
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 28 October 2022
Sec. Radiation Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.1032145
 Yongkai Lu1,2†
Yongkai Lu1,2† Xiaoqin Luo2†
Xiaoqin Luo2† Di Yang3†
Di Yang3† Yi Li1
Yi Li1 Tuotuo Gong1
Tuotuo Gong1 Binglin Li4
Binglin Li4 Jian Cheng4
Jian Cheng4 Ruijuan Chen1,4*
Ruijuan Chen1,4* Xin Guo5*
Xin Guo5* Wei Yuan1*
Wei Yuan1*Objectives: Chemotherapy and radiotherapy generally cause serious adverse side effects in cancer patients, thereby affecting subsequent treatment. Numerous studies have shown that taking probiotics is an option for preventing and treating these side effects. In this investigation, a meta-analysis of the effects of oral probiotics on side effects brought on by radiotherapy, chemotherapy, or chemoradiotherapy treatment will be carried out.
Methods: Two researchers independently and carefully reviewed all pertinent studies that were published before June 30, 2022 and were accessible on PubMed, Embase, Cochrane Library, and the Web of Science. Moreover, the Cochrane Collaboration’s Tool was used to evaluate the risk of bias. Utilizing Review Manager software version 5.4, data were retrieved from eligible studies to evaluate their merits and determine odds ratios (OR) and 95% confidence intervals (CIs) (RevMan 5.4).
Results: 2 097 patients from 16 randomized controlled trials were extracted, and standard meta-analysis methods were used to examine the data. Compared with the placebo groups, oral probiotics significantly reduced the side effects caused by radiotherapy and chemotherapy on various types of cancer, such as head and neck cancer, pelvic and abdominal cancer, breast cancer, lung cancer, etc. (OR: 0.31, 95% CI: 0.20 – 0.48; P < 0.005). Further analysis found that the incidence of diarrhea in patients with pelvic and abdominal cancers (OR: 0.32, 95% CI: 0.16 - 0.65; P < 0.005) and the frequency of oral mucositis in patients with head and neck tumors were also significantly lower (OR: 0.28, 95% CI: 0.18 - 0.43; P < 0.005) after the oral administration of probiotics. This suggests that probiotics have a positive influence on the treatment of side effects after chemoradiotherapy. Additionally, a funnel plot revealed that there was no significant publication bias in this study.
Conclusions: Probiotics may help to reduce the occurrence of cancer therapy-related side effects, especially oral mucositis in head and neck tumors and diarrhea in patients with pelvic and abdominal tumors. However, given the small number of clinical trials involved, additional randomized, double-blind, multicentric trials in a larger population are required. This paper may assist researchers in improving trial design in the selection of probiotic strains and selecting appropriate patients who may benefit from probiotic treatments.
The main cause of sickness and mortality in people is cancer. According to GLOBOCAN 2020 predictions, there were roughly 19.3 million new cases and 10 million cancer deaths in 2020, which were much higher than the previous figures from 2018. Both its incidence and mortality are rising quickly globally (1). The two cornerstones of cancer treatment, radiotherapy and chemotherapy, are frequently hindered in their efficacies and applications by their severe side effects, which can include oral mucositis, diarrhea, proctitis, nausea, vomiting, alopecia, cognitive impairment, etc. (2–5). These conditions have a direct impact on patients’ quality of life and may lead to further complications. Developing new cancer treatment strategies has received a lot of attention in recent decades. However, few studies have focused on minimizing the side effects of these cancer treatments.
By 2025, cancer patients must have access to “accurate cancer diagnosis, excellent multimodal therapy, rehabilitation, and supportive and palliative care services,” according to the World Cancer Declaration, which was signed in 2013. To achieve this, safer therapeutic approaches must take into account both therapeutic advantages and accompanying toxicities (6). More researchers have begun to focus on the role of probiotics in preventing or reducing side effects in patients receiving radiotherapy and chemotherapy treatment in recent years. In 2018, a randomized controlled trial of 54 patients with cervical cancer by Linn et al. (7) showed that the incidence of diarrhea in radiotherapy patients in the probiotic group was significantly lower than in the placebo group (53.8% vs. 82.1%). Jiang et al. (8) designed a randomized controlled trial to investigate the effect of probiotics in reducing the severity of oral mucositis in patients with nasopharyngeal carcinoma after chemoradiotherapy. According to their research, probiotic combinations greatly improved patients’ immune responses and lessened the severity of oral mucositis via altering the gut flora. Recently, Zhang et al. (9) conducted a fascinating study into the application of probiotics. Their team’s randomized double-blind research revealed that probiotic supplements protected chemotherapy-related cognitive impairment in breast cancer patients via altering plasma metabolites, such as p-Mentha-1,8-dien-7-ol. Regarding the use of probiotics in colorectal patients after chemotherapy, Babak et al. (10) revealed that supplementation with probiotic preparations improved the quality of life of colorectal patients after chemotherapy, reduced certain inflammatory biomarkers, and relieved some of the side effects of chemotherapy.
Many studies have paved the way for the application of probiotics in the treatment of the side effects of radiotherapy and chemotherapy. However, these studies generally have the problems of small sample size, many research subjects or types of cancer, different outcome indicators, or different bacterial species selection. In other words, even if the subject of the study is one particular type of cancer, the outcome indicators and the bacteria selected may be inconsistent. These problems may lead to the combined analysis of many research results failing to provide clinicians with reliable evidence-based medical proof. As a result, the goal of this study is to collect as many clinical RCT studies as possible for meta-analysis in order to systematically assess the practical application of probiotic supplementation in the treatment of side effects after radiotherapy and chemotherapy in oncology patients and provide a foundation for clinical decision making.
We searched PubMed, Embase, the Cochrane library, and Web of Science for relevant studies published before June 30, 2022 using a combination of medical topic heading (MeSH) phrases and/or free text words including “cancer,” “probiotic,” “placebo,” and “chemoradiotherapy.” There was no restriction on the language of the papers that were published. In addition, we checked the references of the selected studies by hand.Two investigators independently conducted literature searches and screening, and a third investigator was consulted to resolve any differences.
All included studies adhered to the PICOS guidelines (participants, intervention, comparison, outcomes, and study design). The following were the criteria for inclusion (1): Participants [P]: cancer patients without distant metastases who required radiation or chemotherapy (2); Intervention [I]: probiotics were administered to patients in the experimental group (3); Comparison [C]: control group received placebo (4); Outcomes [O]: the incidence of adverse reactions following radiation or chemotherapy. In order to aggregate the results of as many trials as feasible, we neglected the particular causes of adverse events in this analysis (5); Study design [S]: randomised controlled trials (RCTs) and observational research, such as cohort and case-control studies.
Articles conforming to any of the following criteria were excluded (1): Reviews, case reports, correspondence, and abstracts (2); Poor quality or blatantly irrelevant studies were excluded (3); Research without available data that could be combined.
Two researchers independently retrieved information from the listed studies (Mr. Yang and Mr. Li): Included in the data were the first author, publication year, age range, cancer kind, probiotic type, results, sample size, and treatment plan. A third investigator arbitrated disagreements concerning data extraction. (Mrs. Guo).
Two investigators independently examined and cross-checked the risk of bias in the RCTs (Ms. Guo and Ms. Li). The following characteristics were evaluated using the Cochrane Handbook for Systematic Reviews of Interventions (Version 5.1) to determine the risk of bias: (a) existence of a precise, exact random sequence; (b) use of allocation concealment; (c) anonymisation of researchers and subjects; (d) evaluation of outcomes using a blind procedure; (e) complete data (prevention of probable follow-up loss); (f) study findings are not reported in a biased manner; (g) bias from external sources. These seven biases considered in each study were categorized as “high risk,” “low risk,” or “unclear” if insufficient information was available to determine the possible bias (11).
RevMan software version 5.4 was used to compile the pooled statistics (Cochrane Collaboration, Oxford, UK). The odds ratio (OR) and 95% confidence interval (CI) were selected as the effect indicators to analyze the data on the incidence of adverse events. Using the Cochrane Q test and the I2 statistic, which quantified the fraction of total variation attributable to heterogeneity as opposed to chance, heterogeneity between trials was examined (12). If the P-value of the Q test was greater than 0.10 and I2 was less than 50 percent, a fixed-effects model was applied to non-significantly heterogeneous data. In the absence of this, a random-effects model was applied to data with substantial variability (13, 14). In addition, a sensitivity analysis was conducted to explore the potential impact of a single study on the entire evaluation. This was accomplished by deleting one study at a time and combining the remaining trials. Furthermore, a funnel plot was utilized to assess the publication’s bias. Publication bias is unlikely if the points in a funnel plot are spread symmetrically on both sides of the dashed center line and are concentrated in the center. Otherwise, there is a strong likelihood of publishing bias.
Initial searches in PubMed, Embase, the Cochrane Library, and Web of Science yielded 396 articles after removing 143 duplicates. After this, 93 articles without the necessary qualifications were weeded out by reading the titles and abstracts. The remaining papers were reviewed in full, and 16 qualified articles were evaluated based on their structure and quality (7–10, 15–26). Figure 1 depicts the study selection procedure in detail.
Finally, 16 studies (7–10, 15–26) involving 2,097 cancer patients were included in our meta-analysis. It should be noted that from the statistical data, we classified the outcomes of each study as categorical variables or continuous variables. Only randomized controlled trials with categorical outcomes were included in this study. The papers of Babak et al. (10) and Hilda et al. (26) included outcomes with multiple categorical variables, so in these studies, we only included diarrhea as the study subject. No obvious bias was found in any of the other studies. Eight of the included studies focused on pelvic and abdominal cancers (7, 10, 20, 21, 23–26), five on tumors of the head and neck (8, 15–17, 22), two on breast cancer (9, 18), and one on non-small cell lung cancer (19). In addition, the number and combination of probiotic strains chosen in various clinical trials varied, with four studies (15, 17, 22, 26) using a single strain and the rest studies choosing two or more strains. Table 1 and Figure 2 detail the fundamental characteristics and risk of bias evaluation of the included research.
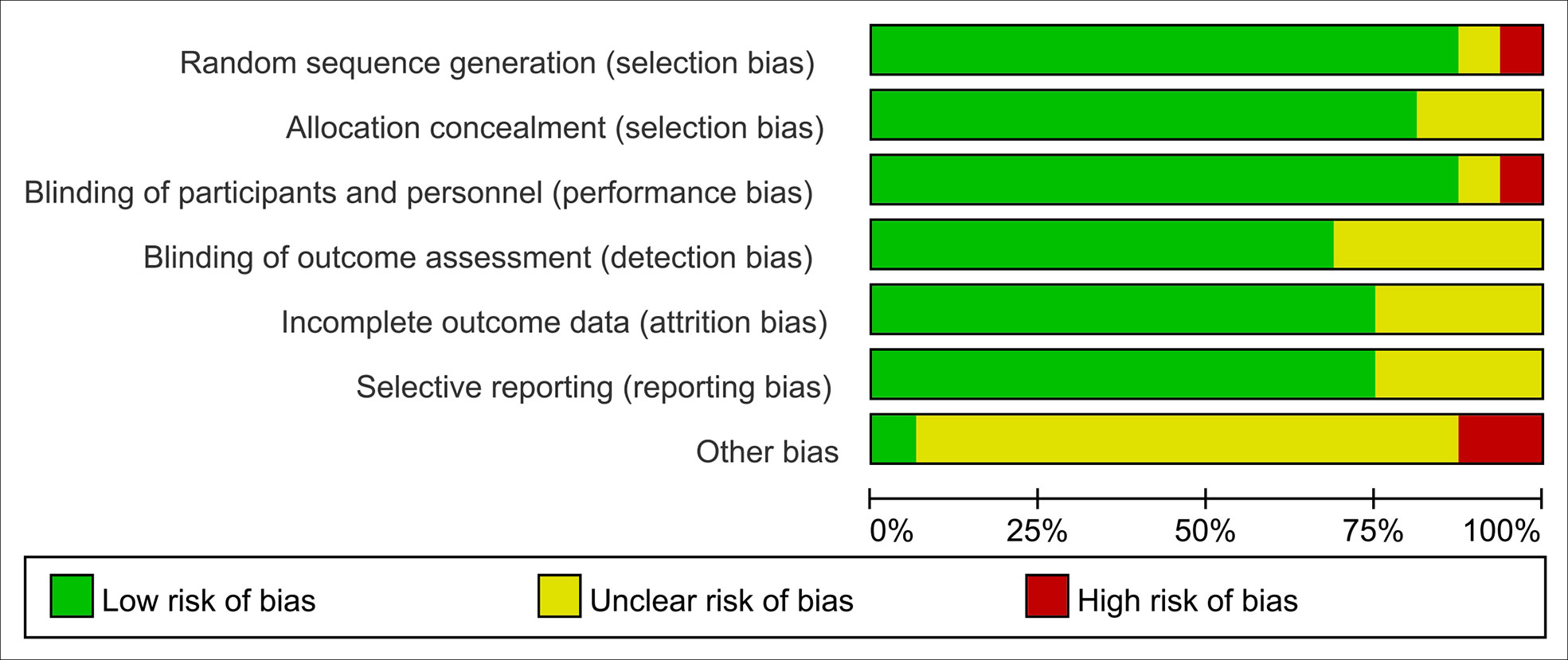
Figure 2 Risk of bias assessment, (risk of bias graph) review authors’ judgements about each risk of bias item presented as percentages across all included studies.
Data on side effects after chemoradiotherapy interventions were extracted from 16 articles including 2,097 patients. Data concerning side effects in this study only included dichotomous variables. In the case of multiple dichotomous variables, the data on the incidence of oral mucositis was preferred for head and neck tumors, and the data on diarrhea for pelvic and abdominal tumors were chosen. Due to the high between-study heterogeneity, the random-effects model was used (I2 ≥ 50%, P ≤ 0.10). Pooled results indicated that there was a significant difference between the probiotic group and the placebo group. By merging the results with clinical data from the included trials, we found that probiotic intervention effectively reduced the occurrence of radiation and chemotherapy-related side effects in various malignancies. As Figure 3 illustrates, the OR, expressed as treatment group vs. control group, was 0.31 (95% CI: 0.20 - 0.48; P < 0.005). No side effects due to probiotic administration were reported in any of the literature included in this part of the study.
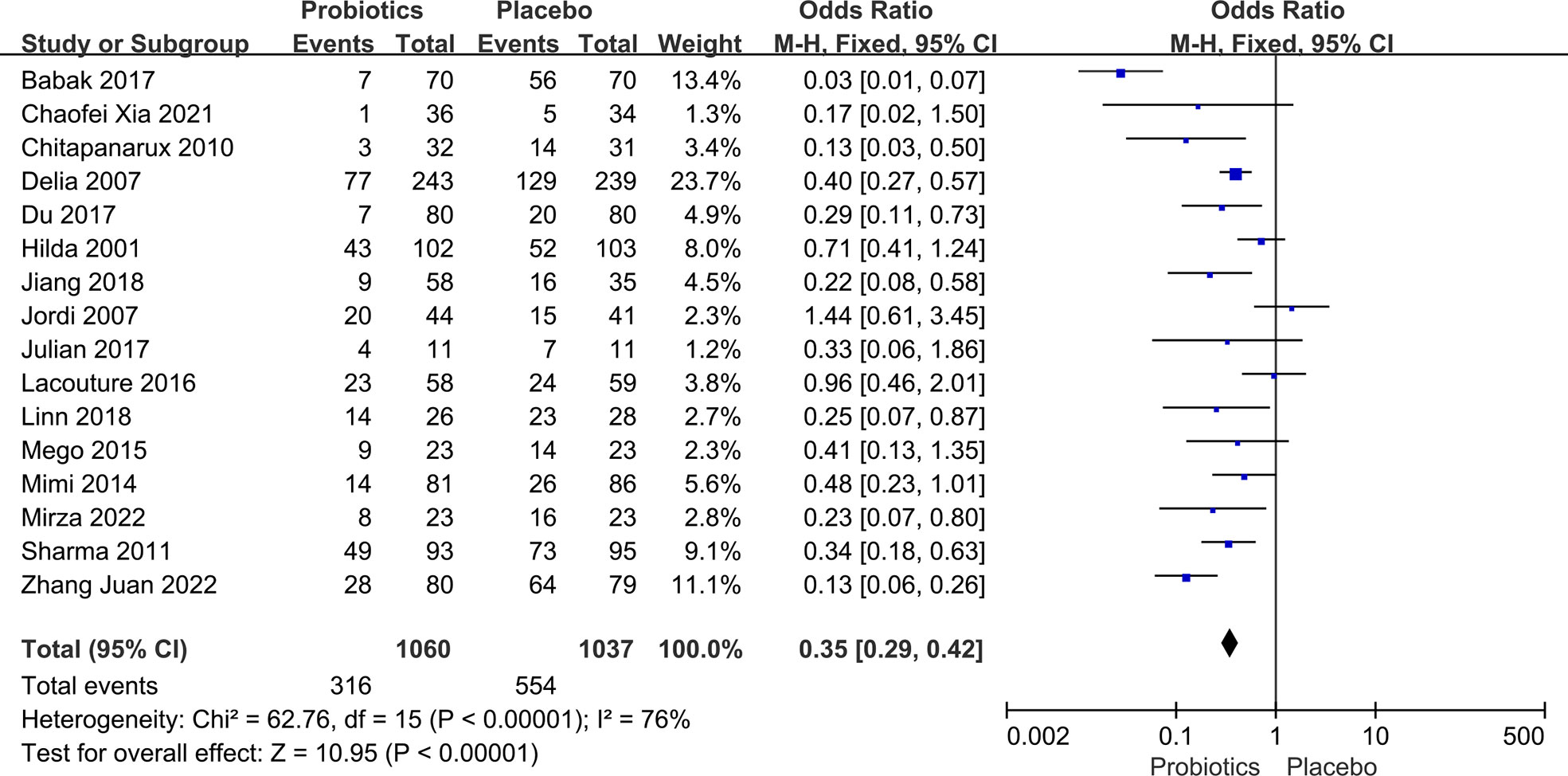
Figure 3 Forest plot of incidence of side effects in probiotic and placebo groups after radiotherapy or chemotherapy for different cancers.
The incidence of diarrhea after chemoradiotherapy for pelvic and abdominal tumors was extracted from eight studies (7, 10, 20, 21, 23–26) with 1,242 patients. The heterogeneity test revealed statistically significant variations between studies. (I2 ≥ 50%, P ≤ 0.10). Thus, a random-effects model was introduced. Compared to the placebo group, pelvic and abdominal cancer patients benefitted greatly from taking probiotics. The data demonstrated that the probability of diarrhea in the probiotic group was significantly lower than in the placebo group (OR: 0.32, 95% CI: 0.16 - 0.65; P < 0.005; Figure 4). In any of the literature that was looked at for this part of the study, there were no reports of side effects from taking probiotics.
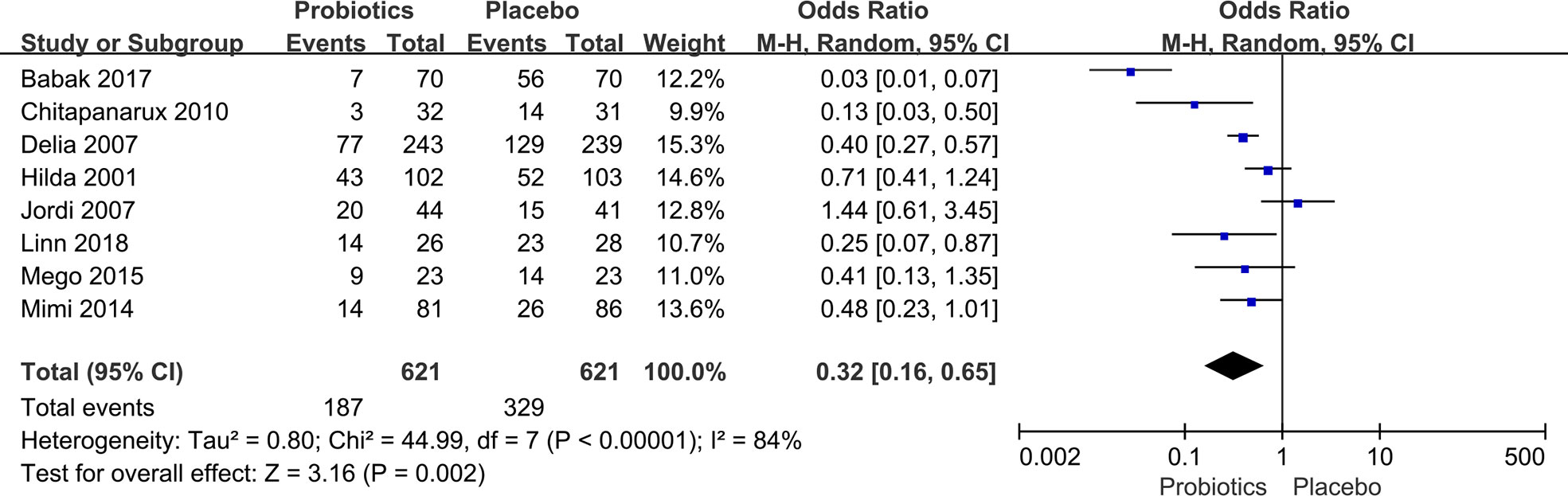
Figure 4 Forest plot of incidence of diarrhea in probiotic and placebo groups after radiation or chemotherapy for pelvic and abdominal cancer.
Five studies (8, 15–17, 22) involving 557 head and neck cancer patients were included in this analysis. During the analysis, we discovered no substantial heterogeneity between studies (I2 = 0%; P = 0.93), so a fixed-effects model was used. Compared to placebo, the use of probiotics decreased the incidence of oral mucositis in patients with head and neck cancer who had chemoradiotherapy (OR: 0.28, 95% CI: 0.18 - 0.43; P < 0.005; Figure 5). There were no reports of side effects from probiotic administration in any of the literature examined in this section of the study.
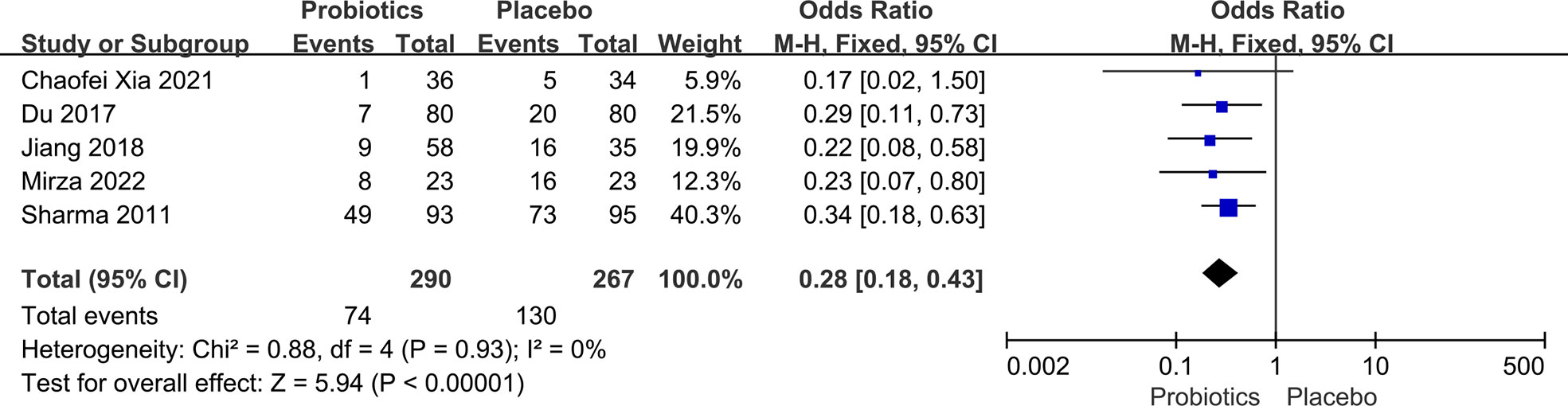
Figure 5 Forest plot of the incidence of oral mucositis in the probiotic and placebo groups after radiation or chemotherapy for head and neck cancer.
Our primary analysis results, as well as those of our sensitivity studies, were not significantly different from one another. The findings of sensitivity analyses for the three indicators of overall bad effects in various malignancies, diarrhea in pelvic and abdominal cancers, and oral mucositis in head and neck cancers, are presented in Appendix Figures S1, S2, and S3, respectively.
Utilizing a funnel plot, publication bias in the literature was evaluated. If there were at least 10 studies included in the meta-analysis, funnel plot asymmetry tests were conducted (27). The funnel plot of various indicators (Figure 6) demonstrates that the point estimates are symmetrically distributed on both sides and centered in the middle. Because of this, there was no proof of publishing bias.
In this study, we identified 16 RCTs that assessed the use of probiotics in the prevention of chemoradiotherapy-induced adverse events. Of these, eight papers reported on diarrhea data in patients with pelvic and abdominal cancers, five studies provided oral mucositis information on patients with head and neck tumors, two RCTs focused on adverse effects in breast cancer patients, and one paper described the side effects after chemoradiotherapy in non-small cell lung cancer patients. In terms of treatment tactics (strains, dosages, and probiotic therapy duration), patient ages, comorbidities, tumor kinds, and measured outcomes, these investigations were varied. This may explain the between-study heterogeneity of the results. For studies where meta-analyses were possible, sensitivity analyses showed no qualitative change in conclusions when changes between studies were assessed. Due to the limited number of heterogeneous studies, subgroup analysis were unable to be conducted. Ultimately, for all cancers included in this study, probiotics showed modest beneficial effects in reducing various side effects of chemoradiotherapy. A more comprehensive investigation revealed that probiotics significantly decreased oral mucositis in individuals with head and neck cancers and diarrhea in patients with pelvic-abdominal tumors following chemoradiotherapy. Consequently, probiotic supplementation may be considered a viable adjuvant therapy for patients who have had radiotherapy and chemotherapy.
In the past five years, three high-quality meta-analyses (28–30) have studied the effectiveness of probiotics in avoiding radiation and chemotherapy-related adverse effects in cancer patients. Feng et al. (28) conducted a pooled study on the incidence of oral mucositis and diarrhea after chemotherapy. Also, Wang et al. (30) studied the incidence of diarrhea after chemoradiotherapy for pelvic and abdominal tumors. Shu et al. (29) also focused on oral mucositis. In contrast, our meta-analysis included more randomized controlled studies with a total of 2,097 cancer patients from 16 publications. Additionally, our research was more diverse and we did not differentiate between the various forms of cancer, types of side effects, and treatments. In terms of treatment methods, cancer treatment is increasingly moving towards comprehensive treatment plans. This means it is necessary to have a deeper understanding of the side effects of radiotherapy and chemotherapy treatment, rather than to study them separately. Finally, the conclusions of this paper are more specific than in existing studies. Firstly, probiotics have a positive effect on most side effects reported after chemoradiotherapy. Moreover, they effectively prevent oral mucositis and diarrhea.
In a pooled analysis of all included studies (7–10, 15–26), each trial studied a variety of outcome indicators. Generally speaking, the indicators can be divided into two categories according to the variable type. One is categorical variables, which mainly include the incidence of certain adverse events, while the other is continuous variables, which comprise occurrence time, cut-off time, and adverse event severity score. Our meta-analysis revealed that for adverse event rates (binary variables), the probiotic group exhibited a significant reduction in side effects (OR: 0.31, 95% CI: 0.20 - 0.48; P < 0.005). Additionally, this summary included heterogeneous factors such as multiple cancer types (e.g., head and neck tumors, pelvic and abdominal tumors, breast cancer, lung cancer), multiple adverse reaction indicators (e.g., diarrhea, mucositis, cognitive impairment, vaginal microbiota changes), and multiple treatment methods (radiotherapy or chemotherapy), and results suggest that the probiotic group is generally superior to the placebo group. The “generally useful” effect of probiotics is explained in a review on the mechanism of probiotics published by Oelschlaeger (31) in 2010. Here, probiotics may modify host defenses, and this method of action is expected to promote the prevention and treatment of inflammation in the digestive tract or its components. In addition, probiotics have direct effects on commensal and/or pathogenic microbes.This idea is crucial for avoiding and treating infections and restoring the microbial balance in the gut in many instances (31).
Studies have also shown that radiation causes disruption of the gut microbiome, alters bacterial flora, disrupts host homeostasis, damages gut microvilli, decreases enzymatic activity, and reduces overall gut transit time, ultimately leading to diarrhea (21, 32–37). Moreover, a number of in vitro and in vivo research demonstrated that chemotherapeutic medicines trigger death in crypt cells, leading to decreased intestinal absorption, altered gut microbiota, impaired gut homeostasis, and ultimately diarrhea (38–40).. In our research, the incidence of diarrhea, defined as the frequency of Grade 3 or 2 diarrhea, was used as the primary outcome. Regarding the incidence of diarrhea in patients with abdominal and pelvic malignancies, this meta-analysis demonstrated a significant reduction in the probiotic group (OR: 0.32, 95% CI: 0.16 - 0.65; P < 0.005). Additionally, we found that among the eight studies (7, 10, 20, 21, 23–26) that included diarrhea data, the results of Urbancsek et al. (26) and Giralt et al. (24) showed larger OR values, at 0.71 and 1.44, respectively. This indicated that the advantage of the probiotic group was not obvious or even nonexistent, which may be related to the fact that they only selected a single species of Lactobacillus rhamnosus or Lactobacillus casei DN-114 001 in clinical trials. In the other studies (7, 10, 20, 21, 23, 25), using multiple probiotic combinations, the probiotic groups showed a significant reduction in diarrhea. These findings are consistent with the study by Feng et al. (28).
In clinical practice, the occurrence of oral mucositis after chemoradiotherapy in patients with head and neck tumors is essentially unavoidable. We included a meta-analysis of five studies involving 557 participants to evaluate the effectiveness of probiotics in the prevention and treatment of oral mucositis caused by cancer treatments, including chemotherapy, radiotherapy, and chemoradiotherapy. Results of the pooled analysis showed that probiotic intervention effectively reduced the incidence of oral mucositis (OR: 0.28, 95% CI: 0.18 - 0.43; P < 0.005). Additionally, there was no significant heterogeneity (I2 = 0, P = 0.93) among the five clinical trials. Interestingly, three researchers (15, 17, 22) used a single strain of probiotic, while two (8, 16) used a combination of multiple strains, but their results all showed that the probiotic group exhibited obvious advantages. These findings contradict the conclusion that a single probiotic strain is ineffective against diarrhea in patients with pelvic and abdominal tumors.
Systemic infections, detrimental metabolic activity, excessive immunological activation, gene transfer, obesity, skin problems, and gastrointestinal side effects have been linked to probiotic use (41).. Due to the heterogeneity of different treatment regimens and malignancies, probiotic-related side effects could not be distinguished. None of the studies included in this paper reported adverse effects caused by probiotics, but Giralt et al. (24) claimed that taking probiotics did not improve post-radiation diarrhea in gynecological cancer patients. Of the 41 patients in the placebo group, 24 suffered Grade 2 or higher diarrhea, while 30 of the 44 patients in the probiotic group had Grade 2 or higher diarrhea. In conclusion, based on the available evidence, we are still unable to estimate whether it is safe for cancer patients to receive probiotics for various side effects due to the numerous variables involved, such as probiotic strain type, dosage, duration of use, and suitability of patients with the right physical condition.
Despite the fact that our results demonstrate that probiotics can lessen various adverse effects of cancer treatment, including oral mucositis and diarrhea, it is important to note some potential limits. First, there are few studies available. Besides, the participants in the study were diagnosed with different types of cancer, which may affect the reliability of the final results. Also, there are no clinical standards for the active ingredients and dosage of probiotics, which leads to different experimental protocols in each study. Finally, most of the studies focused on head and neck, pelvic, and abdominal tumors, with only two studies concerning breast cancer and one study involving lung cancer, making the findings less general. However, since this is a more comprehensive and systematic meta-analysis of the topic, the results are meaningful and clinically valuable. Microorganisms have recently been shown to play an important role in many diseases (42, 43). As with the aforementioned constraints, the choice and mix of probiotics and target demographics varies from study to study, making it challenging to suggest and approve them in clinical usage guidelines. This study may help researchers to select appropriate probiotics and extrapolate their potentially beneficial effects on patients, especially head and neck, pelvic, and abdominal tumors.
This meta-analysis reveals that probiotics may lower the occurrence of side effects caused by cancer therapy, particularly oral mucositis in patients with head and neck malignancies and diarrhea in individuals with pelvic and abdominal tumors. Due to the modest number of clinical trials included in this study, however, additional randomized, double-blind, multicentric trials in a broader population are necessary. This work assists researchers in improving the design of clinical trials involving the selection of probiotic strains and the selection of patients who may benefit from probiotic therapy.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
YKL, DY, and XL: Conceptualization. YL, TG, BL: Data curation and original draft writing. RC, XG, WY: Statistical analysis. YL, JC: Manuscript review and editing. All authors contributed to the article and approved the submitted version.
Xi'an Innovation Capability Strong Foundation Project No. 21XYJ0021; Xi'an Central Hospital Scientific Research Project No. 2022QN06.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1032145/full#supplementary-material
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Maria OM, Eliopoulos N, Muanza T. Radiation-induced oral mucositis. Front Oncol (2017) 7:89. doi: 10.3389/fonc.2017.00089
3. Visich KL, Yeo TP. The prophylactic use of probiotics in the prevention of radiation therapy-induced diarrhea. Clin J Oncol Nurs (2010) 14(4):467–73. doi: 10.1188/10.Cjon.467-473
4. Paydar I, Cyr RA, Yung TM, Lei SY, Collins BT, Chen LN, et al. Proctitis 1 week after stereotactic body radiation therapy for prostate cancer: Implications for clinical trial design. Front Oncol (2016) 6:167. doi: 10.3389/fonc.2016.00167
5. Ouimet LA, Stewart A, Collins B, Schindler D, Bielajew C. Measuring neuropsychological change following breast cancer treatment: An analysis of statistical models. J Clin Exp Neuropsychol (2009) 31(1):73–89. doi: 10.1080/13803390801992725
6. Liu YQ, Wang XL, He DH, Cheng YX. Protection against chemotherapy- and radiotherapy-induced side effects: A review based on the mechanisms and therapeutic opportunities of phytochemicals. Phytomedicine (2021) 80:1–16. doi: 10.1016/j.phymed.2020.153402
7. Linn YH, Thu KK, Win NHH. Effect of probiotics for the prevention of acute radiation-induced diarrhoea among cervical cancer patients: a randomized double-blind placebo-controlled study. Probiotics Antimicrob Proteins (2019) 11(2):638–47. doi: 10.1007/s12602-018-9408-9
8. Jiang C, Wang H, Xia C, Dong Q, Chen E, Qiu Y, et al. A randomized, double-blind, placebo-controlled trial of probiotics to reduce the severity of oral mucositis induced by chemoradiotherapy for patients with nasopharyngeal carcinoma. Cancer (2019) 125(7):1081–90. doi: 10.1002/cncr.31907
9. Zhang J, Jie C, Boni D, Liang YP, Kai L, Ling W, et al. Probiotic supplement attenuates chemotherapy-related cognitive impairment in patients with breast cancer: a randomised, double-blind, and placebo-controlled trial. Eur J Cancer (2022) 161:10–22. doi: 10.1016/j.ejca.2021.11.006
10. Golkhalkhali B, Rajandram R, Paliany AS, Ho GF, Ishak WZW, Johari CS, et al. Strain-specific probiotic (microbial cell preparation) and omega-3 fatty acid in modulating quality of life and inflammatory markers in colorectal cancer patients: a randomized controlled trial. Asia-Pacific J Clin Oncol (2018) 14(3):179–91. doi: 10.1111/ajco.12758
11. Higgins Jpt AD. Chapter 8. assessing risk of bias in included studies. In: Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions (2011). Available from: www.cochrane-handbook.org.
12. Higgins JPT TJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane handbook for systematic reviews of interventions version 6.1 (updated September 2020). Cochrane (2020). www.training.cochrane.org/handbook. doi: 10.1002/9781119536604
13. DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clin trials (1986) 7(3):177–88. doi: 10.1016/0197-2456(86)90046-2
14. Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst (1959) 22(4):719–48.
15. Mirza MA, Aruna D, Irukulla M. Efficacy of bacillus clausii UBBC - 07 spores in the amelioration of oral mucositis in head and neck cancer patients undergoing radiation therapy. Cancer Treat Res Commun (2022) 31:100523–. doi: 10.1016/j.ctarc.2022.100523
16. Xia C, Jiang C, Li W, Wei J, Hong H, Li J, et al. A phase II randomized clinical trial and mechanistic studies using improved probiotics to prevent oral mucositis induced by concurrent radiotherapy and chemotherapy in nasopharyngeal carcinoma. Front Immunol (2021) 12:618150. doi: 10.3389/fimmu.2021.618150
17. Du SX, Jia YR, Ren SQ, Gong XJ, Tang H, Wan-Shui W, et al. The protective effects of bacillus licheniformis preparation on gastrointestinal disorders and inflammation induced by radiotherapy in pediatric patients with central nervous system tumor. Adv Med Sci (2018) 63(1):134–9. doi: 10.1016/j.advms.2017.09.005
18. Marschalek J, Farr A, Marschalek M-L, Domig KJ, Kneifel W, Singer CF, et al. Influence of orally administered probiotic lactobacillus strains on vaginal microbiota in women with breast cancer during chemotherapy: A randomized placebo-controlled double-blinded pilot study. Breast Care (2017) 12(5):335–9. doi: 10.1159/000478994
19. Lacouture ME, Keefe DM, Sonis S, Jatoi A, Gernhardt D, Wang T, et al. A phase II study (ARCHER 1042) to evaluate prophylactic treatment of dacomitinib-induced dermatologic and gastrointestinal adverse events in advanced non-small-cell lung cancer. Ann Oncol (2016) 27(9):1712–8. doi: 10.1093/annonc/mdw227
20. Mego M, Chovanec J, Vochyanova-Andrezalova I, Konkolovsky P, Mikulova M, Reckova M, et al. Prevention of irinotecan induced diarrhea by probiotics: A randomized double blind, placebo controlled pilot study. Complement Ther Med (2015) 23(3):356–62. doi: 10.1016/j.ctim.2015.03.008
21. Demers M, Dagnault A, Desjardins J. A randomized double-blind controlled trial: impact of probiotics on diarrhea in patients treated with pelvic radiation. Clin Nutr (2014) 33(5):761–7. doi: 10.1016/j.clnu.2013.10.015
22. Sharma A, Rath GK, Chaudhary SP, Thakar A, Mohanti BK, Bahadur S. Lactobacillus brevis CD2 lozenges reduce radiation- and chemotherapy-induced mucositis in patients with head and neck cancer: A randomized double-blind placebo-controlled study. Eur J Cancer (2012) 48(6):875–81. doi: 10.1016/j.ejca.2011.06.010
23. Chitapanarux I, Chitapanarux T, Traisathit P, Kudumpee S, Tharavichitkul E, Lorvidhaya V. Randomized controlled trial of live lactobacillus acidophilus plus bifidobacterium bifidum in prophylaxis of diarrhea during radiotherapy in cervical cancer patients. Radiat Oncol (2010) 5:31. doi: 10.1186/1748-717x-5-31
24. Giralt J, Regadera JP, Verges R, Romero J, de la Fuente I, Biete A, et al. Effects of probiotic lactobacillus casei DN-114 001 in prevention of radiation-induced diarrhea: results from multicenter, randomized, placebo-controlled nutritional trial. Int J Radiat Oncol Biol Phys (2008) 71(4):1213–9. doi: 10.1016/j.ijrobp.2007.11.009
25. Delia P, Sansotta G, Donato V, Frosina P, Messina G, De Renzis C, et al. Use of probiotics for prevention of radiation-induced diarrhea. World J Gastroenterol (2007) 13(6):912–5. doi: 10.3748/wjg.v13.i6.912
26. Urbancsek H, Kazar T, Mezes I, Neumann K. Results of a double-blind, randomized study to evaluate the efficacy and safety of antibiophilus (R) in patients with radiation-induced diarrhoea. Eur J Gastroenterol Hepatol (2001) 13(4):391–6. doi: 10.1097/00042737-200104000-00015
27. Sterne JAC, Sutton AJ, Ioannidis JPA, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ (2011) 343:d4002. doi: 10.1136/bmj.d4002
28. Feng J, Gao M, Zhao C, Yang J, Gao H, Lu X, et al. Oral administration of probiotics reduces chemotherapy-induced diarrhea and oral mucositis: A systematic review and meta-analysis. Front Nutr (2022) 9:823288. doi: 10.3389/fnut.2022.823288
29. Shu ZK, Li PJ, Yu BQ, Huang S, Chen YY. The effectiveness of probiotics in prevention and treatment of cancer therapy-induced oral mucositis: A systematic review and meta-analysis. Oral Oncol (2020) 102:1-11. doi: 10.1016/j.oraloncology.2019.104559
30. Wang YH, Yao N, Wei KK, Jiang L, Hanif S, Wang ZX, et al. The efficacy and safety of probiotics for prevention of chemoradiotherapy-induced diarrhea in people with abdominal and pelvic cancer: a systematic review and meta-analysis. Eur J Clin Nutr (2016) 70(11):1246–53. doi: 10.1038/ejcn.2016.102
31. Oelschlaeger TA. Mechanisms of probiotic actions – a review. Int J Med Microbiol (2010) 300(1):57–62. doi: 10.1016/j.ijmm.2009.08.005
32. Scartoni D, Desideri I, Giacomelli I, Di Cataldo V, Di Brina L, Mancuso A, et al. Nutritional supplement based on zinc, prebiotics, probiotics and vitamins to prevent radiation-related gastrointestinal disorders. Anticancer Res (2015) 35(10):5687–92.
33. Bismar MM, Sinicrope FA. Radiation enteritis. Curr Gastroenterol Rep (2002) 4(5):361–5. doi: 10.1007/s11894-002-0005-3
34. Berg RD. Bacterial translocation from the gastrointestinal tract. Adv Exp Med Biol (1999) 473:11–30. doi: 10.1007/978-1-4615-4143-1_2
35. Link-Amster H, Rochat F, Saudan KY, Mignot O, Aeschlimann JM. Modulation of a specific humoral immune response and changes in intestinal flora mediated through fermented milk intake. FEMS Immunol Med Microbiol (1994) 10(1):55–63. doi: 10.1111/j.1574-695X.1994.tb00011.x
36. Famularo G, Mosca L, Minisola G, Trinchieri V, De Simone C. Probiotic lactobacilli: a new perspective for the treatment of inflammatory bowel disease. Curr Pharm Des (2003) 9(24):1973–80. doi: 10.2174/1381612033454207
37. Packey CD, Ciorba MA. Microbial influences on the small intestinal response to radiation injury. Curr Opin Gastroenterol (2010) 26(2):88–94. doi: 10.1097/MOG.0b013e3283361927
38. Gibson RJ, Bowen JM, Inglis MR, Cummins AG, Keefe DM. Irinotecan causes severe small intestinal damage, as well as colonic damage, in the rat with implanted breast cancer. J Gastroenterol Hepatol (2003) 18(9):1095–100. doi: 10.1046/j.1440-1746.2003.03136.x
39. Gibson RJ, Keefe DM. Cancer chemotherapy-induced diarrhoea and constipation: mechanisms of damage and prevention strategies. Support Care Cancer (2006) 14(9):890–900. doi: 10.1007/s00520-006-0040-y
40. McQuade RM, Stojanovska V, Abalo R, Bornstein JC, Nurgali K. Chemotherapy-induced constipation and diarrhea: Pathophysiology, current and emerging treatments. Front Pharmacol (2016) 7:414. doi: 10.3389/fphar.2016.00414
41. Redman MG, Ward EJ, Phillips RS. The efficacy and safety of probiotics in people with cancer: a systematic review. Ann Oncol (2014) 25(10):1919–29. doi: 10.1093/annonc/mdu106
42. Hou J, Zheng H, Li P, Liu H, Zhou H, Yang X. Distinct shifts in the oral microbiota are associated with the progression and aggravation of mucositis during radiotherapy. Radiother Oncol (2018) 129(1):44–51. doi: 10.1016/j.radonc.2018.04.023
Keywords: probiotic, placebo, chemoradiotherapy, cancer, meta-analysis
Citation: Lu Y, Luo X, Yang D, Li Y, Gong T, Li B, Cheng J, Chen R, Guo X and Yuan W (2022) Effects of probiotic supplementation on related side effects after chemoradiotherapy in cancer patients. Front. Oncol. 12:1032145. doi: 10.3389/fonc.2022.1032145
Received: 30 August 2022; Accepted: 11 October 2022;
Published: 28 October 2022.
Edited by:
Valerio Nardone, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Francolini Giulio, University of Florence, ItalyCopyright © 2022 Lu, Luo, Yang, Li, Gong, Li, Cheng, Chen, Guo and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ruijuan Chen, am9uYXNjaGVuMTk4OUB5ZWFoLm5ldA==; Xin Guo, Z3VveDA2QHFxLmNvbQ==; Wei Yuan, eXVhbndlaXdlaWlAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.