
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 28 November 2022
Sec. Thoracic Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.1024833
This article is part of the Research Topic Combination of Immunotherapy and Radiation Therapy for Non-small Cell Lung Cancer: Efficacy and Toxicity, Validated Biomarkers, and Mechanistic Studies View all 6 articles
Introduction: Radiotherapy (RT) is currently the main treatment for brain metastases (BMs) from non-small cell lung cancer (NSCLC). Due to the short survival time and obvious adverse reactions of RT, we urgently need more appropriate treatment. This network meta-analysis reviewed the efficacy and adverse effects of radiotherapy-based combination therapy for patients without targeted epidermal growth factor receptor (EGFR) mutations/anaplastic lymphoma kinase (ALK) gene rearrangement NSCLC BMs, to screen out the therapy with the best efficacy.
Methods: PubMed, Embase, Web of Science, and Cochrane Library were searched from the earliest publication date available to 1 April 2022. STATA15.0 was used to conduct heterogeneity analysis, sensitivity analysis, forest plot analysis, and publication bias analysis.
Results: A total of 28 studies, involving 3707 patients were included in the Bayesian network meta-analysis. In the limited paired meta-analysis for head-to-head comparative trials, compared with RT-based combination therapy, RT combined with Immune checkpoint inhibitors (ICIs) showed significant overall survival (OS) benefit (HR 0.65, 95%CI 0.47–0.9, p<0.01), RT combined with ICIs showed a non-significant difference for intracranial progression-free survival (iPFS) (HR 0.76, 95%CI 0.27–2.27, p<0.01) and progression-free survival (PFS) (HR 0.9, 95%CI 0.36–2.37, p<0.01). In addition, according to the ranking results, compared with RT combined with chemotherapy(CT) or with targeted therapy(TT), RT combined with ICIs might be the best treatment mode for OS(ICIs+RT vs CT+RT vs TT+RT; 91.9% vs. 27.8% vs. 29.3%, iPFS (ICIs+RT vs CT+RT vs TT+RT, 46.9% vs 25.2% vs 25.6%) and PFS (ICIs+RT vs CT+RT vs TT+RT, 36.2% vs 31% vs 36.5%).
Conclusions: RT combined with ICIs might be the best treatment mode to prolong the OS for BMs from NSCLC with non-EGFR mutation/ALK gene rearrangement.
Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022350065, identifier (CRD42022350065)
Brain metastases (BMs) are a common complication of non-small cell lung cancer (NSCLC) with a poor prognosis. According to relevant research statistics, at the time of initial diagnosis, the incidence of BMs in patients with NSCLC is about 12.8%, and this proportion might rise to 25.6% in patients with advanced NSCLC. The median survival of patients with NSCLC is only 7 months (1). Current treatments for BMs typically include surgery (in selected cases for tissue diagnosis, brain decompression, and prolongation of survival), radiation therapy alone, and/or some combinations of systemic drug treatments. Radiotherapy (RT) is still the standard treatment for patients with BMs from NSCLC. However, due to the limitation of radiotherapy, the median survival time is not optimistic, and the median survival time of RPA(recursive partitioning analysis) grade III patients is only 2.3 months (2). Either whole brain radiotherapy (WBRT) or stereotactic radiosurgery (SRS) has certain limitations and adverse effects (3, 4). Therefore, there is an urgent need for optimal treatment for patients with BMs from NSCLC.
In recent years, advances in genomics have led to the development of targeted therapies for NSCLC with specific mutations. Targeted drugs represented by EGFR-TKI significantly improve the survival and prognosis of lung adenocarcinoma (5). However, in patients with advanced lung squamous cell carcinoma, the incidence of EGFR mutation and ALK gene rearrangement is only 2.7% and 1.5-2.5% (6). The benefit of TKI-targeted drug therapy is very limited, which makes it more urgent to explore the ideal treatment plan for patients with wild-type NSCLC. At present, ICIs have achieved certain safety and efficacy in the treatment of patients with wild-type NSCLC. Due to the existence of the “blood-brain barrier”, the role of anti-tumor drugs is generally ignored. Although lymphocytes in the ICIs setting of the normal brain parenchyma and primary central nervous system (CNS) tumors are rare, tumor-infiltrating lymphocytes (TILs) are prominent in BMs. Besides, the density of TILs correlates with PFS and OS in solid tumors, so the consistency of higher TILs density and improved OS supports the use of ICIs for the treatment of systemic and central metastatic disease (7). Several clinical trials have achieved encouraging results. CHECKMATE017 and CHECKMATE057showed that some patients with BMs have significantly improved OS with nivolumab (8).
There are data to suggest that the combination of ICIs and RT may further improve the status of patients with BMs. Many mechanisms have been used to explain this combined effect, such as the indirect modulation of radiation for the expression levels of immune checkpoint on the surface of cancer cells and immune cells in the tumor microenvironment through interferon-γ. A recent study showed that radiation-induced DNA double-strand breaks upregulate Programmed cell death ligand protein-1(PD-L1) expression on tumor cells via ATM/AR/Chk1 kinases (9). Abdulhaleem et al. published a series of studies about patients with BMs from NSCLC. If these patients were treated with ICIs and SRS, their median survival was 40 months, and if they were treated with SRS alone, their median survival was 8 months. Therefore, RT combined with ICIs may be a favorable treatment option for patients with BMs. However, there is currently no large-sample randomized controlled trial data on ICIs combined with RT, and there is still some controversy. In addition, chemotherapy has been reported to benefit patients with BMs by simultaneously treating both primary cancer and BMs. Studies have shown that compared with WBRT alone, temozolomide (TMZ) combined with WBRT in the treatment of patients with BMs from NSCLC has a higher effective rate and longer progression-free survival (10). But other chemotherapeutic drugs generally were not with the ability to cross the blood-brain barrier and reach the targeted lesion. Therefore, there is a certain controversy in the treatment of patients with BMs by chemotherapy.
In conclusion, although RT is the most important treatment for patients with BMs from NSCLC, it is necessary to explore the RT-based combination therapy to prolong the survival of patients, especially for patients without targeted epidermal growth factor receptor (EGFR) mutations/anaplastic lymphoma kinase (ALK) rearrangement. At present, a large number of studies on RT-based combination therapy (such as chemotherapy, Immune checkpoint inhibitors, etc.) are ongoing. Some of the research results have been published, but there is still a lack of head-to-head direct comparison of the efficacy and safety of different combination therapy regimens. Based on data from randomized controlled trials and retrospective cohort studies, this study compared comprehensively and quantitatively the efficacy of RT-based combination therapy in the treatment of BMs from NSCLC with non-EGFR mutation/ALK gene rearrangement. Our use of a Bayesian network meta-analysis allows comparisons between treatments that have never been evaluated in existing trials, and provides new insights into the relative efficacy and established quality advantages.
This study was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA 2020) (11). Besides, this study was registered in PROSPERO (CRD:42022350065).
We conducted a computerized search of PubMed, Web of Science, the Cochrane Library, and Embase, the search strategy strictly followed the Population Intervention Comparative Outcomes Study (PICOS) design framework, including the following fields of Medical Subject Heading (MeSH) terms: “NSCLC” and “RT”,MeSH and subtitles were combined with “AND” or “OR”. The language type of the included studies is English. We included studies about RT-based combination therapy for BMs from NSCLC from May 28, 2002, to February 21, 2022, i.e. articles. No articles in the databases before May 28, 2002, met the inclusion criteria. In addition, we manually searched for relevant reviews and articles with included trials for additional references. Search terms related to “brain metastases”, “ICIs”, “targeted therapy”, “RT” and “chemotherapy” were included. The full set of search terms and strategies for each database were showed in Supplementary Table S1.
References meeting the following criteria were included, Firstly, patients with BMs from NSCLC (in our analysis, “mutation agnostic” studies were defined as all patients with NSCLC, regardless of target mutation status. “Wild-type” studies are those that explicitly include only wild-type (no EGFR mutation/ALK rearrangement) primary patients with NSCLC. Then, comparing at least two independent treatment regimens for BMs from NSCLC; And reporting sufficient information to calculate hazard ratios (HR). References were excluded based on the following criteria: 1.Patients with definite driver gene EGFR/ALK-positive; 2.Letters and abstracts; 3.Single-arm studies; 4.Non-English literature.
We excluded review articles, case series, case reports, guidelines, and conference abstracts; full-text studies that met the inclusion criteria were thoroughly reviewed. Two researchers (WM, JJ) independently reviewed the full text and extracted the study type, sample size, median age of patients, percentage of male/female, treatment plan and specific interventions (including specific methods and doses of radiotherapy), median follow-up time, outcome measures (OS, PFS, iPFS, grade 3/4 adverse events), medians of OS, iPFS and PFS, and the median number of BMs to an electronic database. Any differences among researchers were resolved through discussion and consensus. The risk of bias was assessed by tools from the Cochrane Collaboration (11), and other trials were assessed by Risk If Bias in non-randomized intervention studies (Robins-I) (12).
Our study endpoints were intracranial progression-free survival (iPFS), overall survival (OS), overall progression-free survival (PFS), and grade 3/4 adverse events. iPFS is generally considered to be the median survival time without radiographic intracranial progression or death from any cause (13). Because the number of analyzable co-adverse events from grade 3/4 adverse events was insufficient for statistical analysis, we analyzed only OS, PFS, and iPFS separately, and reported each outcome in the appropriate network. quantitatively only studies reporting comparisons of hazard ratios between interventions were used in the analysis, all other studies were reported qualitatively. Results of OS in analysis were expressed as a HR with a confidence interval (CI) of 95%. P<0.05 was considered a significant level. Heterogeneity was assessed with the I2 statistic. I2 values less than 25% and greater than 50% were considered to be low and high heterogeneity, respectively.
Only studies reporting comparisons of HR between interventions were used in our quantitative analysis, all other studies reported qualitatively. Results of OS in Bayesian network meta-analysis were expressed as a HR with a confidence interval (CI) of 95%. P<0.05 was considered a significant level. Heterogeneity was assessed with the I2 statistic. I2 values less than 25% and greater than 50% were considered to be low and high heterogeneity, respectively (14). When included studies did not report HRs, we estimated them from summary statistics using the method described by Tierney et al. in 2007 (12). We used Getdata Graph Digiamer2.26 (http://www.getdata-graphdigitizer.com) to digitize the Kaplan-Meier curve. We used GeMTC version 0.14.3 (http://drugis.org/software/addis1/gemtc) and employed a random response model for Bayesian network meta-analysis. The parameters of the GeMTC software were chosen as tuning iterations, 20,000; simulation iterations, 50,000. We ranked outcome of the five treatments (RT alone, ICIs alone, RT combined with chemotherapy, RT combined with ICIs, RT combined with targeted therapy) from the best (rank 1) to the worst (rank 5) using the ranking probabilities calculated by the network-consistent model. The rank probability distribution of each treatment was plotted in a histogram.
The histograms showed the ranking probability distribution of each treatment at each possible position. We evaluated the convergence of the model using the potential scale reduction factor (PSRF) of the Brooks-Gelman-Rubin method (13). The closer the PSRF is to 1, the better the convergence of the model. We converted the data format and used STATASE15 software to draw network diagrams and funnel plots to determine whether there was publication bias. As the network diagram did not form a closed loop, the node splitting method was not examined.
We identified 11,179 studies by searching the databases (Figure 1). Duplications were removed, and 7,141 papers were for the title and abstract screening. After excluding studies, such as conference abstracts, non-English papers, and non-related interventions, 28 papers, including 12 randomized controlled trials, were finally included in the Bayesian network meta-analysis. A total of 3703 patients received at least one of the five treatment strategies (Table 1) (15–42).
All eligible studies were published from 2002 to 2022. We used the Cochrane Collaboration tool and Risk If Bias in a non-randomized intervention study (Robins-I) for quality assessment. the results of the quality assessment were shown in Figure 2A and Table 2. Figure 2A | The reviewers judged the risk of bias for each included study, and 6 of the 12 studies were open-label trials (35, 38–42), without blinding in study design. 6 studies recruited less than expected (32, 34, 35, 39–41), 9 studies did not mention random sequence generation (32, 33, 35, 37–42), 3 studies did not mention study blinding design (31–33), and other aspects were assessed as high quality. Table 2 | Of the cohort studies, 12 studies did not specify whether subjects had developed the focused disease (15, 16, 18, 20, 23, 24, 26–30, 36), and were rated as high risk, 25 studies only mentioned part follow-up related data (15–21, 23, 25–30), which is not enough to judge the completeness of the follow-up data, and were rated as high risk, and the rest included studies were low risk. We rated articles with a score of ≥ 6 as high quality, and all included studies were high quality.

Figure 2 (A) risk of bias summary, green represents low risk of bias, yellow represents unclear risk of bias, red represents high risk of bias; (B) network.
Our study compared five interventions: RT combined with ICIs, RT combined with targeted therapy, RT combined with chemotherapy, RT alone, and ICIs alone. The network was shown in Figure 2B. The thickness of each line in the network diagram is proportional to the number of comparisons. Based on DIC values, random-effects models were applied to the PSA-PFS, time to SSE, and OS in the Gleason Score ≥8 subgroups analysis; fixed-effects models were applied to other comparisons.
A total of 28 studies were included in the OS analysis, and ICIs+RT (HR=0.65, 95% confidence interval: 0.47-0.9) had a survival benefit over CT+RT; ICIs+RT (HR=0.66, 95% confidence interval: 0.51-0.85) had a survival benefit over RT alone; ICIs+RT (HR=0.67, 95% confidence interval: 0.46-0.96) had a survival benefit over TT+RT alone. The other interventions were not statistically significant. ICIs+RT was the most effective combination regimen (92%), while the possibility of TT+RT (29%) was the lowest. The pooled HR for OS were shown in Figure 3A-E and the detailed ranking results were shown in Figure 4A.
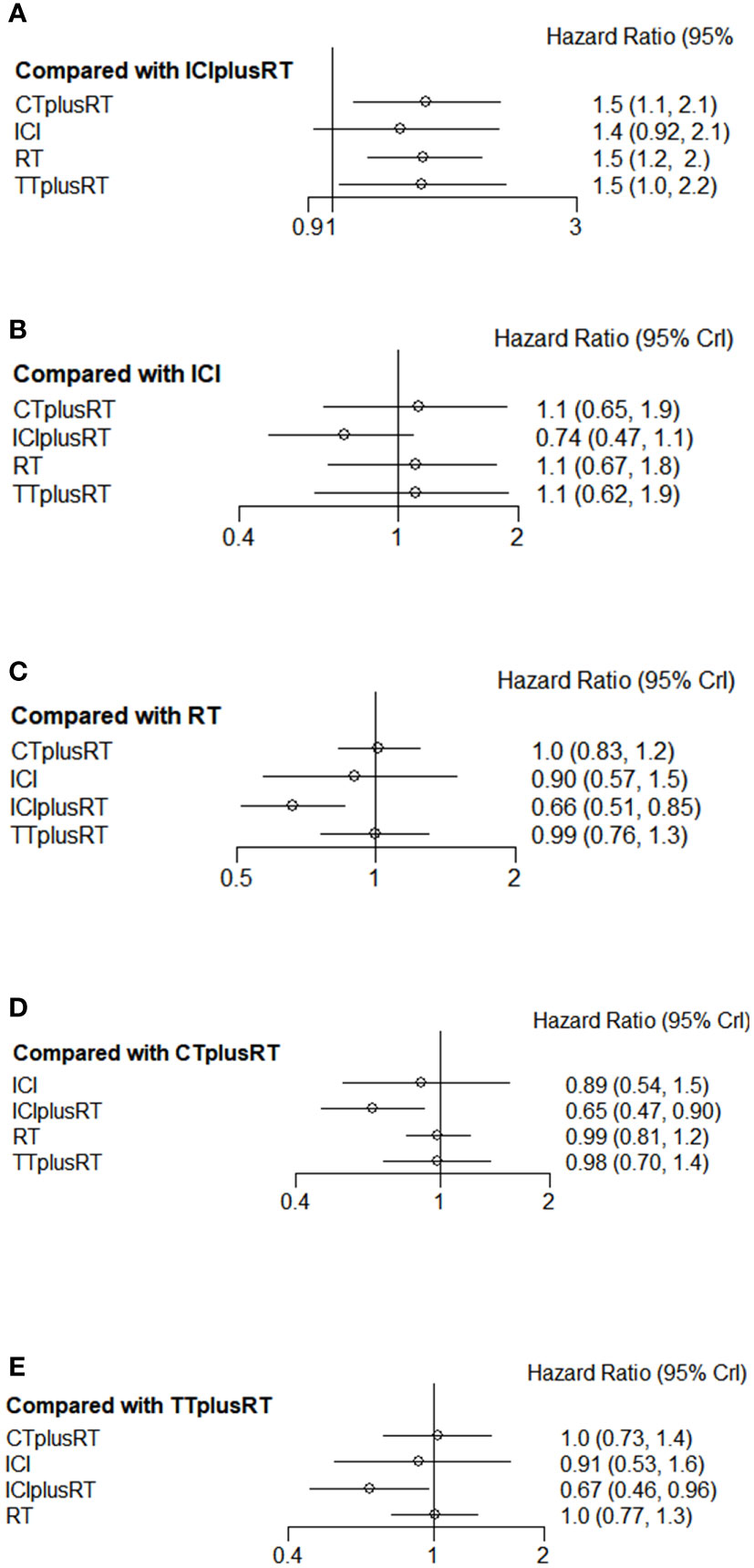
Figure 3 Forest plots of multivariable interventions for OS. (A) Compared with Immune checkpoint inhibitor combined with radiotherapy (ICIplusRT); (B) Compared with Immune checkpoint inhibitor (ICI); (C) Compared with radiotherapy (RT); (D) Compared with chemotherapy combined with radiotherapy(CTplusRT); (E) Compared with targeted therapy combined with radiotherapy (TTplusRT).
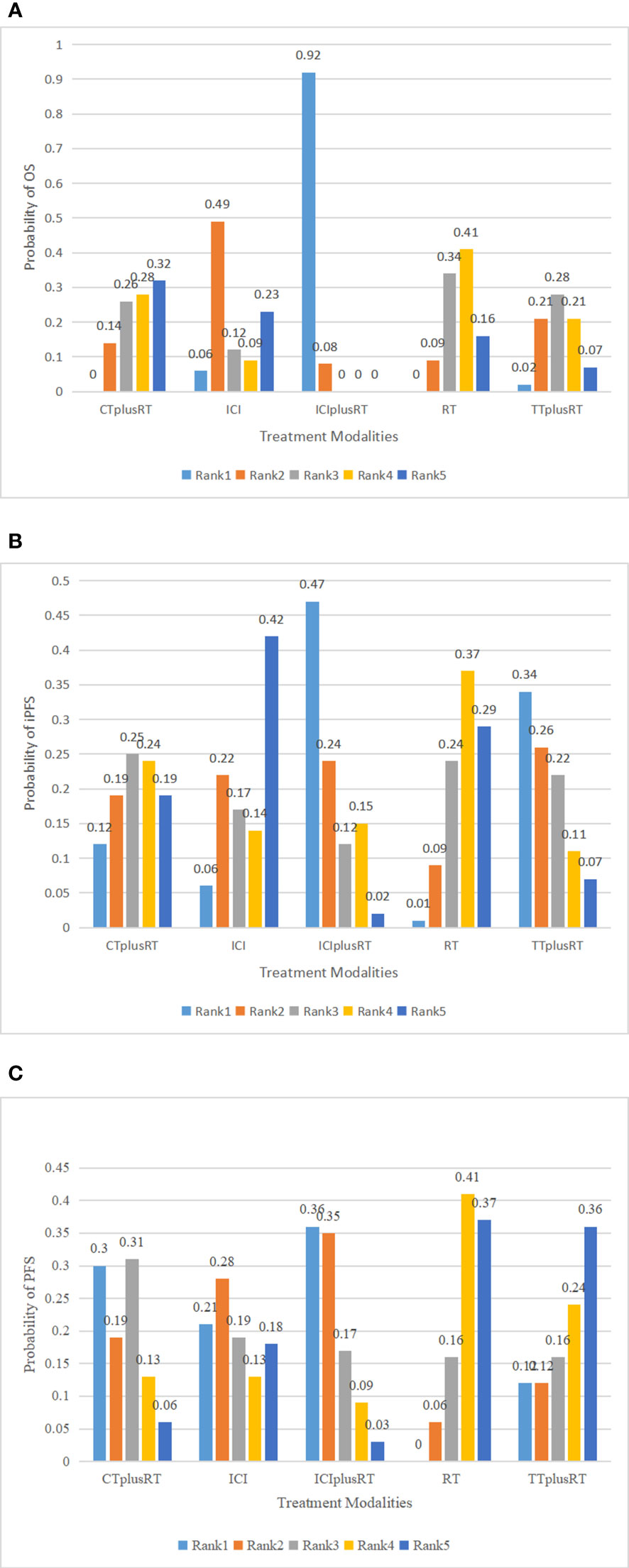
Figure 4 Ranking of treatments in terms of overall survival (OS), progression-free survival (PFS), and intracranial progression-free survival (iPFS). Abbreviations: CT+RT, chemotherapy combined with radiotherapy; ICI, Immune checkpoint inhibitor; ICI+RT, Immune checkpoint inhibitor combined with radiotherapy; RT, radiotherapy; TT+RT, targeted therapy combined with radiotherapy; (A) ranking of treatments to OS; (B) ranking of treatments to iPFS; (C) ranking of treatments to PFS.
Ten studies were included in the iPFS analysis, and there was no statistical significance in the indirect pairwise comparison of the five treatments. In the ranking, ICIs+RT was the most effective combination treatment (45.3%), while ICIs (43.8%) ranked last. The pooled HR for iPFS were shown in Figure 5A-E, and the detailed ranking results were shown in Figure 4B.
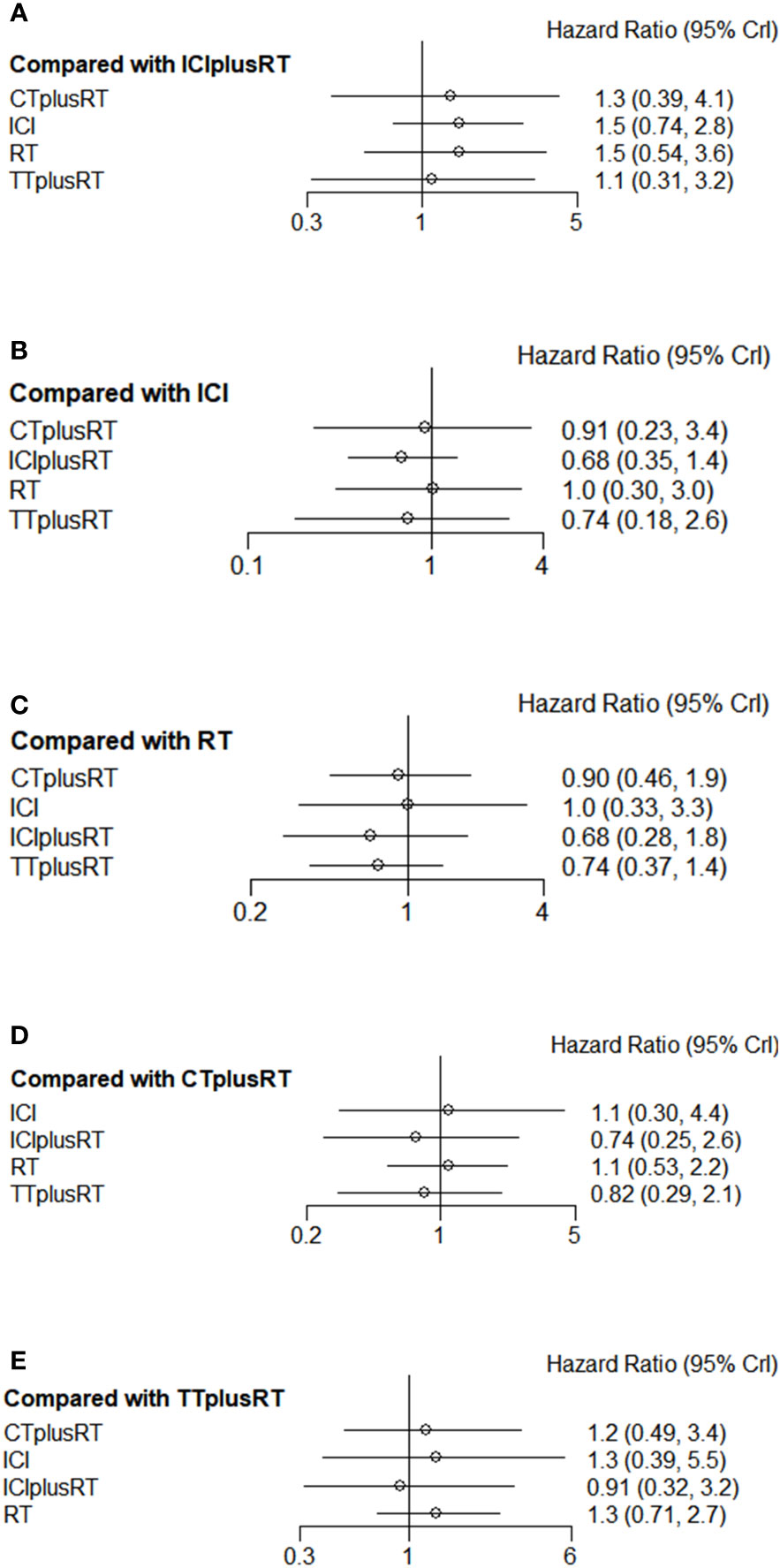
Figure 5 Forest plots of multivariable interventions for iPFS. (A) Compared with Immune checkpoint inhibitor combined with radiotherapy (ICIplusRT); (B) Compared with Immune checkpoint inhibitor (ICI); (C) Compared with radiotherapy (RT); (D) Compared with chemotherapy combined with radiotherapy(CTplusRT); (E) Compared with targeted therapy combined with radiotherapy (TTplusRT).
Twelve studies were included in the PFS analysis, and there was no statistical significance in the indirect pairwise comparison of the 5 treatments. In the ranking, ICIs+RT was the most effective combination regimen (36%), while TT+RT (36.1%) ranked last. The pooled HR for PFS were shown in Figure 6A-E, and the detailed ranking results were shown in Figure 4C.
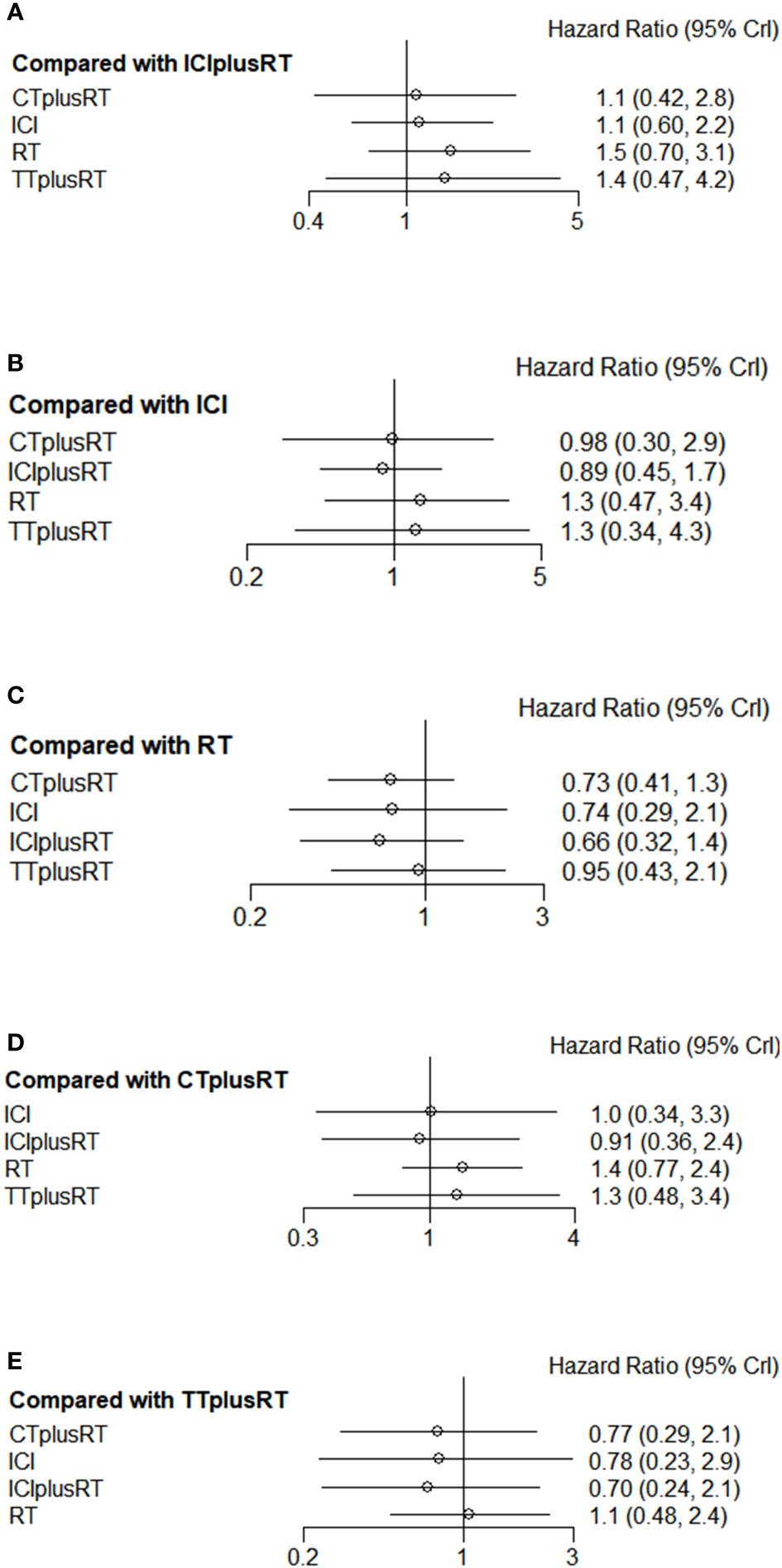
Figure 6 Forest plots of multivariable interventions for PFS. (A)Compared with Immune checkpoint inhibitor combined with radiotherapy (ICIplusRT); (B) Compared with Immune checkpoint inhibitor (ICI); (C) Compared with radiotherapy (RT); (D) Compared with chemotherapy combined with radiotherapy(CTplusRT); (E) Compared with targeted therapy combined with radiotherapy (TTplusRT).
All included studies reported adverse effects. 10 studies reported no 3/4 grade or higher adverse effects (15, 20, 22–27), and the remaining 18 studies reported 839 adverse events, The ICIs combined RT intervention accounted for 65 cases. The reported adverse effects involved different systems and symptoms. The most common adverse effects were on the gastrointestinal tract and CNS. Details of the reported safety concerns were provided in Table 3.
The potential scaling factor was limited to 1, reflecting the good convergence of this study. The funnel plots of included trials were nearly symmetrical, suggesting no apparent publication bias. Considering that there were no closed loops in the network graph, inconsistency evaluation did not apply to our study. The OS heterogeneity analysis of the entire network showed that the value of RT alone versus ICIs combined with RT was 70.1%, and the value of ICIs alone versus ICIs combined with RT was 79.7%. There was high heterogeneity. This may be related to the inclusion of patients with different pathological tumor types than NSCLC. The results of convergence, inconsistency, publication bias, and heterogeneity can be found in the Supplementary Figures.
We conducted a Bayesian network meta-analysis of the efficacy of RT-based combination therapy for BMs from NSCLC with non-EGFR mutation/ALK gene rearrangement. The result showed that, compared with RT, RT combined with chemotherapy and RT combined with target therapy, ICIs combined with RT had a significant OS benefit, regardless of whether OS was counted from the date of diagnosis of BMs or the date of RT. In terms of iPFS and PFS, ICIs combined with RT was also the most effective treatment option, with moderate to high certainty. There were no significant differences in grade 3/4 adverse effects between the ICIs combined with the RT group and the other treatment groups, indicating that ICIs combined with RT was tolerable.
In the era of immunotherapy, the anti-PD-1 antibody Pembrolizumab has been approved as a first-line treatment for PD-L1-positive advanced NSCLC (43), and related mechanisms also support the efficacy of ICIs in patients with BMs from NSCLC. After immunotherapy, the vascular permeability of lymphocytes increases, and a large number of activated T lymphocytes derived from the primary tumor and deep external cervical lymph node tissue penetrate the blood-brain barrier to exert intracranial antitumor activity (44).
In the study by Teixeira et al. (45), comparing ICIs alone with ICIs+RT, no intracranial disease control rate (iDCR) and objective response rate (iORR) were observed in patients with BMs who received RT before the initiation of ICIs. There was a statistical difference between patients with BMs who received RT before ICIs and those who received ICIs alone. Considering radiation necrosis, ICIs alone should be considered the first-choice treatment for patients with active NSCLC with BMs. The above is inconsistent with our conclusions. There may be the following reasons. First, in terms of the sample size of the included population, they only included 566 people, which is much smaller than ours. Then, there is no restriction on the sequence of RT combined ICIs in our study. And the main outcome of our meta-analysis was OS, iPFS, and PFS, while Teixeira’s study did not perform statistical analysis from survival indicators due to not enough data. Finally, the two articles included patients with inconsistent brain metastases, and our study included a population with stable BMs at baseline. In the Keynote-042 study (46), pembrolizumab worked only in patients with untreated or brain metastases 5 to 20 mm in diameter. In addition, the use of ICIs alone in the treatment of patients with BMs from NSCLC is controversial. We analyzed ICIs combined with RT in the treatment of patients with BMs and found that the improvement in OS may be largely due to RT can promote the anti-tumor efficacy (44) of ICIs by inducing T lymphocytes to release tumor antigens and activate antigens. In the study by Kim et al. (47), the local response rate (ORR) of ICIs combined with RT was superior to ICIs monotherapy. There was no difference in the incidence of grade 3/4 CNS related adverse events (5% vs 4%; p=0.93). Compared with ICIs monotherapy, patients treated with the combination of ICIs and RT had better overall survival and intracranial progression-free survival. In addition, in the study by Yang et al. (48), the overall survival (OS) of brain RT combined with ICIs was significantly better than that of brain RT alone compared with the brain RT alone group. In the treatment of patients with NSCLC BMs, RT combined with ICIs has better efficacy. From the studies we included, it can be seen that when combined therapy is given, radiotherapy is mostly SRS and WBRT, with a few studies using stereotactic radiosurgery (SRT) and gamma knife surgery (GKS). The ICIs involved in the studies mainly include Nivolumab, Pembrolizumab, Atezolizumab, Durvalumab, etc. The available evidence suggests that simultaneous combination of ICIs with SRS, Kotecha et al. (49) enrolled 150 BM patients and found that the group receiving SRS in combination with ICIs had a higher objective remission rate than the SRS group alone, and a subgroup analysis concluded that the combination was most effective within one ICIs half-life before and after SRS, so many studies have defined synchronous treatment as receiving RT within one month before and after ICIs, Although there is a lack of prospective high-quality evidence on the optimal timing of radiotherapy combined with immunotherapy and the specific dose of the combination, the available evidence suggests that the combination of ICIs with RT for brain metastases may improve efficacy and survival without a significant increase in radiotherapy-related toxicity, and that patients with non-EGFR mutated/ALK rearranged non-small cell lung cancer BMs with indications for intracranial radiotherapy may be treated with a combination of ICIs and radiotherapy preferably with SRS. No reduction in radiotherapy dose is recommended without clear evidence.
In our study, RT combination chemotherapy in improving OS for patients with BMs from NSCLC with non-EGFR mutation/ALK gene rearrangement was inferior to RT combined with ICIs, although concurrent chemoradiotherapy is currently the first-line guideline for the treatment of such patients (43). The conclusion emphasizes the concept of patients dying of systemic disease (refers to a disease in which multiple systems of the body are involved) and the importance of maintaining cognition for as long as possible time. However, despite the efficacy of ICIs combined with RT could prolong overall survival, it still lacks iPFS, PFS benefit because our study did not specifically differentiate between RT modality. After all, the local control of BMs is mainly achieved through brain RT (48).
Among the grade 3/4 adverse effects, because there is not enough data to support statistical analysis, the grade 3/4 adverse events involved in 28 works of literature were summarized, as shown in Table 3, and no evidence was found. The significant differences between the RT combined with the ICIs group and the other treatment groups further confirm the reliability of our conclusions. In the meta-analysis by Sha et al. (50), which included 51 studies (n=15,398), 35 ICIs alone (n=13,956) and 16 ICIs+RT studies (n=1,442). Results showed that grade 3-4 adverse events were similar in patients receiving ICIs plus RT and ICIs alone. The above indicated that the safety of ICIs combined with RT therapy for patients with BMs from NSCLC is acceptable.
Our meta-analysis has some limitations. First, the studies in this meta-analysis included retrospective cohort studies and randomized controlled studies, and there was bias between treatment groups. Second, the included studies had a large period, RT and ICIs, chemotherapy, and targeted drug types are confounding factors, and this deficiency may have affected the pooled effect size of the data. Finally, the sample size of the included studies was not large enough for subgroup analysis, and the median number of BMs was not high. This also causes certain deviations in judging the efficacy of drugs.
However, in the absence of published articles from prospective randomized controlled trials, there is a lack of convincing evidence to support the efficacy of ICIs combined with RT in patients with BMs from NSCLC with non-EGFR mutation/ALK gene rearrangement. Our analysis is urgently needed to provide a rationale for the design of randomized controlled trials, as well as applications to guide clinical practice.
Several ongoing trials (NCT03391869, NCT04889066, NCT04787185) investigate more detailed information, including timing and sequence of combination therapy and optimal dosing, and these further studies may provide insights into the establishment of new NSCLC brain metastases in specific settings.
In addition, the three major clinical studies of ICIs combined with chemotherapy, Keynote021 (51), Keynote189 (52), and Keynote407 (53), all included patients with stable baseline BMs. Compared with chemotherapy alone, ICIs combined with chemotherapy had significant advantages in OS, PFS, ORR, etc., and the incidence of related adverse events was not significantly different from the chemotherapy group. The enhanced intracranial efficacy of this combination therapy against BMs may depend on the penetration of the blood-brain barrier by a large number of chemotherapeutic drugs, and these cytotoxic drugs induce an active ICIs microenvironment to maximize the efficacy of ICIs (44). Our meta-analysis did not include the group of ICIs combined with chemotherapy, because there are fewer related studies were comparing ICIs combined with chemotherapy and RT in our search scope. And the data from Keynote021, Keynote189 and Keynote407 could not be used in this Bayesian network meta-analysis, because they were only included in the chemotherapy combined with immunotherapy and chemotherapy alone groups and did not share a common association with our data, such as a control group of the same type or an experimental group, Future research should focus on evaluating the efficacy of ICIs combined with RT and ICIs combined with chemotherapy sex, and direct non-inferior face-to-face comparisons.
In conclusion, according to the comprehensive evaluation of Bayesian network meta-analysis, compared with chemotherapy combined with RT and RT alone, ICIs combined with RT significantly improved the OS of patients with BMs from NSCLC, and the grade 3/4 adverse reactions were acceptable. More clinical data will be needed to further determine the long-term efficacy of ICIs combined with RT.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Conceptualization: MW. Methodology: RC, XZ. Supervision: JC. Writing-original draft: MW. Writing-review and editing: JJ, QC. All authors contributed to the article and approved the submitted version.
This work was supported by grants from the Science and Technology Agency of Qinghai Province (Grant No. 2022-ZJ-719), Qinghai Health Committee (Grant No. 2020-wjzd-03).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1024833/full#supplementary-material
Supplementary Table 1 | Search strategy
1. Cagney DN, Martin AM, Catalano PJ, Redig AJ, Lin NU, Lee EQ, et al. Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: a population-based study. Neuro Oncol (2017) 19(11):1511–21. doi: 10.1093/neuonc/nox077
2. Suh JH, Kotecha R, Chao ST, Ahluwalia MS, Sahgal A, Chang EL. Current approaches to the management of brain metastases. Nat Rev Clin Oncol (2020) 17(5):279–99. doi: 10.1038/s41571-019-0320-3
3. Redmond AJ, Diluna ML, Hebert R, Moliterno JA, Desai R, Knisely JP, et al. Gamma knife surgery for the treatment of melanoma metastases: the effect of intratumoral hemorrhage on survival. J Neurosurg (2008) 109 Suppl:99–105. doi: 10.3171/JNS/2008/109/12/S16
4. Brown PD, Jaeckle K, Ballman KV, Farace E, Cerhan JH, Anderson SK, et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: A randomized clinical trial. Jama. (2016) 316(4):401–9. doi: 10.1001/jama.2016.9839
5. Soldera SV, Leighl NB. Update on the treatment of metastatic squamous non-small cell lung cancer in new era of personalized medicine. Front Oncol (2017) 7:50. doi: 10.3389/fonc.2017.00050
6. Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-Small-Cell lung cancer. N Engl J Med (2015) 373(2):123–35. doi: 10.1056/NEJMoa1504627
7. Bhalla N, Brooker R, Brada M. Combining immunotherapy and radiotherapy in lung cancer. J Thorac Dis (2018) 10(Suppl 13):S1447–s60. doi: 10.21037/jtd.2018.05.107
8. Liu Y, Dong Y, Kong L, Shi F, Zhu H, Yu J. Abscopal effect of radiotherapy combined with immune checkpoint inhibitors. J Hematol Oncol (2018) 11(1):104. doi: 10.1186/s13045-018-0647-8
9. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. Bmj. (2009) 339:b2700. doi: 10.1136/bmj.b2700
10. Brar K, Taslimi S, Ellenbogen Y, Deng J, Hou W, Moraes FY, et al. Comparative efficacy of treatments for brain metastases from non-small cell lung cancer without an EGFR-Mutation/ALK-Rearrangement: A systematic review and network meta-analysis. World Neurosurg (2022) 158:e87–e102. doi: 10.1016/j.wneu.2021.10.113
11. Mao L, Jian C, Changzhi L, Dan H, Suihua H, Wenyi T, et al. Cytochrome CYP2C19 polymorphism and risk of adverse clinical events in clopidogrel-treated patients: a meta-analysis based on 23,035 subjects. Arch Cardiovasc Dis (2013) 106(10):517–27. doi: 10.1016/j.acvd.2013.06.055
12. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. (2007) 8:16. doi: 10.1186/1745-6215-8-16
13. Yuan X, Liu WJ, Li B, Shen ZT, Shen JS, Zhu XX. A Bayesian network meta-analysis of whole brain radiotherapy and stereotactic radiotherapy for brain metastasis. Med (Baltimore). (2017) 96(34):e7698. doi: 10.1097/MD.0000000000007698
14. Demaria S, Santori FR, Ng B, Liebes L, Formenti SC, Vukmanovic S. Select forms of tumor cell apoptosis induce dendritic cell maturation. J Leukoc Biol (2005) 77(3):361–8. doi: 10.1189/jlb.0804478
15. Guo T, Chu L, Chu X, Yang X, Li Y, Zhou Y, et al. Brain metastases, patterns of intracranial progression, and the clinical value of upfront cranial radiotherapy in patients with metastatic non-small cell lung cancer treated with PD-1/PD-L1 inhibitors. Transl Lung Cancer Res (2022) 11(2):173–87. doi: 10.21037/tlcr-22-54
16. Abdulhaleem M, Johnston H, D’Agostino R, Lanier C, LeCompte M, Cramer CK, et al. Local control outcomes for combination of stereotactic radiosurgery and immunotherapy for non-small cell lung cancer brain metastases. J Neuro-Oncology. (2022) 157(1):101–7. doi: 10.1007/s11060-022-03951-7
17. Scoccianti S, Olmetto E, Pinzi V, Osti MF, Di Franco R, Caini S, et al. Immunotherapy in association with stereotactic radiotherapy for non-small cell lung cancer brain metastases: results from a multicentric retrospective study on behalf of AIRO. Neuro-Oncology. (2021) 23(10):1750–64. doi: 10.1093/neuonc/noab129
18. Samuel E, Lie G, Balasubramanian A, Hiong A, So Y, Voskoboynik M, et al. Impact of radiotherapy on the efficacy and toxicity of anti-PD-1 inhibitors in metastatic NSCLC. Clin Lung Cancer. (2021) 22(3):e425–e30. doi: 10.1016/j.cllc.2020.06.001
19. Metro G, Gili A, Signorelli D, De Toma A, Garaffa M, Galetta D, et al. Upfront pembrolizumab as an effective treatment start in patients with PD-L1 ≥ 50% non-oncogene addicted non-small cell lung cancer and asymptomatic brain metastases: an exploratory analysis. Clin Trans Oncol (2021) 23(9):1818–26. doi: 10.1007/s12094-021-02588-8
20. Lu L, Mei F, Peng X, Yi Lin W, Jun Y, Huang Y, et al. Stereotactic radiosurgery with whole brain radiotherapy combined with bevacizumab in the treatment of brain metastases from NSCLC. Int J Neurosci (2021) 1–8. doi: 10.1080/00207454.2021.1916490
21. Liao G, Qian Y, Arooj S, Zhao Z, Yan M, Li Z, et al. Radiation plus anti-PD-1 therapy for NSCLC brain metastases: A retrospective study. Front Oncol (2021) 11. doi: 10.3389/fonc.2021.742971
22. Lee MH, Cho KR, Choi JW, Kong DS, Seol HJ, Nam DH, et al. Immune checkpoint inhibitors for non-small-cell lung cancer with brain metastasis: The role of gamma knife radiosurgery. J Korean Neurosurgical Society. (2021) 64(2):271–81. doi: 10.3340/jkns.2020.0135
23. Khan M, Zhao Z, Li X, Liao G. Anti-PD1 therapy plus whole-brain radiation therapy may prolong PFS in selected non-small cell lung cancer patients with brain metastases: A retrospective study. Int J Gen Med (2021) 14:8903–18. doi: 10.2147/IJGM.S333890
24. He Z, Liu J, Ma Y, Jiang H, Cui Z, Wang G, et al. Anlotinib combined with cranial radiotherapy for non-small cell lung cancer patients with brain metastasis: A retrospectively, control study. Cancer Manage Res (2021) 13:6101–11. doi: 10.2147/CMAR.S319650
25. Enright TL, Witt JS, Burr AR, Yadav P, Leal T, Baschnagel AM. Combined immunotherapy and stereotactic radiotherapy improves neurologic outcomes in patients with non-small-cell lung cancer brain metastases. Clin Lung Cancer. (2021) 22(2):110–9. doi: 10.1016/j.cllc.2020.10.014
26. Guénolé M, Lucia F, Bourbonne V, Dissaux G, Reygagne E, Goasduff G, et al. Impact of concomitant systemic treatments on toxicity and intracerebral response after stereotactic radiotherapy for brain metastases. BMC Cancer. (2020) 20(1):991. doi: 10.1186/s12885-020-07491-z
27. Shepard MJ, Xu Z, Donahue J, Eluvathingal Muttikkal TJ, Cordeiro D, Hansen L, et al. Stereotactic radiosurgery with and without checkpoint inhibition for patients with metastatic non-small cell lung cancer to the brain: a matched cohort study. J Neurosurg (2019) 133:1–8. doi: 10.1093/neuros/nyz310_217
28. Lanier CM, Hughes R, Ahmed T, LeCompte M, Masters AH, Petty WJ, et al. Immunotherapy is associated with improved survival and decreased neurologic death after SRS for brain metastases from lung and melanoma primaries. Neurooncol Pract (2019) 6(5):402–9. doi: 10.1093/nop/npz004
29. Chen L, Douglass J, Kleinberg L, Ye X, Marciscano AE, Forde PM, et al. Concurrent immune checkpoint inhibitors and stereotactic radiosurgery for brain metastases in non-small cell lung cancer, melanoma, and renal cell carcinoma. Int J Radiat Oncol Biol Physics. (2018) 100(4):916–25. doi: 10.1016/j.ijrobp.2017.11.041
30. Deng X, Zheng Z, Lin B, Su H, Chen H, Fei S, et al. The efficacy and roles of combining temozolomide with whole brain radiotherapy in protection neurocognitive function and improvement quality of life of non-small-cell lung cancer patients with brain metastases. BMC Cancer (2017) 17:42. doi: 10.1186/s12885-016-3017-3
31. Chabot P, Hsia TC, Ryu JS, Gorbunova V, Belda-Iniesta C, Ball D, et al. Veliparib in combination with whole-brain radiation therapy for patients with brain metastases from non-small cell lung cancer: results of a randomized, global, placebo-controlled study. J Neuro-Oncology. (2017) 131(1):105–15. doi: 10.1007/s11060-016-2275-x
32. Lim SH, Lee JY, Lee MY, Kim HS, Lee J, Sun JM, et al. A randomized phase III trial of stereotactic radiosurgery (SRS) versus observation for patients with asymptomatic cerebral oligo-metastases in non-small-cell lung cancer. Ann Oncol (2015) 26(4):762–8. doi: 10.1093/annonc/mdu584
33. Lee S, Lewanski C, Counsell N, Ottensmeier C, Bates A, Patel N, et al. Randomized trial of erlotinib plus whole-brain radiotherapy for NSCLC patients with multiple brain metastases. J Natl Cancer Institute [Internet] (2014) 106(7):dju151. doi: 10.1002/central/CN-01071325/full
34. Sperduto PW, Wang M, Robins HI, Schell MC, Werner-Wasik M, Komaki R, et al. A phase 3 trial of whole brain radiation therapy and stereotactic radiosurgery alone versus WBRT and SRS with temozolomide or erlotinib for non-small cell lung cancer and 1 to 3 brain metastases: Radiation therapy oncology group 0320. Int J Radiat Oncol Biol Physics. (2013) 85(5):1312–8. doi: 10.1016/j.ijrobp.2012.11.042
35. Hassler MR, Pfeifer W, Knocke-Abulesz TH, Geissler K, Altorjai G, Dieckmann K, et al. Temozolomide added to whole brain radiotherapy in patients with multiple brain metastases of non-small-cell lung cancer: a multicentric Austrian phase II study. Wiener Klinische Wochenschrift. (2013) 125(15-16):481–6. doi: 10.1007/s00508-013-0402-7
36. Ge XH, Lin Q, Ren XC, Liu YE, Chen XJ, Wang DY, et al. Phase II clinical trial of whole-brain irradiation plus three-dimensional conformal boost with concurrent topotecan for brain metastases from lung cancer. Radiat Oncol (2013) 8:238. doi: 10.1186/1748-717X-8-238
37. Grønberg BH, Ciuleanu T, Fløtten Ø, Knuuttila A, Abel E, Langer SW, et al. A placebo-controlled, randomized phase II study of maintenance enzastaurin following whole brain radiation therapy in the treatment of brain metastases from lung cancer. Lung Cancer. (2012) 78(1):63–9. doi: 10.1016/j.lungcan.2012.07.007
38. Chua D, Krzakowski M, Chouaid C, Pallotta MG, Martinez JI, Gottfried M, et al. Whole-brain radiation therapy plus concomitant temozolomide for the treatment of brain metastases from non-Small-Cell lung cancer: A randomized, open-label phase II study. Clin Lung Cancer. (2010) 11(3):176–81. doi: 10.3816/CLC.2010.n.022
39. Neuhaus T, Ko Y, Muller RP, Grabenbauer GG, Hedde JP, Schueller H, et al. A phase III trial of topotecan and whole brain radiation therapy for patients with CNS-metastases due to lung cancer. Br J Cancer. (2009) 100(2):291–7. doi: 10.1038/sj.bjc.6604835
40. Verger E, Miguel G, Yaya R, Vinolas N, Villa S, Pujol T, et al. Temozolomide and concomitant whole brain radiotherapy in patients with brain metastases: A phase II randomized trial. Int J Radiat Oncol Biol Physics. (2005) 61(1):185–91. doi: 10.1016/j.ijrobp.2004.04.061
41. Guerrieri M, Wong K, Ryan G, Millward M, Quong G, Ball DL. A randomised phase III study of palliative radiation with concomitant carboplatin for brain metastases from non-small cell carcinoma of the lung. Lung Cancer. (2004) 46(1):107–11. doi: 10.1016/j.lungcan.2004.02.019
42. Antonadou D, Paraskevaidis M, Sarris G, Coliarakis N, Economou I, Karageorgis P, et al. Phase II randomized trial of temozolomide and concurrent radiotherapy in patients with brain metastases. J Clin Oncol (2002) 20(17):3644–50. doi: 10.1200/JCO.2002.04.140
43. Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A, et al. NCCN guidelines insights: Non-small cell lung cancer, version 2. 2021. J Natl Compr Canc Netw (2021) 19(3):254–66. doi: 10.6004/jnccn.2021.0013
44. Zhou S, Xie J, Huang Z, Deng L, Wu L, Yu J, et al. Anti-PD-(L)1 immunotherapy for brain metastases in non-small cell lung cancer: Mechanisms, advances, and challenges. Cancer Lett (2021) 502:166–79. doi: 10.1016/j.canlet.2020.12.043
45. Teixeira Loiola de Alencar V, Guedes Camandaroba MP, Pirolli R, Fogassa CAZ, Cordeiro de Lima VC. Immunotherapy as single treatment for patients with NSCLC with brain metastases: A systematic review and meta-analysis-the META-L-BRAIN study. J Thorac Oncol (2021) 16(8):1379–91. doi: 10.1016/j.jtho.2021.04.014
46. Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. (2019) 393(10183):1819–30. doi: 10.1016/S0140-6736(18)32409-7
47. Kim DY, Kim PH, Suh CH, Kim KW, Kim HS. Immune checkpoint inhibitors with or without radiotherapy in non-small cell lung cancer patients with brain metastases: A systematic review and meta-analysis. Diagnostics. (2020) 10(12):1098. doi: 10.3390/diagnostics10121098
48. Yang Y, Deng L, Yang Y, Zhang T, Wu Y, Wang L, et al. Efficacy and safety of combined brain radiotherapy and immunotherapy in non-Small-Cell lung cancer with brain metastases: A systematic review and meta-analysis. Clin Lung Cancer. (2022) 23(2):95–107. doi: 10.1016/j.cllc.2021.06.009
49. Kotecha R, Kim JM, Miller JA, Juloori A, Chao ST, Murphy ES, et al. The impact of sequencing PD-1/PD-L1 inhibitors and stereotactic radiosurgery for patients with brain metastasis. Neuro Oncol (2019) 21(8):1060–8. doi: 10.1093/neuonc/noz046
50. Sha CM, Lehrer EJ, Hwang C, Trifiletti DM, Mackley HB, Drabick JJ, et al. Toxicity in combination immune checkpoint inhibitor and radiation therapy: A systematic review and meta-analysis. Radiotherapy Oncol (2020) 151:141–8. doi: 10.1016/j.radonc.2020.07.035
51. Langer CJ, Gadgeel SM, Borghaei H, Papadimitrakopoulou VA, Patnaik A, Powell SF, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol (2016) 17(11):1497–508. doi: 10.1016/S1470-2045(16)30498-3
52. Gadgeel S, Rodríguez-Abreu D, Speranza G, Esteban E, Felip E, Dómine M, et al. Updated analysis from KEYNOTE-189: Pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non-Small-Cell lung cancer. J Clin Oncol (2020) 38(14):1505–17. doi: 10.1200/JCO.19.03136
53. Paz-Ares L, Vicente D, Tafreshi A, Robinson A, Soto Parra H, Mazières J, et al. A randomized, placebo-controlled trial of pembrolizumab plus chemotherapy in patients with metastatic squamous NSCLC: Protocol-specified final analysis of KEYNOTE-407. J Thorac Oncol (2020) 15(10):1657–69. doi: 10.1016/j.jtho.2020.06.015
Keywords: radiotherapy, brain metastasis, NSCLC, bayesian network meta-analysis, neuro-oncology
Citation: Wu M, Jiang J, Zhang X, Chen J, Chang Q and Chen R (2022) RT-based combination therapy for brain metastasis from NSCLC with non-EGFR mutation/ALK gene rearrangement: A network meta-analysis. Front. Oncol. 12:1024833. doi: 10.3389/fonc.2022.1024833
Received: 22 August 2022; Accepted: 07 November 2022;
Published: 28 November 2022.
Edited by:
Ting Xu, University of Texas MD Anderson Cancer Center, United StatesCopyright © 2022 Wu, Jiang, Zhang, Chen, Chang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Jiang, eG5yaGV1bUAxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.