
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 26 October 2022
Sec. Cancer Imaging and Image-directed Interventions
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.1029684
 Jingjie Shang1†
Jingjie Shang1† Huimin You2†
Huimin You2† Chenchen Dong1
Chenchen Dong1 Yingxin Li1
Yingxin Li1 Yong Cheng1
Yong Cheng1 Yongjin Tang1
Yongjin Tang1 Bin Guo1
Bin Guo1 Jian Gong1
Jian Gong1 Xueying Ling1*
Xueying Ling1* Hao Xu1*
Hao Xu1*Objectives: Brain metastases (BMs) are a major cause leading to the failure of treatment management for non-small-cell lung cancer (NSCLC) patients. The purpose of this study was to evaluate the predictive value of baseline metabolic tumor burden on 18F-FDG PET/CT measured with metabolic tumor volume (MTV) and total lesion glycolysis (TLG) for brain metastases (BMs) development in patients with locally advanced non-small-cell lung cancer (NSCLC) after treatment.
Methods: Forty-seven patients with stage IIB-IIIC NSCLC who underwent baseline 18F-FDG PET/CT examinations were retrospectively reviewed. The maximum standardized uptake value (SUVmax), MTV, and TLG of the primary tumor (SUVmaxT, MTVT, and TLGT), metastatic lymph nodes (SUVmaxN, MTVN, and TLGN), and whole-body tumors (SUVmaxWB, MTVWB, and TLGWB) were measured. The optimal cut-off values of PET parameters to predict brain metastasis-free survival were obtained using Receiver operating characteristic (ROC) analysis, and the predictive value of clinical variables and PET parameters were evaluated using Cox proportional hazards regression analysis.
Results: The median follow-up duration was 25.0 months for surviving patients, and 13 patients (27.7%) developed BM. The optimal cut-off values were 21.1 mL and 150.0 g for MTVT and TLGT, 20.0, 10.9 mL and 55.6 g for SUVmaxN, MTVN and TLGN, and 27.9, 27.4 mL and 161.0 g for SUVmaxWB, MTVWB and TLGWB, respectively. In the Cox proportional hazards models, the risk of BM was significantly associated with MTVN and MTVWB or TLGN and TLGWB after adjusting for histological cell type, N stage, SUVmaxN, and SUVmaxWB.
Conclusions: Baseline metabolic tumor burden (MTV and TLG) evaluated from the level of metastatic lymph nodes and whole-body tumors are significant predictive factors for BM development in patients with locally advanced NSCLC.
Brain metastases (BMs) are the most frequent types of failure in patients with locally advanced non-small-cell lung cancer (NSCLC) (1–3), which is related to significant neurocognitive impairment, life quality deficits, poor prognosis, and high mortality (4). Previous studies have shown that prophylactic cranial irradiation (PCI) can delay the onset or reduce the occurrence of BM in locally advanced NSCLC, but failed to demonstrate a significant improvement in overall survival (OS) (5, 6), which suggests that such therapy may not be appropriate for all NSCLC patients. Therefore, identifying potential patients who may benefit from PCI is important to reduce brain relapses and improve their outcomes.
BM develops following the haematogenous spread of tumor cells to the brain microvasculature. Initially, cancerous cells need to separate from the primary tumor, infiltrate into the surrounding tissues, then enter the vasculature and lymphatic system (7). The assessment of tumor aggressiveness is helpful to predict the occurrence of BM (8). For patients with locally advanced NSCLC, the primary tumor status (T), especially the regional lymph node involvement status (N), can reflect the invasiveness and growth characteristics of the tumors (9). However, the conventional T and N stages are based on the size and location of the tumor and could not comprehensively reflect aggressiveness, and tumor-specific factors vary even among patients within the same disease stage, creating a heterogeneous subgroup concerning the prognosis (10). Therefore, BM predictors based on tumor biology are needed to reflect the biological characterization of aggressiveness.
18F-FDG PET/CT is a well-established molecular imaging technology that enables non-invasive quantification of tumor biological characteristics. It is now routinely used for diagnosis, staging, outcome prediction, and response evaluation for NSCLC patients (11). Several studies have reported that the maximum standardized uptake value (SUVmax), a semiquantitative index that reflects tumor metabolic activity, is a significant prognostic imaging marker for NSCLC patients, but it cannot represent uptake for the entire tumor mass (12). More recently, there has been a growing recognition of the metabolic tumor volume (MTV) and total lesion glycolysis (TLG), the volumetric PET parameters which have been explored as measures of metabolic tumor burden, as promising quantitative PET indices. Studies have shown that MTV and TLG predict prognosis better than SUVmax and tumor stage for NSCLC (13–15). Nevertheless, whether volumetric PET parameters could predict BM development in NSCLC patients has not been reported.
Therefore, the aim of our current study was to evaluate the predictive value of baseline MTV and TLG for BM development in locally advanced NSCLC patients and compare them with other predictors.
All patients with newly diagnosed stage IIB-IIIC NSCLC who received baseline 18F-FDG PET/CT examination between November 2012 and November 2020 were retrospectively reviewed. Those patients who had negative baseline brain magnetic resonance imaging (MRI) results, had no history of other prior malignancies, and had complete follow-up information were eligible for the study. The patient’s demographic and clinic pathological variables at the time of diagnosis were recorded, which included age, sex, histology, smoking status, and tumor stage.
Patients were followed up every 3 months for 2 years, and then every 6 months thereafter. The follow-up evaluations consisted of medical history, physical examination, thoracic CT, abdominal ultrasound, and other necessary examinations as clinically indicated. Contrast-enhanced brain MRI was performed if patients had suspicious symptoms or disease progression. The physician determined disease progression based on available information, including clinical assessments, radiologic examinations, and pathology reports. This study was approved by our institutional review board (Number: KY-2021-075) and complied with national legislation and the guidelines of the Declaration of Helsinki. Due to the retrospective nature of this study, informed consent was not required.
All examinations were performed using a standard protocol on GE Discovery PET/CT 690 scanner. Patients were required to fast for at least 6 h with serum glucose concentrations < 200 mg/dL before the examination. Imaging was obtained 50 to 70 min after intravenous injection of 18F-FDG (0.08-0.10 mCi/kg). A whole-body unenhanced CT scan was firstly performed with 130 kV, 100-180 mA modulated based on the patient’s body weight. After the whole-body CT scanning, PET data were acquired using the time-of-flight (TOF) technology. The PET images were attenuated correction in terms of CT data and reconstructed using the TOF together with point spread function (PSF) technology.
pt?>PET volume computer-assisted reading (PET VCAR), an automated segmentation system in the GE Advantage Workstation, was used to calculate the PET parameters of tumors. PET VCAR can separate the target tumor from the background tissue based on a threshold value calculated by an iterative adaptive segmentation algorithm, and our previous studies have described the detailed instructions of this software (16, 17). By drawing a volume of interest around the tumor, the SUVmax, MTV, and TLG of the entire tumor could be measured automatically (Figure 1). Sometimes needed to adjust manually the estimated tumor surface to include the margins of the entire tumor within the volume of interest. All hypermetabolic metastatic lesions were selected for analysis, while hypermetabolic foci explained by physiological activity or inflammation were excluded. The SUVmax, MTV, and TLG of the primary tumor, metastatic lymph nodes, and whole-body tumors were measured and denoted as SUVmaxT, MTVT, TLGT, SUVmaxN, MTVN, TLGN, SUVmaxWB, MTVWB, and TLGWB, respectively.
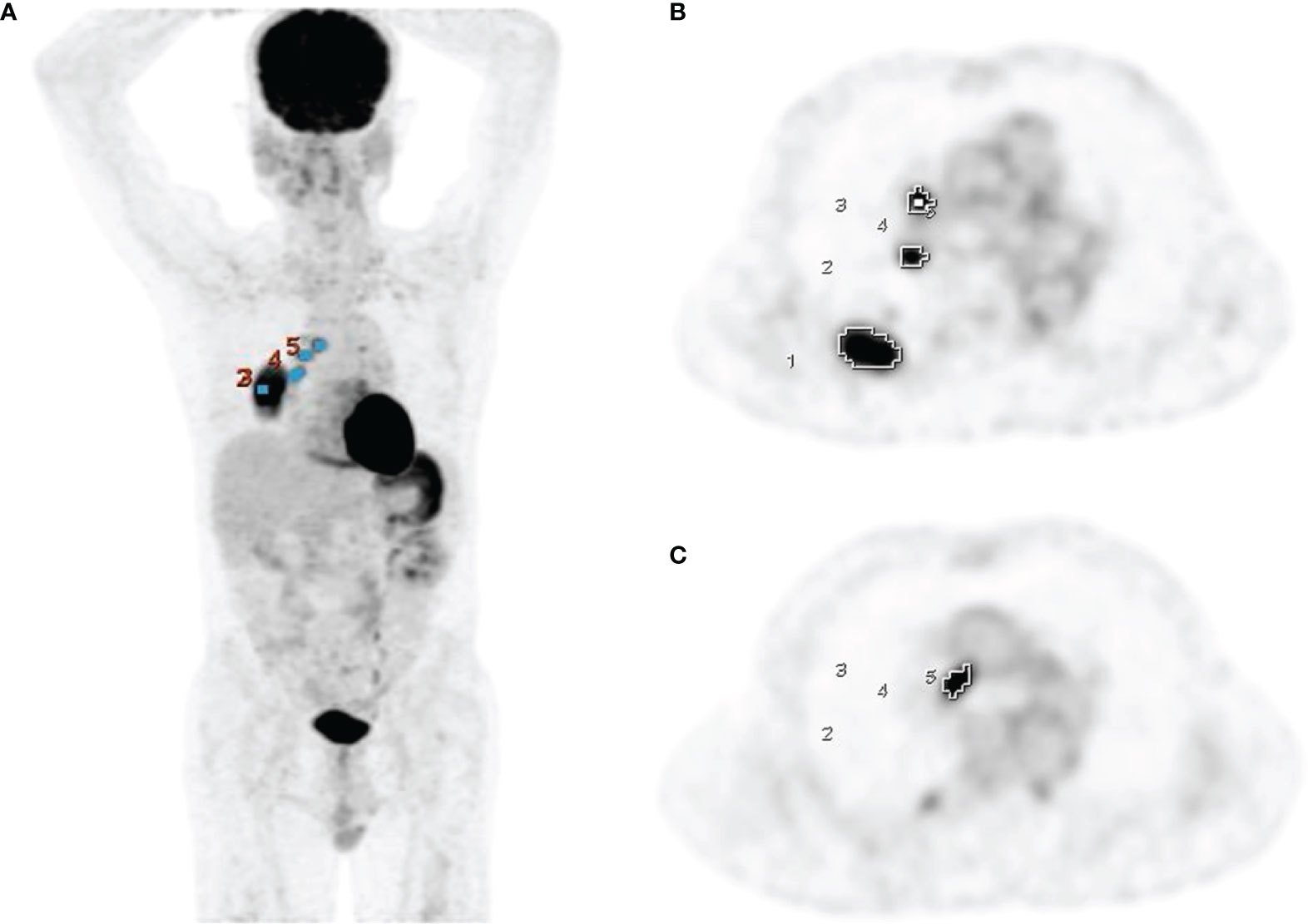
Figure 1 Measurement of PET parameters using PET VCAR on PET coronal (A) and axial images (B, C) in a 69-year-old man with adenocarcinoma.
Brain metastasis-free survival (BMFS), the time from initial therapy to when the BM was confirmed by a brain MRI scan, was recorded as the primary endpoint. OS, the time from treatment initiation to death or final follow-up, was recorded as the secondary endpoint. The Mann-Whitney U test was used to analyze the difference in PET parameter values between the BM-negative and BM-positive groups, and p < 0.05 was considered significant. Significant parameters related to BM were further analyzed using receiver operating characteristic (ROC) analysis to determine the optimal cut-off values to predict BMFS. Patients were dichotomized based on these optimal cut-off values. The BMFS and OS curves of different groups were estimated using the Kaplan–Meier method and compared by the log-rank test, and p < 0.05 was considered significant. Risk factors for BM development, including various PET parameters and other clinical variables, were identified using univariate and multivariate Cox regression analyses. Each factor whose p < 0.1 in the univariate analysis was further analyzed by the multivariate analysis. Two separate multivariate models were used to analyze MTV and TLG because there was multicollinearity between them. All data were processed with SPSS software.
Two hundred and three patients with newly diagnosed stage II B-IIIC NSCLC were retrospectively reviewed. We excluded 128 patients for whom the clinical data were incomplete or lost to follow-up, 15 patients who had a history of other prior malignancy, and 13 patients who were treated with curative surgery. Finally, a total of 47 patients (27 men and 20 women; mean age 64 years, range, 42-85 years) were enrolled in this study, of which 25 were adenocarcinoma, 17 were squamous cell carcinoma and 5 were large cell carcinoma. The tumor stage was IIB in 6 patients, IIIA in 19, IIIB in 12 and IIIC in 10 patients. The treatment approach consisted of chemoradiotherapy (n = 29) and chemotherapy or targeted therapy (n = 18), which was performed according to each patient’s situation and the corresponding physician’s decision. The chemotherapy regimens included cisplatin- or carboplatin-based regimens in combination with gemcitabine, pemetrexed, paclitaxel, or docetaxel. The targeted therapies included gefitinib, osimertinib, or anlotinib. The total dose of radiotherapy varied from 40 Gy in 20 fractions to 70 Gy in 35 fractions, with a mean prescribed dose of 57.1 ± 10.6 Gy. The patient’s demographics and clinical characteristics are summarized in Table 1.
The median follow-up duration among survivors was 25.0 months, with a range of 3.5-105.9 months. At the time of analysis, 21 (44.7%) patients were still alive and 26 (55.3%) patients had died. Overall, 40 patients (85.1%) experienced disease progression: 18 patients (45.0%) had local and/or locoregional recurrence, 9 (22.5%) patients had distant metastases, and 13 (32.5%) patients had both local and/or locoregional and distant recurrence. Thirteen patients (27.7%) experienced BM; of these, 8 patients (61.5%) experienced BM as the first relapse site, and 5 patients (38.5%) developed BM after extracranial disease progression. The median time of BM diagnosis after treatment (BMFS) was 14.3 months (range, 4.1-50.7 months), and the 1-, 3-, and 5-year cumulative rates of BM were 11.5%, 28.4% and 39.8%, respectively. OS was significantly lower among those patients with BM positivity compared with patients without BM positivity (p = 0.01).
The descriptions of the mean and optimal cut-off values of various parameters as determined by ROC curves are provided in Table 2. The differences in SUVmaxT between the BM-negative and BM-positive groups were not statistically significant (p> 0.05). Conversely, MTVT, TLGT, SUVmaxN, MTVN, TLGN, SUVmaxWB, MTVWB, and TLGWB were significantly different between groups (p< 0.05). Furthermore, for 40 patients who experienced disease progression, the differences in MTVT, TLGT, SUVmaxN, MTVN, TLGN, MTVWB, and TLGWB between the BM-negative and BM-positive groups were also statistically significant (p< 0.05). The optimal cut-off values for BMFS derived from the AUC data were 21.1 mL and 150.0 g for MTVT and TLGT, 20.0, 10.9 mL and 55.6 g for SUVmaxN, MTVN and TLGN, and 27.9, 27.4 mL and 161.0 g for SUVmaxWB, MTVWB and TLGWB, respectively. Using these derived optimal cut-off points, the Kaplan–Meier curves of BMFS are shown in Figure 2. Patients with high MTV and TLG at the primary tumor, metastatic lymph nodes, and whole-body tumor levels had significantly shorter BMFS (p< 0.05).
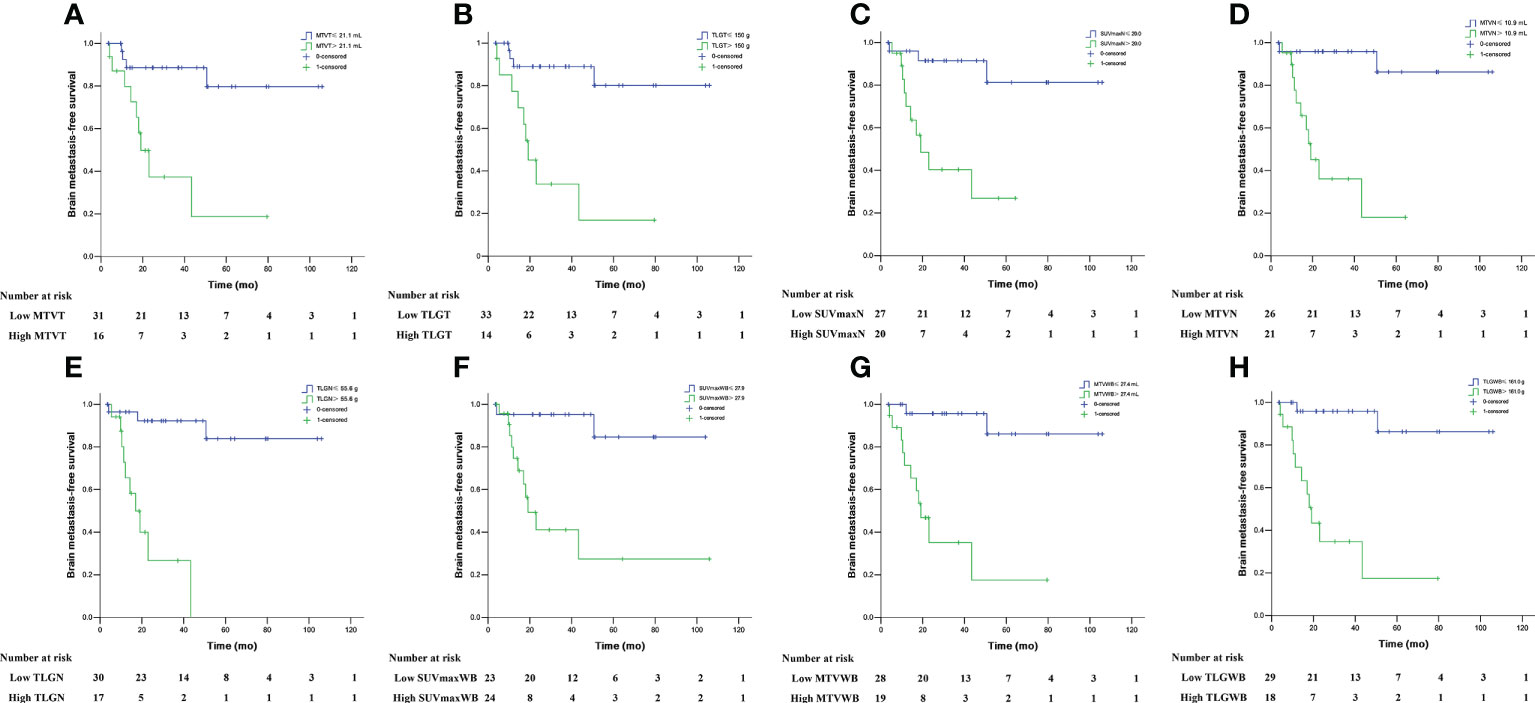
Figure 2 Brain metastasis-free survival in locally advanced NSCLC patients stratified by MTVT (A), TLGT (B), SUVmaxN (C), MTVN (D), TLGN (E), SUVmaxWB (F), MTVWB (G), and TLGWB (H).
The univariate and multivariate analysis results are summarized in Table 3. In univariate analysis, adenocarcinoma histology, high T stage (T2-4), N stage (N3), MTVT> 21.1 mL, TLGT> 150.0 g, SUVmaxN > 20.0, MTVN > 10.9 mL, TLGN > 55.6 g, SUVmaxWB > 27.9, MTVWB > 27.4 mL, and TLGWB > 161.0 g (all p< 0.05) were significantly related to an increased risk of developing brain relapses. Multivariate analysis showed high MTVN (HR = 6.241; 95% CI, 1.037-37.559; p = 0.046) and high MTVWB (HR = 6.936; 95% CI, 1.185-40.604; p = 0.032) remained risk factors for BM after adjusting for histology, T stage, N stage, SUVmaxN, and SUVmaxWB. Furthermore, high TLGN (HR = 5.550; 95% CI, 0.966-31.900; p = 0.055) and high TLGWB (HR = 6.491; 95% CI, 1.029-40.961; p = 0.047) also remained risk factors for BM after adjusting for histology, T stage, N stage, SUVmaxN, and SUVmaxWB.
The present study indicates that the pretreatment metabolic tumor burden of the primary tumor, metastatic lymph nodes, and whole-body tumors as measured by MTV and TLG has a high potential value in predicting BM development in locally advanced NSCLC. Patients with high MTVT, TLGT, MTVN, TLGN, MTVWB and TLGWB were associated with shorter median BMFS. Moreover, the multivariate analysis demonstrated that MTVN and MTVWB or TLGN and TLGWB were significant predictors of an increased risk of relapse with BM, even after adjusting for well-known clinicopathological predictive factors in patients with NSCLC.
BM is a common type of complication in patients with NSCLC, both in early and locally advanced stages after treatment with complete surgical resection and chemoradiotherapy. The occurrence rates of BM in locally advanced NSCLC patients were 25% to 55%, with a median BMFS in the range of 9.0-16.0 months in previous studies (1–3). In most of these patients, BM occurred within 3 years of diagnosis. The results of our study are similar to these observations, with an overall rate of BM of 27.7%, a median BMFS of 14.3 months, and a 3-year actuarial incidence of BM of 28.4%. Furthermore, similar to previous studies (18), we found that the OS of the BM-positive group was significantly lower than that of the BM-negative group.
Several studies have reported that the higher T stages and especially higher N stages of NSCLC were associated with an increased occurrence rate of BM (19–22). In the study by Bajard et al. (19), researchers found that T4 and N2-3 were risk factors for BM development in 305 patients with stage I-III NSCLC. However, with disease development, the accuracy of the T and N stages as a surrogate for tumor burden breaks down due to a wide spectrum of disease severities represented by only a few different stages (10). Some researchers, therefore, proposed using the number or size of metastatic lymph nodes or the ratio of metastatic to examined lymph nodes to predict BM development (23, 24). Ding et al. (24) analyzed 217 patients with stage IIIA-N2 NSCLC and found that the number of metastatic mediastinal lymph nodes ≥ 3 and the ratio of metastatic to examined lymph nodes ≥ 30% were significantly correlated with a risk of BM development.
The volumetric PET parameters MTV and TLG can provide a more complete estimation of the tumor burden and biological aggressiveness. Previous studies have demonstrated that metabolic tumor burden is a significant and independent predictive factor for progression in patients with NSCLC (13–15). However, none of these specifically focused on the development of BM. In the present study, we conducted a comprehensive evaluation of volumetric PET parameters at different levels and estimated optimal cut-off points to propose clinically predictive markers for BM. We found that MTV and TLG of the primary tumor, metastatic lymph nodes, and whole-body tumors were significantly different between the BM-negative and BM-positive groups in all patients and all recurrences. Furthermore, the multivariate analysis showed that MTVN, MTVWB, and TLGWB were independent predictors of BM (all p < 0.05), even after adjusting for histology, T stage, N stage, SUVmaxN, and SUVmaxWB. TLGN also showed a trend as an independent predictor for BM development (p = 0.055). More accurate risk stratification may aid clinician and patient decision-making for optimal treatment choices and better outcome prediction. Patients with high baseline MTV or TLG should be closely monitored after treatment and potentially enrolled in novel treatment approaches. Therefore, incorporating metabolic tumor burden in therapeutic regimens could help select patient groups that would most benefit from PCI therapies.
The current study showed that, unlike volumetric PET parameters, the SUVmax of the primary tumor was not significantly different between the BM-negative and BM-positive groups. Furthermore, the multivariate analysis showed that SUVmaxN and SUVmaxWB were not significant predictors of an increased risk of relapse with BM. This may be partly because SUVmax only represents the measurement from a single spot of the most hypermetabolic area of a tumor mass and does not reflect accurately the metabolic activity of the whole tumor (12). Indeed, multiple studies evaluating the prognostic value of MTV and TLG, which reflect the whole tumor metabolic burden, have shown that these measures are either more accurate than SUVmax or the sole prognostic marker of outcome in NSCLC (13–15). Therefore, MTV and TLG may potentially be better surrogate imaging markers of tumor biology than SUVmax.
The present study showed that the occurrence rate of BM was associated with adenocarcinoma (HR=5.715; p=0.024) according to the univariate analysis. Similar results have been shown in several studies, in which patients with adenocarcinoma or nonsquamous cell carcinoma had a significantly higher incidence of developing BM than squamous cell carcinoma, with a relative risk in the range of 2.1 to 4.1 (25–27). This may be related to the pathological and biological characteristics that the adenocarcinoma subtype is generally more invasive than the squamous cell carcinoma subtype (28).
The current study had some limitations. First, the study design was retrospective and we had a small sample size, and absence of an independent set of patients to perform verification. Furthermore, the heterogeneous treatment protocols may have a confounding effect on prognostication. Further prospective studies with larger homogeneous patient cohorts are warranted to validate the results. Second, due to the limited number of patients enrolled, we did not analyze whether there were differences in PET parameters between patients with BM and other distant metastasis. More patient cohorts were needed to analyze these differences in the next study. However, our results preliminarily indicate that baseline metabolic tumor burden can provide predictive information for BM development. Third, the accuracy of the metabolic tumor burden may be affected by the false-negative and false-positive results of 18F-FDG PET. In this study, we try to reduce such deviation by reading 18F-FDG PET together with diagnostic CT.
Baseline metabolic tumor burden at the level of the metastatic lymph nodes and whole-body tumors as measured with MTV and TLG on 18F-FDG PET are significant predictive factors for BM development independent of T and N stages and are better predictive imaging biomarkers than SUVmax in patients with locally advanced NSCLC. Therefore, the addition of tumor burden measurements can help further stratify patients within each stage and optimize treatment methods. These results will need to be validated in a multicenter prospective study with larger homogeneous patient cohorts.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by The institutional review board of the First Affiliated Hospital of Jinan University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements. Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
JS and HY contributed to the writing of the manuscript. JS, HY, CD, YL and YC collected the original data. JS contributed to the statistical analysis of the data and constructed the figures and tables. JS, YT, BG and JG contributed to the analysis of imaging information. XL and HX conceived the paper layout and modified the paper. All authors had read and approved the final version of the manuscript.
This work was financially supported by the Fundamental Research Funds for the Central Universities (21619352) and Funding by Science and Technology Projects in Guangzhou (202201010168).
We would like to thank the staff members of the Department of Nuclear Medicine and PET/CT-MR Centre, First Affiliated Hospital of Jinan University, for their excellent technical support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sert F, Cosgun G, Yalman D, Ozkok S. Can we define any marker associated with brain failure in patients with locally advanced non-small cell lung cancer? Cancer Radiother (2021) 25(4):316–22. doi: 10.1016/j.canrad.2020.11.002
2. Sun F, Chen Y, Chen X, Sun X, Xing L. CT-based radiomics for predicting brain metastases as the first failure in patients with curatively resected locally advanced non-small cell lung cancer. Eur J Radiol (2021) 134:109411. doi: 10.1016/j.ejrad.2020.109411
3. Chen AM, Jahan TM, Jablons DM, Garcia J, Larson DA. Risk of cerebral metastases and neurological death after pathological complete response to neoadjuvant therapy for locally advanced nonsmall-cell lung cancer: Clinical implications for the subsequent management of the brain. Cancer (2007) 109(8):1668–75. doi: 10.1002/cncr.22565
4. Peters S, Bexelius C, Munk V, Leighl N. The impact of brain metastasis on quality of life, resource utilization and survival in patients with non-small-cell lung cancer. Cancer Treat Rev (2016) 45:139–62. doi: 10.1016/j.ctrv.2016.03.009
5. Gore EM, Bae K, Wong SJ, Sun A, Bonner JA, Schild SE, et al. Phase III comparison of prophylactic cranial irradiation versus observation in patients with locally advanced non-small-cell lung cancer: Primary analysis of radiation therapy oncology group study RTOG 0214. J Clin Oncol (2011) 29(3):272–8. doi: 10.1200/JCO.2010.29.1609
6. Sun A, Bae K, Gore EM, Movsas B, Wong SJ, Meyers CA, et al. Phase III trial of prophylactic cranial irradiation compared with observation in patients with locally advanced non-small-cell lung cancer: Neurocognitive and quality-of-life analysis. J Clin Oncol (2011) 29(3):279–86. doi: 10.1200/JCO.2010.29.6053
7. Achrol AS, Rennert RC, Anders C, Soffietti R, Ahluwalia MS, Nayak L, et al. Brain metastases. Nat Rev Dis Primers (2019) 5(1):5. doi: 10.1038/s41572-018-0055-y
8. Ebben JD, You M. Brain metastasis in lung cancer: Building a molecular and systems-level understanding to improve outcomes. Int J Biochem Cell Biol (2016) 78:288–96. doi: 10.1016/j.biocel.2016.07.025
9. Asamura H, Chansky K, Crowley J, Goldstraw P, Rusch VW, Vansteenkiste JF, et al. The international association for the study of lung cancer lung cancer staging project: Proposals for the revision of the n descriptors in the forthcoming 8th edition of the TNM classification for lung cancer. J Thorac Oncol (2015) 10(12):1675–84. doi: 10.1097/JTO.0000000000000678
10. UyBico SJ, Wu CC, Suh RD, Le NH, Brown K, Krishnam MS. Lung cancer staging essentials: the new TNM staging system and potential imaging pitfalls. Radiographics (2010) 30(5):1163–81. doi: 10.1148/rg.305095166
11. Grootjans W, de Geus-Oei LF, Troost EG, Visser EP, Oyen WJ, Bussink J. PET in the management of locally advanced and metastatic NSCLC. Nat Rev Clin Oncol (2015) 12(7):395–407. doi: 10.1038/nrclinonc.2015.75
12. Pellegrino S, Fonti R, Pulcrano A, Del Vecchio S. PET-based volumetric biomarkers for risk stratification of non-small cell lung cancer patients. Diagnostics (Basel) (2021) 11(2):210. doi: 10.3390/diagnostics11020210
13. Liao S, Penney BC, Wroblewski K, Zhang H, Simon CA, Kampalath R, et al. Prognostic value of metabolic tumor burden on 18F-FDG PET in nonsurgical patients with non-small cell lung cancer. Eur J Nucl Med Mol Imaging (2012) 39(1):27–38. doi: 10.1007/s00259-011-1934-6
14. Soussan M, Chouahnia K, Maisonobe JA, Boubaya M, Eder V, Morere JF, et al. Prognostic implications of volume-based measurements on FDG PET/CT in stage III non-small-cell lung cancer after induction chemotherapy. Eur J Nucl Med Mol Imaging (2013) 40(5):668–76. doi: 10.1007/s00259-012-2321-7
15. Hyun SH, Ahn HK, Kim H, Ahn MJ, Park K, Ahn YC, et al. Volume-based assessment by (18)F-FDG PET/CT predicts survival in patients with stage III non-small-cell lung cancer. Eur J Nucl Med Mol Imaging (2014) 41(1):50–8. doi: 10.1007/s00259-013-2530-8
16. Shang J, Ling X, Zhang L, Tang Y, Xiao Z, Cheng Y, et al. Comparison of RECIST, EORTC criteria and PERCIST for evaluation of early response to chemotherapy in patients with non-small-cell lung cancer. Eur J Nucl Med Mol Imaging (2016) 43(11):1945–53. doi: 10.1007/s00259-016-3420-7
17. Shang J, Tan Z, Cheng Y, Tang Y, Guo B, Gong J, et al. A method for evaluation of patient-specific lean body mass from limited-coverage CT images and its application in PERCIST: comparison with predictive equation. EJNMMI Phys (2021) 8(1):12. doi: 10.1186/s40658-021-00358-7
18. Ernani V, Stinchcombe TE. Management of brain metastases in non-Small-Cell lung cancer. J Oncol Pract (2019) 15(11):563–70. doi: 10.1200/JOP.19.00357
19. Bajard A, Westeel V, Dubiez A, Jacoulet P, Pernet D, Dalphin JC, et al. Multivariate analysis of factors predictive of brain metastases in localised non-small cell lung carcinoma. Lung Cancer (2004) 45(3):317–23. doi: 10.1016/j.lungcan.2004.01.025
20. Mujoomdar A, Austin JH, Malhotra R, Powell CA, Pearson GD, Shiau MC, et al. Clinical predictors of metastatic disease to the brain from non-small cell lung carcinoma: primary tumor size, cell type, and lymph node metastases. Radiology (2007) 242(3):882–8. doi: 10.1148/radiol.2423051707
21. Won YW, Joo J, Yun T, Lee GK, Han JY, Kim HT, et al. A nomogram to predict brain metastasis as the first relapse in curatively resected non-small cell lung cancer patients. Lung Cancer (2015) 88(2):201–7. doi: 10.1016/j.lungcan.2015.02.006
22. Hendriks LEL, Brouns JWM, Amini M, Uyterlinde W, Wijsman R, Bussink J, et al. Development of symptomatic brain metastases after chemoradiotherapy for stage III non-small cell lung cancer: Does the type of chemotherapy regimen matter? Lung Cancer (2016) 101:68–75. doi: 10.1016/j.lungcan.2016.09.008
23. Ceresoli GL, Reni M, Chiesa G, Carretta A, Schipani S, Passoni P, et al. Brain metastases in locally advanced nonsmall cell lung carcinoma after multimodality treatment: risk factors analysis. Cancer (2022) 95(3):605–12. doi: 10.1002/cncr.10687
24. Ding X, Dai H, Hui Z, Ji W, Liang J, Lv J, et al. Risk factors of brain metastases in completely resected pathological stage IIIA-N2 non-small cell lung cancer. Radiat Oncol (2012) 7:119. doi: 10.1186/1748-717X-7-119
25. Gaspar LE, Chansky K, Albain KS, Vallieres E, Rusch V, Crowley JJ, et al. Time from treatment to subsequent diagnosis of brain metastases in stage III non-small-cell lung cancer: a retrospective review by the southwest oncology group. J Clin Oncol (2005) 23(13):2955–61. doi: 10.1200/JCO.2005.08.026
26. Ji Z, Bi N, Wang J, Hui Z, Xiao Z, Feng Q, et al. Risk factors for brain metastases in locally advanced non-small cell lung cancer with definitive chest radiation. Int J Radiat Oncol Biol Phys (2014) 89(2):330–7. doi: 10.1016/j.ijrobp.2014.02.025
27. Zhang Q, Cai XW, Feng W, Yu W, Fu XL. Risk factors of brain metastases as initial failure in completely resected stage IIIA(N2) non-small cell lung cancer. Ann Transl Med (2020) 8(6):374. doi: 10.21037/atm.2020.02.72
Keywords: non-small-cell lung cancer, brain metastases, PET/CT, metabolic tumor volume, predictive value
Citation: Shang J, You H, Dong C, Li Y, Cheng Y, Tang Y, Guo B, Gong J, Ling X and Xu H (2022) Predictive value of baseline metabolic tumor burden on 18F-FDG PET/CT for brain metastases in patients with locally advanced non-small-cell lung cancer. Front. Oncol. 12:1029684. doi: 10.3389/fonc.2022.1029684
Received: 31 August 2022; Accepted: 12 October 2022;
Published: 26 October 2022.
Edited by:
Lijun Lu, Southern Medical University, ChinaReviewed by:
Andrei Fodor, IRCCS San Raffaele Scientific Institute, ItalyCopyright © 2022 Shang, You, Dong, Li, Cheng, Tang, Guo, Gong, Ling and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hao Xu, dHhoQGpudS5lZHUuY24=; Xueying Ling, bGluZ3h1ZXlAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.