
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 21 October 2022
Sec. Neuro-Oncology and Neurosurgical Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.1034944
This article is part of the Research Topic Case Reports in Neuro-Oncology and Neurosurgical Oncology : 2022 View all 32 articles
Background: Extraneural metastasis (ENM) of glioblastoma are rare. However, as patient overall survival improves, the incidence of ENM has gradually increased. Although several risk factors have been proposed, venous sinus invasion was regarded as a very exceptional route for ENM.
Case description: We report a 60-year-old man with glioblastoma in the temporal lobe, invading the transverse and sigmoid venous sinus. After gross total tumor resection, the patient received the standard chemoradiation therapy. Systemic evaluation for persistent shoulder and back pain revealed widespread metastasis to lymph nodes and multiple bones 9 months after surgery. Despite spine radiation therapy, the patient became paraplegic and died 1 year after surgery.
Conclusions: Venous sinus invasion should be kept in mind by physicians, as a risk factor for glioblastoma ENM. Systemic evaluation of these patients with extracranial symptoms should be performed without hesitation.
Glioblastoma is the most common and fatal primary central nervous system (CNS) cancer with highly infiltrative growth. After maximal safe resection, concurrent chemoradiotherapy and adjuvant chemotherapy using temozolomide (TMZ) has been used as standard treatment for initially diagnosed glioblastoma. Despite the improvement of surgical intervention and various treatment strategies, the average survival period is around 15-18 months and 5-year survival rate was reported as 6.8% in the developed country (1).
Unlike most systemic cancers in other organs with high frequency of brain metastasis, the systemic extraneural metastasis (ENM) of glioblastoma was rare (0.4%-0.5% of all cases) possibly due to strong barriers formed by the dura mater and blood-brain barrier (BBB) (1, 2). However, in a systemic review for the published ENM cases, Pietschmann et al. reported a remarkable increase in the number of reported cases per decade over time (3). This increase was possibly related to rising awareness of the ENM along with improvement of imaging diagnosis. Since the majority of ENM developed at the late stage of clinical course with a median interval of 9 months or 2 years from the initial diagnosis (3, 4), the higher chance of ENM was also related to improved local tumor control and prolonged survival of glioblastoma. Additionally, although glioblastoma may be less common in Asian and African countries possibly due to differences in age distribution and diagnostic accessibility (1), the age-adjusted incidence rate of glioblastoma has recently increased in North American and European countries (5, 6). The improvement in diagnostic techniques, lifestyle changes, or environmental factors might be responsible for the increased incidence rate of glioblastoma, leading the increased chance of ENM (7, 8). Therefore, clinician should be aware of the possibility of ENM in glioblastoma and its risk factors to prevent oversight and misdiagnosis.
A 60-year-old man presented with a 2-month history of cognitive impairment. He had hypertension and a history of early gastric cancer surgery 10 years previously. There were no abnormal radiological findings on abdominopelvic or chest computed tomography (CT). Brain magnetic resonance imaging (MRI) showed a large contrast-enhancing (67×48×60 mm) lesion in the left temporal lobe, invading the transverse and sigmoid venous sinuses (Figures 1A–C).

Figure 1 Radiological and pathological images of the initial mass. (A–C) Preoperative Gd-enhanced T1-weighted MRI showing a heterogeneously enhanced mass in the left temporal region with leptomeningeal involvement. Note the lesion invading the transverse and sigmoid sinuses (arrow head). (D, E) Representative microphotos of the primary surgical sample with palisading tumor cell necrosis and GFAP immunopositivity, consistent with a diagnosis of glioblastoma. (F) The removed dural tissue is involved by glioblastoma (D, F: hematoxylin and eosin staining, original magnification ×40, (E): GFAP immunohistochemistry, original magnification ×200).
Suspecting glioma, a craniotomy and gross total tumor resection were performed. At surgery, an infiltrating mass was found invading the dura mater, including a sinus wall component. The pathologic diagnosis was isocitrate dehydrogenase (IDH)-wildtype glioblastoma with O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation. Tumor cell invasion of the dura was confirmed histopathologically (Figures 1D–F). The patient underwent concurrent chemoradiation therapy (60 Gy) and three cycles of adjuvant TMZ. Unfortunately, two remote meningeal enhancing lesions in the left frontal and parietal convexity were found on follow-up MRI 5 months after surgery (Figures 2A, B) and the patient was treated with metronomic TMZ plus radiation therapy (RT, 50 Gy). Since there was no overlap of the radiation fields compared to the initial RT plan, there was little possibility of radiation necrosis (Figures 2C, D). At that time, the patient had intermittent right shoulder and back pain. Chest and shoulder x-rays were performed, but there were no specific radiologic findings.
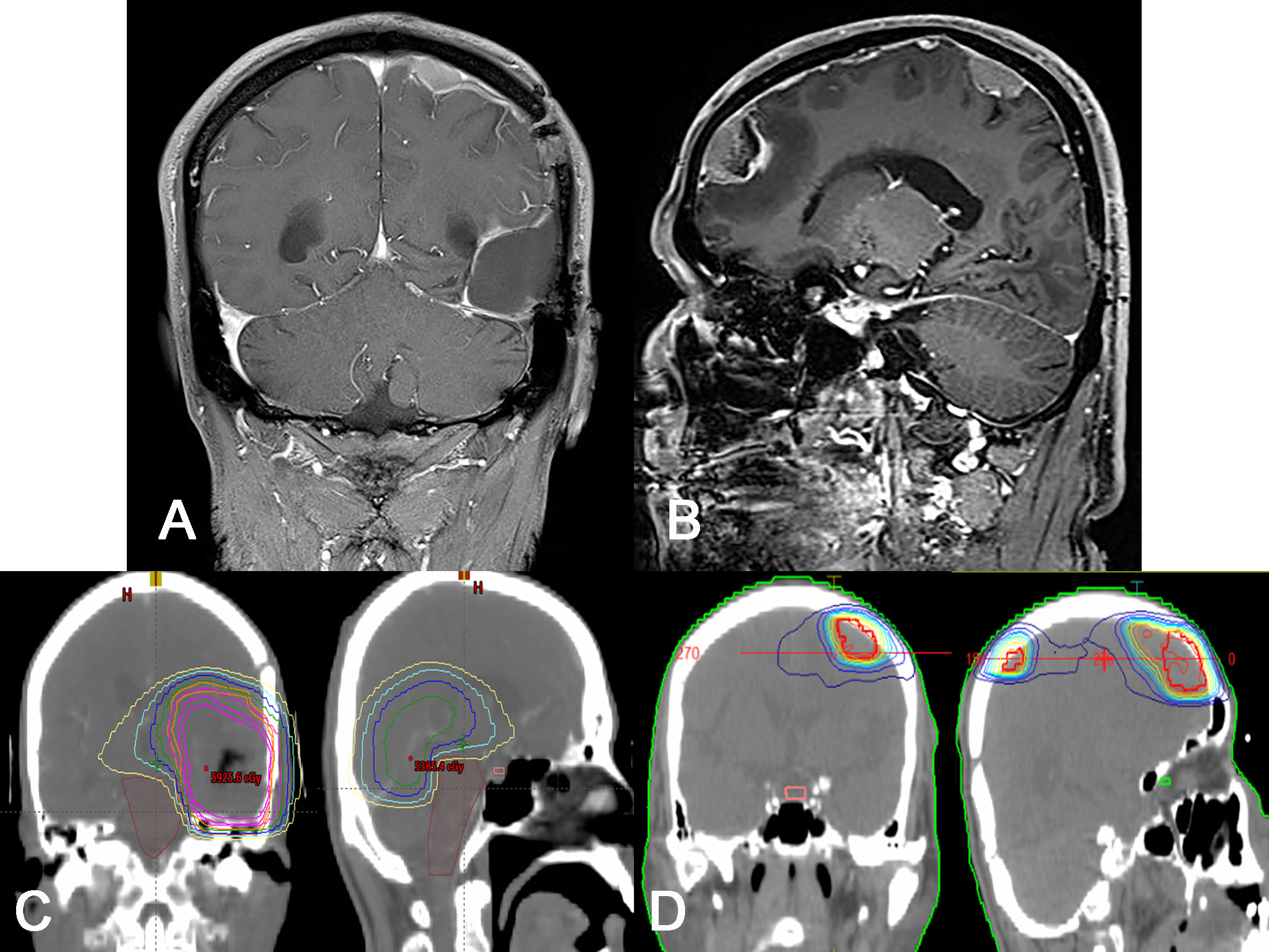
Figure 2 Radiological and treatment plan images for recurred lesions. (A, B) Gd-enhanced T1-weighted MRI showing two enhanced, dural based masses along the dura mater of remote site. Note that there is no definitive recurrence on the initial lesion. (C, D) Representative photos of dose plans for initial radiation (C, 60 Gy) and re-irradiation (D, 50 Gy) treatments. Note that there is no definitive overlap in radiation field in two dose plans.
Due to progressive shoulder and back pain, systemic evaluations including spine MRI, positon emission tomography (PET)-CT, and chest and abdomen CT were done 9 months after surgery and revealed widespread metastases to the chest & cervical lymph nodes and multiple bones, including the vertebrae, scapula, and pelvis (Figure 3). The largest vertebral lesion was biopsied and diagnosed as metastatic glioblastoma as evidenced by glial fibrillary acidic protein (GFAP) immunopositivity (Figure 4). Despite spine RT (24 Gy), the patients became paraplegic, was admitted to palliative care, and died 1 year after surgery.
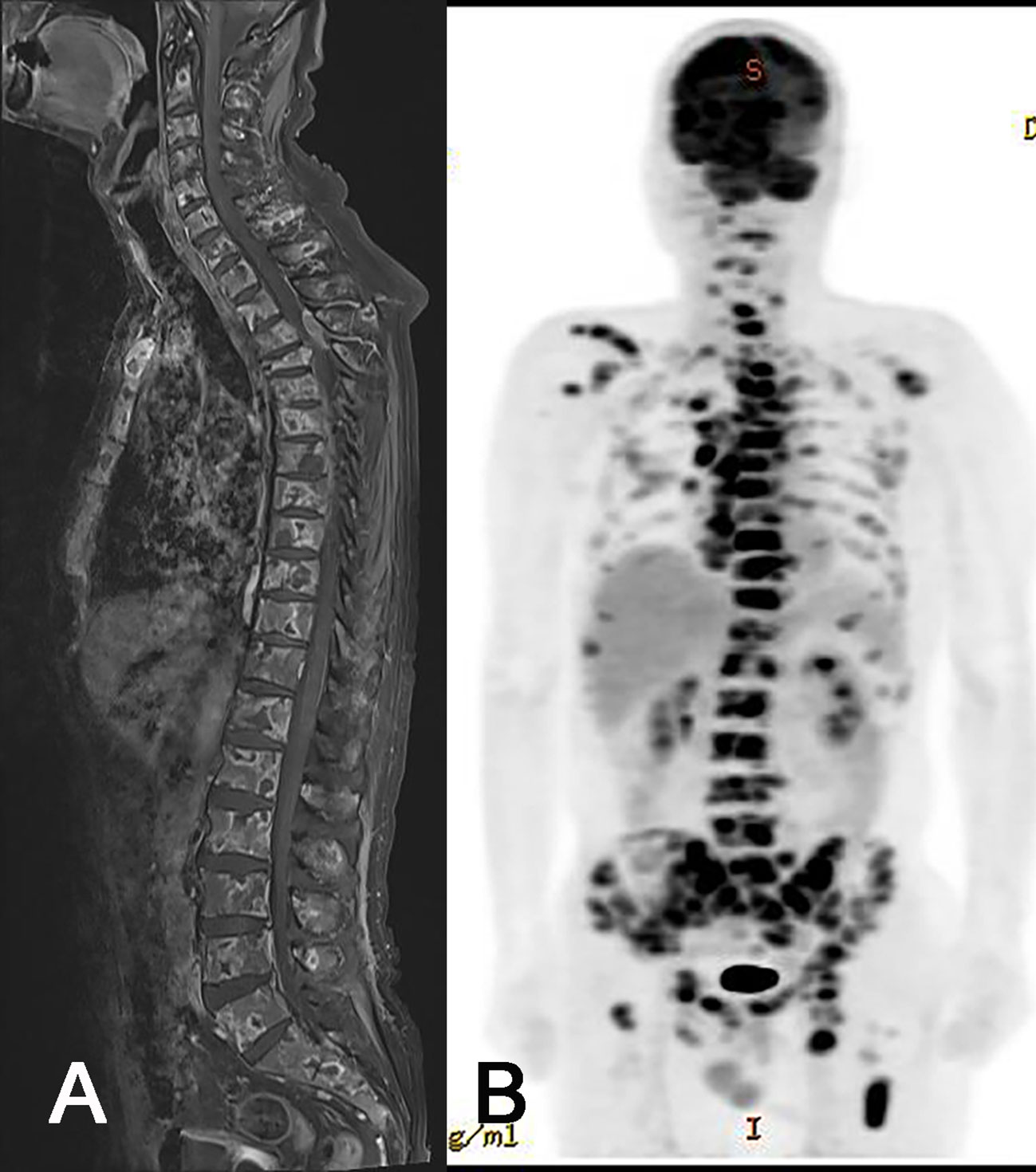
Figure 3 Radiologic images of extraneural metastasis (ENM). Whole spine MRI (A) and PET-CT (B) showing extensive ENM to multiple bones and lymph nodes.
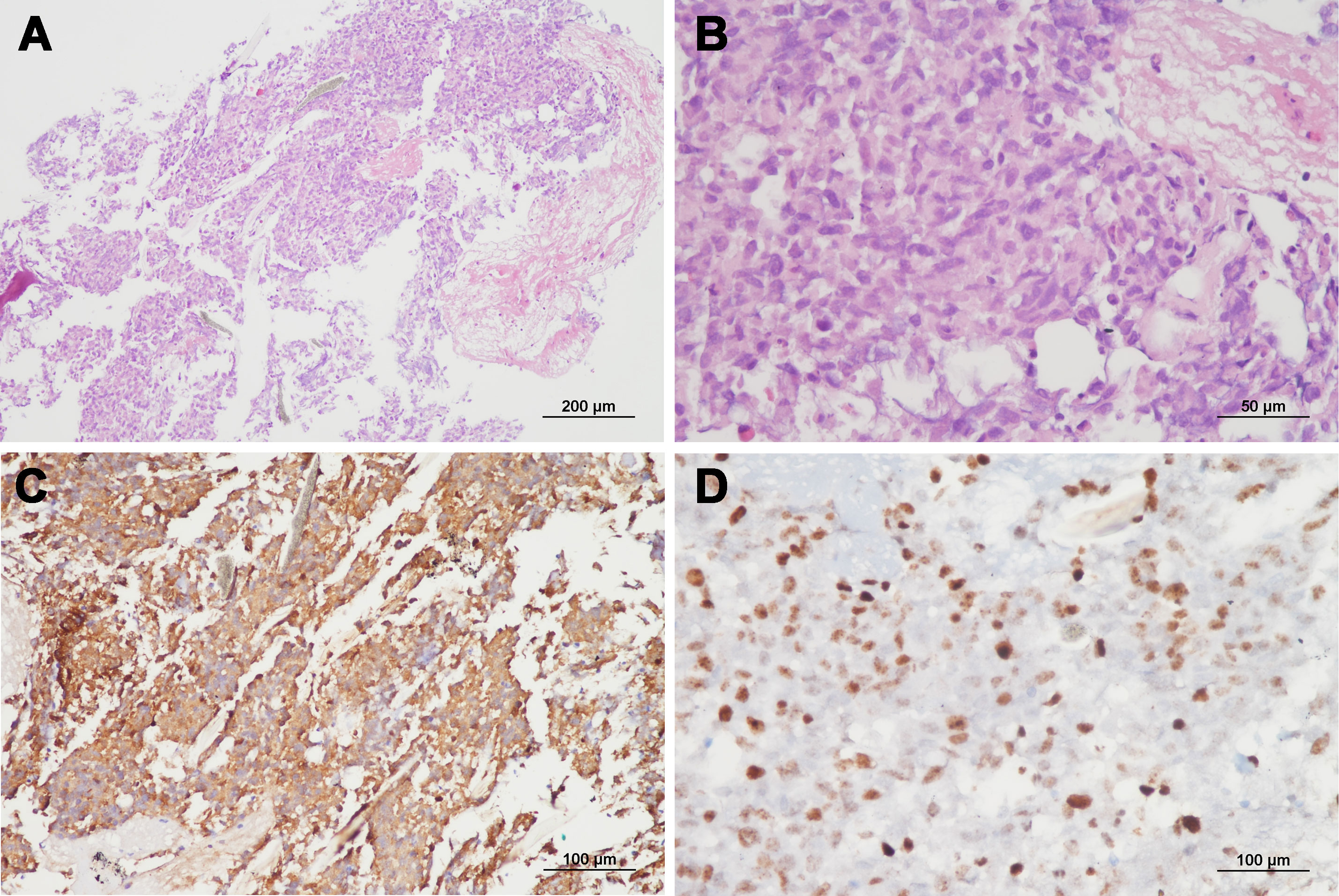
Figure 4 Representative microphotos of a vertebral lesion with GFAP immunopositivity and high Ki-67 proliferation index, consistent with a diagnosis of metastatic glioblastoma. (A, B: hematoxylin and eosin staining, original magnification ×100 & ×400, C: GFAP immunohistochemistry, original magnification ×200, D: Ki-67 staining, original magnification ×400).
Previous studies reported that risk factors for glioblastoma ENM include invasive procedures, RT, young age, prolonged survival time, tumor recurrence, and sarcomatous component (3, 9). Also, ENM occurred much more frequently in males, and the most common tumor location was in the temporal lobe (3, 10). Generally, chemoradiation therapy removes most of the tumors, but can promote the selective evolution of treatment-resistant tumor cell clones, which can result in more aggressive biological behavior. Surgery and RT can also promote ENM by breaking the tight junction of the BBB. As a result, tumor treatment can play a role in helping metastasis to occur more easily. However, about 10% of ENMs occur in patients who have not undergone surgery and there was no significant difference in the circulating tumor cell numbers in patients before or after surgery. Even before treatment, about 20% of glioblastoma patients had circulating tumor cells in the peripheral blood (11). As metastatic precursor cells, circulating tumor cells have more mesenchymal phenotype and stem cell like properties (12). The therapeutic approach may not be an essential factor for metastases and its role in ENM remains to be demonstrated.
Glioblastoma ENM probably have a histopathological and/or genetic predisposition. To develop distant metastases in systemic cancers, epithelial-mesenchymal transition (EMT) can be required. Sarcomatous metaplasia in glioblastoma is an example of EMT. With a sarcomatous phenotype, glioblastoma cells can degrade extracellular matrix proteins, promoting vascular or lymphatic invasion. Some tumor clones have some specific genotypes that predisposes to ENM. In analysis of genetic predisposition, glioblastoma with ENM had a tendency of tumor suppressor gene alteration. Common genomic alteration included TP53, ATRX, PTEN, RB1, TERT, IDH1, and NF1 (13). Among them, TP53 mutation has been most frequently reported mutation (13–16). As p53 plays an important role in DNA repair, TP53 mutation could induce repertory of another genetic mutation that make ENM possible. An aggressive sarcomatous phenotype or other unknown genotype might promote vascular or lymphatic invasion and systemic dissemination of glioblastoma.
Considering common metastatic sites (lung, bone, and lymph node) and wide dissemination feature in ENM, the metastatic route is probably via a vascular or lymphatic pathway (3). In this case, the tumor invaded the venous sinus and surrounding dura mater, and tumor cells were likely shed into the venous blood and circulating blood flow. Since the widespread ENM through the superior sagittal sinus was proposed in postmortem examination for an untreated malignant astrocytoma (17), previous studies showed that venous sinus invasion is a risk factor for metastases in glioblastoma (18, 19, Table 1). In addition, lymphatic vessels in the meninges are a network of narrow channels that run mostly along the sagittal, transverse, and sigmoid venous sinuses. The lymphatic network at the base of the brain is dense and exits the skull along with the cranial nerves (20). In a tumor invading the dura mater around a venous sinus, tumor cells can enter the lymphatic circulation. In this case, the tumor recurrence was along the dura mater without definitive recurrence at the initial site, and there was multiple metastasis at cervical lymph nodes. These findings demonstrate the possibility of ENM along the lymphatic system.
The prognosis of glioblastoma with ENM is dismal. Median overall survival from diagnosis of ENM ranged from 1.5 months to 6 months. Although there was no prognostic significance in age, gender, initial tumor location, or time interval from initial diagnosis of primary tumor to detection of ENM, the presence of sarcomatous component and lung metastasis were possible poor prognostic factors (3, 13, 21). Because of the rarity and unknown pathogenesis of ENM, the treatment strategy for EMN has not been fully established. However, considering that ENM occurs frequently in young patients around the age of 40 (3, 13, 21), it is possible to combine targeted therapy (13, 16) or immunotherapy (22) with conventional treatment strategies (3, 21). Our patient had a glioblastoma with venous sinus invasion, a history of craniotomy and RT, and intracranial tumor recurrence at remote site. Unfortunately, although the patient had multiple risk factors and complained of pain due to ENM, a systemic evaluation was delayed.
Venous sinus invasion should be considered as a possible route for ENM in glioblastoma. In managing patients with such risk factor, clinicians should not hesitate to perform a systemic evaluation when patients have extraneural symptoms.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical review and approval were waived for this study, due to the fact that this is a single case report. Written informed consent was also obtained from the participant for the publication of this manuscript, including any potentially-identifying images or data. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Conceptualization: K-HL & K-SM. Data curation: KA, YK, and K-SM. Analysis and interpretation of data: K-HL & K-SM. Funding acquisition: K-SM. Writing-original draft: KA, YK, and K-SM. Writing-review & editing: K-HL. Manuscript approval: all authors. All authors contributed to the article and approved the submitted version.
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Minist (2020R1C1C1007832 for K-SM, 2022R1A2C1011889 for K-HL). There was no role of the funding bodies in the design of the study, in the collection, analysis, and interpretation of data, or in the writing of the manuscript.
The English in this document has been checked by at least two professional editors, both native speakers of English. For a certificate, please see: (http://www.textcheck.com/certificate/qoCxX6).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
BBB, Blood-brain barrier; CT, Computed tomography; ENM, Extraneural metastasis; MRI, Mmagnetic resonance imaging; TMZ, Temozolomide.
1. Louis DN, Perry A, Aldape KD, Reifenberger G, Capper D, Sarkar C, et al. Glioblastoma, IDH-wildtype. In: Central nervous system tumours. Lyon, France: International Agency of Research on Cancer (2021). p. 59–5. WHO Classification of Tumours Editorial Board.
2. Pasquier B, Pasquier D, N'Golet A, Panh MH, Couderc P. Extraneural metastases of astrocytomas and glioblastomas: clinicopathological study of two cases and review of literature. Cancer (1980) 45(1):112–25. doi: 10.1002/1097-0142(19800101)45:1<112::AID-CNCR2820450121>3.0.CO;2-9
3. Pietschmann S, von Bueren AO, Kerber MJ, Baumert BG, Kortmann RD, Müller K. An individual patient data meta-analysis on characteristics, treatments and outcomes of glioblastoma/ gliosarcoma patients with metastases outside of the central nervous system. PloS One (2015) 10(4):e0121592. doi: 10.1371/journal.pone.0121592
4. Beauchesne P. Extra-neural metastases of malignant gliomas: Myth or reality? Cancers (Basel) (2011) 27(3):461–77. doi: 10.3390/cancers3010461
5. Davis FG, Smith TR, Gittleman HR, Ostrom QT, Kruchko C, Barnholtz-Sloan JS. Glioblastoma incidence rate trends in Canada and the united states compared with England, 1995-2015. Neuro Oncol (2020) 22(2):301–2. doi: 10.1093/neuonc/noz203
6. Ostrom QT, Cioffi G, Gittleman H, Patil N, Waite K, Kruchko C, et al. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the united states in 2012-2016. Neuro Oncol (2019) 21(Suppl 5):v1–v100. doi: 10.1093/neuonc/noz150
7. Volkow ND, Tomasi D, Wang GJ, Vaska P, Fowler JS, Telang F, et al. Effects of cell phone radiofrequency signal exposure on brain glucose metabolism. JAMA (2011) 305(8):808–13. doi: 10.1001/jama.2011.186
8. Philips A, Henshaw DL, Lamburn G, O'Carroll MJ. Brain tumours: Rise in glioblastoma multiforme incidence in England 1995-2015 suggests an adverse environmental or lifestyle factor. J Environ Public Health (2018) 2018:7910754. doi: 10.1155/2018/7910754
9. Beaumont TL, Kupsky WJ, Barger GR, Sloan AE. Gliosarcoma with multiple extracranial metastases: Case report and review of the literature. J Neurooncol (2007) 83(1):39–46. doi: 10.1007/s11060-006-9295-x
10. Cunha MLVD, Maldaun MVC. Metastasis from glioblastoma multiforme: A meta-analysis. Rev Assoc Med Bras (1992) (2019) 65(3):424–33. doi: 10.1590/1806-9282.65.3.424
11. Müller C, Holtschmidt J, Auer M, Heitzer E, Lamszus K, Schulte A, et al. Hematogenous dissemination of glioblastoma multiforme. Sci Transl Med (2014) 6(247):247ra101. doi: 10.1126/scitranslmed.3009095
12. Liu T, Xu H, Huang M, Ma W, Saxena D, Lustig RA, et al. Circulating glioma cells exhibit stem cell-like properties. Cancer Res (2018) 78(23):6632–42. doi: 10.1158/0008-5472.CAN-18-0650
13. Noch EK, Sait SF, Farooq S, Trippett TM, Miller AM. A case series of extraneural metastatic glioblastoma at memorial Sloan Kettering cancer center. Neurooncol Pract (2021) 8(3):325–36. doi: 10.1093/nop/npaa083
14. Rong T, Zou W, Qiu X, Cui W, Zhang D, Wu B, et al. A rare manifestation of a presumed non-osteophilic brain neoplasm: Extensive axial skeletal metastases from glioblastoma with primitive neuronal components. Front Oncol (2021) 11:760697. doi: 10.3389/fonc.2021.760697
15. Mohme M, Maire CL, Schliffke S, Joosse SA, Alawi M, Matschke J, et al. Molecular profiling of an osseous metastasis in glioblastoma during checkpoint inhibition: potential mechanisms of immune escape. Acta Neuropathol Commun (2020) 8(1):28. doi: 10.1186/s40478-020-00906-9
16. Chai M, Shi Q. Extracranial metastasis of glioblastoma with genomic analysis: A case report and review of the literature. Transl Cancer Res (2022) 11(8):2917–25. doi: 10.21037/tcr-22-955
17. Rubinstein LJ. Development of extracranial metastases from a malignant astrocytoma in the absence of previous craniotomy. Case Rep J Neurosurg (1967) 26(5):542–7. doi: 10.3171/jns.1967.26.5.0542
18. Hamilton JD, Rapp M, Schneiderhan T, Sabel M, Hayman A, Scherer A, et al. Glioblastoma multiforme metastasis outside the CNS: Three case reports and possible mechanisms of escape. J Clin Oncol (2014) 32(22):e80–4. doi: 10.1200/JCO.2013.48.7546
19. Ray A, Manjila S, Hdeib AM, Radhakrishnan A, Nock CJ, Cohen ML, et al. Extracranial metastasis of gliobastoma: Three illustrative cases and current review of the molecular pathology and management strategies. Mol Clin Oncol (2015) 3(3):479–86. doi: 10.3892/mco.2015.494
20. Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, et al. Structural and functional features of central nervous system lymphatic vessels. Nature (2015) 523(7560):337–41. doi: 10.1038/nature14432
21. Lun M, Lok E, Gautam S, Wu E, Wong ET. The natural history of extracranial metastasis from glioblastoma multiforme. J Neurooncol (2011) 105(2):261–73. doi: 10.1007/s11060-011-0575-8
Keywords: extracranial, extraneural, glioblastoma, metastasis, venous sinus
Citation: Kim YJ, Ahn KH, Lee K-H and Moon K-S (2022) Case report: Fulminant extraneural metastasis of glioblastoma through venous sinus. Front. Oncol. 12:1034944. doi: 10.3389/fonc.2022.1034944
Received: 02 September 2022; Accepted: 10 October 2022;
Published: 21 October 2022.
Edited by:
Dimitrios N. Kanakis, University of Nicosia, CyprusReviewed by:
Dorte Schou Nørøxe, Copenhagen University Hospital, DenmarkCopyright © 2022 Kim, Ahn, Lee and Moon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kyung-Sub Moon, bW9vbmtzQGNob25uYW0uYWMua3I=; Kyung-Hwa Lee, bWRrYXlsZWVAam51LmFjLmty
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.