- 1Department of Gastroenterology, Beijing Friendship Hospital, Capital Medical University, National Clinical Research Center for Digestive Diseases, Beijing Digestive Disease Center, Beijing, China
- 2Department of Clinical Epidemiology and Evidence-based Medicine, Beijing Friendship Hospital, Capital Medical University, Beijing, China
Background and aims: Colorectal neoplasms (CRN) include colorectal cancer (CRC) and colorectal adenoma (CRA). The relationship between CRN and triglyceride-glucose (TyG) index or between CRN and atherogenic index of plasma (AIP) is unclear. This study aims to investigate the roles of TyG index and AIP in predicting CRN in people without cardiovascular disease (CVD).
Methods: 2409 patients without CVD underwent colonoscopy were enrolled. Clinical information and relevant laboratory test results of these patients were collected and recorded. According to endoscopic and pathological results, all participants were divided into a neoplasms group and a non-neoplasms group. The TyG index was calculated as ln (TGs×FPG/2), while AIP was calculated as log (TGs/HDL-C). We used uni- and multivariate logistic regression and restricted cubic spline (RCS) to analyze the association between the TyG inedx, AIP and CRN, develop predictive models and construct the nomograms. Receiver operating characteristic (ROC) curves were utilized to evaluate the predictive value for CRN.
Results: Participants in the neoplasms group were more likely to be older, have higher TyG index, higher AIP and higher rates of fecal occult blood test positivity, and were more likely to be male, smokers and those with the family history of CRC (P < 0.05). The higher TyG index was related to the higher risk of CRN [OR (95% CI): 1.23 (1.08 - 1.41), P = 0.003]. The higher AIP was related to the higher risk of CRN [OR (95% CI): 1.55 (1.16 - 2.06), P = 0.003]. These two indicators are better for predicting CRN in women than men. The combined use of the TyG index and other independent risk factors (age, sex, smoking status, family history and FOBT) to distinguish CRN was effective, with a sensitivity of 61.0%, a specificity of 65.1% and an AUC of 0.669 (95%CI, 0.639 - 0.698). Likewise, the combined use of the AIP and other independent risk factors to distinguish CRN was also effective, the model had an overall 56.3% sensitivity and 68.7% specificity with an AUC of 0.667 (95%CI, 0.638 - 0.697).
Conclusion: This study showed that the TyG index and the AIP might be biomarkers that could be used to predict the risk of CRN in patients without CVD.
Introduction
Colorectal cancer (CRC) is recognized as the world’s fourth most deadly cancer with almost 900,000 deaths annually (1). Colorectal adenoma (CRA) is usually regarded to be precursor lesion for most CRC (2, 3). Both are collectively referred to as colorectal neoplasms (CRN), which are major public health problems that result in significant economic burdens for governments. There is an urgent need for usable and reliable non-invasive markers to make an early diagnosis of patients at risk of colorectal neoplasms in the general population, and then encourage those at risk to undergo a colonoscopy to reduce CRC morbidity and mortality (4).
Recently, the triglyceride-glucose (TyG) index and the atherosclerotic index of plasma (AIP) have attracted extensive attention and medical research. The TyG index, a parameter derived from the fasting blood glucose and triglyceride levels, has been evaluated as a reliable surrogate for insulin resistance (IR) (5–7). Numerous studies have shown that TyG index is an autonomous risk factor for the incidence of cardiovascular disease (CVD) (8–11). It is also associated with an increased risk of diabetes (12), hypertension (13) and nonalcoholic fatty liver disease (14). The atherogenic index of plasma (AIP) is the logarithmically transformed ratio of triglyceride (TG) to high-density lipoprotein-cholesterol (HDL-C) in molar concentration (mmol/L) (15). It has been proven to be a reliable biomarker for the prediction of various vascular diseases (16). AIP is also closely related to insulin resistance, diabetes and metabolic syndrome (17–19). Although these two indicators have demonstrated an outstanding performance in diagnosing CVD and metabolic disease, the association between either TyG index or AIP and CRN still needs further exploration. To date few relevant studies have been scheduled to investigate this correlation.
Considering that the TyG index and AIP are closely related to CVD, we chose to explore the role of these two indicators in predicting CRN in those people without CVD. We chose this approach in order to exclude the effect of CVD on these two indicators.
Methods
Participants
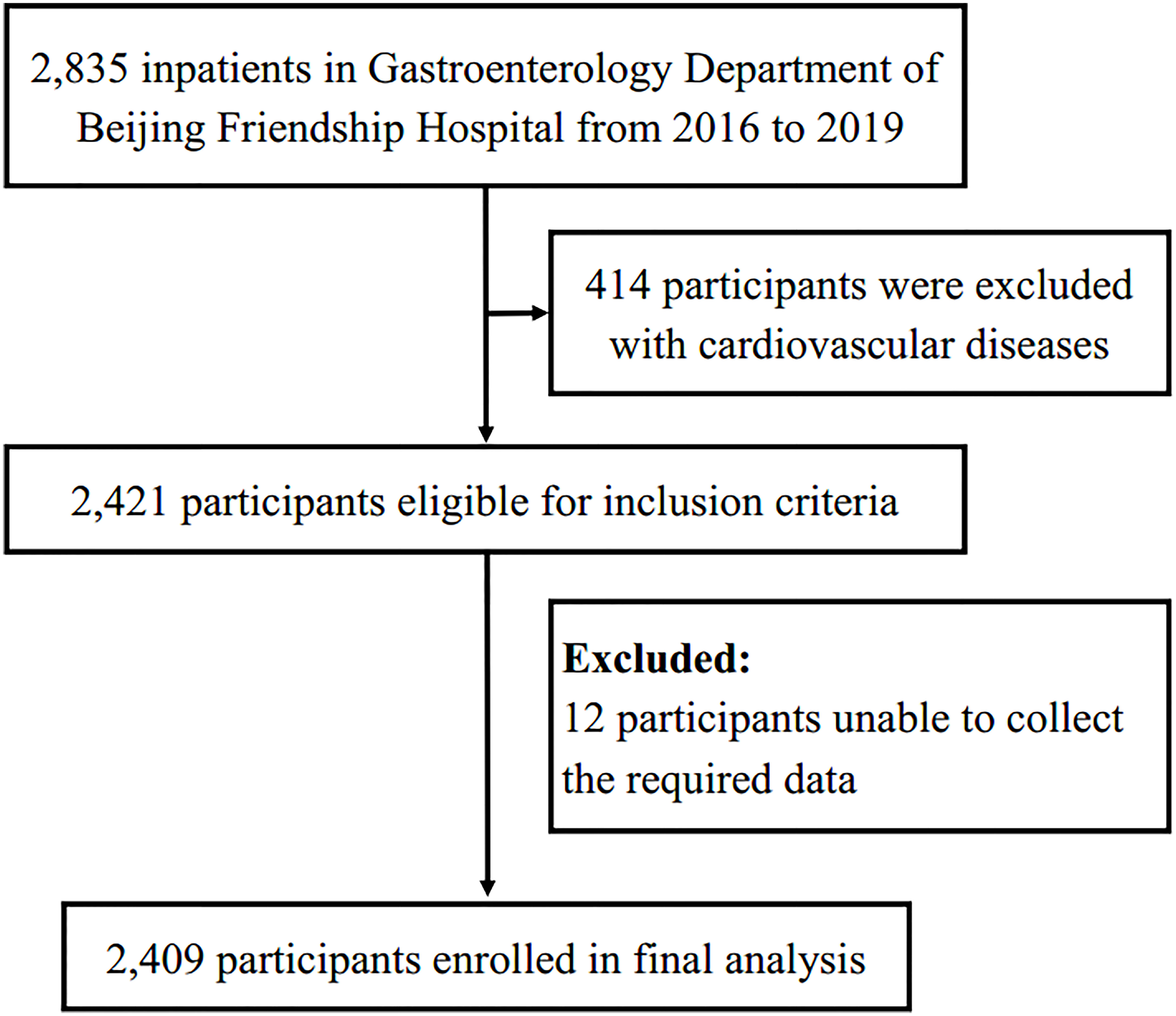
This is a single-center retrospective analysis of 2835 patients (aged ≥ 18 years) that were admitted to Beijing Friendship Hospital. They underwent a colonoscopy during the period January 1, 2016 to December 31, 2019. Participants with a reported previous history of cardiovascular diseases (n=414) and participants with missing baseline data (n=12) were excluded. In total, 2409 participants were finally included in the current study (Figure 1). This study was in accordance with the principles of the Declaration of Helsinki and approved by the ethics committee of Beijing Friendship Hospital, Capital Medical University (No.2021-P3-138-01). Given the retrospective nature of the present research, no informed consent was required.
Data collection and definitions
Data of demographic and clinical characteristics, including sex, age, body mass index (BMI), history of smoking and drinking, and family history of CRC were extracted from the electronic medical recording system of the Beijing Friendship Hospital. On admission, blood samples from the median cubital vein were collected early in the morning after an overnight fast (> 8 h). We analyzed fasting triglyceride (TGs), fasting plasma glucose (FPG) and high-density lipoprotein-cholesterol (HDL-C) levels immediately after sampling. Fecal samples were taken for fecal occult blood testing (FOBT) within 24 hours before the scheduled colonoscopy. The TyG index was calculated as ln [fasting TGs (mg/dL)×FPG (mg/dL)/2] (20). AIP was analytically calculated from log (TGs/HDL-C), where TGs and HDL-C were expressed in millimole per liter (15). CVD are defined as pathological conditions involving the cardiovascular system including the heart, the blood vessels, or the pericardium.
A colonoscopy was performed by endoscopists that had performed a minimum of 500 colonoscopies. All detected polyps were biopsied or removed. The histopathology was determined by experienced gastrointestinal pathologists. According to endoscopic and pathological results, all participants were divided into a neoplasms group and a non-neoplasms group. The neoplasms group consisted of patients with CRC or CRA. The non-neoplasms group consisted of patients without any evidence of elevated lesions under endoscopy, as well as patients with non-adenomatous colorectal polyps.
Statistical analysis
Normally distributed continuous variables were reported as mean ± standard deviation (SD), and categorical variables were represented as frequencies and percentages. The Student t-test was applied for the continuous variables and the Chi-square test was applied for categorical variables to compare the differences between the two groups. Univariate and multivariate logistic regression models were used to evaluate the associations between different indices and neoplasms in non-CVD participants. Model 1: adjusted for age; Model 2: adjusted for age and sex; Model 3: adjusted for age, sex, family history and FOBT. The restricted cubic splines with 4 knots were applied to determine the nonlinear relationship between different indices and the risk of neoplasms in non-CVD participants. A multivariate binary logistic regression was used to construct diagnostic models for neoplasms. Receiver operating characteristic (ROC) curves and the area under the curve (AUC) were used to assess the diagnostic performance of the models. To facilitate the clinical application of diagnostic model 3, a nomogram was developed based on AIP, TyG and other potential risk factors of neoplasms. A data analysis was performed using SPSS 25.0 (IBM Corporation, New York, USA) and R version 4.1.2 (R core Team 2017). All reported P values were two-tailed, and P < 0.05 was considered to be statistically significant.
Results
Baseline characteristics
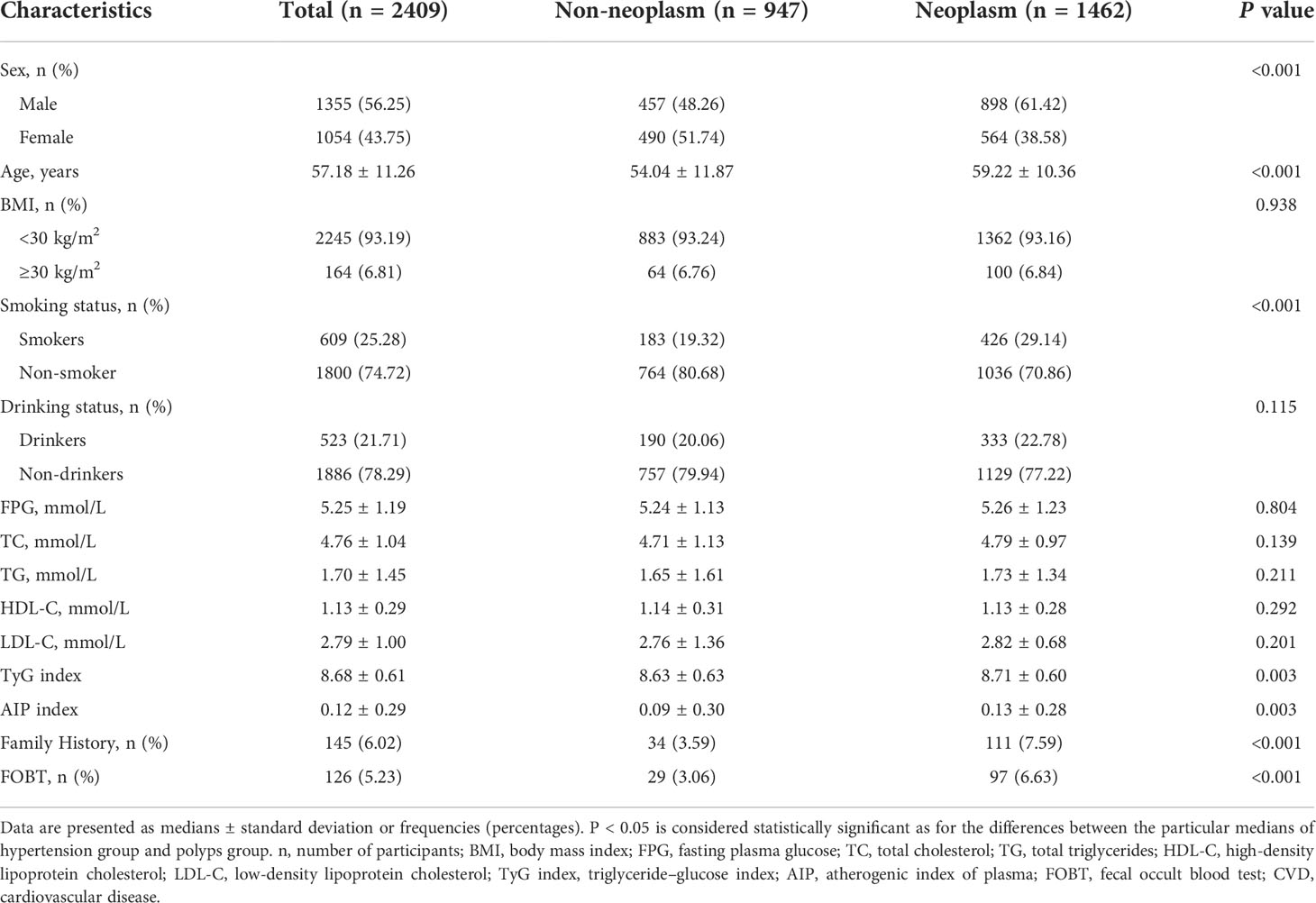
The 2409 participants (56.25% men, mean age 57.18 ± 11.26 years) were divided into a non-neoplasms group (n=947) and a neoplasms group (n=1462). Baseline characteristics of the total population and groups are presented in Table 1. Participants in the neoplasms group were more likely to be older, have higher TyG index and AIP, and have higher rates of fecal occult blood test (FOBT) positivity. They were also more likely to be male, smokers and have a family history of colorectal cancer (P < 0.05).
Associations between TyG index, AIP and neoplasm
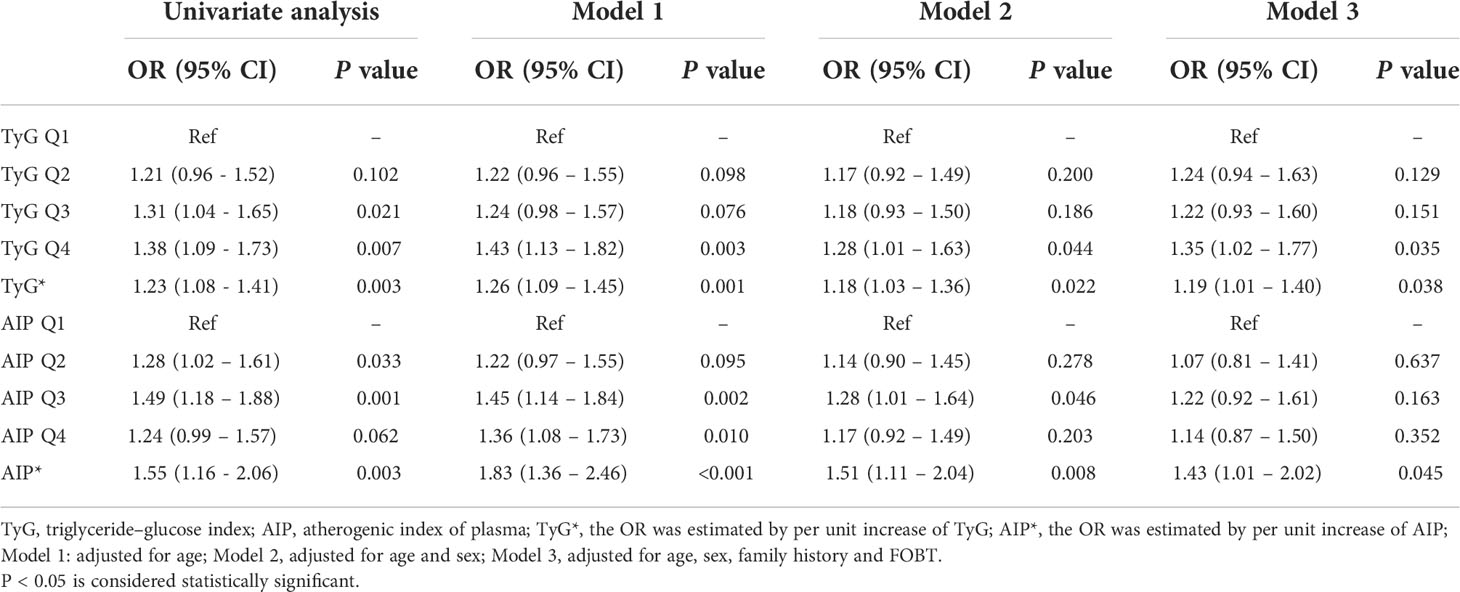
As shown in Table 2, as a ranking variable, the TyG index in the highest quartile (the lowest quartile as the reference) was associated with a higher risk of CRN [OR (95% CI): 1.38 (1.09 - 1.73), P = 0.007]. As a continuous variable, the higher TyG index was independently related to the higher risk of CRN [OR (95% CI): 1.23 (1.08 - 1.41), P = 0.003]. In multi-variable adjusted models, the associations between TyG index and any CRN did not attenuate. Similarly, the higher AIP was related to the higher risk of CRN [OR (95% CI): 1.55 (1.16 - 2.06), P = 0.003], and the association between AIP and CRN was still strong (P < 0.05) with an increase in other risk factors (age, sex, family history and FOBT). In the age-adjusted model, individuals in the highest quartile of AIP had a higher risk of developing CRN [OR (95% CI): 1.36 (1.08 - 1.73), P = 0.010] when the lowest quartile of AIP was used as a reference.
Restricted cubic spline
In Figure 2, we used the restricted cubic splines to visualize the relationship between TyG index, AIP and CRN across the different sexes. Within the normal AIP range, female individuals with a higher AIP had an increased prevalence of CRN when the AIP value was set to 0.041, as a reference. Analogously, in the normal TyG range, when a TyG value of 8.551 was used as a reference, female individuals with higher TyG had an increased prevalence of CRN. However, these associations were less pronounced in males.

Figure 2 Restricted cubic spline plot of the association between TyG, AIP index and neoplasm in non-CVD participants. (A) the association between AIP and neoplasm in total participants. (B) the association between TyG index and neoplasm in total participants. (C) the association between AIP and neoplasm in male participants. (D) the association between TyG index and neoplasm in male participants. (E) the association between AIP and neoplasm in female participants. (F) the association between TyG index and neoplasm in female participants.
The development of the diagnostic models and the nomogram
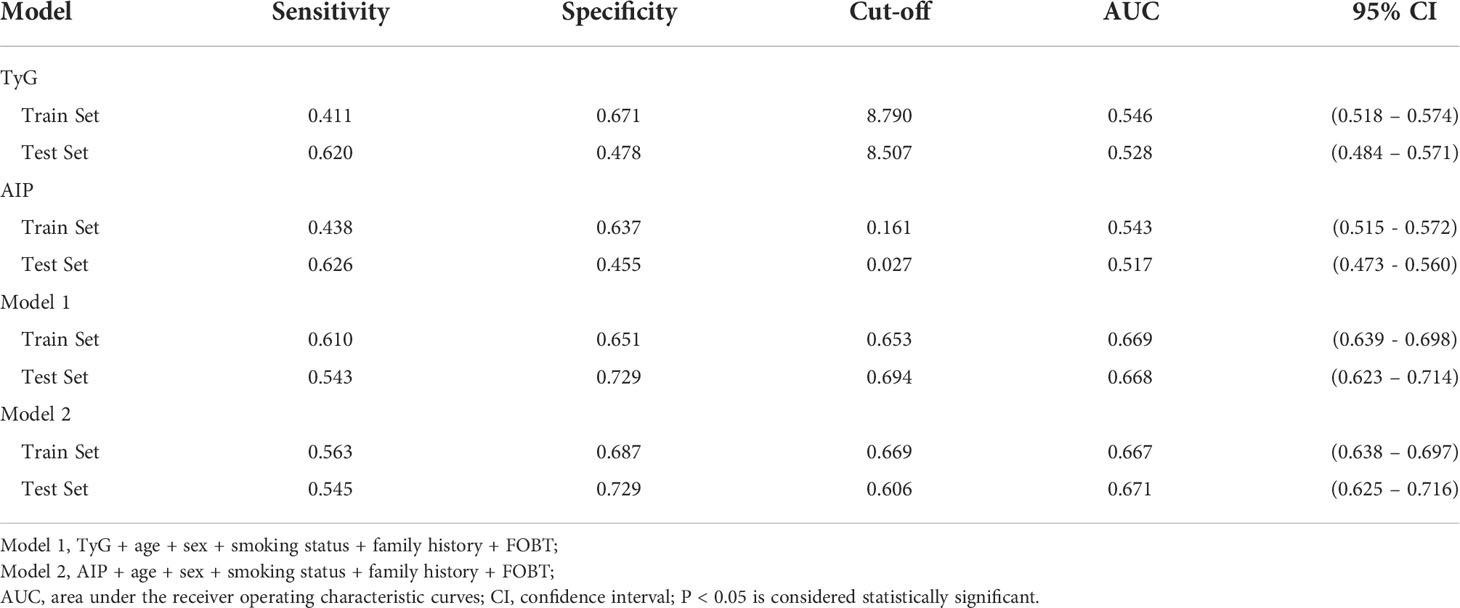
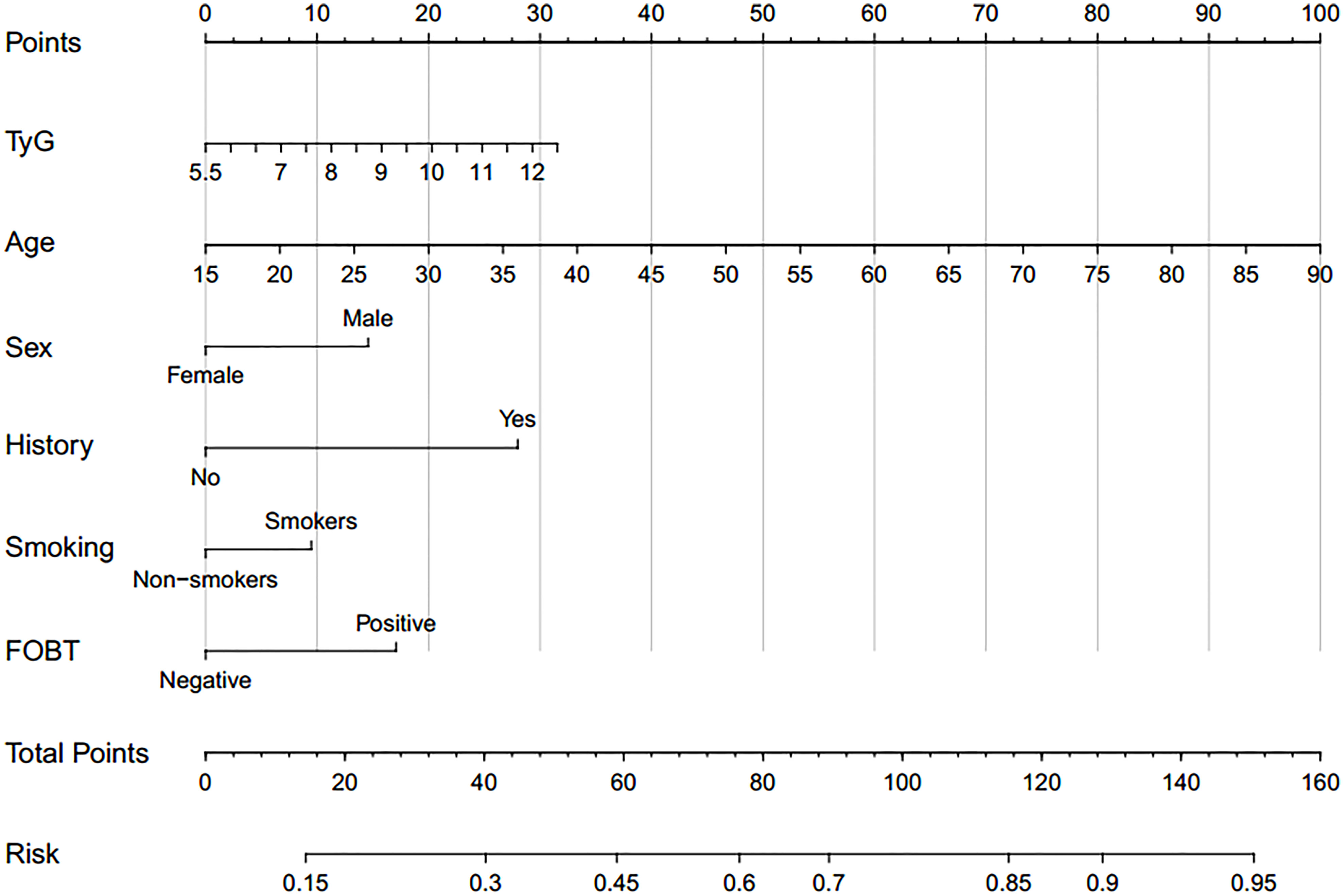
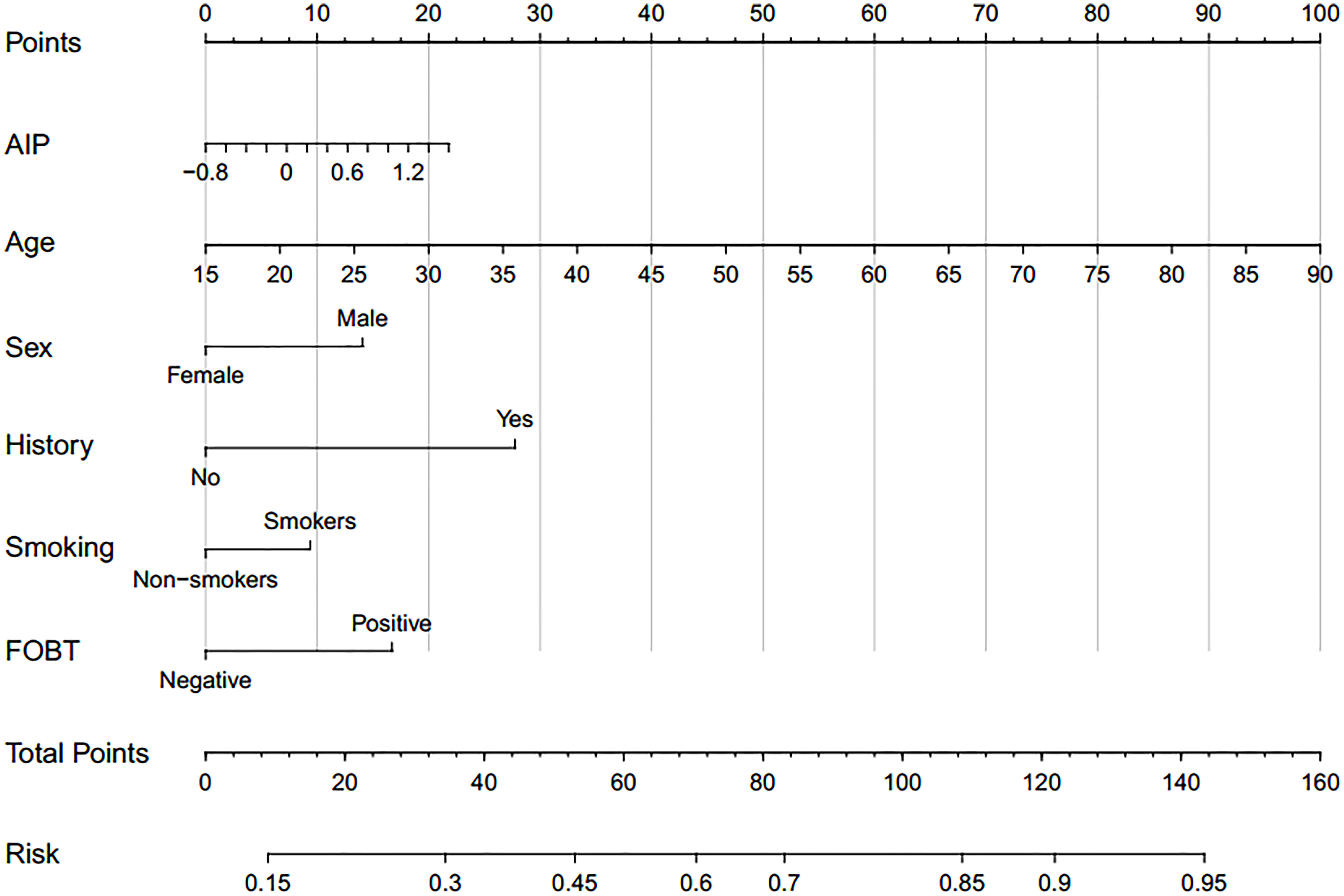
Based on TyG index and AIP respectively, and referring to the logistic regression results, the CRN prediction models were constructed based on the training set consisting of two-thirds of the total population. It was subsequently validated in the test set consisting of the remaining one-third of the population. The accuracies of the TyG-based model and the AIP-based model in diagnosing CRN are shown in Table 3. Model 1 (TyG, age, sex, smoking status, family history, FOBT) had an overall 61.0% sensitivity and 65.1% specificity with an AUC of 0.669 (95%CI, 0.639 - 0.698). Model 2 (AIP, age, sex, smoking status, family history, FOBT) had an overall 56.3% sensitivity and 68.7% specificity with an AUC of 0.667 (95%CI, 0.638 - 0.697). The predictive efficacies for the diagnosis of CRN were evaluated using ROC curves (Figure 3). All significant factors of CRN occurrence were integrated into the nomograms of model 1 (Figure 4) and model 2 (Figure 5), respectively. This was done in order to calculate the probability of an individual without cardiovascular disease having CRN.
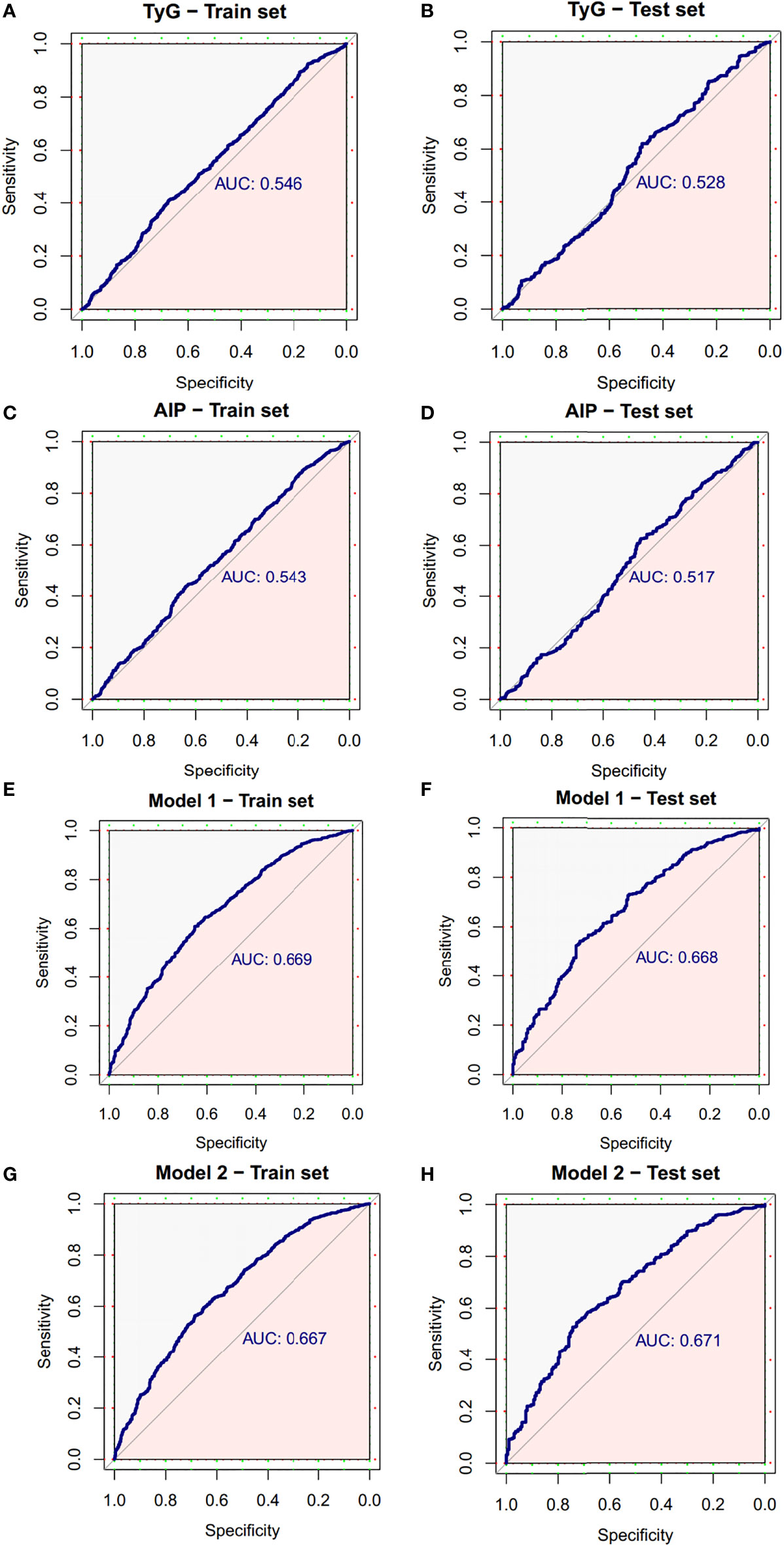
Figure 3 ROC curve of the diagnostic models for neoplasms. (A) ROC curve of the TyG index for diagnosis of neoplasms in train set. (B) ROC curve of the TyG index for diagnosis of neoplasms in test set. (C) ROC curve of AIP for diagnosis of neoplasms in train set. (D) ROC curve of AIP for diagnosis of neoplasms in test set. (E) ROC curve of model 1 for diagnosis of neoplasms in train set. (F) ROC curve of model 1 for diagnosis of neoplasms in test set. (G) ROC curve of model 2 for diagnosis of neoplasms in train set. (H) ROC curve of model 2 for diagnosis of neoplasms in test set. AUC, area under the curve; CI, confidence interval; ROC, receiver operating characteristic.
Discussion
In this retrospective case-control study, we identified both the TyG index and the AIP can be used to predict the presence of CRN in patients without CVD. In these individuals, the higher the value of TyG index, the higher the incidence of CRN (which consist of CRC and CRA). Similarly, a high AIP index was associated with a high prevalence of CRN. Furthermore, our results shown that there were differences between genders in the prediction effects of TyG index and AIP on CRN. The correlation between each of these two indicators and CRN is more pronounced in women than in men.
The medical and academic interest in the effects of the TyG index and the AIP have been increasing. The TyG index is considered a simple and useful surrogate for IR in clinical practice (21). Studies have shown that its predictive value for IR is even better than the homeostasis model assessment of insulin resistance (HOMA-IR) (22), which is the gold standard method for measuring IR. Most of the current research on the TyG index is concentrated in the field of CVD. As an independent risk factor for CVD (23), the TyG index can predict CVD risk in the general population (24). Several studies have also reported that the TyG index may predict acute coronary syndromes, symptomatic CVD and ischaemic stroke (9, 25–28). An elevated TyG index is significantly associated with a higher risk of arterial stiffness (29, 30), it is also used as a valuable biomarker for the development of type 2 diabetes mellitus (22, 31, 32) and metabolic syndrome (33). Moreover, Huihui Ren et al. identified that the TyG index was closely associated with the severity and morbidity in COVID-19 patients (34). As for AIP, it is a substitute for small, dense low-density lipoprotein-cholesterol (sdLDL-C) particles and is inversely related to the LDL-C particle diameter (35). As a marker of plasma atherogenicity, AIP has proven to be a good predictive and prognostic biomarker for cardiovascular events as well as the best predictor of hypertension and diabetes (36–38). It has also recently been proved to be a novel factor more closely related to non-alcoholic fatty liver disease than other lipid parameters in adults (39).
Although the exact mechanism causal to the correlation between the two indicators of the TyG index and the AIP and CRN has not yet been elucidated, it may be related to IR. Former studies have shown that IR is an important risk factor for the development of early colorectal neoplasia (39). It is also associated with a poorer response to anti-CRC therapy (40). IR and secondary hyperinsulinemia with an increase in insulin growth factor (IGF) levels promoted intestinal tumor proliferation through mechanisms that overlap with CRN progression (41). These include the PI3K/Akt/mTOR/S6K signaling pathway (42), the RAS-Raf-MAPK signaling pathway, and the c-JunN-terminal kinase (JNK) signaling pathway (43). As the key features in the development of IR (44), increased visceral and ectopic fat deposition can induce immune cell infiltration and macrophage activation in adipose tissue (45). This can trigger disturbances in the balance of inflammatory-regulatory cytokines, resulting in systemic persistent low-degree inflammation (46), which might promote tumorigenesis. Visceral fat can also secrete a substantial amount of heterogeneous adipocytokines (47), which further influences neoplasm development through its role in the physiological and pathological processes such as angiogenesis, cell proliferation and apoptosis (48, 49). In addition, IR contributes to subcellular component abnormalities, including lipotoxicity, oxidative stress, mitochondrial dysfunction, endoplasmic reticulum (ER) stress, and impaired calcium signaling (45, 50–52). These abnormalities might promote the development of neoplasms.
It is recognized that abnormalities of lipid metabolism are closely related to tumor cell growth, proliferation and differentiation. Previous studies have shown that both TG and LDL-C were significantly associated with an increasing prevalence of colorectal neoplasia (53), and there was an inverse correlation between HDL-C levels and colon cancer risk (54). However, compared with quantitative changes of a routine lipid profile, TyG index and AIP can better reflect the level of blood lipid metabolism in the body. Okamura et al. demonstrated that TyG index could predict the incidence of CRC, with the cut-off value of 8.272 (55). Liu T et al. found that elevated TyG index and TG/HDL-C ratio were associated with a higher risk of developing CRC (56). Stevanovic et al. demonstrated that the determination of the LDL-C particle diameter or the ratio of sdLDL-C was more meaningful in assessing the risk of CRC development than the measurement of total LDL-C concentration (57). The diameter of the sdLDL-C particle is smaller, so it is more easily oxidized to produce oxidized LDL-C (oxLDL-C), which significantly contributes to the malignant transformation of colorectal polyps (58). This suggests that the AIP can also be a useful risk marker of CRC. However, previous studies focused only on CRC, ignoring its precancerous lesions, CRA. CRA often has the tendency to undergo malignant transformation, but can be completely removed by endoscopy. Undoubtedly, far more people currently suffer from CRN than from CRC. Compared to previous studies, we enrolled a large number of patients with CRA rather than only CRC patients, and demonstrated for the first time that both the TyG index and the AIP are reliable blood-based biomarkers for CRN.
We also used a restricted cube analysis to present the effect of small changes in the TyG index and AIP on the OR value of the dependent variable as a continuous curve. Within a certain range, the OR value of CRN in females amplified with increasing AIP or increasing TyG index. Notably, no similar apparent linear or nonlinear relationship existed in the males. To the best of our knowledge, this is the first study to demonstrate such a correlation. Many researchers have demonstrated that men have higher rates of colorectal polyps and carcinoma than women (59–61), and men have more risk factors associated with metabolic disease than women. For example, men are more likely to be smokers and drinkers and have higher serum uric acid (62). Therefore, the associations of TyG index and AIP with CRN in men may be influenced by more confounding relationships, and the prediction effects of these two indicators on CRN are limited. Further prospective large-scale studies are required to confirm our findings.
In recent years, an increasing number of prognostic nomogram models for CRC have been established. However, to our knowledge, this is the first study to build predictive models for CRN based on TyG index and AIP. The models we developed had good sensitivity and accuracy, and the nomograms were constructed based on the models. Anyone without CVD can use our nomograms to conveniently assess their risk of CRN by referring to their own usual known easy parameters. Our research results can guide the targeted screening of colonoscopies in this specific population, which can increase the detection rate of CRA and prevent the occurrence of CRC. This could ameliorate a long-term prognosis and benefit more potential CRA patients, especially those who do not have medical conditions or are unwilling to undergo routine colonoscopy. On the other hand, more effective prioritization testing using colonoscopies for those with high TyG index or high AIP among non-CVD patients, could also reduce the economic and clinical burdens of current screening methods.
Nevertheless, there are several limitations need to be considered when interpreting our findings. Firstly, due to the cross-sectional study design, causality cannot be established based on the results of this single study. Secondly, although we avoided the possible effect of CVD on outcomes by selecting study subjects, we still cannot exclude the possibility of residual or unmeasured variables given the observational study design of the present analysis. Thirdly, although the number of patients in the case group is sufficient, the participants were recruited in one hospital, which may limit the generalizability of the results to other populations. More research across ethnicities and demographics is needed, and relevant prospective studies are required to confirm our findings. These additional studies will provide more information regarding the potential of TyG index and AIP in predicting neoplasms.
Conclusion
In conclusion, the data presented in this study demonstrates that non-CVD patients with higher TyG index or AIP are more likely to have CRN. Based on the results of this innovative research, we posit that both TyG index and AIP are reliable indicators for predicting CRN in people without CVD.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the ethics committees of Beijing Friendship Hospital, Capital Medical University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
Conceived and designed the research: MH, HS and PL. Performed the research: MH and SY. Analyzed the data: HW and GZ. Manuscript preparation: MH. Manuscript revisions: SZ, HS and PL. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the National Natural Science Foundation of China (82070575), Beijing Municipal Administration of Hospitals’ Youth Programme (QML20190104), Beijing Nova Program (Z201100006820147), Beijing Municipal Science & Technology Commission (Z181100001718221).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chan AO, Jim MH, Lam KF, Morris JS, Siu DC, Tong T, et al. Prevalence of colorectal neoplasm among patients with newly diagnosed coronary artery disease. JAMA (2007) 298(12):1412–9. doi: 10.1001/jama.298.12.1412
2. Voutsinas J, Wilkens LR, Franke A, Vogt TM, Yokochi LA, Decker R, et al. Heterocyclic amine intake, smoking, cytochrome P450 1A2 and n-acetylation phenotypes, and risk of colorectal adenoma in a multiethnic population. Gut (2013) 62(3):416–22. doi: 10.1136/gutjnl-2011-300665
3. Bleijenberg AGC, van Leerdam ME, Bargeman M, Koornstra JJ, van Herwaarden YJ, Spaander MC, et al. Substantial and sustained improvement of serrated polyp detection after a simple educational intervention: results from a prospective controlled trial. Gut (2020) 69(12):2150–8. doi: 10.1136/gutjnl-2019-319804
4. Atkin W, Wooldrage K, Brenner A, Martin J, Shah U, Perera S, et al. Adenoma surveillance and colorectal cancer incidence: a retrospective, multicentre, cohort study. Lancet Oncol (2017) 18(6):823–34. doi: 10.1016/S1470-2045(17)30187-0
5. Abbasi F, Reaven GM. Comparison of two methods using plasma triglyceride concentration as a surrogate estimate of insulin action in nondiabetic subjects: triglycerides × glucose versus triglyceride/high-density lipoprotein cholesterol. Metabolism (2011) 60(12):1673–6. doi: 10.1016/j.metabol.2011.04.006
6. Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord (2008) 6(4):299–304. doi: 10.1089/met.2008.0034
7. Du T, Yuan G, Zhang M, Zhou X, Sun X, Yu X. Clinical usefulness of lipid ratios, visceral adiposity indicators, and the triglycerides and glucose index as risk markers of insulin resistance. Cardiovasc Diabetol (2014) 13:146. doi: 10.1186/s12933-014-0146-3
8. Vega GL, Barlow CE, Grundy SM, Leonard D, DeFina LF. Triglyceride-to-high-density-lipoprotein-cholesterol ratio is an index of heart disease mortality and of incidence of type 2 diabetes mellitus in men. J Investig Med (2014) 62(2):345–9. doi: 10.2310/JIM.0000000000000044
9. Sánchez-Íñigo L, Navarro-González D, Fernández-Montero A, Pastrana-Delgado J, Martínez JA. The TyG index may predict the development of cardiovascular events. Eur J Clin Invest (2016) 46(2):189–97. doi: 10.1111/eci.12583
10. Salazar MR, Carbajal HA, Espeche WG, Aizpurúa M, Dulbecco CA, Reaven GM. Comparison of two surrogate estimates of insulin resistance to predict cardiovascular disease in apparently healthy individuals. Nutr Metab Cardiovasc Dis (2017) 27(4):366–73. doi: 10.1016/j.numecd.2016.12.002
11. Li S, Guo B, Chen H, Shi Z, Li Y, Tian Q, et al. The role of the triglyceride (triacylglycerol) glucose index in the development of cardiovascular events: a retrospective cohort analysis. Sci Rep (2019) 9(1):7320. doi: 10.1038/s41598-019-43776-5
12. Zhang M, Wang B, Liu Y, Sun X, Luo X, Wang C, et al. Cumulative increased risk of incident type 2 diabetes mellitus with increasing triglyceride glucose index in normal-weight people: the rural Chinese cohort study. Cardiovasc Diabetol (2017) 16:30. doi: 10.1186/s12933-017-0514-x
13. Sánchez-Íñigo L, Navarro-González D, Pastrana-Delgado J, Fernández-Montero A, Martínez JA. Association of triglycerides and new lipid markers with the incidence of hypertension in a Spanish cohort. J Hypertens (2016) 34(7):1257–65. doi: 10.1097/HJH.0000000000000941
14. Zhang S, Du T, Zhang J, Lu H, Lin X, Xie J, et al. The triglyceride and glucose index (TyG) is an effective biomarker to identify nonalcoholic fatty liver disease. Lipids Health Dis (2017) 16(1):15. doi: 10.1186/s12944-017-0409-6
15. Qin Z, Zhou K, Li Y, Cheng W, Wang Z, Wang J, et al. The atherogenic index of plasma plays an important role in predicting the prognosis of type 2 diabetic subjects undergoing percutaneous coronary intervention: results from an observational cohort study in China. Cardiovasc Diabetol (2020) 19(1):23. doi: 10.1186/s12933-020-0989-8
16. Kwon HS, Ko JS, Lee JH, Kwon KY, Han JH. A positive association between the atherogenic index of plasma and white matter hyperintensity. Korean J Fam Med (2022) 43:193–8. doi: 10.4082/kjfm.21.0129
17. Zhu XW, Deng FY, Lei SF. Meta-analysis of atherogenic index of plasma and other lipid parameters in relation to risk of type 2 diabetes mellitus. Prim Care Diabetes (2015) 9:60–7. doi: 10.1016/j.pcd.2014.03.007
18. Zhou K, Qin Z, Tian J, Cui K, Yan Y, Lyu S. The atherogenic index of plasma: a powerful and reliable predictor for coronary artery disease in patients with type 2 diabetes. Angiology (2021) 72:934–41. doi: 10.1177/00033197211012129
19. Zhang X, Zhang X, Li X, Feng J, Chen X. Association of metabolic syndrome with atherogenic index of plasma in an urban Chinese population: a 15-year prospective study. Nutr Metab Cardiovasc Dis (2019) 29:1214–9. doi: 10.1016/j.numecd.2019.07.006
20. Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, Martínez-Abundis E, Ramos-Zavala MG, Hernández-González SO, et al. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab (2010) 95(7):3347–51. doi: 10.1210/jc.2010-0288
21. Won KB, Park EJ, Han D, Lee JH, Choi SY, Chun EJ, et al. Triglyceride glucose index is an independent predictor for the progression of coronary artery calcification in the absence of heavy coronary artery calcification at baseline. Cardiovasc Diabetol (2020) 19(1):34. doi: 10.1186/s12933-020-01008-5
22. Low S, Khoo K, Irwan B, Sum CF, Subramaniam T, Lim SC, et al. The role of triglyceride glucose index in development of type 2 diabetes mellitus. Diabetes Res Clin Pract (2018) 143:43–9. doi: 10.1016/j.diabres.2018.06.006
23. Liu Q, Cui H, Ma Y, Han X, Cao Z, Wu Y. Triglyceride-glucose index associated with the risk of cardiovascular disease: the kailuan study. Endocrine (2022) 75(2):392–9. doi: 10.1007/s12020-021-02862-3
24. Wang A, Tian X, Zuo Y, Chen S, Meng X, Wu S, et al. Change in triglyceride-glucose index predicts the risk of cardiovascular disease in the general population: a prospective cohort study. Cardiovasc Diabetol (2021) 20(1):113. doi: 10.1186/s12933-021-01305-7
25. Alizargar J, Bai CH, Hsieh NC, Wu SV. Use of the triglyceride-glucose index (TyG) in cardiovascular disease patients. Cardiovasc Diabetol (2020) 19(1):8. doi: 10.1186/s12933-019-0982-2
26. Da Silva A, Caldas APS, Hermsdorff HHM, Bersch-Ferreira ÂC, Torreglosa CR, Weber B, et al. Triglyceride-glucose index is associated with symptomatic coronary artery disease in patients in secondary care. Cardiovasc Diabetol (2019) 18(1):89. doi: 10.1186/s12933-019-0893-2
27. Shi W, Xing L, Jing L, Tian Y, Yan H, Sun Q, et al. Value of triglyceride-glucose index for the estimation of ischemic stroke risk: Insights from a general population. Nutr Metab Cardiovasc Dis (2020) 30(2):245–53. doi: 10.1016/j.numecd.2019.09.015
28. Wang L, Cong HL, Zhang JX, Hu YC, Wei A, Zhang YY, et al. Triglyceride-glucose index predicts adverse cardiovascular events in patients with diabetes and acute coronary syndrome. Cardiovasc Diabetol (2020) 19(1):80. doi: 10.1186/s12933-020-01054-z
29. Wu Z, Zhou D, Liu Y, Li Z, Wang J, Han Z, et al. Association of TyG index and TG/HDL-c ratio with arterial stiffness progression in a non-normotensive population. Cardiovasc Diabetol (2021) 20(1):134. doi: 10.1186/s12933-021-01330-6
30. Zhao S, Yu S, Chi C, Fan X, Tang J, Ji H, et al. Association between macro- and microvascular damage and the triglyceride glucose index in community-dwelling elderly individuals: the northern shanghai study. Cardiovasc Diabetol (2019) 18(1):95. doi: 10.1186/s12933-019-0898-x
31. Navarro-González D, Sánchez-Íñigo L, Pastrana-Delgado J, Fernández-Montero A, Martinez JA. Triglyceride-glucose index (TyG index) in comparison with fasting plasma glucose improved diabetes prediction in patients with normal fasting glucose: the vascular-metabolic CUN cohort. Prev Med (2016) 86:99–105. doi: 10.1016/j.ypmed.2016.01.022
32. Chamroonkiadtikun P, Ananchaisarp T, Wanichanon W. The triglyceride-glucose index, a predictor of type 2 diabetes development: A retrospective cohort study. Prim Care Diabetes (2020) 14(2):161–7. doi: 10.1016/j.pcd.2019.08.004
33. Shin KA, Kim YJ. Usefulness of surrogate markers of body fat distribution for predicting metabolic syndrome in middle-aged and older Korean populations. Diabetes Metab Syndr Obes (2019) 12:2251–9. doi: 10.2147/DMSO.S217628
34. Ren H, Yang Y, Wang F, Yan Y, Shi X, Dong K, et al. Association of the insulin resistance marker TyG index with the severity and mortality of COVID-19. Cardiovasc Diabetol (2020) 19(1):58. doi: 10.1186/s12933-020-01035-2
35. Kim SH, Cho YK, Kim YJ, Jung CH, Lee WJ, Park JY, et al. Association of the atherogenic index of plasma with cardiovascular risk beyond the traditional risk factors: a nationwide population-based cohort study. Cardiovasc Diabetol (2022) 21(1):81. doi: 10.1186/s12933-022-01522-8
36. Baliarsingh S, Sharma N, Mukherjee R. Serum uric acid: marker for atherosclerosis as it is positively associated with "atherogenic index of plasma". Arch Physiol Biochem (2013) 119(1):27–31. doi: 10.3109/13813455.2012.732580
37. Marwaha RK, Tandon N, Garg MK, Kanwar R, Sastry A, Narang A, et al. Dyslipidemia in subclinical hypothyroidism in an Indian population. Clin Biochem (2011) 44:1214–7. doi: 10.1016/j.clinbiochem.2011.07.003
38. Onat A, Can G, Kaya H, Hergenç G. "Atherogenic index of plasma" (log10 triglyceride/high-density lipoprotein-cholesterol) predicts high blood pressure, diabetes, and vascular events. J Clin Lipidol (2010) 4:89–98. doi: 10.1016/j.jacl.2010.02.005
39. Liu J, Zhou L, An Y, Wang Y, Wang G. The atherogenic index of plasma: A novel factor more closely related to non-alcoholic fatty liver disease than other lipid parameters in adults. Front Nutr (2022) 9:954219. doi: 10.3389/fnut.2022.954219
40. Vigneri PG, Tirrò E, Pennisi MS, Massimino M, Stella S, Romano C, et al. The Insulin/IGF system in colorectal cancer development and resistance to therapy. Front Oncol (2015) 5:230. doi: 10.3389/fonc.2015.00230
41. Lin J, Gill A, Zahm SH, Carter CA, Shriver CD, Nations JA, et al. Metformin use and survival after non-small cell lung cancer: A cohort study in the US military health system. Int J Cancer (2017) 141:254–63. doi: 10.1002/ijc.30724
42. Khan KH, Yap TA, Yan L, Cunningham D. Targeting the PI3K-AKT-mTOR signaling network in cancer. Chin J Cancer (2013) 32(5):253–65. doi: 10.5732/cjc.013.10057
43. Bardou M, Barkun AN, Martel M. Obesity and colorectal cancer. Gut (2013) 62(6):933–47. doi: 10.1136/gutjnl-2013-304701
44. Da Silva IV, Rodrigues JS, Rebelo I, Miranda JPG, Soveral G. Revisiting the metabolic syndrome: the emerging role of aquaglyceroporins. Cell Mol Life Sci (2018) 75(11):1973–88. doi: 10.1007/s00018-018-2781-4
45. Nishida K, Otsu K. Inflammation and metabolic cardiomyopathy. Cardiovasc Res (2017) 113(4):389–98. doi: 10.1093/cvr/cvx012
46. Bloomgarden ZT. Inflammation and insulin resistance. Diabetes Care (2003) 26(5):1619–23. doi: 10.2337/diacare.26.5.1619
47. Dalamaga M, Diakopoulos KN, Mantzoros CS. The role of adiponectin in cancer: a review of current evidence. Endocr Rev (2012) 33(4):547–94. doi: 10.1210/er.2011-1015
48. Cabia B, Andrade S, Carreira MC, Casanueva FF, Crujeiras AB. A role for novel adipose tissue-secreted factors in obesity-related carcinogenesis. Obes Rev (2016) 17(4):361–76. doi: 10.1111/obr.12377
49. Sahin-Efe A, Katsikeris F, Mantzoros CS. Advances in adipokines. Metabolism (2012) 61(12):1659–65. doi: 10.1016/j.metabol.2012.09.001
50. Witteles RM, Fowler MB. Insulin-resistant cardiomyopathy clinical evidence, mechanisms, and treatment options. J Am Coll Cardiol (2008) 51(2):93–102. doi: 10.1016/j.jacc.2007.10.021
51. Aroor AR, Mandavia CH, Sowers JR. Insulin resistance and heart failure: molecular mechanisms. Heart Fail Clin (2012) 8(4):609–17. doi: 10.1016/j.hfc.2012.06.005
52. Van de Weijer T, Schrauwen-Hinderling VB, Schrauwen P. Lipotoxicity in type 2 diabetic cardiomyopathy. Cardiovasc Res (2011) 92(1):10–8. doi: 10.1093/cvr/cvr212
53. Tian Y, Wang K, Li J, Wang J, Wang Z, Fan Y, et al. The association between serum lipids and colorectal neoplasm: a systemic review and meta-analysis. Public Health Nutr (2015) 18(18):3355–70. doi: 10.1017/S1368980015000646
54. Schaefer EJ, Anthanont P, Asztalos BF. High-density lipoprotein metabolism, composition, function, and deficiency. Curr Opin Lipidol (2014) 25(3):194–9. doi: 10.1097/MOL.0000000000000074
55. Okamura T, Hashimoto Y, Hamaguchi M, Obora A, Kojima T, Fukui M. Triglyceride-glucose index (TyG index) is a predictor of incident colorectal cancer: a population-based longitudinal study. BMC Endocr Disord (2020) 20(1):113. doi: 10.1186/s12902-020-00581-w
56. Liu T, Zhang Q, Wang Y, Ma X, Zhang Q, Song M, et al. Association between the TyG index and TG/HDL-c ratio as insulin resistance markers and the risk of colorectal cancer. BMC Cancer. (2022) 22(1):1007. doi: 10.1186/s12885-022-10100-w
57. Stevanovic M, Vekic J, Bogavac-Stanojevic N, Janac J, Stjepanovic Z, Zeljkovic D, et al. Significance of LDL and HDL subclasses characterization in the assessment of risk for colorectal cancer development. Biochem Med (Zagreb) (2018) 28(3):030703. doi: 10.11613/BM.2018.030713
58. Crespo-Sanjuán J, Calvo-Nieves MD, Aguirre-Gervás B, Herreros-Rodríguez J, Velayos-Jiménez B, Castro-Alija MJ, et al. Early detection of high oxidative activity in patients with adenomatous intestinal polyps and colorectal adenocarcinoma: myeloperoxidase and oxidized low-density lipoprotein in serum as new markers of oxidative stress in colorectal cancer. Lab Med (2015) 46(2):123–35. doi: 10.1309/LMZJJU6BC86WUDHW
59. McCashland TM, Brand R, Lyden E, de Garmo P, CORI Research Project. Gender differences in colorectal polyps and tumors. Am J Gastroenterol (2001) 96(3):882–6. doi: 10.1111/j.1572-0241.2001.3638_a.x
60. Chen W, Wang M, Jing X, Wu C, Zeng Y, Peng J, et al. High risk of colorectal polyps in men with non-alcoholic fatty liver disease: A systematic review and meta-analysis. J Gastroenterol Hepatol (2020) 35(12):2051–65. doi: 10.1111/jgh.15158
61. Seo JY, Bae JH, Kwak MS, Yang JI, Chung SJ, Yim JY, et al. The risk of colorectal adenoma in nonalcoholic or metabolic-associated fatty liver disease. Biomedicines (2021) 9(10):1401. doi: 10.3390/biomedicines9101401
Keywords: triglyceride glucose index, Atherogenic index of plasma, colorectal neoplasms, cardiovascular disease, insulin resistance
Citation: Han M, Wang H, Yang S, Zhu S, Zhao G, Shi H and Li P (2022) Triglyceride glucose index and Atherogenic index of plasma for predicting colorectal neoplasms in patients without cardiovascular diseases. Front. Oncol. 12:1031259. doi: 10.3389/fonc.2022.1031259
Received: 29 August 2022; Accepted: 27 October 2022;
Published: 14 November 2022.
Edited by:
Marwa Matboli Sayed, Ain Shams University, EgyptCopyright © 2022 Han, Wang, Yang, Zhu, Zhao, Shi and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haiyun Shi, c2hpaGFpeXVuMTAxNkBnbWFpbC5jb20=; Peng Li, bGlwZW5nQGNjbXUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Muzhou Han
Muzhou Han Hao Wang2†
Hao Wang2† Siying Zhu
Siying Zhu Guiping Zhao
Guiping Zhao Haiyun Shi
Haiyun Shi Peng Li
Peng Li