- 1Department of Oncology, Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan, Shandong, China
- 2Department of Radiation Oncology, Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan, Shandong, China
Background: Interventional embolization is a common treatment for hemoptysis, one of the complications of lung cancer. However, there are no official guidelines for the use of this method in antitumor therapy.
Case Description: Herein, we describe a case of a patient who was pathologically diagnosed as central squamous cell lung cancer. The patient received chemotherapy, interventional embolization and radiotherapy successively. The tumor regressed rapidly within 48 hours of receipt of interventional embolization. Furthermore, the tumor decreased by more than 50% in size within 7 days during radiotherapy. Unfortunately, the patient has since developed lymph node metastases and remains under treatment.
Conclusions: Thus, finding the suitable blood vessel embolized may be a suitable option to reduce the local tumor load and can be considered as antitumor therapy in combination with other treatments. The patient’s theoretical hypoxia state after interventional therapy still produced a good tumor regression after radiotherapy. However, so far, no related studies have reported the changes of tumor immune microenvironment in human body after intervention and radiotherapy.
Introduction
Interventional embolization is a minimally invasive treatment that aims at blocking blood flow by placing embolic materials to achieve the purpose of treatment. It is often used to treat patients with hemoptysis (1, 2) but is rarely used as an antitumor therapy. However, based on its mechanism of action, bronchial arterial embolization (BAE) as an auxiliary means of local antitumor therapy may be capable of providing a good response (3–8). Here, we report a case of tumor remission achieved through this intervention when used in combination with other treatments. Although it is not a conventional clinical treatment, considerable therapeutic effect was seen in this case. We present the following case in accordance with the CARE reporting checklist.
Case presentation
A 57-year-old Chinese man was admitted to the respiratory department at a regional hospital owing to hemoptysis and cough, no obvious cause was identified, and the patient denied having chest pain, fever, shiver, and other concomitant symptoms. The patient’s social history was as follows: 60-pack years and 30 years of significant alcohol intake; however, he had been sober for 10 years. There was no other relevant medical history of note, and the patient revealed no genetic, congenital, or developmental abnormalities. Unfortunately, the family genetic history was not provided. The patient had not received any prior treatment for these symptoms. Physical examination revealed that the superficial lymph nodes of the whole body were not palpable or enlarged. The breathing sounds of both lungs were clear, and no coarse or fine crackles were heard. Computed tomography (CT) scan revealed an irregular large soft tissue mass in the right hilar region, invading the right main bronchus, narrowing, and truncating with atelectasis. The tumor diameter was 52.85 mm (mediastinal window images were uniformly used for measurement and comparison) with atelectasis (Figure 1A). Histological examination of a transbronchial specimen confirmed that the tumor was a squamous cell carcinoma (SCC). No lymph node metastases or distant organ metastases were identified. Based on the 8th Edition Lung Cancer Stage Classification, it was diagnosed as right central lung SCC with right upper lobe atelectasis (stage cT3N0M0, IIB) (9).
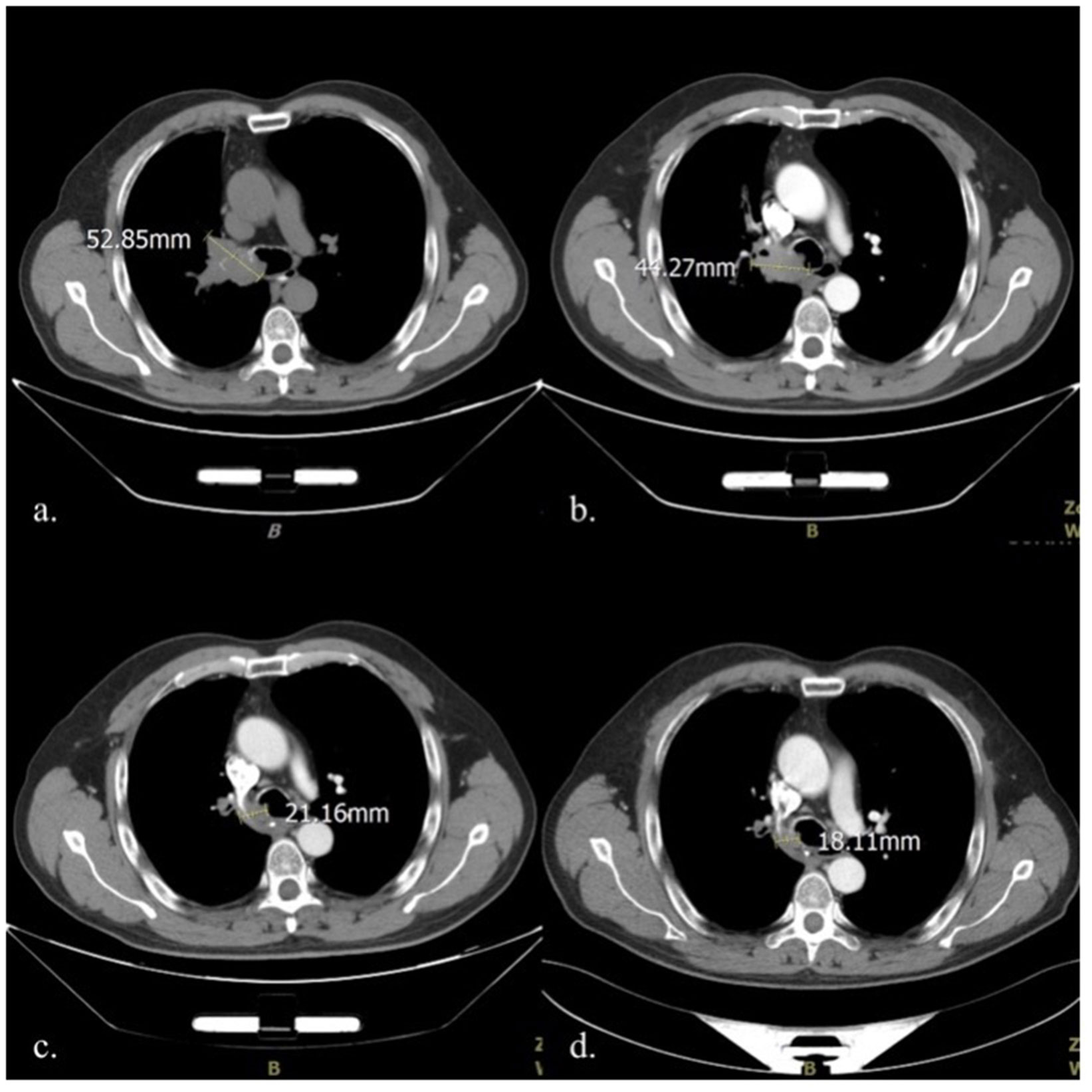
Figure 1 Comparison of mediastinal window CT images in four different periods: (A) Before treatment initiation (2020-10-12); (B). After two cycles of chemotherapy (2020-11-25); (C) At the end of the 5th fractionation of radiotherapy (2020-12-9); (D) At the end of the 10th fractionation of radiotherapy (2020-12-16). CT, computed tomography.
As the tumor was close to the carina, a multidisciplinary team (MDT) consultation was undertaken; the team consisted of a medical oncologist, a thoracic surgeon, a radiologist, and a pathologist. As the MDT concluded that the risks associated with the surgical resection of the tumor were very high, it was not considered at the time. A treatment plan was developed for induction chemotherapy with sequential thoracic radiotherapy since the large diameter of the tumor and vascular invasion. Paclitaxel (albumin-bound) 400 mg D1 combined with carboplatin 500 mg D1 on a 21-day cycle was used for induction chemotherapy. The first treatment cycle began on October 16, 2020, and the two-cycle efficacy was evaluated as stable disease (Figure 1B).
At the end of the first round of chemotherapy, the patient again presented with cough with hemoptysis in the early morning of the 3rd day; the blood was dark red and of approximately 10-15 ml. Routine blood test revealed that the bleeding was not caused by thrombocytopenia, a common adverse reaction to chemotherapy. Bronchial artery embolization was performed twice during chemotherapy to address the symptoms. The first embolization was performed on October 19, 2020. The interventional doctor injected the contrast medium into the thickened artery— the trunk of the right middle and lower lobe bronchial artery; this helped to clearly stain the tumor, and the staining disappeared after poly vinyl alcohol (PVA) microspheres measuring 300–500 mm in diameter was injected into the arteries. However, the results were unsatisfactory, and the patient’s hemoptysis did not reduce in intensity following the procedure. A second embolization was performed 6 weeks later (December 2, 2020). The PVA microspheres with a diameter of 100-300mm and two coils with diameter 2cm/crimp diameter 2cm (2-2) were injected into the right upper lobe bronchial artery; and four coils with diameter 2cm/crimp diameter 2cm (2-2) were injected into the right middle lobe bronchial artery. The patient’s hemoptysis disappeared; no chemotherapeutic drugs were used in either procedure. The patient tolerated the intervention, and there were no adverse reactions, such as fever, headache, nausea, or vomiting. On the second day after embolization, intensity-modulated radiation therapy (IMRT) was initiated at a dose of 60 Gy for 30 fractions. On December 9, 2020, enhanced CT performed during radiotherapy to reposition the patient revealed that the tumor had significantly reduced in size to 21.16 mm (Figure 1C). On December 16, 2020, after the 10th radiotherapy fractionation, an enhanced CT scan revealed that the tumor had reduced to 18.11 mm in size. It remained this size for 4 months (Figure 1D). Unfortunately, the patient has since developed lymph node metastases (occurred 3 months after radiotherapy) and remains under treatment. The patient provided informed consent for publication of this case report.
Discussion
In this case, the SCC of the lung markedly reduced after interventional therapy. Albumin-bound paclitaxel-carboplatin combination is a standard chemotherapy regimen for SCC of the lung. The patient first received two cycles of this regimen as induction chemotherapy, with resultant stabilization of disease (10). The hemoptysis symptoms worsened during chemotherapy, indicating that the tumor was not satisfactorily controlled; two BAEs were therefore performed. Different blood vessels were used in both embolization processes. The first embolization was performed on the right lower lobe bronchial artery. Although the interventional doctor identified the responsible vessels on injection of contrast media before and after embolization, the hemoptysis was not completely relieved. Angiography of the tumor and its vasculature was repeated; it was noted that the tumor was located at the junction of the upper and middle lobes of the right lung (Figure 2). Therefore, the small right upper lobe bronchial artery was embolized the second time, and the embolization of the middle lobe bronchial artery was improved. On the second day after the surgery, the patient expectorated soft, grayish-red tissue (December 4, 2020). This was pathologically confirmed as SCC, and it was reported as being necrotic and friable. This suggests that the rapid reduction of the tumor likely occurred within 48 hours after the interventions discussed in this case study. Successive imaging during radiotherapy also confirmed this.
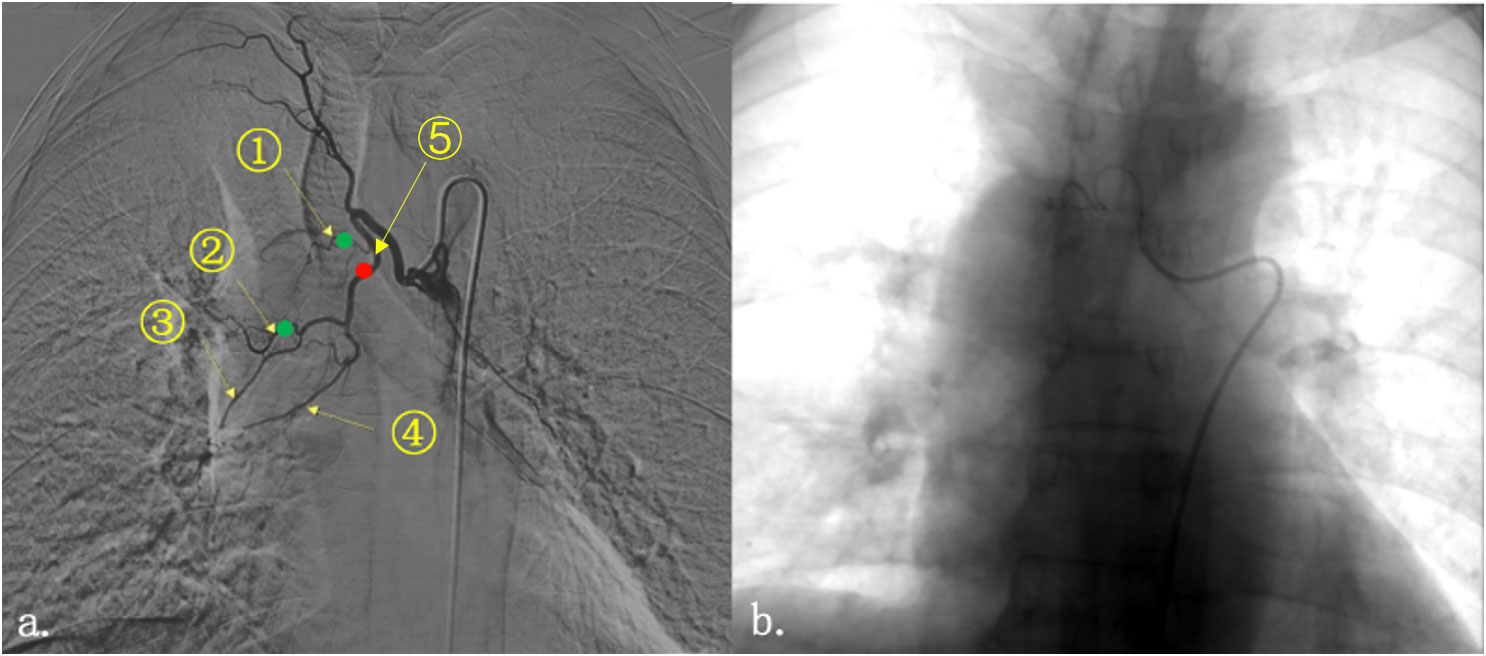
Figure 2 Imaging of tumor under contrast medium perfusion. Right bronchial artery angiography showed that the tumor at the right hilum was mainly supplied by two blood vessels (A). They are (1) the right upper lobe bronchial artery; (2) the right middle lobe bronchial artery; (3) the right bronchial artery flowing through part of the middle lobe and lower lobe; (4) the right inferior lobe bronchial artery; and (5) the trunk of the right middle and lower lobe bronchial artery. The red dot represented the general location of the first embolization; and the green dot represented the location of the second one. (B) The bronchial artery was embolized by injection of microspheres and coils until the vascular enhancement image disappeared.
The maximum diameter of the tumor decreased by more than half (from 44.27 mm to 21.16 mm) on day 7 after therapeutic intervention following the administration of only 5 fractions of radiation (cumulative dose: 10 Gy), considerably less than the current, effective antitumor dose recommended (11). Therefore, we hypothesize that the short-term effect on the tumor is related to bronchial artery embolization. Vascular occlusions are caused by placing embolic material upstream of the target blood vessels to occlude it and prevent hemoptysis. In fact, interrupting the blood supply can block the supply of nutrients necessary for tumor growth and enable necrosis. However, it is difficult to block the blood supply entirely in the clinical setting. Thus, embolization is typically used as a treatment for hemoptysis, and it is rarely used in local antitumor therapy.
Tumors can develop new vascular branches when the primary blood supply is blocked. These new branches are often very small and difficult to observe with the current imaging techniques. Even if a blood vessel is identified, its small lumen diameter makes the surgical procedure challenging. In a study by Fujita et al. (7), bleeding did not stop immediately in 18% patients with hemoptysis who received BAE. This was due to the incomplete embolization of the contributory vessels. Some large tumors invade the mediastinum and the thoracic wall, precluding complete embolization of the contributory arteries (7).
Furthermore, it is challenging to choose the appropriate embolus. Arteries are elastic, and if the embolization procedure is not secure, blood may flow through, resulting in embolization failure. Many types of embolic agents are clinically used, each with distinct properties. Because of its small diameter, ethiodized oil injection can cause complications, such as spinal cord injury and cerebral embolism. There is also the disadvantage of incomplete embolization. Gelatin sponge particles have good flexibility, excellent transport ability, and better fusion with the target vessels; thus, embolization is thorough. However, due to their biodegradability, blood flow is easily restored (12, 13), necessitating multiple interventional therapies to be performed (14, 15).
In China, some physicians perform palliative treatment through interventional therapy combined with chemoembolization by administering chemotherapeutic drugs directly into the tumor-feeding artery. This can significantly increase the local drug concentration in the tumor and reduce systemic adverse reactions (16). Zhao RF et al. (17) studied 50 patients with lung carcinoma who were administered chemotherapeutic drugs with Embosphere® Microspheres (Merit Medical, Utah, USA) into target vessels. The efficacy of the intervention was evaluated one month after the third treatment. An overall effective rate of 88% was observed—9 patients (18%) had complete responses, 35 (70%) had partial responses, and 6 (12%) had no change. A prospective study also achieved good responses. Liu S et al. (18) used CalliSpheres drug- eluting beads-bronchial artery chemoembolization embolization (DEB-BACE) to treat 21 refractory non-small cell lung cancer (NSCLC). After treatment the quality of life was significantly improved, and no serious adverse events such as spinal cord injury and cerebral embolism during the perioperative period. However, in the current case, the patient was significantly relieved only by simple embolization, which suggests that finding specific target vessels is of vital importance for killing tumors. Combined chemotherapy can achieve the effect of icing on the cake.
In addition, the patient received IMRT on day 2 following the intervention, and the tumor continued to regress during radiotherapy. After the 10th radiotherapy session, the maximum diameter of the tumor was 20 mm. Theoretically, tumor cell hypoxia is exacerbated by embolization, which could promote the development of dormant carcinoma stem cells, sustain their potential for proliferation and differentiation, and reduce tumor sensitivity to radiotherapy. Further research is required to explore the process of continuous tumor regression in patients undergoing radiotherapy and how embolization alters the tumor microenvironment (19).
Although BAE is not a routine treatment per the National Comprehensive Cancer Network Guidelines for non-small cell lung cancer, its anticancer effect could be underestimated. Local interventional embolization combined with systemic therapy could be an excellent strategy to control the overall tumor load. The selection of suitable blood vessels is imperative. The timing of embolization and the mode of combination of constituents are topics for further research.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Shandong Cancer Hospital and Institute. The patient provided his written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
XH and YM contributed to conception and design of the report. SZ wrote the first draft of the manuscript. JZ, XM, XH and YM wrote sections of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China [grant numbers 82172720 and 81972864].
Acknowledgments
Thanks to Editage Inc. for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor YL declared a shared parent affiliation with the authors JZ, XM, YM, XH at the time of review.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1026087/full#supplementary-material
Abbreviations
- BAE, Bronchial artery embolization; CT, Computed tomography; IMRT, Intensity-modulated radiation therapy; SCC, Squamous cell carcinoma; MDT, Multidisciplinary team; NSCLC, Non-small cell lung cancer; PVA, Poly vinyl alcohol.
References
1. Kalva SP. Bronchial artery embolization. Tech Vasc Interv Radiol (2009) 12:130–8. doi: 10.1053/j.tvir.2009.08.006
2. Chun JY, Morgan R, Belli AM. Radiological management of hemoptysis: a comprehensive review of diagnostic imaging and bronchial arterial embolization. Cardiovasc Intervent Radiol (2010) 33(2):240–50. doi: 10.1007/s00270-009-9788-z
3. Hayakawa K, Tanaka F, Torizuka T, Mitsumori M, Okuno Y, Matsui A, et al. Bronchial artery embolization for hemoptysis: immediate and long-term results. Cardiovasc Intervent Radiol (1992) 15(3):154–8; discussion 158. doi: 10.1007/BF02735578
4. Park HS, Kim YI, Kim HY, Zo JI, Lee JH, Lee JS. Bronchial artery and systemic artery embolization in the management of primary lung cancer patients with hemoptysis. Cardiovasc Intervent Radiol (2007) 30(4):638–43. doi: 10.1007/s00270-007-9034-5
5. Wang GR, Ensor JE, Gupta S, Hicks ME, Tam AL. Bronchial artery embolization for the management of hemoptysis in oncology patients: utility and prognostic factors. J Vasc Interv Radiol (2009) 20(6):722–9. doi: 10.1016/j.jvir.2009.02.016
6. Garcia-Olivé I, Sanz-Santos J, Centeno C, Andreo F, Muñoz-Ferrer A, Serra P, et al. Results of bronchial artery embolization for the treatment of hemoptysis caused by neoplasm. J Vasc Interv Radiol (2014) 25(2):221–8. doi: 10.1016/j.jvir.2013.09.017
7. Fujita T, Tanabe M, Moritani K, Matsunaga N, Matsumoto T. Immediate and late outcomes of bronchial and systemic artery embolization for palliative treatment of patients with nonsmallcell lung cancer having hemoptysis. Am J Hosp Palliat Care (2014) 31(6):602–7. doi: 10.1177/1049909113499442
8. Xie K, Wang YL, Chen L, Peng ZQ, Liu Q. Clinical comparative analysis of two embolization methods in interventional treatment of hemoptysis. Lin Chuang Fang She Xue Za Zhi (2018) 6:1034–9. doi: 10.13437/j.cnki.jcr.2018.06.039
9. Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT. The eighth edition lung cancer stage classification. Chest. (2017) 151:193–203. doi: 10.1016/j.chest.2016.10.010
10. Aupérin A, Le Péchoux C, Pignon JP, Koning C, Jeremic B, Clamon G, et al. Concomitant radio-chemotherapy based on platin compounds in patients with locally advanced non-small cell lung cancer (NSCLC): A meta-analysis of individual data from 1764 patients. Ann Oncol (2006) 17(3):473–83. doi: 10.1093/annonc/mdj117
11. Bezjak A, Temin S, Franklin G, Giaccone G, Govindan R, Johnson ML, et al. Definitive and adjuvant radiotherapy in locally advanced non-small-cell lung cancer: American society of clinical oncology clinical practice guideline endorsement of the American society for radiation oncology evidence-based clinical practice guideline. J Clin Oncol (2015) 33(18):2100–5. doi: 10.1200/JCO.2014.59.2360
12. Seki A, Hori S, Sueyoshi S, Hori A, Kono M, Murata S, et al. Transcatheter arterial embolization with spherical embolic agent for pulmonary metastases from renal cell carcinoma. Cardiovasc Intervent Radiol (2013) 36(6):1527–35. doi: 10.1007/s00270-013-0576-4
13. White RI Jr. Bronchial artery embolotherapy for control of acute hemoptysis: analysis of outcome. Chest (1999) 115(4):912–5. doi: 10.1378/chest.115.4.912
14. Han K, Yoon KW, Kim JH, Kim GM. Bronchial artery embolization for hemoptysis in primary lung cancer: A retrospective review of 84 patients. J Vasc Interv Radiol (2019) 30(3):428–34. doi: 10.1016/j.jvir.2018.08.022
15. Zhao GS, Liu Y, Zhang Q, Li C, Zhang YW, Ren ZZ, et al. Transarterial chemoembolization combined with huaier granule for the treatment of primary hepatic carcinoma: Safety and efficacy. Med (Baltimore) (2017) 96(29):e7589. doi: 10.1097/MD.0000000000007589
16. Seki A, Shimono C. Transarterial chemoembolization for management of hemoptysis: initial experience in advanced primary lung cancer patients. Jpn J Radiol (2017) 35(9):495–504. doi: 10.1007/s11604-017-0659-2
17. Zhao RF, Cheng LZ, Wang D, Zhao ZJ. Application value of embolization with embosphere microspheres in bronchogenic carcinoma. Zhong Guo Yi Yao Ke Xue (2021) 10:199–202. doi: 10.3969/j.issn.2095-0616.2021.10.051
18. Liu S, Wang QD, Li Q, Yu GJ, Xu HC. CalliSpheres drug-eluting beads trans-bronchial artery chemoembolization for 21 cases of refractory non-small cell lung cancer. Zhong Guo Lin Chuang Yan Jiu (2021) 01):56–60. doi: 10.13429/j.cnki.cjcr.2021.01.012
Keywords: lung cancer, hemoptysis, interventional embolization, combined treatment, case report
Citation: Zhou S, Zhang J, Meng X, Meng Y and Han X (2022) Case Report: Bronchial artery embolization and chemoradiotherapy for central squamous cell lung carcinoma with rapid regression. Front. Oncol. 12:1026087. doi: 10.3389/fonc.2022.1026087
Received: 23 August 2022; Accepted: 29 November 2022;
Published: 14 December 2022.
Edited by:
Yuliang Li, The Second Hospital of Shandong University, ChinaReviewed by:
Phurich Janjindamai, Prince of Songkla University, ThailandHitoshi Takeuchi, Fukujuji Hospital, Japan
Copyright © 2022 Zhou, Zhang, Meng, Meng and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao Han, aHh6YmIxOTgzQDE2My5jb20=; Yingtao Meng, bWVuZ3l0MjAyMTA4MTZAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Siqi Zhou1,2
Siqi Zhou1,2 Xue Meng
Xue Meng Xiao Han
Xiao Han