- 1Department of Otolaryngology, Head and Neck Surgery, Beijing Tongren Hospital, Capital Medical University, Key Laboratory of Otolaryngology Head and Neck Surgery, Ministry of Education, Capital Medical University, Beijing, China
- 2Department of Otolaryngology, the First Affiliated Hospital of Anhui University of Chinese Medicine, Hefei, Anhui, China
Objective: Adenoid cystic carcinoma of the head and neck mainly occurs in the major salivary glands, of which the parotid gland and submandibular gland are the most common. The purpose of this study was to clarify the site-specific differences in prognosis and molecular expression characteristics of the patients and to achieve stratified risk management of the clinical prognosis.
Materials: By performing a single-centre retrospective analysis combined with analyses of the Surveillance, Epidemiology, and End Results (SEER) database, cBioPortal and GEO databases, the clinical prognostic characteristics and the differences in molecular expression patterns of ACC in the submandibular gland and parotid gland were analysed. Cox regression analysis, the chi-square test, Fisher’s test and the log-rank test were used to compare the significance of differences.
Results: Compared with patients with parotid gland ACC, the submandibular gland ACC is more likely to have metastases in the cervical lymph node (21.7% vs. 3.3%) and shows a higher rate of distant metastasis within 1 year after the primary site diagnosis (47.8% vs. 23.3%), a worse overall prognosis, more frequent mutations of MYB/MYBL1 (50% vs. 25%) and abnormal upregulation of the phosphatidylinositol-3 kinase (PI3K) pathway.
Conclusions: Submandibular gland ACC is associated with site-specific early cervical lymph node metastasis and hidden distant metastasis, along with rapid progression and a poor prognosis. A high MYB/MYBL1 mutation rate and abnormal upregulation of the PI3K pathway with MYB involvement were identified.
1. Introduction
Adenoid cystic carcinoma (ACC), a rare malignant tumor originating from secretory glands, occurs most commonly in the head and neck. The main clinical features of ACC are relentless and slow growth, perineural invasion, and a high distant metastasis rate. ACC is composed of the epithelial and myoepithelial cells, which are arranged into three pathological subtypes: cribriform, tubular and solid (most of which are mixed). Therefore, the pathological manifestation of ACC is biphasic differentiation. Surgery combined with radiotherapy is the conventional treatment for the primary tumor. Distant metastasis developed in 52% of patients, mainly within the first 5 years following diagnosis, and the median time to metastasis was only 31.5 months (1). The most common site of distant metastasis is the lung, accounting for 67-85.9% of cases, followed by the bone and liver (2, 3). The 5-year overall survival rate(OS) is 68%, and once metastasis occurs, the median survival time is only 20-32 months (4). The special pathological subtypes (solid or high-grade transformation), T/N stages, and treatment of the primary sites are common clinical risk factors for ACC lung metastasis (5–8). The high incidence rate and uncontrollable continuous progression of distant metastasis are challenges in the treatment of this disease.
Achieving population stratification by screening high-risk factors and adopting different clinical intervention measures are very important. Renata Ferrarotto (2021) analysed two ACC molecular subtypes defined by MYC and P63 using RNA sequencing (RNA-seq) and revers-phase protein microarray (RPPA). The high-risk type of ACC-I showed substantial overexpression of MYC and MYC target genes, mRNA splicing, and enrichment for NOTCH-activating mutations, resulting in shorter median survival than patients with ACC-II (3.44 years vs. 23.2 years) (9). In addition to molecular typing, clinical characteristics might also identify high-risk groups for simple and effective risk stratification and management.
The parotid gland and submandibular gland are the most common salivary glands in the head and neck (64.8%) (10). The salivary glands site was a prognostic risk factor in the nomogram model developed by Xiaoli Mu (11), but the specific classification of salivary glands was not performed. Jason Tasoulas et al. found that the overall prognosis of patients with submandibular gland ACC in stage IV was worse than that of patients with ACC in the parotid gland and minor salivary glands (10), but the specific differences in the clinical prognosis presentation and molecular expression patterns were not clarified. We further confirmed whether the prognosis of patients with submandibular gland ACC and parotid gland ACC differed in a large multicentre sample, and analysed the reasons for the difference. Therefore, based on the retrospective case data from our hospital combined with the SEER, cBioPortal and GEO data, we analysed the specific clinical manifestations and molecular characteristics of submandibular gland ACC by comparing it with parotid gland ACC which has a similar gene expression background and acinar structure with submandibular gland (12).
2. Materials and methods
2.1. Data sources
2.1.1. SEER database
The dataset “Incidence-SEER Research Plus Data, 18 Registries, Nov 2020 Sub (2000-2018)” was downloaded from the SEER database using SEER Stat 8.4.0 software. Case screening criteria were based on the study by Jason Tasoulas et al. (10), as follows:
1. The screened tumor type was ACC in the classification of salivary gland tumors determined by the World Health Organization (WHO). The SEER database code was based on the International Classification of Cancer Diseases, the third edition (ICD-O-3) system (ACC = 8200), and patients with ACC were initially screened.
2. The main specific information included age, sex, race, primary site, TNM stage, surgery (primary site and cervical lymph node), radiotherapy, chemotherapy, SEER cause-specific death classification, survival in months, and extent of disease—SEER Combined Mets to DX-lung/liver/bone/brain.
3. As TNM staging was not available in the data before 2004, data collected after 2004 were selected. Cases from 2004 to 2009 were graded according to the American Joint Committee on Cancer (AJCC) 6th edition, while cases from 2010 and later were graded according to the AJCC 7th edition. The staging criteria for major salivary gland tumors were not significantly revised in the 7th edition; cases classified according to the 6th and 7th editions of the AJCC were merged. Invalid cases, which have multiple primary tumors, ambiguous survival time, or non-specific death classification, were removed. (Figure 1).
2.1.2. Collection of clinical data from patients
A retrospective analysis was performed on patients (including outpatients and inpatients) who were diagnosed with head and neck ACC in Beijing Tongren Hospital from January 2005 to March 2022, and the primary sites were the parotid gland and submandibular gland. The basic information was complete. Clinical and pathological characteristics, such as sex, symptoms at first diagnosis, TNM stage (based on the eighth edition of AJCC), treatment method, pathological grade, perineural invasion, and the Ki67 index, were collected. The pathological grading criteria used by Szanto et al. (13) were uniformly modified to grade I-II and grade III. Pulmonary metastases were diagnosed by two or more lung CT reports (at intervals of 3 months or more) with the persistent progression of pulmonary nodules and after the exclusion of other diseases, and the diagnosis of extrapulmonary metastasis was made by performing a PET-CT evaluation.
2.1.3. Mutation and RNA-seq analyses
Data on mutations in parotid and submandibular glands ACC, including the mutation frequency and mutation type, were obtained from the cBioPortal database (https://www.cbioportal.org/). ACC was searched and the following datasets were selected: (J Clin Invest 2019), (Fmi. Am J Surg Pathl.2014), (JHU, Cancer Prev Res 2016), (MDA. Clin Cancer Res 2015), (MGH. Nat Gen 2016), (MSKCC. NAT Genet 2013), and (Sanger/Mda.jCI 2013). The information collected included the site, age, histological type, and perineural invasion. The histological type was uniformly modified into grade I-II and grade III according to (13) the pathological grading criteria. The mutational landscape was mapped using Microsoft Office Home and Student 2019. The parotid gland and submandibular gland were selected as the “Tumor Disease Anatomic Site”. “Cancer Gene” was screened as defined by OncoKB from mutated genes/structural variant genes, and the number of mutations in each sample should be greater than 2.
The GSE88804 and GSE34816 datasets were screened in the GEO database and corrected for batch effects, and the parts were selected as parotid gland and submandibular gland ACC for the differential expression analysis. The differential expression analysis was performed using R 4.2.0 and the R limma package (|log fold change| > 0.585, P value < 0.05). Differentially expressed genes were selected for a Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis using the HIPLOT enrichment database (public/db/kegg/hsa_kegg_20220424.rds) (https://hiplot.com.cn/). Bubble charts were drawn using the web tool Sangerbox3.0 (http://vip.sangerbox.com/home.html). Gene set enrichment analysis (GSEA) was performed using a web tool (http://www.webgestalt.org/). Venn diagrams were constructed and analysed using the web tool (https://hiplot.com.cn/basic/).
2.1.4. Statistical analysis
Cox regression analysis, the chi-square test, Fisher’s test, and the log-rank test were performed on the data using SPSS 22.0 and GraphPad Prism 8.0 software. Survival curves were drawn using the Kaplan-Meier method. P<0.05 (*), P<0.01 (**), and P<0.001 (***) indicate a significant difference. The web tool Sangerbox3.0 was used to draw the forest map (http://vip.sangerbox.com/home.html).
3. Results
3.1. Epidemiological characteristics and prognosis of patients with submandibular gland ACC based on the SEER database
After downloading the dataset and performing the initial screening (Figure 1, Exclusion criteria 1), a total of 5077 ACC patients were screened, among which the patients with ACC of the major salivary glands accounted for 34%. The submandibular gland (41.0%, 712/1738) and parotid gland (48.2%, 838/1438) were the most common sites of tumors in the major salivary glands (Figure 2A). The age group with the highest proportion of ACC in the submandibular gland was 40-54 years old, and the incidence of ACC in the submandibular gland was higher in younger patients (P<0.05, Figure 2B, Table S1). The incidence rate of ACC in the major salivary glands and submandibular gland in females was approximately 1.5 times that in males, and white people were the most frequently affected (Figures 2C, D).
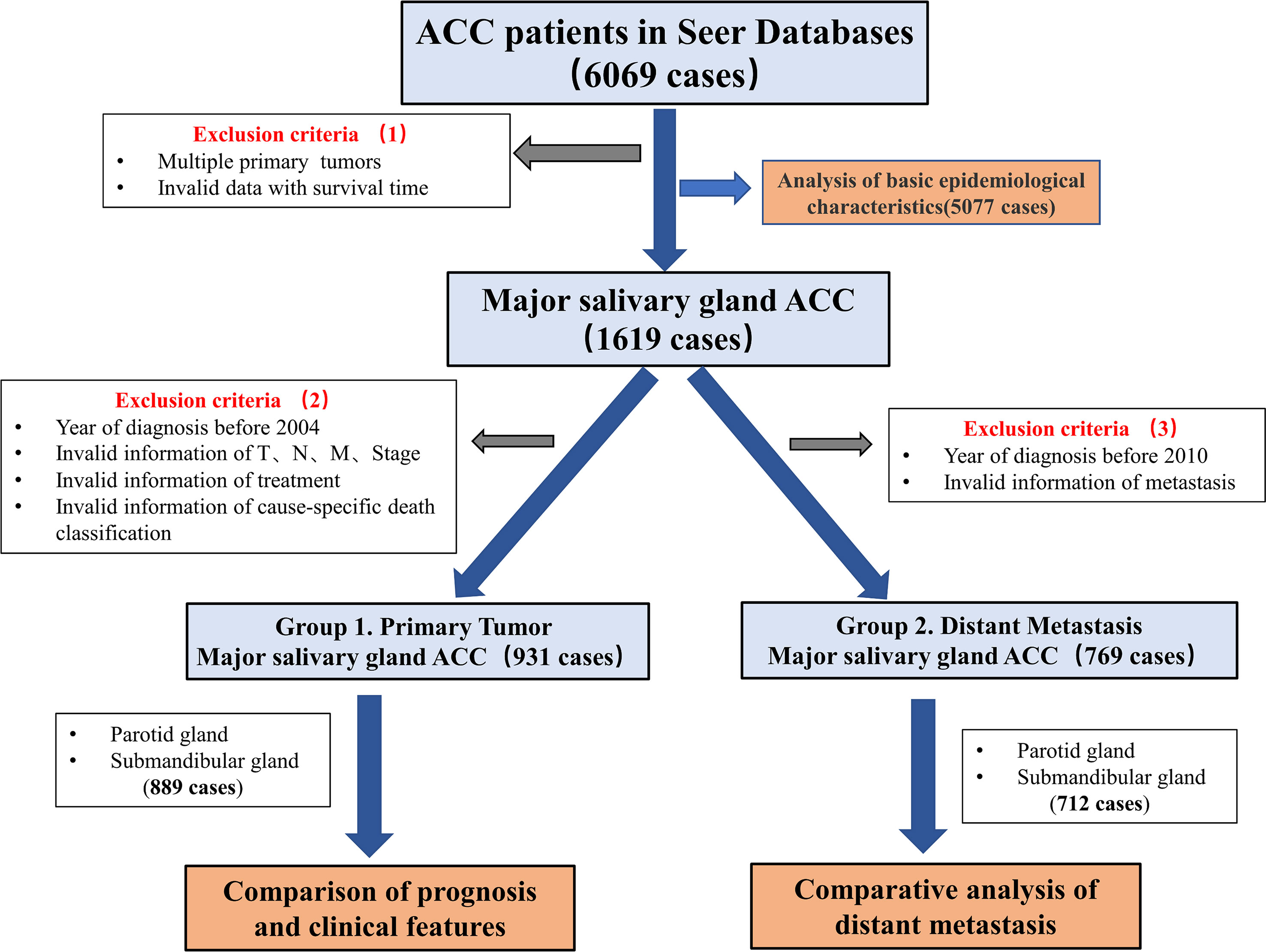
Figure 1 Flow chart showing the screening process for data from the SEER database. The dataset “Incidence-SEER Research Plus Data, 18 Registries, Nov 2020 Sub (2000-2018)”, including a total of 6069 patients with ACC, was selected based on screening criteria 1. The basic epidemiological characteristics of 5077 ACC patients with complete information were analysed. Then, a total of 1619 patients with major salivary glands ACC were selected. According to screening criteria 2, patients with missing basic clinical characteristics were excluded from the 1619 patients for the analysis of the differences in clinical characteristics to determine the prognosis. According to screening criteria 3, 769 patients were selected from the original 1619 patients, and distant metastasis was analysed.
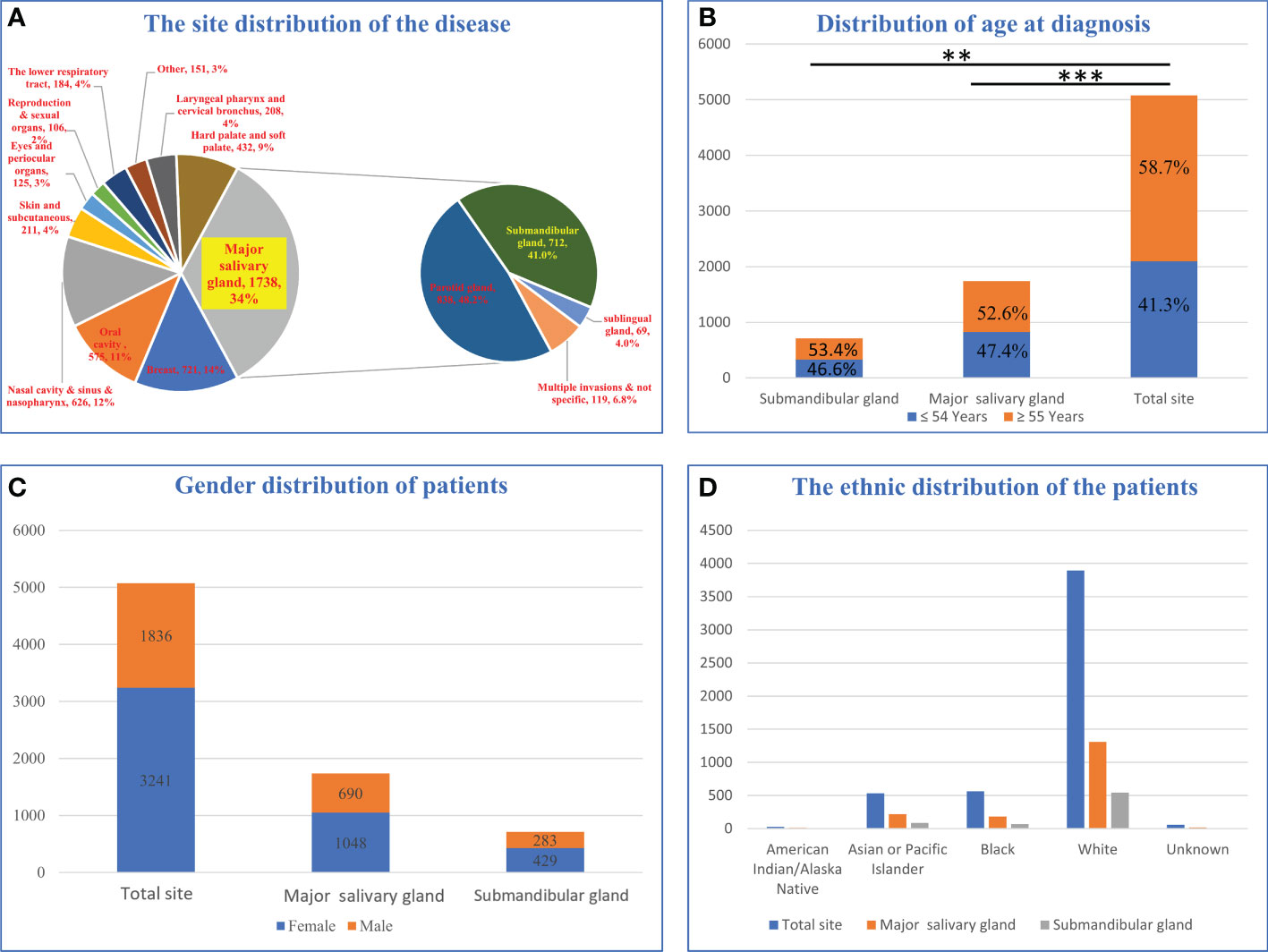
Figure 2 Epidemiological analysis of patients with submandibular gland ACC. (A) Distribution of disease sites in 5077 patients. (B) Age distribution of submandibular gland ACC compared with the major salivary glands and all parts of the body. (C) Sex distribution of patients with submandibular gland ACC compared with major salivary glands and body parts. (D) The racial distribution of submandibular gland ACC compared with major salivary glands and body parts (** P-value <0.01; *** P-value <0.001).
Two groups of data were obtained after screening based on two criteria (Figure 1). Group 1 was used to compare the clinical characteristics of 931 patients with primary site tumors after removing 42 patients with incomplete data. A total of 889 patients were obtained, including 477 patients with parotid gland ACC and 412 patients with submandibular gland ACC. The median follow-up times were 93 months and 100 months, respectively (the mean follow-up times were 95 months and 100 months, respectively).
The Cox regression analysis showed that age, site, T stage, N stage, M stage and chemotherapy were the related factors affecting the prognosis (P<0.05), and the overall prognosis of patients with submandibular gland ACC was poor (P<0.05) (Figures 3 and 4A). Basic clinical features were analysed and the log-rank test revealed that age, tumor stage, T stage, N stage, M stage, and surgical treatment at the primary site correlated with the prognosis (P<0.05, Table S2). However, differences in the prognosis of patients with parotid gland ACC and submandibular gland ACC were significant only in patients with stage IV tumors (P<0.001, Figure 4B), consistent with the results reported by Jason Tasoulas et al. (10). Further analysis of the distribution characteristics of the stage IV population showed that compared with patients with parotid gland ACC, the proportion of patients with stage T4 submandibular gland ACC was lower (50% vs. 87.8%), while the rates of lymph node metastasis (58.3% vs. 35.4%) and distant metastasis (35.7% vs. 17.1%) were higher (P<0.01, Figure 4C). Compared with patients with parotid gland ACC, patients with submandibular gland ACC showed mainly stage I-III tumors (79.6% vs. 65.6%) and T1-3 tumors (89.8% vs. 69.8%) (Table 1, P<0.05). Distant metastasis was evaluated in Group 2 (post-2010 dataset), including 383 patients with parotid gland ACC (mean follow-up time: 49 months, median follow-up time: 44 months) and 329 patients with submandibular gland ACC (mean follow-up time: 51 months, median follow-up time: 50 months). The overall distant metastasis rate of patients with submandibular gland ACC was higher than that of patients with parotid gland ACC (8.81% vs. 5.22%, Figure 4D), and the lung metastasis rate was higher (7% vs. 3%). In conclusion, compared with parotid gland ACC, submandibular gland ACC has a worse prognosis in general, with a higher rate of distant metastasis, mainly lung metastasis, at an early stage (within 1 year).
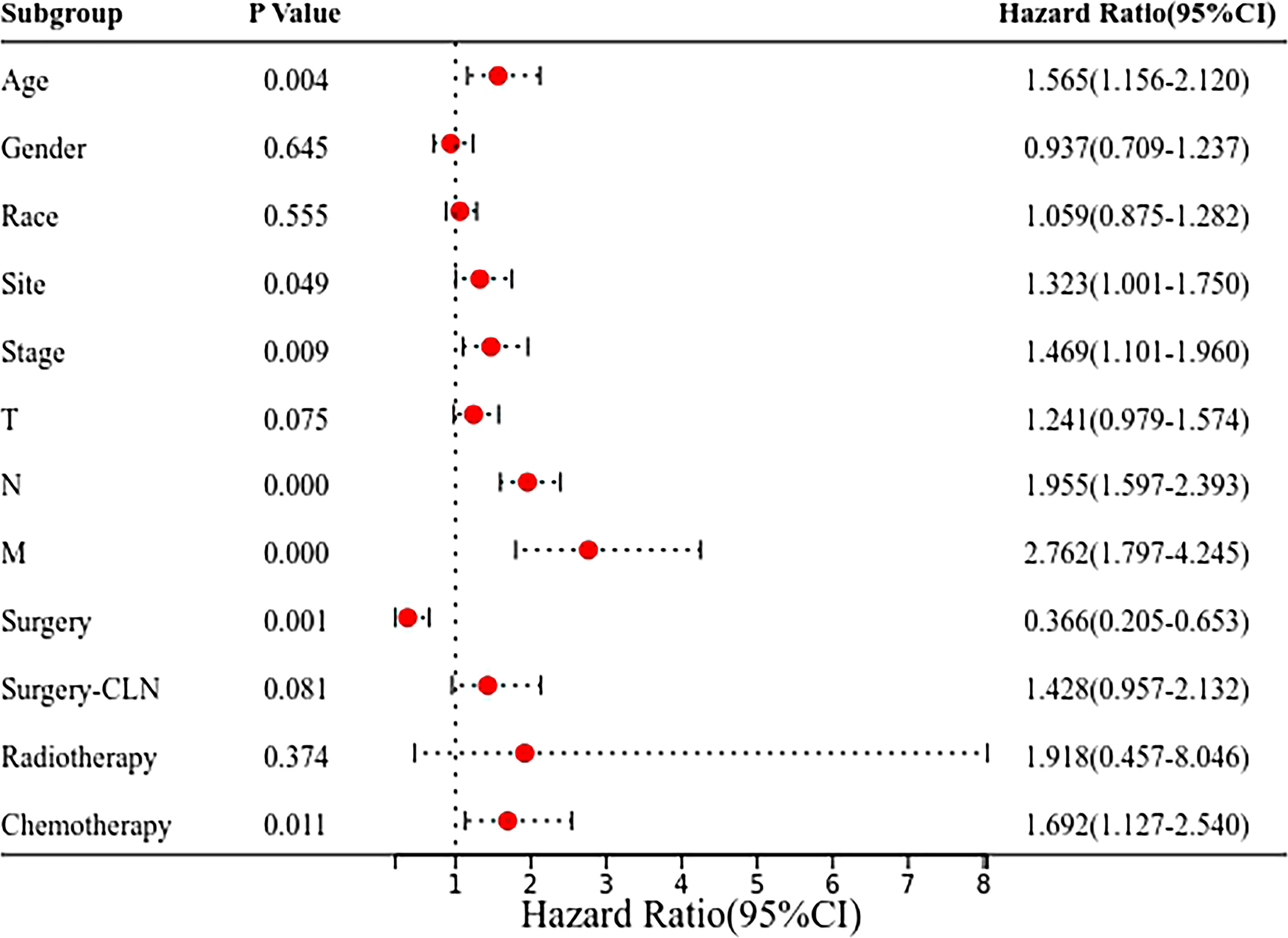
Figure 3 Forest plot of the Cox regression analysis of 899 patients with submandibular/parotid glands ACC in the SEER database. The clinical prognosis of 899 patients with parotid gland and submandibular gland ACC was determined using the Cox regression model to analyse the factors influencing the prognosis, such as the tumor site.
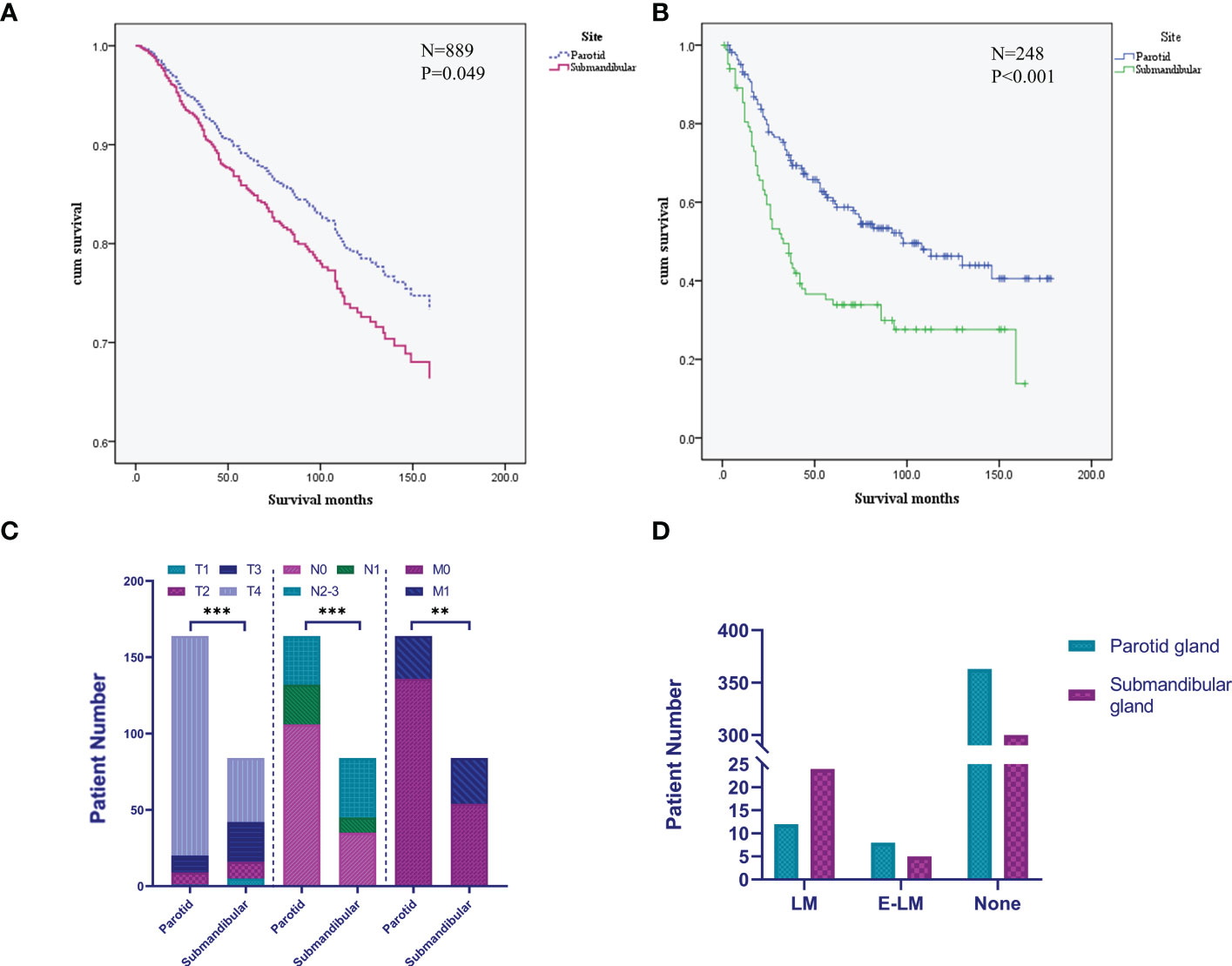
Figure 4 Differences in the prognosis and clinical characteristics of patients with submandibular/parotid gland ACC in the SEER database. (A) Survival curves for patients with submandibular and parotid glands ACC analysed using the Cox regression model. (B) Survival curves for patients with stage IV submandibular and parotid glands ACC analysed using the Kaplan-Meier method. (C) Difference in the distribution of TNM staging in patients with stage IV submandibular and parotid glands ACC. (D) Distribution of distant metastases of submandibular and parotid glands ACC. LM, lung metastasis; E-LM, extrapulmonary metastasis; None, no distant metastasis found (** P-value <0.01; *** P-value <0.001).
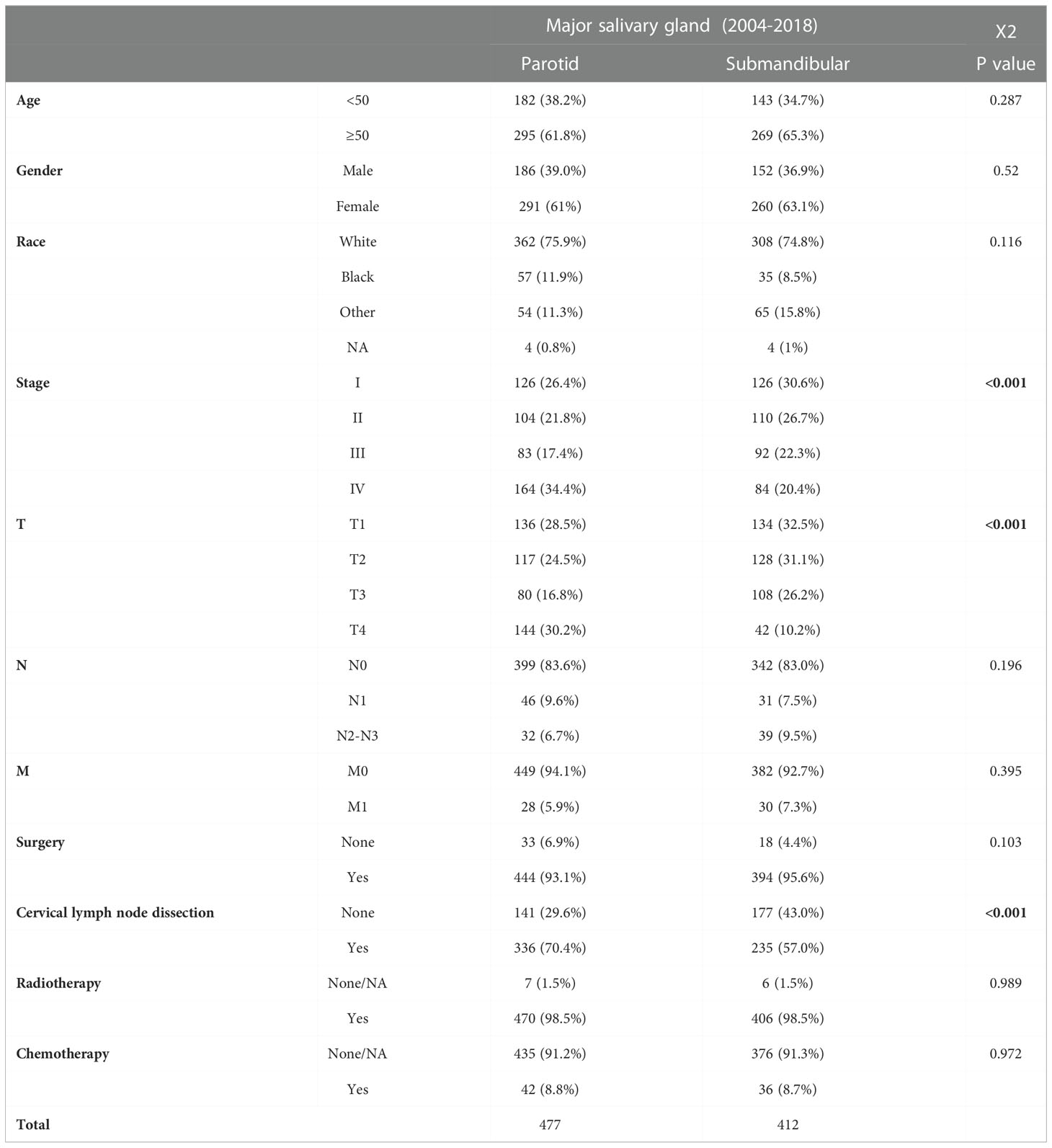
Table 1 Differences in the clinical characteristics of patients with submandibular and parotid glands ACC from the SEER database.
3.2. Clinicopathological features and manifestations of lung metastases of ACC of the submandibular gland (retrospective analysis of patients from a single centre)
Seventy-six patients with ACC (parotid gland ACC (30) and submandibular gland ACC (46)) were included in our analysis. The mean follow-up time was 66 months (median 55 months) for patients with parotid gland ACC and 58 months (median 47 months) for patients with submandibular gland ACC. Compared with patients with parotid gland ACC, more patients with submandibular gland ACC were older than 50 years of age (45.7%, 21/46) and had a higher rate of cervical lymph node metastasis (21.7% vs. 3.3%). No significant differences were observed in the pathological grade, neurotropic growth, Ki67 index or treatment methods (including general treatment, radiotherapy and neck dissection) (Table 2).
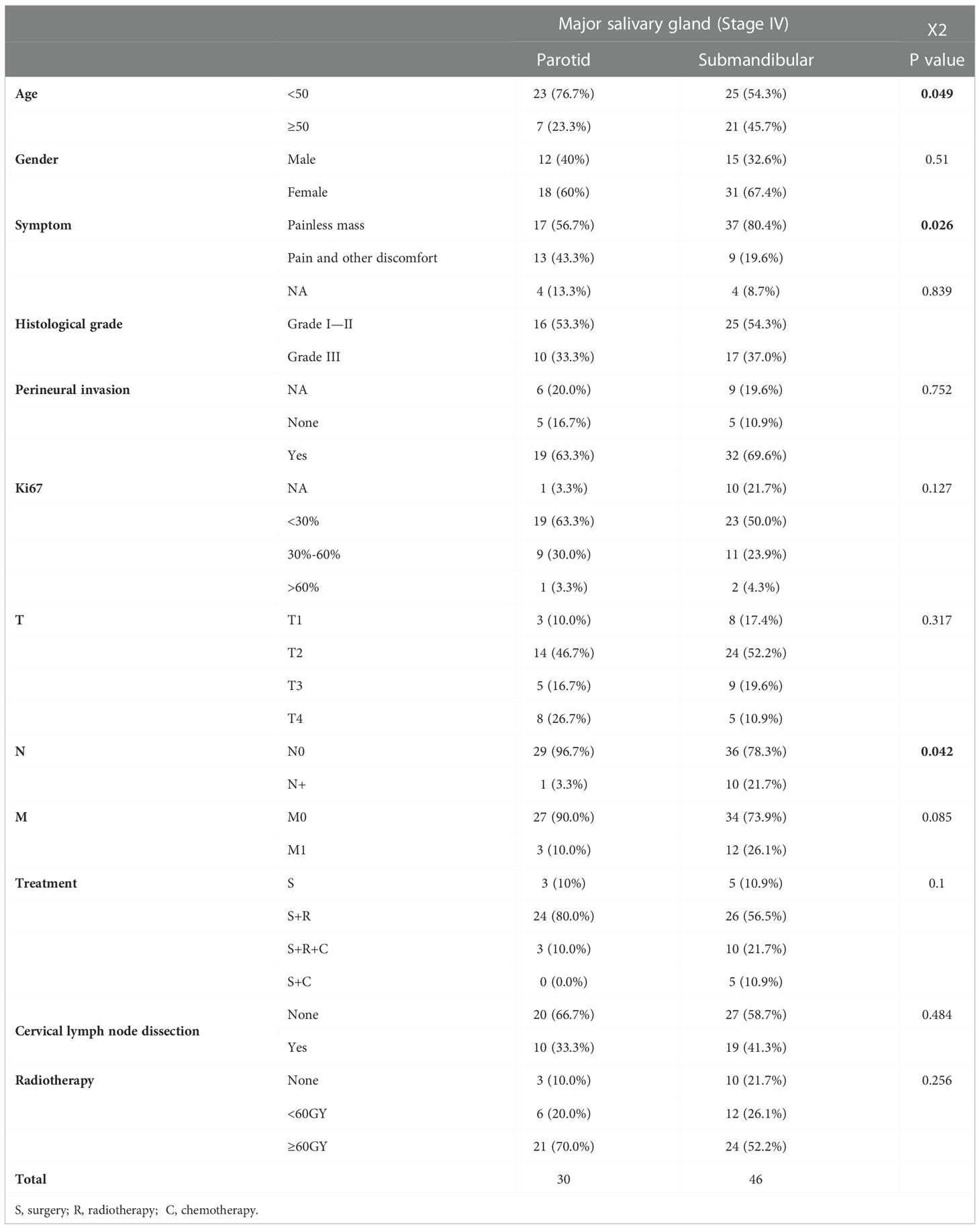
Table 2 Differences in the overall clinical characteristics of patients with submandibular and parotid glands ACC based on a single-centre retrospective analysis.
When comparing distant metastases at the first diagnosis and follow-up, patients with submandibular gland ACC had a mean distant metastasis-free survival (MFS) of 32 months (median 14 months), while those with parotid gland ACC had a mean value of 44 months (median 24 months); however, the log-rank test did not reveal a significant difference (P>0.05, Figure 5A). The rate of distant metastasis in patients with submandibular gland ACC (47.8%) was higher than that in patients with parotid gland ACC (23.3%) within 1 year after the primary diagnosis (P<0.05, Figure 5B). Compared with parotid gland ACC and submandibular gland ACC in the early stage (T1-2), the rate of distant metastasis of submandibular gland ACC was significantly higher at the first diagnosis (P<0.05), and the rate of cervical lymph node metastasis (N+) was slightly higher (Figure 5C).
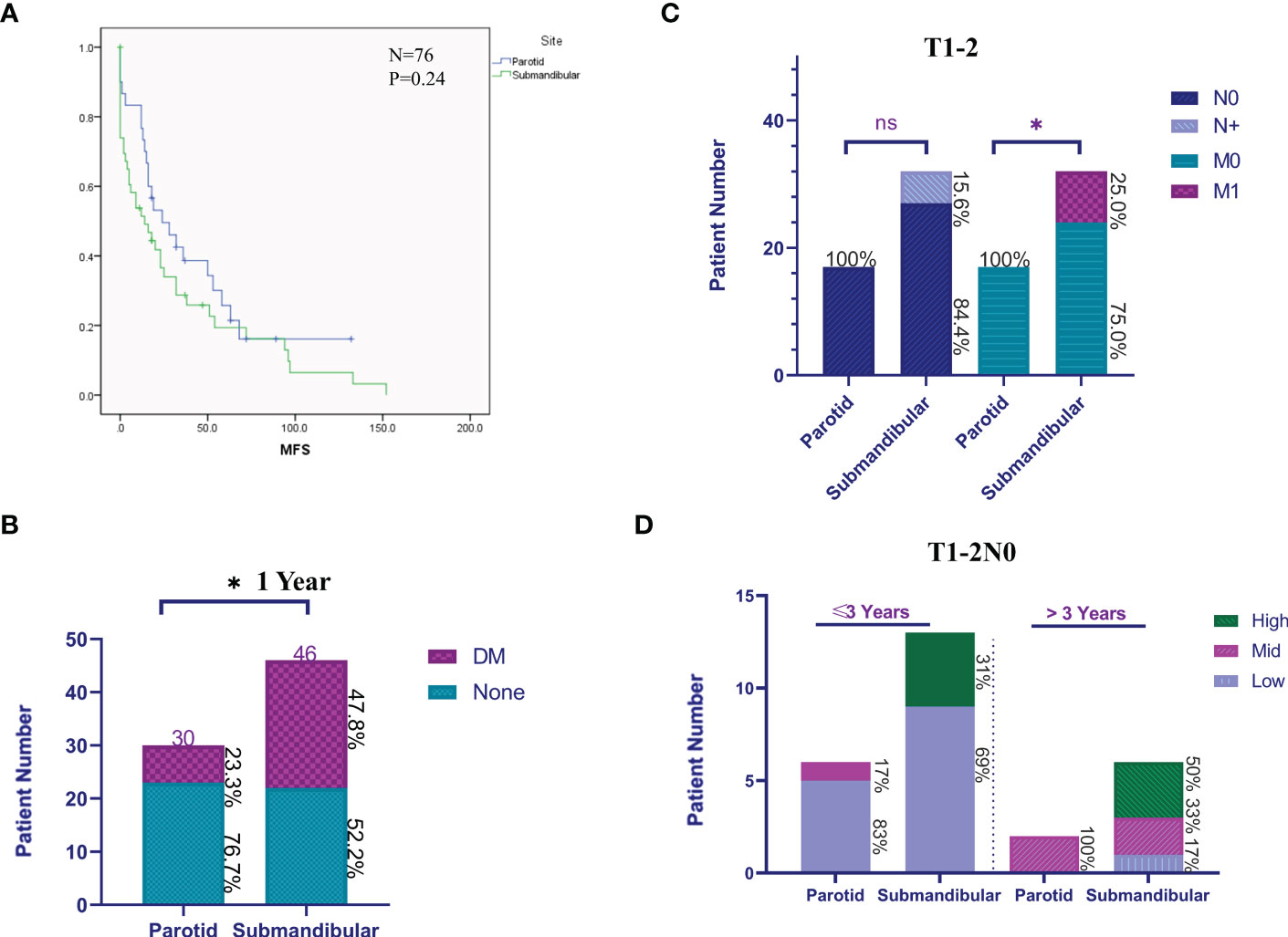
Figure 5 Clinical features of patients with submandibular/parotid glands ACC from a single-centre retrospective analysis. (A) The Kaplan-Meier method was used to analyse the differences in distant metastases from ACC of the submandibular and parotid glands. (B) The difference in distant metastases (DM) within 1 year after the primary diagnosis of submandibular and parotid glands ACC. (C) Cervical lymph node and distant metastasis ratio at the first diagnosis of early (T1-2) submandibular and parotid glands ACC. (D) Early (T1-2N0) differences in the rate of progression of distant metastases of submandibular and parotid glands ACC. High: prognostic high-risk status for distant metastasis; Mid: prognostic medium-risk status for distant metastasis; and Low: prognostic low-risk status for distant metastasis (ns: P-value> 0.05; * P-value <0.05).
We subsequently screened and analysed patients with complete information on distant metastasis identified at the first diagnosis of primary early-stage (T1-2N0) ACC, including 8 patients with parotid gland ACC (62%, 8/17) and 19 patients with submandibular gland ACC (73%, 19/27) to compare the difference in disease status at the time of the first diagnosis of distant metastasis. We identified a significant difference in the risk level of the disease course of the first diagnosis of distant metastasis. The risk classification is as follows: low-risk, multiple nodules in both lungs and the largest diameter is <1 cm, without extrapulmonary metastasis; medium-risk, multiple nodules in both lungs and the largest is 1-3 cm in diameter, without extrapulmonary metastasis; and high-risk, multiple nodules in both lungs with a maximum diameter greater than 3 cm or with extrapulmonary metastasis. The analysis of the distribution of risk grades of distant metastasis in patients with parotid gland ACC and submandibular gland ACC within 3 years and after 3 years showed that the high-risk grade of distant metastasis in patients with submandibular gland ACC was 31% and 50%, respectively, while the high-risk grade of distant metastasis in patients with parotid gland ACC was 0 (Figure 5D). In conclusion, patients with ACC of the submandibular gland are prone to early occult distant metastasis, and the disease progresses rapidly.
3.3. Mutation map and expression characteristics of ACC oncogene in submandibular gland
The dataset was searched using the cBioPortal database, and 22 cases of submandibular gland ACC and 36 cases of parotid gland ACC were screened for gene mutation analysis (Figure 6). The mutant genes were divided into four categories: MYB/MYBL1-NFIB-related fusion genes, Notch pathway-related genes, epigenetic modification-related genes, and others. The MYB/MYBL1-NFIB fusion had a higher mutation frequency in submandibular gland ACC compared with submandibular gland ACC (50% vs. 25%), and the fusion mutation of MYBL1 only appeared in submandibular gland ACC (9%). NOTCH1 mutation did not occur in ACC of the parotid gland, accounting for 9% of ACC of the submandibular gland; meanwhile, mutations in SPEN (a negative regulator of the Notch pathway) did not appear in ACC of the submandibular gland, and the mutation frequency in ACC of the parotid gland was 11%. Epigenetic modification-related genes included ARID1A, ARID5B, SMARCA2, CHD2, and SF3B1. Except for CHD2 (9%), all of them were detected in parotid and submandibular gland ACC, and all of them had mutually exclusive mutations.
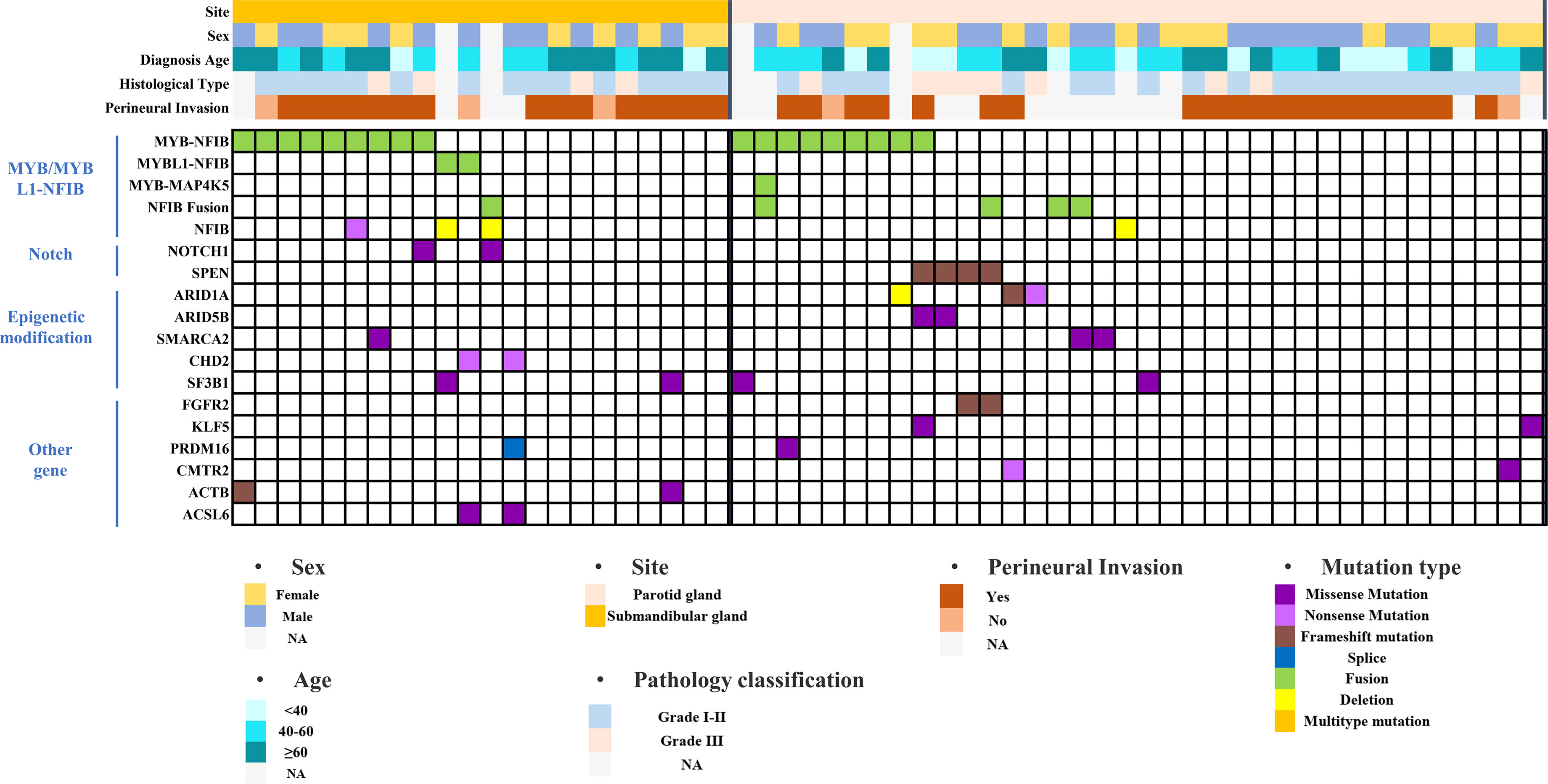
Figure 6 Mutation landscape of submandibular gland and parotid gland ACC based on the cBioPortal database.
We further studied the differences in the molecular expression patterns of ACC tumor tissues in the parotid gland and submandibular gland and analysed the abnormal pathways in submandibular gland ACC by screening upregulated and downregulated genes in 10 cases of submandibular gland ACC and 16 cases of parotid gland ACC from the two GEO datasets. MYB gene expression was significantly upregulated in submandibular gland ACC compared with parotid gland ACC (Figure 7A). KEGG enrichment analysis of genes with P<0.05 and |log FC|>0.585 showed that genes were significantly enriched in the PI3K pathway, including the PDGFA, MYB, ITGA2, FN1, EGF, COL6A3, COL1A2, and COL1A1 genes (Figure 7B). According to GSEA, the activity of the PI3K pathway was upregulated (Figure 7C). Next, we aimed to exclude differences in the expression of genes related to the PI3K pathway in normal parotid and submandibular glands tissues, and 6311 differentially expressed genes (P<0.05, |log FC|>1) in the normal parotid gland and submandibular gland tissues were downloaded from the supplementary materials of the study by Marie Saitou et al. (12) and intersected with the differentially expressed genes in the submandibular gland and parotid gland ACC obtained from the GEO dataset. No intersecting genes were identified (Figure 7D). This result excluded the possibility that differentially expressed genes in tumor genes were caused by differences in the genetic background of normal tissues. Therefore, based on the results from the cBioPortal and GEO databases, submandibular gland ACC has a higher frequency of MYB/MYBL1 mutations, and genes in the PI3K pathway, including MYB, are upregulated.

Figure 7 RNA sequencing results from the submandibular gland and parotid gland ACC based on the GEO database. (A) Volcano plot showing differentially expressed genes. Red dots and green dots indicate upregulated and downregulated genes, respectively, in submandibular gland ACC compared with parotid gland ACC. (B) Bubble diagram of the KEGG enrichment analysis showing the results for differentially expressed genes. The colour and size of bubbles correspond to the P value and the number of enriched genes, respectively. (C) GSEA of differentially expressed genes revealed that the PI3K signaling pathway was upregulated in ACC of the submandibular gland. (D) Venn diagram of gene set intersections: set 1 is the differentially expressed genes in the normal parotid gland and submandibular gland tissues, and set 2 is the differentially expressed genes in the parotid gland and submandibular gland ACC tissues.
4. Discussion
ACC is a rare malignant tumor of glandular origin that may occur systemically but is more common in the head and neck. The development of uncontrolled distant metastases after primary surgery has become a major challenge in disease treatment. Risk stratification based on differences in prognosis is a prerequisite for individualized treatment of the disease. Renata Ferrarotto proposed in 2017 that patients with NOTCH1 mutations should be defined as a population with a poor prognosis who are prone to have extrapulmonary metastasis (14); In 2021, they proposed two risk subtypes defined by MYC and TP63: ACC-I (37%) and ACC-II (63%). High-risk ACC-I is characterized by the enrichment of NOTCH-activating mutations and overexpression of MYC target genes, and mRNA splicing. The continuous improvement of molecular typing is helpful for the precise treatment of diseases, and risk stratification based on clear clinical features can simply and effectively assist with clinical individual treatment. Moreover, molecular typing should be further defined and improved based on molecular expression characteristics in populations with varying clinical prognostic performance.
Identifying the site-specific clinical prognosis of patients with submandibular gland ACC is helpful for simple and effective clinical treatment stratification and an accurate risk assessment. In the nomogram prediction model constructed by Ian Ganly, tumors in the nasal cavity and paranasal sinuses had the highest risk, followed by the major salivary glands, while tumors in the larynx/pharynx/oral cavity had the lowest risk. However, none of the studies further analysed the prognostic risk weights for the three major salivary glands (15, 16). A log-rank univariate analysis performed by Jason Tasoulas using SEER data revealed that the prognosis of patients with submandibular gland ACC was worse than that of patients with parotid gland and minor salivary glands ACC which was only showed in stage IV. But the specific manifestation of the clinical prognosis difference has not been compared and expounded in detail (10). We screened 5077 patients with ACC from the SEER database, a large sample clinical database. Using Cox regression analysis, patients with submandibular gland ACC were found to have a worse prognosis than patients with parotid gland ACC, consistent with the research conclusions described above. Because normal tissues of the parotid and submandibular glands have similar gene expression patterns (12), the submandibular gland ACC can be compared with the parotid gland ACC to determine the specific clinical prognosis and abnormal molecular expression. Similar to the study by Jason Tasoulas et al, the same analysis showed that the prognosis of patients with stage IV submandibular gland ACC was significantly worse than the patients with parotid gland ACC (10). Further analysis and comparison indicated that ACC of the parotid gland was characterized by a higher T stage, while ACC of the submandibular gland was characterized by a higher rate of cervical lymph node metastasis and distant metastasis. Although the log-rank test showed that a higher TNM stage and T stage are high-risk factors, the finding that the proportion of stage IV and T4 stage parotid gland ACC was higher than that in the submandibular gland ACC seems paradoxical, as the parotid gland has a larger space for invasion inside and outside the envelope and is prone to a later T stage. Subsequently, a dataset with complete distant metastasis information was selected for further analysis, and submandibular gland ACC had a higher overall metastasis rate than parotid gland ACC, and the lung was the main site of metastasis. In conclusion, compared with parotid gland ACC, submandibular gland ACC has a higher metastasis rate and worse prognosis.
In addition, the aforementioned study based on the SEER database found that the submandibular ACC was more likely to have lymph node metastasis and earlier distant metastasis than the parotid gland in stage IV. Because clinical staging adopts a mixed grading method compared with TNM staging, it cannot reflect the inherent law of tumor occurrence and development. Moreover, the SEER database lacks the specific time and disease progression status of distant metastasis. Therefore, we expanded the sample size as much as possible in a single-centre retrospective cohort to analyse the specific characteristics of distant metastases in submandibular gland ACC and to analyse the difference in the progression rate of lymph node and distant metastases of submandibular gland ACC compared with parotid gland ACC. Limited by the heterogeneity and the small size of the population, we did not observe a significant difference in the distant metastasis time of the two sites of ACC tumors using the log-rank test; however, within 1 year after diagnosis, submandibular gland ACC had a higher rate of distant metastasis (P<0.05). At the same time, in patients with T1-2 stage, submandibular gland ACC had a higher rate of cervical lymph node metastasis and distant metastasis than parotid gland ACC at the first diagnosis (P<0.05). Further comparison of the metastatic status of patients with T1-2N0 stage parotid gland ACC and submandibular gland ACC showed that patients with submandibular gland ACC had a higher risk level of distant metastasis (pulmonary metastatic nodules >3 cm or extrapulmonary metastasis). In conclusion, compared with patients with parotid gland ACC, patients with submandibular gland ACC have a risk of early occult metastasis and rapid disease progression, resulting in a poor site-specific prognosis.
To further explore the molecular expression characteristics of ACC in the submandibular gland with high metastasis and poor prognosis, we analyzed the gene mutation characteristics based on the cBioPortal dataset and found that there was a high MYB/MYBL1-NFIB fusion ratio in the submandibular gland. Differentially expressed genes were analysed using the GEO database to further verify the differences in expression and abnormal pathways, and we found that the MYB-dominated PI3K pathway was also significantly enriched and upregulated in the submandibular gland ACC. However, the upregulation of genes in the PI3K pathway, including MYB, in the submandibular gland ACC was not due to a difference in expression between normal tissues of the parotid and submandibular glands (Figure 7D). MYB fusion mutations (the most common fusion NFIB) are hallmark molecular events in the development of ACC, with a mutation frequency of 16-100% (17–19), and high expression of MYB is closely related to the poor prognosis (20). Relevant studies have found that the genes of the PI3K pathway amplified and mutated in various tumors, such as breast cancer and gastric cancer, and this signaling pathway plays a role in cell survival, angiogenesis, lymph node metastasis, and distant metastasis (21, 22). In salivary gland cancers, aggressive tumor types have higher genomic alterations in the PI3K pathway (23). Therefore, the upregulation of the PI3K pathway may be the main reason for the higher rate of lymph node and distant metastasis of submandibular gland ACC than parotid gland ACC. One study found that the administration of PI3K inhibitors to the ACC xenograft mouse model effectively reduces the primary tumor burden and lung metastasis (24). Target therapies against abnormal genetic alterations have shown varying degrees of promise in the clinic as precision medicines of ACC.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
Conception and design: MZ and TM. Data analysis: XW, SZ, and GY. Manuscript writing: MZ and TM. Manuscript revision: RS and XC. Funding support: XC. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the National Natural Science Foundation of China (grant no. 82173312) and the Capital Health Development Research Project (grant no. 2022-2-2057).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1021169/full#supplementary-material
Supplementary Table 1 | Analysis of the age distribution of patients with submandibular gland ACC in the SEER database.
Supplementary Table 2 | Overall clinical characteristics of 889 patients with submandibular/parotid glands ACC in the SEER database.
References
1. van Weert S, Bloemena E, van der Waal IC, de Bree R, Rietveld DHF, Kuik JD, et al. Adenoid cystic carcinoma of the head and neck: A single-center analysis of 105 consecutive cases over a 30-year period. Oral Oncol (2013) 49(8):824–9. doi: 10.1016/j.oraloncology.2013.05.004
2. Ouyang DQ, Liang LZ, Zheng GS, Ke ZF, Weng DS, Yang WF, et al. Risk factors and prognosis for salivary gland adenoid cystic carcinoma in southern china: A 25-year retrospective study. Medicine (2017) 96(5):e5964. doi: 10.1097/MD.0000000000005964
3. Bhayani MK, Yener M, El-Naggar A, Garden A, Hanna EY, Weber RS, et al. Prognosis and risk factors for early-stage adenoid cystic carcinoma of the major salivary glands. Cancer (2012) 118(11):2872–8. doi: 10.1002/cncr.26549
4. van der Wal JE, Becking AG, Snow GB, van der Waal I. Distant metastases of adenoid cystic carcinoma of the salivary glands and the value of diagnostic examinations during follow-up. Head Neck (2002) 24(8):779–83. doi: 10.1002/hed.10126
5. Bobbio A, Copelli C, Ampollini L, Bianchi B, Carbognani P, Bettati S, et al. Lung metastasis resection of adenoid cystic carcinoma of salivary glands. Eur J Cardiothorac Surg (2008) 33(5):790–3. doi: 10.1016/j.ejcts.2007.12.057
6. Xu MJ, Wu TJ, van Zante A, El-Sayed IH, Algazi AP, Ryan WR, et al. Mortality risk after clinical management of recurrent and metastatic adenoid cystic carcinoma. J Otolaryngol Head Neck Surg (2018) 47(1):28. doi: 10.1186/s40463-018-0273-z
7. Seok J, Lee DY, Kim WS, Jeong W-J, Chung E-J, Jung YH, et al. Lung metastasis in adenoid cystic carcinoma of the head and neck. Head Neck (2019) 41(11):3976–83. doi: 10.1002/hed.25942
8. Seethala RR, Hunt JL, Baloch ZW, Livolsi VA, Leon Barnes E. Adenoid cystic carcinoma with high-grade transformation: a report of 11 cases and a review of the literature. Am J Surg Pathol (2007) 31(11):1683–94. doi: 10.1097/PAS.0b013e3180dc928c
9. Ferrarotto R, Mitani Y, McGrail DJ, Li K, Karpinets TV, Bell D, et al. Proteogenomic analysis of salivary adenoid cystic carcinomas defines molecular subtypes and identifies therapeutic targets. Clin Cancer Res (2021) 27(3):852–64. doi: 10.1158/1078-0432.CCR-20-1192
10. Tasoulas J, Divaris K, Theocharis S, Farquhar D, Shen C, Hackman T, et al. Impact of tumor site and adjuvant radiotherapy on survival of patients with adenoid cystic carcinoma: A SEER database analysis. Cancers (2021) 13(4):589. doi: 10.3390/cancers13040589
11. Megwalu UC, Sirjani D. Risk of nodal metastasis in major salivary gland adenoid cystic carcinoma. Otolaryngol Head Neck Surg Off J Am Acad Otolaryngol Head Neck Surg (2017) 156(4):660–4. doi: 10.1177/0194599817690138
12. Saitou M, Gaylord EA, Xu E, May AJ, Neznanova L, Nathan S, et al. Functional specialization of human salivary glands and origins of proteins intrinsic to human saliva. Cell Rep (2020) 33(7):108402. doi: 10.1016/j.celrep.2020.108402
13. Szanto PA, Luna MA, Tortoledo ME, White RA. Histologic grading of adenoid cystic carcinoma of the salivary glands. Cancer (1984) 54(6):1062–9. doi: 10.1002/1097-0142(19840915)54:63.0.CO;2-E
14. Ferrarotto R, Mitani Y, Diao L, Guijarro I, Wang J, Zweidler-McKay P, et al. Activating NOTCH1 mutations define a distinct subgroup of patients with adenoid cystic carcinoma who have poor prognosis, propensity to bone and liver metastasis, and potential responsiveness to Notch1 inhibitors. J Clin Oncol (2017) 35(3):352–60. doi: 10.1200/JCO.2016.67.5264
15. Ganly I, Amit M, Kou L, Palmer FL, Migliacci J, Katabi N, et al. Nomograms for predicting survival and recurrence in patients with adenoid cystic carcinoma. Int Collab Study Eur J Cancer (Oxford Engl 1990) (2015) 51(18):2768–76. doi: 10.1016/j.ejca.2015.09.004
16. Mu X, Li Y, He L, Guan H, Wang J, Wei Z, et al. Prognostic nomogram for adenoid cystic carcinoma in different anatomic sites. Head Neck (2021) 43(1):48–59. doi: 10.1002/hed.26443
17. de Almeida-Pinto YD, Costa S, de Andrade BAB, Altemani A, Vargas PA, Abreu LG, et al. t(6;9)(MYB-NFIB) in head and neck adenoid cystic carcinoma: A systematic review with meta-analysis. Oral Dis (2019) 25(5):1277–82. doi: 10.1111/odi.12984
18. Mitani Y, Liu B, Rao PH, Borra VJ, Zafereo M, Weber RS, et al. Novel MYBL1 gene rearrangements with recurrent MYBL1-NFIB fusions in salivary adenoid cystic carcinomas lacking t(6;9) translocations. Clin Cancer Res (2016) 22(3):725–33. doi: 10.1158/1078-0432.CCR-15-2867-T
19. Gonda TJ, Ramsay RG. Adenoid cystic carcinoma can be driven by MYB or MYBL1 rearrangements: New insights into MYB and tumor biology. Cancer Discovery (2016) 6(2):125–7. doi: 10.1158/2159-8290.CD-15-1470
20. Mitani Y, Li J, Rao PH, Zhao Y-J, Bell D, Lippman SM, et al. Comprehensive analysis of the MYB-NFIB gene fusion in salivary adenoid cystic carcinoma: Incidence, variability, and clinicopathologic significance. Clin Cancer Res (2010) 16(19):4722–31. doi: 10.1158/1078-0432.CCR-10-0463
21. Mishra R, Patel H, Alanazi S, Kilroy MK, Garrett JT. PI3K inhibitors in cancer: Clinical implications and adverse effects. Int J Mol Sci (2021) 22(7):3464. doi: 10.3390/ijms22073464
22. Chen Y, Mao B, Peng X, Zhou Y, Xia K, Guo H, et al. A comparative study of genetic profiles of key oncogenesis-related genes between primary lesions and matched lymph nodes metastasis in lung cancer. J Cancer (2019) 10(7):1642–50. doi: 10.7150/jca.28266
23. Ross JS, Gay LM, Wang K, Vergilio JA, Suh J, Ramkissoon S, et al. Comprehensive genomic profiles of metastatic and relapsed salivary gland carcinomas are associated with tumor type and reveal new routes to targeted therapies. Ann Oncol (2017) 28(10):2539–46. doi: 10.1093/annonc/mdx399
Keywords: adenoid cystic carcinoma, lung metastasis, MYB, parotid gland, submandibular gland
Citation: Zhou M, Ma T, Wang X, Zhang S, Yang G, Song R and Chen X (2022) High-risk subtype: Clinical manifestations and molecular characteristics of submandibular gland adenoid cystic carcinoma. Front. Oncol. 12:1021169. doi: 10.3389/fonc.2022.1021169
Received: 17 August 2022; Accepted: 30 November 2022;
Published: 16 December 2022.
Edited by:
Ester Orlandi, National Center of Oncological Hadrontherapy, ItalyReviewed by:
Huan Li, Sun Yat-sen University Cancer Center (SYSUCC), ChinaTobias Ettl, University Medical Center Regensburg, Germany
Copyright © 2022 Zhou, Ma, Wang, Zhang, Yang, Song and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ruohui Song, c29uZ18wNTE1QDEyNi5jb20=; Xiaohong Chen, dHJjaHhoQDE2My5jb20=
†These authors have contributed equally to this work
 Mengjiao Zhou
Mengjiao Zhou Tingyao Ma
Tingyao Ma Xuelian Wang
Xuelian Wang Shujing Zhang
Shujing Zhang Guoliang Yang
Guoliang Yang Ruohui Song
Ruohui Song Xiaohong Chen
Xiaohong Chen