
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 27 October 2022
Sec. Gynecological Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.1024173
This article is part of the Research Topic Methods in Gynecological Oncology View all 10 articles
Superficial myofibroblastoma (SMF) of the lower female genital tract is a relatively rare benign mesenchymal tumor. The diagnosis is usually challenging as it shares several similar clinicopathological features with other tumors. Herein, we present a case of a 71-year-old Chinese female patient with postmenopausal vaginal bleeding. Colposcopy imaging revealed a well-circumscribed mass in the vagina with a wide pedicle, resembling a mushroom. The patient underwent surgery, and the tumor was histologically diagnosed as SMF. To the best of our knowledge, this is the first report of colposcopic imaging of a superficial vaginal myofibroblastoma. In this case study, we review the clinicopathological features of SMF of the lower female genital tract reported in the literature to improve the understanding of the disease.
In 2001, Laskin et al. described a distinctive tumor of the cervix and vagina, which they named “superficial cervicovaginal myofibroblastoma (SCVM)” (1). In 2005, Ganesan et al. proposed that the term ‘‘superficial myofibroblastoma (SMF) of the lower female genital tract’’ be used instead of SCVM as some neoplasms have a vulvar location (2, 3). SMFs of the lower female genital tract are an unusual type of benign mesenchymal tumor (4). Only 57 cases have been reported in the literature, among which 43 cases arose in the vagina; the clinical manifestations were not specific, and the lesions were not typical, which makes pre-pathological diagnoses of patients challenging. Here, we present the diagnosis and treatment of a patient with superficial vaginal myofibroblastoma presenting with postmenopausal vaginal bleeding. To further clarify the location, scope, and nature of the lesion, we performed colposcopy on the patient prior to surgery. In addition, we review the literature on the clinicopathological features of this distinctive tumor.
The patient was a 71-year-old postmenopausal woman (gravida 2, para 2). On December 17, 2020, the patient visited our hospital for “a small amount of vaginal bleeding for 1 day.” Upon gynecological examination, a 2.0 cm × 2.0 cm mass was found in the upper part of the right wall of the vagina, protruding from the vaginal wall with a smooth surface. On December 22, 2020, a pelvic ultrasound showed endometrial thickening (0.7 cm) and an uneven echo. A 1.2 cm × 0.6 cm × 0.5 cm mass was observed on the anterior wall of the vagina, with a fuzzy boundary, and a hypoechoic inside with multiple strong echoes. Color Doppler flow imaging (CDFI) could detect a few blood flow signals. On December 23, 2020, pelvic enhancement magnetic resonance (MR) was performed, which indicated endometrial thickening and uneven internal signal and enhancement. An oval equal T1 mixed T2 signal shadow was observed on the anterior wall of the upper segment of the vagina, approximately 1.7 cm × 1.4 cm × 1.9 cm, with obvious ring reinforcement (Figures 1A-C). We suggested that the patient be hospitalized for surgery, but the patient was not hospitalized in time owing to atrial fibrillation. Therefore, the patient was regularly reviewed using pelvic ultrasound while treating her atrial fibrillation. Ultrasound showed that the vaginal mass gradually increased in size, and there was effusion with a medium echo mass in the uterine cavity. On December 11, 2021, a pelvic ultrasound showed that the thickness of the single-layer endometrium was approximately 0.3 cm, and the echo was uneven. A liquid area with a depth of approximately 0.7 cm was observed in the uterine cavity. The mass on the anterior wall of the vagina grew to be 2.1 cm × 1.5 cm × 1.4 cm in size. CDFI could detect blood flow signals (Figures 1D-F).
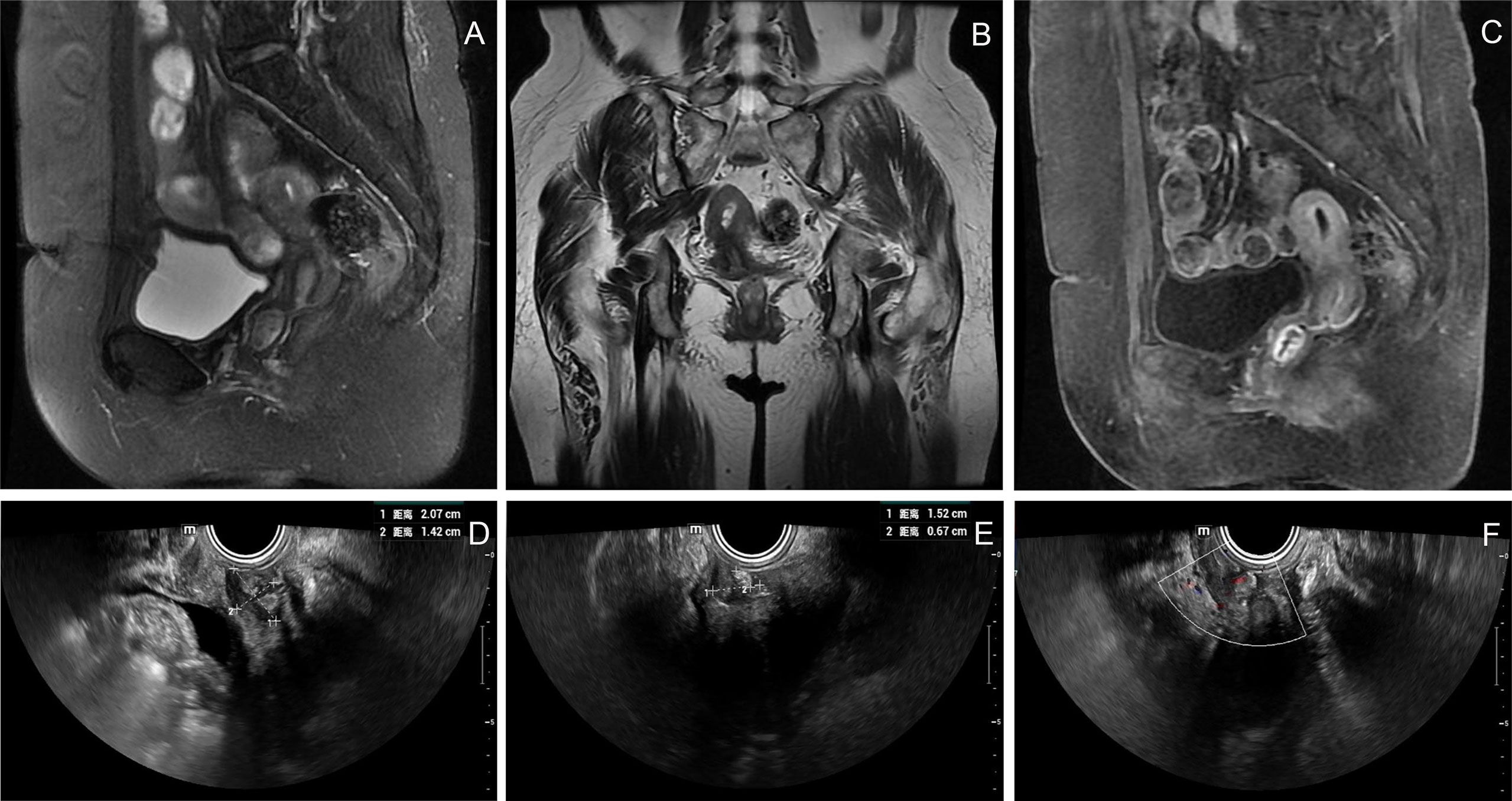
Figure 1 Imaging and ultrasound findings of the patient. (A–C) Pelvic enhancement MR: an oval equal T1 mixed T2 signal shadow was observed on the anterior wall of the upper segment of the vagina, approximately 1.7 cm × 1.4 cm × 1.9 cm, with obvious ring reinforcement; (D–F) Pelvic ultrasound: a 2.1 cm × 1.5 cm × 1.4 cm mass was observed on the anterior wall of the vagina, with a fuzzy boundary and a hypoechoic inside with multiple strong echoes. CDFI could detect blood flow signals.
The patient did not visit our department for hospitalization until June 22, 2022. She was not on any current medications and had no history of tamoxifen or hormonal therapy. On June 23, 2022, the pelvic ultrasound showed that the mass on the anterior wall of the vagina increased to 2.9 cm × 1.7 cm × 1.5 cm in size. Before surgery, we performed a colposcopy to further clarify the location, scope, and nature of the mass. Under the colposcope, a mass was observed on the right anterior wall of the upper part of the vagina, approximately 3.0 cm × 3.0 cm × 1.0 cm in size, pink, tough in texture, smooth on the surface, small vesicles visible inside the mass, a wide pedicle connected to the vaginal wall, and the shape similar to a mushroom (Figures 2A-C). There was no significant change in the tumor epithelium after staining with 5% acetic acid (Figures 2D, E), and the tumor epithelium appeared brownish-black after staining with 5% Lugol’s solution (an iodine-containing solution) (Figure 2F). Therefore, we speculated that this mass was a benign tumor with normal squamous epithelium on its surface. On June 28, 2022, the patient underwent vaginal mass resection and hysteroscopic endometrial polypectomy under general anesthesia. The vaginal mass was completely removed during the surgery, and the frozen section was pathologically determined to be benign. The patient recovered well and was discharged on the third postoperative day. The pathological results of the vaginal wall tumor indicated that it was a superficial vaginal myofibroblastoma. The gross pathological examination showed that the size of the tumor was approximately 3.2 cm × 2.8 cm × 0.8 cm, the texture was tough and slightly soft, and the color was pink white (Figure 3A). Microscopic examination revealed that the tumor tissue was located under the squamous epithelium (Figure 3B) and was composed of spindle and stellate-shaped cells and contained abundant thin-walled blood vessels, with some mast cells scattered in the tissue (Figures 3C, D). Immunohistochemical analysis showed that the tumor was positive for caldesmon, desmin, CD34, estrogen receptor (ER), and progesterone receptor (PR) (Figures 3E-I); was focally positive for α-smooth muscle actin (SMA) (Figure 3J); and was approximately 3% positive for Ki-67 (Figure 3K) and negative for cytokeratin (CK), epithelial membrane antigen (EMA), and S-100 (Figures 3L-N). The scattered mast cells were positive for CD117 (Figure 3O). No abnormalities were found in the outpatient follow-up 1 month postoperatively.
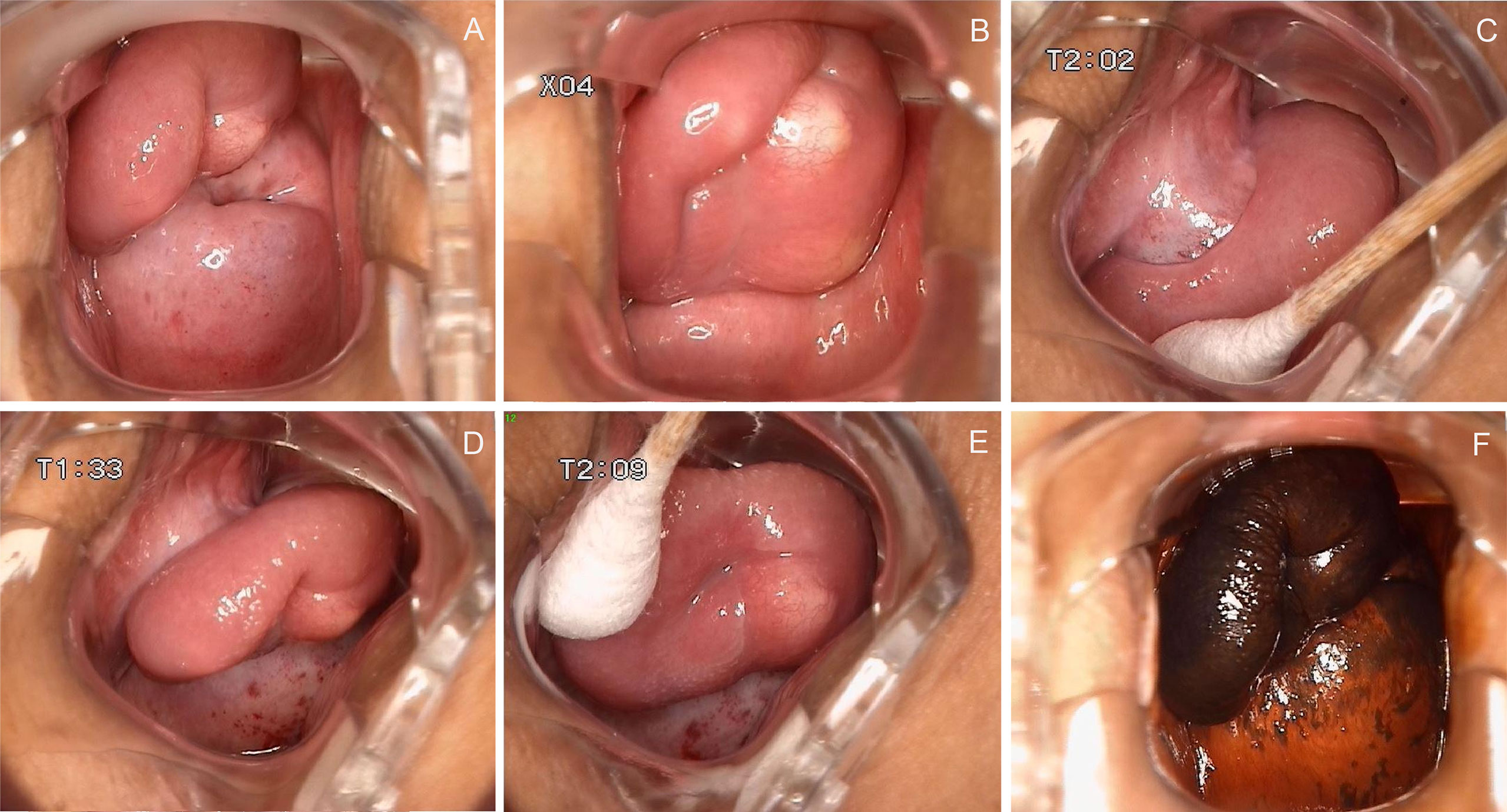
Figure 2 Colposcopy findings of the patient. (A–C) A mass was observed on the right anterior wall of the upper part of the vagina, approximately 3.0 cm × 3.0 cm × 1.0 cm in size, pink, tough in texture, smooth on the surface, small vesicles visible inside the mass, a wide pedicle connected to the vaginal wall, and the shape similar to a mushroom; (D, E) There was no significant change in the tumor epithelium after staining with 5% acetic acid; (F) The tumor epithelium appeared brownish-black after staining with 5% Lugol’s solution.
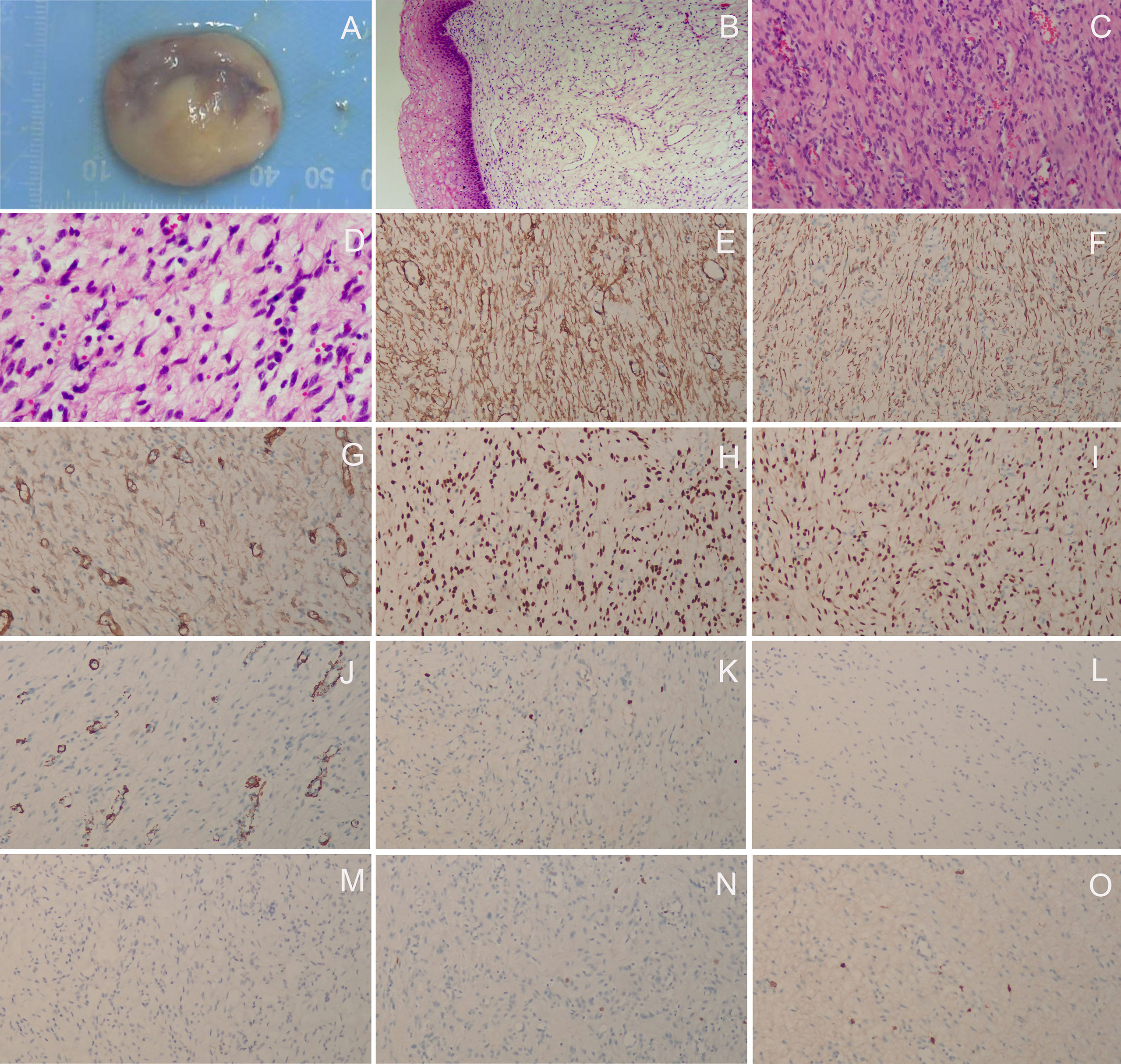
Figure 3 Histopathological and immunohistochemical staining findings. (A) The gross pathological examination showed that the size of the tumor was approximately 3.2 cm × 2.8 cm × 0.8 cm, the texture was tough and slightly soft, and the color was pink white; (B) The tumor tissue was located under the squamous epithelium (H&E staining, ×40); (C, D) The tumor tissue was composed of spindle and stellate-shaped cells and contained abundant thin-walled blood vessels, with some mast cells scattered in the tissue (H&E staining, C, ×100; D, × 200); (E) caldesmon (+), (F) desmin (+), (G) CD34 (+), (H) ER (+), (I) PR (+), (J) αSMA (focally +), (K) Ki-67 (approximately 3% +), (L) CK (–), (M) EMA (–), (N) S-100 (–); (O) The scattered mast cells were positive for CD117 (E-O, ×100).
SMF has been recognized as a mesenchymal neoplasm arising from the superficial portion of the lower genital tract in women (5). The exact etiology and pathogenic mechanisms underlying tumor development remain unclear (5, 6). Liu et al. suggested no association between an SMF tumor and infection with human papilloma virus, Epstein-Barr virus, or human herpesvirus 8 (6). Many gynecologists and pathologists may not be familiar with the disease owing to its rarity. We performed a PubMed-indexed, English-language literature search that yielded 57 reported cases of SMF of the lower female genital tract (Table 1). Among them, 75.4% (43/57) occurred in the vagina, 12.3% (7/57) in the cervix, and 12.3% (7/57) in the vulva. The five non-classical myofibroblastoma cases reported by Magro et al. (9) were excluded because it was not clear if the tumors were histologic variants or other types of myofibroblastoma. In the reported cases, 19.5% (8/41) of patients had been taking tamoxifen for whom a history of drug use was available, raising the possibility of a hormone-responsive neoplasm.
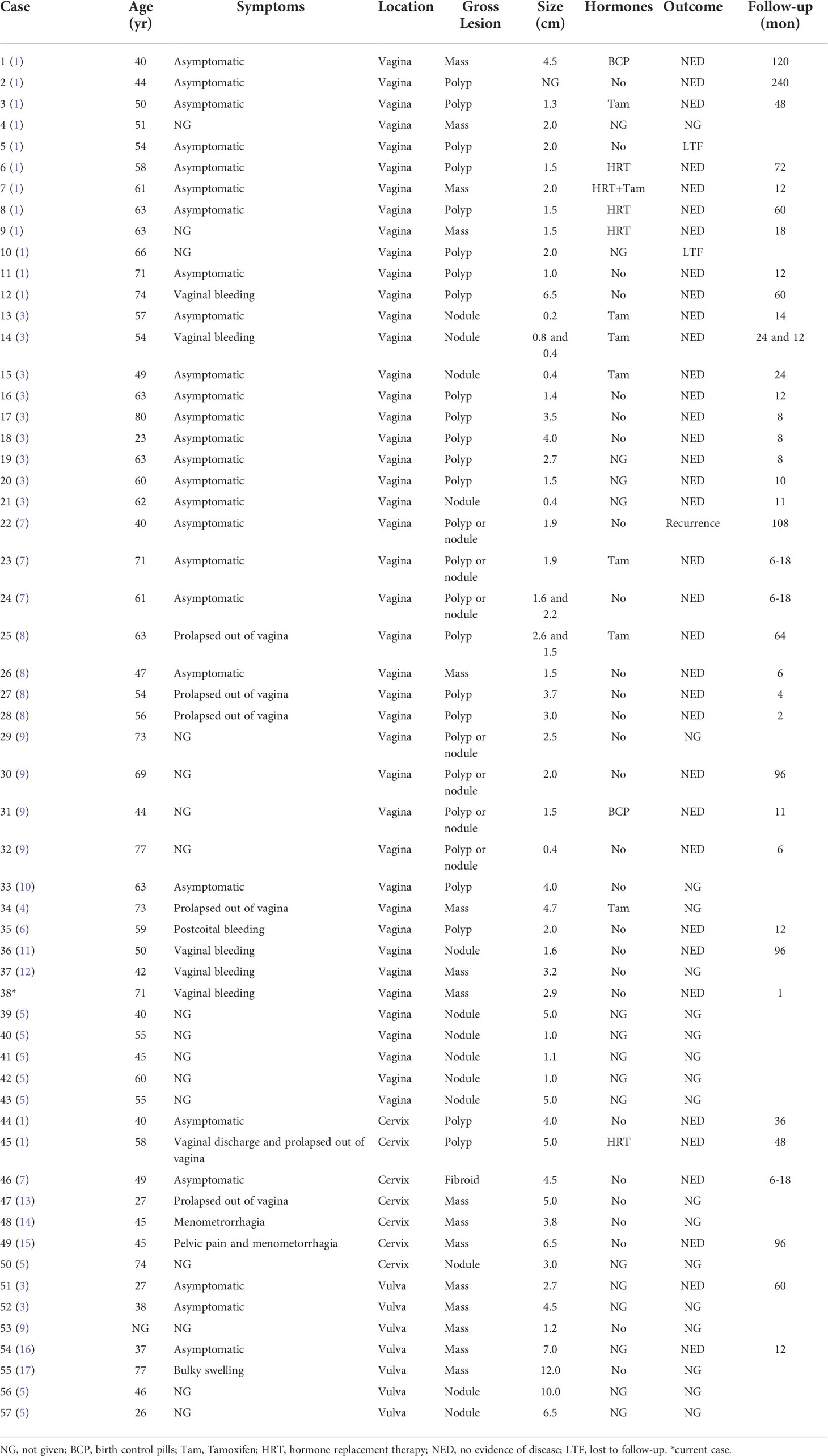
Table 1 Cases of superficial myofibroblastoma of the lower female genital tract in the English language literature.
The symptoms in patients with SMF were non-specific. The main clinical manifestations were asymptomatic polypoid or nodular masses of varying sizes (0.2–12 cm). Occasionally, the mass may prolapse out of the vagina or manifest as abnormal vaginal bleeding. Only five of the 57 patients had two lesions, and the rest had single lesions. The patients ranged in age from 23–80 years, with a mean age of 54.7 and a median age of 55.5 years. Only six patients were younger than 40 years of age, and only one of them was pregnant. In our case, a vaginal mass was found during gynecological examination due to postmenopausal vaginal bleeding, which may have been caused by endometrial polyps.
The diagnosis of SMF of the lower female genital tract is usually challenging because it is rare and shares many clinicopathological features with other mesenchymal tumors, such as fibroepithelial stromal polyps, angiomyofibroblastoma, mammary-type myofibroblastoma, cellular angiofibroma, and aggressive angiomyxoma (12, 18). The diagnosis should be combined with gynecological examination, ultrasound, and MRI, and the final diagnosis still depends on histopathology. Histological examination shows a well-circumscribed, yet unencapsulated lesion, covered by unremarkable or hyperplastic squamous epithelium (19). Tumors usually present with spindle and stellate-shaped cells within a collagenous stroma, showing an expansive growth pattern with a grenz zone of uninvolved tissue (12). Multiple patterns, including lace-like, sieve-like, and fascicular, are characteristic features, as are myxoid or edematous foci, and few mitotic figures (19). Cells are positive for vimentin and usually for desmin. CD34 and αSMA are positive in some cases, and most neoplasms are positive for ER and PR. Tumors are negative for S100, EMA, and CK (19). The case described in this report is consistent with the above histopathological features, and the histopathological features of other mesenchymal lesions have been described in detail previously (12, 18, 19).
As described in the literature, SMF often presents as a well-circumscribed polypoid or nodular mass. Our case also presented as a nodular mass, and we used colposcopy to magnify the lesion to visualize a more intuitive manifestation of this mass. Simultaneously, we observed a wide pedicle that connected the mass to the vaginal wall, similar to that reported by Tomita et al. (12) and Adams et al. (13). Owing to the small number of cases, it is unclear whether this phenomenon is unique to SMF. To the best of our knowledge, this is the first report of colposcopy as an aid in the diagnosis and evaluation of SMF. Colposcopy is a procedure in which a lighted, magnifying instrument called a colposcope is used to examine the cervix, vagina, and vulva (20). Colposcopy is important in the diagnosis of cervical lesions. It also plays a significant role in the diagnosis of vaginal lesions, because the shape, location, and scope of the lesions can be clearly observed, and the reaction of the lesion epithelium to 5% acetic acid and Lugo’s solution can be applied in this small space. In the present case, no abnormal changes were observed after the use of acetic acid or Lugol’s solution. Dysplastic cells dehydrate and turn densely white with the application of acetic acid (20). Lugol’s solution may also be used to highlight the dysplastic area, where the dysplastic cells remain yellow owing to a lack of absorption of the solution (20). Although definitive diagnosis should be based on histopathological assessment, colposcopic features such as well-defined morphology and coverage with normal squamous epithelium may contribute to differentiating from more aggressive entities such as malignant tumors.
Surgical resection is the main clinical treatment for SMF; among 37 patients with available follow-up information (1 month to 20 years), only one case had local recurrence 9 years after initial incomplete excision (7), indicating a good prognosis and low recurrence rate, although long-term follow-up is still recommended (7, 11). The patient in this case study completed the treatment and remained in good condition without recurrence.
SMF of the lower female genital tract is a relatively rare benign mesenchymal tumor most likely to occur in the vagina. The age range of SMF patients is wide; however, it mainly occurs in perimenopausal and postmenopausal women. Here, we report the diagnosis and treatment of superficial vaginal myofibroblastoma in a postmenopausal woman. For the first time, colposcopy was used for auxiliary diagnosis and evaluation before surgery. The lesion was covered with normal squamous epithelium with a wide pedicle and a mushroom-like appearance. The patient had a good prognosis and experienced no recurrence after surgical treatment.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Institutional Review Board of Shengjing Hospital of China Medical University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
YW was responsible for drafting of the manuscript. MS analyzed the literature. JW was responsible for the data collection and critical revision of the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the Shengjing Hospital of China Medical University 345 Talent Project (No. 30B).
We would like to thank the patient for her permission to present this case report to sensitize practitioners. We also thank all the medical staff who participated in the diagnosis and treatment of this patient.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Laskin WB, Fetsch JF, Tavassoli FA. Superficial cervicovaginal myofibroblastoma: fourteen cases of a distinctive mesenchymal tumor arising from the specialized subepithelial stroma of the lower female genital tract. Hum Pathol (2001) 32(7):715–25. doi: 10.1053/hupa.2001.25588
2. McCluggage WG. A review and update of morphologically bland vulvovaginal mesenchymal lesions. Int J Gynecol Pathol (2005) 24(1):26–38. doi: 10.1097/01.pgp.0000148336.77264.6e
3. Ganesan R, McCluggage WG, Hirschowitz L, Rollason TP. Superficial myofibroblastoma of the lower female genital tract: Report of a series including tumours with a vulval location. Histopathology (2005) 46(2):137–43. doi: 10.1111/j.1365-2559.2005.02063.x
4. Smith SA, Doyle V, Rutherford E, Elliot V, Blaquiere RM. Superficial myofibroblastoma of the lower female genital tract with description of the MRI features. BJR Case Rep (2017) 3(1):20160052. doi: 10.1259/bjrcr.20160052
5. Tajiri R, Shiba E, Iwamura R, Kubo C, Nawata A, Harada H, et al. Potential pathogenetic link between angiomyofibroblastoma and superficial myofibroblastoma in the female lower genital tract based on a novel MTG1-CYP2E1 fusion. Mod Pathol (2021) 34(12):2222–8. doi: 10.1038/s41379-021-00886-8
6. Liu JL, Su TC, Shen KH, Lin SH, Wang HK, Hsu JC, et al. Vaginal superficial myofibroblastoma: a rare mesenchymal tumor of the lower female genital tract and a study of its association with viral infection. Med Mol Morphol (2012) 45(2):110–4. doi: 10.1007/s00795-011-0566-z
7. Stewart CJ, Amanuel B, Brennan BA, Jain S, Rajakaruna R, Wallace S. Superficial cervico-vaginal myofibroblastoma: a report of five cases. Pathology (2005) 37(2):144–8. doi: 10.1080/00313020500058284
8. Wang QF, Wu YY, Wang J. Superficial cervicovaginal myofibroblastoma: report of four cases and literature review. Chin Med J (Engl) (2010) 123(8):1093–6. doi: 10.3760/cma.j.issn.0366-6999.2010.08.022
9. Magro G, Caltabiano R, Kacerovská D, Vecchio GM, Kazakov D, Michal M. Vulvovaginal myofibroblastoma: expanding the morphological and immunohistochemical spectrum. a clinicopathologic study of 10 cases. Hum Pathol (2012) 43(2):243–53. doi: 10.1016/j.humpath.2011.04.027
10. Olinici CD, Crişan D, Zolog A, Puşcaş M. Vaginal superficial myofibroblastoma. case report and review of the literature. Rom J Morphol Embryol (2007) 48(2):165–70.
11. Atinga A, El-Bahrawy M, Stewart V, Bharwani N. Superficial myofibroblastoma of the genital tract: a case report of the imaging findings. BJR Case Rep (2019) 5(1):20180057. doi: 10.1259/bjrcr.20180057
12. Tomita Y, Takabayashi E, Yuzawa S, Okizaki A. Superficial myofibroblastoma of the vagina with a stalk: Case report of a rare vaginal tumor with notable radiological findings. Radiol Case Rep (2021) 16(12):3690–4. doi: 10.1016/j.radcr.2021.08.064
13. Adams B, Fogarty P, McKenna M, McManus D. Superficial myofibroblastoma of the lower female genital tract: First case report of a pregnant patient. J Obstet Gynaecol (2008) 28(6):657–8. doi: 10.1080/01443610802421668
14. Abdelaziz M, Eziba N, Sharma S, Kleven D, Al-Hendy A. Cervical superficial myofibroblastoma: Case report and review of the literature. SAGE Open Med Case Rep (2017) 5:2050313x17726936. doi: 10.1177/2050313x17726936
15. Cinel L, O'Hara B, Prestipino A. Superficial myofibroblastoma of the lower female genital tract in the uterine cervix showing focal pseudosarcomatous morphology. Pathology (2009) 41(7):691–3. doi: 10.3109/00313020903305837
16. Peng WX, Wada R, Kure S, Fukunaga M, Naito Z. Superficial myofibroblastoma in the vulva mimicking aggressive angiomyxoma: A case report and review of the literature. Case Rep Pathol (2019) 2019:1582714. doi: 10.1155/2019/1582714
17. Patrizi L, Borelli B, Di Prete M, Bruno V, Mauriello A, Piccione E, et al. A rare case of vulvar superficial myofibroblastoma associated with ambigous and unusual differential diagnosis. Gynecol Oncol Rep (2020) 34:100637. doi: 10.1016/j.gore.2020.100637
18. Schoolmeester JK, Fritchie KJ. Genital soft tissue tumors. J Cutan Pathol (2015) 42(7):441–51. doi: 10.1111/cup.12507
19. McCluggage WG. Recent developments in vulvovaginal pathology. Histopathology (2009) 54(2):156–73. doi: 10.1111/j.1365-2559.2008.03098.x
Keywords: superficial myofibroblastoma, vagina, mesenchymal tumor, colposcope, diagnosis
Citation: Wang Y, Sun M and Wang J (2022) Superficial vaginal myofibroblastoma with mushroom-like appearance: A case report with colposcopic findings and literature review. Front. Oncol. 12:1024173. doi: 10.3389/fonc.2022.1024173
Received: 21 August 2022; Accepted: 17 October 2022;
Published: 27 October 2022.
Edited by:
Federico Ferrari, University of Brescia, ItalyReviewed by:
Brannan B. Griffin, Vanderbilt University Medical Center, United StatesCopyright © 2022 Wang, Sun and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiao Wang, d2FuZ2ppYW84ODExMzBAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.