitations to be noted in the present study. First, all involved studies were retrospective in nature, which may increase the risk of bias, and more prospective studies and randomized controlled trials are required to further investigate this issue. Second, due to the limited number of included studies, the value of the PIV dynamics in survival outcomes needs to be further clarified. Third, the cut-off value of PIV varies greatly among studies, which might affect the clinical utility of these findings. Finally, we were also unable to compare the prognostic predictability of PIV with other biomarkers in colorectal cancer patients, with few data eligible.
5 Conclusions
The findings of the meta-analysis suggested that the PIV is of great significance in predicting long-term survival results in patients with colorectal cancer. However, further research is still required to validate the value of PIV in colorectal malignancy.
Author contributions
X-CY wrote the manuscript. X-CY and HL performed the data search and data analysis. X-CY and HL and D-CL prepared figures and tables. All authors reviewed the manuscript. X-WL and R-HC approved the final manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1036890/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71 (3):209–49. doi: 10.3322/caac.21660
2. Deng Y, Sun Y, Lin Y, Huang Y, Chi P. Clinical implication of the advanced lung cancer inflammation index in patients with right-sided colon cancer after complete mesocolic excision: A propensity score-matched analysis. World J Surg Oncol (2022) 20(1):246. doi: 10.1186/s12957-022-02712-0
3. Ren H, Bösch F, Pretzsch E, Jacob S, Westphalen CB, Holch JW, et al. Identification of an EMT-related gene signature predicting recurrence in stage II/III colorectal cancer - a retrospective study in 1780 patients. Ann Surg (2022) 276(5):897–904. doi: 10.1097/SLA.0000000000005644
4. Gusakov EA, Topchu IA, Mazitova AM, Dorogan LV, Bulatov ER, Serebriiskii IG, et al. Design, synthesis and biological evaluation of 2-quinolyl-1,3-tropolone derivatives as new anti-cancer agents. RSC advances (2021) 11(8):4555–71. doi: 10.1039/d0ra10610k
5. Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: Links to genetic instability. Carcinogenesis (2009) 30(7):1073–81. doi: 10.1093/carcin/bgp127
6. Xia J, Chen Y, Wen S, Du X, Shen B. Peripheral blood inflammation indicators as predictive indicators in Immunotherapy of advanced non-small cell lung cancer. Zhongguo fei ai za zhi = Chin J Lung cancer (2021) 24(9):632–45. doi: 10.3779/j.issn.1009-3419.2021.103.10
7. Jafri SH, Shi R, Mills G. Advance lung cancer inflammation index (ALI) at diagnosis is a prognostic marker in patients with metastatic non-small cell lung cancer (NSCLC): A retrospective review. BMC cancer (2013) 13:158. doi: 10.1186/1471-2407-13-158
8. Watanabe K, Noma D, Masuda H, Masuda M. Preoperative inflammation-based scores predict early recurrence after lung cancer resection. J Thorac disease (2021) 13(5):2812–23. doi: 10.21037/jtd-20-3458
9. Li Q-Q, Lu Z-H, Yang L, Lu M, Zhang XT, Li J, et al. Neutrophil count and the inflammation-based Glasgow prognostic score predict survival in patients with advanced gastric cancer receiving first-line chemotherapy. Asian Pacific J Cancer Prev (2014) 15(2):945–50. doi: 10.7314/APJCP.2014.15.2.945
10. Zhang X, Cui MM, Fu S, Li LL, Liu YS, Liu ZP, et al. Platelet distribution width correlates with prognosis of gastric cancer. Oncotarget (2017) 8(12):20213–9. doi: 10.18632/oncotarget.15561
11. Xu BB, Xu Y, Lu J, Wu Y, Wang JB, Lin JX, et al. Prognostic significance of combined lymphocyte-monocyte ratio and tumor-associated macrophages in gastric cancer patients after radical resection. J Cancer (2020) 11(17):5078–87. doi: 10.7150/jca.44440
12. Lotfinejad P, Asghari Jafarabadi M, Abdoli Shadbad M, Kazemi T, Pashazadeh F, Sandoghchian Shotorbani S, et al. Prognostic role and clinical significance of tumor-infiltrating lymphocyte (TIL) and programmed death ligand 1 (PD-L1) expression in triple-negative breast cancer (TNBC): A systematic review and meta-analysis study. Diagnostics (Basel Switzerland) (2020) 10(9):704. doi: 10.3390/diagnostics10090704.
13. Fornarini G, Rebuzzi SE, Banna GL, Calabrò F, Scandurra G, Giorgi De U, et al. Immune-inflammatory biomarkers as prognostic factors for immunotherapy in pretreated advanced urinary tract cancer patients: An analysis of the Italian SAUL cohort. Esmo Open (2021) 6(3):100118. doi: 10.1016/j.esmoop.2021.100118.
14. Şahin AB, Cubukcu E, Ocak B, Deligonul A, Orhan SO, Tolunay S, et al. Low pan-immune-inflammation-value predicts better chemotherapy response and survival in breast cancer patients treated with neoadjuvant chemotherapy. Sci Rep (2021) 11(1):14662. doi: 10.1038/s41598-021-94184-7
15. Zeng R, Liu F, Fang C, Yang J, Luo LF, Yue P, et al. PIV and PILE score at baseline predict clinical outcome of anti-PD-1/PD-L1 inhibitor combined with chemotherapy in extensive-stage small cell lung cancer patients. Front Immunol (2021) 12:724443. doi: 10.3389/fimmu.2021.724443
16. Chen X, Hong X, Chen G, Xue JH, Huang J, Wang F, et al. The pan-Immune-Inflammation value predicts the survival of patients with anaplastic lymphoma kinase-positive non-small cell lung cancer treated with first-line ALK inhibitor. Trans Oncol (2022) 17:101338–8. doi: 10.1016/j.tranon.2021.101338
17. Lin F, Zhang LP, Xie SY, Huang HY, Chen XY, Jiang TC, et al. Pan-Immune-Inflammation value: A new prognostic index in operative breast cancer. Front Oncol (2022) 12:830138. doi: 10.3389/fonc.2022.830138
18. Guven DC, Sahin TK, Erul E, Kilickap S, Gambichler T, Aksoy S. The association between the pan-Immune-Inflammation value and cancer prognosis: A systematic review and meta-analysis. Cancers (Basel). (2022) 14(11):2675. doi: 10.3390/cancers14112675
19. Lin Y, Liu Z, Qiu Y, Zhang JY, Wu HN, Liang R, et al. Clinical significance of plasma d-dimer and fibrinogen in digestive cancer: A systematic review and meta-analysis. Eur J Surg oncol: J Eur Soc Surg Oncol Br Assoc Surg Oncol (2018) 44(10):1494–503. doi: 10.1016/j.ejso.2018.07.052
20. Papakonstantinou A, Gonzalez NS, Pimentel I, Suñol A, Zamora E, Ortiz C, et al. Prognostic value of ctDNA detection in patients with early breast cancer undergoing neoadjuvant therapy: A systematic review and meta-analysis. Cancer Treat Rev (2022) 104:102362. doi: 10.1016/j.ctrv.2022.102362
21. Corti F, Lonardi S, Intini R, Salati M, Fenocchio E, Belli C, et al. The pan-Immune-Inflammation value in microsatellite instability-high metastatic colorectal cancer patients treated with immune checkpoint inhibitors. Eur J Cancer (2021) 150:155–67. doi: 10.1016/j.ejca.2021.03.043
22. Efil SC, Guner G, Guven DC, Celikten B, Celebiyev E, Taban H, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in combination with systemic inflammatory markers in colon cancer. Ann Oncol (2021) 32(Supplement 5):S561. doi: 10.1016/j.annonc.2021.08.977
23. Fucà G, Guarini V, Antoniotti C, Morano F, Moretto R, Corallo S, et al. The pan-Immune-Inflammation value is a new prognostic biomarker in metastatic colorectal cancer: Results from a pooled-analysis of the Valentino and TRIBE first-line trials. Br J Cancer (2020) 123(3):403–9. doi: 10.1038/s41416-020-0894-7
24. Pérez-Martelo M, González-García A, Vidal-Ínsua Y, Blanco-Freire C, Brozos-Vázquez EM, Abdulkader-Nallib I, et al. Clinical significance of baseline pan-Immune-Inflammation value and its dynamics in metastatic colorectal cancer patients under first-line chemotherapy. Sci Rep (2022) 12(1):6893. doi: 10.1038/s41598-022-10884-8
25. Sato R, Oikawa M, Kakita T, Okada T, Abe T, Tsuchiya H, et al. A decreased preoperative platelet-to-lymphocyte ratio, systemic immune-inflammation index, and pan-immune-inflammation value are associated with the poorer survival of patients with a stent inserted as a bridge to curative surgery for obstructive colorectal cancer. Surg Today (2022). doi: 10.1007/s00595-022-02575-8
26. Sato S, Shimizu T, Ishizuka M, Suda K, Shibuya N, Hachiya H, et al. The preoperative pan-immune-inflammation value is a novel prognostic predictor for with stage I-III colorectal cancer patients undergoing surgery. Surg Today (2022) 52(8):1160–9. doi: 10.1007/s00595-021-02448-6
27. Fucà G, Beninato T, Bini M, Mazzeo L, Guardo LD, Cimminiello C, et al. The pan-Immune-Inflammation value in patients with metastatic melanoma receiving first-line therapy. Targeted Oncol (2021) 16(4):529–36. doi: 10.1007/s11523-021-00819-0
28. Gambichler T, Said S, Abu Rached N, Scheel CH, Susok L, Stranzenbach R, et al. Pan-immune-inflammation value independently predicts disease recurrence in patients with merkel cell carcinoma. J Cancer Res Clin Oncol (2022) 148(11):3183–9. doi: 10.1007/s00432-022-03929-y
29. Susok L, Said S, Reinert D, Mansour R, Scheel CH, Becker JC, et al. The pan-immune-inflammation value and systemic immune-inflammation index in advanced melanoma patients under immunotherapy. J Cancer Res Clin Oncol (2022) 148(11):3103–8. doi: 10.1007/s00432-021-03878-y
30. Yan M, Zheng M, Niu R, Yang XH, Tian SF, Fan LL, et al. Roles of tumor-associated neutrophils in tumor metastasis and its clinical applications. Front Cell Dev Biol (2022) 10:938289. doi: 10.3389/fcell.2022.938289
31. Gneo L, Rizkalla N, Hejmadi R, Mussai F, de Santo C, Middleton G. TGF-β orchestrates the phenotype and function of monocytic myeloid-derived suppressor cells in colorectal cancer. Cancer immunol immunotherapy: CII. (2022) 71(7):1583–96. doi: 10.1007/s00262-021-03081-5
32. Shibutani M, Maeda K, Nagahara H, Fukuoka T, Nakao S, Matsutani S, et al. The peripheral monocyte count is associated with the density of tumor-associated macrophages in the tumor microenvironment of colorectal cancer: a retrospective study. BMC cancer (2017) 17(1):404. doi: 10.1186/s12885-017-3395-1
33. Olsson AK, Cedervall J. The pro-inflammatory role of platelets in cancer. Platelets (2018) 29(6):569–73. doi: 10.1080/09537104.2018.1453059
34. Wan S, Lai Y, Myers RE, Li BS, Hyslop T, London J, et al. Preoperative platelet count associates with survival and distant metastasis in surgically resected colorectal cancer patients. J gastrointestinal cancer (2013) 44(3):293–304. doi: 10.1007/s12029-013-9491-9
35. Shen Y, Guan Y, Hummel JJ, Shyu CR, Mitchem JB. Immunogenomic pathways associated with cytotoxic lymphocyte infiltration and survival in colorectal cancer. BMC cancer (2020) 20(1):124. doi; 10.1186/s12885-020-6513-4
36. Pang H, Zhang W, Liang X, Zhang ZQ, Chen XL, Zhao LY, et al. Prognostic score system using preoperative inflammatory, nutritional and tumor markers to predict prognosis for gastric cancer: A two-center cohort study. Adv Ther (2021) 38(9):4917–34. doi: 10.1007/s12325-021-01870-z
Keywords: colorectal cancer, pan-immune-inflammation value, overall survival (OS), progression-free survival (PFS), meta-analysis
Citation: Yang X-C, Liu H, Liu D-C, Tong C, Liang X-W and Chen R-H (2022) Prognostic value of pan-immune-inflammation value in colorectal cancer patients: A systematic review and meta-analysis. Front. Oncol. 12:1036890. doi: 10.3389/fonc.2022.1036890
Received: 09 September 2022; Accepted: 05 December 2022;
Published: 22 December 2022.
Edited by:
Kanjoormana Aryan Manu, Amala Cancer Research Centre, IndiaReviewed by:
Juha P. Väyrynen, University of Oulu, FinlandEmil Bulatov, Kazan Federal University, Russia
Copyright © 2022 Yang, Liu, Liu, Tong, Liang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xian-Wen Liang, bHh3em5keEAxNjMuY29t; Ri-Hui Chen, YzE5OTBjaGVucmlodWlAMTI2LmNvbQ==
†These authors share first authorship
 Xiao-Chuan Yang1†
Xiao-Chuan Yang1† Xian-Wen Liang
Xian-Wen Liang