
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 09 January 2023
Sec. Radiation Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.1025455
Background: Immune checkpoint inhibitors (ICIs) are used to treat locally-advanced and metastatic lung cancer, which can lead to severe immunogenic-related cardiotoxicities. We assessed the risk of cardiotoxicity in ICI-treated lung cancer patients with or without cardiac radiation from thoracic radiotherapy.
Methods: Retrospective data was collected on Stage III-IV lung cancer patients who received ICIs between 2015 and 2018. All cardiotoxicities associated with ICI were assessed in correlation with the timing of radiotherapy (RT) in relation to ICI, and the mean RT heart dose. The rate of cardiac events in relation to RT timing and heart dose was compared using multiple logistic regression including the Framingham risk score and steroid use prior to ICI therapy.
Results: Of 194 ICI-treated patients evaluated, 55.2% (n=107/194) patients had received thoracic RT at a median dose of 60.4 Gy (range, 15-75). Cardiotoxicities such as non-ST elevated myocardial infarction and new onset supraventricular tachycardias were observed in 13 (12.2%) of those who had thoracic RT versus 9 (10.3%) who did not (p=0.87). 38 patients who received RT concurrently with ICI did not develop any cardiotoxicity whereas 14.1% (n=22/156) of those who did not receive concurrent RT developed cardiotoxicities (univariate, p=0.030; multivariate, p=0.055). There were no significant differences in the mean heart RT dose, Framingham risk score, and steroid treatment between patients that received concurrent RT with ICI versus non-concurrent RT/ICI.
Conclusion: ICI-related cardiotoxicities were not significantly associated with patients who received concurrent thoracic radiotherapy in this retrospective review. Further validation of prospective studies is needed to confirm these results.
Immune checkpoint inhibitors (ICIs) and chimeric antigen receptor T-cell therapy have been successful in treating a wide variety of cancers including melanoma, lymphoma, and lung cancer (1). ICIs amplify T-cell-mediated immune responses against cancer cells by blocking intrinsic immune down-regulators such as anti-cytotoxic T-lymphocyte associated protein 4 (CTLA-4), programmed cell death protein-1 (PD-1), and programmed cell death ligand-1b (PD-L1) (1). While surgical resection remains the standard of care for early-stage non-small-cell lung cancer (NSCLC), chemoradiation has been a mainstay of treatment for locally advanced NSCLC. Consolidation immunotherapy has emerged as the new standard with improved survival (2) in this subset of patients after conventional chemoradiation, with the regimens including CTLA-4, PD-1, and PD-L1 checkpoint inhibitors.
Radiation in high doses may potentiate an immune response called the abscopal effect, in which local treatment of radiation therapy (RT) induces a systemic response that could target distant sites of the disease (3). Conversely, radiation may also induce immunosuppression through the T-cell inhibition (4). Concurrent chemoradiotherapy in patients with metastatic disease may reduce the time to recurrence and may improve overall survival, however, with a potentially increased risk for immune-related adverse events (irAEs) (3, 5). One of the rare irAEs in combination with RT is ICI-related myocarditis, a potential therapy-limiting form of cardiotoxicity with a high rate of associated significant morbidity and mortality (1).
Du et al. demonstrated strong preclinical evidence that radiation-induced cardiotoxicity is modulated by the PD-1 axis by delivering cardiac irradiation in a mouse model concurrently with PD-1 blockade (6). Radiation was delivered in a single dose of 20 Gy or in fractionated cardiac irradiation of 30 Gy delivered over five fractions. Acute mortality of 30% within 2 weeks after cardiac irradiation plus anti–PD-1 antibody compared with 0% from cardiac irradiation plus immunoglobulin G (p=0.023) was observed, which was also associated with decreased cardiac output, increased lymphocytic infiltration, and fibrosis (6). There is a paucity of data reporting cardiotoxicity in patients receiving both RT and immunotherapy, especially in the real-world setting. The objective of this study was to assess if the interaction between cardiac heart dose from thoracic RT and immunotherapy in lung cancer patients was correlated with different clinical outcomes.
Patients with a diagnosis of NSCLC and small-cell lung cancer (SCLC) who received ICIs in the past 3 years between February 2015 and February 2018 were identified from the Tumor Registry at Vidant Medical Center/East Carolina University. Patients were included in the study if they were ≥ 18 years old, completed at least 1 infusion of ICI therapy, and received treatment at our institution. RT was delivered between cycles of immunotherapy for patients who received RT concurrently with ICI. Concurrent RT with ICI has been delivered within our institution especially if the patient required palliative therapy. Usually, acute radiation side effects can last up to 12 weeks after radiation (7), and the timing of cardiotoxicity typically occurs early after initiation of ICI with a median time of 2 months with the majority of the cases occurring within 3 months (8). Therefore, we looked to see when acute toxicities are most likely to be present by comparing the variability in the overlaps between radiation therapy and immunotherapy.
Demographic data including age, sex, body mass index, and ethnicity were collected. Information regarding the patient’s lung cancer type, prior and concomitant chemotherapy, ICI therapy, medical history, cardiac medications prior to initiation of therapy, steroid use prior to initiation of therapy, and cardiac radiation dose were retrospectively reviewed. Thoracic radiation parameters collected included prescription dose, dose per fraction, and mean heart dose. Dosimetric parameters V5, V25, and V30 were also collected.
ICI-related cardiotoxicities (iRC) (+) patients were defined as 1) non-ST elevated myocardial infarction (NSTEMI) in the setting of acute cardiac syndrome symptoms, 2) new onset supraventricular tachycardias (SVT) including atrial fibrillation (AF), 3) myocarditis or 4) pericardial disorders (acute pericarditis and/or non-malignant pericardial effusion) in the absence of sepsis, electrolyte disorders or other confounding medical problems. A clinical diagnosis of myocarditis included 2 or more clinical presentations of: 1) new-onset (0 days up to 3 months) or subacute/chronic (>3 months of worsening dyspnea at rest/exertion and/or fatigue with left and/or right heart failure signs and/or imaging findings of new right and/or left ventricular dysfunction, or elevations of natriuretic peptides or troponin 2) cardiac death or aborted cardiac death 3) new I to III degree atrioventricular block or bundle branch block, sinus arrest, ventricular tachycardia or fibrillation and asystole, or 4) tissue characterization by cardiac magnetic resonance imaging (MRI) showing edema or late gadolinium enhancement. iRC (-) patients were those who did not experience a cardiac-related irAEs and were further subcategorized into patients who did not experience any irAEs, experienced non-cardiac irAEs, or who had disease progression (could not complete 4 cycles of ICI due to tumor progression).
Cardiotoxicity that could be attributed to ICI was determined by cardiologists and medical oncologists and graded based on the common terminology for clinical adverse events (CTCAE) (Table 1). The cohort used in the analysis is a subset of patients described in a prior report on cardiotoxicity and ICI that did not analyze the interaction with radiotherapy. The methodology of grading toxicity is described in this publication (9). The Framingham risk score for predicting 10-year coronary heart disease risk was calculated according to the modified model in 2008 (10). Chi-square was used for data with binary inputs. Student’s t-test was used for continuous data including baseline age and BMI. Mann-Whitney U-test was used for non-parametric data including mean heart dose and Framingham Risk Score. Multiple regression was used for multivariate analysis. All statistical analyses were performed using MedCalc version 12.6.0 (MedCalc Software, Ostend, Belgium).
Among 194 patients reviewed retrospectively, 176 patients had NSCLC and 18 patients had SCLC. Patients had a median age of 64 (IQR of 36 to 88 years old), and were predominantly Caucasian (n=123, 63.4%) and male (n=112, 57.7%). Patients had either stage III (n=66, 34.02%) or stage IV (n=128, 65.98%) lung cancer. The median Framingham score for our patient population was a 22.34% risk of a cardiac event in 10 years. Some of the predominant cardiac comorbidities included diabetes in 29.38% of patients, coronary artery disease/coronary artery bypass graft in 17.01% of patients, hypertension in 62.89% of patients, hyperlipidemia in 30.41% of patients, and chronic obstructive pulmonary disease in 36.59% of patients. Medications included steroids, beta-blockers, calcium channel blockers, diuretics, renin-angiotensin-aldosterone-system inhibitors, and statins (Table 2).
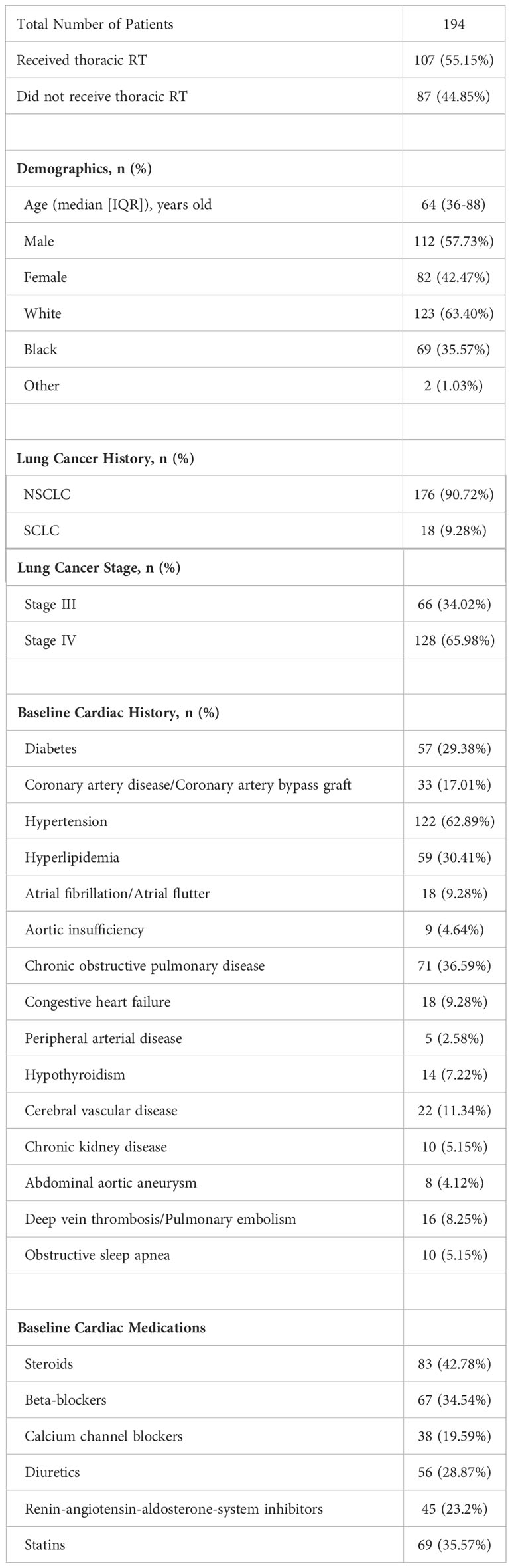
Table 2 Baseline patient characteristics include demographics, cancer history, co-morbidities, cardiac medical history, and medications prior to initiation of therapy.
Patients received different chemotherapy regimens with the majority receiving alkylating agents (n=135, 69.59%). Majority of the patients received nivolumab (n=135, 69.59%) while remaining patients received pembrolizumab (n=48, 24.74%) and atezolizumab (n=11, 5.67%). The median total number of ICI cycles patients received was 4, range of 1-27. In patients who developed cardiotoxicity, the median number of ICI cycles they received prior to the cardiotoxic event was 3.5 cycles, range of 1-8. The average period of monitoring for toxicity after the initiation of ICI was 5.6 months, counting after the date of the first infusion, and 22.2 months, counting after the last date of the thoracic RT. The average total prescription dose of thoracic RT was 60.4 Gray (Gy). The mean dose per fraction of thoracic RT was 1.8 Gy, and the range was 1.3-12 Gy. The average global heart dose was 9.53 Gy, range of 0-37.74 Gy. The average maximum point dose to the heart was 63.38 Gy. The mean heart dosimetric parameters were 36.85 (range 0-100) for V5, 15.22 (range 0-67) for V25, and 11.51 for V30 (range 0-55) (Table 3).
There were 107 patients who received thoracic RT before, concurrently, or after ICI infusion, while 87 patients did not receive any thoracic RT (Figure 1). Among the 194 ICI-treated patients, 22 patients (11.3%) developed cardiotoxicity (Figure 2). Table 4 describes the different type of cardiac toxic events that were described in these 22 patients. The two most common cardiac toxicities included myocarditis and cardiac arrhythmias such as SVT/AF. Among the patients who received thoracic RT, 38 (19.59%) patients received thoracic RT concurrently with ICI (Figure 3). Table 5 describes the baseline patient characteristics between the cohorts of patients treated concurrently and non-concurrently. There was no statistical significance between the two groups regarding their age, gender, smoking status, coexisting cardiovascular disease including diabetes and the treatment for HTN, and prior known vascular disease. There was a statistical difference in their baseline BMI (p=0.008). Cardiotoxicity occurred in 13 patients (12.2%) out of 107 patients who received any thoracic RT versus 9 (10.3%) out of 87 patients who did not receive thoracic RT (p=0.87) (Figure 2). All 38 patients who received concurrent RT with ICI did not develop cardiotoxicity compared to 22 out of 156 patients (14.1%) who did not receive concurrent RT (p=0.030) (Figure 3). There was no significant difference in mean heart dose between the concurrent RT/ICI group versus the non-concurrent RT/ICI group (p=0.96) (Figure 4). There was no significant difference in the Kaplan-Meier survival analysis of the overall survival between the non-concurrent RT+ICI group and concurrent RT+ICI group (p=0.21) (Figure 5).
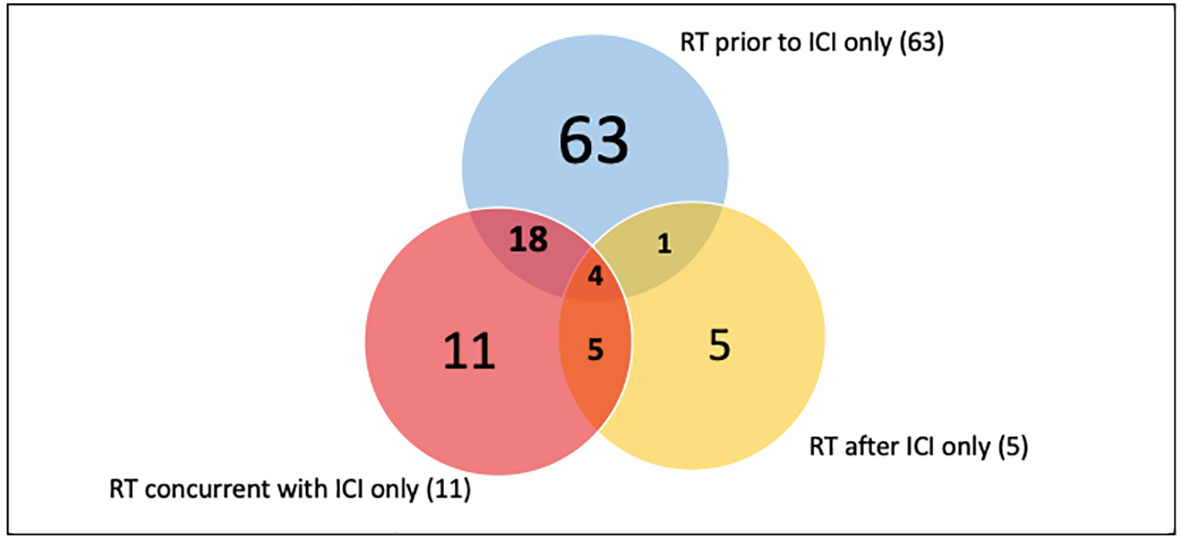
Figure 1 Thoracic RT and ICI treatment schedule. The number indicates a number of patients treated with thoracic RT in regards to the ICI schedule.
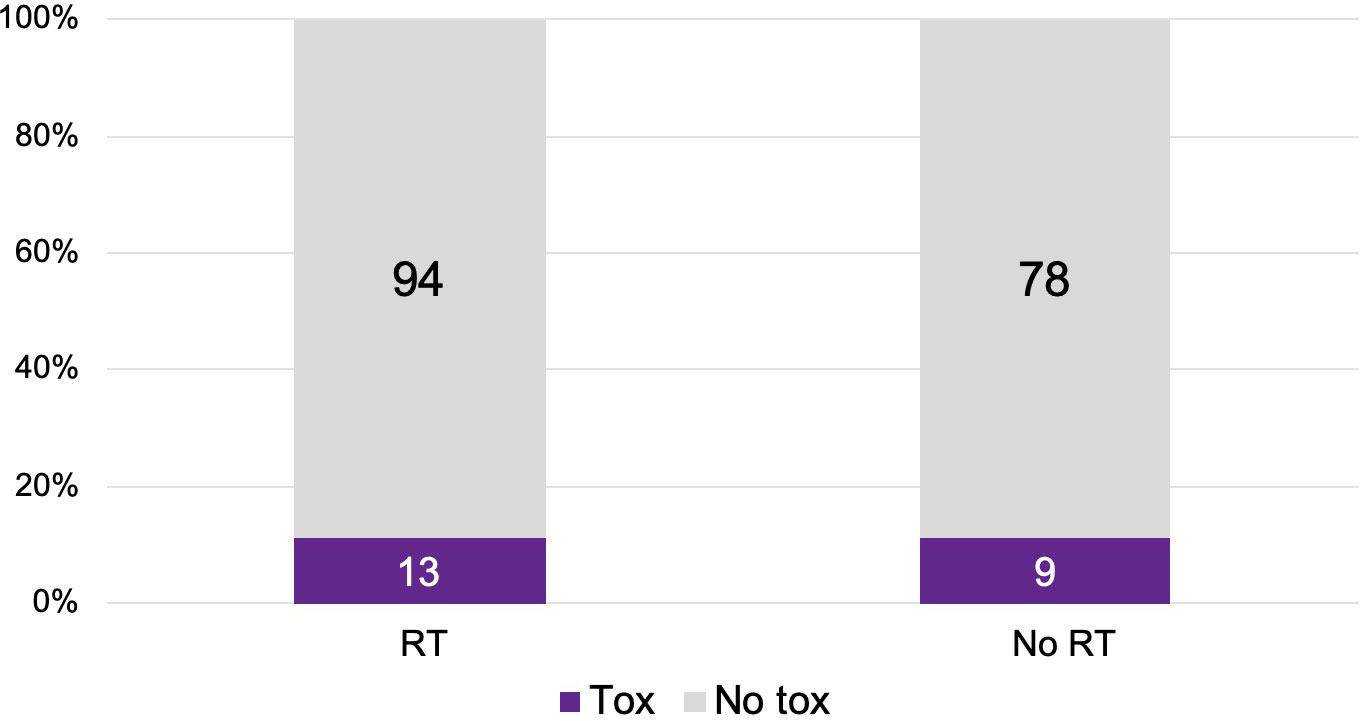
Figure 2 The plot of cardiotoxic events between the 107 patients who received thoracic RT versus 87 patients who did not receive thoracic RT. The numbers indicate patients who did (purple) or did not (gray) experience a cardiotoxic event. There was no significant difference in the percentage of patients with cardiotoxic events between the 2 groups (p=0.87).
Figure 3 The plot of cardiotoxic events in patients who received ICI concurrent with thoracic RT versus ICI not concurrently with thoracic RT. Patients who received ICI non-concurrently with thoracic RT had higher rates of cardiotoxicity (p=0.030).
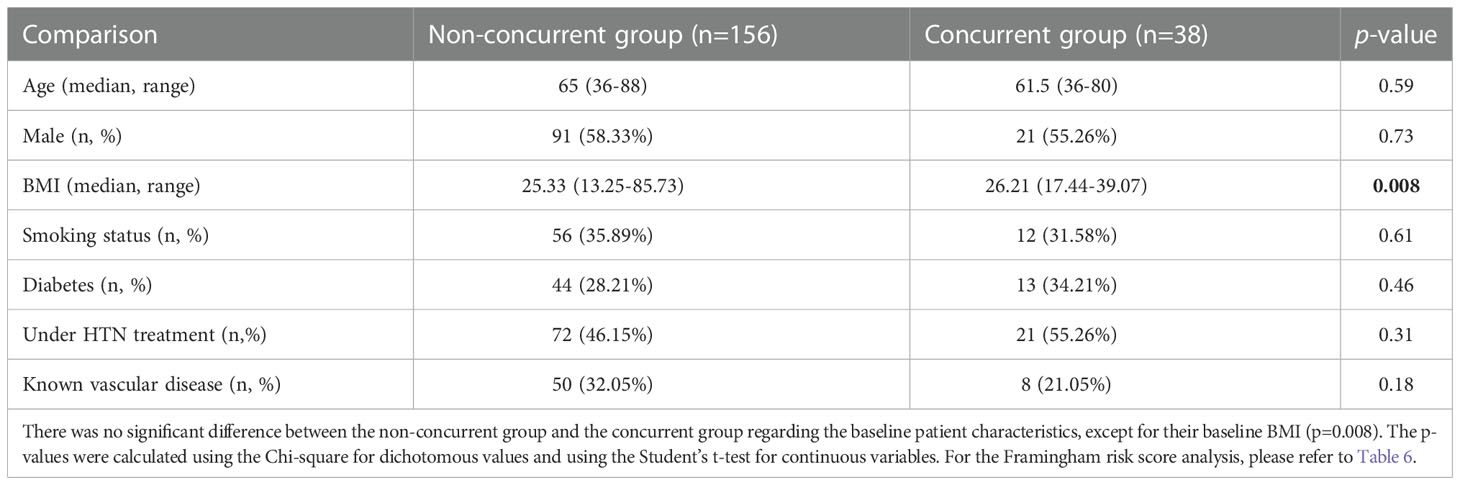
Table 5 Baseline patient characteristic comparison between the non-concurrent group and the concurrent group.
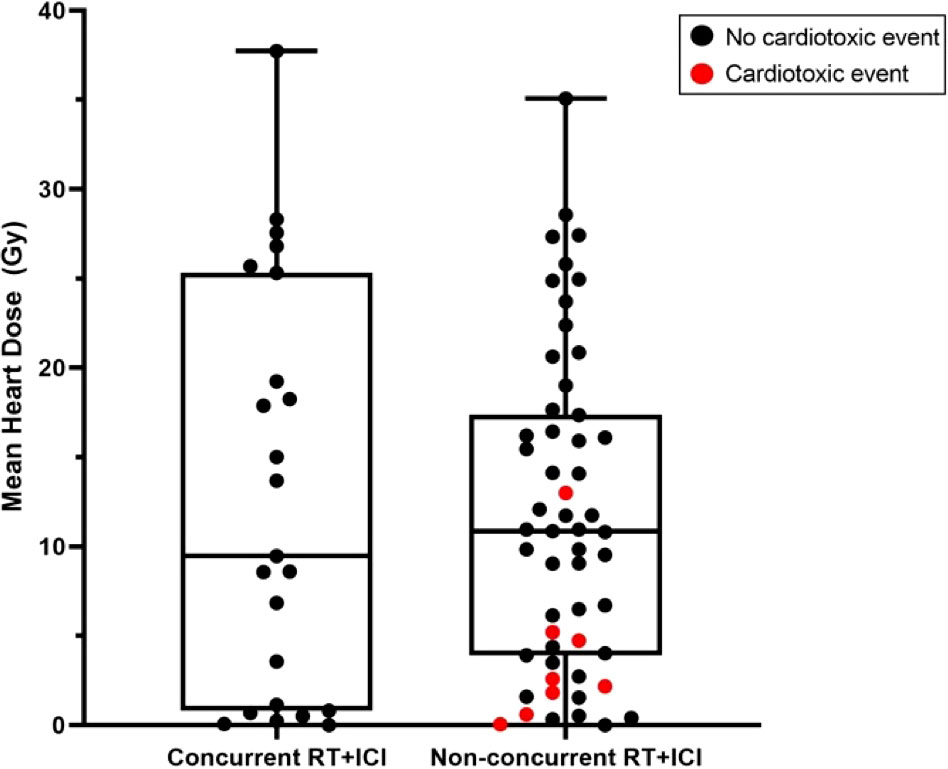
Figure 4 Box and whisker plots of mean heart dose of subjects who received radiotherapy concurrently with ICI (concurrent RT+ICI) versus not (non-concurrent RT+ICI). Each point represents a patient, plotted against the range, interquartile range, and median. There was no significant difference in mean heart dose between the groups (p=0.96).
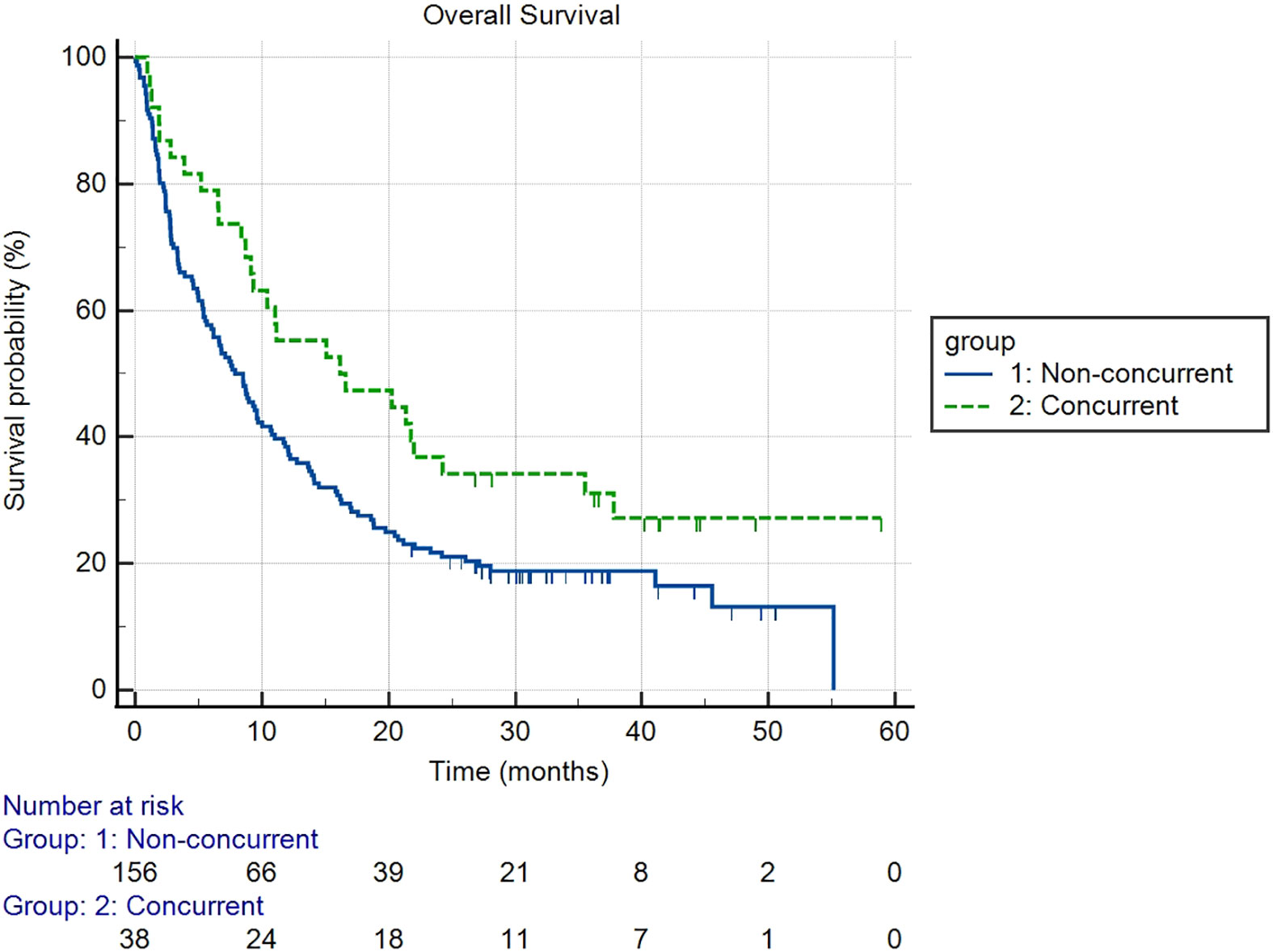
Figure 5 Kaplan-Meier survival analysis of the overall survival between the non-concurrent RT+ICI and concurrent RT+ICI. There was no significant difference in overall survival between the two groups (p=0.21).
Two different cohorts were used for univariable analysis between patients who received ICI concurrently and non-concurrently with thoracic RT. Cohort 1 included patients who received some form of RT (n=107) while cohort 2 included all the patients, those with or without RT (n=194). In Cohort 1, there was no significant difference in mean heart RT dose (p=0.96), Framingham risk score (p=0.56), and prior steroid treatment (p=0.59) between patients that received concurrent RT with ICI versus non-concurrent RT/ICI. However, patients who received ICI non-concurrently with thoracic RT had significantly increased cardiotoxicity (p=0.044) (Table 7). In Cohort 2, there were no significant differences in mean heart RT dose (p=0.68), Framingham risk score (p=0.78), and prior steroid treatment (p=0.72) between patients that received concurrent RT with ICI versus non-concurrent RT/ICI. However, patients who received ICI non-concurrently with thoracic RT also had significantly increased cardiotoxicity (p=0.030) in this cohort (Table 6). In a multiple logistic regression model, steroid treatment was independently predictive of cardiotoxicity (p<0.001), and the lack of concurrent RT/ICI approached significance (p=0.055).
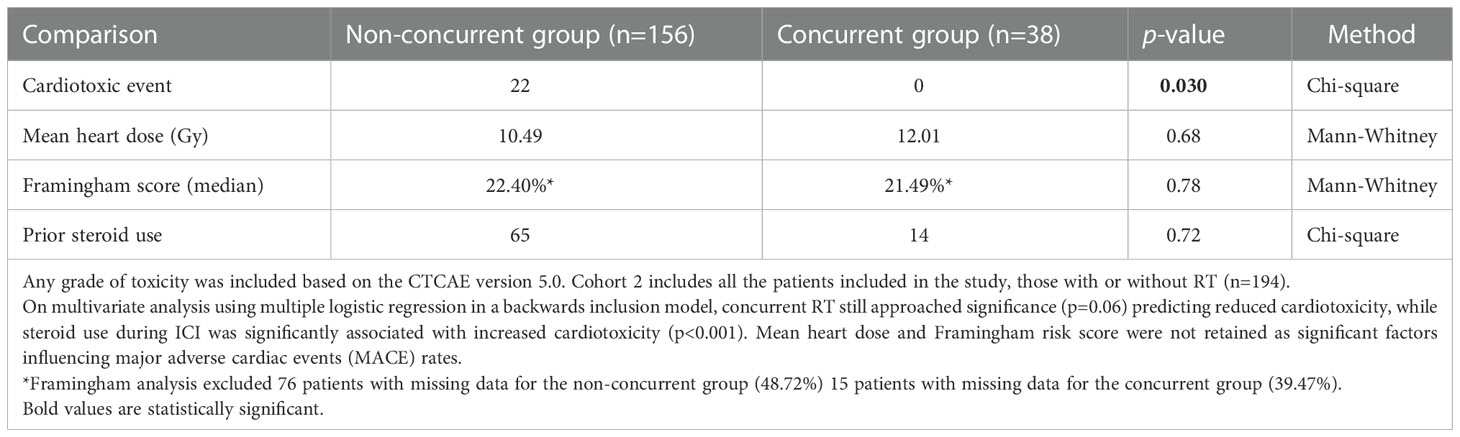
Table 6 Univariate analysis between patients who received ICI concurrently versus non-concurrently with thoracic RT.
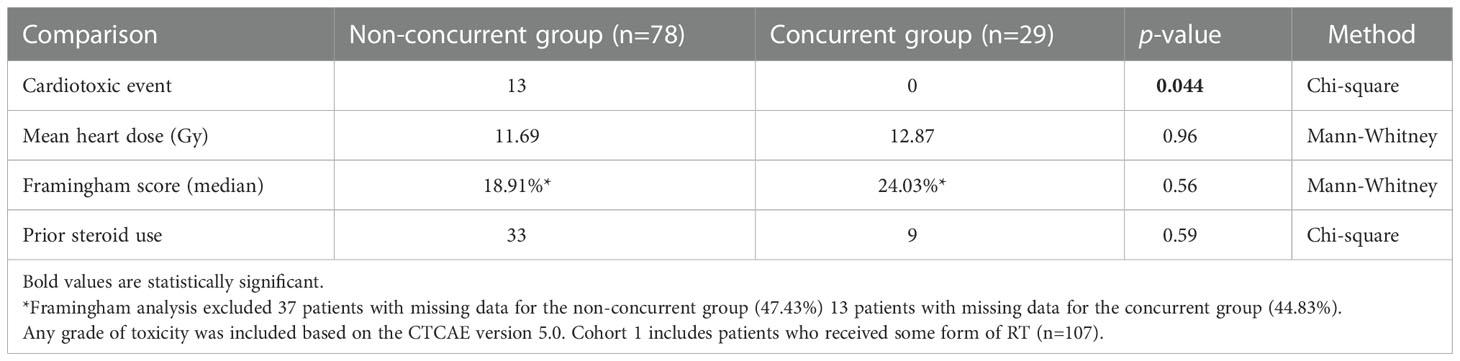
Table 7 Univariate analysis between patients who received ICI concurrently versus non-concurrently with thoracic RT.
We assessed the relationship between cardiac radiation and risk of cardiotoxicity in NSCLC and SCLC patients treated with combined ICI and thoracic radiotherapy. Our results were not consistent with our initial hypothesis that concurrent treatment of RT with ICI worsens cardiotoxicity in lung cancer patients. In fact, the results showed that no MACEs occurred in patients who received thoracic radiotherapy concurrently with ICI. This suggests that thoracic radiation concurrently delivered with ICI does not necessarily increase the risk of cardiotoxicity. The fewer cardiac events in our cohort with thoracic radiotherapy ICI–treated patients were independent of the cardiac radiation dose, Framingham risk score, and prior steroid treatment. The baseline BMI was statistically different between the concurrent and non-concurrent groups but the higher BMI in the concurrent group, which would predict a higher risk of a cardiac event in that group, was not shown in our results.
The PACIFIC trial was the first phase III prospective randomized controlled trial to show an increase in progression-free survival (PFS) and overall survival (OS) using consolidation durvalumab following concurrent chemoradiation (2). The initial analysis showed a significantly improved PFS compared to the placebo (16.8 months compared to 5.6 months, p<0.001), which led to the FDA and EMA approval of duravalumab for use in the consolidative therapy (2). Upon the success of chemoradiation followed by immunotherapy in treating locally advanced NSCLC, many trials are investigating the incorporation of immunotherapy with concurrent chemoradiotherapy in the definitive management of locally advanced NSCLC. The preliminary safety and efficacy results of the PD-L1 inhibitor durvalumab in combination with conventional radiotherapy were assessed by number of small Phase I/II trials. Levy et al. recently showed that this combination was well tolerated in a small cohort of patients in their single center subset analysis in France (11). In a completed trial by Jabbour et al., concurrent PD-1 treatment with chemoradiotherapy was well tolerated with encouraging PFS of 69.7% at 12 months, however, with potentially an increased risk of pneumonitis using concurrent PD-1 checkpoint blockade (5). Therefore, much remains to be addressed about this combination, including the best sequences to follow when combining RT with ICIs and the best radiation dose for maximal efficacy (12, 13). Additionally, complicating the use of thoracic RT and ICI’s is that cardiac radiation has a dose dependent association with an increased risk of MACEs (14, 15).
The synergy of combining RT and immunotherapy to enhance clinical benefit through abscopal effects has been widely investigated in both pre-clinical and clinical settings (3). The local treatment of RT stimulates expression of MHC I, which primes T-cell response via release of tumor-specific antigens and immunomodulatory cytokines (16). The release of cytokines activates the systemic immune response against tumors even in the non-irradiated lesions, culminating into an abscopal effect. The addition of immunotherapy is thought to further enhance the abscopal effects by activating the immune system, which involves increase in lymphocytes. Immunotherapy such as immune checkpoint inhibitor (ICI) blocks the inhibition of T cells on antigen presenting cells, thereby unleashing T cells to react against tumor cells. ICIs are frequently administered intravenously, which allows for systemic activation of T cells, and when combined with RT-induced T cell priming effects, could potentiate antitumor immunity via abscopal effects, although this is not an effect that has been clearly demonstrated in human trials (17). However, radiation therapy-induced lymphopenia in patients treated with ICI may decrease the abscopal effects and leads to poor overall survival outcomes (18, 19). Chen et al. reported that patients with absolute lymphocyte count above median value despite RT had greater abscopal response and improved survival in response to ICI (20). In pancreatic cancer, Wild et al. (19) demonstrated that stereotactic body radiation therapy (SBRT), compared to conventional RT (CRT), delivers radiation dose in a smaller target volume with fewer fractions, suggesting lymphocytes in normal tissue could be preserved. Menon et al. also suggested patients who received low-dose radiation to metastatic lesions in combination with high-dose stereotactic ablative radiotherapy and ICI therapy may be capable of enhancing an immune response leading to abscopal effects (21). Our study did not distinguish between CRT and SBRT in association with the observed cardioprotective effects in ICI-treated lung cancer patient.
An underlying mechanism of immunotherapy-related cardiotoxicity is upregulation of CD8+T cells and CD68+ macrophages, involving hyperactivated cytotoxic T-cells (22). Although the mechanism is not completely understood, there is evidence that ICI increases T cells activities against common antigens shared by tumor and heart, leaving cardiac cells susceptible to cytotoxic T-cells (22). Therefore, RT may be effective in reversing such cardiotoxicity by decreasing lymphocyte count (22) as well as neutralizing immune activities by TGFβ induction as described by Vanpouille-Box et al. (4).
Most studies on combining RT and ICI therapy have focused on overall safety and efficacy. Implications specifically on cardiotoxicity with the combination of RT and ICI warrants further investigation on a molecular level. By better understanding the immunological mechanism of the combination in respect to cardiotoxicity may help formulate a safer treatment option for lung cancer patients who are good candidates for ICI therapy.
Given the retrospective study design, there may be under-reporting of cardiac events due to competing risk of patients’ metastatic, locally advanced disease diagnoses. The metastatic disease may interfere with patients’ attribution of cardiac symptoms as true cardiac symptoms, as most patients, especially if they received palliative treatments, may have symptoms that are attributed to their metastatic disease. It should be noted that this study was conducted with small sample size. The decrease in MACEs in patients receiving concurrent RT during ICI was based on univariate and multivariate (approaching significance) analyses. This may be due in part to insufficient data for a portion of the subjects leading to incomplete Framingham risk scoring, thus a proper multiple logistic regression model could not be performed. Our comparison of the patients who received concurrent RT+ICI vs. all those who did not means that the group that did not receive concurrent therapy was fairly heterogeneous. This would be one of the issues that multiple logistic regressions in a larger cohort could potentially address, but given the limitations of a retrospective review, we feel as though this was the best approach we could take with the current data that is available to us. A larger prospective cohort study is planned to further investigate the potential cardioprotective effect of thoracic RT given during ICI.
This retrospective review does not show an increase in cardiac events with thoracic radiation given concurrently with ICI. In our limited study, as compared to those who received non-concurrent RT, patients with concurrent RT and ICI had a significantly lower rate of cardiac events in our cohort. This may suggest a potential cardioprotective effect of thoracic RT given during ICI, although this needs to be validated in a larger prospective study with a strong statistical design, and the underlying molecular mechanisms of this effect needs to be explored using basic models. If this possible cardioprotective effect is rigorously validated, then this might influence the findings of future clinical trials.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
CS wrote the manuscript and submitted the data. MM helped obtain and verify the cardiac toxicity data. AN and PW obtained and verified the immunotherapy data. AJ and MP obtained and verified the radiotherapy data and helped with the data analysis. All authors contributed to the article and approved the submitted version.
Stipend was provided for the first author of the manuscript for the work of this project from Brody School of Medicine for Summer Scholars Research Program 2020.
We would like to thank Vidant Radiation Oncology for cooperation with the study as an organization.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ICI, Immune checkpoint inhibitors; CTLA-4, cytotoxic T-lymphocyte associated protein 4; PD-1, programmed cell death protein-1; PD-L1, programmed cell death ligand-1b; NSCLC, non-small-cell lung cancer; RT, radiation therapy; irAE, immune checkpoint inhibitor-related adverse events; SCLC, small-cell lung cancer; iRC, ICI-related cardiotoxicities; NSTEMI, non-ST elevated myocardial infarction; SVT, supraventricular tachycardias; AF, atrial fibrillation; CTCAE, common terminology for clinical adverse events; MACE, major adverse cardiac events; PFS, progression-free survival; OS, overall survival; SBRT, stereotactic body radiation therapy; CRT, conventional radiation therapy.
1. Ganatra S, Parikh R, Neilan TG. Cardiotoxicity of immune therapy. Cardiol Clinics (2019) 37(4):385–97. doi: 10.1016/j.ccl.2019.07.008
2. Modi C, Berim L, Isserow L, Malhotra J, Patel M, Langenfeld J, et al. Combining radiation therapy and immunotherapy for lung cancers: A narrative review. Shanghai Chest (2021) 5:10–0. doi: 10.21037/shc-20-66
3. Cushman TR, Gomez D, Kumar R, Likacheva A, Chang JY, Cadena AP, et al. Combining radiation plus immunotherapy to improve systemic immune response. J Thorac Dis (2018) 10(S3):S468–79. doi: 10.21037/jtd.2018.01.130
4. Vanpouille-Box C, Diamond JM, Pilones KA, Zavadil J, Babb JS, Formenti SC, et al. TGFβ is a master regulator of radiation therapy-induced antitumor immunity. Cancer Res (2015) 75(11):2232–42. doi: 10.1158/0008-5472.CAN-14-3511
5. Jabbour SK, Berman AT, Decker RH, Lin Y, Feigenberg SJ, Gettinger SN, et al. Phase 1 trial of pembrolizumab administered concurrently with chemoradiotherapy for locally advanced non–small cell lung cancer: A nonrandomized controlled trial. JAMA Oncol (2020) 6(6):848. doi: 10.1001/jamaoncol.2019.6731
6. Du S, Zhou L, Alexander GS, Park K, Yang L, Wang N, et al. PD-1 modulates radiation-induced cardiac toxicity through cytotoxic T lymphocytes. J Thorac Oncol (2018) 13(4):510–20. doi: 10.1016/j.jtho.2017.12.002
7. Arroyo-Hernández M, Maldonado F, Lozano-Ruiz F, Muñoz-Montaño W, Nuñez-Baez M, Arrieta O. Radiation-induced lung injury: current evidence. BMC Pulm Med (2021) 21(1):9. doi: 10.1186/s12890-020-01376-4
8. Patel RP, Parikh R, Gunturu KS, Tariq RZ, Dani SS, Ganatra S, et al. Cardiotoxicity of immune checkpoint inhibitors. Curr Oncol Rep (2021) 23(7):79. doi: 10.1007/s11912-021-01070-6
9. Moey MYY, Tomdio AN, McCallen JD, Vaughan LM, O’Brien K, Naqash AR, et al. Characterization of immune checkpoint inhibitor-related cardiotoxicity in lung cancer patients from a rural setting. JACC: CardioOncol (2020) 2(3):491–502. doi: 10.1016/j.jaccao.2020.07.005
10. D’Agostino RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: The framingham heart study. Circulation (2008) 117(6):743–53. doi: 10.1161/CIRCULATIONAHA.107.699579
11. Levy A, Massard C, Soria JC, Deutsch E. Concurrent irradiation with the anti-programmed cell death ligand-1 immune checkpoint blocker durvalumab: Single centre subset analysis from a phase 1/2 trial. Eur J Cancer (2016) 68:156–62. doi: 10.1016/j.ejca.2016.09.013
12. Zhang TW, Snir J, Boldt RG, Rodrigues GB, Louie AV, Gaede S, et al. Is the importance of heart dose overstated in the treatment of non-small cell lung cancer? a systematic review of the literature. Int J Radiat Oncol Biol Phys (2019) 104(3):582–9. doi: 10.1016/j.ijrobp.2018.12.044
13. Wang K, Pearlstein KA, Patchett ND, Deal AM, Mavroidis P, Jensen BC, et al. Heart dosimetric analysis of three types of cardiac toxicity in patients treated on dose-escalation trials for stage III non-small-cell lung cancer. Radiother Oncol (2017) 125(2):293–300. doi: 10.1016/j.radonc.2017.10.001
14. Atkins KM, Rawal B, Chaunzwa TL, Lamba N, Bitterman DS, Williams CL, et al. Cardiac radiation dose, cardiac disease, and mortality in patients with lung cancer. J Am Coll Cardiol (2019) 73(23):2976–87. doi: 10.1016/j.jacc.2019.03.500
15. Dess RT, Sun Y, Matuszak MM, Sun G, Soni PD, Bazzi L, et al. Cardiac events after radiation therapy: Combined analysis of prospective multicenter trials for locally advanced non–Small-Cell lung cancer. JCO (2017) 35(13):1395–402. doi: 10.1200/JCO.2016.71.6142
16. Wan S, Pestka S, Jubin RG, Lyu YL, Tsai Y-C, Liu LF. Chemotherapeutics and radiation stimulate MHC class I expression through elevated interferon-beta signaling in breast cancer cells. PloS One (2012) 7(3):e32542. doi: 10.1371/journal.pone.0032542
17. Schoenfeld JD, Giobbie-Hurder A, Ranasinghe S, Kao KZ, Lako A, Tsuji J, et al. Durvalumab plus tremelimumab alone or in combination with low-dose or hypofractionated radiotherapy in metastatic non-small-cell lung cancer refractory to previous PD(L)-1 therapy: an open-label, multicentre, randomised, phase 2 trial. Lancet Oncol (2022) 23(2):279–91. doi: 10.1016/ S1470-2045(21)00658-6
18. Venkatesulu BP, Mallick S, Lin SH, Krishnan S. A systematic review of the influence of radiation-induced lymphopenia on survival outcomes in solid tumors. Crit Rev Oncology/Hematol (2018) 123:42–51. doi: 10.1016/j.critrevonc.2018.01.003
19. Wild AT, Herman JM, Dholakia AS, Moningi S, Lu Y, Rosati LM, et al. Lymphocyte-sparing effect of stereotactic body radiation therapy in patients with unresectable pancreatic cancer. Int J Radiat OncologyBiologyPhys (2016) 94(3):571–9. doi: 10.1016/j.ijrobp.2015.11.026
20. Chen D, Verma V, Patel RR, Barsoumian HB, Cortez MA, Welsh JW. Absolute lymphocyte count predicts abscopal responses and outcomes in patients receiving combined immunotherapy and radiation therapy: Analysis of 3 phase 1/2 trials. Int J Radiat Oncol Biol Phys (2020) 108(1):196–203. doi: 10.1016/j.ijrobp.2020.01.032
21. Menon H, Chen D, Ramapriyan R, Verma V, Barsoumian HB, Cushman TR, et al. Influence of low-dose radiation on abscopal responses in patients receiving high-dose radiation and immunotherapy. J Immunother Cancer (2019) 7(1):237. doi: 10.1186/s40425-019-0718-6
Keywords: immune checkpoint inhibitors (ICI), immunotherapy, radiotherapy, cardiotoxicity, cardiac radiation dose
Citation: Son C, Moey MYY, Walker PR, Naqash AR, Peach MS and Ju AW (2023) Cardiac toxicity in patients with lung cancer receiving thoracic radiotherapy and immunotherapy. Front. Oncol. 12:1025455. doi: 10.3389/fonc.2022.1025455
Received: 22 August 2022; Accepted: 13 December 2022;
Published: 09 January 2023.
Edited by:
Etienne Giroux Leprieur, Hôpital Ambroise-Paré, FranceReviewed by:
Sam Makhoul, Central Arkansas Radiation Therapy Institute Foundation, United StatesCopyright © 2023 Son, Moey, Walker, Naqash, Peach and Ju. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrew W. Ju, anVhQGVjdS5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.