- 1Department of Gynecology, Shanghai University of Medicine & Health Sciences Affiliated Zhoupu Hospital, Shanghai, China
- 2Department of Gynecology, Shanghai University of Medicine & Health Sciences, Shanghai, China
Introduction: Hydrometra is a common gynecological disease, especially in postmenopausal women. However, its epidemiology, harmfulness, and value in predicting gynecological tumors have not been clearly elucidated.
Methods: In this study, the prevalence rate of and risk factors for hydrometra were investigated in 3,903 women who underwent screening for gynecological diseases at Zhoupu Hospital in Shanghai from 1 January to 31 December 2021. In addition, pathological distribution of hydrometra and its predictive value in gynecological tumors were studied in another 186 patients in whom hydrometra was diagnosed sonographically at Zhoupu Hospital, from 1 January 2020 to 31 December 2021, and who underwent hysteroscopy and postoperative pathological examination.
Results: The observed prevalence rate of hydrometra was 10.86%, which was higher than the prevalence of other gynecological diseases. Univariate and multivariate analysis indicated that advanced age (OR 1.11) and vaginitis (OR 3.18) were independent risk factors for hydrometra. Among 186 patients with a sonographic diagnosis of uterine fluid, simple hydrometra accounted for 34.41% of cases, inflammation accounted for 16.23%, and hematometra accounted for 2.15%, while gynecological tumors accounted for 5.91%. Moreover, univariate and multivariate analysis indicated that a higher body mass index (>23.92 kg/m2), greater hydrometra volume (i.e., distance between the two layers of endometrium>4.75 mm), and abnormal vaginal bleeding were high-risk predictive factors for gynecological tumors.
Discussion: In conclusion, hydrometra is a common disease, and is a risk factor for endometrial cancer and cervical cancer, especially in patients with higher hydrometra volume, higher BMI, and abnormal vaginal bleeding. It is necessary to pay more attention to hydrometra.
Introduction
Hydrometra is a common disease, with a reported prevalence rate of 14.1% (1). Inflammation is considered to be the most important cause, especially in postmenopausal women, and is rare in premenopausal women (2, 3). Estrogen stimulation or treatment with tamoxifen also contributes to hydrometra development (4), and obstruction of the cervix, vagina, or fallopian tubes is thought to play an important role (5). Other factors associated with high risk for hydrometra include advanced age and long postmenopausal time, while hormone replacement therapy was once thought to be a potential treatment for hydrometra (1).
Moreover, previous studies have considered hydrometra as a barrier to reproduction, accounting for reduced pregnancy rates in women undergoing in vitro fertilization (IVF) (5–7). However, this view remains controversial. Mehtap Polat etal. (8) reported that transient intrauterine fluid accumulation was not detrimental to IVF if it was not due to hydrosalpinx or any identifiable pelvic pathology.
Hydrometra has been considered as a typical symptom of genital tract tumors, and this is especially true of fallopian tube carcinoma, ovarian cancer, and endometrial carcinoma (9–11). Other researchers believe that hydrometra can be used as a prognostic factor in cervical cancer and endometrial carcinoma (12, 13). However, most previous studies were case reports, and systematic studies on hydrometra are still lacking. Moreover, hydrometra, being a common symptom, is usually ignored as a warning of potential adverse effects on women’s health, especially cancer. There remains a lack of large-scale epidemiological studies of hydrometra to clarify its incidence, risk factors, and value in predicting in tumors. Moreover, studies of the pathogenesis of hydrometra have been mainly carried out in animals, so risk factors for hydrometra are still unknown.
In this study, we attempted to determine the epidemiology of and risk factors for hydrometra through regional screening for gynecological diseases. Furthermore, the pathological distribution of 186 cases of hydrometra after hysteroscopy was analyzed retrospectively to determine its perniciousness. In addition, we discuss factors that determine the role of hydrometra in gynecological tumor prediction.
Methods
Gynecological diseases screening information
To investigate the prevalence rate and risk factors of hydrometra, we studied 4,140 women who underwent screening for gynecological diseases in Zhoupu Hospital from 1 January to 31 December 2021. Of these, 237 women were excluded from this study because they had had a hysterectomy, leaving 3,903 women included in the study. All 3,903 patients had undergone all screening tests for gynecological diseases and provided complete data for study. All gynecological disease screening was performed by senior attending physicians or more senior doctors.
Uterine fibroids, ovarian cysts, hydrometra, and endometrial lesions were certified by transvaginal ultrasound examination with a LOGIQ E9 scanner (GE, USA) using a 4- to 10-Hz transducer. The structure and thickness of the endometrium were recorded for each woman. Abnormal endometrium was defined as an endometrial thickness of > 5 mm on ultrasound in a postmenopausal woman or > 15 mm in a premenopausal woman (14). Uterine fibroids were diagnosed by ultrasound as previously described by Fascilla etal. (15). Ovarian cysts were diagnosed by ultrasound as reported by Granberg and Wikland (16) and Vitale etal. (17). Adenomyosis was diagnosed by ultrasound as described by Andres etal. (18). The volume of hydrometra was determined by measuring the distance between the two layers of endometrium.
Prolapse of the uterus was diagnosed in accordance with Guideline No. 413 (19), and was certified by two experienced gynecologists.
Cervical lesions were screened using a ThinPrep cytologic test (TCT). The results of TCTs were determined by two experienced pathologists in accordance with the 2001 Bethesda System (20). Cervical polyps were diagnosed as described previously (21).
Vaginitis was detected in accordance with the relevant American College of Obstetricians and Gynecologists (ACOG) Practice Bulletin (22). If cleanliness of leukorrhea was more than 3 degree or the presence of Trichomonas, mycetes, or clue cells was found, then vaginitis was diagnosed.
Information on patients with hydrometra
To study the pathological outcome of hydrometra and its relationship with gynecological tumors, another resident patient sample with hydrometra was employed, this time comprising 186 patients who received a sonographic diagnosis of hydrometra in Zhoupu Hospital from 1 January 2020 to 31 December 2021. All patients were divided into two groups according to the pathological findings: a positive group (i.e., those with cervical cancer, high-grade squamous intraepithelial lesion of the cervix, atypical hyperplasia of the endometrium, or endometrial cancer) and a negative control (NC) group (i.e., those patients with none of the above lesions).
All patients underwent hysteroscopy and postoperative pathological examination by an experienced gynecologist and pathologist. Moreover, all patients underwent cervical canal and uterine cavity sampling during hysteroscopy. The presence of hydrometra was certified by hysteroscopy, the presence of an intrauterine device (IUD) was determined by hysteroscopy and ultrasound, and the presence of intrauterine occupation was determined by pathological findings. Body mass index (BMI) was calculated using the formula weight/height2.
Ethics statement
The protocol for this research project was approved by Zhoupu Hospital Ethics Committee and conformed to the provisions of the Declaration of Helsinki of 1995 (as revised in Brazil, 2013). All patient information was exported from the hospital information system (HIS) electronic medical record system. All patients provided written informed consent and patient anonymity was preserved.
Statistical methods
All statistical analyses were performed using SPSS software (version 22). Depending on the sample size, quantitative data were analyzed using the chi-squared test, the chi-squared test with continuous correction, or Fisher’s exact probability method. In the case of measurement data, the two independent-samples Student’s t-test, a corrected t-test, or the Wilcoxon rank-sum test was used to compare differences between the two groups. Multivariate analysis was performed using two-factor logistic regression analysis. Values of 0 or 1 were assigned to dichotomous variables, 0 for “no” and 1 for “yes”. For continuous variables, original data were used for the regression analyses. Differences were considered significant at a two-sided p-value < 0.05.
Results
Analysis of prevalence of hydrometra
In this study, the prevalence rate of different gynecological diseases among women undergoing screening in Pudong New Area in Shanghai was determined. The clinical and demographic characteristics of the 3,903 women who participated are shown in Table S1. Among the 3,903 women who underwent gynecological screening, the lesion with the highest prevalence rate was hydrometra, reaching 10.86% (Figure 1A). This was followed by vaginitis (prevalence rate 10.76%), cervical polyps (prevalence rate 8.28%), abnormal endometrium (prevalence rate 3.61%), uterine fibroids (prevalence rate 1.82%), prolapse of uterus (prevalence rate 1.43%), and adenomyosis (prevalence rate 0.51%) (Figure 1A). Moreover, it was found that the mean (SD) age of women with hydrometra was higher than that of women without hydrometra (62.07 ± 0.25 years, compared with 58.12 ± 0.12 years, respectively; p < 0.0001; Figure 1B). The age distribution of women with and without hydrometra was also analyzed. It was found that the prevalence rate of hydrometra was highest in the ≥ 60 years age group (63.68%) and lowest in the ≤ 50 years age group (2.83%) (Figure 1C). In contrast, it was found that the prevalence rate of no hydrometra was lower in the ≥ 60 years age group (37.65%) (Figure 1C). These data suggest that hydrometra is a common disease in women, especially in those aged ≥ 60 years.
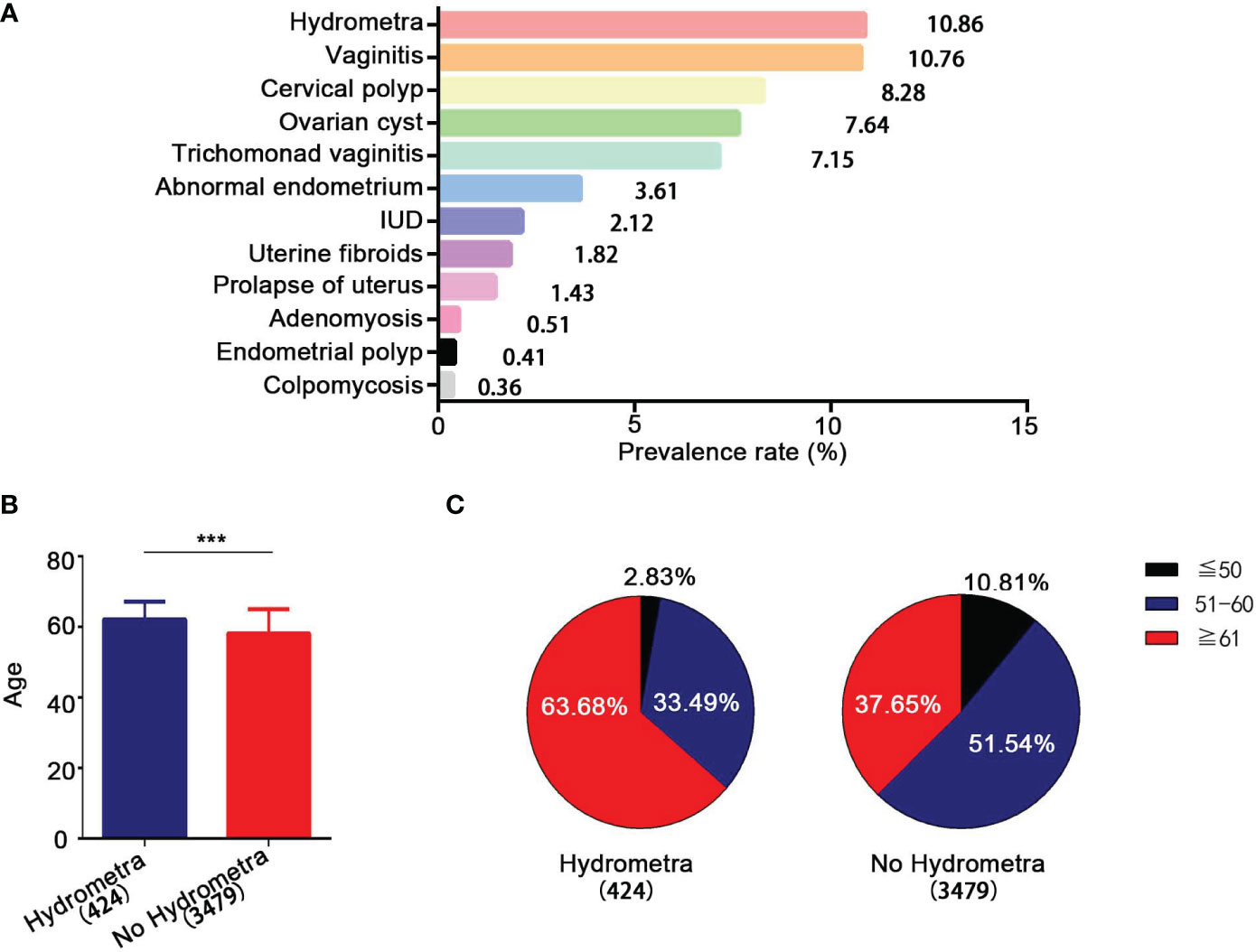
Figure 1 Epidemiological analysis of hydrometra. (A) Prevalence rate of different gynecological diseases. The y-axis represents different gynecological diseases, and the x-axis represents prevalence rate. (B) Age comparison between women with and without hydrometra. (C) Age distribution comparison between women with hydrometra and without hydrometra. ***p<0.001.
Risk factors analysis for hydrometra
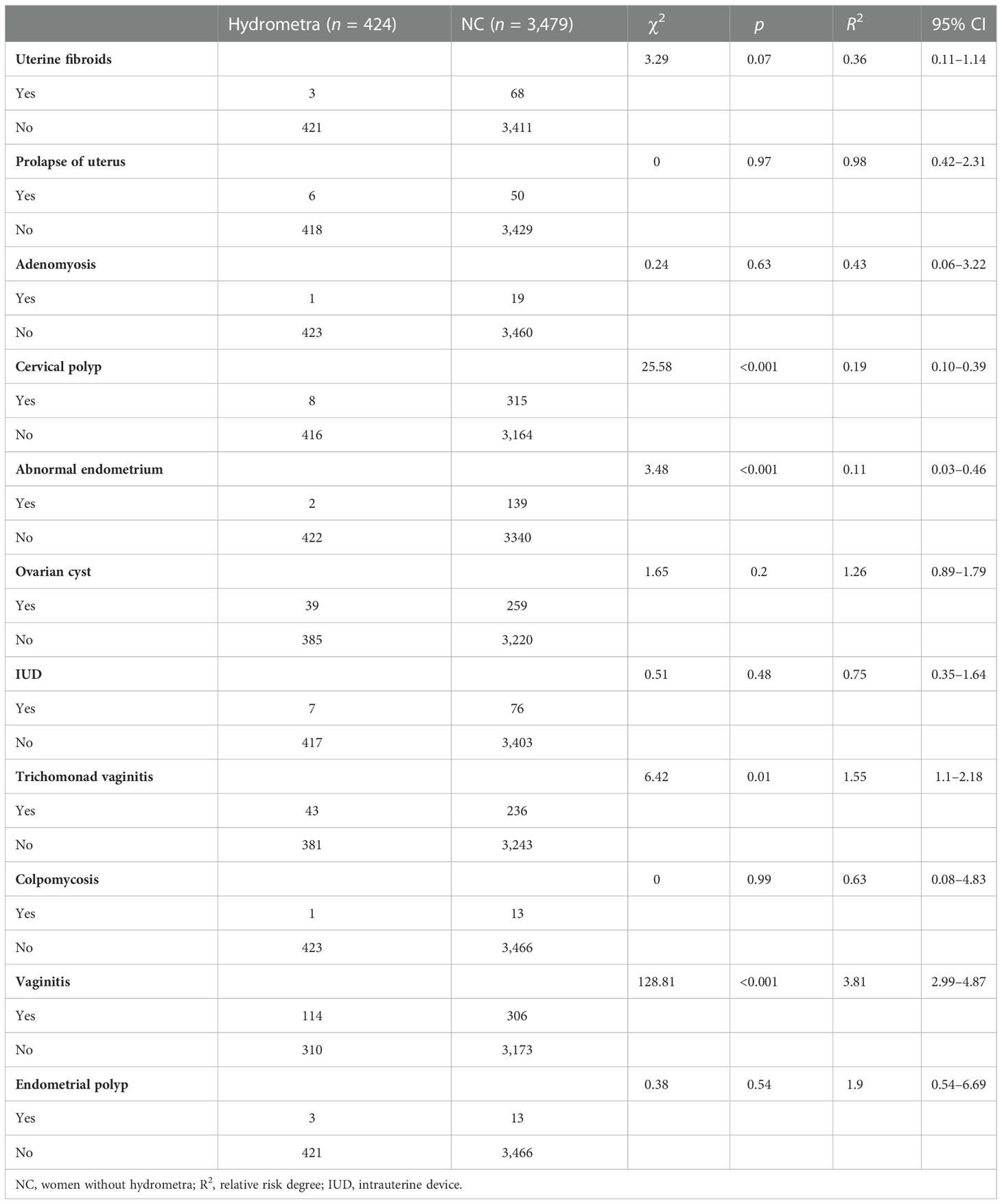
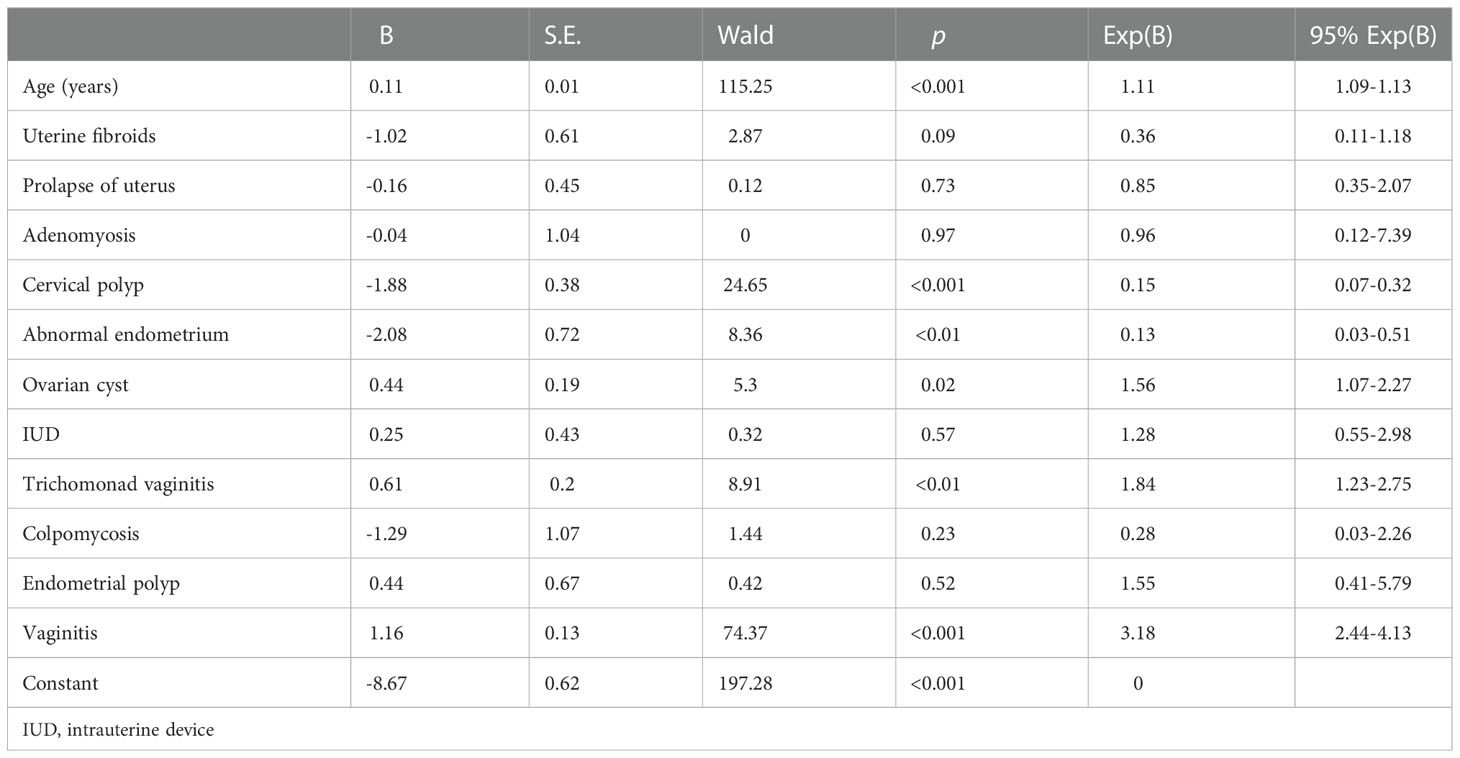
Using the gynecological disease screening data, univariate and multivariate analyses were performed to assess risk factors for hydrometra. As shown in Table 1, vaginitis was found to be highly associated with hydrometra (OR 3.81; p <0.001). Moreover, stratified analysis showed that trichomonad vaginitis was highly associated with hydrometra (OR 1.55), while there was no significant correlation between colpomycosis and hydrometra. Moreover, it was found that, somewhat counterintuitively, abnormal endometrial thickening and cervical polyps were protective factors for hydrometra (Table 1). In addition, multivariate analysis showed that advanced age, vaginitis, and ovarian cyst are factors associated with high risk for hydrometra (Table 2). In contrast, abnormal endometrial thickening and cervical polyp are factors that protect against hydrometra (Table 2). These results imply that advanced age and vaginitis are positively associated with hydrometra, and that timely treatment of vaginitis may be beneficial in reducing the prevalence of hydrometra to a certain extent.
Pathological distribution analysis of hydrometra
To study the pathological distribution analysis of hydrometra, we studied a further 186 patients in whom hydrometra was diagnosed by sonography at Zhoupu Hospital from 1 January 2020 to 31 December 2021, and who underwent hysteroscopic operation and postoperative pathological examination. The clinical and demographic characteristics of these patients are shown in Table S2. The results indicate that the prevalence rate of gynecological tumors among women with hydrometra was 5.91%. Among the 186 patients, there were five cases of endometrial cancer, four cases of atypical hyperplasia of endometrium, one case of cervical cancer, and one case of high-grade squamous intraepithelial lesion of the cervix (Figure 2A). Moreover, the prevalence rate of inflammation was 16.23%. However, the prevalence rate of hydrometra was only 34.41%, while the negative rate was 36.56% (Figure 2A). These data imply that hydrometra is a high-risk factor for gynecological tumor.
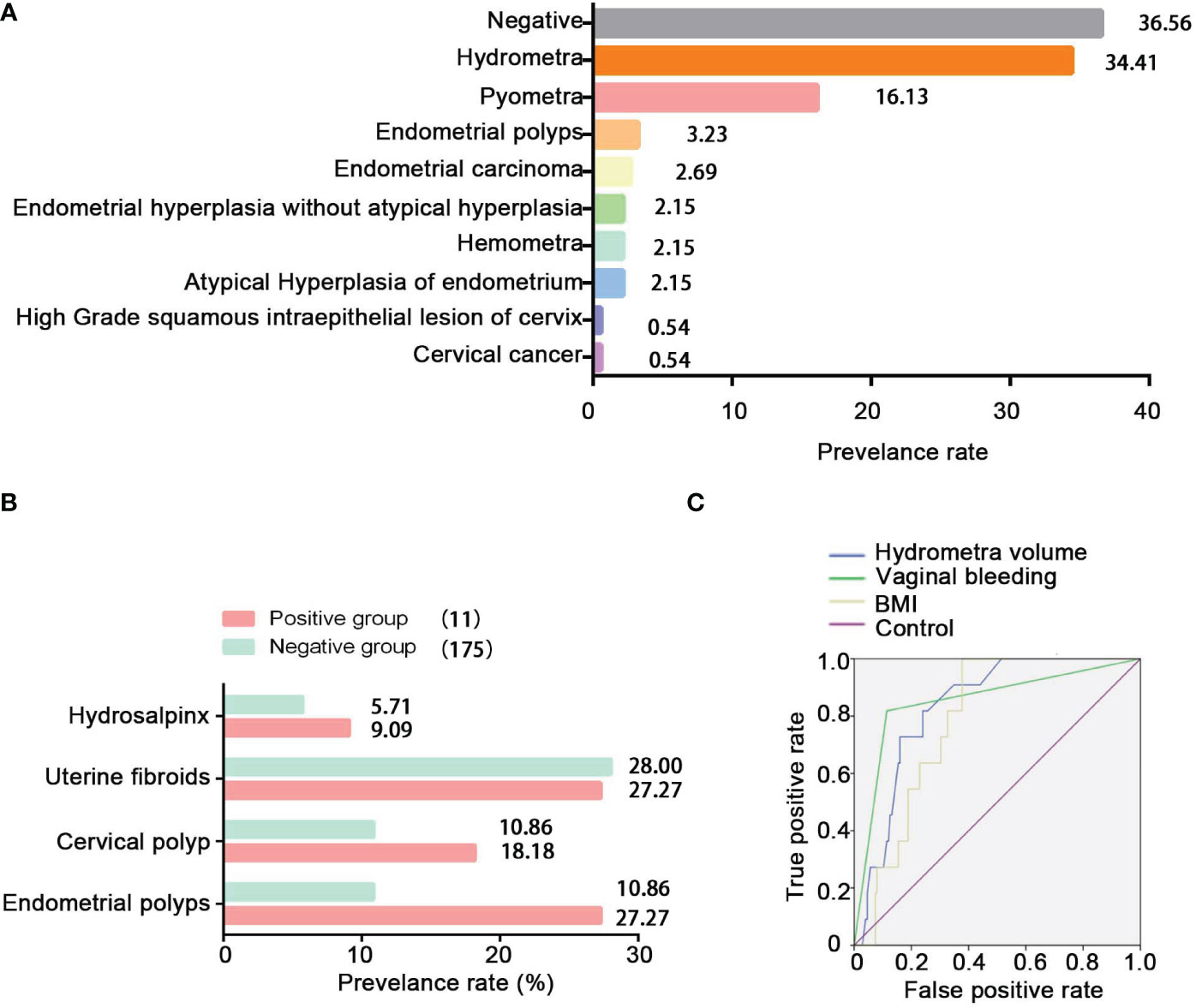
Figure 2 Pathological distribution of hydrometra and its predictive value in gynecological tumors. (A) Pathological distribution of sonographic diagnosis of hydrometra. The y-axis represents different pathological diagnoses, and the x -axis represents prevalence rate. (B) Comparison of hydrosalpinx, uterine fibroids, cervical polyp, and endometrial polyps between the positive group and the NC group. (C) ROC analysis to assess the predictive value of hydrometra volume, hydrometra complicated with BMI, or hydrometra complicated with abnormal vaginal bleeding in gynecological tumors.
Analysis of high-risk factors of hydrometra complicated with gynecological tumors
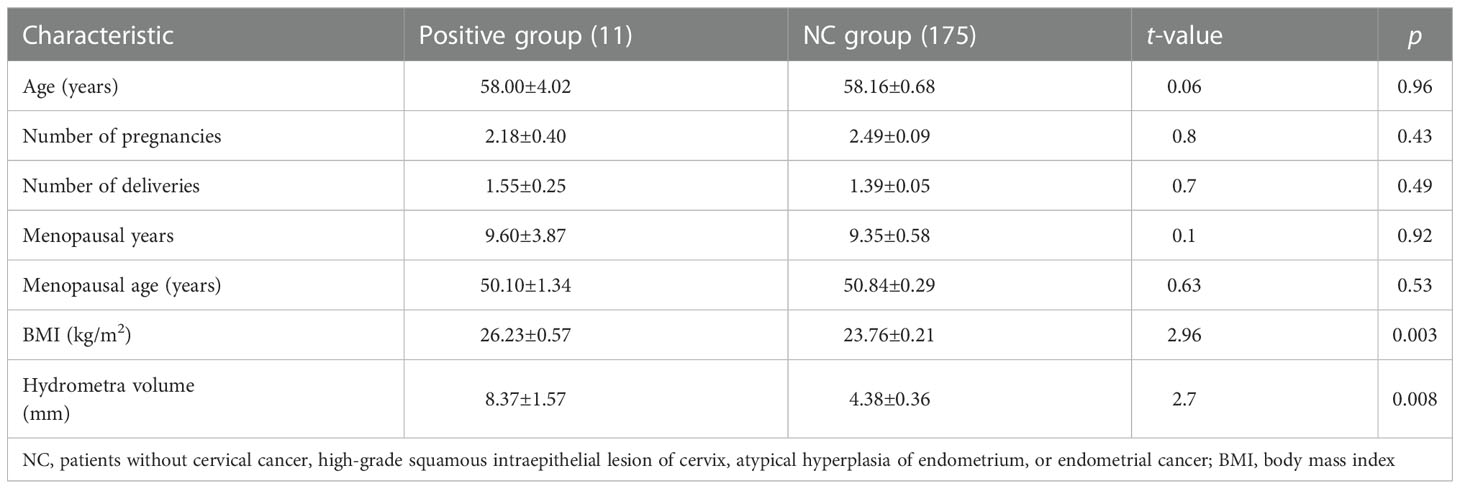
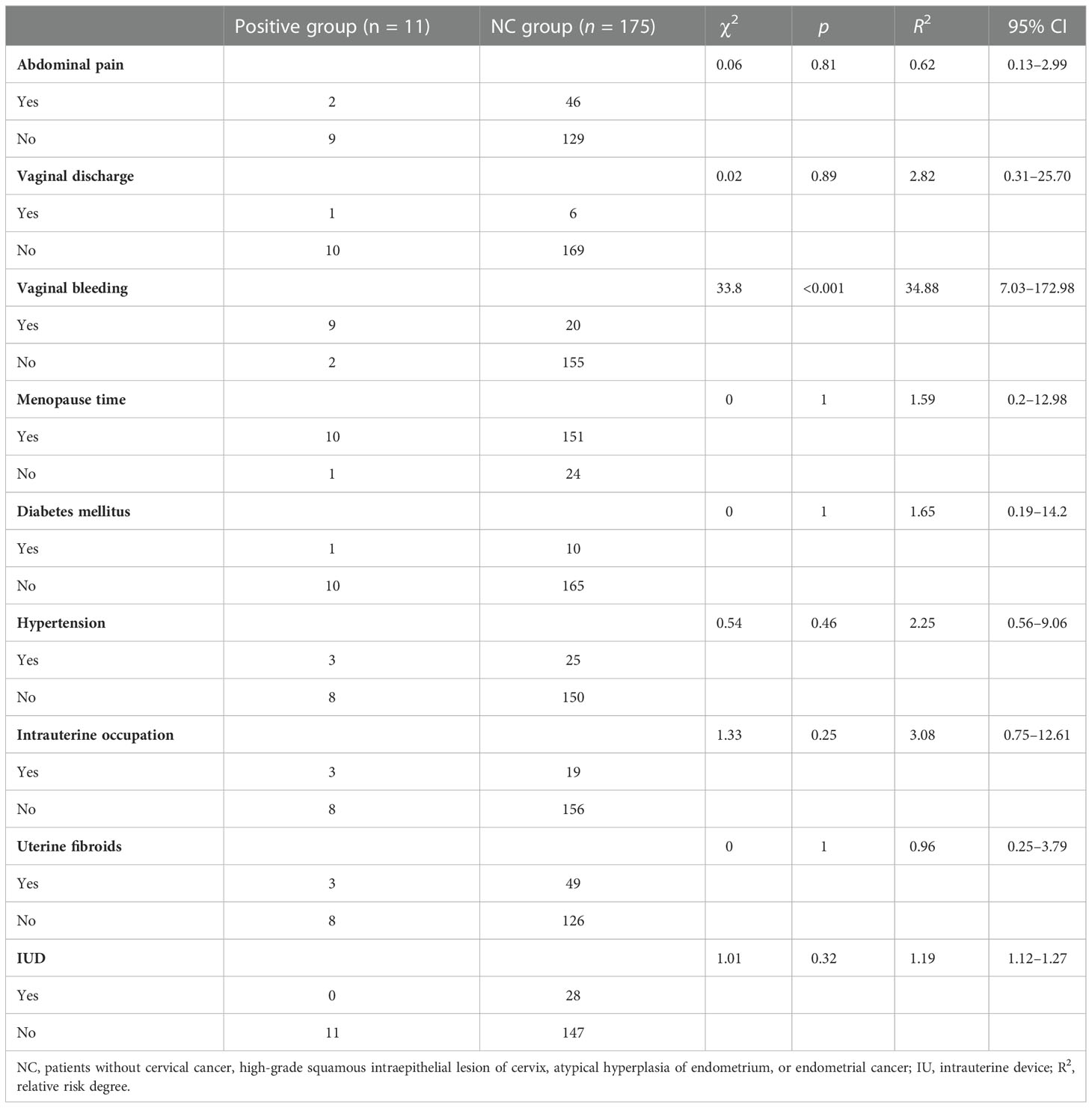
In this study, the research data showed that the prevalence rate of hydrosalpinx, cervical polyp, or endometrial polyp was higher in the positive group than in the negative group (Figure 2B). Furthermore, it can be seen that BMI and hydrometra volume were higher in patients in the positive group than in those in the NC group, which implies that high BMI and hydrometra are risk factors for hydrometra complicated by gynecological tumors (Table 3). Further univariate analysis showed that abnormal vaginal bleeding was a high-risk factor for hydrometra to predict gynecological tumor (OR 34.88; Table 4). What is more, two-factor logistic regression analysis also indicated that BMI, hydrometra volume, and abnormal vaginal bleeding were high-risk factors for hydrometra to predict gynecological tumor (Table 5). However, diabetes mellitus, menopausal age, vaginal discharge, and menopausal years were not predictive of gynecological tumors in hydrometra patients (Table 5). These data indicate that hydrometra complicated by obesity, higher hydrometra volume, or abnormal vaginal bleeding is positively associated with gynecological tumors.
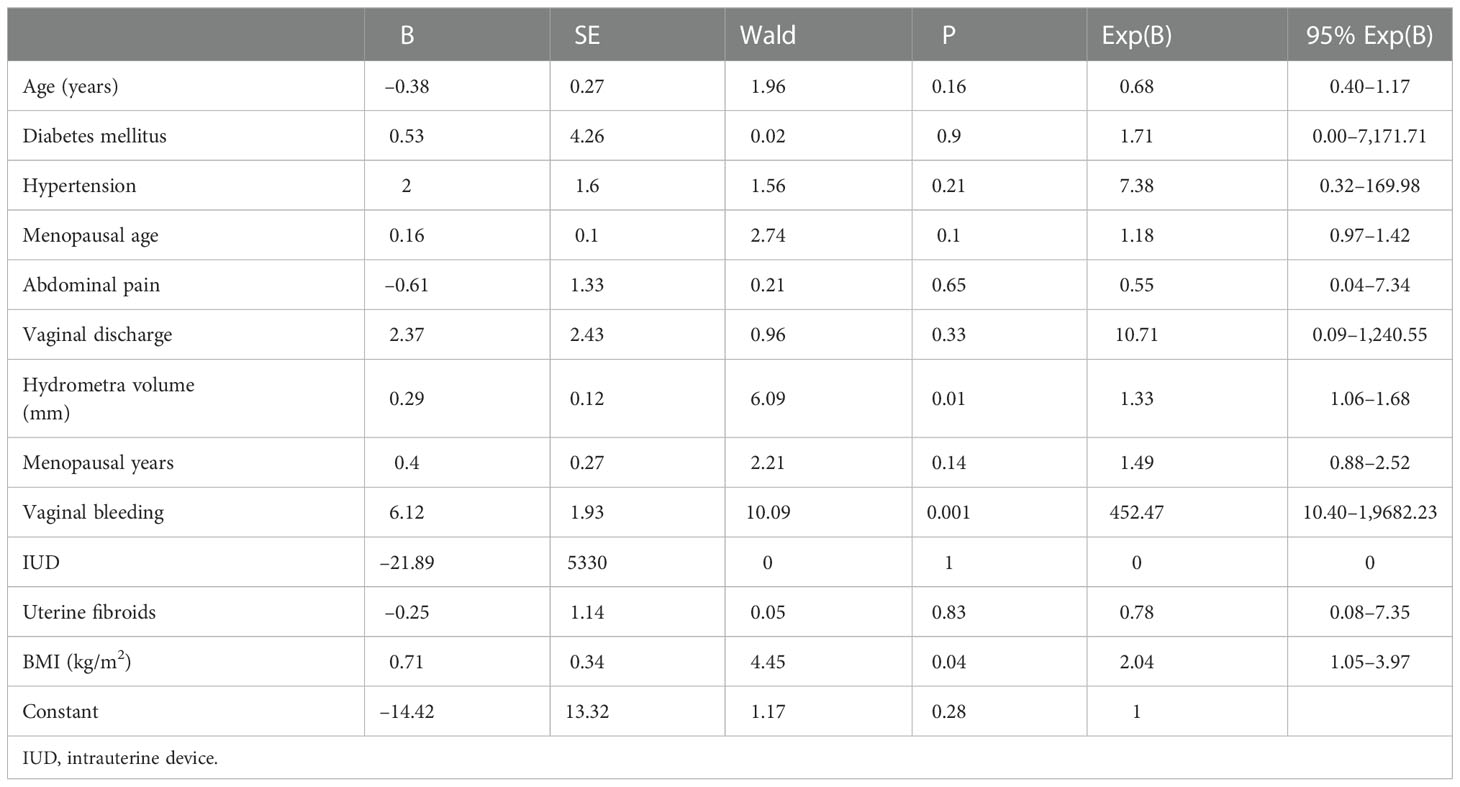
Table 5 Multivariate analysis for risk factors of hydrometra complicated with gynecological cancer (sample size: 186).
Analysis of the predictive value of hydrometra in gynecological tumors
To study the predictive value of abnormal vaginal bleeding, hydrometra volume, and BMI in gynecological tumors, receiver operating characteristic (ROC) curve analysis was performed. The area under the curve (AUC) was used to assess the sensitivity of different risk factors. The results showed that the AUC of hydrometra complicated by abnormal vaginal bleeding (0.852) was higher than the AUC of hydrometra volume (0.834), while the AUC of hydrometra complicated by obesity (0.784) was the lowest of the three factors (Figure 2C). We also considered the cut-off value of hydrometra volume and BMI in gynecological tumor prediction. The results showed that, at a cut-off value for hydrometra volume > 4.75 mm, the diagnostic sensitivity was 81.8% and the false-positive rate was 24%, with the largest difference value between the sensitivity rate and false-positive rate. We also found that, when a BMI > 23.92 kg/m2 was taken as the cut-off value, the diagnostic sensitivity was 100% and the false-positive rate was 37.7%, with the largest difference value between the sensitivity rate and false-positive rate. Furthermore, when abnormal vaginal bleeding was considered as a gynecological tumor predictor factor, the diagnostic sensitivity was 81.8% and the false-positive rate was 11.4%. These data imply that a hydrometra volume >4.75 mm, hydrometra associated with a BMI > 23.92 kg/m2 , and hydrometra associated with abnormal vaginal bleeding are all of predictive value for gynecological tumors.
Discussion
Hydrometra is a common gynecological disease. For a long time, it was regarded as only a benign disease, without recognizing its harmfulness and value in the tumor prediction. In this study, we observed that the prevalence rate of hydrometra was 10.86%, similar to the rate reported by Bar-Hava etal. (1). Moreover, the prevalence rate of cancer and precancerous lesions among hydrometra patients was 5.91%. These data suggest that hydrometra is not only a benign disease, but also an important manifestation of gynecological tumors. Therefore, hydrometra cannot be ignored, and should receive more attention.
Many studies have investigated the cause of hydrometra. Antonson etal. (23) reported that increased serum estrogen levels resulted in hydrometra in mice. Moreover, increased levels of lactoferrin, complement C3, and chitinase 3-like 1 (CHI3L1) have been found to be associated with hydrometra (24). López Rivero etal. (25) reported that an isthmocele due to a cesarean section was also associated with persistent hydrometra. In addition, abnormal development of the urogenital tract has been reported to be a cause of hydrometra (26). In addition, McQueen etal. (27) reported that increased triggering receptor expressed on myeloid cells 1/3 (TREM-1/3) infected mice through promoting transepithelial neutrophil migration in the uterus and uterine glands. However, most current studies have focused on animals, with limited data from humans. Therefore, high-risk factors for hydrometra development in humans are still unknown. In this study, advanced age, vaginitis, and ovarian cyst were found to be high-risk factors for hydrometra. It is speculated that elderly women with uterine atrophy and degeneration are more likely to experience tract obstruction, which contributes to hydrometra, and that vaginitis, which is prone to upward spread, can also induce hydrometra. Similarly, ovarian cysts may affect fallopian tube peristalsis and reduce patency, thus inducing hydrometra. However, it is difficult to believe that abnormal endometrial thickening and cervical polyps, which are thought to reduce the patency of the cervix and uterine cavity, could protect against hydrometra, as found in this study. This requires further investigation.
Inflammation is another important cause of hydrometra. Yeung etal. (28) reported that pyometra accounted for 47.8% of cases of intrauterine fluid in 228 patients, with hydrometra accounting for 43.0% and hematometra for the remaining 9.2%. However, these findings differ from our data. In our study, we found that pyometra accounted for only 16.13% of cases of intrauterine fluid in 186 patients, whereas hydrometra accounted for 34.41% and hematometra for 2.15%. These results might be ascribed to differences between regions and different populations. These data suggest that inflammation is not the most important cause of hydrometra, which would explain the fact that many patients with hydrometra do not respond to anti-inflammatory therapy in clinical practice. Moreover, Sik Wing Yeung etal. (28) found that advanced age (>75 years) was an independent risk factor for pyometra, whereas we found no independent risk factor for pyometra.
Other studies have reported that further factors associated with endometrial cancer, notably an endometrial stripe ≥ 2 cm, higher body mass index and waist-to-hip ratio, excessive unopposed exposure of the endometrium to estrogen, increasing age, obesity, hypertension, diabetes mellitus, and abnormal gene expression (Table S3) (29–36). In addition, potential risk factors for cervical cancer include human papillomavirus infection, a family history of cancer, vaginal bleeding, hypertension, multiple sexual partners, initiation of sex at a young age, smoking, and hormonal contraceptive use (Table S4) (37–43). However, the role of hydrometra in endometrial cancer and cervical cancer is still unknown. In this study, it was found that hydrometra was a risk factor for endometrial cancer and cervical cancer. We believe that this is a new discovery. More importantly, it was found that higher hydrometra volume, higher BMI, and abnormal vaginal bleeding, when complicating hydrometra, are potential predictive factors for endometrial cancer and cervical cancer. This finding is of clinical value and warrants further study.
However, our study had some limitations. For example, owing to the relatively small sample size, multivariate analysis result in high odds ratios and wide 95% CIs. Therefore, our results need to be validated in a large sample. In addition, the study was retrospective and was carried out in a single center. The results should be confirmed in multicenter prospective studies.
Conclusions
In conclusion, hydrometra is a common symptom in women, especially in advanced age women. Advanced age, vaginitis and ovarian cyst have found to be responsible for hydrometra, while anti-inflammation might be beneficial to prevent the occurrence of hydrometra. Moreover, hydrometra is a risk factor for endometrial cancer and cervical cancer, especially with higher hydrometra volume, higher BMI, and abnormal vaginal bleeding. Therefore, timely hysteroscopy and histopathological examination for hydrometra patients will be beneficial for early detection of endometrial and cervical tumors, with important clinical value.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by Zhoupu Hospital Ethics Committee. The patients/participants provided their written informed consent to participate in this study.
Author contributions
Study Design: JFW and SHW; Data Interpretation: JFW, SQW,and ZL; Manuscript Preparation: JFW and SHW; Literature Search: LZ and SHW; Funds collections: JFW. All authors contributed to the article and approved the submitted version.
Funding
The study was jointly supported by Academic Leader Training Program of Pudong New Area Health System in Shanghai (Grant No. PWRd2021–13), Shanghai Municipal Health Commission (Grant No. 201940222) and Top-100 Talent Cultivation Plan of Shanghai University of Medical and Health Sciences (Grant No. B3–0200–20–311008).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1028886/full#supplementary-material
References
1. Bar-Hava I, Orvieto R, Ferber A, Krissi H, Rath-Wolfson L, Gal R, et al. Asymptomatic postmenopausal intrauterine fluid accumulation: characterization and significance. Climacteric (1998) 1:279–83. doi: 10.3109/13697139809085555
2. Mori KM, Epstein HD, Roossin MC, Goldstein BH. A rare case of idiopathic pyometra in a premenopausal patient. J Menopausal Med (2020) 26:169–72. doi: 10.6118/jmm.20021
3. Balas Şener, Yılmaz KB, Yıldırım SAkçalar, Açıkgöz B, Tatar İdilGüneş, Bayar B, et al. Spontaneous perforation of pyometra: A rare cause of acute abdomen and sepsis. Turk J Surg (2018) 34:342–5. doi: 10.5152/turkjsurg.2017.3203
4. Kalampokas T, Sofoudis C, Anastasopoulos C, Boutas I, Melloy S, Kondi-Pafiti A, et al. Effect of tamoxifen on postmenopausal endometrium. Eur J Gynaecol Oncol (2013) 34:325–8.
5. Chien L-W, Au H-K, Xiao J, Tzeng C-R. Fluid accumulation within the uterine cavity reduces pregnancy rates in women undergoing IVF. Hum Reprod (2002) 17:351–6. doi: 10.1093/humrep/17.2.351
6. Mansour RT, Aboulghar MA, Serour GI, Riad R. Fluid accumulation of the uterine cavity before embryo transfer: a possible hindrance for implantation. J In Vitro Fert Embryo Transf (1991) 8:157–9. doi: 10.1007/BF01131707
7. He Q, Tsang LL, Ajonuma LC, Chan HC. Abnormally up-regulated cystic fibrosis transmembrane conductance regulator expression and uterine fluid accumulation contribute to chlamydia trachomatis-induced female infertility. Fertil Steril (2010) 93:2608–14. doi: 10.1016/j.fertnstert.2010.01.040
8. Mehtap Polat, Boynukalin FKübra, Yarali I, Erdoğan BDoğanay, Bozdağ Gürkan, Yaralı H. Transient intrauterine fluid accumulation not due to hydrosalpinx or any identifiable pelvic pathology is not detrimental to IVF outcome. Arch Gynecol Obstet (2014) 290:569–73. doi: 10.1007/s00404-014-3245-1
9. Gomes FV, Dias JoãoL, Lucas R, Cunha TM. Primary fallopian tube carcinoma: review of MR imaging findings. Insights Imaging (2015) 6:431–9. doi: 10.1007/s13244-015-0416-y
10. Lu J, Gao Li, Yu H, Xia L, Guo X, Zheng F. A patient from zhejiang, China, with synchronous mucinous metaplasia and neoplasms of the female genital tract: A case report. . J Obstet Gynaecol Res (2019) 45:1382–5. doi: 10.1111/jog.13959
11. Yong SL, Dahian S, Ramlan AH, Kang M. The diagnostic challenge of ovarian carcinoma in normal-sized ovaries: a report of two cases. Horm Mol Biol Clin Investig (2018) 35:20180043. doi: 10.1515/hmbci-2018-0043
12. Pardo J, Kaplan B, Nitke S, Ovadia J, Segal J, Neri A. Postmenopausal intrauterine fluid collection: correlation between ultrasound and hysteroscopy. Ultrasound Obstet Gynecol (1994) 4:224–6. doi: 10.1046/j.1469-0705.1994.04030224.x
13. van den Tillaart SAHM, Srámek A, Wasser MNJM, Trimbos JBMZ. Barrel index of bulky cervical tumours and intrauterine fluid determined by MRI as additional prognostic factors for survival. Eur J Gynaecol Oncol (2013) 34:208–12.
14. Wolfman W, Leyland N, Heywood M, Singh SS, Rittenberg DA, Soucy R, et al. Asymptomatic endometrial thickening. J Obstet Gynaecol Can (2010) 32:990–9. doi: 10.1016/s1701-2163(16)34690-4
15. Fascilla FD, Cramarossa P, Cannone R, Olivieri C, Vimercati A, Exacoustos C. Ultrasound diagnosis of uterine myomas. Minerva Ginecol (2016) 68:297–312.
16. Granberg S, Wikland M. Ultrasound in the diagnosis and treatment of ovarian cystic tumours. Hum Reprod (1991) 6:177–85. doi: 10.1093/oxfordjournals.humrep.a137301
17. Vitale SG, Haimovich S, Laganà AS, Alonso L, Di Spiezio Sardo A, Carugno J, et al. Endometrial polyps An evidence-based diagnosis and management guide. . Eur J Obstet Gynecol Reprod Biol (2021) 260:70–7. doi: 10.1016/j.ejogrb.2021.03.017
18. Andres MP, Borrelli GM, Ribeiro J, Baracat EC, Abrão MS, Kho RM. Transvaginal ultrasound for the diagnosis of adenomyosis: Systematic review and meta-analysis. J Minim Invasive Gynecol (2018) 25:257–64. doi: 10.1016/j.jmig.2017.08.653
19. Geoffrion R, Larouche M. Guideline no. 413: Surgical management of apical pelvic organ prolapse in women. J Obstet Gynaecol Can (2021) 43:511–523.e1. doi: 10.1016/j.jogc.2021.02.001
20. Alrajjal A, Pansare V, Choudhury MSR, Khan MYA, Shidham VB. Squamous intraepithelial lesions (SIL: LSIL, HSIL, ASCUS, ASC-h, LSIL-h) of uterine cervix and Bethesda system. Cytojournal (2021) 18:16. doi: 10.25259/Cytojournal_24_2021
21. Budak A, Kanmaz AG. Role of endometrial sampling in cases with asymptomatic cervical polyps. J Gynecol Obstet Hum Reprod (2019) 48:207–11. doi: 10.1016/j.jogoh.2019.01.005
22. Paavonen JA, Brunham RC. Vaginitis in nonpregnant patients: ACOG practice bulletin number 215. Obstet Gynecol (2020) 135:1229–30. doi: 10.1097/AOG.0000000000003857
23. Antonson P, Matic M, Portwood N, Kuiper RV, Bryzgalova G, Gao H, et al. aP2-cre-mediated inactivation of estrogen receptor alpha causes hydrometra. PloS One (2014) 9:e85581. doi: 10.1371/journal.pone.0085581
24. Antonson P, Nalvarte I, Varshney M, Xu Li, Windahl SH, Humire P, et al. Identification of proteins highly expressed in uterine fluid from mice with hydrometra. Biochem Biophys Res Commun (2015) 466:650–5. doi: 10.1016/j.bbrc.2015.09.099
25. López Rivero LP, Jaimes M, Camargo F, López-Bayghen E. Successful treatment with hysteroscopy for infertility due to isthmocele and hydrometra secondary to cesarean section: A case report. World J Clin Cases (2019) 7:753–8. doi: 10.12998/wjcc.v7.i6.753
26. Fujita Tsuboi AM, Uchida K, Nishimura R. Complex malformations of the urogenital tract in a female dog: Gartner duct cyst, ipsilateral renal agenesis, and ipsilateral hydrometra. . Jpn J Vet Res (2016) 64:147–52.
27. McQueen BE, Kollipara A, Gyorke CE, Jr CWA, Ezzell A, Darville T, et al. Reduced uterine tissue damage during chlamydia muridarum infection in TREM-1,3-Deficient mice. . Infect Immun (2021) 89:e0007221. doi: 10.1128/IAI.00072-21
28. Yeung SW, Cheung CW, Wong ASW, Fan HL, Chan JHY, Sahota DS, et al. Epidemiology and spectrum of positive bacteriological culture in intrauterine fluid collected from women with postmenopausal bleeding. Menopause (2014) 21:794–8. doi: 10.1097/GME.0000000000000182
29. Cavaliere AF, Perelli F, Zaami S, Piergentili R, Mattei A, Vizzielli G, et al. Towards personalized medicine: Non-coding RNAs and endometrial cancer. Healthcare (Basel) (2021) 9(8):965. doi: 10.3390/healthcare9080965
30. Braun MM, Overbeek-Wager EA, Grumbo RJ. Diagnosis and management of endometrial cancer. Am Fam. Phys (2016) 93:468–74.
31. Piergentili R, Zaami S, Cavaliere AF, Signore F, Scambia G, Mattei A, et al. Non-coding RNAs as prognostic markers for endometrial cancer. Int J Mol Sci (2021) 22(6):3151. doi: 10.3390/ijms22063151
32. Vetter MH, Smith B, Benedict J, Hade EM, Bixel K, Copeland LJ, et al. Preoperative predictors of endometrial cancer at time of hysterectomy for endometrial intraepithelial neoplasia or complex atypical hyperplasia. Am J Obstet Gynecol (2020) 222:60.e1–7. doi: 10.1016/j.ajog.2019.08.002
33. Gao Yu, Zhai P, Jiang F, Zhou F, Wang X. Association between coffee drinking and endometrial cancer risk: A meta-analysis. J Obstet Gynaecol Res (2022) 48:774–95. doi: 10.1111/jog.15139
34. Doherty MT, Sanni OB, Coleman HG, Cardwell CR, Glenn McCluggage W, Quinn D, et al. Concurrent and future risk of endometrial cancer in women with endometrial hyperplasia: A systematic review and meta-analysis. PloS One (2020) 15:e0232231. doi: 10.1371/journal.pone.0232231
35. Raglan O, Kalliala I, Markozannes G, Cividini S, Gunter MJ, Nautiyal J, et al. Risk factors for endometrial cancer: An umbrella review of the literature. Int J Cancer (2019) 145:1719–30. doi: 10.1002/ijc.31961
36. Stelloo E, Nout RA, Osse EM, Jürgenliemk-Schulz IJ, Jobsen JJ, Lutgens LC, et al. Improved risk assessment by integrating molecular and clinicopathological factors in early-stage endometrial cancer-combined analysis of the PORTEC cohorts. Clin Cancer Res (2016) 22(16):4215–24. doi: 10.1158/1078-0432.CCR-15-2878
37. Pimple S, Mishra G. Cancer cervix: Epidemiology and disease burden. Cytojournal (2022) 19:21. doi: 10.25259/CMAS_03_02_2021
38. De Strooper LMA, Berkhof J, Steenbergen RDM, Lissenberg-Witte BI, Snijders PJF, Meijer CJLM, et al. Cervical cancer risk in HPV-positive women after a negative FAM19A4/mir124-2 methylation test: A post hoc analysis in the POBASCAM trial with 14 year follow-up. Int J Cancer (2018) 143(6):1541–8. doi: 10.1002/ijc.31539
39. Holloway SB, Colon GR, Zheng W, Lea JS. Tumor necrotic debris and high nuclear grade: Newly identified high-risk factors for early-stage endocervical adenocarcinoma. Am J Clin Oncol (2021) 44:162–8. doi: 10.1097/COC.0000000000000798
40. Hansen B, Campbell S, Nygård M. Regional differences in cervical cancer incidence and associated risk behaviors among Norwegian women: a population-based study. BMC Cancer (2021) 21:935. doi: 10.1186/s12885-021-08614-w
41. Becker TM, Wheeler CM, McGough NS, Parmenter CA, Stidley CA, Jamison SF, et al. Cigarette smoking and other risk factors for cervical dysplasia in southwestern Hispanic and non-Hispanic white women. Cancer Epidemiol Biomarkers Prev (1994) 3(2):113–9.
42. Castle PE, Walker JL, Schiffman M, Wheeler CM. Hormonal contraceptive use, pregnancy and parity, and the risk of cervical intraepithelial neoplasia 3 among oncogenic HPV DNA-positive women with equivocal or mildly abnormal cytology. Int J Cancer (2005) 117(6):1007–12. doi: 10.1002/ijc.21279
43. McIntyre-Seltman K, Castle PE, Guido R, Schiffman M, Wheeler CM, ALTS Group. Smoking is a risk factor for cervical intraepithelial neoplasia grade 3 among oncogenic human papillomavirus DNA-positive women with equivocal or mildly abnormal cytology. Cancer Epidemiol Biomarkers Prev (2005) 14(5):1165–70. doi: 10.1158/1055-9965.EPI-04-0918
Keywords: hydrometra, endometrial cancer, cervical cancer, inflammation, risk factor
Citation: Wu J, Wang S, Zhang L, Wu S and Liu Z (2023) Epidemiological analysis of hydrometra and its predictive value in gynecological tumors. Front. Oncol. 12:1028886. doi: 10.3389/fonc.2022.1028886
Received: 26 August 2022; Accepted: 05 December 2022;
Published: 05 January 2023.
Edited by:
Dana Kristjansson, Norwegian Institute of Public Health (NIPH), NorwayReviewed by:
Federica Perelli, Santa Maria Annunziata Hospital, ItalyCharlotte Sun, University of Texas MD Anderson Cancer Center, United States
Copyright © 2023 Wu, Wang, Zhang, Wu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Suqin Wu, d3Vfc3VfcWluZ0AxMjYuY29t; Zhou Liu, enB5eWZja0AxMjYuY29t
†These authors have contributed equally to this work
 Jianfa Wu
Jianfa Wu Sihong Wang
Sihong Wang Li Zhang
Li Zhang Suqin Wu
Suqin Wu Zhou Liu
Zhou Liu