
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 16 January 2023
Sec. Gastrointestinal Cancers: Colorectal Cancer
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.1026825
This article is part of the Research Topic Case Reports in Gastrointestinal Cancers : 2022 View all 33 articles
Background: Perivascular epithelioid cell tumor of the gastrointestinal tract (GI PEComa) is a rare mesenchymal neoplasm. GI PEComa is mostly observed in the colon and has a marked middle-aged female predominance. PEComa has no typical clinical or imaging manifestations or endoscopic characteristics. Therefore, the diagnosis of this disease mostly relies on pathological findings. HMB-45 is a sensitive immune marker of PEComa.
Case presentation: We reported a case of a middle-aged female with sigmoid colon PEComa. To exclude carcinogenesis, the large basal polyp in the sigmoid colon was removed by endoscopic mucosal resection (EMR). Immunohistochemistry analysis results showed that this lesion expressed HMB-45, which is a characteristic melanin marker of PEComa. Finally, the lesion was diagnosed as sigmoid colon PEComa. At the time of submission of this report, surgical resection was the primary treatment for PEComa. Though the characteristics of tumor biology and clinical behavior in PEComa are not clear, the boundary is clear, and the tumor can be completely removed. However, close follow-up is required after the surgery because of the lesion’s undetermined benign and malignant nature.
Conclusion: The present case study emphasizes the importance of pathological diagnosis. Therefore, upon finding gastrointestinal polyps with a mucosal ulcer under endoscopy, the GI PEComa diagnosis should be considered. It is necessary to detect the characteristic melanin markers of PEComa. Due to the rarity of these cases, challenges are faced in diagnosing and treating PEComa.
PEComa is a family of rare mesenchymal neoplasms with a marked female predominance. This disease’s peak onset is observed in 40-49 years of age. In addition, a few cases occur in children or adolescents (1). In 1992, Bonetti et al. used “perivascular epithelioid cells (PEC)” to describe certain epithelioid cells with perivascular distribution and expression of melanocyte markers (2). In 2002, the WHO defined PEComa as “a mesenchymal tumor composed of histologically and immunohistochemically distinctive perivascular epithelioid cells” (3). The PEComa family mainly includes angiomyolipoma (AML), lymphangioleiomyomatosis (LAM), clear-cell “sugar” tumor (CCST) of the lung, and malignant ligament clear cell tumor (CCMT) (4, 5). In addition, a rare group of PEComas called “PEComas–not otherwise specified” (PEComas-NOS), with similar morphology and immunophenotype, may arise in soft tissues (such as retroperitoneal, abdominopelvic, and cutaneous) and visceral sites (such as gastrointestinal, gynecologic, and genitourinary) (6, 7). In immunohistochemistry, virtually all PEComas express melanocytic markers, such as HMB-45 and Melan-A (5, 8, 9). PEComas exhibit neither characteristic clinical nor typical imaging and endoscopic manifestations. Therefore, PEComas are generally diagnosed using typical histological and immunohistochemical findings. PEComas-NOS manifests a broad biological behavior including benign, uncertain malignant potential, and malignant features. Folpe et al. proposed that “tumor size >5 cm, infiltrative growth pattern, high nuclear grade, necrosis, and mitotic activity >1/50 HPF” could predict malignant behavior of PEComas (10) Moreover, these features are the key to the prognostication of PEComa. Currently, wide-margin surgical resection is the best treatment method for PEComas. Additionally, some targeted molecular therapies are under the exploratory phase. Here, we present a case of PEComa of gastrointestinal tract located in the sigmoid colon of a female patient. The clinical and endoscopic findings and pathological features of this case are described to deepen our understanding of GI PEComa so as to improve its diagnostic accuracy and therapeutic effect (Figure 1).
A 47-year-old female was admitted to Binzhou Medical university hospital. The patient had no tumor-related family history or medical history of tuberous sclerosis complex (TSC), inflammatory bowel diseases, or malignant melanoma. Her physical examination results were within normal ranges. The blood and biochemical parameters, including the tumor markers such as the levels of serum CEA, CA19-9, AFP, CA15-3, and CA-125, were also within the normal ranges. The patient underwent a colonoscopy at the outpatient department of our hospital for constipation. The examination revealed a 1.5 cm × 2.0 cm polyp with surface erosion in the colon, 18 cm away from the anus. The polyp had a large basal lesion with an eroded surface. The patient was referred to our inpatient department for polyp resection. Although we considered the possibility of neoplastic polyps, we did not consider the possibility of GI PEComa initially.
The well-circumscribed tumor was 2.0 cm in maximum diameter and had a wide base (Figures 2A–C). Because the tumor had a wide base, we used endoscopic mucosal resection (EMR) to remove the lesion (Figures 2D, E) and improve postoperative pathology.
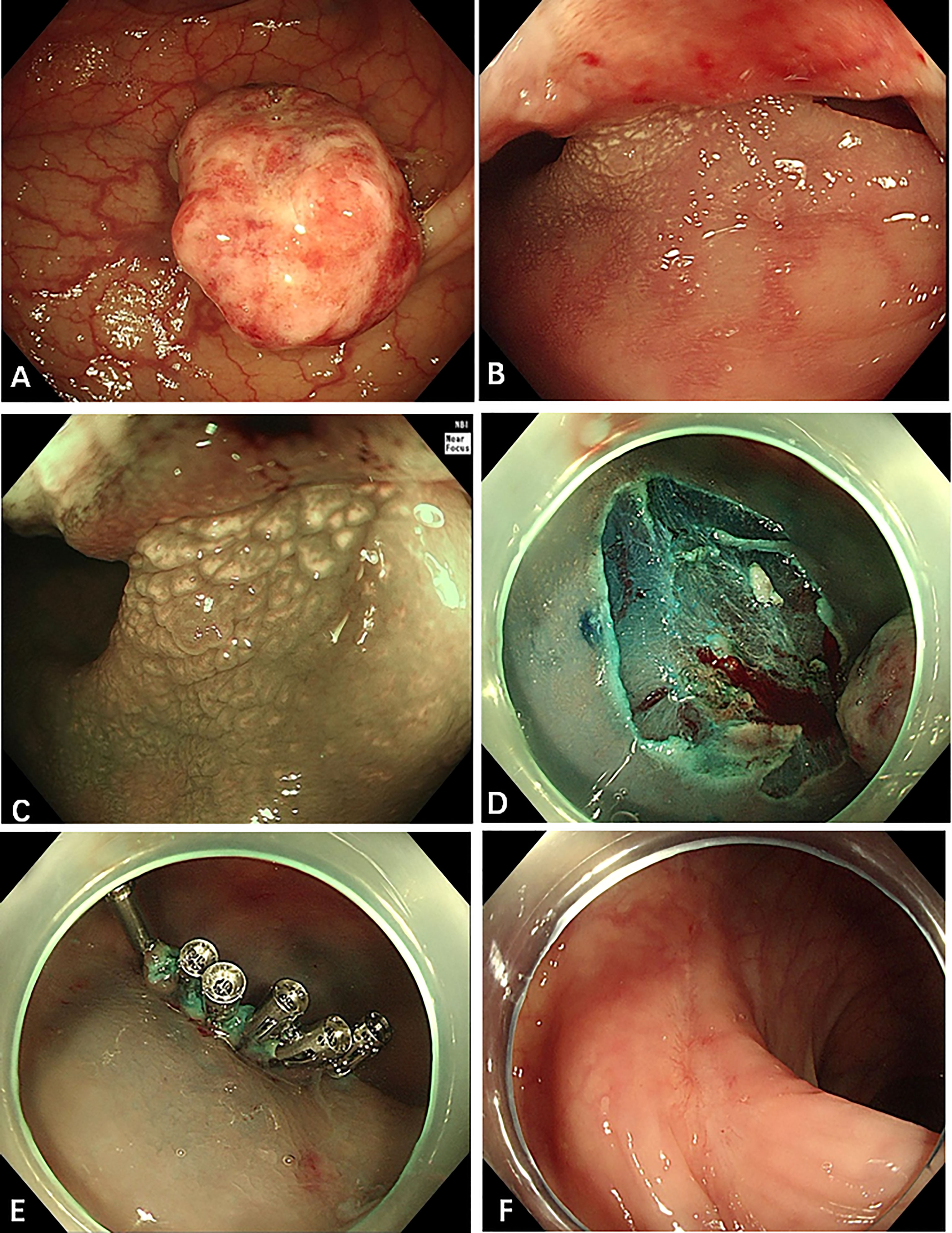
Figure 2 Endoscopic appearance of the sigmoid colon tumor. (A–C) There was a polypoid tumor in the sigmoid colon, 18 cm away from the anus, with a diameter of 1.5 cm × 2 cm and with a wide base. The surface of the polyp is eroded, and the gland tube and blood vessel under (Narrow Band Imaging, NBI) are not clearly observed. But the gland tube at the base is still regular. (D, E) A little blood oozed from the wound after EMR. We applied six endoscopic hemoclips to stop the bleeding. (F) Three months after the EMR, the wound was observed to be healed well.
Microscopically, the tumor is composed of nests of round, oval, or polygonal epithelioid cells with abundant clear eosinophilic granular cytoplasm. In our case, the nests were separated by thin fibrovascular septa (Figure 3A). The tumor cells had large nuclei with prominent nucleoli and the obvious local atypia of tumor cells (Figure 3B). The mitotic rate was >1/50HPF and about 20% of the cells expressed Ki67 (Figure 3E). In addition, the tumor showed invasive growth and foci of coagulation necrosis.
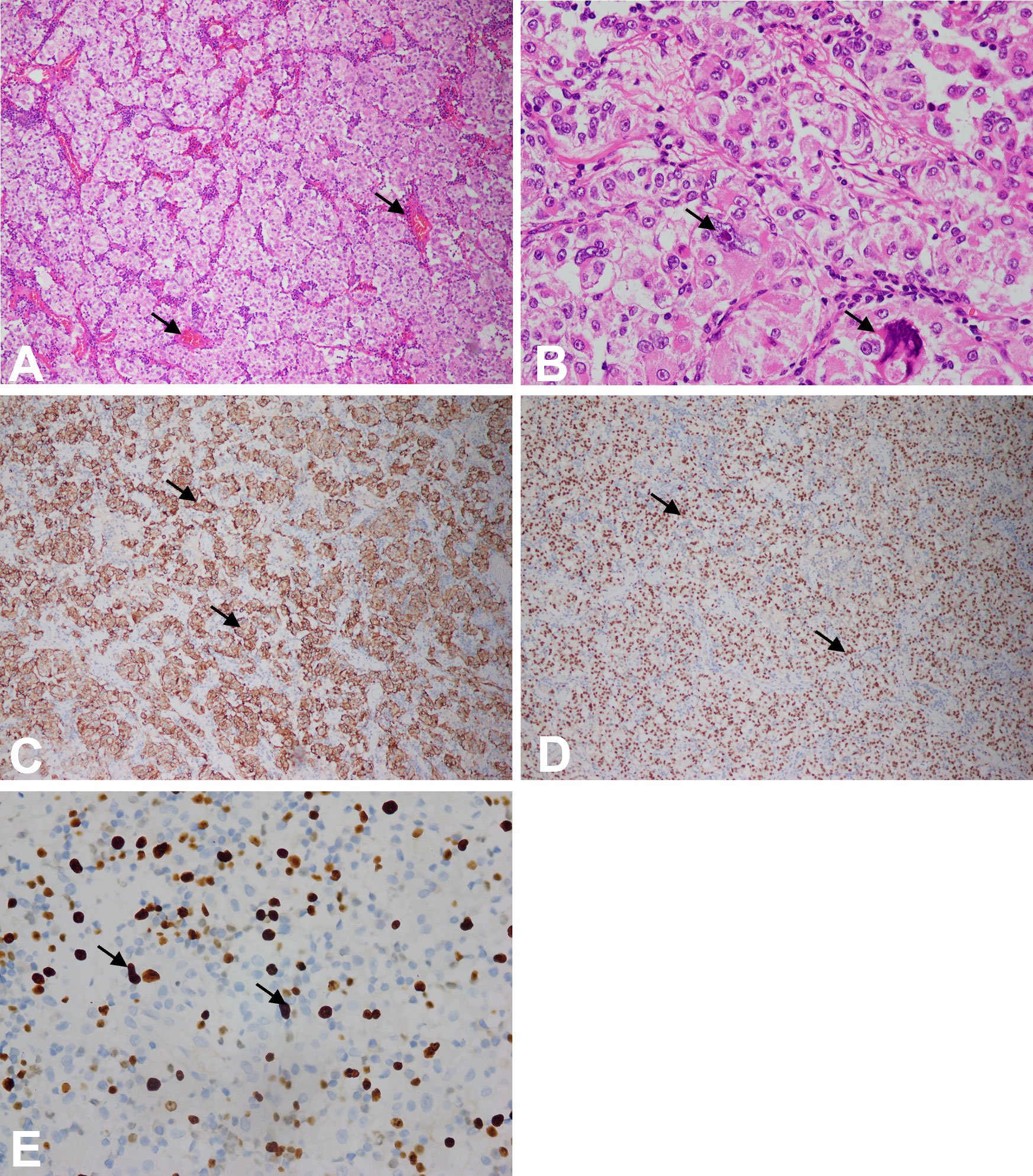
Figure 3 (A) Microscopic features of the tumor at 10× magnification using microscope. The tumor consisted of nests of epithelioid cells with clear-to-eosinophilic granular cytoplasm, as pointed by arrowheads. (B) Microscopic features of the tumor at 40× magnification using microscope. The tumor cells had large nuclei with prominent nucleoli, and the local atypia of tumor cells was obvious, as pointed by arrowheads. (C) The tumor cells were immunoreactive for HMB-45, and yellow was positive in the cytoplasm, as pointed by arrowheads (at 10× magnification using microscope). (D) The tumor cells were immunoreactive for TFE-3, and yellow was positive in the nucleus, as pointed by arrowheads (at 10x magnification using microscope). (E) Ki67 immunohistochemistry at 40× magnification using microscope.
Immunohistochemically, the cells stained positive for HMB-45 (Figure 3C) and TFE-3 (Figure 3D). However, they were negative for Melan-A, desmin, smooth muscle actin (SMA), S-100, CD117, and CK (Figure 4). Therefore, depending on the endoscopy, histology, and immunohistochemistry results, we diagnosed the tumor as GI PEComa. Of note, we did not find any vascular invasion and base incision margin involvement in the postoperative pathology, which showed complete curative resection.
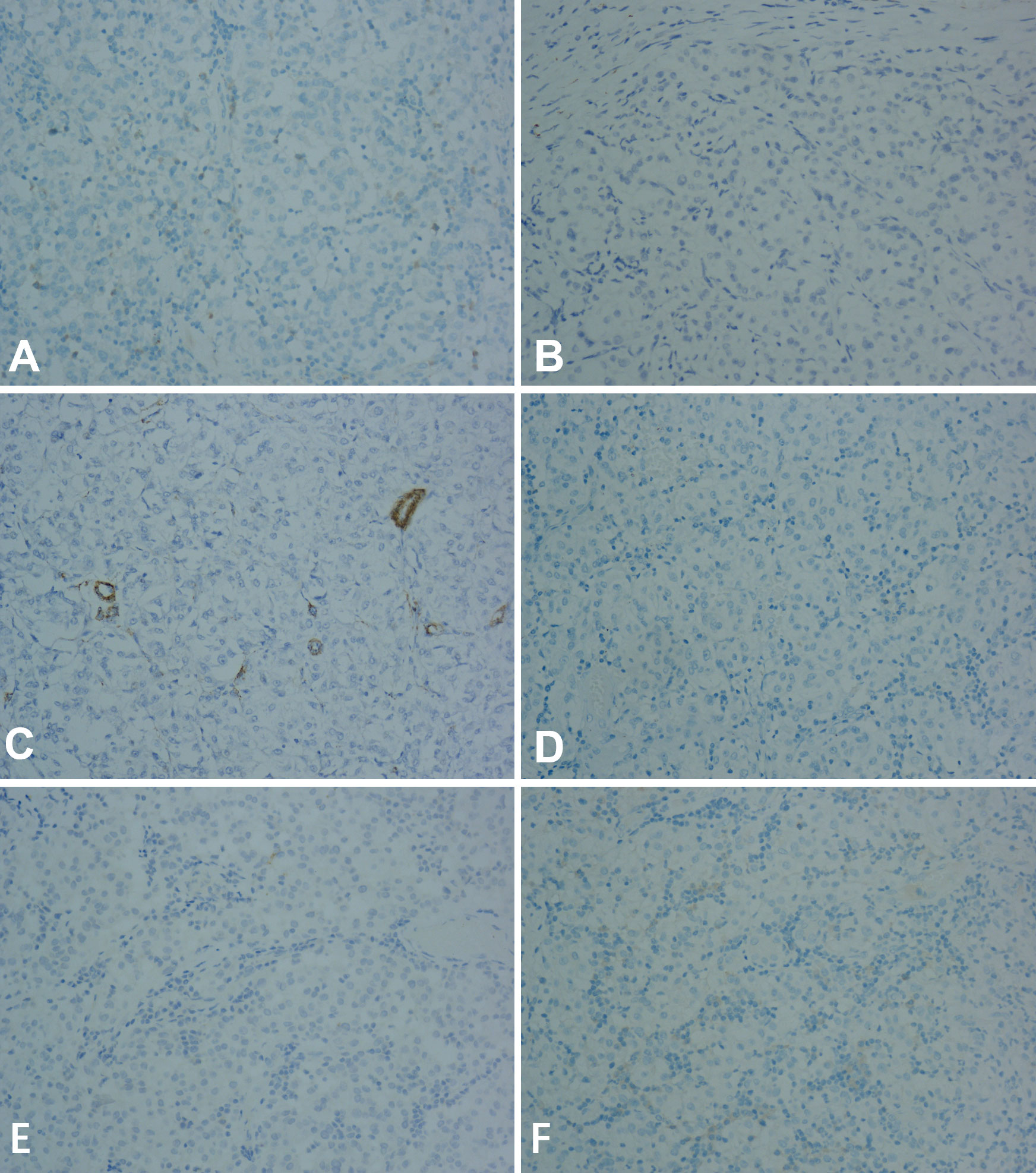
Figure 4 The tumor was negative for Melan-A, desmin, smooth muscle actin (SMA), S-100, CD117, and CK at 20x magnification using microscope. (A) Melan-A. (B) desmin. (C) smooth muscle actin (SMA). (D) S-100. (E) CK. (F) CD117.
Given the tumor invasive growth, obvious local atypia, focal coagulation necrosis, mitotic rate >1/50HPF, and the high expression of Ki67, the possibility of malignancy was high. Therefore, we used chest X-ray, total abdominal CT, and gastroscopy to exclude metastasis.
We also conducted relevant laboratory and imaging examinations. The examinations did not show any signs of malignancy or metastasis. Combined with pathological findings, the case was diagnosed as GI PEComa with unclear benign and malignant origin. Therefore, a long-term follow-up was necessary to exclude tumor malignant transformation, recurrence, and metastasis. Three months after EMR, colonoscopy showed that the postoperative healing was good (Figure 2F). The laboratory examinations and abdominal and pelvic CT showed no signs of recurrence and metastasis.
The gastrointestinal tract is the second most common location, after the uterus, of PEComa, accounting for 20-25% of the cases. The colon is the most common site of GI PEComa, followed by the small intestine, rectum, and stomach (11). Although PEComa shows a significant female predominance, the frequency of GI PEComa is similar in female and male patients (12). Based on previous case reports, the biological behavior of GI PEComa varies from benign to malignant. Most GI PEComas are benign or have uncertain malignant potential. However, a few cases show malignant behavior. Compared to other body parts, the malignancy rate of GI PEComa is relatively high (10–15).
Upon endoscopy examination, most GI PEComas exhibit clear edges, mostly polypoid lesions, necrosis, and mucosal ulcer. In previous reports, some GI PEComas were limited to the mucosa and submucosa, however, some extended to the muscular propria or even into the mesentery (12). Therefore, we could not diagnose GI PEComa only by the general manifestations observed during endoscopy. The final diagnosis depended on the pathological findings. Histologically, PEComas are composed of nests of epithelioid cells, have abundant granular eosinophilic to clear cytoplasm, and are surrounded by a delicate capillary vasculature (16). In most cases, the tumor shows a nested, trabecular, or sheet-like architecture. In addition, the tumors often show prominent nuclear pleomorphism, including coarse chromatin, hyperchromasia, prominent nucleoli, and pleomorphism (12). Immunohistochemically, PEComas express both melanocyte and muscle markers (5). Of note, the most sensitive markers of melanocytes are HMB-45 and Melan-A (17). In our case, the tumor was described as a polyp or polypoid, was well-circumscribed, and had no specific diagnostic value. Initially, we did not consider the possibility of GI PEComa. However, sigmoid colon PEComa was diagnosed after the pathological biopsy due to its positive characteristic immunohistochemical features. PEComa may have TSC mutation and TFE3 gene fusion. However, no cytogenetic and genomic analyses of the tumor were performed.
Differential diagnosis with other gastrointestinal tumors is important. A benign submucosal tumor and gastrointestinal stromal tumor (GIST) have similar endoscopic and histological appearances as GI PEComa. However, the melanin (HBM45 and Melan-A) expression is typically negative in these tumors but always positive in PEComas. In addition, PEComas must be distinguished from melanoma, liomyoma, etc based on the results of the immunohistochemical analysis. Our case showed that the possibility of GI PEComa could not be ignored for polypoid lesions. Therefore, it was essential to perform histological and immunohistochemical examinations.
Most GI PEComas are benign or have uncertain malignant potential and do not metastasize. However, malignant PEComas demonstrate local invasion and/or metastatic spread. The optimal approach to treat PEComa is not yet clear (18). National Comprehensive Cancer Network (NCCN) guidelines indicate that surgical resection is the mainstay of treatment of GI PEComas, particularly for benign tumors (19). Moreover, in the current guidelines, no standardized regimen is provided to avoid its recurrence after surgery. In the current case report, malignant PEComa with metastasis was treated with adjuvant chemotherapy (20–23); however, a standard chemotherapeutic regimen is not established for advanced PEComa.
Presently, the knowledge about the molecular genetic alterations in PEComas is limited. In a previous report, two different molecular groups were identified, including the classic marker of TSC mutation and TFE3 gene fusion. Further, molecular genetic studies revealed the deletion of 16p at the locus of the TSC2 gene in PEComas. TSC1 and TSC2 genes negatively regulated the activation of mTOR (24). Therefore, for the palliative therapy of PEComas, mTOR inhibitors, such as sirolimus and everolimus, can be used in patients with TSC mutations (25). Also, targeting the VEGF/VEGFR signaling pathway may play an important role in the inhibition of tumorigenesis. Furthermore, it may be a viable treatment option for TFE3-related malignant PEComas. Studies have shown that VEGFR-2 inhibitor apatinib has therapeutic potential in PEComa patients with TFE3 rearrangement (26). Therefore, targeting the VEGF/VEGFR signaling pathway may be a novel therapeutic option for TFE3-associated malignant PEComas. However, the clinical cases are limited. Thus, combination-targeted therapy needs further exploration. Recently, Maren Schmiester found that the TSC1/2-mTOR pathway and TFE3 overexpression can promote tumorigenesis of PEComa (27).
We used endoscopic mucosal resection (EMR) to remove the tumor. EMR is a new endoscopic minimally invasive treatment with the advantages of lesser trauma, complete resection of lesion mucosa, and fewer complications. Although EMR has several advantages, such as fewer procedural complications, its use for PEComas is not the first treatment recommendation. Moreover, EMR for PEComa is not validated, and prospective data of PEComa patients are lacking. By differentiating between benign and malignant PEComas, EMR can potentially undertreat more aggressive diseases. Nonetheless, we did not find signs of malignancy and metastasis in our case; thus, no additional surgery or other adjuvant treatment was required.
Due to the rarity and the lack of standardized biological manifestation of PEComas, the diagnostic criteria of malignant PEComas are not fully agreed upon internationally. Therefore, the prognosis of PEComas, too, remains uncertain (28–30). However, the malignant behavior may be predicted by histological features, such as size more than 5 cm, invasive growth, high differentiation, mitotic rate ≥1/50HPF, necrosis, and vascular infiltration (13). Though the tumor size was less than 5 cm in the present case, coagulative necrosis was observed, showing that the PEComa had potential invasive growth with an uncertainty of being malignant. Therefore, a long-term follow-up with the patient is essential, throughout which endoscopy and imaging examinations will be performed regularly to exclude tumor recurrence and distant metastasis. So far, the patient has no specific symptoms of discomfort. No recurrence or distant metastases were observed during follow-up of 3 mo.
GI PEComas have no typical clinical and imaging manifestations or endoscopic characteristics. Thus, it is difficult to diagnose the tumor using these parameters and even ignore the possibility of PEComas. In the case of gastrointestinal polyps, especially mucosal ulcers, the possibility of GI PEComa should be considered. It is necessary to perform a pathological biopsy and immunohistochemical analysis of the excised tissues to assess the characteristic melanin markers of PEComas and confirm the benign or malignant nature of the lesion. Due to the rarity of GI PEComas and the limitation of clinical cases, we face challenges in diagnosing and treating them.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
HY: Primary author (wrote most of the paper). YB and KL: Author of the manuscript, gastroenterologist involved in clinical management. GG and YL: Provided grammatical corrections to the manuscript. YZ was the pathologists responsible for the pathological diagnosis. CL: Provided reviews to the scientific content of the manuscript. NS: provided revisions to the scientific content of the manuscript. All authors contributed to the article and approved the submitted version.
We would like to express our gratitude to the patient for consenting to the publication of this report. We also thank all who helped writing the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Martignoni G, Pea M, Reghellin D, Zamboni G, Bonetti F. PEComas: the past, the present and the future. Virchows Arch (2008) 452(2):119–32. doi: 10.1007/s00428-007-0509-1
2. Bonetti F, Pea M, Martignoni G, Zamboni G. PEC and sugar. Am J Surg Pathol (1992) 16(3):307–8. doi: 10.1097/00000478-199203000-00013
3. Folpe AL, McKenney JK, Li Z, Smith SJ, Weiss SW. Clear cell myomelanocytic tumor of the thigh: Report of a unique case. Am J Surg Pathol (2002) 26(6):809–12. doi: 10.1097/00000478-200206000-00018
4. Pizzi M, di Lorenzo I, d’Amore ES, D’Angelo P, Alaggio R. Pediatric gastrointestinal PEComas: a diagnostic challenge. Pediatr Dev Pathol (2014) 17(5):406–8. doi: 10.2350/14-06-1501-CR.1
5. Thway K, Fisher C. PEComa: morphology and genetics of a complex tumor family. Ann Diagn Pathol (2015) 19(5):359–68. doi: 10.1016/j.anndiagpath.2015.06.003
6. Zhao LJ, Yang YJ, Wu H, Huang SM, Liu K. Perivascular epithelioid cell tumor of the liver: A case report and literature review. Eur Rev Med Pharmacol Sci (2013) 17(12):1665–8.
7. Armah HB, Parwani AV. Perivascular epithelioid cell tumor. Arch Pathol Lab Med (2009) 133(4):648–54. doi: 10.5858/133.4.648
8. Jungbluth AA, Busam KJ, Gerald WL, Stockert E, Coplan KA, Iversen K, et al. A103: An anti-melan-a monoclonal antibody for the detection of malignant melanoma in paraffin-embedded tissues. Am J Surg Pathol (1998) 22(5):595–602. doi: 10.1097/00000478-199805000-00011
9. Acosta AM, Adley BP. Predicting the behavior of perivascular epithelioid cell tumors of the uterine corpus. Arch Pathol Lab Med (2017) 141(3):463–9. doi: 10.5858/arpa.2016-0092-RS
10. Chen Z, Han S, Wu J, Xiong M, Huang Y, Chen J, et al. A systematic review: Perivascular epithelioid cell tumor of gastrointestinal tract. Med (Baltimore). (2016) 95(28):e3890. doi: 10.1097/MD.0000000000003890
11. Lu B, Wang C, Zhang J, Kuiper RP, Song M, Zhang X, et al. Perivascular epithelioid cell tumor of gastrointestinal tract: Case report and review of the literature. Med (Baltimore). (2015) 94(3):e393. doi: 10.1097/MD.0000000000000393
12. Doyle LA, Hornick JL, Fletcher CD. PEComa of the gastrointestinal tract: clinicopathologic study of 35 cases with evaluation of prognostic parameters. Am J Surg Pathol (2013) 37(12):1769–82. doi: 10.1097/PAS.0b013e31829caab3
13. Folpe AL, Mentzel T, Lehr HA, Fisher C, Balzer BL, Weiss SW. Perivascular epithelioid cell neoplasms of soft tissue and gynecologic origin: A clinicopathologic study of 26 cases and review of the literature. Am J Surg Pathol (2005) 29(12):1558–75. doi: 10.1097/01.pas.0000173232.22117.37
14. Saluja K, Thomas J, Zhang S, Sturgis EM, Jain KS, Prieto VG, et al. Malignant perivascular epithelioid cell tumor of the oropharynx with strong TFE3 expression mimicking alveolar soft part sarcoma: A case report and review of the literature. Hum Pathol (2018) 76:149–55. doi: 10.1016/j.humpath.2017.11.016
15. Shi HY, Wei LX, Sun L, Guo AT. Clinicopathologic analysis of 4 perivascular epithelioid cell tumors (PEComas) of the gastrointestinal tract. Int J Surg Pathol (2010) 18(4):243–7. doi: 10.1177/1066896908330481
16. Yanai H, Matsuura H, Sonobe H, Shiozaki S, Kawabata K. Perivascular epithelioid cell tumor of the jejunum. Pathol Res Pract (2003) 199(1):47–50. doi: 10.1078/0344-0338-00353
17. Chang KL, Folpe AL. Diagnostic utility of microphthalmia transcription factor in malignant melanoma and other tumors. Adv Anat Pathol (2001) 8(5):273–5. doi: 10.1097/00125480-200109000-00004
18. Wagner AJ, Ravi V, Riedel RF, Ganjoo K, Van Tine BA, Chugh R, et al. Nab-sirolimus for patients with malignant perivascular epithelioid cell tumors. J Clin Oncol (2021) 39(33):3660–70. doi: 10.1200/JCO.21.01728
19. Klompenhouwer AJ, Verver D, Janki S, Bramer WM, Doukas M, Dwarkasing RS, et al. Management of hepatic angiomyolipoma: A systematic review. Liver Int (2017) 37(9):1272–80. doi: 10.1111/liv.13381
20. Cheng HC, Kuo CY, Huang CW, Shih HH, Lin CH, Wang JY. Unusual paediatric sigmoid perivascular epithelioid cell tumour with regional lymph node metastasis treated using gemcitabine and docetaxel: a case report and literature review. J Int Med Res (2021) 49(9):3000605211041509. doi: 10.1177/03000605211041509
21. Starbuck KD, Drake RD, Budd GT, Rose PG. Treatment of advanced malignant uterine perivascular epithelioid cell tumor with mTOR inhibitors: Single-institution experience and review of the literature. Anticancer Res (2016) 36(11):6161–4. doi: 10.21873/anticanres.11208
22. Scheppach W, Reissmann N, Sprinz T, Schippers E, Schoettker B, Mueller JG. PEComa of the colon resistant to sirolimus but responsive to doxorubicin/ifosfamide. World J Gastroenterol (2013) 19(10):1657–60. doi: 10.3748/wjg.v19.i10.1657
23. Subbiah V, Trent JC, Kurzrock R. Resistance to mammalian target of rapamycin inhibitor therapy in perivascular epithelioid cell tumors. J Clin Oncol (2010) 28(24):e415. doi: 10.1200/JCO.2010.29.4678
24. Planelles M, Macias L, Peiro G, Bulimbasic S, Hes O, Robles A, et al. Rheb/mTOR/p70s6k cascade and TFE3 expression in conventional and sclerosing PEComas of the urinary tract. Appl Immunohistochem Mol Morphol. (2016) 24(7):514–20. doi: 10.1097/PAI.0000000000000209
25. Razak OA, Varela C, Nassr MMA, Jang M, Han YD. A case of caecal “PECOMA”: An uncommon entity. Int J Surg Case Rep (2022) 90:106689. doi: 10.1016/j.ijscr.2021.106689
26. Xu J, Gong XL, Wu H, Zhao L. Case report: Gastrointestinal PEComa with TFE3 rearrangement treated with anti-VEGFR TKI apatinib. Front Oncol (2020) 10:582087. doi: 10.3389/fonc.2020.582087
27. Schmiester M, Dolnik A, Kornak U, Pfitzner B, Hummel M, Treue D, et al. TFE3 activation in a TSC1-altered malignant PEComa: Challenging the dichotomy of the underlying pathogenic mechanisms. J Pathol Clin Res (2021) 7(1):3–9. doi: 10.1002/cjp2.187
28. Waters PS, Mitchell DP, Murphy R, McKenna M, Waldron RP. Primary malignant gastric PEComa - diagnostic and technical dilemmas. Int J Surg Case Rep (2012) 3(2):89–91. doi: 10.1016/j.ijscr.2011.11.003
29. Arribas Jurado M, Revollo I, Rubio Fernandez A, Galeano Diaz F, Blanco Fernandez G. Primary liver PEComa. Cir Esp. (2015) 93(9):600–1. doi: 10.1016/j.ciresp.2014.07.011
Keywords: perivascular epithelioid cell tumors, sigmoid colon, HMB-45, treatment, case report
Citation: Yan H, Zhang S, Ba Y, Li K, Gao G, Li Y, Zhang Y, Liu C and Shi N (2023) Case Report: Perivascular epithelioid tumors of the gastrointestinal tract. Front. Oncol. 12:1026825. doi: 10.3389/fonc.2022.1026825
Received: 24 August 2022; Accepted: 28 December 2022;
Published: 16 January 2023.
Edited by:
Giovanni Crisafulli, IFOM - The FIRC Institute of Molecular Oncology, ItalyReviewed by:
Wenpeng Huang, First Affiliated Hospital of Zhengzhou University, ChinaCopyright © 2023 Yan, Zhang, Ba, Li, Gao, Li, Zhang, Liu and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ning Shi, c2hpbmluZzEyMDVAMTI2LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.