- 1Department of Urology, Kaohsiung Medical University Hospital, Kaohsiung, Taiwan
- 2Department of Urology, China Medical University and Hospital, Taichung, Taiwan
- 3School of Medicine, China Medical University, Taichung, Taiwan
- 4Department of Urology, China Medical University Beigang Hospital, Yunlin, Taiwan
- 5Division of Urology, Department of Surgery, Chi Mei Medical Center, Tainan, Taiwan
- 6Department of Medical Science Industries, College of Health Sciences, Chang Jung Christian University, Tainan, Taiwan
- 7School of medicine, Kaohsiung Medical University, Kaohsiung, Taiwan
- 8Division of Urology, Department of Surgery, Taichung Veterans General Hospital, Taichung, Taiwan
- 9Institute of Medicine, Chung Shan Medical University, Taichung, Taiwan
- 10Department of Urology, MacKay Memorial Hospital, Taipei, Taiwan
- 11Department of Medicine, Mackay Medical College, Taipei, Taiwan
- 12Institute of Biomedical Informatics, National Yang Ming Chiao Tung University, Taipei, Taiwan
- 13Division of Urology, Department of surgery, Taipei Tzuchi Hospital, The Buddhist Tzu Chi Medical Foundation, New Taipei City, Taiwan
- 14School of Medicine, Buddhist Tzu Chi University, Hualien, Taiwan
- 15Department of Urology, National Taiwan University Hospital, College of Medicine, National Taiwan University, Taipei, Taiwan
- 16Divisions of Urology, Department of Surgery, Far Eastern Memorial Hospital, New Taipei City, Taiwan
- 17Department of Electrical Engineering, Yuan Ze University, Taoyuan, Taiwan
- 18Department of Biomedical Engineering, National Taiwan University, Taipei, Taiwan
- 19Department of Urology, Hualien Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation and Tzu Chi University, Hualien, Taiwan
- 20Department of Urology, Taiwan Adventist Hospital, Taipei, Taiwan
- 21Division of Urology, Department of Surgery, Kaohsiung Veterans General Hospital, Kaohsiung, Taiwan
- 22Department of surgery, Taipei Tzu chi Hospital, The Buddhist Tzu Chi Medical Foundation, New Taipei City, Taiwan
- 23Department of Urology, Taipei Medical University Hospital, Taipei Medical University, Taipei, Taiwan
- 24Division of Urology, Department of Surgery, Taipei City Hospital Renai Branch, Taipei, Taiwan
- 25Department of Urology, School of Medicine, National Yang Ming Chiao Tung University, Taipei, Taiwan
- 26College of Medicine, Fu-Jen Catholic University, New Taipei City, Taiwan
- 27Department of Urology, Cardinal Tien Hospital, New Taipei City, Taiwan
- 28Department of Life Science, College of Science, National Taiwan Normal University, Taipei, Taiwan
- 29Department of Urology, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan
- 30Department of Urology, Shuang Ho Hospital, Taipei Medical University, New Taipei City, Taiwan
- 31TMU Research Center of Urology and Kidney (TMU-RCUK), Taipei Medical University, Taipei, Taiwan
- 32Division of Urology, Department of Surgery, Chang Gung Memorial Hospital at Chiayi, Chiayi, Taiwan
- 33Chang Gung University of Science and Technology, Chiayi, Taiwan
- 34Department of Medicine, College of Medicine, Chang Gung University, Taoyuan, Taiwan
- 35Department of Urology, Ditmanson Medical Foundation Chiayi Christian Hospital, Chiayi, Taiwan
- 36Department of Health and Nutrition Biotechnology, Asian University, Taichung, Taiwan
- 37Division of Urology, Department of Surgery, Chang Gung Memorial Hospital, Linkou Branch, Taoyuan, Taiwan
- 38Department of Urology, School of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan
- 39Graduate Institute of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan
- 40Department of Urology, Kaohsiung Municipal Ta-Tung Hospital, Kaohsiung, Taiwan
Purpose: This study aimed to evaluate the oncological outcome of delayed surgical wait time from the diagnosis of upper tract urothelial carcinoma (UTUC) to radical nephroureterectomy (RNU).
Methods: In this multicenter retrospective study, medical records were collected between 1988 and 2021 from 18 participating Taiwanese hospitals under the Taiwan UTUC Collaboration Group. Patients were dichotomized into the early (≤90 days) and late (>90 days) surgical wait-time groups. Overall survival, disease-free survival, and bladder recurrence-free survival were calculated using the Kaplan–Meier method and multivariate Cox regression analysis. Multivariate analysis was performed using stepwise linear regression.
Results: Of the 1251 patients, 1181 (94.4%) were classifed into the early surgical wait-time group and 70 (5.6%) into the late surgical wait-time group. The median surgical wait time was 21 days, and the median follow-up was 59.5 months. Our study showed delay-time more than 90 days appeared to be associated with worse overall survival (hazard ratio [HR] 1.974, 95% confidence interval [CI] 1.166−3.343, p = 0.011), and disease-free survival (HR 1.997, 95% CI 1.137−3.507, p = 0.016). This remained as an independent prognostic factor after other confounding factors were adjusted. Age, ECOG performance status, Charlson Comorbidity Index (CCI), surgical margin, tumor location and adjuvant systemic therapy were independent prognostic factors for overall survival. Tumor location and adjuvant systemic therapy were also independent prognostic factors for disease-free survival.
Conclusions: For patients with UTUC undergoing RNU, the surgical wait time should be minimized to less than 90 days. Prolonged delay times may be associated with poor overall and disease-free survival.
1. Introduction
Upper tract urothelial carcinoma (UTUC) is a rare malignant tumor, which accounts for 5–10% of all urothelial carcinomas, with an estimated annual incidence of 1–2 cases per 100,000 in Western countries (1). However, this can vary between different geographical regions, age, occupation, and other factors (2). In Taiwan, according to the 2018 Cancer Registry Annual Report published by the Health Promotion Administration Ministry of Health and Welfare, a high incidence of UTUC was discovered, which represented 43% of UCs, especially in the southwest coast region (3).
Radical nephroureterectomy (RNU) with bladder cuff resection is the standard treatment for UTUC (4). Prior to surgery, besides high diagnostic accuracy of computed tomography (CT), ureteroscopy (URS) is still regarded an important step in the diagnosis of UTUC (5). Hence, a patient with localized UTUC receives at least two surgeries (URS and RNU) during the clinical management (6). Compared with bladder cancer, UTUC shows more aggressive nature, and over 60% of patients have invasive disease at the time of diagnosis (7). Urologists recommend that it is necessary for patients with a definite diagnosis to receive surgery immediately (8).
Inevitably, there are variables, such as preoperative evaluation, the pursuit of additional therapeutic opinions, limitations of surgical schedules, and patient-related reasons, that may lead to a delay from symptom onset to diagnosis, and later to surgical treatment (9). Also, the outbreak of coronavirus disease-19 (COVID-19) has had a profound global impact on all aspects of urology health care, in which non-emergency operations were postponed or cancelled. Generally, surgeons believe that prolonged surgical wait time (SWT) may have a negative impact on the patient’s clinical outcome because of the invasiveness of UTUC (8). Some studies have shown that a delay of >3months in treatment for bladder cancer may cause worse survival (10, 11). However, there are still conflicting reports on UTUC. In this study, we evaluated the impact of the delay from diagnosis to radical nephroureterectomy (RNU) on the oncological outcomes of UTUC.
2. Methods
2.1. Patient population
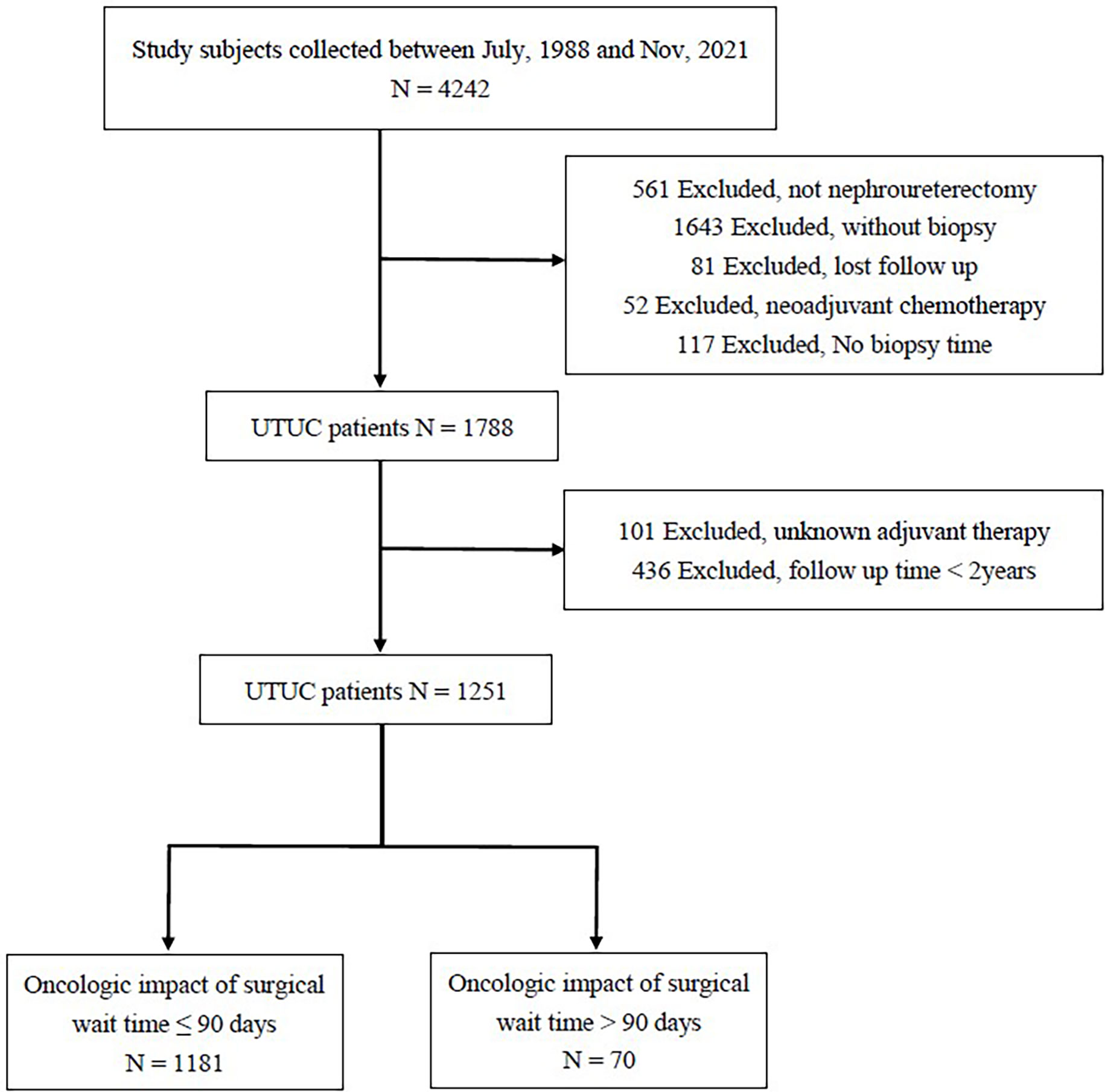
This study was approved by our institutional review board [KMUHIRB-E(I)-20180214] and meet the guidelines of the responsible governmental agency. We retrospectively reviewed the medical records of 18 participating Taiwanese hospitals under the Taiwan UTUC Collaboration Group and identified 4242 UTUC patients. The following patients were excluded from the analysis (Figure 1): those without nephroureterectomy (n = 561), without biopsy (n = 1643), without biopsy time (n = 117), without regular follow-up (n = 81), and treated with neoadjuvant chemotherapy (n = 52). In addition, we excluded patients with unknown adjuvant therapy (n = 101) and a follow-up time of <2 years (n = 436). We finally included 1251 patients who underwent RNU between July 1988 and November 2021. The patient biopsy dates were from February 14, 2000, to March 2, 2021. The patients’ operation periods were from February 22, 2000, to March 23, 2021. According to different SWTs, the patients were divided into early and late groups.
Various parameters were collected for analysis, including sex, age, ECOG performance status, Charlson Comorbidity Index (CCI), cell type, tumor location, tumor size, and important pathological features such as pathological T stage, pathological N stage, adjuvant systemic therapy, lymphovascular invasion (LVI), surgical margin, and preoperative hydronephrosis.
2.2. Statistical methods
Differences between the groups were compared using the two-sample Pearson chi-square test for categorical variables. Continuous variables were tested for normality using the Kolmogorov–Smirnov test. The Kaplan–Meier estimator was used to estimate the rates of prognostic outcomes, and survival curves were compared using the stratified log-rank test. The Cox proportional hazard model was used to assess the effect of the surgical approach on the prognostic outcomes, with and without adjusting for potential confounders. This study used stepwise regression, a method of fitting regression models in which the predictive variables were chosen using an automatic procedure. This study analyzed multiple factors affecting follow-up time and adjuvant use using stepwise linear regression. All relevant covariates, significant and non-significant, in the univariate analysis were included in the variable list to be selected. The significance levels for entry and stay were set to 0.05 and 0.1. The best regression model was then identified manually by reducing the significance levels to 0.05, corresponding to the chosen level. All statistical assessments were two-tailed and considered statistically significant at p <0.05. Statistical analyses were performed using IBM SPSS statistical software version 26.
3. Results
3.1. Population characteristics
A total 1251 patients with UTUC who underwent RNU were enrolled in the study, including 519 men (41.5%) and 732 women (58.5%). The median SWT was 21 days [interquartile range (IQR): 11.00–32.0]. The median follow-up duration was 59.5 months [IQR: 39.3–87.7]. The median age was 67.4 years [IQR: 60.6–75.1].
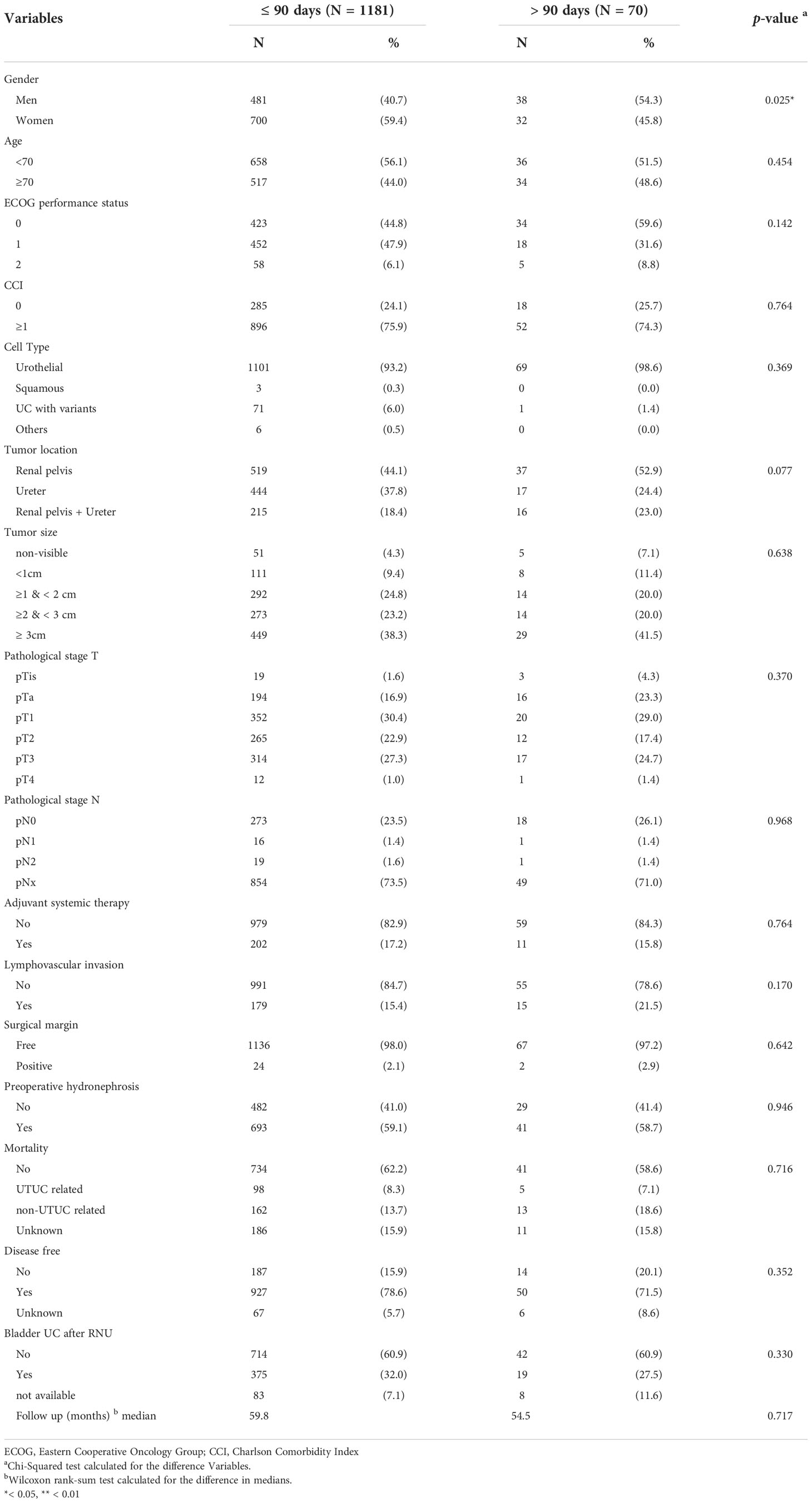
Clinicopathological characteristics showing the association of time from diagnosis to RNU are shown in Table 1. Our study cohort were then classified according to the time from the date of URS biopsy to the date of RNU (≤90 days vs. >90 days), which was defined as SWT. There were 1181 (94.4%) patients in early SWT group and 70 (5.6%) patients in late SWT group. There were no significant differences between the two groups in terms of age, ECOG performance status, CCI, tumor pathological type, tumor location, tumor size, pathological T-stage, pathological N-stage, adjuvant systemic therapy, LVI, surgical margin, preoperative hydronephrosis, mortality, disease-free status, bladder UC after RNU, or follow-up time, except for sex (p = 0.025). Men could delay surgery more easily than women.
At the time of surgery, 621 patients (49.6%) had muscle-invasive (MI) disease (T2); however, no significant differences were noted between the two groups.
3.2. Survival
3.2.1. Overall survival
In total, 633 patients died during the follow-up period. The 5-year OS rates were 84% in the early SWT group and 79% in the late SWT groups, with no significant difference (p = 0.145).
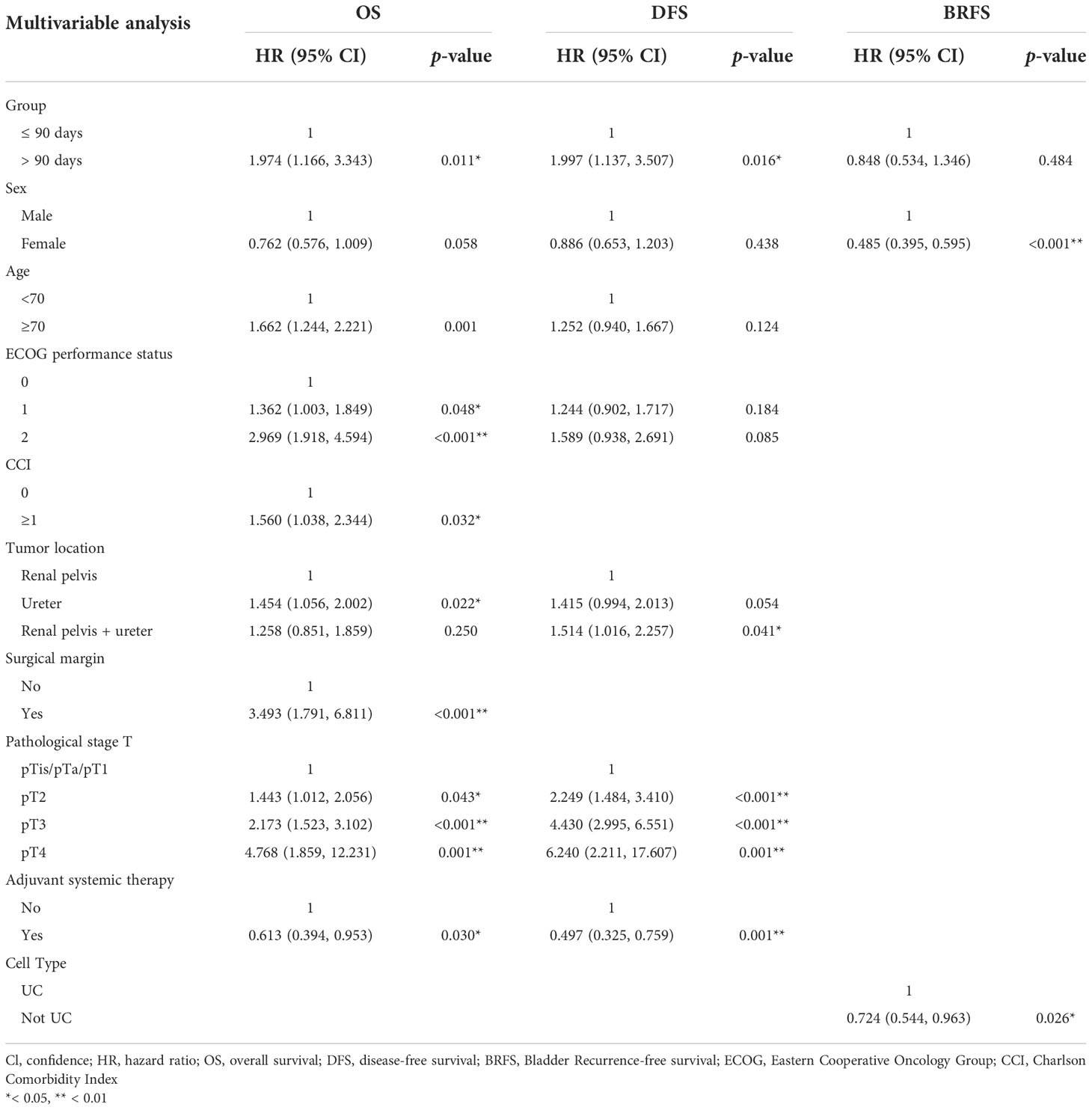
Univariate analyses showed that age (p < 0.001), ECOG performance status (2, p < 0.001), CCI (p < 0.001), tumor location (ureter, p = 0.030), preoperative hydronephrosis (p = 0.012), LVI (p < 0.001), surgical margin (p < 0.001), and pathological T stage (p < 0.001) were associated with poor OS (Table 2). Multivariate analysis revealed that SWT (p = 0.011), age (p = 0.001), ECOG performance status (1 and 2, p = 0.048 and p < 0.001, respectively), CCI (p = 0.032), tumor location (ureter tumor, p = 0.022), surgical margin (p < 0.001), pathological T-stage (pT2, pT3 and pT4, p = 0.043, p < 0.001 and p = 0.001, respectively), and adjuvant systemic therapy (p = 0.030) were independent predictors of OS (Table 3).
3.2.2. Disease-free survival
DFS for patients who had RNU ≤90 days and >90 days after diagnosis were 83% and 79% at 5 years after the surgery, respectively. Univariate analyses showed that age (p = 0.028), ECOG performance status (2, p = 0.026), tumor location (ureter and ureter + renal pelvis, p = 0.023 and p = 0.010, respectively), LVI (p < 0.001), surgical margin (p < 0.001), and pathological T stage (p < 0.001) were associated with poor DFS. Multivariate analysis revealed that SWT (p = 0.016), tumor location (renal pelvic and ureter tumor, p = 0.041), pathological T stage (pT2, pT3 and pT4, p < 0.001, p < 0.001 and p = 0.001, respectively), and adjuvant systemic therapy (p = 0.001) were independent predictors of DFS.
3.2.3. Bladder recurrence free survival
In univariate analyses, sex (p < 0.001) and tumor location (renal pelvic and ureter tumor, p = 0.025) were associated with poor BRFS. Multivariate analysis revealed that sex (p < 0.001) and pathological tumor type (p = 0.026) were independent predictors of BRFS.
3.2.4. Kaplan–Meier analysis
The Kaplan–Meier curve of non-muscle invasive and muscle invasive groups with different surgical wait times are compared in Figure 2. Regarding low stage tumor, Kaplan–Meier analysis showed no statistical intergroup differences for OS, DFS (p = 0.274, 0.554, respectively). Muscle invasive tumors also revealed no statistical differences in OS between the early and late SWT groups (p = 0.111), whereas the early SWT group had a better DFS than the late SWT group (p = 0.033). There was no difference between the two groups with respect to BRFS, regardless of the stage.
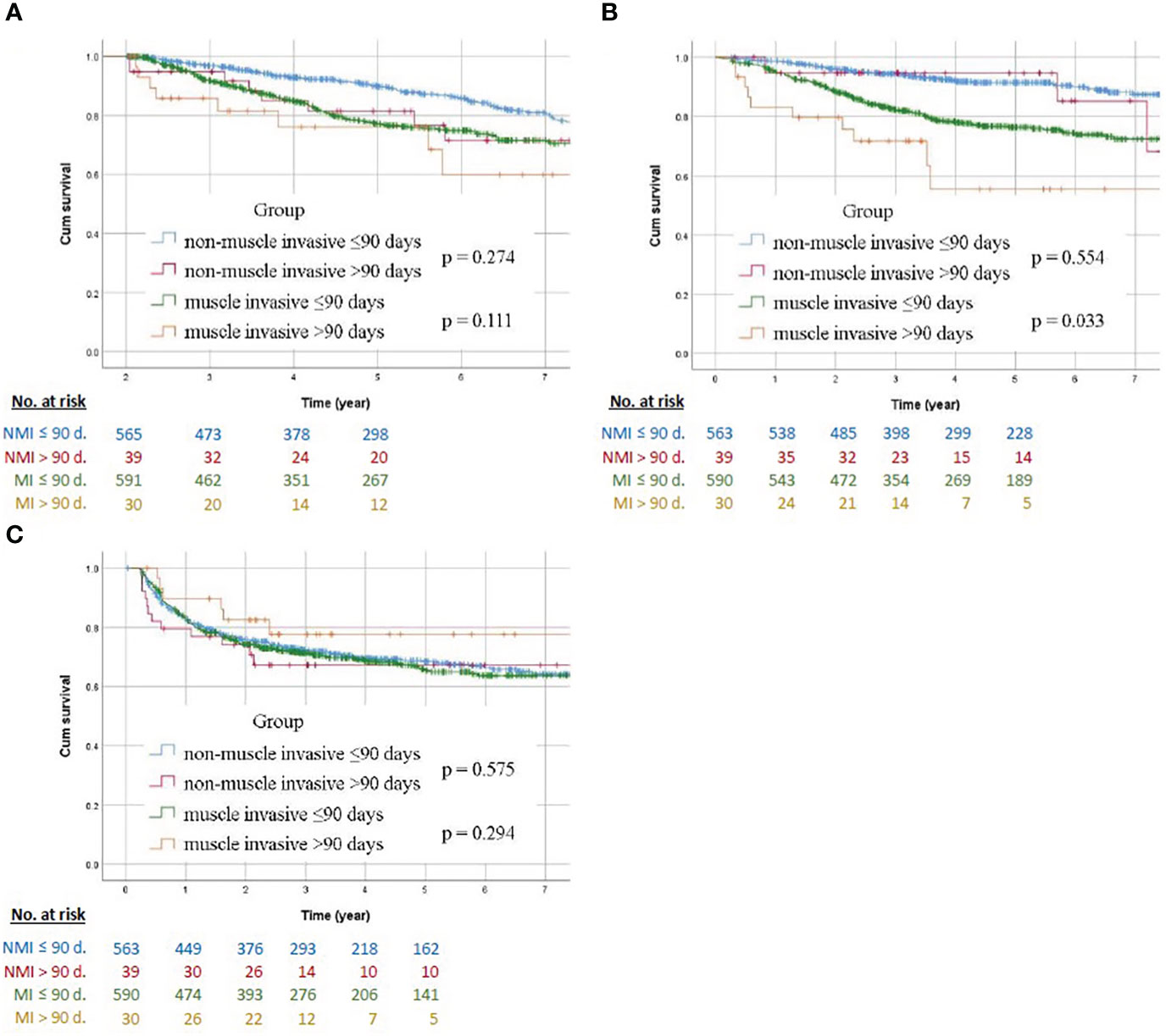
Figure 2 Survival Kaplan–Meier curves in patients with non-muscle invasive and muscle invasive UTUC comparing early and late surgical wait time to nephroureterectomy (A) overall survival, (B) disease-free survival, and (C) bladder recurrence-free survival. NMI, non-muscle invasive; MI, muscle invasive; d., day.
4. Discussion
In Asia, especially in Taiwan, UTUC has a higher prevalence than that in Western countries. In this multicenter retrospective study of a population-based Taiwanese database, the SWT no more than 90 days between diagnosis and radical nephroureterectomy for the UTUC is associated with a better OS and DFS. It is helpful for urologists to recognize the related risk factors. The major carcinogenic factors are occupational exposure to chemical agents and tobacco consumption. Aristolochic acid exposure (Chinese herb nephropathy) is a specific risk factor as well (12). Preoperative (such as tobacco consumption, tumor location, and multi-focality, American Society of Anesthesiology score) and postoperative factors (such as tumor stage and grade, lymph node involvement, and LVI) have been acknowledged to be prognostic factors of UTUC. Once a UTUC lesion is suspected and diagnosed, surgery should be arranged as soon as possible following the guidelines. To our best knowledge, this is the first multicenter large-scale study in Asia to evaluate the oncologic impact of SWT for the UTUC population.
In a previous study, extended SWT beyond a particular threshold has an adverse impact on the patient’s quality of life and psychological health, and even worse clinical outcomes between different urological neoplasms (8). There are some possible factors for the prolonged SWT. Objective factors, such as preoperative evaluation, limitations of the health care system, and seeking second opinions, can contribute to the delay of the surgery, while disease-related factors, may also cause prolonged interval between diagnosis and surgery. In addition, current COVID-19 pandemic has led to significant delay in urological surgeries as well (13). Many large volume hospitals are busy dealing with the pandemic. Lee et al. reported that despite the serious situation of COVID-19, we should still try to avoid delaying the operation (6). Hence, it is important to clarify how SWT affects the oncologic impact and prognosis of UTUC (14).
There is still no consensus regarding SWT for UTUC in previously published studies. This may be due to different inclusion criteria and research methods. Most studies set the cutoff time to 3 months based on previous experience with bladder cancer. Decreased OS and disease-specific survival were observed when the interval between diagnosis and cystectomy was more than 3 months (11). In a literature review, six studies were found to report on the oncologic impact of SWT on UTUC patients. Among studies using 3 months as the cutoff time, Lee et al. and Zhao et al. found worse OS and CSS after a 3-month delay in the RNU group (6, 15). However, some studies have shown contradictory results. Waldert et al. and Sundi et al. showed no significant effect of SWT on CSS and recurrence-free survival (RFS) (9, 16). Furthermore, other time intervals have also been discussed in previous publications. Lee et al. included 138 patients with a cutoff time of 1 month. The results showed that worse CSS and RFS were related to greater SWT in ureter tumor subgroup rather than overall UTUC patients (17). Xia et al. divided the cohort into 6 surgical wait-time groups, from less than 7 days to between 120 and 180 days. A surgical wait time of >120 days was correlated with worse OS (18). In the present study, a delay time of more than 90 days appeared to be associated with worse OS and DFS, and this remained an independent prognostic factor after adjusting for other confounding factors.
The possible reason for no consensus for SWT may be related to different inclusion time among the studies mentioned above. Some involved patients’ first presentation to the outpatient department, whereas others recruited patients with initial CT imaging or URS biopsy. Hematuria may be treated with conservative treatment at first, and further surveys will be conducted. CT is a useful diagnostic tool for UTUC and can be used to detect the detailed anatomy of the urinary tract. It can be used to visualize tumors of the distal ureter and renal pelvis, but calyceal tumors could sometimes be missed according to a previous comprehensive analysis (19). CT scan was correlated to final histopathology with a sensitivity of 89% and an overall accuracy of 74% in 148 patients. URS had similar sensitivity but significantly greater specificity and accuracy when compared with CT (20). Even though diagnostic ureteroscopy seems to increase the time to RNU, previous study showed no statistical differences in CSS, RFS and metastasis-free survival (21). Hence, URS still plays an important role in the diagnosis of UTUC.
In line with previous studies, there was a significant correlation between biopsy grade and surgical tumor grade, and high grade was strongly associated with invasive tumor stage (pT2–T4) (22, 23). In the present study, we found that higher pathological stage T was associated with poor OS and DFS. In addition, the survival curve revealed that a higher tumor stage leads to poor survival outcomes. Our cohort included low-grade and high-grade patients on biopsy. Hence, we analyzed subgroup for the patients with high-grade disease on biopsy. The results revealed SWT more than 90 days appeared to be associated with worse OS (HR 2.147, 95% CI 1.164−3.959, p = 0.014), and DFS (HR 2.445, 95% CI 1.259−4.748, p = 0.008) in multivariable survival analysis. This remained as an independent prognostic factor after other confounding factors were adjusted. The result was corresponded to all UTUC patients in our study. Hence, if biopsy results indicate a high-grade tumor, the surgery plan should not be delayed because of the association with invasive tumor stage. We strongly recommend that patients with higher grade undergo surgery as soon as possible. Besides, the undergrading and understaging rates were 32% and 46%, respectively (23), so we should not underestimate the low-grade tumor as well and manage the disease within the threshold of 90 days.
Our analysis also showed that age, ECOG performance status, CCI, surgical margin, tumor location and adjuvant systemic therapy were independent prognostic factors for overall survival. Tumor location and adjuvant systemic therapy were independent prognostic factors for disease-free survival. In a subgroup analysis of ureteral urothelial carcinoma by Lee et al., there was a statistically significant difference in CSS and RFS of 1-month SWT to surgery. In accordance with the literature, ureteral location seems to be an independent factor for worse CSS and RFS compared to the renal pelvis (24). The rich blood vessels and lymphatics in the surrounding layer of the ureter may lead to distant metastasis.
In our analysis, no difference was observed in BRFS between the ≤90 and >90 days groups, while sex and cell type were the independent prognostic factors. Bladder recurrence tended to occur more in men, which is consistent with previous studies (25, 26). The meta-analysis has identified significant predictors, such as patient-, tumor-, and treatment-specific factors, of bladder recurrence after RNU. We should also be aware of the possibility of metachronous bladder tumor, and patients should be urged for regular follow-up.
Our study has several limitations. First, it was of retrospective design with some inherent limitations. There was no information on the reasons for the delay in surgical time. Second, there were definite losses during the time of data collection due to the long follow-up time. Moreover, despite the large number of cases, multiple institutions across two decades were involved in this study. Inclusion of diverse backgrounds and surgeons with various levels of experience and possible lack of generalizability of their work due to potentially endemic etiology of UTUC in Taiwan were inevitable, causing potential introduction of bias.
In conclusion, for patients with UTUC undergoing RNU, the SWT should be minimized to less than 90 days. Prolonged wait time may be associated with poor OS and DFS. Further research is required to corroborate our results.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
H-LK, Y-CT contributed to conception and design of the study. Y-CT, K-HW performed the statistical analysis. K-HW wrote draft of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by grants from the Ministry of Health and Welfare and Kaohsiung Medical University Hospital (MOHW110-TDU-B-212-124006), Kaohsiung Medical University Hospital (KMUH-DK(C)110006), Kaohsiung Medical University Regenerative Medicine and Cell Therapy Research Center Grant (KMU-TC109A02), Center for Liquid Biopsy and Cohort Research (KMU-TC109B05), and the Ministry of Science and Technology, Taiwan (MOST 110-2321-B-037-002).
Acknowledgments
We thank Dr. Yu-Tsai Li for statistical assistance and all members of the Taiwan Upper Tract Urothelial Carcinoma Collaboration Group. This study was supported by grants from the Ministry of Health and Welfare and Kaohsiung Medical University Hospital (MOHW110-TDU-B-212-124006), Kaohsiung Medical University Hospital (KMUH-DK(C)110006), Kaohsiung Medical University Regenerative Medicine and Cell Therapy Research Center Grant (KMU-TC109A02), Center for Liquid Biopsy and Cohort Research (KMU-TC109B05), and the Ministry of Science and Technology, Taiwan (MOST 110-2321-B-037-002-). We thank all members of the Taiwan Upper Tract Urothelial Carcinoma Collaboration group: Allen W. Chiu, Bing-Juin Chiang, Chao-Hsiang Chang, Chao-Yuan Huang, Cheng-Huang Shen, Cheng-Kuang Yang, Cheng-Ling Lee, Chen-Hsun Ho, Che-Wei Chang, Chia-Chang Wu, Chieh-Chun Liao, Chien-Hui Ou, Chih-Chen Hsu, Chih-Chin Yu, Chih-Hung Lin, Chih-Ming Lu, Chih-Yin Yeh, Ching-Chia Li, Chi-Ping Huang, Chi-Rei Yang, Chi-Wen Lo, Chuan-Shu Chen, Chung-Hsin Chen, Chung-You Tsai, Chung-Yu Lin, Chun-Hou Liao, Chun-Kai Hsu, Fang-Yu Ku, Hann-Chorng Kuo, Han-Yu Weng, Hao-Han Chang, Hong-Chiang Chang, Hsiao-Jen Chung, Hsin-Chih Yeh, Hsu-Che Huang, Ian-Seng Cheong, I-Hsuan Alan Chen, Jen-Kai Fang, Jen-Shu Tseng, Jen-Tai Lin, Jian-Hua Hong, Jih-Sheng Chen, Jungle Chi-Hsiang Wu, Kai-Jie Yu, Keng-Kok Tan, Kuan-Hsun Huang, Kun-Lin Hsieh, Lian-Ching Yu, Lun-Hsiang Yuan, Hao-Lun Luo, Marcelo Chen, Min-Hsin Yang, Pai-Yu Cheng, Po-Hung Lin, Richard Chen-Yu Wu, See-Tong Pang, Shin-Hong Chen, Shin-Mei Wong, Shiu-Dong Chung, Shi-Wei Huang, Shuo-Meng Wang, Shu-Yu Wu, Steven Kuan-Hua Huang, Ta-Yao Tai, Thomas Y. Hsueh, Ting-En Tai, Victor Chia-Hsiang Lin, Wei-Chieh Chen, Wei-Ming Li, Wei-Yu Lin, Wen-Hsin Tseng, Wen-Jeng Wu, Wun-Rong Lin, Yao-Chou Tsai, Yen-Chuan Ou, Yeong-Chin Jou, Yeong-Shiau Pu, Yi-Chia Lin, Yi-Hsuan Wu, Yi-Huei Chang, Yi-Sheng Lin, Yi-Sheng Tai, Yu-Khun Lee, Yuan-Hong Jiang, Yu-Che Hsieh, Yu-Chi Chen, Yu-Ching Wen, Yung-Tai Chen, Zhe-Rui Yang.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Munoz JJ, Ellison LM. Upper tract urothelial neoplasms: incidence and survival during the last 2 decades. J Urol. (2000) 164:1523–5. doi: 10.1016/S0022-5347(05)67019-X
2. Soualhi A, Rammant E, George G, Russell B, Enting D, Nair R, et al. The incidence and prevalence of upper tract urothelial carcinoma: A systematic review. BMC Urol (2021) 21(1):110. doi: 10.1186/s12894-021-00876-7
3. Cancer registry annual report, 2018. Taiwan: Health Promotion Administration Ministry of Health and Welfare (2020).
4. Roupret M, Zigeuner R, Palou J, Boehle A, Kaasinen E, Sylvester R, et al. European Guidelines for the diagnosis and management of upper urinary tract urothelial cell carcinomas: 2011 update. Eur Urol (2011) 59(4):584–94. doi: 10.1016/j.eururo.2010.12.042
5. Jinzaki M, Kikuchi E, Akita H, Sugiura H, Shinmoto H, Oya M. Role of computed tomography urography in the clinical evaluation of upper tract urothelial carcinoma. Int J Urol (2016) 23(4):284–98. doi: 10.1111/iju.13032
6. Lee HY, Chan EO, Li CC, Leung D, Li WM, Yeh HC, et al. How to manage patients with suspected upper tract urothelial carcinoma in the pandemic of covid-19? Urol Oncol (2021) 39(10):733.e11–e16. doi: 10.1016/j.urolonc.2021.06.007
7. Margulis V, Shariat SF, Matin SF, Kamat AM, Zigeuner R, Kikuchi E, et al. Outcomes of radical nephroureterectomy: A series from the upper tract urothelial carcinoma collaboration. Cancer (2009) 115(6):1224–33. doi: 10.1002/cncr.24135
8. Bourgade V, Drouin SJ, Yates DR, Parra J, Bitker MO, Cussenot O, et al. Impact of the length of time between diagnosis and surgical removal of urologic neoplasms on survival. World J Urol (2014) 32(2):475–9. doi: 10.1007/s00345-013-1045-z
9. Waldert M, Karakiewicz PI, Raman JD, Remzi M, Isbarn H, Lotan Y, et al. A delay in radical nephroureterectomy can lead to upstaging. BJU Int (2010) 105(6):812–7. doi: 10.1111/j.1464-410X.2009.08821.x
10. Gore JL, Lai J, Setodji CM, Litwin MS, Saigal CS. Urologic diseases in America p. mortality increases when radical cystectomy is delayed more than 12 weeks: Results from a surveillance, epidemiology, and end results-Medicare analysis. Cancer (2009) 115(5):988–96. doi: 10.1002/cncr.24052
11. Lee CT, Madii R, Daignault S, Dunn RL, Zhang Y, Montie JE, et al. Cystectomy delay more than 3 months from initial bladder cancer diagnosis results in decreased disease specific and overall survival. J Urol (2006) 175(4):1262–7; discussion 7. doi: 10.1016/S0022-5347(05)00644-0
12. Soria F, Shariat SF, Lerner SP, Fritsche HM, Rink M, Kassouf W, et al. Epidemiology, diagnosis, preoperative evaluation and prognostic assessment of upper-tract urothelial carcinoma (Utuc). World J Urol (2017) 35(3):379–87. doi: 10.1007/s00345-016-1928-x
13. Teoh JY, Ong WLK, Gonzalez-Padilla D, Castellani D, Dubin JM, Esperto F, et al. A global survey on the impact of covid-19 on urological services. Eur Urol (2020) 78(2):265–75. doi: 10.1016/j.eururo.2020.05.025
14. Mian BM, Siddiqui S, Ahmad AE. Management of urologic cancers during the pandemic and potential impact of treatment deferrals on outcomes. Urol Oncol (2021) 39(5):258–67. doi: 10.1016/j.urolonc.2020.10.013
15. Zhao F, Qi N, Zhang C, Xue N, Li S, Zhou R, et al. Impact of surgical wait time on survival in patients with upper urinary tract urothelial carcinoma with hydronephrosis. Front Oncol (2021) 11:698594. doi: 10.3389/fonc.2021.698594
16. Sundi D, Svatek RS, Margulis V, Wood CG, Matin SF, Dinney CP, et al. Upper tract urothelial carcinoma: Impact of time to surgery. Urol Oncol (2012) 30(3):266–72. doi: 10.1016/j.urolonc.2010.04.002
17. Lee JN, Kwon SY, Choi GS, Kim HT, Kim TH, Kwon TG, et al. Impact of surgical wait time on oncologic outcomes in upper urinary tract urothelial carcinoma. J Surg Oncol (2014) 110(4):468–75. doi: 10.1002/jso.23589
18. Xia L, Taylor BL, Pulido JE, Guzzo TJ. Impact of surgical waiting time on survival in patients with upper tract urothelial carcinoma: A national cancer database study. Urol Oncol (2018) 36(1):10.e5– e22. doi: 10.1016/j.urolonc.2017.09.013
19. Abouelkheir RT, Elawdy MM, Taha DE, El-Hamid MA, Osman Y, El-Diasty T. The accuracy of computed tomography in the diagnosis of upper urinary tract urothelial carcinoma in correlation with the final histopathology: A retrospective study in 275 patients at a tertiary urology institute. Urol Ann (2021) 13(4):356–61. doi: 10.4103/UA.UA_32_20
20. Grahn A, Melle-Hannah M, Malm C, Jaderling F, Radecka E, Beckman M, et al. Diagnostic accuracy of computed tomography urography and visual assessment during ureterorenoscopy in upper tract urothelial carcinoma. BJU Int (2017) 119(2):289–97. doi: 10.1111/bju.13652
21. Nison L, Roupret M, Bozzini G, Ouzzane A, Audenet F, Pignot G, et al. The oncologic impact of a delay between diagnosis and radical nephroureterectomy due to diagnostic ureteroscopy in upper urinary tract urothelial carcinomas: Results from a Large collaborative database. World J Urol (2013) 31(1):69–76. doi: 10.1007/s00345-012-0959-1
22. Tai Y-S, Chiang I-N, Huang C-Y, Tai H-C, Pu Y-S. Effectiveness of different diagnostic tools for upper urinary tract urothelial carcinoma. Urological Sci (2014) 26:57–60. doi: 10.1016/j.urols.2014.07.004
23. Subiela JD, Territo A, Mercade A, Balana J, Aumatell J, Calderon J, et al. Diagnostic accuracy of ureteroscopic biopsy in predicting stage and grade at final pathology in upper tract urothelial carcinoma: Systematic review and meta-analysis. Eur J Surg Oncol (2020) 46(11):1989–97. doi: 10.1016/j.ejso.2020.06.024
24. Yafi FA, Novara G, Shariat SF, Gupta A, Matsumoto K, Walton TJ, et al. Impact of tumour location versus multifocality in patients with upper tract urothelial carcinoma treated with nephroureterectomy and bladder cuff excision: A homogeneous series without perioperative chemotherapy. BJU Int (2012) 110(2 Pt 2):E7–13. doi: 10.1111/j.1464-410X.2011.10792.x
25. Seisen T, Granger B, Colin P, Leon P, Utard G, Renard-Penna R, et al. A systematic review and meta-analysis of clinicopathologic factors linked to intravesical recurrence after radical nephroureterectomy to treat upper tract urothelial carcinoma. Eur Urol (2015) 67(6):1122–33. doi: 10.1016/j.eururo.2014.11.035
Keywords: urinary tract urothelial carcinoma, surgical wait time, nephroureterectomy, ureteroscopy, survival
Citation: Wu K-H, Chang C-H, Wu H-C, Huang SK, Liu C-L, Yang C-K, Li J-R, Tseng J-S, Lin W-R, Yu C-C, Lo C-W, Huang C-Y, Chen C-H, Tsai C-Y, Cheng P-Y, Jiang Y-H, Lee Y-K, Chen Y-T, Yeh T-C, Lin J-T, Tsai Y-C, Hsueh TY, Chiang B-J, Chiang Y-D, Lin W-Y, Jou Y-C, Pang S-T and Ke H-L (2022) Oncologic impact of delay between diagnosis and radical nephroureterectomy. Front. Oncol. 12:1025668. doi: 10.3389/fonc.2022.1025668
Received: 23 August 2022; Accepted: 28 November 2022;
Published: 14 December 2022.
Edited by:
Fumitaka Koga, Tokyo Metropolitan Komagome Hospital, JapanReviewed by:
Hiroaki Matsumoto, Yamaguchi University, JapanFahad Quhal, Medical University of Vienna, Austria
Copyright © 2022 Wu, Chang, Wu, Huang, Liu, Yang, Li, Tseng, Lin, Yu, Lo, Huang, Chen, Tsai, Cheng, Jiang, Lee, Chen, Yeh, Lin, Tsai, Hsueh, Chiang, Chiang, Lin, Jou, Pang and Ke. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hung-Lung Ke, aHVuZ2x1bmdrZUBnbWFpbC5jb20=
 Kuan-Hsien Wu
Kuan-Hsien Wu Chao-Hsiang Chang
Chao-Hsiang Chang Hsi-Chin Wu2,3,4
Hsi-Chin Wu2,3,4 Cheng-Kuang Yang
Cheng-Kuang Yang Jian-Ri Li
Jian-Ri Li Chi-Wen Lo
Chi-Wen Lo Chao-Yuan Huang
Chao-Yuan Huang Chung-Hsin Chen
Chung-Hsin Chen Chung-You Tsai
Chung-You Tsai Pai-Yu Cheng
Pai-Yu Cheng Yung-Tai Chen
Yung-Tai Chen Ting-Chun Yeh
Ting-Chun Yeh Jen-Tai Lin
Jen-Tai Lin Yao-Chou Tsai
Yao-Chou Tsai Bing-Juin Chiang
Bing-Juin Chiang Wei-Yu Lin
Wei-Yu Lin Hung-Lung Ke
Hung-Lung Ke