- 1Department of Hematology, Capital Medical University Affiliated Beijing Friendship Hospital, Beijing, China
- 2Department of Hematology, The Fifth Medical Center of Chinese PLA General Hospital, Beijing, China
- 3Department of Hematology, Northern Theater General Hospital, Shenyang, China
Primary central nervous system lymphoma (PCNSL) is a highly aggressive brain tumor with poor prognosis if no treatment. The activation of the NF-κB (nuclear factor kappa-B) is the oncogenic hallmark of PCNSL, and it was driven by B cell receptor (BCR) and Toll-like receptor (TLR) signaling pathways. The emergence of Bruton’s tyrosine kinase inhibitors (BTKis) has brought the dawn of life to patients with PCNSL. This review summarizes the management of PCNSL with BTKis and potential molecular mechanisms of BTKi in the treatment of PCNSL. And the review will focus on the clinical applications of BTKi in the treatment of PCNSL including the efficacy and adverse events, the clinical trials currently being carried out, the underlying mechanisms of resistance to BTKi and possible solutions to drug resistance.
Introduction
Primary central nervous system lymphoma (PCNSL) is a rare and highly aggressive non-Hodgkin lymphoma (NHL) with poor prognosis. PCNSL is characterized by specific extra-nodal sites affecting brain and other CNS including spinal cord, eye or cerebrospinal fluid (CSF). It accounts for approximately 4-6% of extra-nodal NHL with a male: female ratio of 1.21:1 (1). PCNSL was recognized as primary diffuse large B-cell lymphoma of the CNS by the World Health Organization (WHO) 2016 classification of hematopoietic and lymphoid tumors (2). The 2022 WHO classification revision arises an entity named as primary large B-cell lymphoma of immune-privileged sites based on their respective anatomical structures including PCNSL, primary vitreoretinal lymphoma (PVRL) and primary testicular lymphoma (PTL) (3). This new entity now combines a group of aggressive B-cell lymphomas which share similar immunophenotypic and molecular features in the immunocompetent older patients with male predominance (4). With the understanding of the pathophysiology of PCNSL, relevant biomarkers from CSF by liquid chromatography/mass spectrometry establish a biological correlation with tumor microenvironment (5). The existence of aberrant somatic hypermutation mutation analyses in CSF showed tumor-associated genes may provide an alternative pathway for PCNSL development (6). The consequences of potential pathogenetic and relevant genomic landscape in PCNSL have been uncovered in recent years (7). Based on this background, we focus on a new potential oral inhibitor of Bruton’s tyrosine kinase (BTK), which has been explored as a novel therapeutic option against CNS lymphomas.
B-cell receptor (BCR)/NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) signaling axis supports the survival and proliferation of malignant B cells (8). BTK is centrally involved in BCR/NF-κB signaling pathway and BTK inhibitors (BTKis) were developed as promising novel agents of B-cell lymphoma (9). BTK contains five domains and all five approved BTKis (ibrutinib, acalabrutinib and zanubrutinib, tirabrutinib and orelabrutinib) target cysteine 481 (C481) domain (10). Ibrutinib is a first-generation BTKi, and it was approved for the treatment of chronic lymphocytic leukemia (CLL), mantle cell lymphoma (MCL), marginal zone lymphoma (MZL), chronic GVHD (graft-versus-host disease) and Waldenström’s macroglobulinemia (WM) by the FDA (U.S. Food and Drug Administration) (11). However, in real world studies, estimated 19-49% of patients discontinued ibrutinib, off-target binding was the most common reason for discontinuation (12). To reduce toxicity and side effects, next-generation BTK inhibitors improve the tolerability and reduce bleeding and cardiac arrhythmia with more selective kinase inhibition. Acalabrutinib and zanubrutinib were approved by FDA in 2017 and 2019, respectively and zanubrutinib was granted conditional approval in 2020 in China, while tirabrutinib was approved only in Japan (13). Unfortunately, BTK resistance limited the long-time use in B-cell malignancies, and the most common acquired resistance caused by BTK C481 point mutation and phospholipase C gamma 2 (PLCG2) mutation (14, 15). To overcome resistance, noncovalent and reversible BTKis including vecabrutinib and LOXO-305 or ARQ-531 were found to be effective despite presence of mutations within C481S or PLCG2 (16, 17). Some studies show that primary resistance to BTK inhibitors is due to epigenetic rather than genetic changes (14). BTK inhibitors have been investigated in the treatment of CNS lymphomas in clinical trials and showed promising therapeutic options (18, 19). Based on impressive responses, National Comprehensive Cancer Network (NCCN) guidelines were updated in 2018 to include BTKi for the management of relapsed/refractory (R/R) PCNSL (20). Tirabrutinib is the first approved BTKi for the treatment of R/R PCNSL in Japan (21, 22).
The present review will focus on potential molecular mechanisms and the applications of BTKis in PCNSL. This review includes five main topics: i) an update on management of PCNSL; ii) the BCR/BTK signaling pathway in PCNSL; iii) the clinical application of BTKi in PCNSL; iv) the mechanism of resistance to BTKi and management; v) the side effects of BTKi.
Overview of PCNSL
Most PCNSLs (over 90%) are histologically classified as DLBCL, followed by rare Burkitt, lymphoblastic T-cell and low-grade lymphomas (23, 24). Imaging choice in brain tumor including magnetic resonance imaging (MRI) or 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) is crucial for diagnosis and surgical planning (25, 26). Noninvasive imaging detect provides prognostic insight and minimal residual disease (MRD) assessment as well (27, 28). Typical brain parenchyma involvement is found in more than 90% of the cases, and most frequent locations are cerebral hemispheres, corpus callosum and deep gray matter (29, 30). leptomeningeal or ocular involvement is detected in 10-20% of patients at diagnosis (31). A systematic review of 1481 patients with PCNSL showed brain biopsy was the preferred method of diagnosis in 95% of patients, and preoperative CSF analysis obviated 7.4% of cases suffering surgery (32).
According to the Hans algorithm, most PCNSLs are classified as non-germinal center B-cell-like (non-GCB) phenotype (78-96%), which could interpret the poor prognosis in PCNSL (33–35). By using immunohistochemistry (IHC), double-expressing lymphomas (DELs) are defined as co-expression of 2 oncogenes (MYC and BCL2) (36). The prognosis of PCNSL patients with DEL was controversial (37, 38). Although they reported that patients with MYC expression or BCL2 expression were significantly associated with poor overall survival (OS) respectively (37, 38). A meta-analysis of the literature showed a significantly high frequency of concurrent mutations with MYD88 L265P and cluster of differentiation 79B (CD79B) in PCNSL (39). MYD88 L265P mutation is most common mutation associated with activated B-cell-like (ABC) subtype DLBCL arising in immune-privileged sites. In mutational spectrum analysis of PVRL using next-generation sequencing (NGS), MYD88 and CD79B mutations were reported in 74% and 55% of patients, respectively. There is a considerable overlap between PCNSL/PVRL and MCD genetic subtype (based on the co-occurrence of MYD88 and CD79B mutations) DLBCL (40). PD-L1 amplifications in PCNSLs were identified and PD-1/PD-L1 inhibitors probably will be a treatment option (41). In Nayyar N et al. study, PD-L1 expression was detected in 30% patients using whole-exome sequencing (WES), which may be a mechanism for immune evasion in PCNSL (42).
With the elucidation of the molecular properties, there has been significant progress in the management of PCNSL during the last two decades. There is no standard induction or consolidation treatment for PCNSL patients due to the paucity of phase 3 randomized clinical study. The optimal treatment strategy for PCNSL includes induction, consolidation and/or and maintenance therapy (43). Age and performance status (PS) should be considered in the choice of treatment and are prognostic factors as well (44). High-dose methotrexate (HD-MTX)-based chemotherapy is the standard initial induction treatment (45, 46). Polychemotherapy regimens including HD-MTX plus cytarabine/thiotepa/temozolomide provided improved treatment outcomes compared to MTX monotherapy (47, 48). The progression-free (PFS) and OS were significantly better in patients undergoing upfront autologous stem cell transplant (ASCT) as consolidation strategy, which might be especially beneficial for high-risk PCNSL patient (49). Whole brain radiation therapy (WBRT) followed by HD-MTX only achieved the PFS benefit, but is associated with long-term neurotoxicity (50, 51). Unfortunately, relapse rate is high particularly in elderly or frail high-risk PCNSL patient within the first two years after diagnosis (50). Maintenance therapy is also being explored in clinical trials in elderly or relapsed patients (52). Maintenance group with HD-MTX every three months improved OS compared with HD-MTX followed by WBRT (53). Lenalidomide maintenance delays WBRT in relapsed PCNSL (54).
Although improved treatment response has been achieved in the PCNSL patients, but the management of R/R PCNSL remains difficult. The small-molecule targeted agents have been investigated with identification of the molecular properties in many R/R PCNSL. Immunomodulatory imide drugs (IMiDs) have been investigated in PCNSL due to dose-dependent CSF penetration of IMiDs (55, 56). Inhibitors for the phosphatidylinositol-3 kinase (PI3K)/AKT/mTOR pathway had synergistic effects with BTK inhibitor in a preclinical study (57). Immune checkpoint inhibitors (ICIs) could be possible therapeutic options. Pembrolizumab was shown encouraging outcomes achieving CR in three of five patients, and PFS >13 months (58). Anti-CD19 Chimeric antigen receptor T (CAR-T) cells yielded significant potential and treatment responses in R/R PCNSL (59, 60). With the development of targeted therapies, emerging data demonstrate BTK inhibitors are very promising treatment strategy targeted BCR/BTK signaling pathway (61, 62).
BCR/BTK signaling pathway in PCNSL
PCNSL is characterized by aberrant activation of BCR/NF-κB and Toll-like receptors (TLR)/NF-κB signaling pathways (40). These two signaling axises have been identified as central signaling pathways in PCNSL and potential targets for molecular therapy (63). Besides these, immune escape, PI3K/AKT/mTOR and JAK/STAT signaling pathways are important mechanisms in R/R PCNSL, which could be the targets of BTKi resistance treatment. MYD88 mutation plays a crucial role in TLR signaling pathway activation, which could activate NF-κB via interleukin-1 receptor-activated kinase 4 (IRAK4) (64). BCR signaling pathway is activated via BTK phosphorylation after binding of the antigen to the extracellular domain of CD79B (65, 66). The activation of both pathways could lead to increased NF-κB signaling. Caspase recruitment domain family member 11 (CARD11) is a downstream member of BCR signaling pathway. CARD11 mutation may contribute to NF-κB and associate with the resistance to single-agent ibrutinib (42). BTKi targeting BCR/NF-κB pathway has led to breakthrough treatment in PCNSL. Targeting PI3K may downregulate upstream pathway. Proteosome inhibitors and IMiDs may prevent release of NF-κB function, and affect downstream pathway. Anti-CD79B CAR-T cells and anti-CD79B antibody-drug conjugates (ADCs) have been investigated as the new therapeutic approaches targeting CD79B (67, 68).
BCR/NF-κB and TLR/NF-κB pathways gene mutations could be identified by different sequencing projects in PCNSL, and also have association with clinical characteristics. CD79B and MYD88 mutations are more frequently observed in PCNSL. Two previous studies involving a large number of PCNSL patients reported mutations in CD79B (83%) and MYD88 (79%) in 71 patients, and mutations in CD79B (41%) and MYD88 (58%) in 177 patients, respectively (69, 70). MYD88 mutation has been found in 38-79% of PCNSL patients, and CD79B mutation was reported in 30-83% of PCNSL patients (65, 69–75). Overall, BCR/NF-κB and/or TLR/NF-κB signaling pathways were altered in >90% of PNCSL patients (71). CARD11 mutation was found in about 11-30% of cases of PCNSL (70, 71). Genetic alterations in PCNSL suggested similar pathogenesis in immune-privileged sites including PTL and PVRL (40, 41, 72).
BTK inhibitors in the treatment of PCNSL
Ibrutinib in the treatment of R/R PCNSL
In 2017, ibrutinib is used for the first time in the treatment of 18 patients with R/R PCNSL (76). The dose of ibrutinib was 560-840 mg, and it was treated with DA-TEDDi-R (rituximab, liposomal doxorubicin, temozolomide, etoposide and dexamethasone). Efficacy above complete response unconfirmed (CRu) was achieved in 12 patients, and eight patients remained in remission until 15.5 months. The median PFS was 15.3 months, and median OS was not reached (76). In the same year, a phase I study of ibrutinib monotherapy (560-840mg) for the treatment of 13 patients with R/R PCNSL (70). Three patients were refractory to prior treatments including HD- MTX-based chemotherapy and radiotherapy. Clinical responses were achieved in 10 patients, with 5 patients in complete response (CR) and 5 patients in partial response (PR). The median follow-up time was 479 days, and the median PFS and OS was 4.6 months and 15 months, respectively (70). One year later, the same research team reported a phase II study of ibrutinib monotherapy (560-840mg) for the treatment of 29 patients with R/R PCNSL (77). The median follow-up time was 22 months, and the overall response rate (ORR) was 81%, the median PFS and OS was 4 months and 19.5 months, respectively (77). Considering clinical responses of ibrutinib monotherapy are often transient or incomplete, the research team conducted three phase I trials of ibrutinib combined with chemotherapy for the treatment R/R PCNSL. One is rituximab and HD-MTX in combination with ibrutinib (560-840mg) in 9 patients, the ORR was 89%, while 50% of patients had no disease progression after 19.7 months of follow-up (78). The second trial is the ibrutinib (560mg) combined with pan-PI3K inhibitor copanlisib in 6 patients. After a median follow-up of 180 days, the ORR was 67%, including 1 CR, 3 PR, 1 SD (stable disease) and 1 PD (progressive disease) as best response (79). The third trial is designed as ibrutinib (560-840mg) in combination with lenalidomide and rituximab for the treatment of 15 patients with R/R PCNSL or secondary CNSL (SCNSL). 11 patients achieved clinical response, including 4 CR, 7 PR, 2 SD and 1 PD, and while 50% of patients were disease free at 3.03 months after 6.9 months of follow-up (80).
In the same time, the lymphoma study association (LYSA) and the French oculo-cerebral lymphoma (LOC) network reported a phase II trial of 44 patients with R/R PCNSL or PVRL treated with ibrutinib (560mg) monotherapy (18). 27 patients obtained disease control after 2 months of treatment, including 10 CR, 17 PR and 5 SD. The median follow-up time was 25.7 months, and the median PFS was 4.8 months, while 50% of patients survived at 25.7 months (18). Subsequently, a phase I trial reported ibrutinib was combined with temozolomide, etoposide, liposomal doxorubicin, dexamethasone and rituximab (TEDDI-R) in 13 patients with R/R PCNSL. 92% patients responded after receiving only 1 cycle in evaluable 12 patients. 8 patients achieved CR after at least 4 cycles and the remaining 4 patients were continuing treatment. After a median following-up of 5.2 months, the 1-year PFS and OS estimated was 60.0% and 100%, respectively (81). And a retrospective study of 5 patients with R/R PCNSL treated with ibrutinib monotherapy or ibrutinib-based chemotherapy (82). Although 4 patients underwent ASCT, the ORR was 80% and 2 patients received ibrutinib maintenance after achieving CR (82). In another two retrospective studies, patients were treated with ibrutinib (560mg) in combination with chemotherapy. Although the ORR was more than 80%, the median PFS was only 6 months (83, 84). Then in a prospectively registered 14 patients with R/R PCNSL, 11/14 patients were refractory to first line treatment, 5/14 patients had previously received ASCT. All 14 patients received the regimen of ibrutinib, rituximab and lenalidomide (IR2), and achieved 4 CR, 4 PR, 3 SD, 3 PD (85). In another prospective data from phase I/II clinical trials, 33 patients with R/R PCNSL (n=9) or SCNSL (n=24) received ibrutinib monotherapy or ibrutinib-based chemotherapy. Four of 9 patients achieved CR and the median PFS and OS were both 3.1 months (86). Based on a single center experience of single-agent ibrutinib for the treatment of R/R PCNSL, ibrutinib could be an effective bridge-to-transplant treatment. Two of 3 patients achieved CR after receiving ibrutinib, and were eligible for ASCT (87). The published studies with ibrutinib monotherapy and combination treatment for R/R PCNSL were summarized in Table 1.
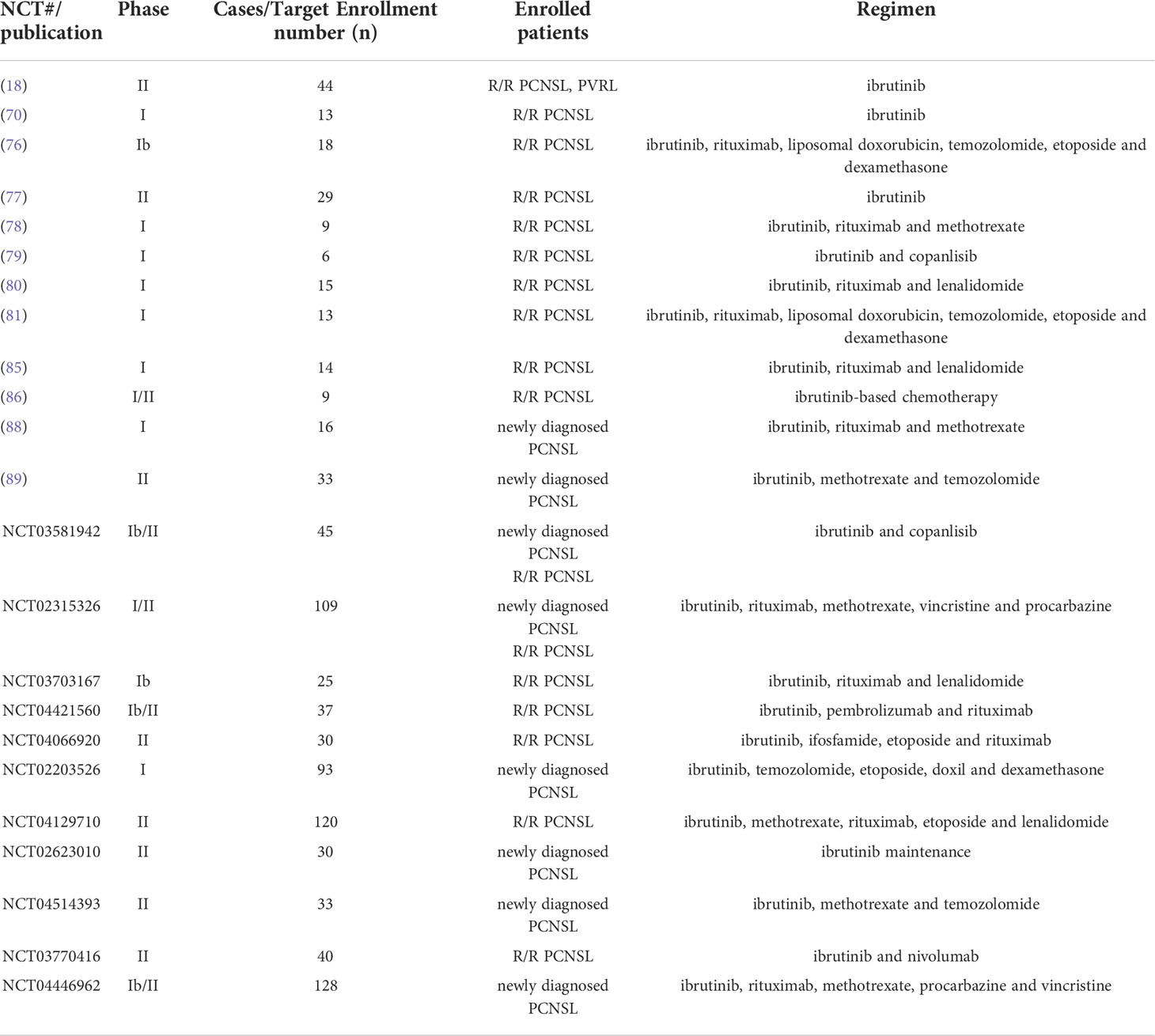
Table 1 Summary of published and ongoing ibrutinib monotherapy and combination treatments for R/R PCNSL or newly diagnosed PCNSL.
Ibrutinib in the treatment of newly diagnosed PCNSL
A retrospective analysis reported ibrutinib in combination with MTX for the treatment of 11 patients with newly diagnosed PCNSL. 9 patients achieved response, including 7 CR and 2 PR. After a follow-up of 11.6 months, the median PFS was 7.4 months while the median OS was not reached (90). A phase I study investigated the combination of ibrutinib, rituximab and HD-MTX (I-RM) for the treatment of 16 newly diagnosed PCNSL patients. 15 patients achieved response, including 8 CR, 7 PR and 1 PD. The median PFS and OS were not achieved after a median follow-up of 24.5 months (88). A phase II study explored the ibrutinib in combination with HD-MTX and temozolomide in 33 newly diagnosed PCNSL patients. 9 patients were enrolled until April 2021. At a median follow-up of 7 months, the 1-year PFS and 1-year OS were 88.9% and 100%, respectively (89). Another phase II trial evaluated ibrutinib maintenance therapy in elderly newly diagnosed PCNSL patients after achieving PR or CR. Four patients with PR improved to CR after maintenance therapy. At median follow-up of 29 months, the 2-year PFS and 2-year OS were 72.6% ± 10.6% and 89% ± 7.5%, respectively (91). With the continuous encouraging study reports, there are still ongoing clinical trials for the treatment of PCNSL with ibrutinib (Table 1).
Zanubrutinib in the treatment of PCNSL
Zanubrutinib is a second-generation BTKi. The first retrospective study evaluated the efficacy of zanubrutinib-containing regimens in 4 patients with newly diagnosed and 4 patients with R/R PCNSL. All newly diagnosed PCNSL patients achieved CR and 75% of R/R PCNSL patients achieved CR (92). Another retrospective study reported 3 newly diagnosed and 8 relapsed patients with PVRL treated with zanubrutinib monotherapy. At a median follow-up of 7.5 months, 4 patients had disease progression and 7 patients achieved durable CR (93).
Acalabrutinib in the treatment of PCNSL
Acalabrutinib is another second-generation BTK inhibitor. Although there are no data about acalabrutinib in PCNSL, there are four ongoing studies. One (NCT04688151) is a phase I trial of dose escalation of acalabrutinib combined with durvalumab in R/R PCNSL or SCNSL. The second (NCT04462328) is a phase Ib trial of acalabrutinib, rituximab and durvalumab in R/R PCNSL. The other two ongoing trials (NCT04906902, NCT04548648) involve single-agent acalabrutinib in R/R PCNSL.
Orelabrutinib in the treatment of PCNSL
Orelabrutinib is second-generation irreversible BTKi with high CSF/plasma ratio (94). A retrospective analysis evaluated the efficacy and safety of orelabrutinib-based chemotherapy in 15 patients with R/R PCNSL. 86.7% patients achieved treatment response and the CR rate was 73.3% (95). Another retrospective analysis of orelabrutinib-based regimen for the treatment of PCNSL (newly diagnosed or R/R). 4/4 patients with newly diagnosed PCNSL achieved response, and were in remission until 6 months. 60% (9/15) patients with R/R PCNSL achieved response, and the 6-month PFS and OS rate were 67.7% and 70%, respectively (96).
In a phase II trial of orelabrutinib in combination with anti-PD-1 monoclonal antibody in R/R PCNSL patients, 61.5% patients achieved response, 1-year PFS rate estimated was 66.7% (97). In addition, there are three ongoing studies, one (NCT04961515) is a phase Ib/II study of orelabrutinib combined with sintilimab in R/R PCNSL, another (NCT05021770) is a phase Ib/II study of orelabrutinib combined with thiotepa in R/R PCNSL, the third (NCT05209620) is a single arm phase II study to explore the combination of orelabrutinib and pemetrexed for the treatment R/R PCNSL.
Tirabrutinib in the treatment of PCNSL
Tirabrutinib is a second-generation BTK inhibitor. In March 2020, tirabrutinib was approved for the treatment R/R PCNSL patients. Takeshi et al. reported a patient had acute progression of PCNSL after tirabrutinib treatment and he was recovered from coma with 3 days methylprednisolone treatment. MRI lesion in the right thalamus disappeared after the treatment of 3 months (98). Then a phase I/II study reported tirabrutinib in 44 patients with R/R PCNSL. 64% patients achieved response and median PFS was 2.9 months, while median OS was not reached (99). Noriharu et al. reported a case of relapsed PCNSL treated with tirabrutinib. The brain lesion was ameliorated after four-week tirabrutinib treatment. The patient achieved CR after ten- week treatment (100). Subsequently, Hiroko et al. reported a PVRL patient refractory to HD-MTX-based chemotherapy and whole-brain radiotherapy. Tirabrutinib was selected as salvage treatment for this patient. Surprisingly, after two weeks, MRI showed total tumor removal and complete disappearance of the peritumoral signal abnormality (101).
BTK inhibitors resistance and management
Although the efficacy of BTKi in the treatment of PCNSL is promising, for ibrutinib, about 1/3 of patients develop primary drug resistance, while a large proportion of patients develop acquired drug resistance (102). CARD11 mutation is a known primary ibrutinib resistance mechanism in PCNSL (70). Resistant mutation is reported in CLL and MCL including C481S and R665W mutations in BTK and PLCG2 mutation in the downstream of BTK. Drug resistance is also evolved by amplification of alternative signaling pathways, cancer stem cells (CSCs) and extrinsic tumor microenvironment (TME). Mechanisms of ibrutinib resistance could be as follows (Figure 1).
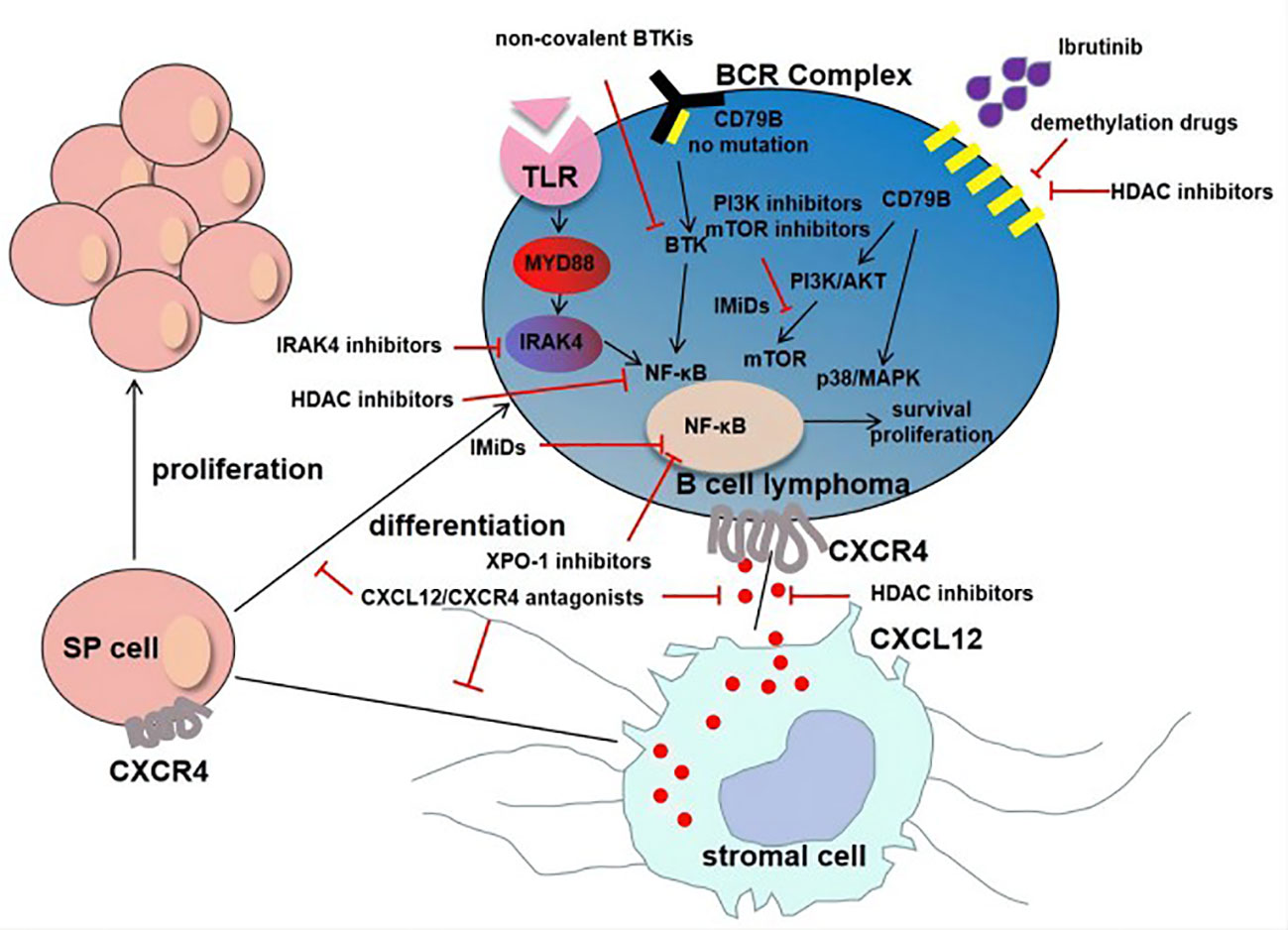
Figure 1 Mechanisms of ibrutinib resistance in PCNSL. For tumor cells without CD79B mutation, ibrutinib resistance was mainly due to MYD88-dependent survival signaling pathway (MYD88→IRAK4→NF-κB). Another important mechanism of resistance is adaptive activation of alternative signaling pathways involving CD79B overexpression and the activation of PI3K/Akt/mTOR pathway and p38/MAPK. The interaction of CXCL12 secreted by stromal cells and CXCR4 secreted by tumor cells promotes tumor growth and resistance. In addition, the existence of SP cells and extrinsic tumor microenvironment are also the mechanisms of drug resistance.
Genetic mechanisms and BTKi resistance
Mutations in BTK, PLCG2 and CARD11 have been found in MCL, WM and CLL during ibrutinib therapy, and these are key genetic mechanisms. Tumors without CD79B mutation revealed that MYD88-dependent survival signaling was the main signaling for these tumors in PCNSL (103). A subsequent study revealed that there was a high frequency mutation of KLHL14 in ABC-DLBCL, which promoted assemblage of the MYD88-TLR9-BCR complex, eventually induced resistance to ibrutinib (104).
Non-genetic adaptive mechanisms of BTKi resistance
Previous studies have shown that amplification of alternative signaling pathways is closely related to acquired drug resistance. The study of Kim et al. revealed that ibrutinib- resistance ABC-DLBCL cell lines could be generated through co-culture with ibrutinib (105). Moreover, transcriptomic analysis revealed the CD79B overexpression and the activation of PI3K/Akt/mTOR pathway and p38/MAPK in ibrutinib-resistance cell lines (105). BCL2, MYC and XPO1 overexpression may rescue the absence of BCR activity and lead to B cell survival despite BTK inhibition (106).
TME and BTKi resistance
TME is a special environment for tumor cells to survive, containing a variety of cells (fibroblasts, endothelial cells, immune cells, etc.) and biochemical factors (cytokines, growth factors, chemokines, etc.) system. Mesenchymal stromal cells (MSCs) are multipotent and major constituent of stromal niches of TME in various tissues and organs. The interaction of tumor cells and the TME mediates the development of drug resistance. Ibrutinib resistance involving TME has been reported in CLL and MCL (107–109). CXCR4 is overexpressed on Tregs and CXCR4 antagonists favor T effector access to TME. Targeting CXCR4 and PD-1 synergistically reduced cell growth in murine tumor models (110).
Side population (SP) cells and BTKi resistance
CSCs exhibit stem cell function, including the ability of self-replication and multiple cell differentiation. Previous studies have identified SP cells in various malignancies, which are CSC-like cells and resistant to radiation and chemotherapy. SP cells could be detected in Hodgkin lymphoma, DLBCL and follicular lymphoma (FL) cell lines (111, 112). There was an interaction between SP cells and TME through CXCL12/CXCR4 signaling, which may allow SP cells to evade chemotherapy and targeted therapy (112). CXCL12/CXCR4 antagonist could be an attractive approach to target SP cells (112).
Management of BTK inhibitor resistance
Many studies have been performed to explore how to overcome resistance to BTKi in hematological malignancies. Our question arising from this section is whether the new investigational agents penetrate the CNS blood‐brain barrier (BBB). The outcomes and clinical benefits are investigated in patients receiving novel targeted therapeutic approaches and immune checkpoint inhibitors. Optimizing combination of active therapies could be a challenge in the management of BTki resistance.
PI3K/AKT/mTOR inhibitors
An acquired ibrutinib resistance could be the activation of alternative pathways independent of BCR. Then Kapoor et al. identified that ibrutinib resistance can be overcome by PI3K isoform inhibitors and the mechanism was targeting the activation of PI3K/AKT/mTOR signaling pathway (113, 114). CARD11, CD79A/B, TNFAIP3, and MYD88 are BCR signaling components, mutation of any of them would drive alternative signaling pathway activation. PI3K isoform inhibitors shrank the tumor by targeting the PI3K pathway in ibrutinib-resistant ABC-DLBCL in vivo models (115). GDC-0084, a small molecule inhibitor of PI3K and mTOR, was detected that its total and free drug of brain tumor tissue/plasma ratio was>1 and>0.5, respectively, which suggested that it can cross BBB (116). Temsirolimus is a mTOR inhibitor which was used in the treatment of 37 patients with R/R PCNSL and achieved response in 20 patients including 5 CR, 3 CRu and 12 PR (117).
Reversing the reduced BTK expression
Jain et al. observed the total BTK expression of ABC-DLBCL lines was significantly reduced after chronically exposure to ibrutinib (114). Down-regulation or loss of drug-target expression is another way of acquired drug resistance of tumor cells. The possible mechanisms are the selective reduction of BTK expressing cells and the epigenetic changes due to long-term exposure to ibrutinib (118). Therefore, trying to cooperate with demethylation drugs or deacetylase inhibitors may increase the expression of BTK.
BCL2 inhibitors
Jain et al. reported that BCL2 expression could be upregulated in resistant cells under chronic ibrutinib exposure (114). Kuo et al. also have confirmed that synergistic anti-proliferative activity of ibrutinib and BCL2 inhibitor venetoclax in resistant ABC-DLBCL lines (119). It is believed that the venetoclax with the molecular weight of 868.44 may not cross BBB, but Ahmed et al. have confirmed that it can pass through the BBB in 66 samples of relapsed or refractory acute myeloid leukemia or acute lymphoblastic leukemia (120).
IRAK4 inhibitors
IRAK4 belongs to the IRAK family and plays an important role in tumor growth and progression. IRAK4 is a TLR adapter protein and activation of TLR/NF-κB signaling pathway is mediated by IRAK4 kinase. The study of Kelly et al. and Schaffer et al. revealed the synergistic anti-proliferative activity of IRAK4 inhibitor and ibrutinib in ABC-DLBCL cell lines with MYD88 mutation (121, 122). There is an ongoing clinical trial of CA-4948 (an oral IRAK4 inhibitor) combined with ibrutinib (NCT03328078) in patients with R/R hematologic malignancies, of which part B included the newly diagnosed PCNSL and ibrutinib-resistance R/R PCNSL (123).
Histone deacetylase (HDAC) inhibitors
In ABC-DLBCLs, primary ibrutinib resistance could be induced by MYD88 mutation, Mondello et al. reported that HDAC inhibitor panobinostat could control transcriptionally the expression of MYD88 and synergize with ibrutinib in ABC-DLBCL cell lines with MYD88 mutattion (124). Singleton et al. found that water-soluble panobinostat intraparenchymal administration could be effective in the CNS tumor via surgically implanted microcatheters (125).
Bromodomain and extraterminal domain-containing proteins (BETs) inhibitors
JQ1, a selective BET-inhibitor, has shown some synergistic activity with BTKi in ABC-DLBCL (126). BRD4 belongs to BET family member and preclinical studies with BRD4 inhibitors demonstrate the suppression of MYC expression in MYC-driven Burkitt’s lymphoma cell lines (127). JQ1 has been reported quantification of 5 and 0.5 ng/mL for plasma and brain microdialysate due to good CNS penetration in the mouse model (128).
Non-covalent (Reversible) BTK inhibitors
Pirtobrutinib is highly potent non-covalent BTKi and inhibits both wild type and C481-mutated BTK due to reversible bind. Some clinical trials demonstrated promising efficacy of pirtobrutinib in previously treated CLL/SLL and MCL including covalent BTKi-resistance patients (129). Pirtobrutinib was used in 52 BTKi pre-treated MCL patients (ORR 52%) and it was well tolerated (130).
IMiDs: lenalidomide and pomalidomide
Lenalidomide and pomalidomide (POM) are next-generation IMiDs, and both have been shown the CNS permeability, especially pomalidomide was shown to have higher CNS penetration than lenalidomide (40% vs.11%) (56). The molecular mechanisms of IMiDs include inhibition of NF-κB and PI3K/AKT axis, as well as interferon regulatory factor 4 (IRF4) (131). Lenalidomide is active as monotherapy in relapsed PCNSL, and maintenance treatment prolongs response duration after salvage (54). The combination of POM and dexamethasone has significant therapeutic activity against R/R PCNSL and PVRL in 29 patients (ORR: 48%; median PFS: 5.3 months) (56).
XPO1 inhibitors: Selinexor
Selinexor (KPT-330) is a selective nuclear export inhibitor of exportin-1 (CRM1/XPO1). FOXO3a/PTEN-dependent resistance could be restored in ibrutinib-resistant cells by a nuclear export inhibitor (113). Selinexor was firstly reported as reversing resistance through the combination of ibrutinib and selinexor in CLL ibrutinib- refractory cells with BTK C481S mutation (132). The synergy effect was also observed for ibrutinib-resistant ABC-DLBCL cells (113). Selinexor crosses the BBB and favorable CNS penetration property was investigated with brain: plasma ratio 0.72 in rats (133). In pre-clinical mouse models of PCNSL, the combination of selinexor and ibrutinib was showed as promising therapeutic option (134).
Immune checkpoint inhibitors
Immune checkpoint molecules including PD-1 and PD-L1 may result in tumor cell escape in some solid tumors and hematological malignancy. Increased PD-L1 expression in tumor tissue or high PD-1 expression on tumor-infiltrating lymphocytes may predict treatment response to PD-1 blockade. High PD-L1 expression (>5% staining) was found in 18/48 patients (37.5%), PD-1 expression was found in 12/14 (85.7%) of PCNSL tumor specimens (135). In a study of 71 patients with PCNSL, PD-1 expression and PD-L1 expression were found in 16/71 and 42/71 patients, respectively (136). In the meanwhile, preclinical results indicate 50% of CNSL model mice treated with anti-PD1 achieved CR and potential cure in long-time surviving mice (137). Based on growing evidence, immune checkpoint inhibitors may be used as monotherapy or in combination with other treatments in R/R patients with PCNSL. In a case series study of five patients with R/R CNSL (4/5 PCNSL and 1/5 SCNSL), all patients achieved clinical response to PD-1 inhibitor nivolumab (CR:4 patients; PFS:13-17 months) (138). Almost the consistent treatment response was seen in Graber et al. study. All five patients (4/5 PCNSL and 1/5 SCNSL) were treated with PD-1 inhibitor pembrolizumab, two patients were observed durable remission (58). In Ambady et al. study, six R/R CNSL patients (3/6 PCNSL and 3/6 SCNSL) were treated with pembrolizumab in combination with rituximab, 3 patients achieved CR and maintained for more than six months (139). These small series studies support that immune checkpoint inhibitor could be a promising treatment option in CNS lymphoma.
BTK inhibitors adverse events
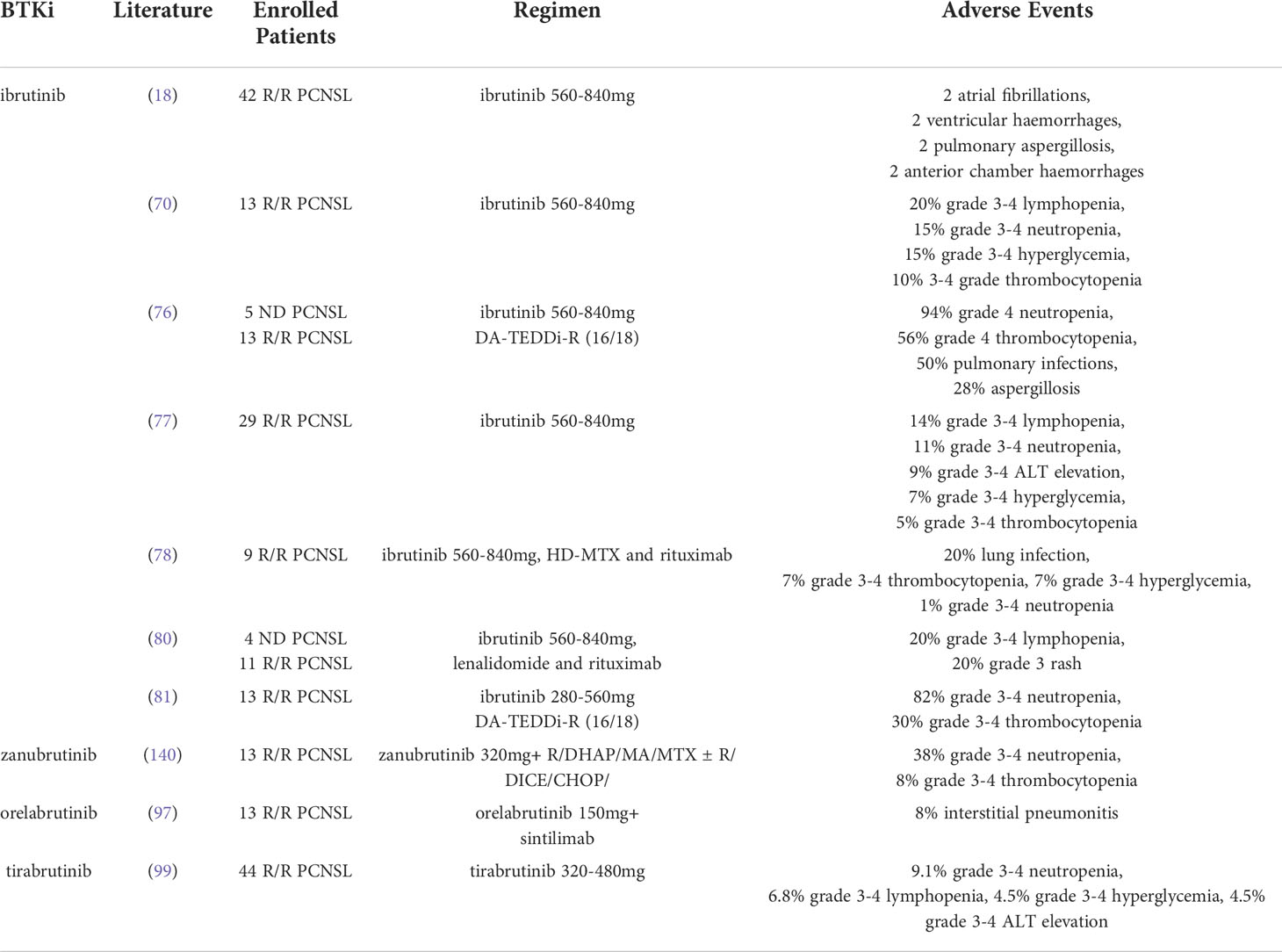
The adverse events of BTKis are often associated with immunosuppression and hematological toxicities including neutropenia, lymphopenia, anemia and thrombocytopenia, opportunistic infections such as aspergillosis and pneumocystis carinii pneumonia (PCP). In addition, BTKis also bind to other homologous kinases including TEC kinase family, interleukin-2-inducible T-cell kinase (ITK) and epidermal growth factor receptor (EGFR). The off-target effects of ibrutinib may induce atrial fibrillation, bleeding events, dermatological toxicities, diarrhoea, etc. According to the current literature, the adverse events of BTKi in PCNSL were summarized in Table 2.
Conclusion
BTK inhibitor monotherapy or in combination with other treatments have shown good clinical efficacy in newly diagnosed and R/R PCNSL and are well tolerated, but BTKi resistance remains an unavoidable problem. The mechanisms of drug resistance and how to overcome drug resistance have been preliminarily explored, and some definite conclusions have been obtained. The combination of BTK inhibitors with other treatments may reverse BTKi resistance and have a long-term survival benefit in patients with PCNSL.
Author contributions
All authors listed have made substantial and intellectual contribution in the preparation of the manuscript and final version was approved for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ostrom QT, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the united states in 2014-2018. Neuro Oncol (2021) 23(S3):iii1–105. doi: 10.1093/neuonc/noab200
2. Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the world health organization classification of lymphoid neoplasms. Blood (2016) 127(20):2375–90. doi: 10.1182/blood-2016-01-643569
3. Alaggio R, Amador C, Anagnostopoulos I, Attygalle AD, Oliveira Araujo IB, Berti E, et al. The 5th edition of the world health organization classification of haematolymphoid tumours: Lymphoid neoplasms. Leukemia (2022) 36(7):1720–19. doi: 10.1038/s41375-022-01613-1
4. Attarbaschi A, Abla Q, Ronceray L, Bansil S, Bomken S, Burkhardt B, et al. Primary central nervous system lymphoma: Initial features, outcome, and late effects in 75 children and adolescents. Blood Adv (2019) 3(24):4291–7. doi: 10.1182/bloodadvances.2019001062
5. Roy S, Josephson SA, Fridlyand J, Karch J, Kadoch C, Karrim J, et al. Protein biomarker identification in the CSF of patients with CNS lymphoma. J Clin Oncol (2008) 26(1):96–105. doi: 10.1200/JCO.2007.12.1053
6. Montesinos-Rongen M, Roost DV, Schaller C, Wiestler OD, Deckert M. Primary diffuse Large b-cell lymphomas of the central nervous system are targeted by aberrant somatic hypermutation. Blood (2004) 103(5):1869–75. doi: 10.1016/j.canlet.2021.02.025
7. Hua Y, Li W, Kaminska B. Emerging insights into origin and pathobiology of primary central nervous system lymphoma. Cancer Lett (2021) 509:121–9. doi: 10.1016/j.canlet.2021.02.02
8. Robak T, Witkowska M, Smolewski P. The role of bruton's kinase inhibitors in chronic lymphocytic leukemia: Current status and future directions. Cancers (Basel) (2022) 14(3):771. doi: 10.3390/cancers14030771
9. McDonald C, Xanthopoulos C, Kostareli E. The role of bruton's tyrosine kinase in the immune system and disease. Immunology (2021) 164(4):722–36. doi: 10.1111/imm.13416
10. Liu JK, Chen CJ, Wang DM, Zhang J, Zhang TT. Emerging small-molecule inhibitors of the bruton's tyrosine kinase (BTK): Current development. Eur J Med Chem (2021) 217:113329. doi: 10.1016/j.ejmech.2021.113329
11. Wen TY, Wang JS, Shi YK, Qian HL, Liu P. Inhibitors targeting bruton's tyrosine kinase in cancers: Drug development advances. Leukemia (2021) 35(2):312–32. doi: 10.1038/s41375-020-01072-6
12. Mato AR, Nabhan C, Thompson MC, Lamanna N, Brander DM, Hill B, et al. Toxicities and outcomes of 616 IIbrutinib-treated patients in the united states: A real-world analysis. Haematologica (2018) 103(5):874–9. doi: 10.3324/haematol.2017.182907
13. Estupiñán HY, Berglöf A, Zain R, Edvard Smith CI. Comparative analysis of BTK inhibitors and mechanisms underlying adverse effects. Front Cell Dev Biol (2021) 9:630942. doi: 10.3389/fcell.2021.630942
14. Shaffer AL, Phelan JD, Wang JQ, Huang DW, Wright GW, Kasbekar M, et al. Overcoming acquired epigenetic resistance to BTK inhibitors. Blood Cancer Discovery (2021) 2(6):630–47. doi: 10.1158/2643-3230.BCD-21-0063
15. Wang HR, Zhang WT, Yang JY, Zhou KS. The resistance mechanisms and treatment strategies of BTK inhibitors in b-cell lymphoma. Hematol Oncol (2021) 39(5):605–15. doi: 10.1002/hon.2933
16. Reiff SD, Muhowski EM, Guinn D, Lehman A, Fabian CA, Cheney C, et al. Noncovalent inhibition of C481S bruton tyrosine kinase by GDC-0853: A new treatment strategy for ibrutinib-resistant CLL. Blood (2018) 132(10):1039–49. doi: 10.1182/blood-2017-10-809020
17. Bond DA, Woyach JA. Targeting BTK in CLL: Beyond ibrutinib. Curr Hematol Malig Rep (2019) 14(3):197–205. doi: 10.1007/s11899-019-00512-0
18. Soussain C, Choquet S, Blonski M, Leclercq D, Houillier C, Rezai K, et al. Ibrutinib monotherapy for relapse or refractory primary CNS lymphoma and primary vitreoretinal lymphoma: Final analysis of the phase II 'Proof-of-Concept' iLOC study by the lymphoma study association (LYSA) and the French oculo-cerebral lymphoma (LOC) network. Eur J Cancer (2019) 117:121–30. doi: 10.1016/j.ejca.2019.05.024
19. Grommes C, Tang SS, Wolfe J, Kaley TJ, Daras M, Pentsova EI, et al. Phase 1b trial of an ibrutinib-based combination therapy in Recurrent/Refractory CNS lymphoma. Blood (2019) 133(5):436–45. doi: 10.1182/blood-2018-09-875732
20. Ferreri AJM, Holdhoff M, Nayak L, Rubenstein JL. Evolving treatments for primary central nervous system lymphoma. Am Soc Clin Oncol Educ Book (2019) 39:454–66. doi: 10.1200/EDBK_24254
21. Dhillon S. Tirabrutinib: First approval. Drugs (2020) 80(8):835–40. doi: 10.1007/s40265-020-01318-8
22. Munakata W, Tobinai K. Tirabrutinib hydrochloride for b-cell lymphomas. Drugs Today (Barc) (2021) 57(4):277–89. doi: 10.1358/dot.2021.57.4.3264113
23. Villano JL, Koshy M, Shaikh H, Dolecek TA, McCarthy BJ. Age, gender, and racial differences in incidence ad survival in primary CNS lymphoma. Br J Cancer (2011) 105(9):1414–18. doi: 10.1038/bjc.2011.357
24. Tang DS, Chen Y, Shi YY, Tao H, Tao SD, Zhang QE, et al. Epidemiologic characteristics, prognostic factors, and treatment outcomes in primary central nervous system lymphoma: A SEER-based study. Front Oncol (2022) 12:817043. doi: 10.3389/fonc.2022.817043
25. Tsang M, Cleveland J, Rubenstein JL. On point in primary CNS lymphoma. Hematol Oncol (2020) 38(5):640–7. doi: 10.1002/hon.2761
26. Mayerhoefer ME, Umutlu L, Schöder H. Functional imaging using radiomic features in assessment of lymphoma. Methods (2021) 188:105–11. doi: 10.1016/j.ymeth.2020.06.020
27. Wiggins RH, Hoffman JM, Fine GC, Covington MF, Salem AE, Koppula BR, et al. PET-CT in clinical adult oncology-v. head and neck and neuro oncology. Cancers (Basel) (2022) 14(11):2726. doi: 10.3390/cancers14112726
28. Sakai M, Higashi M, Fujiwara T, Uehira T, Shirasaka T, Nakanishi K, et al. MRI Imaging features of HIV-related central nervous system diseases: Diagnosis by pattern recognition in daily practice. Jpn J Radiol (2021) 39(11):1023–38. doi: 10.1007/s11604-021-01150-4
29. Küker W, Nagele T, Korfel A, Heckl S, Thiel E, Bamberg M, et al. Primary central nervous system lymphomas (PCNSL): MRI features at presentation in 100 patients. J Neurooncol (2005) 72(2):169–77. doi: 10.1007/s11060-004-3390-7
30. Scheichel F, Pinggera D, Popadic B, Sherif C, Marhold F, Freyschlag CF. An update on neurosurgical management of primary CNS lymphoma in immunocompetent patients. Front Oncol (2022) 12:884724. doi: 10.3389/fonc.2022.884724
31. Paydas S. Primary central nervous system lymphoma: Essential points in diagnosis and management. Med Oncol (2017) 34(4):61. doi: 10.1007/s12032-017-0920-7
32. Morell AA, Shah AH, Cavallo C, Eichberg DG, Sarkiss CA, Benveniste R, et al. Diagnosis of primary central nervous system lymphoma: A systematic review of the utility of CSF screening and the role of early brain biopsy. Neurooncol Pract (2019) 6(6):415–23. doi: 10.1093/nop/npz015
33. Camilleri-Broët S, Criniére E, Broët P, Delwail V, Mokhtari K, Moreau A, et al. A uniform activated b-Cell-Like immunophenotype might explain the poor prognosis of primary central nervous system lymphomas: Analysis of 83 cases. Blood (2006) 107(1):190–6. doi: 10.1182/blood-2005-03-1024
34. Lin CH, Kuo KT, Chuang SS, Kuo SH, Chang JH, Chang KC, et al. Comparison of the expression and prognostic significance of differentiation markers between diffuse Large b-cell lymphoma of central nervous system origin and peripheral nodal origin. Clin Cancer Res (2006) 12(4):1152–6. doi: 10.1158/1078-0432.CCR-05-1699
35. Liu J, Wang YM, Liu YT, Liu Z, Cui Q, Ji N, et al. Immunohistochemical profile and prognostic significance in primary central nervous system lymphoma: Analysis of 89 cases. Oncol Lett (2017) 14(5):5505–12. doi: 10.3892/ol.2017.6893
36. Swerdlow SH. Diagnosis of 'Double hit' diffuse Large b-cell lymphoma and b-cell lymphoma, unclassifiable, with features intermediate between DLBCL and burkitt lymphoma: When and how, FISH versus IHC. Hematol Am Soc Hematol Educ Program (2014) 2014(1):90–9. doi: 10.1182/asheducation-2014.1.90
37. Shi QY, Feng X, Bao W, Ma J, Lv JH, Wang X, et al. MYC/BCL2 Co-expression is a stronger prognostic factor compared with the cell-of-Origin classification in primary CNS DLBCL. J Neuropathol Exp Neurol (2017) 76(11):942–8. doi: 10.1093/jnen/nlx083
38. Makino K, Nakamura H, Shinojima K, Kuroda JI, Yano S, Mikami Y, et al. BCL2 expression is associated with a poor prognosis independent of cellular origin in primary central nervous system diffuse Large b-cell lymphoma. J Neurooncol (2018) 140(1):115–21. doi: 10.1007/s11060-018-2940-3
39. Garcia-Reyero J, Magunacelaya NM, Pereña AG, Gonzalez SM, Teran-Villagra N, Azueta A, et al. Clonal evolution in primary diffuse Large b-cell lymphoma of the central nervous system. Appl Immunohistochem Mol Morphol (2020) 28(8):e68–71. doi: 10.1097/PAI.0000000000000655
40. Bonzheim I, Sander P, Salmerón-Villalobos J, Süsskind D, Szurman P, Gekeler P, et al. The molecular hallmarks of primary and secondary vitreoretinal lymphoma. Blood Adv (2022) 6(5):1598–607. doi: 10.1182/bloodadvances.2021004212
41. Chapuy B, Roemer MGM, Stewart C, Tan Y, Abo RP, Zhang L, et al. Targetable genetic features of primary testicular and primary central nervous system lymphomas. Blood (2016) 127(7):869–81. doi: 10.1182/blood-2015-10-673236
42. Nayyar N, White MD, Gill CM, Lastrapes M, Bertalan M, Kaplan A, et al. MYD88 L265P mutation and CDKN2A loss are early mutational events in primary central nervous system diffuse Large b-cell lymphomas. Blood Adv (2019) 3(3):375–83. doi: 10.1182/bloodadvances.2018027672
43. Calimeri T, Steffanoni S, Gagliardi F, Chiara A, Ferreri AJM. How we treat primary central nervous system lymphoma. ESMO Open (2021) 6(4):100213. doi: 10.1016/j.esmoop.2021.100213
44. Rubenstein JL, Gupta NK, Mannis GN, LaMarre AK, Treseler P. How I treat CNS lymphomas. Blood (2013) 122(14):2318–30. doi: 10.1182/blood-2013-06-453084
45. Ferreri AJM, Cwynarski K, Pulczynski E, Fox CP, Schorb E, Rosée PL, et al. Whole-brain radiotherapy or autologous stem-cell transplantation as consolidation strategies after high-dose methotrexate-based chemoimmunotherapy in patients with primary CNS lymphoma: Results of the second randomisation of the international extranodal lymphoma study group-32 phase 2 trial. Lancet Haematol (2017) 4(11):e510–23. doi: 10.1016/S2352-3026(17)30174-6
46. Bromberg JEC, Issa S, Bakunina K, Minnema MC, Seute T, Durian M, et al. Rituximab in patients with primary CNS lymphoma (HOVON 105/ALLG NHL 24): A randomised, open-label, phase 3 intergroup study. Lancet Oncol (2019) 20(2):216–28. doi: 10.1016/S1470-2045(18)30747-2
47. Ferreri AJM, Cwynarski K, Pulczynski E, Ponzoni M, Deckert M, Politi LS, et al. Chemoimmunotherapy with methotrexate, cytarabine, thiotepa, and rituximab (MATRix regimen) in patients with primary CNS lymphoma: Results of the first randomisation of the international extranodal lymphoma study group-32 (IELSG32) phase 2 trial. Lancet Haematol (2016) 3(5):e217–27. doi: 10.1016/S2352-3026(16)00036-3
48. Ferreri AJM, Reni M, Foppoli M, Martelli M, Pangalis GA, Frezzato M, et al. High-dose cytarabine plus high-dose methotrexate versus high-dose methotrexate alone in patients with primary CNS lymphoma: A randomised phase 2 trial. Lancet (2009) 374(9700):1512–20. doi: 10.1016/S0140-6736(09)61416-1
49. Cho H, Chang JH, Kim YR, Kim SJ, Chung H, Park H, et al. The role of upfront autologous stem cell transplantation in high-risk younger patients with primary central nervous system lymphoma. Br J Haematol (2016) 174(3):444–53. doi: 10.1111/bjh.14069
50. Schaff LR, Grommes C. Primary central nervous system lymphoma. Blood (2021) 140(9):971–79. doi: 10.1182/blood.2020008377
51. Thiel E, Korfel A, Martus P, Kanz L, Griesinger F, Rauch M, et al. High-dose methotrexate with or without whole brain radiotherapy for primary CNS lymphoma (G-PCNSL-SG-1): A phase 3, randomised, non-inferiority trial. Lancet Oncol (2010) 11(11):1036–47. doi: 10.1016/S1470-2045(10)70229-1
52. Faivre G, Butler MJ, Le I, Brenner A. Temozolomide as a single agent maintenance therapy in elderly patients with primary CNS lymphoma. Clin Lymphoma Myeloma Leuk (2019) 19(10):665–9. doi: 10.1016/j.clml.2019.05.012
53. Nakajima K, Mizobuchi Y, Fujihara T, Azumi M, Takagi Y. Continued-maintenance therapy with high-dose methotrexate improves overall survival of patients with primary central nervous system lymphoma. J Med Invest (2021) 68(3.4):286–91. doi: 10.2152/jmi.68.286
54. Rubenstein JL, Geng H, Fraser EJ, Formaker P, Chen LJ, Sharma J, et al. Phase 1 investigation of Lenalidomide/Rituximab plus outcomes of lenalidomide maintenance in relapsed CNS lymphoma. Blood Adv (2018) 2(13):1595–607. doi: 10.1182/bloodadvances.2017014845
55. Rubenstein JL, Geng H, Vu K, Mannis G, Formaker P, Hwang J, et al. Maintenance lenalidomide in primary CNS lymphoma. Ann Oncol (2019) 30(8):1397–8. doi: 10.1093/annonc/mdz142
56. Tun HW, Johnston PB, DeAngelis LM, Atherton PJ, Pederson LD, Koenig PA, et al. Phase 1 study of pomalidomide and dexamethasone for Relapsed/Refractory primary CNS or vitreoretinal lymphoma. Blood (2018) 132(21):2240–8. doi: 10.1182/blood-2018-02-835496
57. Tateishi K, Miyake Y, Nakamura T, Yamamoto T. Primary central nervous system lymphoma: Clinicopathological and genomic insights for therapeutic development. Brain Tumor Pathol (2021) 38(3):173–82. doi: 10.1007/s10014-021-00408-z
58. Graber JJ, Plato B, Mawad R, Moore DJ. Pembrolizumab immunotherapy for relapsed CNS lymphoma. Leuk Lymphoma (2020) 61(7):1766–8. doi: 10.1080/10428194.2020.1742903
59. Tiberghien P, Deconinck E, Adotevi O. More on anti-CD19 CAR T cells in CNS diffuse Large-B-Cell lymphoma. N Engl J Med (2017) 377(21):2101–2. doi: 10.1056/nejmc1712460
60. Siddiqi T, Wang X, Blanchard MS, Wagner JR, Popplewell LL, Budde LE, et al. CD19-directed CAR T-cell therapy for treatment of primary CNS lymphoma. Blood Adv (2021) 5(20):4059–63. doi: 10.1182/bloodadvances.2020004106
61. Grommes C, Nayak L, Tun HW, Batchelor TT. Introduction of novel agents in the treatment of primary CNS lymphoma. Neuro Oncol (2019) 21(3):306–13. doi: 10.1093/neuonc/noy193
62. Low JT, Peters KB. Ibrutinib in primary central nervous system diffuse Large b-cell lymphoma. CNS Oncol (2020) 9(1):Cns51. doi: 10.2217/cns-2019-0022
63. Ollila TA, Olszewski AJ. Extranodal diffuse Large b cell lymphoma: Molecular features, prognosis, and risk of central nervous system recurrence. Curr Treat Options Oncol (2018) 19(8):38. doi: 10.1007/s11864-018-0555-8
64. Wu KL, Zhang HH, Fu YJ, Zhu YZ, Kong LJ, Chen L, et al. TLR4/MyD88 signaling determines the metastatic potential of breast cancer cells. Mol Med Rep (2018) 18(3):3411–20. doi: 10.3892/mmr.2018.9326
65. Cetin GO, Baris IC, Caner V, Sarikepe B, Turk NS, Tepeli E, et al. Mutational status of EZH2 and CD79B hot spots in mature b-cell non-hodgkin's lymphomas: Novel CD79B variations have been revealed. Eur Rev Med Pharmacol Sci (2016) 20(5):830–6.
66. Schmitz R, Wright GW, Huang DW, Johnson CA, Phelan JD, Wang JQ, et al. Genetics and pathogenesis of diffuse Large b-cell lymphoma. N Engl J Med (2018) 378(15):1396–407. doi: 10.1056/NEJMoa1801445
67. Bourbon E, Salles G. Polatuzumab vedotin: An investigational anti-CD79b antibody drug conjugate for the treatment of diffuse Large b-cell lymphoma. Expert Opin Investig Drugs (2020) 29(10):1079–88. doi: 10.1080/13543784.2020.1800638
68. Yin ZX, Zhang Y, Wang X. Advances in chimeric antigen receptor T-cell therapy for b-cell non-Hodgkin lymphoma. biomark Res (2021) 9(1):58. doi: 10.1186/s40364-021-00309-5
69. Nakamura T, Tateishi K, Niwa T, Matsushita Y, Tamura K, Kinoshita M, et al. Recurrent mutations of CD79B and MYD88 are the hallmark of primary central nervous system lymphomas. Neuropathol Appl Neurobiol (2016) 42(3):279–90. doi: 10.1111/nan.12259
70. Grommes C, Alessandro P, Nicolaos P, Sarah ST, Carl C, Derrek S, et al. Ibrutinib unmasks critical role of bruton tyrosine kinase in primary CNS lymphoma. Cancer Discovery (2017) 7(9):1018–29. doi: 10.1158/2159-8290.CD-17-0613
71. Braggio E, Wier SV, Ojha J, McPhail E, Asmann YW, Egan J, et al. Genome-wide analysis uncovers novel recurrent alterations in primary central nervous system lymphomas. Clin Cancer Res (2015) 21(17):3986–94. doi: 10.1158/1078-0432.CCR-14-2116
72. Kraan W, Horlings HM, van Keimpema M, Schilder-Tol EJM, Oud MECM, Scheepstra C, et al. High prevalence of oncogenic MYD88 and CD79B mutations in diffuse Large b-cell lymphomas presenting At immune-privileged sites. Blood Cancer J (2013) 3(9):e139. doi: 10.1038/bcj.2013.28
73. Gonzalez-Aguilar A, Idbaih A, Boisselier B, Habbita N, Rossetto M, Laurenge A, et al. Recurrent mutations of MYD88 and TBL1XR1 in primary central nervous system lymphomas. Clin Cancer Res (2012) 18(19):5203–11. doi: 10.1158/1078-0432.CCR-12-0845
74. Montesinos-Rongen M, Schmitz R, Brunn A, Gesk S, Richter J, Hong K, et al. Mutations of CARD11 but not TNFAIP3 may activate the NF-κB pathway in primary CNS lymphoma. Acta Neuropathol (2010) 120(4):529–35. doi: 10.1007/s00401-010-0709-7
75. Bruno A, Boisselier B, Labreche K, Marie Y, Polivka M, Jouvet A, et al. Mutational analysis of primary central nervous system lymphoma. Oncotarget (2014) 5(13):5065–75. doi: 10.18632/oncotarget.2080
76. Lionakis MS, Dunleavy K, Roschewski M, Widemann BC, Butman JA, Schmitz R, et al. Inhibition of b cell receptor signaling by ibrutinib in primary central nervous system lymphoma. Cancer Cell (2017) 31(6):833–43. doi: 10.1016/j.ccell.2017.04.012
77. Grommes C, Wolfe J, Gavrilovic J, Kaley T, Stone J, Daras M, et al. II of single-agent ibrutinib in Recurrent/Refractory primary (PCNSL) and secondary CNS lymphoma (SCNSL). Blood (2018) 132(S1):2965. doi: 10.1182/blood-2018-99-118538
78. Grommes C, Tang SS, Wolfe J, Kaley TJ, Daras M, Pentsova EI, et al. Phase 1b trial of an ibrutinib-based combination therapy in Recurrent/Rrefractory CNS lymphoma. Blood (2019) 133(5):436–45. doi: 10.1182/blood-2018-09-875732
79. Grommes C, Gavrilovic I, Miller AM, Stone JB, Kaley T, Madzsar JT, et al. Phase ib of copanlisib in combination with ibrutinib in Recurrent/Rrefractory primary CNS lymphoma (PCNSL). Blood (2019) 134(S1):1598. doi: 10.1182/blood-2019-126214
80. Grommes C, Piotrowski A, Pentsova E, Nolan C, Francis J, DeAngelis L, et al. Phase ib trial with dose expansion of the bruton's tyrosine kinase (BTK) inhibitor, ibrutinib, in combination with rituximab and lenalidomide in patients with Refractory/Recurrent primary central nervous system lymphoma (PCNSL) and Refractory/Recurrent secondary central nervous system lymphoma (SCNSL). Blood (2020) 136(S1):48. doi: 10.1182/blood-2020-143075
81. Roschewski M, Melani C, Lakhotia R, Pittaluga S, Phelan JD, Peer C, et al. Phase 1 study of escalating doses of ibrutinib and temozolomide, etoposide, liposomal doxorubicin, dexamethasone, rituximab (TEDDI-r) with isavuconazole for relapsed and refractory primary CNS lymphoma. Blood (2020) 136(S1):12–3. doi: 10.1182/blood-2020-137637
82. Lauer EM, Waterhouse M, Braig M, Mutter J, Bleul S, Duque-Afonso J, et al. Ibrutinib in patients with Relapsed/Refractory central nervous system lymphoma: A retrospective single-centre analysis. Br J Haematol (2020) 190:e95–125. doi: 10.1111/bjh.16759
83. Chen YD, Liu HG, Sun XF, Bai XY, Cui Q, Xing RX, et al. Preliminary exploration of ibrutinib combined with chemotherapy in the treatment of Relapsed/Refractory primary CNS lymphoma. Chin J Neurosurg (2020) 36:1047–51.
84. Escure G, Tilmont R, Barbieux S, Wemeau M, Hieulle J, Boyle EM. Franck Morschhauser treatment with temozolomide and ibrutinib in Recurrent/Rrefractory primary (PCNSL) and secondary CNS lymphoma (SCNSL). Eur J Haematol (2021) 107:370–3. doi: 10.1111/ejh.13667
85. Houillier C, Moluçon-Chabrot C, Moles MP, Willems L, Ahle G, Waultier A, et al. Combination of rituximab-Lenalidomide-Ibrutinib in Relapsed/Refractory primary CNS lymphoma: A cohort study of the loc network. Hematol Oncol (2021) 39(S2):233. doi: 10.1002/hon.73-2880
86. Lewis KL, Chin CK, Manos K, Casey J, Hamad N, Crawford J, et al. Ibrutinib for central nervous system lymphoma: the Australasian lymphoma Alliance/MD Anderson cancer center experience. Br J Haematol (2021) 192(6):1049–53. doi: 10.1111/bjh.16946
87. Deak-Mihaly D, Iluta S, Pasca S, Jitaru C, Roman A, Andries A, et al. Ibrutinib monotherapy as bridge-to-Transplant for Relapsed/Refractory primary oculo-cerebral lymphoma. J Clin Med (2021) 10:4483–93. doi: 10.3390/jcm10194483
88. Guo YX, Lan XX, Chang XL, Wang GX, Zou DM, Su L, et al. Ibrutinib in combination with rituximab and high-dose methotrexate in newly diagnosed primary central nervous system LymphomaLymphoma patients. Blood (2021) 138(S1):1416–18. doi: 10.1182/blood-2021-147742
89. Wang XX, Gao Y, Yu SS, Bai B, Ou QX, Zhu LQ, et al. Preliminary results of a phase II study of methotrexate in combination with ibrutinib and temozolomide (MIT) in newly diagnosed primary CNS lymphoma. Blood (2021) 138(S1):2481–83. doi: 10.1182/blood-2021-150658
90. Chen FL, Pang DW, Guo HG, Ou QX, Wu X, Jiang XM. Clinical outcomes of newly diagnosed primary CNS lymphoma lymphoma treated with ibrutinib-based combination therapy: A real-world experience of off-label ibrutinib use. Cancer Med (2020) 9(22):8676–84. doi: 10.1002/cam4.3499
91. Bairey O, Taliansky A, Amiel AB, Yust-Katz S, Gurion R, Zektser M, et al. Phase II study of ibrutinib maintenance for elderly patients with primary CNS lymphoma following first-line treatment with high dose methotrexate-based chemotherapy. EHA (2021).
92. Zhang Y, Li YN, Zhuang Z, Wang W, Wei C, Zhao DQ, et al. Preliminary results of zanubrutinib-containing regimens in DLBCL and cerebrospinal fluid distribution of zanubrutinib. Blood (2021) 138(S1):4556. doi: 10.1182/blood-2021-150949
93. Wang L, Guan WX, Liu XD, Wang H, Peng XY. Targeting bruton tyrosine kinase for the treatment of vitreoretinal lymphoma: Report of 11 consecutive patients. Blood (2021) 138(S1):1421. doi: 10.1182/blood-2021-144657
94. Song YQ, Deng LJ, Zhang B, Luo H, Zhao RB. Preliminary results of drug concentrations in peripheral blood and cerebrospinal fluid of orelabrutinib in patients with relapsed or refractory primary or secondary central nervous system lymphoma. CSCO (2021).
95. Yang C, Cui Y, Ren X, Li M, Yu K, Shen S, et al. Orelabrutinib combined with lenalidomide and immunochemotherapy for Relapsed/Refractory primary central nervous system lymphoma: A retrospective analysis of case series. Front Oncol (2022) 12:901797. doi: 10.3389/fonc.2022.901797
96. Wu JJ, Wang WH, Dong M, Ma SS, Zhang XD, Zhu LN, et al. Orelabrutinib-bruton tyrosine kinase inhibitor-based regimens in the treatment of central nervous system lymphoma: A retrospective study. Invest New Drugs (2022) 40(3):650–9. doi: 10.1007/s10637-022-01219-5
97. Zhang Y, Wang W, Zhao DQ, Zhang W, Zhou DB. Preliminary results of a phase II study of orelabrutinib in combination with anti-PD-1 monoclonal antibody in refractory of relapsed primary CNS lymphoma. EHA (2022).
98. Satow T, Horiguchi S, Komuro T. Recovery from coma of a patient having acute progression of primary central nervous system lymphoma using tirabrutinib and methylprednisolone. Neurooncol Adv (2020) 2(1):1–3. doi: 10.1093/noajnl/vdaa164
99. Narita Y, Nagane M, Mishima K, Terui Y, Arakawa Y, Yonezawa H, et al. Phase I/II study of tirabrutinib, a second-generation bruton's tyrosine kinase inhibitor, in Relapsed/Refractory primary central nervous system lymphoma. Neuro Oncol (2021) 23(1):122–33. doi: 10.1093/neuonc/noaa145
100. Nakagawa N, Yamano R, Kajikawa S, Kondo Y, Okumura H. Successful bridging therapy with tirabrutinib before ASCT for relapsed primary DLBCL of the CNS complicated with PBC, cirrhosis, and pancytopenia. Leuk Res Rep (2022) 17:100331. doi: 10.1016/j.lrr.2022.100331
101. Iizuka-Honma H, Takizawa H, Mitsumori T, Okura H, Ishii H, Noguchi M. Refractory primary vitreoretinal lymphoma involving the spinal cord with a temporary complete response to tirabrutinib: A case report. Intern Med (2022). doi: 10.2169/internalmedicine.9591-22
102. Valla K, Flowers CR, Koff JL. Targeting the b cell receptor pathway in non-Hodgkin lymphoma. Expert Opin Investig Drugs (2018) 27(6):513–22. doi: 10.1080/13543784.2018.1482273
103. Wilson WH, Young RM, Schmitz R, Yang Y, Pittaluga S, Wright G, et al. Targeting b cell receptor signaling with ibrutinib in diffuse Large b cell lymphoma. Nat Med (2015) 21:922–26. doi: 10.1038/nm.3884
104. Choi J, Phelan JD, Wright GW, Haupl B, Huang DW, Shaffer AL, et al. Regulation of b cell receptor-dependent NF-κB signaling by the tumor suppressor KLHL14. Proc Natl Acad Sci USA (2020) 117(11):6092–102. doi: 10.1073/pnas.1921187117
105. Kim JH, Kim WS, Ryu K, Kim SJ, Park C. CD79B limits response of diffuse Large b cell lymphoma to ibrutinib. Leuk Lymphoma (2016) 57(6):1413–22. doi: 10.3109/10428194.2015.1113276
106. Ondrisova L, Mraz M. Genetic and non-genetic mechanisms of resistance to BCR signaling inhibitors in b cell malignancies. Front Oncol (2020) 10:591577. doi: 10.3389/fonc.2020.591577
107. Guan J, Huang D, Yakimchuk K, Okret S. P110alpha inhibition overcomes stromal cell-mediated ibrutinib resistance in mantle cell lymphoma. Mol Cancer Ther (2018) 17(5):1090–100. doi: 10.1158/1535-7163.MCT-17-0784
108. Rudelius M, Rosenfeldt MT, Leich E, Rauert-Wunderlich H, Solimando AG, Beilhack A, et al. Inhibition of focal adhesion kinase overcomes resistance of mantle cell lymphoma to ibrutinib in the bone marrow microenvironment. Haematologica (2018) 103(1):116–25. doi: 10.3324/haematol.2017.177162
109. Guo A, Lu P, Coffey G, Conley P, Pandey A, Wang YL. Dual SYK/JAK inhibition overcomes ibrutinib resistance in chronic lymphocytic leukemia: Cerdulatinib, but not ibrutinib, induces apoptosis of tumor cells protected by the microenvironment. Oncotarget (2017) 8(No.8):12953–67. doi: 10.18632/oncotarget.14588
110. D'Alterio C, Buoncervello M, Ieranò C, Napolitano M, Portella L, Rea G, et al. Targeting CXCR4 potentiates anti-PD-1 efficacy modifying the tumor microenvironment and inhibiting neoplastic PD-1. J Exp Clin Cancer Res (2019) 38(1):432. doi: 10.1186/s13046-019-1420-8
111. Lee MR, Ju HJ, Kim BS, Ko YH, Kim WS, Kim SJ. Isolation of side population cells in b-cell non-hodgkin’s lymphomas. Acta Haematol (2013) 129:10–7. doi: 10.1159/000341284
112. Lee CG, Das B, Lin TL, Grimes C, Zhang X, Lavezzi T, et al. A rare fraction of drug-resistant follicular lymphoma cancer stem cells interacts with follicular dendritic cells to maintain tumourigenic potential. Br J Haematol (2012) 158(1):79–90. doi: 10.1111/j.1365-2141.2012.09123.x
113. Kapoor I, Li Y, Sharma A, Zhu H, Bodo J, Xu W, et al. Resistance to BTK inhibition by ibrutinib can be overcome by preventing FOXO3a nuclear export and PI3K/AKT activation in b-cell lymphoid malignancies. Cell Death Dis (2019) 10:924. doi: 10.1038/s41419-019-2158-0
114. Jain N, Havranek O, Singh RK, Khashab T, Shirazi F, Sehgal L, et al. Overcoming ibrutinib resistance by targeting phosphatidylinositol-3-Kinase signaling in diffuse Large b-cell lymphoma. Biorxiv (2019). doi: 10.1101/523761
115. Paul J, Soujon M, Wengner AM, Zitzmann-Kolbe S, Sturz A, Haike K, et al. Simultaneous inhibition of PI3Kδ and PI3Kα induces ABC-DLBCL regression by blocking BCR-dependent and -independent activation of NF-κB and AKT. Cancer Cell (2017) 31(1):64–78. doi: 10.1016/j.ccell.2016.12.003
116. Wen PY, Cloughesy TF, Olivero AG, Morrissey KM, Wilson TR, Lu XY, et al. First-in-Human phase I study to evaluate the brain-penetrant PI3K/mTOR inhibitor GDC-0084 in patients with progressive or recurrent high-grade glioma. Clin Cancer Res (2020) 26(8):1820–8. doi: 10.1158/1078-0432.CCR-19-2808
117. Korfel A, Schlegel U, Herrlinger U, Dreyling M, Schmidt C, Von Baumgarten L, et al. Phase II trial of temsirolimus for relapsed/refractory primary CNS lymphoma. J Clin Oncol (2016) 34(No.15):1757–63. doi: 10.1200/JCO.2015.64.9897
118. Holmes KB, Sadreev II, Rawstron AC, Munir T, Westhead DR, Hillmen P, et al. Ibrutinib induces chromatin reorganisation of chronic lymphocytic leukaemia cells. Oncogenesis (2019) 8(5):32. doi: 10.1038/s41389-019-0142-2
119. Kuo HP, Ezell SA, Schweighofer KJ, Cheung LWK, Hsieh S, Apatira M, et al. Combination of ibrutinib and ABT-199 in diffuse Large b-cell lymphoma and follicular lymphoma. Mol Cancer Ther (2017) 16(7):1246–56. doi: 10.1158/1535-7163.MCT-16-0555
120. Salem AH, Badawi MA, Place AE, Palenski TL, Arrendale R, Kim SY, et al. Venetoclax crosses the blood brain barrier: A pharmacokinetic analysis of the cerebrospinal fluid in pediatric leukemia patients. Blood (2020) 136(S1):30–1. doi: 10.1182/blood-2020-137197
121. Kelly PN, Romero DL, Yang Y, Shaffer AL, Chaudhary D, Robinson S, et al. Selective interleukin-1 receptor-associated kinase 4 inhibitors for the treatment of autoimmune disorders and lymphoid malignancy. J Exp Med (2015) 212(13):2189–201. doi: 10.1084/jem.20151074
122. Schaffer M, Chaturvedi S, Davis C, Aquino R, Stepanchick E, Versele M, et al. Identification of potential ibrutinib combinations in hematological malignancies using a combination high-throughput screen. Leuk Lymphoma (2018) 59(4):931–40. doi: 10.1080/10428194.2017.1349899
123. Nowakowski GS, Leslie LA, Joffe E, Rosenthal AC, Lunning MA, Patel K, et al. A multi-center, dose-finding study to assess safety, tolerability, pharmacokinetics and preliminary efficacy of a noval IRAK4 inhibitor CA-4948 in combination with ibrutinib, in patients with relapsed or refractory hematologic malignancies. Blood (2020) 136(S1):49–50. doi: 10.1182/blood-2020-140884
124. Mondello P, Brea EJ, De Stanchina E, Toska E, Chang AY, Fennell M, et al. Panobinostat acts synergistically with ibrutinib in diffuse Large b cell lymphoma cells with MyD88 L265P mutations. JCI Insight (2017) 2(6):e90196. doi: 10.1172/jci.insight.90196
125. Singleton WGB, Bienemann AS, Woolley M, Johnson D, Lewis O, Wyatt MJ, et al. The distribution, clearance, and brainstem toxicity of panobinostat administered by convection-enhanced delivery. J Neurosurg Pediatr (2018) 22(3):288–96. doi: 10.3171/2018.2.PEDS17663
126. Ceribelli M, Kelly PN, Shaffer AL, Wright GW, Xiao W, Yang Y, et al. Blockade of oncogenic IκB kinase activity in diffuse Large b-cell lymphoma by bromodomain and extraterminal domain protein inhibitors. Proc Natl Acad Sci USA (2014) 111(31):11365–70. doi: 10.1073/pnas.1411701111
127. Lu J, Qian Y, Altieri M, Dong H, Wang J, Raina K, et al. Hijacking the E3 ubiquitin ligase cereblon to efficiently target BRD4. Chem Biol (2015) 22(6):755–63. doi: 10.1016/j.chembiol.2015.05.009
128. Nair S, Davis A, Campagne O, Schuetz JD, Stewart CF. Development and validation of an LC-MS/MS method to quantify the bromodomain and extra-terminal (BET) inhibitor JQ1 in mouse plasma and brain microdialysate: Application to cerebral microdialysis study. J Pharm BioMed Anal (2021) 204:114274. doi: 10.1016/j.jpba.2021.114274
129. Cohen JB, Shah NN, Alencar AJ, Gerson JN, Patel MR, Fakhri B, et al. MCL-133 pirtobrutinib, a highly selective, non-covalent (Reversible) BTK inhibitor in previously treated mantle cell lymphoma: Updated results from the phase 1/2 BRUIN study. Clin Lymphoma Myeloma Leuk (2022) 22(S2):394–5. doi: 10.1016/S2152-2650(22)01569-5
130. Coombs CC, Pagel JM, Shah NN, Lamanna N, Munir T, Lech-Maranda E, et al. CLL-120 pirtobrutinib, a highly selective, non-covalent (Reversible) BTK inhibitor in previously treated CLL/SLL: Updated results from the phase 1/2 BRUIN study. Clin Lymphoma Myeloma Leuk (2022) 22(S2):68–9. doi: 10.1016/S2152-2650(22)01327-1
131. Wirsching HG, Weller M, Balabanov S, Roth P. Targeted therapies and immune checkpoint inhibitors in primary CNS lymphoma. Cancers (Basel) (2021) 13(12):3073. doi: 10.3390/cancers13123073
132. Hing ZA, Mantel R, Beckwith KA, Guinn D, Williams E, Smith LL, et al. Selinexor is effective in acquired resistance to ibrutinib and synergizes with ibrutinib in chronic lymphocytic leukemia. Blood (2015) 125(20):3128–32. doi: 10.1182/blood-2015-01-621391
133. Green AL, Ramkissoon SH, McCauley D, Jones K, Perry JA, Hsu JH, et al. Preclinical antitumor efficacy of selective exportin 1 inhibitors in glioblastoma. Neuro Oncol (2015) 17(5):697–707. doi: 10.1093/neuonc/nou303
134. Iménez I, Carabia J, Bobillo S, Palacio C, Abrisqueta P, Pagès C, et al. Repolarization of tumor infiltrating macrophages and increased survival in mouse primary CNS lymphomas after XPO1 and BTK inhibition. J Neurooncol (2020) 149(1):13–25. doi: 10.1007/s11060-020-03580-y
135. Ou A, Sumrall A, Phuphanich S, Spetzler D, Gatalica Z, Xiu J, et al. Primary CNS lymphoma commonly expresses immune response biomarkers. Neurooncol Adv (2020) 2(1):1–8. doi: 10.1093/noajnl/vdaa018
136. Monabati A, Nematollahi P, Dehghanian A, Safaei A, Sadeghipour A, Movahedinia S, et al. Immune checkpoint molecules in primary diffuse Large b-cell lymphoma of the central nervous system. Basic Clin Neurosci (2020) 11(4):491–8. doi: 10.32598/bcn.11.4.2542.1
137. Qiu Y, Li Z, Pouzoulet F, Vishnu P, Copland JA, Knutson KL, et al. Immune checkpoint inhibition by anti-PDCD1 (anti-PD1) monoclonal antibody has significant therapeutic activity against central nervous system lymphoma in an immunocompetent preclinical model. Br J Haematol (2018) 183(4):674–8. doi: 10.1111/bjh.15009
138. Nayak L, Iwamoto FM, La Casce A, Mukundan S, Roemer MGM, Chapuy B, et al. PD-1 blockade with nivolumab in Relapsed/Refractory primary central nervous system and testicular lymphoma. Blood (2017) 129(23):3071–3. doi: 10.1182/blood-2017-01-764209
139. Ambady P, Szidonya L, Firkins J, James J, Johansson K, White T, et al. Combination immunotherapy as a non-chemotherapy alternative for refractory or recurrent CNS lymphoma. Leuk Lymphoma (2019) 60(2):515–8. doi: 10.1080/10428194.2018.1480771
Keywords: PCNSL, BTKi, bruton’s tyrosine kinase inhibitors, primary central nervous system lymphoma, ibrutinib
Citation: Shen J and Liu J (2022) Bruton’s tyrosine kinase inhibitors in the treatment of primary central nervous system lymphoma: A mini-review. Front. Oncol. 12:1034668. doi: 10.3389/fonc.2022.1034668
Received: 01 September 2022; Accepted: 31 October 2022;
Published: 17 November 2022.
Edited by:
Xiao-Tang Kong, University of California, Irvine, United StatesReviewed by:
Monica Balzarotti, Humanitas Research Hospital, ItalyCaroline Besson, Université de Versailles Saint-Quentin-en-Yvelines, France
Copyright © 2022 Shen and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinghua Liu, bXRsamg3NjQ2QDE2My5jb20=
 Jing Shen
Jing Shen Jinghua Liu
Jinghua Liu